Abstract
We present the discovery in TMC-1 of allenyl acetylene, H2CCCHCCH, through the observation of nineteen lines with a signal-to-noise ratio ~4-15. For this species, we derived a rotational temperature of 7±1K and a column density of 1.2±0.2×1013 cm–2. The other well known isomer of this molecule, methyl diacetylene (CH3C4H), has also been observed and we derived a similar rotational temperature, Tr=7.0±0.3 K, and a column density for its two states (A and E) of 6.5±0.3×1012 cm–2. Hence, allenyl acetylene and methyl diacetylene have a similar abundance. Remarkably, their abundances are close to that of vinyl acetylene (CH2CHCCH). We also searched for the other isomer of C5H4, HCCCH2CCH (1.4-Pentadiyne), but only a3σ upper limit of 2.5×1012 cm–2 to the column density can be established. These results have been compared to state-of-the-art chemical models for TMC-1, indicating the important role of these hydrocarbons in its chemistry.
The rotational parameters of allenyl acetylene have been improved by fitting the existing laboratory data together with the frequencies of the transitions observed in TMC-1.
Keywords: molecular data, line: identification, ISM: molecules, ISM: individual (TMC-1), astrochemistry
1. Introduction
More than 200 different chemical species have been detected in space. Most of them have a large dipole moment which permits an easy search for their rotational transitions through radio astronomical observations. However, only a few pure hydrocarbons, CnHm (with m≥2), have been detected so far, and their role in the chemistry of cold dark clouds is poorly understood. Molecules such as C2H2, C2H4, and C2H6, which lack a permanent dipole moment, are studied through their derivatives, mainly through the replacement of a hydrogen atom by the CN radical. In this context, it is worth noting that while CH2CHCN was detected in the early years of millimeter radio astronomy, its equivalent with the CCH group, vinyl acetylene, has been detected only recently towards the cold dark cloud TMC-1 with an abundance that is twice that of the cyanide derivative (Cernicharo et al. 2021a). This is mainly due to the huge difference in the dipole moments of CH2CHCN (μa=3.821 D, Kraśnicki & Kisiel 2011) and CH2CHCCH (μa=0.43D, Sobolev et al. 1962). Hence, only a few hydrocarbons with a low dipole moment have been found so far in the interstellar medium (ISM). Among them are propene (Marcelino et al. 2007), with a dipole moment of 0.36 D (Lide & Mann 1957), and deuterated methane, which has been tentatively detected towards the low mass protostar IRAS 04368+2557 (Sakai et al. 2012). The later species has a very low dipole moment indeed, 0.0056 D (Ozier et al. 1969). Pure unsaturated hydrocarbons radicals, CnH, and their anions, have moderate to very large dipole moments and have been detected up to n = 8 in interstellar and circumstellar clouds (Cernicharo & Guélin 1996; Remijan et al. 2007; Kawaguchi et al. 2007).
In this letter, we report on the discovery of allenyl acetylene, H2CCCHCCH (also named ethynyl allene), through nineteen well detected rotational transitions. This species was spectroscopically characterised (McCarthy et al. 2020), but never detected in space. It is one of the possible isomers with molecular formula C5H4. We compare the derived abundance with that of other acetylenic species such as CH3C4H (another C5H4 isomer), CH3CCH, and CH2CHCCH (the two latter species are C4H4 isomers). We also searched for another C5H4 isomer, HCCCH2CCH, but only upper limits are obtained. These results are analysed in the context of a state-of-the-art chemical model of a cold dark cloud.
2. Observations
New receivers, which were built within the Nanocosmos project1 and installed at the Yebes 40m radiotelescope, were used for the observations of TMC-1. The Q-band receiver consists of two high electron mobility transistor cold amplifiers, covering the 31.0-50.3 GHz range with horizontal and vertical polarisations. Receiver temperatures vary from 22 K at 32 GHz to 42 K at 50 GHz. Eight 2.5 GHz wide fast Fourier transform spectrometers (FFTs), with a spectral resolution of 38.15 kHz, provide the whole coverage of the Q-band in each polarization. The main beam efficiency varies from 0.6 at 32 GHz to 0.43 at 50 GHz. A detailed description of the system is given by Tercero et al. (2020).
The line survey of TMC-1 (αJ2000 = 4h41m41.9s and δJ2000 = +25°41’27.0”) in the Q-band was performed in several sessions. Previous results for the detection of C3N– and C5N– (Cernicharo et al. 2020b), HC5NH+ (Marcelino et al. 2020), HC4NC (Cernicharo et al. 2020c), and HC3O+ (Cernicharo et al. 2020a) were based on two observing runs performed in November 2019 and February 2020. Two different frequency coverages were achieved, 31.08-49.52 GHz and 31.98-50.42 GHz, in order to check that no spurious spectral ghosts were produced in the down-conversion chain, which downconverts the signal from the receiver to 1-19.5 GHz and then splits it into 8 2.5 GHz bands which are finally analysed by the FFTs. Additional data were taken in October and December 2020. A final observing run was performed in January 2021 to improve the line survey and to further check the consistency of all observed spectral features. These new data have allowed the detection of HC3S+ (Cernicharo et al. 2021b) along with the acetyl cation, CH3CO+ (Cernicharo et al. 2020c), HDCCN (Cabezas et al. 2021), the isomers of C4H3N (Marcelino et al. 2021), and vinyl acetylene (Cernicharo et al. 2021a).
The observations were carried out using frequency switching with a frequency throw of 10 MHz for the first two runs and of 8 MHz for the later ones. The intensity scale, antenna temperature (), was calibrated using two absorbers at different temperatures and the atmospheric transmission model (ATM; Cernicharo 1985; Pardo et al. 2001). Calibration uncertainties have been adopted to be 10 %. The nominal spectral resolution of 38.15 kHz was used for the final spectra. The sensitivity varies across the Q-band from 0.3 to 2.0 mK. All data have been analysed using the GILDAS package2.
3. Results and discussion
The sensitivity of our observations towards TMC-1 (see section 2) is a factor of 10-20 better than in previously published line surveys of this source at the same frequencies (Kaifu et al. 2004). This large improvement has allowed us to detect a forest of weak lines. In fact, it has been possible to detect many individual lines (Marcelino et al. 2021) from molecular species that were reported previously only by stacking techniques (Marcelino et al. 2021). Taking into account the large abundance found in TMC-1 for cyanide derivatives of abundant species, and of the presence of nearly saturated hydrocarbons, such as CH3CHCH2 (Marcelino et al. 2007) and CH2CHCCH (Cernicharo et al. 2021a), we searched for similar species containing CCH such as H2CCCHCCH and HCCCH2CCH. We compared the derived abundances with that of the well know species in this source methyl diacetylene, CH3C4H (MacLeod et al. 1984; Walmsley et al. 1984). Line identifications in this TMC-1 survey were performed using the MADEX catalogue (Cernicharo 2012), the Cologne Database of Molecular Spectroscopy catalogue (CDMS; Müller et al. 2005), and the JPL catalogue (Pickett et al. 1998).
3.1. Allenyl acetylene, H2CCCHCCH
Allenyl acetylene is one of the possible C5H4 isomers. We calculated the relative energies of its three most stable isomers, namely H2CCCHCCH, CH3C4H, and HCCCH2CCH. Structural optimisation calculations for the lowest energy conformers of each isomer were done using the Møller-Plesset post-Hartree-Fock method (Møller & Plesset 1934) and explicitly electron correlation effects through perturbation theory up to the second and the Dunning’s consistent polarised valence triple-ζ basis set (MP2/cc-pVTZ) (Dunning 1989). These calculations were performed using the Gaussian 09 programme package (Frisch et al. 2009). Our results show that CH3C4H is the global minimum, while H2CCCHCCH and HCCCH2CCH lie at 1.44 kJ mol–1 and 1.62 kJ mol–1, respectively, over CH3C4H.
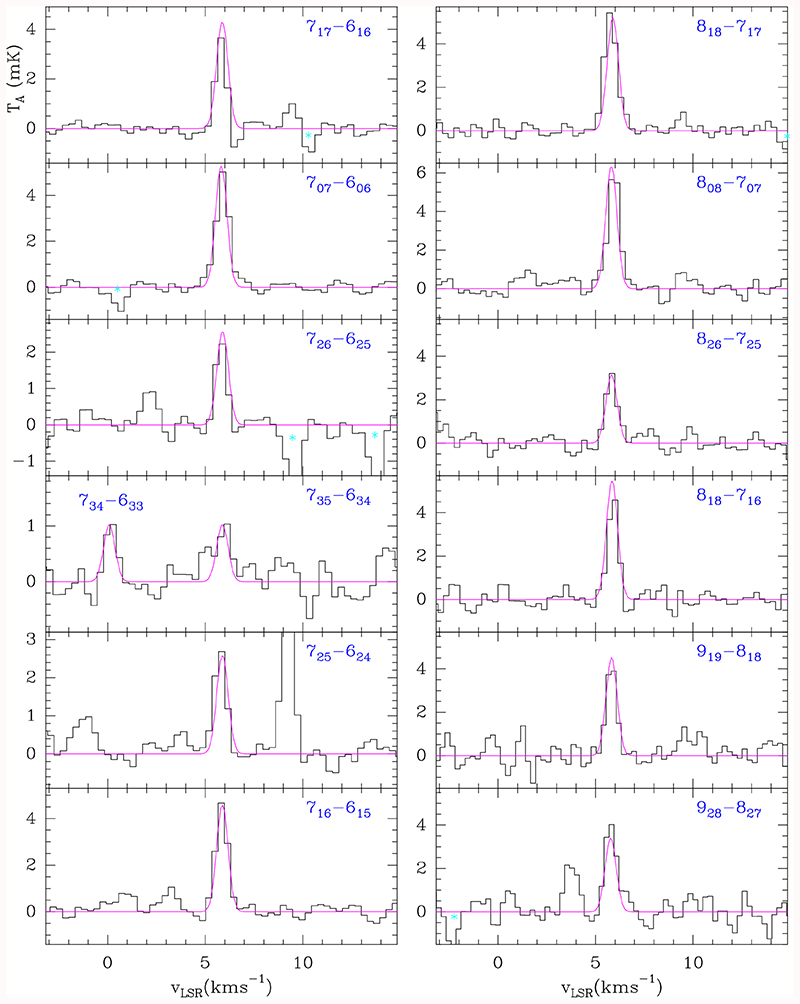
Spectroscopic constants for H2CCCHCCH were derived from a fit to the lines reported by McCarthy et al. (2020) and implemented in the MADEX code (Cernicharo 2012). Nineteen lines with Ka=0, 1, 2, and 3 have been detected in TMC-1. A selected sample of them is shown in Fig. 1. Derived frequencies and line parameters are given in Table A.1. All lines of allenyl acetylene that are not blended with lines from other species and with expected intensities above 1 mK were detected in our survey. Four additional features, with expected intensities of 1-3 mK, fall in the middle of a forest of lines produced by H2CCN (Cabezas et al. 2021) so that deriving their frequencies and intensities was unreliable.
Fig. 1.
Observed transitions of H2CCCHCCH towards TMC-1. The abscissa corresponds to the rest frequency of the lines assuming a local standard of rest velocity of the source of 5.83 km s–1. Frequencies and intensities for the observed lines are given in Table A.1. The ordinate is the antenna temperature, corrected for atmospheric and telescope losses, in millikelvin. The violet line shows the computed synthetic spectrum for this species for Tr=7 K and a column density of 1.2×1013 cm–2. Cyan stars indicate the position of negative features produced in the folding of the frequency switching observations.
In order to compute column densities, we calculated the electric dipole moment components of H2CCCHCCH at the MP2/cc-pVTZ level of theory. The |μa| and |μa| derived values are 0.630 D and 0.011 D, respectively. They are in agreement with those previously reported by Lee & McCarthy (2019), which were obtained using a density functional theory level of theory (M05/6-31G(d)).
An analysis of the observed intensities through a rotational diagram provides a rotational temperature of 9±1K. We performed a model fitting directly to the observed line profiles as described by Cernicharo et al. (2021a), with the result that the best match between the computed synthetic spectrum and the observations was obtained for Tr=7K and N(H2CCCHCCH)=(1.2±0.2)×1013 cm–2. Figure 1 shows the synthetic spectrum. Only the transition 919 – 81,8 required a correction intensity by a factor of 0.8, while for all the other lines the model matches the observations perfectly. Using the H2 column density derived by Cernicharo et al. (1987), the abundance of H2CCCHCCH relative to H2 towards TMC-1 is 1.2×10–9. This abundance is similar to that of vinyl acetylene (Cernicharo et al. 2021a), about ~3 below that of propylene (CH3CHCH2; Marcelino et al. 2007), and a factor of ten below that of methyl acetylene (CH3CCH; Cabezas et al. 2021). Hence, allenyl acetylene is one of the most abundant hydrocarbons in TMC-1, and probably, together with CH3C4H (see Sect. 3.2), the most abundant compound containing five carbon atoms. It is interesting to compare the abundance of allenyl acetylene with that of cyano allene (H2CCCHCN). This species has been recently analysed by Marcelino et al. (2021), resulting in a rotational temperature of 5.5±0.3 K and a column density of (2.7±0.2)×1012 cm–2. Consequently, the abundance ratio of H2CCCHCCH over H2CCCHCN is ~4.5, that is to say the acetylenic derivative of allene is slightly more abundant than the cyanide one. A similar value (~1.8) was obtained for the abundance ratio between CH2CHCCH and CH2CHCN (Cernicharo et al. 2021a).
The measured frequencies of the lines observed in TMC-1 can be used to improve the rotational and distortion constants of H2CCCHCCH. We used the fitting code FITWAT described in Cernicharo et al. (2018). Table 1 provides the results obtained by fitting the laboratory data of McCarthy et al. (2020) alone, and those obtained from a fit to the merged laboratory plus the TMC-1 frequencies. A significant improvement in the uncertainty in the rotational and distortion constants was obtained. The fit to the laboratory data alone results in exactly the same constants as those obtained by McCarthy et al. (2020). The merged fit is recommended to predict the frequency of the rotational transitions of this species above 50 GHz. Predictions could be accurate enough up to 150 GHz to allow for a search of this species in the 3-mm domain. Table B.1 provides the observed and calculated frequencies and their differences.
Table 1. Rotational and distortion constants of H2CCCHCCH.
| Constant | Laboratorya | This work |
| A (MHz) | 25963.54(166) | 25961.178(785) |
| B (MHz) | 2616.375797(314) | 2616.376200(221) |
| C (MHz) | 2412.573364(286) | 2412.573306(194) |
| ΔJ (kHz) | 1.15462(391) | 1.15734(112) |
| ΔJK (kHz) | -85.5217(356) | -85.4904(279) |
| δJ (kHz) | 0.28161(457) | 0.28598(132) |
| Number of lines | 14 | 33 |
| σ(kHz) | 1.0 | 8.5 |
| Jmax, Kamax | 5, 2 | 10,3 |
| νmax (GHz) | 25.144 | 49.218 |
Notes.
Laboratory frequencies from McCarthy et al. (2020).
3.2. Methyl diacetylene, CH3C4H
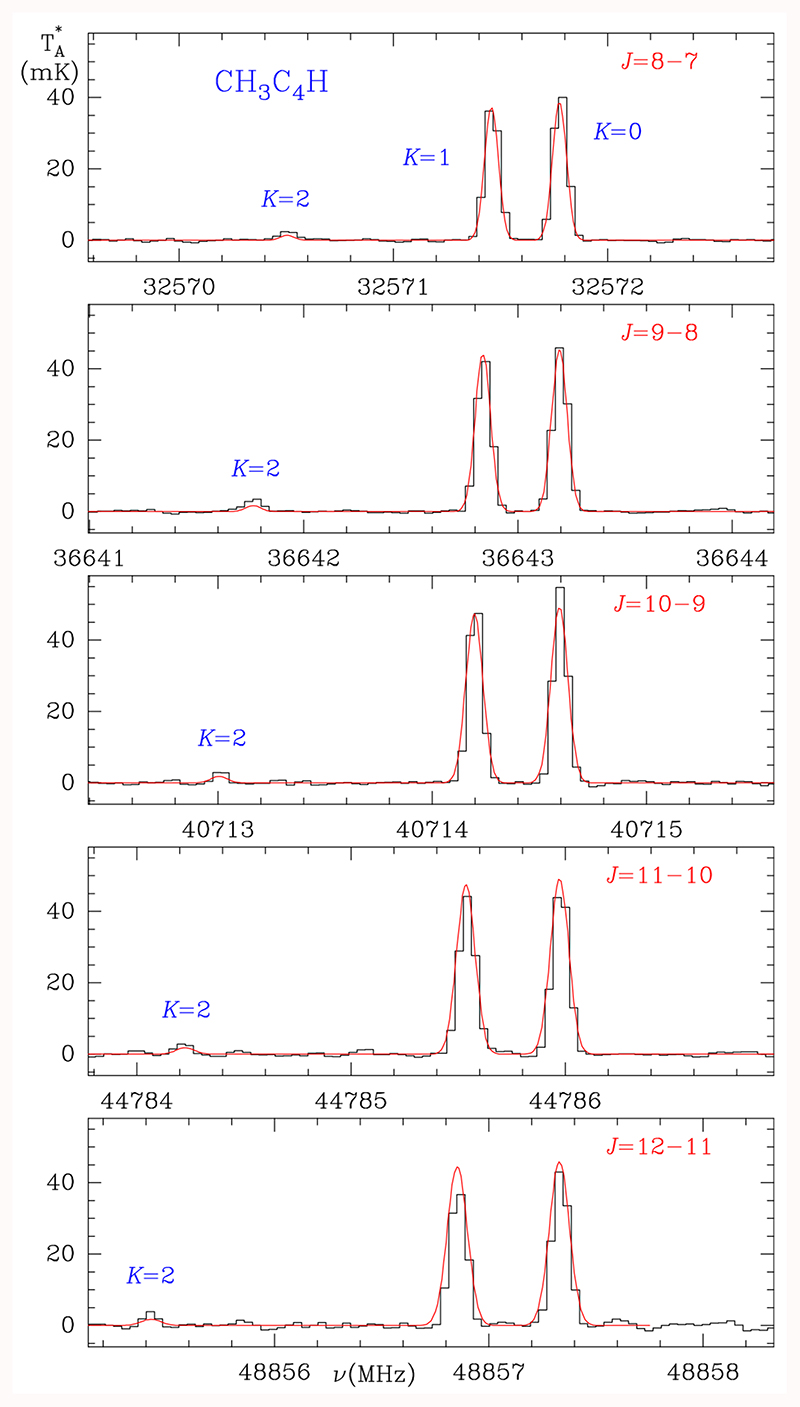
This C5H4 isomer was found in TMC-1 by MacLeod et al. (1984) and Walmsley et al. (1984). We used spectroscopic information from Bester et al. (1984), Heath et al. (1955), and Cazzoli & Puzzarini (2008) to obtain the rotational constants that were implemented in MADEX (Cernicharo 2012). The dipole moment, 1.207 D, was measured by Bester et al. (1984). The constants A and DK are taken from Müller et al. (2002). Five rotational transitions, from Ju = 8 up Ju = 12, lie within our line survey. The K = 0, 1, and 2 components of these transitions were observed as shown in Fig. 2. Derived line parameters are given in Table A.2. The detection of the K=2 component allows for a good determination of the rotational temperature. As for H2CCCHCCH, we assumed a uniform brightness source with a radius of 40” (Fossé et al. 2001). In a first step, we derived Tr from a rotational diagram of the lines of the A and E species. A similar rotation temperature of 7±1 K was obtained for both symmetry species. Then, we produced a synthetic spectrum that was compared with the observed line profiles, allowing us to refine the derived parameters. We found that a synthetic spectrum with Tr=7 K and N(A-CH3C4H)=N(E-CH3C4H)=(6.5±0.2)×1012cm–2 matches the observed spectra very well (see Fig. 2). The synthetic spectrum was corrected for beam dilution and beam efficiency. Consequently, the total column density of methyl diacetylene is (1.3±0.4)×1013 cm–2. This value is in good agreement with those derived by Walmsley et al. (1984) and MacLeod et al. (1984) when corrected for the different dipole moment used by these authors (1.0, and 0.9 D, respectively). Hence, both species, allenyl acetylene and methyl diacetylene, have similar abundances in TMC-1. They are only three times less abundant than propene (Marcelino et al. 2007) and ten times less abundant than methyl acetylene (Cabezas et al. 2021).
Fig. 2.
Observed transitions of CH3C4H towards TMC-1. The abscissa corresponds to the rest frequency of the lines assuming a local standard of rest velocity of TMC-1 of 5.83 km s–1. Line parameters for the observed transitions are given in Table A.1. The ordinate is the antenna temperature, corrected for atmospheric and telescope losses, in millikelvin. The red line shows the computed synthetic spectrum for this species for Tr=7 K and a column density of 6.5×1012 cm–2 for each of the A and E states of vinyl diacetylene.
3.3. HCCCH2CCH
We note that 1,4-Pentadiyne, HCCCH2CCH, is another C5H4 isomer. Its rotational spectrum was measured in the laboratory by Kuczkowski et al. (1981). Its dipole moment, 0.52 D, was measured by the same authors. We searched for it through more than ten rotational transitions falling in the 31-50 GHz range. None of them were detected. We derived a 3σ upper limit to its column density of 4×1012 cm–2. This moderate upper limit is due to the relatively low dipole moment of this molecule.
3.4. Discussion
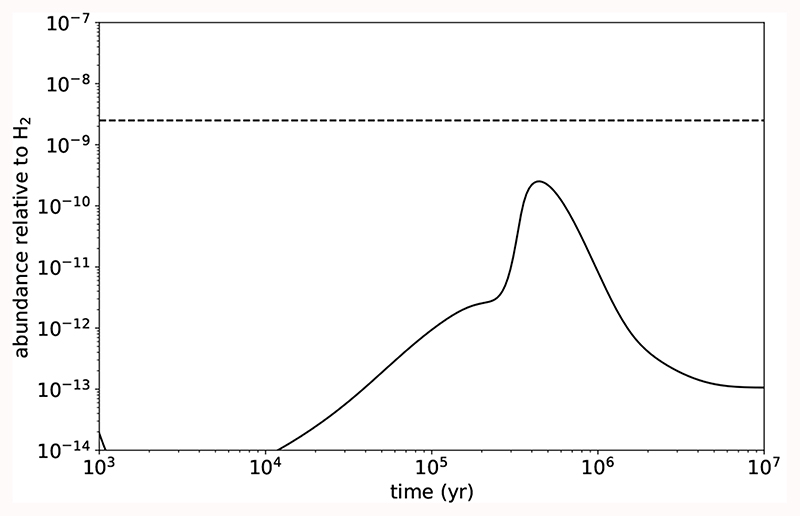
As a matter of fact, the two most stable C5H4 isomers were detected in TMC-1 and both have similar abundances. To learn about the formation of these molecules under cold dark cloud conditions, we carried out chemical model calculations similar to those presented in Marcelino et al. (2021). For chemical model purposes, we considered that the species with molecular formula C5H4 accounts for the various possible isomers. According to our chemical model, the peak abundance of C5H4 isomers under cold dark cloud conditions is 2.5 × 10–10 relative to H2, which is ten times smaller than the sum of the observed abundances of CH3C4H and H2CCCHCCH in TMC-1 (see Fig. 3). While the difference between the calculated and observed abundance is significant, it is interesting to inspect which are the main formation routes in the chemical model. Reactions of the CCH radical with methyl acetylene (CH3CCH) and allene (H2CCCH2)
| (1a) |
| (1b) |
| (2a) |
| (2b) |
account for most of the C5H4 isomers formation. These reactions were experimentally found to be rapid at low temperatures, down to 63 K (Carty et al. 2001), and they are probably also fast at temperatures around 10 K. The reaction of C2 with propylene, which has also been measured to be rapid down to 77 K (Daugey et al. 2008), is also an important source of C5H4 isomers, while a third route involving the dissociative recombination of the ion does also contribute to their formation.
Fig. 3.
Calculated fractional abundance of C5H4 (allowing for various isomers) as a function of time. The horizontal dashed line corresponds to the sum of the observed abundances of the two C5H4 isomers (CH3C4H and H2CCCHCCH) detected in TMC-1.
Although the branching ratios of reactions (1) and (2) are not precisely known, methyl acetylene and allenyl acetylene are the most likely products (Kaiser et al. 2001; Zhang et al. 2009; Goulay et al. 2011). In fact, it would be interesting to verify if CH3C4H is the preferred product of reaction (1) and if H2CCCHCCH is preferentially formed in reaction (2); this would allow one to probe the abundance of the non-polar hydrocarbon allene, which is expected to be large. The chemical scheme depicted by reactions (1) and (2) is similar to that driving the formation of C4H3N isomers in which reactions of the CN radical with CH3CCH and H2CCCH2 are at the heart of the synthesis of the various C4H3N isomers, as discussed by Marcelino et al. (2021). In fact, it is worth noting that the abundance ratio C5H4/C4H3N of 3.5 (Marcelino et al. 2021 and this study) observed in TMC-1 is not far from the CCH/CN abundance ratio of 10 observed in this source (Pratap et al. 1997).
Supplementary Material
Acknowledgements
We thank Ministerio de Ciencia e Innovación of Spain (MI-CIU) for funding support through projects AYA2016-75066-C2-1-P, PID2019-106110GB-I00, PID2019-107115GB-C21 / AEI / 10.13039/501100011033, and PID2019-106235GB-I00. We also thank ERC for funding through grant ERC-2013-Syg-610256-NANOCOSMOS. M.A. thanks MICIU for grant RyC-2014-16277.
Footnotes
References
- Agúndez M, Wakelam V. Chem Rev. 2013;113 doi: 10.1021/cr4001176. 8710. [DOI] [PubMed] [Google Scholar]
- Bester M, Yamada K, Winnewisser G, et al. A&A. 1984;137:L20. [Google Scholar]
- Cabezas C, Endo Y, Roueff E, et al. A&A. 2021;646:L1. [Google Scholar]
- Carty D, Le Page V, Sims IR, Smith IWM. Chem Phys Lett. 2001;344:310. [Google Scholar]
- Cazzoli G, Puzzarini C. A&A. 2008;487 1197. [Google Scholar]
- Cernicharo J. Internal IRAM report. IRAM; Granada: 1985. [Google Scholar]
- Cernicharo J, Guélin M, Hein H, Kahane C. A&A. 1987;181:L9. [Google Scholar]
- Cernicharo J, Guélin M. A&A. 1996;309:L27. [Google Scholar]
- Cernicharo J. In: Stehl C, Joblin C, d’Hendecourt L, editors. ECLA 2011: Proc of the European Conference on Laboratory Astrophysics, EAS Publications Series; Cambridge: Cambridge Univ. Press; 2012. p. 251. 2012 https://nanocosmos.iff.csic.es/?page_id=1619. [Google Scholar]
- Cernicharo J, Kisiel Z, Tercero B, et al. A&A. 2016;587:L4. doi: 10.1051/0004-6361/201527531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernicharo J, Guélin M, Agúndez M, et al. A&A. 2018;618:A4. doi: 10.1051/0004-6361/201833335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernicharo J, Cabezas C, Pardo JR, et al. A&A. 2019;630:L2. doi: 10.1051/0004-6361/201936372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernicharo J, Marcelino N, Agúndez M, et al. A&A. 2020a;642:L17. doi: 10.1051/0004-6361/202039351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernicharo J, Marcelino N, Pardo JR, et al. A&A. 2020b;641:L9. doi: 10.1051/0004-6361/202039231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernicharo J, Marcelino N, Agúndez, et al. A&A. 2020c;642:L8. doi: 10.1051/0004-6361/202039274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernicharo J, Agúndez M, Cabezas C, et al. A&A. 2021a submitted. [Google Scholar]
- Cernicharo J, Cabezas C, Bailleux S, et al. A&A. 2021b in press, 2021arXiv210104603C. [Google Scholar]
- Cernicharo J, Cabezas C, Endo Y, et al. A&A. 2021c in press, 2021arXiv210105163C. [Google Scholar]
- Daugey N, Caubet P, Bergeat A, et al. PCCP. 2008;10:729. doi: 10.1039/b710796j. [DOI] [PubMed] [Google Scholar]
- Dunning TH. J Chem Phys. 1989;90 1007. [Google Scholar]
- Fossé D, Cernicharo J, Gerin M, Cox P. ApJ. 2001;552:168. [Google Scholar]
- Frisch MJ, Trucks GW, Schlegel HB, et al. Gaussian 09, revision D.01. 2009 [Google Scholar]
- Goulay F, Soorkia S, Meloni G, et al. PCCP. 2011;13 doi: 10.1039/c1cp22609f. 20820. [DOI] [PubMed] [Google Scholar]
- Heath GA, Thomas LF, Sherrard EI, et al. Discussions Farad Soc. 1955;19:38. [Google Scholar]
- Kaifu N, Ohishi M, Kawaguchi K, et al. PASJ. 2004;56:69. [Google Scholar]
- Kaiser RI, Chiong CC, Asvany O, et al. J Chem Phys. 2001;114:3488. [Google Scholar]
- Kawaguchi K, Fujimori R, Sayaka A. PASJ. 2007;59:L47. [Google Scholar]
- Kraśnicki A, Kisiel Z. J Mol Spectrosc. 2011;270:83. [Google Scholar]
- Kuzckowski RL, Lovas FL, Suenram RD, et al. J Mol Struct. 1981;72:143. [Google Scholar]
- Lee KLK, McCarthy M. J Phys Chem Lett. 2019;10:2408. doi: 10.1021/acs.jpclett.9b00586. [DOI] [PubMed] [Google Scholar]
- Lide DR, Jr, Mann DE. J Chem Phys. 1957;27:868. [Google Scholar]
- MacLeod JM, Avery LW, Broten NW. ApJ. 1984;282:L89. doi: 10.1086/184181. [DOI] [PubMed] [Google Scholar]
- Marcelino N, Cernicharo J, Agúndez M. ApJ. 2007;665:L127. [Google Scholar]
- Marcelino N, Agúndez M, Tercero B, et al. A&A. 2020;643:L6. doi: 10.1051/0004-6361/202039251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelino N, Tercero B, Agúndez M, Cernicharo J. A&A. 2021 doi: 10.1051/0004-6361/202040177. in press, arxiv.org/abs/2101.08516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MC, et al. J Phys Chem A. 2020;124:5170. doi: 10.1021/acs.jpca.0c02919. [DOI] [PubMed] [Google Scholar]
- Møller C, Plesset MS. Phys Rev. 1934;46:618. [Google Scholar]
- Müller HSP, Pracna P, Horneman V-M. J Mol Spectrosc. 2002;216:397. [Google Scholar]
- Müller HSP, Schlöder F, Stutzki J, Winnewisser G. J Mol Struct. 2005;742:215. [Google Scholar]
- Ozier I, Ho W, Birnbaum G. J Chem Phys. 1969;51 4873. [Google Scholar]
- Pardo JR, Cernicharo J, Serabyn E. IEEE Trans Antennas and Propagation. 2001;49:12. [Google Scholar]
- Pickett HM, Poynter RL, Cohen EA, et al. J Quant Spectrosc Radiat Transfer. 1998;60:883. [Google Scholar]
- Pratap P, Dickens JE, Snell RL, et al. ApJ. 1997;486:862. doi: 10.1086/304553. [DOI] [PubMed] [Google Scholar]
- Remijan AJ, Hollis JM, Lovas FJ. ApJ. 2007;664:L47. [Google Scholar]
- Sakai N, Shirley Y, Sakai T, et al. ApJ. 2012;758:L4. [Google Scholar]
- Sobolev GA, Shcherbakov AM, Akishin PA. Opt Spectrosc. 1962;12 [Google Scholar]
- Tercero F, López-Pérez JA, Gallego, et al. A&A. 2021;645:A37. [Google Scholar]
- Walmsley CM, Jewell PR, Snyder LE, Winnewisser G. A&A. 1984;184:L11. [Google Scholar]
- Zhang F, Kim S, Kaiser RI. PCCP. 2009;11 doi: 10.1039/b822366a. 4707. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.