Abstract
Neurotechnological devices are failing to deliver on their therapeutic promise because of the time it takes to translate them from bench to clinic. In this Perspective, we reflect on lessons learned from medical device successes and failures and consider how such lessons might shape a strategic vision for translating neurotechnologies in the future. We articulate how the intentional design and deployment of “scientific platforms,” from the technology stack of hardware and software through the supporting ecosystem, could catalyse a new wave of innovation, discovery, and therapy. We also identify specific actions that could promote future neurotechnology roadmaps and industrial-academic-government collaborative activities. We believe that community-supported neurotechnology platforms will prove to be transformational in accelerating ideas from bench to bedside, maximizing scientific discovery and improving patient care.
Introduction
The translation of neurotechnology from the bench to the bedside can be a tortuous process. While bioelectronics are hyped as an alternative to drug interventions, the reality is that the translation timelines for medical devices – and their success rates as therapeutic tools – mirror the slow and costly development of new pharmaceuticals, rather than mirroring the lean, accelerated development of new electronics for the consumer market.
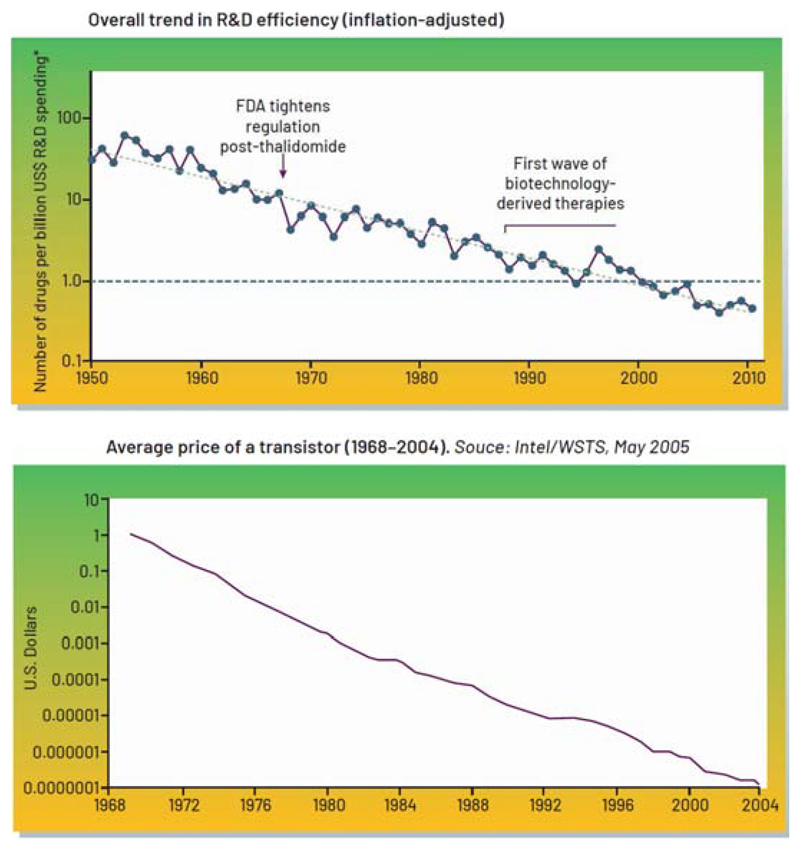
This issue matters because the socioeconomic burden of neurological and psychiatric disorders is significant. In the UK alone, the cost of brain disorders surpasses £100B per annum (Fineberg et al., 2013), and pharmaceuticals are struggling to fill the innovation gap. The inefficiency of bringing new drugs to market is dubbed “Eroom’s” law, given the exponentially increasing cost of drug release – in contrast to Moore’s law, originally referring to the number of transistors on a microchip doubling every two years though the cost of computers is halved, but more generally illustrating the exponential growth for technologies over time (Scannell et al., 2012) (Figure 1). The relative success of microelectronic innovation motivates scientists, physicians, and engineers to consider how lessons and strategy from the consumer electronics domain might be applied to confront the complexity of treating brain disorders, either alone or in combination with pharmacological solutions.
Figure 1.
“Eroom’s” versus Moore’s Law. (Top panel) Inflation-adjusted R&D costs from 1950-2010 pharma R&D efficiency (reproduced with permission from (Scannell et al., 2012). (Lower panel) The decrease in the cost of a transistor over time, following Moore’s projection (Intel/WSTS, 2005(David Brock, 2006)). Note that FDA-related events and biotechnology innovations appear to have a modest impact on the major trend over several decades.
In fact, electrical stimulation and pharmaceutical solutions have co-existed and complemented each other for decades. For example, deep b rain stimulation (DBS) for Parkinson’s disease helps to mitigate the dyskinesia side-effects of levodopa by stimulating the basal ganglia (Deuschl et al., 2013). Similarly, sensing-enabled epilepsy stimulation devices, while initially designed to abort detected seizures (Sun and Morrell, 2014a), have led to a deeper understanding of circadian and multiday rhythms of seizure risk that should prove to be useful for informing pharmaceutical and stimulation-based chronotherapy (Baud et al., 2018).
The pathway for medical device approval is a relatively streamlined process compared to that for pharmaceuticals. Unlike the three-phase testing protocol for pharmaceuticals, which require thousands of patients, medical devices can be tested in smaller trials with fewer patients. As such, the timelines and costs for a device trial can be less expensive by orders of magnitude, especially if technology and surgical procedures are repurposed. In some cases, such as transitioning a device from treating Parkinson’s disease to dystonia, a new therapy application can simply consist of changes made to software code, instructional labelling, and brain target.
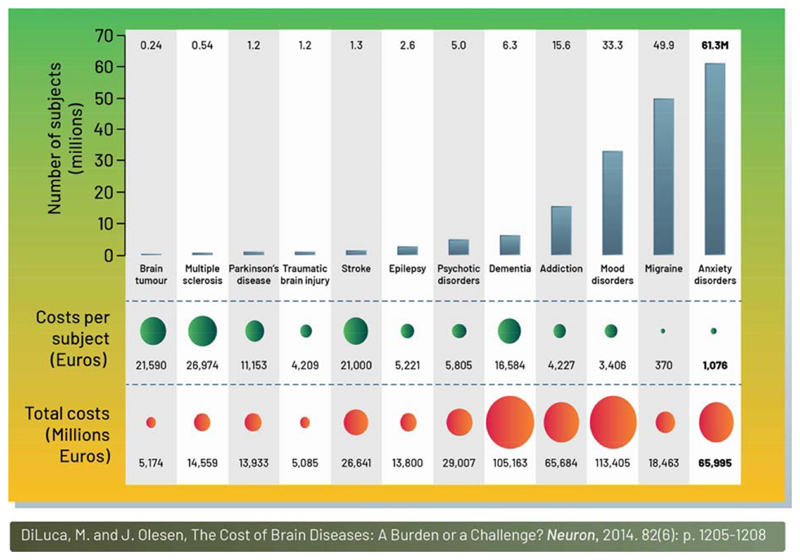
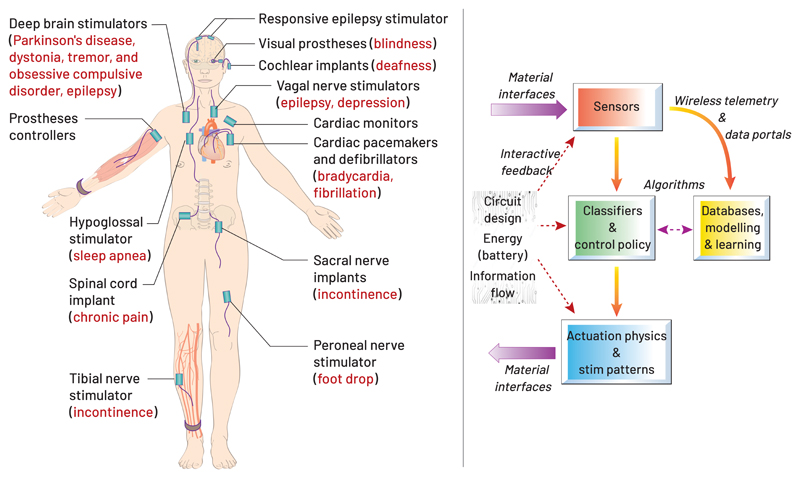
Yet the apparent simplicity of expanding a device’s indication masks important challenges. As with pharmaceuticals, the application of neurotechnology to treat disorders such as dementia, addiction, mood disorders, and anxiety (Figure 2) (DiLuca and Olesen, 2014), which collectively represent a tremendous clinical and economic burden, has been slow. Clinical results to date demonstrate equivocal (Holtzheimer et al., 2017, Dougherty et al., 2015) or limited improvement (Vicheva et al., 2020). From a translational perspective, the efficiency of medical device innovation still has much more in common with pharma R&D than it does with Moore’s law and consumer electronics.
Figure 2.
The economic burden of neurological disorders in the EU. This schematic shows the costs of treating a range of different brain disorders in the EU. It is noteworthy that implanted devices are used to treat only tens of thousands of patients with Parkinson’s disease and epilepsy globally, and are still often seen as a therapy of last resort. The limited penetration of invasive neurotechnology will benefit from continued technological advances and efficacy improvements. Minimally invasive technologies, such as transcranial magnetic stimulation (Fox et al., 2012, Fitzgerald et al., 2003) was recently approved for depression, and already serves hundreds of thousands of patients per year – an order of magnitude greater than even the most successful invasive therapies. (Reproduced with permission from (DiLuca and Olesen, 2014).
The challenges to expanding neurotechnology beyond initial successes in areas such as brain stimulation and cochlear implants are complex. The introduction of new neurotechnology faces multiple barriers: healthcare and R&D economics, the scientific unknowns related to mechanisms of action, regulatory barriers, and ultimately clinician adoption and patient acceptance. For example, even 20 years after its regulatory approval, less than 15% of eligible patients receive DBS therapy for Parkinson’s disease. That said, new applications in neurotechnology could leverage concurrent developments in consumer electronic innovation, rapid progress in machine learning, advances in understanding disease mechanisms, and public awareness of smart technologies such as “the Internet of Things.”
In this Perspective, we consider the role of neurotechnology platforms in speeding the development of therapies for patients and in overcoming the regulatory and translational hurdles that slow the translation of neurotechnologies. We highlight the role of platforms in this regard, and how systems-level platform design might be as important for translation as conventional technological advances are. We also discuss the need to foster the modularity of platforms, to accelerate the innovation of sub-components and limit the “winner takes all” attributes of platforms, and to instead promote collaboration. As we discuss, well-designed platforms could also enable new business models to help coordinate research activities and to increase translational efficiency. We also highlight how this optimistic perspective must also be balanced with the need for platform governance principles that are aligned with ethical guidelines required for medical research. In light of the outstanding unmet need and the potential clinical impacts of neurotechnology, actions like these that catalyse the efficient translation of neurotechnologies to the clinic warrant close consideration.
Barriers and opportunities for successful neurotechnology translation
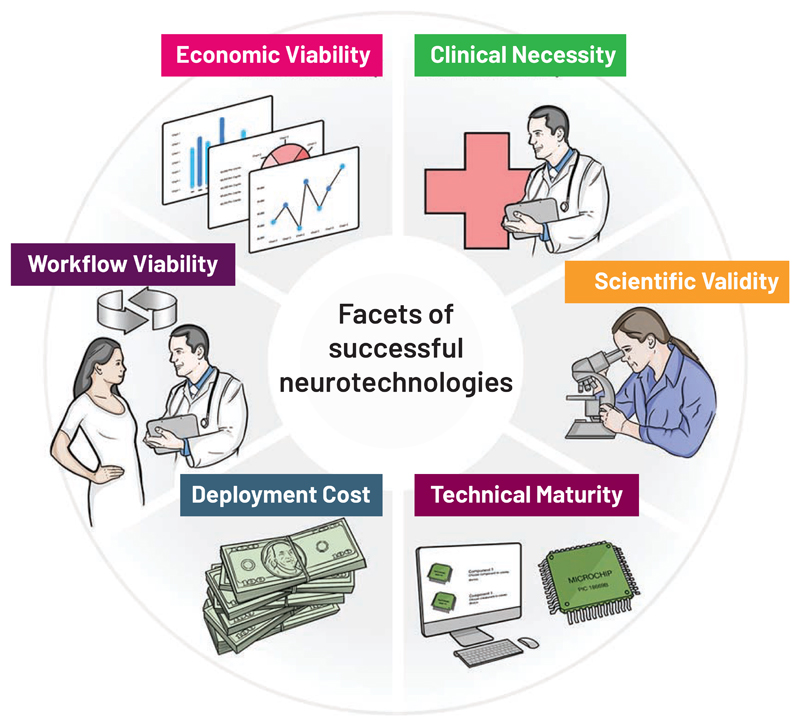
There are multiple elements that contribute to the successful translation of technology to the clinic: clinical need, science and technology, economics, clinical workflow and regulatory strategy. A clear view of these requirements highlights the challenges they present to successful translation. As depicted in Figure 3, we portray these as an interdependent system; as with a wheel, removing one spoke makes for a bumpy ride and can hinder progress all together.
Figure 3.
The interdependent system considerations that impact the clinical translation of neurotechnology. The facets cross multiple disciplines, and are drawn as a wheel to convey that each segment is required for successful translation.
Many readers are familiar with the “technical” right-hand side of Figure 3, where: 1) neurotechnology must be matured to a state where it can be manufactured with high quality, reliability and at acceptable cost; 2) clinical studies demonstrate the scientific validity of the intervention through randomised clinical trials; and 3) there is clear alignment with an unmet clinical need. In complex disease states, the lack of understanding of a therapy’s mechanism of action can be a key hurdle, as seen with nearly all DBS-based therapies, and most strikingly in neuropsychiatric illnesses. It is important to remember that brain stimulation for tremor and Parkinson’s disease was placed within an established lesion target, and the static lesion could be readily replaced with the available technology for tonic stimulation. Brain stimulation also addressed a key unmet clinical need for managing dyskinesia associated with medication. Expanding into diseases with poorly understood disease mechanisms and networks, such as depression, entails uncertainty in the selection of stimulation targets and parameters. Often, stimulation parameters are necessarily best-estimate extrapolations from other successful but unrelated therapies. Even when neurotechnologies finally mature from a science and engineering perspective, they might not address a clinical need any better than existing therapeutic options.
Key reasons for limited translation also lie in the “practical” left-hand side of the wheel in Figure 3. Many neurotechnologies are never translated because they were not developed with an integrated consideration of their associated economics, clinical workflows, patient user needs, or regulatory requirements. A central economic consideration, especially in light of the time required for neurotechnology development and testing (often 5-10 years), is the “time value of money (TVM, see Box 1),” which as stated by Stieglitz (Stieglitz, 2020), is a hurdle that can severely restrict investment in high-risk ventures. As important a barrier, however, and one ultimately linked with the time-value trade-off through impacts on economic costs, is the possible absence of a compelling clinical value proposition relative to the cost of the intervention. This assessment varies between health care systems, and must consider whether the economic model for assessing new technology will be “value-based” or “fee-for-service.” An example of the value-based model is the National Health Service (NHS) in the UK, where an innovator must work with “NICE” (the National Institute for Health and Care Excellence) to demonstrate that their new technology meets a threshold for cost effectiveness. A key metric is defined as Quality-Adjusted-Life-Year (QALY, see Box 1)/£. Using this metric, the interventional cost must meet a hurdle of approximately £25k/QALY. If the new intervention cannot meet this value threshold, it will struggle to be adopted by the NHS. The US still favors the fee-for-service model, where healthcare providers are reimbursed on the basis of the type and number of services they provide. For example, the procedure for implantation of a brain stimulation device is broken into discrete tasks and hardware for remuneration. Device economics in the US are considered by the Centers for Medicare and Medicaid Services and by private insurers. While arguably the metric is not as transparent as, for example, QALY/£, the centrality of the clinical value proposition is still reflected in reimbursement decisions under fee-for-service models. But focusing on process versus outcome can delay therapeutic innovations if a new technology does not align with an existing reimbursement “code.” Such reimbursement constraints can also skew incentives, with tangible impacts on translation. For example, many brain-implant procedures are staged in discrete steps to maximize the reimbursement codes used; a new implant that eliminates the need for staging might shorten implant times and avoid side-effects, such as neck pain from lead tunnelling, but until a code is established for this novel approach, it will not be economically viable. As such we need to recognize the impact of skewed incentives, as reflected in the move to transition to a more value-based approach in the US (Langenbrunner JC, 2009).
Box 1: Glossary of terms.
Time Value of Money (TVM): TVM is the concept that the money you have now is worth more than the identical sum in the future due to its potential earning capacity. This core principle of finance holds that any amount of money is worth more the sooner it is received, provided it can earn interest (Investopedia). In medical technology, the issue is that the extended time to develop and approve a therapy looks unattractive compared to alternative investments.
-
Platforms (Inoue and Tsujimoto, 2018, Porter, 2014)
Product: an architecture that enables products to be generated off a common platform, such as multiple car models derived from a common chassis.
Intermediary: an architecture that more efficiently connects to groups who can benefit from an interaction. For example, ridesharing apps that link drivers and riders.
Ecosystem: an architecture that provides a system for building multiple products and applications off a common central platform. For example, the App store model for smart phones.
Bioelectronics, Bioelectronic Medicines: the use of an electrical interface to the nervous system to modulate signals and to restore health. Historically, these terms are often used interchangeably with the concept of neuromodulation . Currently, these interfaces reinforce precision in stimulation targeting, both spatially and in time, and use adaptation of the body’s natural signalling to provide therapy. For example, stimulation of the vagus nerve to modulate the inflammatory response. (Famm et al., 2013)
Internet of Things: the interconnection via the Internet of computing devices embedded in everyday objects, enabling them to send and receive data (Oxford Dictionary)
Quality Adjusted Life Years (QALY): A measure of the state of the health of a person or group in which the benefits, in terms of length of life, are adjusted to reflect the quality of life. One QALY is equal to 1 year of life in perfect health. (NICE, UK)
International Standards Organization (ISO): ISO releases standards for specific technical domains of medical devices.
Quality Management System (QMS): A QMS is a collection of business processes focused on achieving quality policy and quality objectives to meet customer requirements. These processes are used in a wide range of businesses, including in the manufacture of medical devices. (fda.gov)
Design Master File: A Device Master File typically provides proprietary data about a material, a component, or a manufacturing process that the holder of the Master File wishes to make available to FDA on behalf of the customer, without relinquishing control of the contents to the customer. (fda.gov)
Technology Stack: A typical “technology stack” consists of multiple layers, including new product hardware, embedded software, connectivity, a product cloud consisting of software running on remote servers, a suite of security tools, a gateway for external information sources, and integration with enterprise business systems. From (Porter, 2014)
Right of Reference: Right of reference or use is the authority to rely upon, and otherwise use, an investigation for the purpose of obtaining approval, including the ability to make available the underlying raw data from the investigation for FDA audit, if necessary. (fda.gov)
Network Effects: a phenomenon whereby a product or service gains additional value as more people use it. For example, instant messaging is a market with strong network effects. (Oxford Dictionary)
Multi-homing Costs: costs that imply the expenses of maintaining a presence on multiple platforms at the same time. For example, the cost of maintaining two smart phones using different operating systems. https://hbr.org/2006/10/strategies-for-two-sided-markets
Clinical workflow is another area often overlooked. New technologies that solve one problem, such as advanced electrodes for improved brain interfacing, might create new hurdles, e.g. for the programming neurologist. Expanding the number of contacts from four to eight adds complexity for the clinician programming the device by increasing the factorial combinations of dipole stimulation options. These combinatorics drive the need for support algorithms to realize the potential of the new technology as electrode channels scale up in number (Rebelo et al., 2018), and a need to maximize the return-on-investment for the parameter optimization process (Contarino et al., 2016). Furthermore, the capacity for greater programming complexity, such as adaptive and responsive neurostimulation, shows promise for improving efficacy but can place additional burden both on the programming clinician and the broader care team (Nair et al., 2020). While the costs and impacts of these practical frictions can limit the adoption of new technology, they also provide an opportunity for neurotechnology if a systems mind-set – one that is empathetic to the constraints of a clinical workflow – is applied.
Finally, regulatory requirements can also limit efficient translation. New materials, materials processing, and algorithms need to be assessed against a wide range of international standards for everything from device biocompatibility (ISO 10993) to algorithms for physiologic controllers (ISO 60601-1-10) to software controls (62304, class 3) that are significantly more burdensome than seen in consumer electronic systems. In addition, the need for an International Standards Organization (ISO, Box 1)-compliant quality management system – which provides a vitally important industry-standard audit trail for the entire design, verification and validation process – can be a significant barrier, especially in university settings, where documentation overhead is not recognized as an “academic output.” The generation of a new regulatory master file for an FDA investigational device exemption (IDE) can be highly burdensome for a single protocol, whether developed in industry or academia.
While these challenges to translation can pose seemingly daunting barriers, we believe that a well-designed and well-governed platform ecosystem can significantly lower such barriers to translation. Indeed, it is our view that historical translations of neurotechnology successfully leveraged platform technology, platform ecosystems and platform economics. Innovators should therefore consider how neurotechnology platforms might be designed to benefit from the tailwinds of consumer technology and government research investment, while addressing the key barriers that clinical neuroscience and medical device translation face. As we will argue, platforms provide unique economic opportunities to share costs and to mitigate risks, while also leveraging trends in digital technology to catalyse team-based science and clinical studies in practical environments. However, platforms also require coordination and buy-in across an ecosystem – by academia, industry and government – which presents new challenges and mitigations. Before we discuss this proposition further, we provide a brief introduction to the types of platform we discuss in this review.
Platforms: their different types and applications
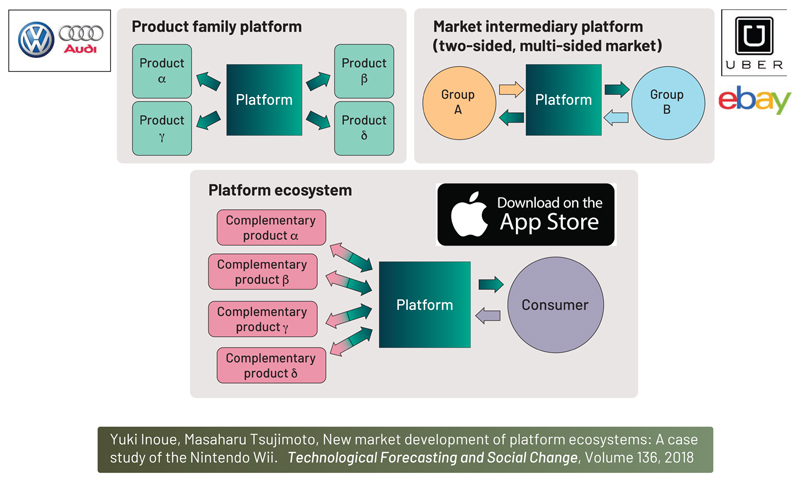
The term “platform” as used here requires definition for clarity. Many modern technologies refer to themselves as platforms, and the word is used interchangeably for concepts that capture modularity, transaction dynamics, and ecosystem development. We first provide a framework for thinking about platforms in neurotechnology, as they can take many forms. As illustrated in Figure 4, derived from (Inoue and Tsujimoto, 2018), we define a taxonomy of platforms that includes three general types:
Figure 4.
A taxonomy of three product platforms relevant for neurotechnology
Product family
This is a foundational platform architecture that enables a series of derivative products to be created, with each product consisting of a slightly modified variant of the core architecture. A common example of a product family is an automotive platform, where cars of a given manufacturer share a common chassis, and stylistic modifications are used to create derivative models. In the case of neurotechnology, the product platform is often a device technology, such as a battery, electronic sub-system, or electrode design that can be modified to address different disease states.
Examples of Product Platforms
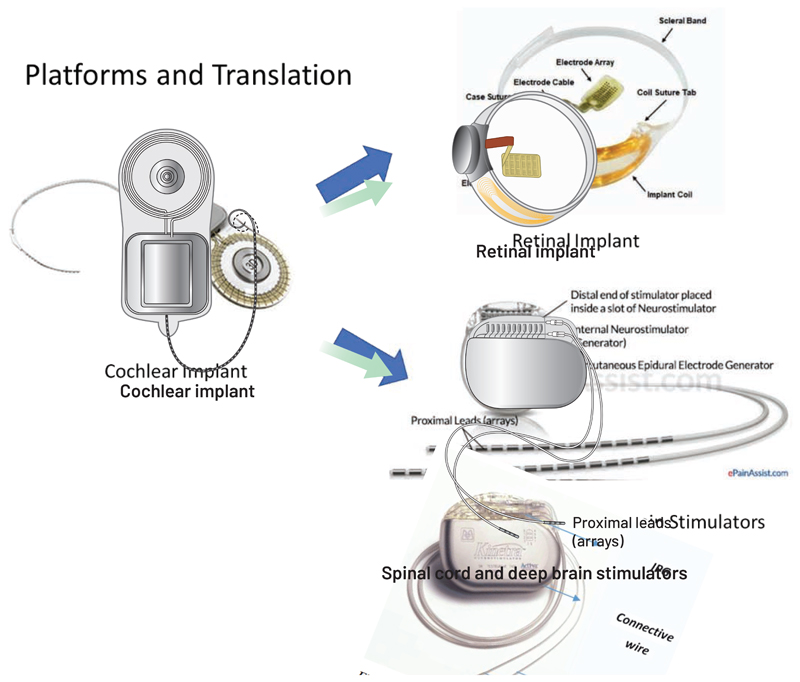
Platforms have to date played a successful role in neurotechnology (Stieglitz, 2020), with dominant commercial clinical technologies based on underlying neurotechnology “product family” platforms. One such example is the family tree of Advanced Bionics’ cochlear implant, which now spans multiple disease states and locations in the nervous system. The platform was originally built to advance cochlear implants, which require a high-channel-count neurostimulator to restore hearing. Once established in the cochlear prosthetics field, the implant acted as a platform for therapeutic applications that would benefit from high-fidelity neural stimulation. The resulting product derivatives include spinal cord stimulators (e.g. Boston Scientific’s Vercise), the first-in-human retinal implants (e.g. Second Sight’s Argus) (Chuang et al., 2014), and deep-brain stimulators that use current steering and directional electrodes (e.g. Boston Scientific’s Precision). The repurposing of product technology is a recurring theme in successful commercial and research systems, where modifications of embedded software can enable a spinal cord stimulator to metamorphose into a deep brain stimulator, or an existing spinal cord stimulator to be translated into a spinal prosthesis (Capogrosso et al., 2016, Wagner et al., 2018) or into a bi-directional closed-loop device (Khanna et al., 2015).
Market intermediary platform
This term describes a platform that helps to connect two user groups with complementary goals. The aim of the intermediary is to remove the barriers that prevent their interaction. The market intermediary platform is often called “two-sided,” as each side of the platform faces a different customer. Examples of this platform include dominant technology companies seen today, such as: 1) Uber, which connects car owners willing to provide a ride with consumers in need of a ride; 2) eBay, which connects sellers and buyers in a virtual flea market; and 3) Google/Facebook, which connects users desiring free services with advertisers. An example of an intermediary platform in neurotechnology is the US National Institutes of Health (NIH) BRAIN public-private partnership (PPP), which we discuss in more detail below.
Examples of Intermediary Platforms
Established neurotechnologies have also created market intermediary platforms for clinical research. For example, commercial systems have helped to facilitate the expansion of therapies by providing the design file for researchers to reference when submitting their investigational applications to regulators. Intermediary platforms, such as the NIH’s PPP for BRAIN (Brain Research through Advancing Innovative Neurotechnologies) and SPARC (Stimulating Peripheral Activity to Relieve Conditions) can connect clinical researchers with research devices available as variants of commercial systems. The PPP processes also helped give rise to customised research tools (Stanslaski et al., 2018, Rouse et al., 2011), which also serve as proof-of-concept prototypes for future products (Malekmohammadi et al., 2016, Kremen et al., 2018, Herron et al., 2017, Swann et al., 2018). In addition to acting as an intermediary, the government funding that supports such PPPs helps to reduce the risk of exploratory research for commercial companies, accelerating the pace and scope of scientific discovery and innovation. A key consideration when designing an intermediate platform is how each side is treated relative to the other. For example, while users of Google acquire services for free, the advertisers must pay for access. The NIH PPP structure arguably sought to balance the benefits and trade-offs of each participant in the construction of the agreements, funding requirements, and terms of engagement; the inclusion of all major device manufacturers, dozens of active protocols, and diverse coverage of disorders suggests the approach was successful for research facilitation.
Platform ecosystem
This term describes a platform that enables many complementary products to be created, with which the user interacts via the platform, forming an “ecosystem.” As such, the platform might constitute a marketplace for these complementary products. Examples include application stores supported by a common hardware infrastructure and distribution network, such as the Apple AppStore, and the video game developers that support Nintendo’s motion-activated Wii controller. Arguably, platform ecosystems are still nascent in neurotechnology, but commercial entities help to support these through established clinical workflows and the scale of deployment, making participation in a platform ecosystem financially attractive. Academic-based ecosystems, including openEEG and BCI2000 (Schalk et al., 2004), can help facilitate research. While much effort is focused on preclinical studies and software, a few small ecosystems have invested in supporting the translation of implantable systems for humans, including regulatory design controls. Two recent examples include the BrainGate consortium (www.braingate.org) (Ajiboye et al., 2017) and the Activa PC+S (Afshar et al., 2012). Nascent start-ups that aspire to build platform ecosystems include the Brain Interchange by Cortec Neuro and the Bioinduction-Oxford DyNeuMo Research System by Bioinduction Ltd.
Examples of Platform Ecosystems
While still in their nascent state, successful commercial devices can expand to support platform ecosystems. The most common examples are software tools to help with the surgical planning of device placement (e.g. BrainLab or Stealth station), or model-based programming assistance to plan surgery (Riva-Posse et al., 2017, Miocinovic et al., 2007) and to set optimal stimulation parameters. On the horizon, additional ecosystem components include: surgical robotics that support initial device placement; wearable diagnostic monitors that can track implant outcomes, such as in epilepsy (Regalia et al., 2019, Baldassano et al., 2018) or movement disorders (Pulliam et al., 2015); and cloud-based diagnostic systems (Baldassano et al., 2018). Platform ecosystems can also create strong inertia that favors the use of an existing system; this is often called “stickiness.” As an example of this concept, Medtronic’s use of an existing brain stimulation infrastructure for treating epilepsy helped to lower the company’s production costs and clinical workflow barriers to adopting the device for a new application. By contrast, the Neuropace RNS (responsive neurostimulation) required a new procedure and clinical workflows. Given the current similarity in clinical outcomes for epilepsy provided by vagal nerve stimulation, DBS and RNS, practitioners might look to other elements of the platform to help guide product choice (Jobst, 2010, Fisher and Velasco, 2014).
It is, nevertheless, worth noting that the use of a platform approach does not guarantee commercial success. At the time of writing, for example, Second Sight, the retinal prosthetics developer of Argus II, is auctioning its assets and its commercial activities are on hold. The Alpha-IMS retinal implant has also ceased commercial activities, reinforcing the challenge of entering this market. Undaunted, dozens of neurotechnology start-up companies remain poised to release technological advances with high ambitions for translational success. The next two sections highlight both the benefits and limitations of platforms for translating neurotechnology.
The Benefits of Platforms for Translating Neurotechnology
As discussed in the preceding sections and as illustrated in Figure 3, platforms have both implicit and explicit benefits that have motivated their historical use. When addressing clinical necessity (see Figure 3, right), system engineers might work with clinicians to identify a common “technology stack” that serves multiple disorders with a common toolset. To establish scientific validity, a well-designed platform can flexibly facilitate clinical investigations with marginal investment, much as DBS for tremor was repurposed to treat multiple disorders, such as Parkinson’s, Obsessive Compulsive Disorder and epilepsy. In such cases, the existing device was used to gather new clinical evidence by making only small changes (e.g. to stimulation parameters, electrode spacing, and targets) required for therapy customization. A common platform can also help to increase manufacturing volume, which in turn helps with yields, process reliability, and lower costs, leading to technical maturity. A platform device might also “evolve” by leveraging digital, reprogrammable firmware technology via wireless telemetry, which allows algorithms to be updated as clinical practice matures (Khanna et al., 2015).
In addition to mitigating clinical and technology constraints, platforms can also help with healthcare economic considerations and the requirements of regulatory processes (see Figure 3, left). In terms of regulatory consideration, a device that derives from a common platform foundation facilitates the statistical assessment of its performance and risks. This knowledge can facilitate regulatory approval being granted for a device applying incremental innovation to treat new disease states or adjunctive features. In addition, the “right of reference” regulatory process in the US means that platforms can be used in early feasibility investigational studies; thus, investigators can leverage the significant predicate information in the Master Files and focus on the novel elements of their protocol (EU regulators, take note!). Platforms can also help to streamline workflows. Another benefit of a common platform is that the user (e.g. clinician) has a consistent experience in interacting with the system. For example, the algorithmic process of stereotactic neurosurgery helps to lower barriers to exploring novel neural targets for neurological and psychiatric disorders across multiple structures in the brain, as long as the core steps of the implant procedure are preserved (Koulousakis et al., 2019, Holtzheimer et al., 2017). In addition, a community of users can form around a platform and ease the barrier of entry for other investigators.
Finally, platforms help with the economic viability of neurotechnology. Together, platforms can amortize the fixed costs of developing and deploying a system across a larger pool of applications. This in turn lowers investment requirements and renders the exploration of new disease states, via the enhancement or alteration of an existing system, a marginal expense with potentially lower risk. Nevertheless, as we discuss below, these platforms also come with some significant limitations.
Platform Limitations and Potential Mitigations
While platforms bring advantages to translation, they also have significant limitations that require consideration. For example, the definition of a “universal” platform for neural interfaces is probably not scientifically, technically or economically tenable. One way to mitigate this issue is through modularity in design. For example, the hardware electronic subsystem of a device might employ flexible connectors with the neural interface. Flexible connectors allow for target-specific electrodes to be designed for specific applications – including surgical placement – but leverage a common hardware architecture. Modularity is a common design trait of cardiac pacemakers, which include parts that are interchangeable even among manufacturers. Modularity is useful for research as well; Medtronic’s brain and spinal cord stimulators have allowed researchers to explore cortical sensing and closed-loop systems. Even though the devices serve different disease applications, the modularity of electrodes and interconnections allowed spinal epidural paddles to be repurposed into investigational cortical electrodes (Swann et al., 2018, Swann et al., 2016, Pels et al., 2019, Vansteensel et al., 2016). The trade-off of modularity for manufacturers is that competitive differentiation can be harder to achieve when standards are enforced; innovation might require alternative goods or services beyond simple system integration.
The extreme diversity in requirements across clinical indications and applications means that products might need to break from the platform to meet key thresholds of clinical utility. For example, the retinal implant started as a 16-channel derivative of the cochlear implant, with innovation focused on the retinal electrode, new sensory coding scheme, and the demonstration of feasibility. While useful as a proof-of-concept, the need for greater visual acuity motivated an upgrade to a 60-channel device for the device’s trial and commercialization (Argus II) (Chuang et al., 2014, Stronks and Dagnelie, 2014). The strategic challenge of this customization helped to undermine the economics of cost amortization across disease states; in the case of Argus II, the full costs had to be covered by one low-prevalence disease state, severe retinitis pigmentosa. Similar challenges arose when DBS technology developed to treat movement disorders (with clear targets based on lesioning therapy and immediate objective measurements to guide therapy optimization) was extended to treat epilepsy and depression. Both disorders involve more complex networks, are more challenging when it comes to measuring outcomes, and pose challenges to rational parameter optimization. In depression alone, two separate industry trials were unsuccessful using simple extensions of existing DBS technology (Holtzheimer et al., 2017, Dougherty et al., 2015).
Another challenge for all types of platforms is the need for coordination between industry, regulatory agencies, and researchers. Successful platform development requires buy-in from commercial interests (toolsets); commitment to the selective, transparent sharing of information to support long-term goals (scope); and the judicious allocation of resources to establish “interfaces” that accelerate system modularity and maximize discovery. Many incentives in the neurotechnology ecosystem – finance, grants, publication, and intellectual property protection – place conflicting constraints on this development process, which can limit the broad collaboration required to develop a successful platform ecosystem.
The key takeaway here, from our perspective, is that platforms can provide a strategic pathway by which to explore new therapies due to their sometimes favourable economic, regulatory, and workflow efficiencies. Once a therapy is established, device adoption and cash flow might justify a device’s customization and refinement for that specific disease state. If customization occurs too soon it can bankrupt the venture if significant capital is not available (note that ventures backed by new entrants, such as Elon Musk’s Neuralink, Kernel, and Facebook might provide interesting exceptions to the classical financing constraints in medical technology (Hanson et al., 2019)). The central challenge is to define a platform that meets the criteria for broad utility while avoiding as many limitations as possible. As described below, this challenge is tangibly captured in the design of the technology stack.
Technology stacks and their design
We believe platforms can accelerate the translation of neurotechnology. Yet, to realize the benefits of a platform, care must go into the design of a “technology stack” (Porter, 2014). As illustrated in Figure 6, the technology stack encompasses the key subcomponents of the system, including: the “direct loop” that interfaces with the nervous system; the system’s sensors, actuators and algorithms (e.g. classifiers, control policies) that adapt actuation based on sensed inputs; and a “supporting loop”, including supporting databases and an analytics infrastructure, to train the algorithms and to optimize patient-specific stimulation parameters.
Figure 6.
Supporting the large number of potential therapies that might benefit (left) motivates the design of the next-generation technology stack (right)
To support a platform mind-set, the stack needs to consider the scope of disease states that are of interest, with potential applications that can interface with the brain, fiber tracts, spinal cord, or with peripheral nerves. Balancing such a breadth of applications with potential clinical efficacy tradeoffs is a key challenge for the neurotechnologist and medical device designer; focusing too much on one indication might limit a platform’s extensibility, while broadening its scope too much might undermine clinical utility. Modular designs will almost certainly be required to help support this balance and to provide device hardware that is scalable to span a range of applications. Classical system engineering considerations must also be factored into platform design, specifically the management of interfaces, information, and energy. These requirements raise several questions for consideration, as discussed below.
Sensing neural interfaces
For sensing neural interfaces, what is the spatial and temporal scale of neural interaction and what are the channel numbers required? Constraints also include surgical placement, fixation, and easy retrieval if needed. Long-term reliability, together with technical maturity, surgical workflow, and the availability of clinical workflows for long-term patient management, are key considerations in the choice of scale. The reliability of sensing interfaces remains an active area of research, which should factor into the selection of sensing methodology (Pels et al., 2019, Milekovic et al., 2018, Gilja et al., 2015).
Actuating neural interfaces
In addition to considering the scale of interaction for actuating neural interfaces, we also need to consider what the physics of actuation (electrical, optical, etc.) and the stimulation parameters are. Stimulation modes extend from sub-Hz modulatory impulses to kHz nerve blocks (Kilgore and Bhadra, 2014). The choice of actuation method has a significant impact on the energy consumption of the device, which can in turn set the size of the implant based on battery physics or its requirement for continuous powering (Liu et al., 2020).
How do actuation and sensing scope determine scale and energy consumption?
All implantable systems must be packaged to ensure their long-term safety and function. Hermetic enclosures, often titanium, have existed as a platform technology for many years, but new packaging materials are urgently needed for future platforms to improve device size and channel count (Nurmikko et al., 2010). How many interconnections are necessary to achieve the broad sensing and actuating features of a device? At the time of this writing, 60-channel titanium-ceramic feedthrough assemblies are available as a platform subcomponent (Stronks and Dagnelie, 2014), yet it remains unclear how this can scale to more channels. New technologies are also needed to support future translational applications that increase channel count while maintaining reliability (Chiang et al., 2020). With respect to energy consumption, rechargeable systems do help to release the constraint on power usage but at the expense of user burden. Similarly, cochlear implants use continuous inductive coupling for energy supply but at the cost of a wearable sub-component. Platforms such as the Brain Interchange (Cortec Inc.) propose similar methods, but limit the potential use cases to those where a continuous power and information link can be established. What works well for a cochlear prosthesis might not translate to a Parkinson’s or heart-failure treatment.
Algorithm support and data collection
For algorithm support and data collection, what level of complexity is required for computation, and does the system have the computational resources to support the algorithm? Should the system embed algorithms inside the device, in local algorithm support devices (e.g. smart phone), the cloud or via a combination of these, in a way that balances performance and user burden, to maintain a telemetry link (Porter, 2014, Ahmadi et al., 2019, Kremen et al., 2018)? Similar to energy management, what burden or constraints does user compliance place on the algorithm architecture? The distribution of algorithm functions involves many trade-offs that are relevant for economics and business models as well (Porter, 2014).
Using stacks to support therapy development
As neurotechnology platforms link with data science, how does a stack include support for therapy development through databases, modelling and algorithms? An example of such a system is the NeuroPace RNS for epilepsy (Sun and Morrell, 2014b). The RNS system can gather longitudinal data records from epilepsy patients, including stimulation parameters, multi-channel brain activity, and recorded events from the intracranial electronics and embedded bioelectrical sensing electronics (Sun and Morrell, 2014a, Denison et al., 2015). With over 2000 patients implanted, the collected data can be mined for associations between patient brain dynamics and optimal parameter settings. The potential ability to provide patient-specific treatment algorithms based on population-level analysis through machine learning exemplifies the network and scaling benefits of platforms that are digitally connected and instrumented with diagnostic sensors.
The design of the technology stack as presented here is a series of sub-components that each have specific constraints and technical attributes. Yet it is critical to also consider how the stack comes together as a complete system, and the unique features that the aggregate design can enable.
From Stack to Platform
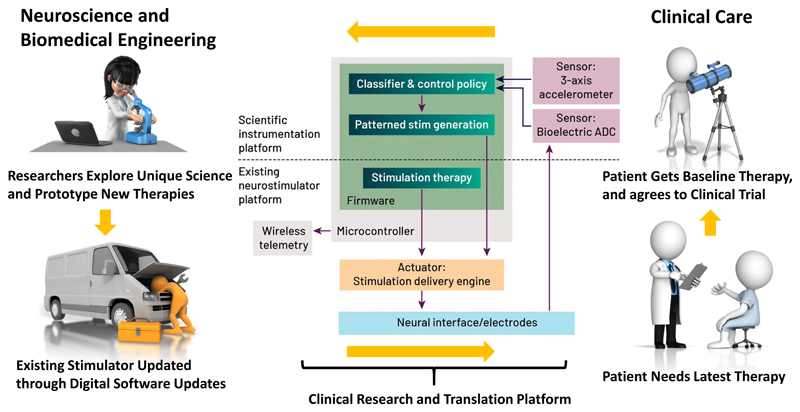
The technology stack is a key enabler of platforms, particularly when serving as a foundation for a product platform architecture. But additional opportunities to enhance translation can be achieved by exploiting the platform ecosystem. For example, a rate-limiting step of neuroscience translation can be access to data and ideas. With this infrastructure in place, a system can be configured for neuroscience discovery. As shown in Figure 7, the device platform can be partitioned into: 1) an “an existing neurostimulator platform” that supports existing therapies, justifies the implant, and streamlines regulatory and ethical approvals; and 2) a “scientific instrumentation platform” that contains additional scientific instrumentation that allows for clinical neuroscience and therapy prototyping in controlled studies. This system partitioning can provide a scheme for continuous therapy refinement. The Activa PC+S and Nexus research tools developed by Medtronic were early prototypes of this strategy (Afshar et al., 2012), and have given rise to systems with more capable sensing, algorithm and prototyping support (Stanslaski et al., 2018). As Figure 7 illustrates, a well-architected, modular system can create a positive feedback loop for clinical neuroscience translation. Building on this notion of a feedback loop, platforms also enable partnerships between commercial and scientific interests, and it is conceivable that an “App Store model” for therapy development could be supported with the research platform acting as a market intermediary and ecosystem. For example, a company could provide a research tool and infrastructure for the research community to use to explore clinical therapeutics; the most promising discoveries from these activities could then be brought to market using the tool and company sponsorship. Galvani pursued this approach when catalysing the field of bioelectronic medicines (Famm et al., 2013).
Figure 7.
Using therapy platforms to drive discovery, translation and application to unmet patient needs in a reinforcement loop. Starting from the bottom right, a patient requires a therapy and has access to it from the “existing” platform capability. However, the patient can also be enrolled in a study and the “scientific instruments” unlocked in a clinical trial. Researchers can then apply this toolkit to explore new therapies and mechanisms of action. The most promising concepts are applied in updates to the system, which then improve the baseline therapy for future patients.
Platforms also provide an opportunity for community-driven science. As mentioned earlier, the NIH PPP for BRAIN, SPARC and HEAL each serve as an intermediary with the additional benefit of providing funding, template contract agreements, and industry-supplied research tools as a community resource. To make the most of this, additional investment should be made into platform support tools that help to leverage the full capability of the system, while avoiding redundant efforts to generate equivalent support. Such programs can at times appear trivial, lacking direct scientific merit, but should be viewed within the context of supporting discovery, translation, and the application of devices to unmet clinical need. We argue that coordinated, complex, and deeply investigative effort is required to successfully orchestrate platform development and dissemination.
Platform governance and ethics
While platforms bring opportunity, several companies are currently in the news for anti-trust, privacy violations, and for applying unfair business practices. To effectively implement a platform ecosystem for neurotechnology, we must also consider the need for platform governance. The interests of industry, academic, and government stakeholders may not always align. Dealing with these issues in a transparent way is critical for ethical research (Hendriks et al., 2019).
For example, the current NIH PPP is an excellent (n=1) example of government-brokered research device availability, but the sustainability of industry’s engagement with the research community is uncertain. Key questions facing the electrical brain stimulation field concern the care of patients, especially over the long term (Lazaro-Munoz et al., 2018). Research studies based on platform ecosystems and scientific questions will eventually end – with the winding down of grant support, device obsolescence or other factors – yet the study participants will be left with implanted neurotechnology that may require care or maintenance that is not covered by insurance or by other conventional support sources. Who should be responsible for long-term patient care?
Platforms also raise issues of open versus closed platform ecosystems. Should such a platform emulate Linux or Windows, Android or Apple? Considerations include the business perspective on sharing value and user-/third-party participation, but also the consideration of governance in a highly regulated environment involving human subjects, where the aggregate system must be verified and validated for performance. On the one hand, platform ecosystems lower the cost and barriers of care as more clinical staff are trained on a single system, with a common toolset for monitoring and configuring devices across multiple disease states. On the other hand, a large diversity in devices can also be envisioned, given the modularity of an ecosystem and the goal to optimize care within a specific disease state. This might lead to a mix of devices that clinicians will lack familiarity with and an absence of a critical mass for driving adoption. Alternatively, devices may be discontinued, and long-term care knowledge and technical capability may simply be lost.
Unlike consumer platforms, translational neurotechnology also requires government approval and post-market surveillance to protect research subjects, and ultimately the patient/consumers once the device is commercially available. The platform ecosystem can help to streamline these procedures for innovators, but they still must be adopted. Furthermore, in terms of resource availability, all clinical investigational device exemptions (IDEs) require quality management systems. The maintenance of such systems require structural support (i.e. funds, technical staff, and administrative personnel) during and beyond individual studies. The maintenance of ongoing regulatory compliance should form part of a platform’s support. In practice, regulatory law, ethics, and medical risk management will likely (and should) limit the ability to have a completely open platform, and a governance mechanism for quality control will be required to assure a platform’s safe translation and regulatory approval.
More broadly, as with innovation elsewhere (although naturally heightened in the realm of neuro-implantable devices), the potential for new interventions that are enabled by platform neurotechnology raises novel ethical questions that must be collectively anticipated and addressed (Hendriks et al., 2019). Is there a platform corollary to support this fundamental work? The concept of the NIH PPP – extended to encompass clinical and lay stakeholders – suggests a powerful organizational principle. Significant investments by the NIH in BRAIN 2.0, for example “Neuroethics of aDBS Systems Targeting Neuropsychiatric and Movement Disorders” at Baylor, and “Achieving Ethical Integration in the Development of Novel Neurotechnologies” at UCSF, should help to guide best practice in platform governance in the future (Zuk et al., 2018, Ramos et al., 2019).
To ultimately translate a neurotechnology, these opportunities, challenges, and trade-offs need to be transferred from abstract principles to specific design actions. The next section describes our efforts to prototype a discovery tool that draws upon the three platform classes described above: product, intermediary, and ecosystem.
Exploring Platform Prototypes to Develop a Discovery Ecosystem
The “OpenMind” academic consortium was formed by the authors to support BRAIN Initiative researchers by accelerating cooperation and innovation in the use of implantable neuromodulation hardware platforms. In addition to disseminating tools that enable currently available neurotechnology to be exploited, the OpenMind consortium also aims to develop a platform ecosystem that is suited to future needs in the field. These include the rapid translation of innovative devices, turn-key solutions for investigational research utilizing innovative devices, and the reduction of costs for new therapy exploration. Many next-generation therapeutic DBS devices now incorporate the sensing of cortical and subcortical field potential activity, as well as the capability for wireless data to be streamed from the internal device to external computers over a wide range of time scales (from days and weeks to months and years). This creates an opportunity to maximize scientific discovery using the unique human neuroscience data that is being collected. Further, it is expected that more devices will come to both research and clinical markets that have sensing and stimulation capabilities and that have potential applications in even broader markets than brain science (e.g. the peripheral nervous system). Thus, the near-term goal of the OpenMind group is to provide data scientists, neuroscientists, clinicians and device developers with the building blocks required to launch clinical studies and that maximize the potential for scientific discovery in the era of responsive neuromodulation. As we discuss below, our review of the existing research ecosystem highlighted two complementary gaps in the research tool environment.
A library of vetted, device-agnostic open source software elements
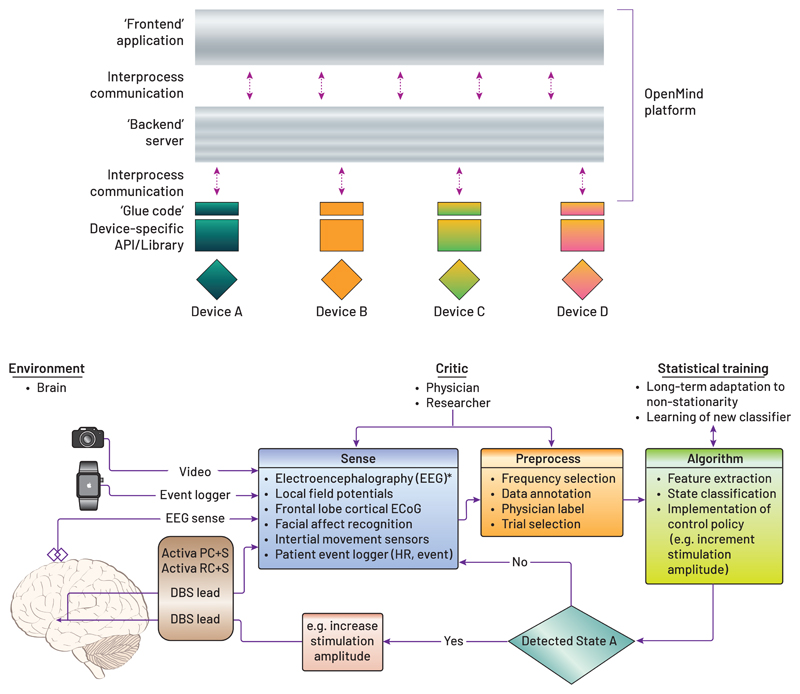
As shown in Figure 8a, we propose a “microservice” architecture that abstracts communications between devices and client application to enable a high degree of code reuse, portability between clinical research sites, and community-driven (and ideally, shared) analytics. Core blocks of code, developed under design controls and service-specific risk management, should provide basic communication interfaces for neural sensing, as well as for data endpoint control, graphical displays, integration with peripheral monitors, closed-loop algorithms, and stimulation updates. The current OpenMind toolkit provides these core blocks as a “getting started” package for new users. The microservice approach offers the modularity that would enable users to rapidly develop their own use-cases (i.e. a specific collection of microservices needed for the specific research study) with minimal component and integration testing needed to meet basic safety standards for investigational studies. Microservices connect and work seamlessly with other Microservices in the OpenMind platform ecosystem.
Figure 8.
Microservice architectures and implementation in biomarker discovery A) Open Mind microservice architecture is a software platform that is agnostic to the neurotechnology hardware. B) Prototype of the microservice architecture for an OCD trial (Provenza et al., 2019) implemented using the Open Mind resources.
A streamlined regulatory framework
Risk management and legal responsibility fall on the sponsor/sponsor-investigator of a study. However, many sensing and stimulation devices will have a similar protocol structure and risk profile, and so could be supported using a common regulatory template, in the spirit of a product platform that serves multiple protocol applications. Such a framework would ensure that developers have addressed all necessary elements of a risk-management and quality-systems management plan. With this in mind, the OpenMind group is developing an “Open Source Quality Management System” (or, OS-QMS) that provides processes and templates to help academic teams to efficiently implement design controls for their Investigational Device Exemptions (IDEs). By opening up the QMS to the community, we can start to transfer to a platform ecosystem that draws on the best modules among the community of users. For example, a risk template might be optimized and shared by one researcher, with templates for verification protocols provided by another group. While this effort is currently focused on research, the core elements are also applicable to commercial interests; regulations do not discriminate between academics and industry, as they are based on patient risk.
To explore these concepts, we have built an initial platform ecosystem using the Medtronic investigational Summit RC+S as a pilot system, which itself was derived from a product platform at Medtronic, the Intellis, by adding sensing capabilities to an electrical stimulation device (Kremen et al., 2017, Stanslaski et al., 2018). The Summit RC+S came with a complete software interface infrastructure (an Application Programming Interface, or API) to facilitate the customization of the device for specific therapy protocols. While an API provides flexibility, the burden of device configuration was transferred to academics, as software developers within their teams must build research software using the Summit API. Such development can be extensive, costly, and device-specific (a “monolithic” software architecture). Thus, OpenMind team members set out to develop a “microservice” software architecture that is robust to device changes, agnostic to disease application, and modular to analytical pipelines. Our value proposition is that the OpenMind software architecture enables industry segments to focus on their interests and expertise while facilitating collaboration. In this approach:
Data scientists can build on- and offline analytical tools without concern for data sourcing, enabling a very high-degree of code sharing / reuse;
Neuroscientists/clinicians can pick and choose which software building blocks suit their specific study needs (e.g. sensing-only subsystems; closed-loop control policies; integration with peripheral sensors, etc.) and then use them “out of the box” to support IDE development and research study aims;
Device developers (commercial or academic) can make modest investments (i.e. coding and the implementation of an application interface to OpenMind microservices shown in Figure 8) and have immediate access to secure data handling, graphical interfaces for configuring devices, data displays, and to the streaming of data to data servers, as well as access to a community of users already in the ecosystem.
The OpenMind streamlined regulatory framework, OS-QMS, has already enabled the rapid development of several clinical study protocols using the Summit RC+S, including a cloud-based epilepsy control system (Kremen et al., 2018), a movement disorders responsive neuromodulation system (Gilron et al., 2020), and a neuropsychiatric exploratory research platform (Provenza et al., 2019), which is illustrated in Figure 8B. The OS-QMS framework can also immediately be applied to other system disorders (e.g. bladder sensors for incontinence) and to other device platforms (e.g. NeuroPace RNS, the DyNeuMo Research System from Oxford and Bioinduction (Zamora et al., 2020), and the Cortec Brain Interchange).
With an open software and regulatory platform ecosystem for neurotechnology development and implementation, one can begin to envision a new modus operandi for research where hospital staff can interact with a unified interface for patient therapy, reducing clinical burden of uptake, and where clinical researchers can perform both multi-site and “multi-device” studies, leveraging device-agnostic data collection and therapy deployment architectures. This, of course, requires cooperation among stakeholders that is still at a nascent stage but which can be facilitated by major programs, such as the NIH PPP linked to major initiatives such as BRAIN and SPARC.
The OpenMind consortium is just one example of developing infrastructures for lowering the barriers for translation by attempting to engage the community. Yet the design of platforms can sometimes have unintended consequences resulting from their design and governance, which the community needs to consider as we propose new methods of interaction.
Avoiding Platform Wars
Platforms can have attributes that lead to disproportionate market share if certain criteria are met (see for example, (Inoue and Tsujimoto, 2018, Sun and Tse, 2007)). While this is not a problem in its own right, the implications and mitigations of market dominance are interesting to consider for neurotechnology translation. We highlight some key questions below.
Do strong network effects exist?
Network effects describe the direct and indirect effects of adding participants to the network. Network effects in neurotechnology might include gaining a critical mass of patients for data aggregation and analysis (as per the NeuroPace Inc. RNS example above), or pulling in complementary products or services that support the network’s activities, such as data science tools. Of note, large companies that can support the full lifecycle of a neurotechnology – by bundling surgical tools and planning, devices, field support and care optimization -- might gain a disproportionate advantage from such network effects.
Are there multi-homing costs?
Multi-homing costs arise when multiple systems need to be maintained and can be an issue for neurotechnology, particularly in clinical environments. A physician or hospital must keep track of multiple systems for parameter programming and for device stocking, and can suffer from a lack of volume to benefit from bulk purchasing discounts. It is worth noting that irritation with multi-device support helped to drive modularity in cardiac pacemakers to lower the burden for clinicians.
Can the unique capabilities of competitors be neutralized?
At this time, many differentiating features arguably provide incremental improvements for therapy rather than foundational improvements that significantly improve QALY levels or that shift care from being palliative to restorative. For example, if a platform can meet the minimum threshold on performance criteria, such as MRI compatibility, then higher performance claims might not qualify as being a differentiating feature. Perhaps the best way to neutralize competitor capability is to adopt modularity, which can limit the ability to generate differentiating features. Manufacturers are aware of this point and might therefore resist the uptake of modular designs.
In sum, the translational neurotechnology space has many attributes of a winner-takes-all platform market that might ultimately favour a few dominant participants. The existing neuromodulation market is controlled largely by a few major commercial entities, who acquire technology to build out their respective product platforms. Translational neural engineers, academics, and entrepreneurs should carefully consider the implications of these competitive dynamics when setting strategies that seek to balance the demands of vibrant innovation and practical market forces.
Concluding remarks
The high patient, clinical, and socio-economic burdens associated with neurological and psychiatric disorders are spurring the development of new therapeutic options, including those that leverage device-based technology. However, the current pace of translation from discovery to new patient therapies is very slow. To accelerate the successful translation and implementation of new neurotechnologies requires us all in the field, and particularly academic clinical researchers, to meet a daunting list of requirements.
Here we propose and discuss how community-supported neurotechnology platforms could prove to be essential for accelerating ideas from the bench to the bedside, maximizing scientific discovery and improving patient care. But this community requires coordination among academics, industry, and government stakeholders. Efforts such as the NIH PPP for the BRAIN initiative have started an organic movement based on user needs; the next steps include maturing this ecosystem and expanding the translational scope of activities, all with an aim to address society’s largest health care challenges.
Figure 5.
Cochlear implants: the “trunk of a neurotechnology family tree.” This schematic shows how the cochlear implant led to derivative products, ranging from a retinal prosthesis to spinalcord and deep brain stimulators.
Acknowledgements
DB received support from NIH UH3NS100549. HD received support from NIH UH3NS109556 and UH3NS115631. PS received support from NIH UH3NS100544 and UH3NS109556. GW received support from UH2&3 NS095495, Neurophysiologically Based Brain State Tracking & Modulation in Focal Epilepsy and R01-NS92882, Reliable Seizure Prediction Using Physiological Signals and Machine Learning. TD received support from the MRC Brain Network Dynamics Unit and the Royal Academy of Engineering. All authors receive funding from NIH 1U24NS113637.
Declarations
DB declares research support from Medtronic and holds a patent in the field of neurotechnology licensed to Blackrock Microsystems, HD declares no competing interests, PS declares research support from Medtronic Inc and Boston Scientific Inc, GW declares interests in Cadence Neuroscience Inc. (Licensed technology) and NeuroOne Inc (Licensed technology and holds stock), TD declares consulting agreements with Cortec Neurotechnology, Synchron, and has stock and research support with Medtronic and Bioinduction. TD has multiple patents in the field of neurotechnology, all licensed to Medtronic.
In their Perspective, Borton et al. describe the opportunities and challenges of scientific platforms to help catalyze translation of clinical neuroscience to therapeutic interventions.
Contributor Information
David A. Borton, School of Engineering and the Carney Institute for Brain Science, Brown University, Providence, RI 02906 USA;VA RR&D Center for Neurorestoration and Neurotechnology, Providence VA Medical Center, Providence, RI, USA
Heather E. Dawes, Department of Neurological Surgery, UCSF, San Francisco, CA 94143 USA; Weill Institute for Neurosciences, UCSF, San Francisco, CA 94143 USA
Gregory A. Worrell, Department of Neurology, Mayo Clinic, Rochester, MN, 55902 USA; Department of Physiology and Biomedical Engineering, Mayo Clinic, Rochester, MN, 55902 USA
Philip A. Starr, Department of Neurological Surgery, UCSF, San Francisco, CA 94134 USA; Weill Institute for Neurosciences UCSF, San Francisco, CA 94134 USA.
Timothy J. Denison, Department of Engineering Science, University of Oxford, OX3 7DQ UK; MRC Brain Network Dynamics Unit, University of Oxford, OX3 7DQ UK.
References
- Afshar P, Khambhati A, Stanslaski S, Carlson D, Jensen R, Linde D, Dani S, Lazarewicz M, Cong P, Giftakis J, Stypulkowski P, et al. A translational platform for prototyping closed-loop neuromodulation systems. Front Neural Circuits. 2012;6:117. doi: 10.3389/fncir.2012.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi N, Cavuto ML, Feng P, Leene LB, Maslik M, Mazza F, Savolainen O, Szostak KM, Bouganis C, Ekanayake J, Jackson A, et al. Towards a Distributed, Chronically-Implantable Neural Interface. 2019 9th International IEEE/EMBS Conference on Neural Engineering (NER); 2019. [20-23 March 2019]. pp. 719–724. [Google Scholar]
- Ajiboye AB, Willett FR, Young DR, Memberg WD, Murphy BA, Miller JP, Walter BL, Sweet JA, Hoyen HA, Keith MW, Peckham PH, et al. Restoration of reaching and grasping movements through brain-controlled muscle stimulation in a person with tetraplegia: a proof-of-concept demonstration. Lancet. 2017;389:1821–1830. doi: 10.1016/S0140-6736(17)30601-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassano S, Zhao X, Brinkmann BH, Kremen V, Bernabei J, Cook MJ, Denison TJ, Worrell GA, Litt B. Cloud computing for seizure detection in implanted neural devices. J Neural Eng. 2018 doi: 10.1088/1741-2552/aaf92e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud MO, Kleen JK, Mirro EA, Andrechak JC, King-Stephens D, Chang EF, Rao VR. Multi-day rhythms modulate seizure risk in epilepsy. Nat Commun. 2018;9:88. doi: 10.1038/s41467-017-02577-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capogrosso M, Milekovic T, Borton D, Wagner F, Moraud E, Mignardot J-B, Buse N, Gandar J, Barraud Q, Xing D, Rey E, et al. A Brain-Spinal Interface Alleviating Gait Deficits after Spinal Cord Injury in Primates. 2016 doi: 10.1038/nature20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C-H, Won SM, Orsborn AL, Yu KJ, Trumpis M, Bent B, Wang C, Xue Y, Min S, Woods V, Yu C, et al. Development of a neural interface for high-definition, long-term recording in rodents and nonhuman primates. Science Translational Medicine. 2020;12 doi: 10.1126/scitranslmed.aay4682. eaay4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang AT, Margo CE, Greenberg PB. Retinal implants: a systematic review. Br J Ophthalmol. 2014;98:852–6. doi: 10.1136/bjophthalmol-2013-303708. [DOI] [PubMed] [Google Scholar]
- Contarino MF, Brinke TRT, Mosch A, Lelieveld W, Postma M, Odekerken VJ, Steendam-Oldekamp TE, Van Laar T, Kuijf ML, Tjepkema-Cloostermans MC, Schuurman PR. How Many Patients would Benefit from Steering Technology for Deep Brain Stimulation? Brain Stimulation. 2016;9:144–145. doi: 10.1016/j.brs.2015.10.003. [DOI] [PubMed] [Google Scholar]
- David Brock E. Understanding Moore’s Law: Four Decades of Innovation. 2006;70 [Google Scholar]
- Denison T, Morris M, Sun F. Building a bionic nervous system. IEEE Spectrum. 2015;52:32–39. [Google Scholar]
- Deuschl G, Schupbach M, Knudsen K, Pinsker MO, Cornu P, Rau J, Agid Y, Schade-Brittinger C. Stimulation of the subthalamic nucleus at an earlier disease stage of Parkinson’s disease: concept and standards of the EARLYSTIM-study. Parkinsonism Relat Disord. 2013;19:56–61. doi: 10.1016/j.parkreldis.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Diluca M, Olesen J. The Cost of Brain Diseases: A Burden or a Challenge? Neuron. 2014;82:1205–1208. doi: 10.1016/j.neuron.2014.05.044. [DOI] [PubMed] [Google Scholar]
- Dougherty DD, Rezai AR, Carpenter LL, Howland RH, Bhati MT, O’Reardon JP, Eskandar EN, Baltuch GH, Machado AD, Kondziolka D, Cusin C, et al. A Randomized Sham-Controlled Trial of Deep Brain Stimulation of the Ventral Capsule/Ventral Striatum for Chronic Treatment-Resistant Depression. Biological Psychiatry. 2015;78:240–248. doi: 10.1016/j.biopsych.2014.11.023. [DOI] [PubMed] [Google Scholar]
- Famm K, Litt B, Tracey KJ, Boyden ES, Slaoui M. Drug discovery: a jump-start for electroceuticals. Nature. 2013;496:159–61. doi: 10.1038/496159a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg NA, Haddad PM, Carpenter L, Gannon B, Sharpe R, Young AH, Joyce E, Rowe J, Wellsted D, Nutt DJ, Sahakian BJ. The size, burden and cost of disorders of the brain in the UK. Journal of Psychopharmacology (Oxford, England) 2013;27:761–770. doi: 10.1177/0269881113495118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RS, Velasco AL. Electrical brain stimulation for epilepsy. Nat Rev Neurol. 2014;10:261–70. doi: 10.1038/nrneurol.2014.59. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Brown TL, Marston NAU, Daskalakis ZJ, De Castella A, Kulkarni J. Transcranial Magnetic Stimulation in the Treatment of Depression: A Double-blind, Placebo-Controlled Trial. Archives of General Psychiatry. 2003;60:1002–1008. doi: 10.1001/archpsyc.60.9.1002. [DOI] [PubMed] [Google Scholar]
- Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilja V, Pandarinath C, Blabe CH, Nuyujukian P, Simeral JD, Sarma AA, Sorice BL, Perge JA, Jarosiewicz B, Hochberg LR, Shenoy KV, et al. Clinical translation of a high-performance neural prosthesis. Nat Med. 2015;21:1142–5. doi: 10.1038/nm.3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilron RE, Little S, Perrone R, Wilt R, De Hemptinne C, Yaroshinsky MS, Racine CA, Wang S, Ostrem JL, Larson PS, Wang DD, et al. Chronic wireless streaming of invasive neural recordings at home for circuit discovery and adaptive stimulation. bioRxiv. 2020 doi: 10.1038/s41587-021-00897-5. 2020.02.13.948349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson TL, Diaz-Botia CA, Kharazia V, Maharbiz MM, Sabes PN. The “sewing machine” for minimally invasive neural recording. bioRxiv. 2019 578542. [Google Scholar]
- Hendriks S, Grady C, Ramos KM, Chiong W, Fins JJ, Ford P, Goering S, Greely HT, Hutchison K, Kelly ML, Kim SYH, et al. Ethical Challenges of Risk, Informed Consent, and Posttrial Responsibilities in Human Research With Neural Devices: A Review. JAMA Neurol. 2019 doi: 10.1001/jamaneurol.2019.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron JA, Thompson MC, Brown T, Chizeck HJ, Ojemann JG, Ko AL. Chronic electrocorticography for sensing movement intention and closed-loop deep brain stimulation with wearable sensors in an essential tremor patient. J Neurosurg. 2017;127:580–587. doi: 10.3171/2016.8.JNS16536. [DOI] [PubMed] [Google Scholar]
- Holtzheimer PE, Husain MM, Lisanby SH, Taylor SF, Whitworth LA, Mcclintock S, Slavin KV, Berman J, Mckhann GM, Patil PG, Rittberg BR, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry. 2017;4:839–849. doi: 10.1016/S2215-0366(17)30371-1. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Tsujimoto M. New market development of platform ecosystems: A case study of the Nintendo Wii. Technological Forecasting and Social Change. 2018;136:235–253. [Google Scholar]
- Jobst BC. Electrical stimulation in epilepsy: vagus nerve and brain stimulation. Curr Treat Options Neurol. 2010;12:443–53. doi: 10.1007/s11940-010-0087-4. [DOI] [PubMed] [Google Scholar]
- Khanna P, Stanslaski S, Xiao Y, Ahrens T, Bourget D, Swann N, Starr P, Carmena JM, Denison T. Enabling closed-loop neurostimulation research with downloadable firmware upgrades. 2015 IEEE Biomedical Circuits and Systems Conference (BioCAS); 2015. [22-24 Oct. 2015]. pp. 1–6. [Google Scholar]
- Kilgore KL, Bhadra N. Reversible nerve conduction block using kilohertz frequency alternating current. Neuromodulation : journal of the International Neuromodulation Society. 2014;17:242–255. doi: 10.1111/ner.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulousakis P, Andrade P, Visser-Vandewalle V, Sesia T. The Nucleus Basalis of Meynert and Its Role in Deep Brain Stimulation for Cognitive Disorders: A Historical Perspective. Journal of Alzheimer’s Disease. 2019;69:905–919. doi: 10.3233/JAD-180133. [DOI] [PubMed] [Google Scholar]
- Kremen V, Brinkmann BH, Kim I, Chang S, Gompel JJV, Herron JA, Baldassano S, Patterson EE, Litt B, Denison T, Worrell GA. Continuous active probing and modulation of neural networks with a wireless implantable system. 2017 IEEE Biomedical Circuits and Systems Conference (BioCAS); 2017. [19-21 Oct. 2017]. pp. 1–4. [Google Scholar]
- Kremen V, Brinkmann BH, Kim I, Guragain H, Nasseri M, Magee AL, Attia TP, Nejedly P, Sladky V, Nelson N, Chang S, et al. Integrating Brain Implants With Local and Distributed Computing Devices: A Next Generation Epilepsy Management System. IEEE Journal of Translational Engineering in Health and Medicine. 2018;6:1–12. doi: 10.1109/JTEHM.2018.2869398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenbrunner Jc CC, O’Dougherty S. Designing and Implementing Provider Payment Systems: How to Manuals. Washington, DC: The World Bank. 2009 [Google Scholar]
- Lazaro-Munoz G, Yoshor D, Beauchamp MS, Goodman WK, Mcguire AL. Continued access to investigational brain implants. Nat Rev Neurosci. 2018;19:317–318. doi: 10.1038/s41583-018-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Urso A, Ponte RMD, Costa T, Valente V, Giagka V, Serdijn WA, Constandinou TG, Denison T. Bidirectional Bioelectronic Interfaces: System Design and Circuit Implications. IEEE Solid-State Circuits Magazine. 2020;12:30–46. [Google Scholar]
- Malekmohammadi M, Herron J, Velisar A, Blumenfeld Z, Trager MH, Chizeck HJ, Bronte-Stewart H. Kinematic Adaptive Deep Brain Stimulation for Resting Tremor in Parkinson’s Disease. Mov Disord. 2016;31:426–8. doi: 10.1002/mds.26482. [DOI] [PubMed] [Google Scholar]
- Milekovic T, Sarma AA, Bacher D, Simeral JD, Saab J, Pandarinath C, Sorice BL, Blabe C, Oakley EM, Tringale KR, Eskandar E, et al. Stable long-term BCI-enabled communication in ALS and locked-in syndrome using LFP signals. J Neurophysiol. 2018;120:343–360. doi: 10.1152/jn.00493.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miocinovic S, Noecker AM, Maks CB, Butson CR, Mcintyre CC. Cicerone: stereotactic neurophysiological recording and deep brain stimulation electrode placement software system. Acta Neurochir Suppl. 2007;97:561–7. doi: 10.1007/978-3-211-33081-4_65. [DOI] [PubMed] [Google Scholar]
- Nair DR, Laxer KD, Weber PB, Murro AM, Park YD, Barkley GL, Smith BJ, Gwinn RP, Doherty MJ, Noe KH, Zimmerman RS, et al. Nine-year prospective efficacy and safety of brain-responsive neurostimulation for focal epilepsy. Neurology. 2020 doi: 10.1212/WNL.0000000000010154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH_PPP https://braininitiative.nih.gov/brain-programs/public-private-partnerships#:~:text=The%20goal%20of%20BRAIN%20Initiative,conduct%20clinical%20research%20in%20the.
- Nurmikko AV, Donoghue JP, Hochberg LR, Patterson WR, Song Y, Bull CW, Borton DA, Laiwalla F, Park S, Ming Y, Aceros J. Listening to Brain Microcircuits for Interfacing With External World-Progress in Wireless Implantable Microelectronic Neuroengineering Devices. Proceedings of the IEEE. 2010;98:375–388. doi: 10.1109/JPROC.2009.2038949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pels EGM, Aarnoutse EJ, Leinders S, Freudenburg ZV, Branco MP, Van Der Vijgh BH, Snijders TJ, Denison T, Vansteensel MJ, Ramsey NF. Stability of a chronic implanted brain-computer interface in late-stage amyotrophic lateral sclerosis. Clin Neurophysiol. 2019;130:1798–1803. doi: 10.1016/j.clinph.2019.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter ME, A H, J E. How Smart, Connected Products Are Transforming Competition. Harvard Business Review. 2014 [Google Scholar]
- Provenza NR, Matteson ER, Allawala AB, Barrios-Anderson A, Sheth SA, Viswanathan A, Mcingvale E, Storch EA, Frank MJ, Mclaughlin NCR, Cohn JF, et al. The Case for Adaptive Neuromodulation to Treat Severe Intractable Mental Disorders. Front Neurosci. 2019;13:152. doi: 10.3389/fnins.2019.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulliam CL, Heldman DA, Orcutt TH, Mera TO, Giuffrida JP, Vitek JL. Motion sensor strategies for automated optimization of deep brain stimulation in Parkinson’s disease. Parkinsonism Relat Disord. 2015;21:378–82. doi: 10.1016/j.parkreldis.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos KM, Grady C, Greely HT, Chiong W, Eberwine J, Farahany NA, Johnson LSM, Hyman BT, Hyman SE, Rommelfanger KS, Serrano EE, et al. The NIH BRAIN Initiative: Integrating Neuroethics and Neuroscience. Neuron. 2019;101:394–398. doi: 10.1016/j.neuron.2019.01.024. [DOI] [PubMed] [Google Scholar]
- Rebelo P, Green AL, Aziz TZ, Kent A, Schafer D, Venkatesan L, Cheeran B. Thalamic Directional Deep Brain Stimulation for tremor: Spend less, get more. Brain Stimul. 2018;11:600–606. doi: 10.1016/j.brs.2017.12.015. [DOI] [PubMed] [Google Scholar]
- Regalia G, Onorati F, Lai M, Caborni C, Picard RW. Multimodal wrist-worn devices for seizure detection and advancing research: Focus on the Empatica wristbands. Epilepsy Research. 2019;153:79–82. doi: 10.1016/j.eplepsyres.2019.02.007. [DOI] [PubMed] [Google Scholar]
- Riva-Posse P, Choi KS, Holtzheimer PE, Crowell AL, Garlow SJ, Rajendra JK, Mcintyre CC, Gross RE, Mayberg HS. A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. Molecular Psychiatry. 2017;23:843. doi: 10.1038/mp.2017.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse AG, Stanslaski SR, Cong P, Jensen RM, Afshar P, Ullestad D, Gupta R, Molnar GF, Moran DW, Denison TJ. A chronic generalized bi-directional brain-machine interface. J Neural Eng. 2011;8 doi: 10.1088/1741-2560/8/3/036018. 036018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannell JW, Blanckley A, Boldon H, Warrington B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat Rev Drug Discov. 2012;11:191–200. doi: 10.1038/nrd3681. [DOI] [PubMed] [Google Scholar]
- Schalk G, Mcfarland DJ, Hinterberger T, Birbaumer N, Wolpaw JR. BCI2000: a general-purpose brain-computer interface (BCI) system. IEEE Trans Biomed Eng. 2004;51:1034–43. doi: 10.1109/TBME.2004.827072. [DOI] [PubMed] [Google Scholar]
- Stanslaski S, Herron J, Chouinard T, Bourget D, Isaacson B, Kremen V, Opri E, Drew W, Brinkmann B, Gunduz A, Adamski T, et al. A Chronically-Implanted Neural Coprocessor for Exploring Treatments for Neurological Disorders. IEEE Trans Biomed Circuits Syst. 2018 doi: 10.1109/TBCAS.2018.2880148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieglitz T. Of Man and Mice: Translational Research in Neurotechnology. Neuron. 2020;105:12–15. doi: 10.1016/j.neuron.2019.11.030. [DOI] [PubMed] [Google Scholar]
- Stronks HC, Dagnelie G. The functional performance of the Argus II retinal prosthesis. Expert review of medical devices. 2014;11:23–30. doi: 10.1586/17434440.2014.862494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun FT, Morrell MJ. Closed-loop neurostimulation: the clinical experience. Neurotherapeutics. 2014a;11:553–63. doi: 10.1007/s13311-014-0280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun FT, Morrell MJ. The RNS System: responsive cortical stimulation for the treatment of refractory partial epilepsy. Expert Rev Med Devices. 2014b;11:563–72. doi: 10.1586/17434440.2014.947274. [DOI] [PubMed] [Google Scholar]
- Sun M, Tse E. When Does the Winner Take All in Two-Sided Markets? Review of Network Economics. 2007;6 [Google Scholar]
- Swann NC, De Hemptinne C, Miocinovic S, Qasim S, Wang SS, Ziman N, Ostrem JL, San Luciano M, Galifianakis NB, Starr PA. Gamma Oscillations in the Hyperkinetic State Detected with Chronic Human Brain Recordings in Parkinson’s Disease. J Neuroscience. 2016;36:6445–6458. doi: 10.1523/JNEUROSCI.1128-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann NC, De Hemptinne C, Thompson MC, Miocinovic S, Miller AM, Gilron R, Ostrem JL, Chizeck HJ, Starr PA. Adaptive deep brain stimulation for Parkinson’s disease using motor cortex sensing. J Neural Eng. 2018;15 doi: 10.1088/1741-2552/aabc9b. 046006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansteensel MJ, Pels EGM, Bleichner MG, Branco MP, Denison T, Freudenburg ZV, Gosselaar P, Leinders S, Ottens TH, Van Den Boom MA, Van Rijen PC, et al. Fully Implanted Brain-Computer Interface in a Locked-In Patient with ALS. N Engl J Med. 2016;375:2060–2066. doi: 10.1056/NEJMoa1608085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicheva P, Butler M, Shotbolt P. Deep brain stimulation for obsessive-compulsive disorder: A systematic review of randomised controlled trials. Neuroscience & Biobehavioral Reviews. 2020;109:129–138. doi: 10.1016/j.neubiorev.2020.01.007. [DOI] [PubMed] [Google Scholar]
- Wagner FB, Mignardot J-B, Le Goff-Mignardot CG, Demesmaeker R, Komi S, Capogrosso M, Rowald A, SeÁÑEz I, Caban M, Pirondini E, Vat M, et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature. 2018;563:65–71. doi: 10.1038/s41586-018-0649-2. [DOI] [PubMed] [Google Scholar]
- Zamora M, Toth R, Ottaway J, Gillbe T, Martin S, Benjaber M, Lamb G, Noone T, Nairac Z, Constandinou TG, Herron J, et al. DyNeuMo Mk-1: A Fully-Implantable, Motion-Adaptive Neurostimulator with Configurable Response Algorithms. bioRxiv. 2020 2020.09.10.292284. [Google Scholar]
- Zuk P, Torgerson L, Sierra-Mercado D, LÁZaro-MuÑOz G. Neuroethics of Neuromodulation: An Update. Current opinion in biomedical engineering. 2018;8:45–50. doi: 10.1016/j.cobme.2018.10.003. In their Perspective, Borton et al. describe the opportunities and challenges of scientific platforms to help catalyze translation of clinical neuroscience to therapeutic interventions. [DOI] [PMC free article] [PubMed] [Google Scholar]