Abstract
Dearomative cycloaddition reactions represent an ideal means of converting flat arenes into three dimensional architectures of increasing interest in medicinal chemistry. Quinolines, isoquinolines and quinazolines, despite containing latent diene and alkene subunits, are scarcely applied in cycloaddition reactions, due to inherent low reactivity of aromatic systems and selectivity challenges. Here we disclose an energy-transfer mediated, highly regio- and diastereoselective intermolecular [4+2] dearomative cycloaddition reaction of these bicyclic azaarenes with a plethora of electronically-diverse alkenes. This approach bypasses the general reactivity and selectivity issues, thereby providing various bridged polycycles which previously have been inaccessible or required elaborate synthetic efforts. Computational studies with density functional theory elucidate the mechanism and origins of the observed regio- and diastereoselectivities.
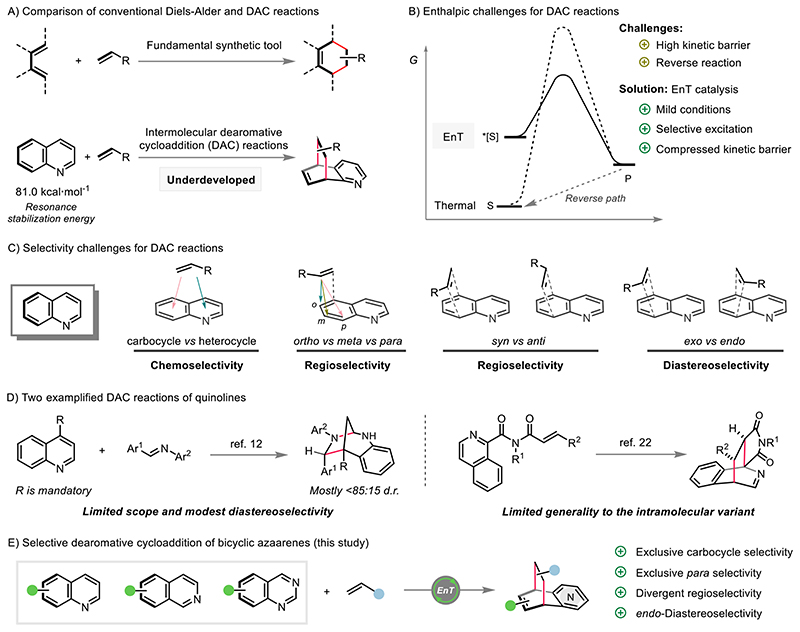
Advancing efficient and selective catalytic processes to access modular structural complexity is a central theme of modern organic synthesis (1, 2). Cycloaddition reactions are among the most synthetically useful means to meet this end by leveraging the construction of sophisticated architectures from readily available feedstocks, and feature excellent step/atom economy with predictable and exclusive stereoselectivity (Fig. 1A) (3, 4). Therefore, cycloaddition reactions have played a prominent role in synthetic chemistry and constitute a major theme in chemistry education (5). The classical substrates employed in cycloaddition reactions are unsaturated hydrocarbons such as 1,3-butadienes, alkenes, alkynes and other related compounds. Industrially, these unsaturated hydrocarbons are produced stepwise via petroleum refining to afford saturated hydrocarbons, followed by cracking or dehydrogenation under harsh conditions (6). However, the initial refining can also directly afford unsaturated N-heterocycles, such as quinoline, isoquinoline and quinaldine. Despite containing both latent diene and alkene subunits, these bicyclic azaarenes have shown dramatically limited applications in cycloaddition reactions. This phenomenon can be attributed both to the severe reactivity challenges and selectivity issues towards the intermolecular dearomative cycloaddition (DAC) reaction (7–10). First, overcoming the kinetic barrier of DAC reactions has traditionally required harsh conditions (Fig. 1B, dashed curve) or an extremely reactive or tailored cycloaddend (11–14). Second, the dearomative cycloadditions are endergonic processes due to the breaking of aromaticity, 81.0 kcal·mol-1 for quinoline and 76.5 kcal·mol-1 for quinazoline (15), thus under thermally induced reaction conditions the starting materials will be favored and a reverse reaction is feasible (Fig. 1B, dashed curve). Last, even if the kinetic and thermodynamic issues could be overcome, the inherent severe chemo-, regio- and diastereoselectivity challenges would still diminish the broad utility of such methods (Fig. 1C).
Fig. 1. Dearomative cycloaddition reactions.
A) Comparison of conventional Diels-Alder and DAC reactions. B) Enthalpic challenge for DAC reactions. C) Selectivity challenges for DAC reactions of bicyclic azaarenes. D) Two exemplified DAC reactions of quinolines. E) Selective [4+2] DAC of quinolines, isoquinolines and quinazolines (this study). EnT = energy transfer. S = substrate. P = product.
In this context, an energy transfer (EnT) approach paves the way towards the solution of the aforementioned reactivity and selectivity problems (16–18). Initially, the ground state azaarene could be selectively excited to a higher energy triplet state by EnT process, thus leading to a compressed kinetic barrier compared to the thermally controlled pathway (Fig. 1B, solid curve vs dashed curve). Moreover, the combination of significantly higher triplet energies in the terminal dearomatization products (which are thus not amenable to further EnT) and the sufficiently mild conditions prevents the reverse reaction. Compared to the conventional ground state chemistry, the unconventional triplet state intermediate could potentially lead to unexpected chemo-, regio- and diastereoselectivities. Recent works have demonstrated the application of EnT process for enabling dearomative cycloaddition reactions, but have faced large limitations. As such, the reaction has mostly been limited to electron-rich arenes or their prefunctionalized derivatives, and intramolecular variants (Fig. 1D), which require multi-step substrate synthesis and offer limited generality (19–27). Additionally, inferior selectivity has been observed in both the intra- and intermolecular DAC reactions (Fig. 1D). All these limitations are also commonly encountered under the thermally-controlled DAC reactions (28–33). Herein, we apply EnT process in combination with Brønsted or Lewis acid mediators to enable the selective DAC reaction of simple quinolines, isoquinolines and quinazolines with a broad variety of alkenes (Fig. 1E). This reaction features exclusive carbocyclic [4+2] cycloaddition, high/divergent regioselectivity and excellent endo-diastereoselectivity.
Evaluation of reaction conditions
Initial experiments involved the reaction of quinoline 1a and 1-hexene 2a in the presence of the commercially available photosensitizer [Ir-F] ([Ir(dF(CF3)ppy)2(dtbbpy)](PF6), 1 or 2 mol%) under the irradiation of blue LEDs (Fig. 2A-2B and fig. S1-S2). Under condition A with HFIP as the solvent, both regiomers 3a (syn: R2 close to the nitrogen of product) and 3b (anti: the reverse cycloaddition order) were isolated in comparable yields of 41% and 46%, but with excellent endo-diastereoselectivities (entry 1, Fig. 2B). By applying condition B with CH2Cl2 as the solvent and BF3·OEt2 (1.25 equiv.) as an additive, 3b was obtained as the predominant regiomer over 3a (entry 2). Next, when using 6-methyl quinoline 1b, under condition B, two regiomers 4a and 4b were obtained (entry 4). Interestingly, back to condition A, a single regiomer 4a was isolated in excellent yield (88%) and as a single endo-diastereomer (entry 5), while the other regiomer 4b was not detected by crude 1H NMR analysis. By using 4-methyl-1-pentene (2b), a single regiomer 5a was also obtained with good outcome (entry 6). Styrene (2c) was also compatible and provided a single regioisomeric product 6a with comparable results (entry 7). Both the unactivated alkene 2a-b and the activated one 2c lead to the consistent endo- diastereoselectivity of products 3-6. Excluding the acidic components, BF3·OEt2 or HFIP, respectively, completely suppressed product formation (entries 3 and 8). Overall, regarding both the reaction efficiency and selectivity, condition A with HFIP as Brønsted acid mediator was assessed to be optimal for the substituted quinoline 1b while condition B with BF3·OEt2 as Lewis acid mediator was more suitable for the unsubstituted substrate (1a).
Fig. 2. Reaction development.
A) Reaction scheme. B) Conditions evaluation (see table S1-S7). One representative enantiomer of the racemic product is presented for all thorough the text. HFIP = 1,1,1,3,3,3-hexafluoro-propan-2-ol.
Mechanistic investigation
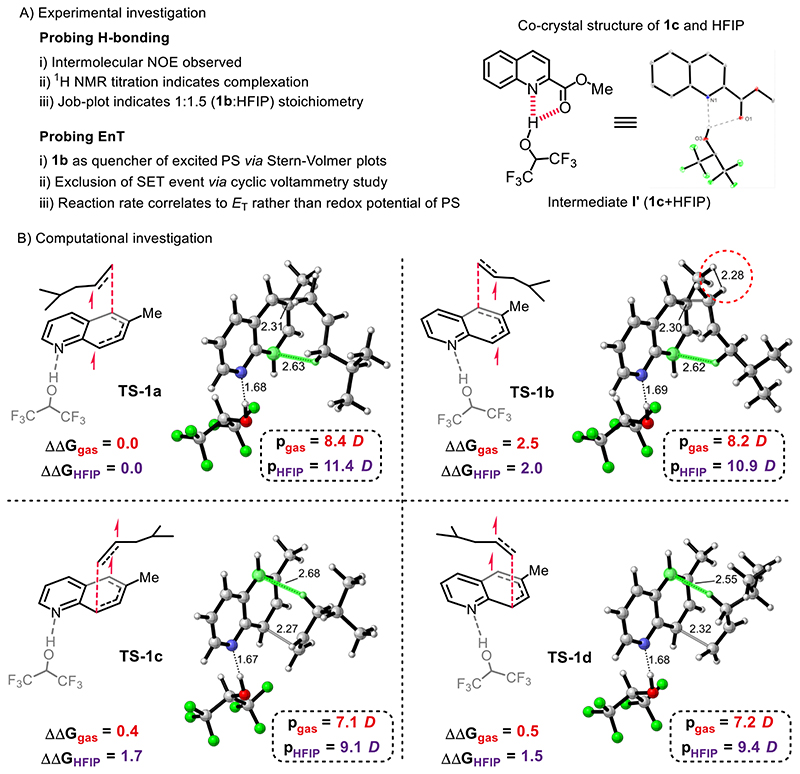
The influence of the acidic solvent HFIP (34–35) and the additive BF3·OEt2 on both the reaction efficiency and selectivities indicates the pivotal role of the Brønsted (36) and Lewis (37–38) acids. Taking this into account, we hypothesized that either the solvent HFIP or the additive BF3·OEt2 was binding to the quinolines 1a and 1b through hydrogen-bonding or Lewis acid/base interaction, respectively, to give an intermediate I, characterized by markedly decreased triplet energy (Fig. 2A). Intermediate I can then be selectively excited to the corresponding triplet state (II) by EnT process. The resultant highly reactive biradical intermediate II would then add across an alkene in a regio- and stereoselective fashion, thus affording the [4+2] DCA products 3-6. A number of mechanistic experiments support this scenario (Fig. 3A, see supplementary materials for details). First, control experiments using H-bonding acceptor co-solvents such as 1,4-dioxane, acetonitrile and N,N-dimethylacetamide (DMA) with HFIP, led to remarkable decrease of the reaction efficiency (table S8). Second, the H-bonding between HFIP and quinoline 1b in solution is supported by a series of NMR experiments including 1H NMR titration, NOESY and Job-plot analysis (fig. S3–S9). Third, in the solid state, a dual H-bonding interaction is directly observed in the co-crystal structure of quinoline-2-carboxylate (1c) and HFIP (Fig. 3A and fig. S22). These observations strongly support an H-bonding interaction between the nitrogen atom of quinoline and the alcoholic proton of HFIP. The Lewis acid/base interaction between BF3·OEt2 and quinolines has been previously disclosed (39). The proposal of these interactions to decrease the singlet-triplet energy gap was confirmed by density functional theory (DFT) calculations. Accordingly, triplet energy of 1a is calculated to be 61.7 kcal·mol-1. In contrast, after complexation with HFIP, protonation by HCl or interaction with BF3, decreased triplet energies of 61.2, 57.7 and 58.9 kcal·mol-1 are respectively predicted, rendering these adducts more amenable to energy transfer by the photosensitizer [Ir-F] (60.8 kcal·mol-1) (fig. S14-S15). This effect of Brønsted and Lewis acids on the triplet energy of quinolines is consistent with the experimental results (Fig. 2B, entries 3 and 8). Next, the proposed EnT activation of quinoline 1b by the excited photosensitizer [Ir-F] was supported by Stern-Volmer luminescence quenching analysis, since a competitive single electron transfer (SET) event was found to be thermodynamically unfeasible by cyclic voltammetry study (fig. S10-12). Conversely, the suggested energy transfer (EnT) process was further corroborated by the correlation of the reaction rate to triplet energies rather than the redox potentials of various photosensitizers (table S10). Overall, both the experimental and computational studies suggest that the ground state quinolines can be activated through Brønsted/Lewis acids mediated EnT process, which is the key for success of the [4+2] DAC reaction.
Fig. 3. Mechanistic investigation.
A) Experimental investigation (see fig. S3-S12 and table S8-S10). B) Computational investigation. Calculated C–C forming TS geometries, energies and dipole moments for the [4+2] DCA reaction between triplet 1b and 2b at the ωB97X-D/6-311++G(d,p), SMD (HFIP)//ωB97X-D/6-31G(d), SMD (HFIP) level of theory. Energies are in kcal·mol-1. Interatomic distances are in Å. Green highlights indicate interactions between developing radical SOMOs and allylic C–H bonds. NOE = nuclear overhauser effect. PS = photosensitizer. SET = single electron transfer. D = Debye.
The high levels of regio- and stereocontrol observed for unactivated alkenes were of particular interest. We initially hypothesized that spin densities of the substrate in the triplet state would have a significant impact on free energy barriers. While calculated spin densities showed that N-protonation and coordination generally lead to increased spin density at the 5-position for quinoline 1b in the triplet state (fig. S13), rate-determining C–C forming transition states (TSs) for the formal [4+2] reaction between quinoline 1b and alkene 2b exhibited low regioselectivity (ΔΔG‡ 0.4–0.5 kcal·mol-1) when calculated in the gas-phase (Fig. 3B), indicating that regiocontrol is not solely due to differences in spin densities. The inclusion of an implicit solvent model for HFIP greatly increases the predicted regioselectivity to levels in good agreement with experimental observations (ΔΔG‡ 1.5-1.7 kcal·mol-1). These results suggest that TS polarity and solvent stabilization play an important role in enforcing regiocontrol. Calculated TS dipole moments confirm that TS-1a, the TS leading to the major product isomer 5a, is significantly more polar (11.4 Debye in HFIP) compared to regioisomeric TSs TS-1c (9.1 Debye) and TS-1d (9.4 Debye). In all four TSs, a close, stabilizing interaction (2.55–2.68 Å) is observed between the developing SOMO of quinoline 1b and one of the allylic C–H bonds on 2b, which anchors the approaching alkene and shapes the lowest-energy TS geometry. As a result, a destabilizing steric clash (H...H distance 2.28 Å) exists between the approaching alkene and the methyl substituent on quinoline 1b in TS-1b. This steric clash is absent from the TS-1a, contributing to the high diastereoselectivity (ΔΔG‡ 2.0 kcal·mol-1). The effects of dispersion were calculated to be insignificant for this reaction (see supplementary material). Calculated selectivities for the reaction between 1b and styrene (2c) found similarly good agreement with experimental observations, with π-π stacking as an important determining factor of TS geometries and energies (fig. S20).
Scope of the [4+2] DAC reaction
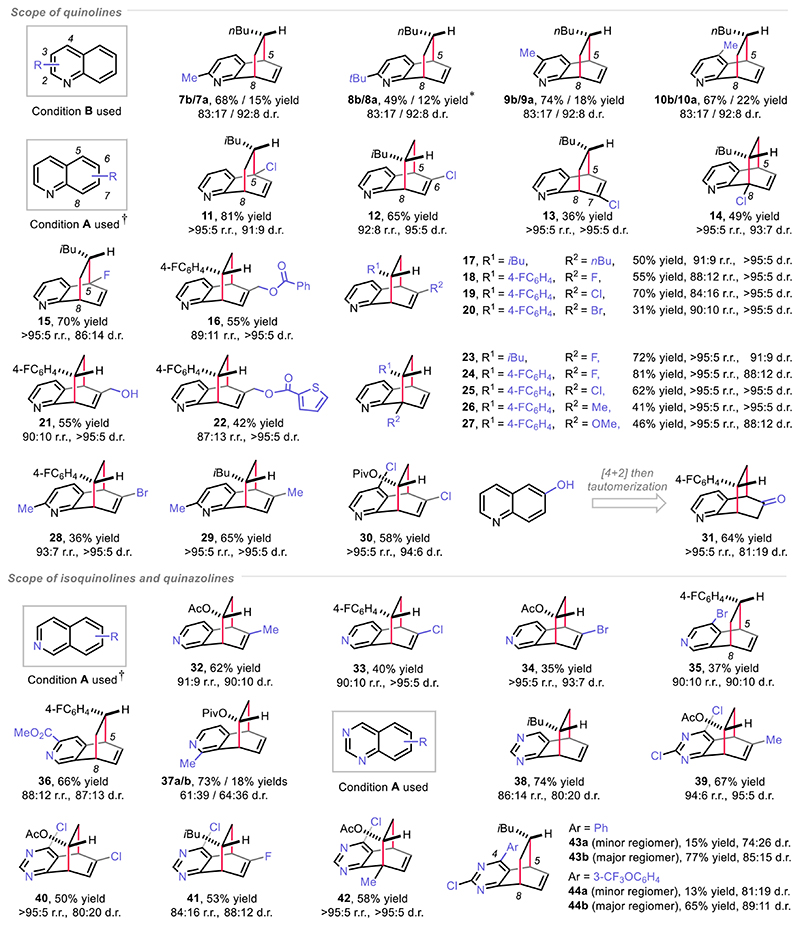
Having assessed the feasibility of the dearomative approach, the reaction scope with respect to the bicyclic azaarenes was evaluated (Fig. 4). In general, the quinolines bearing substituents at each of the 2-8 positions are compatible in this [4+2] dearomative cycloaddition reaction. Accordingly, under condition B, 2, 3 or 4-alkyl substituted quinolines were converted to the corresponding dearomatization products 7-10 in good yields, regio- and diastereoselectivities. Condition A was applied for the 5, 6, 7 or 8-substituted quinolines, thus giving the products 11-31 with remarkable regio- and diastereocontrol. The anti-regioselectivity was observed as predominant when using 2, 3, 4, 5 or 7-substituted quinolines (products 7-11, 13 and 15). A reverse order of addition (svn-regioselectivitv) occurred when using 6 or 8-substituted quinolines (products 12, 14 and 16-31). HCl (2 equiv.) was added to accelerate the reaction or to promote the conversion of the less reactive 5 or 8 positions substituted quinolines (products 11, 14 and 15, table S5-S7). 6-Hydroxyquinoline proved amenable to the [4+2] DAC reaction by providing a ketone 29 through the tautomerization of an enol intermediate. Likewise, both isoquinolines and quinazolines showed good compatibility in the [4+2] DAC reaction (products 32-44). As a limitation, when 1-methyl isoquinoline was applied in this transformation, two separable regiomers 37a and 37b were afforded with modest levels of diastereoselectivity. With respect to quinazolines, the syn-regioselectivity was commonly observed (products 38-42). However, when 4-aryl quinazolines were used as substrates, the reverse anti-regiomers became predominant (43b/44b vs 43a/44a).
Fig. 4. Scope of bicyclic azaarenes.
*NMR yield of minor regiomer 8a is presented. †HCl (2 equiv.) was used. Regioisomeric ratio (r.r.) and diastereomeric ratio (d.r.) are determined by crude 1H NMR analysis. For experimental details, see supplementary materials.
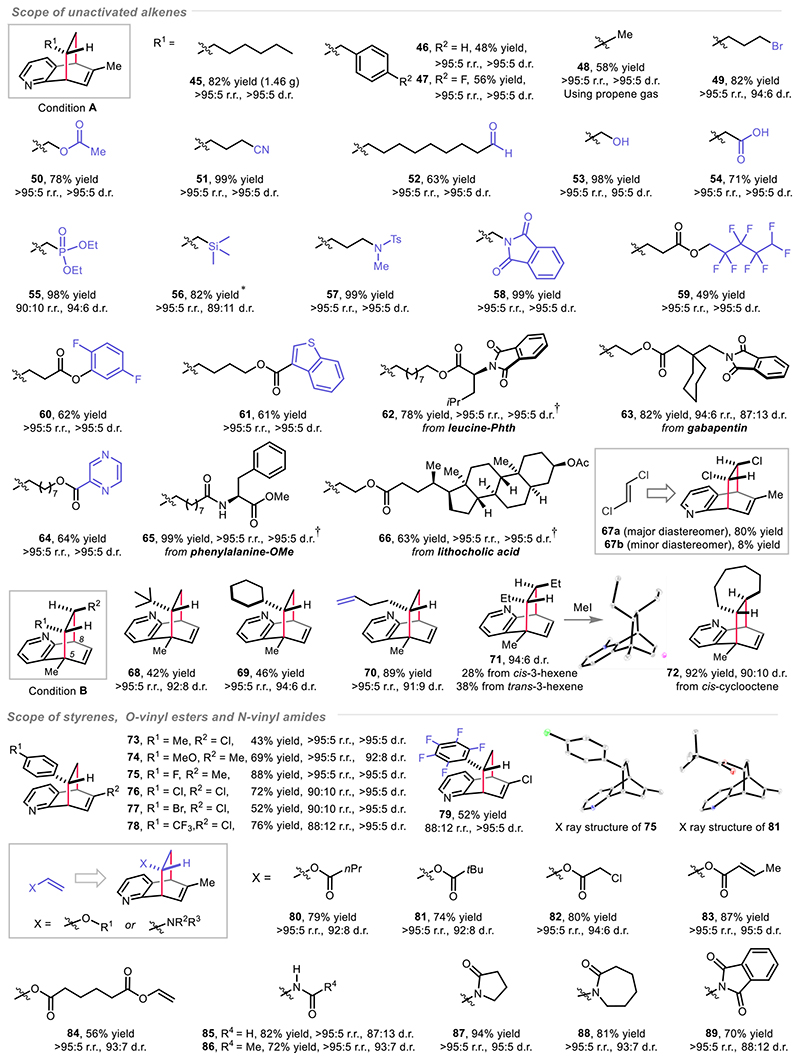
A quite broad scope of unactivated and activated alkenes proved amenable substrates, as presented in Fig. 5. Excellent functional group compatibility was observed, such as bromo (49), esters (50), nitrile (51), aldehyde (52), hydroxyl (53), carboxylic acid (54), phosphate (55), silane (56), sulfonamide (57), phthalimide (58), poly-fluorinated alkane or arene (59, 60), benzothiophene (61), pyrazine (64), and many bioactive motifs (62-63 and 65-66). The [4+2] DAC reaction also proceeded efficiently with gaseous propene (2 bar), thus giving 48 in 58% yield as a single regiomer and diastereomer. A gram scale reaction was performed using 6-methyl quinoline and 1-octene to afford 1.46 g of 45 (5.73 mmol, 82% yield, >95:5 r.r. and >95:5 d.r.), (see detailed step-by-step protocol in the supplementary materials). An internal alkene, trans-1,2-dichloroethylene was also compatible and gave the target product as two separable diastereomers 67a and 67b. Aiming at the construction of challenging all-carbon quaternary stereocenters, 5-methyl quinoline was coupled with a plethora of alkenes under condition B thus providing the corresponding products (68-70) with good yields and selectivities. Stereoconvergence was observed when using trans- or cis-3-hexene, respectively, thus consistently leading to the product 71 with excellent trans-diastereoselectivity. cis-Cyclooctene was applicable to provide 72 in 92% yield and with good trans-diastereoselectivity (90:10). In contrast, unsymmetrical 1,2-disubstituted alkenes displayed decreased selectivity towards the corresponding products. Trisubstituted and tetrasubstituted alkenes proved not compatible with this transformation (table S11-S12). Various electronically diverse styrenes, O-vinyl esters and N-vinvl amides are also applicable in the [4+2] DAC reaction (products 73-89), providing excellent levels of both regio- and diastereocontrol. X-ray structures of 75 and 81 are presented to confirm the configuration of the products. A complementary scope of alkenes is available in the supplementary materials (table S13). Additionally, to improve the user-friendliness and enhance the reproducibility of this method, an assessment of the condition-based sensitivity was performed (40). As a result, this reaction was found to be comparatively sensitive to oxygen and high temperature. A slightly decreased yield was observed when the reaction was scaled up by 70 times, albeit under reduced photosensitizer loading. In contrast, the concentration of substrates, moisture and light intensity did not show any remarkable influence on the reaction (fig. S21).
Fig. 5. Scope of alkenes.
*Photosensitizer Ir(dF-Me-ppy)2(dtbpy)(PF6) was used. †The d.r. value refers to the [4+2] cycloaddition resulted motif of the product. HCl (2 equiv.) was used for obtaining 46-47, 57-60, 62, 64-65, 75-84 and 88-89. Regioisomeric ratio (r.r.) and diastereomeric ratio (d.r.) are determined by crude 1H NMR analysis. For experimental details, see supplementary materials.
Synthetic applications
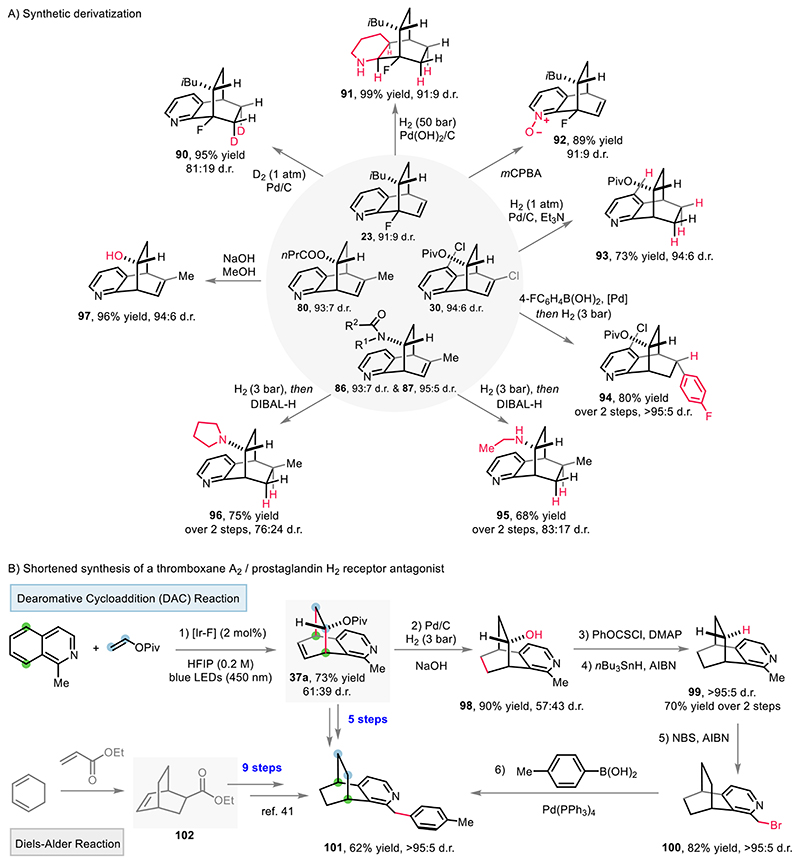
Next, derivatization of the obtained [4+2] DAC products towards relevant three-dimensional scaffolds was conducted (Fig. 6A). Starting from 23, chemoselective reduction were performed under D2 atmosphere (1 atm) or H2 (50 bar), thus affording a deuterated tetrahydro 5,8-ethanoquinoline 90 or a fully saturated decahydro 5,8-ethanoquinoline 91, respectively. Compound 23 could also be converted to the pyridine N-oxide 92 by chemoselective meta-chloroperbenzoic acid (mCPBA) oxidation, without epoxidation of the remaining C=C double bond. All these motifs are accompanied by a pharmaceutically appealing fluorinated quaternary stereocenter which has been readily introduced by the [4+2] DAC reaction using commercial 8-fluoroquinoline. The poly-chlorinated dearomatization product 30 can undergo a dechlorination-reduction reaction to give 93. The alkenyl chloride of 30 was chemoselectively coupled with 4-fluorophenylboronic acid — overcoming the presence of the heteroaryl chloride — under palladium catalysis. Followed by facile hydrogenation, 94 was afforded in 80% yield over two steps and as a single diastereomer. Starting from the amide-containing dearomatization products 86 and 87, a sequential two-step reduction reaction provided a secondary amine 95 and a tertiary one 96, respectively. Likewise, an alcohol 97 was produced by a convenient hydrolysis of compound 80. To emphasize the applicability of this [4+2] DAC reaction towards biologically active compounds, the shortened synthesis of a thromboxane A2/prostaglandin H2 receptor antagonist (121) is presented (Fig. 6B) (41). Accordingly, the dearomatization product 37a was reduced and hydrolyzed in a one-pot reaction to give 98. A subsequent Barton-McCombie deoxygenation gave 99 as a single diastereomer. Following benzylic bromination, a palladium-catalyzed cross-coupling delivered the target compound 101. Compared to the original 9-step synthesis from a conventional Diels-Alder reaction product 102, only 5 steps are needed by starting from the dearomatization product 37a to access the terminal product 101. Overall, the [4+2] DAC reaction could provide a direct and efficient access to various functionalized bridged polycycles which previously have been inaccessible or required strenuous synthetic efforts.
Fig. 6. Synthetic applications.
A) Synthetic derivatization of the [4+2] dearomative cycloaddition products. B) Shortened synthesis of a thromboxane A2/prostaglandin H2 receptor antagonist 101. Diastereomeric ratio (d.r.) displayed herein were determined by 1H NMR analysis of purified compounds. DIBAL-H = diisobutylaluminum hydride. NBS = N-bromosuccinimide. AIBN = azobisisobutyronitrile.
Given the emerging interest in bridged polycycles emerging in medicinal chemistry (42, 43), we anticipate this method will find substantial use in facilitating the efficient synthesis of such scaffolds.
Supplementary Material
One Sentence Summary.
Photochemical cycloaddition reactions of quinolines, isoquinolines and quinazolines with alkenes are an efficient and selective route to polycyclic heterocycles.
Acknowledgments
J.M. thanks Dr. X. Huang (UIUC), Dr. J. Li, S. Mondal, T. Pinkert, T. Paulisch and F. Strieth-Kalthoff (all WWU) for assistance and discussions.
Funding
Generous financial support by the European Research Council (ERC Advanced Grant Agreement no. 788558) and the Deutsche Forschungsgemeinschaft (SFB 858, Leibniz Award) is gratefully acknowledged. M.K.B. and R.G. thank National Institutes of Health (R35GM131755) for financial support. K.N.H thanks the National Science Foundation (Grant CHE-1764328) for financial support. Calculations were performed on the Hoffman2 cluster at the University of California, Los Angeles, and the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by the National Science Foundation (Grant OCI-1053575).
Footnotes
Author contributions
F.G., M.K.B. and J.M., R.G. conceived the project. J.M. and R.G. discovered the HFIP and BF3·OEt2 conditions, independently. J.M., P.B., R.G., F.S., A.H. and X.Z. performed the synthetic experiments. S.C. performed the DFT calculations. C.D. analyzed the X-ray structures. J.M., S.C., M.K.B., K.N.H. and F.G. supervised the research and wrote the manuscript with contributions from all authors.
Competing interests
The authors declare no competing interests.
Data and materials availability
Characterization of new compounds and detailed reaction optimization and mechanistic studies are provided in the supplementary material; crystallographic data are available free of charge under CCDC reference numbers 2041010 (intermediate I’), 2060268 (71·MeI), 2041012 (75), 2041013 (81) and 2041014 (89·HCl).
References and Notes
- 1.Trost B. The atom economy-a search for synthetic efficiency. Science. 1991;254:1471–1477. doi: 10.1126/science.1962206. [DOI] [PubMed] [Google Scholar]
- 2.Wender PA, Verma VA, Paxton TJ, Pillow TH. Function-oriented synthesis, step economy, and drug design. Acc Chem Res. 2008;41:40–49. doi: 10.1021/ar700155p. [DOI] [PubMed] [Google Scholar]
- 3.Jørgensen KA. Cycloaddition reactions in organic synthesis. John Wiley & Sons; 2002. [Google Scholar]
- 4.Carruthers W. Cycloaddition reactions in organic synthesis. Elsevier; 2013. [Google Scholar]
- 5.Nicolaou KC, Snyder SA, Montagnon T, Vassilikogiannakis G. The Diels-Alder reaction in total synthesis. Angew Chem Int Ed. 2002;41:1668–1698. doi: 10.1002/1521-3773(20020517)41:10<1668::aid-anie1668>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 6.Weissermel K, Arpe H-J. Industrial organic chemistry. John Wiley & Sons; 2008. [Google Scholar]
- 7.Atherton J, Jones S. Diels-Alder reactions of anthracene, 9-substituted anthracenes and 9,10-disubstituted anthracenes. Tetrahedron. 2003;46:9039–9057. [Google Scholar]
- 8.Remy R, Bochet CG. Arene-alkene cycloaddition. Chem Rev. 2016;116:9816–9849. doi: 10.1021/acs.chemrev.6b00005. [DOI] [PubMed] [Google Scholar]
- 9.Zheng C, You S-L. Catalytic asymmetric dearomatization by transition-metal catalysis: a method for transformations of aromatic compounds. Chem. 2016;1:830–857. [Google Scholar]
- 10.Wertjes WC, Southgate EH, Sarlah D. Recent advances in chemical dearomatization of nonactivated arenes. Chem Soc Rev. 2018;47:7996–8017. doi: 10.1039/c8cs00389k. [DOI] [PubMed] [Google Scholar]
- 11.Siddiqi Z, Wertjes WC, Sarlah D. Chemical equivalent of arene monooxygenases: dearomative synthesis of arene oxides and oxepines. J Am Chem Soc. 2020;142:10125–10131. doi: 10.1021/jacs.0c02724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leitch JA, Rogova T, Duarte F, Dixon DJ. Dearomative photocatalytic construction of bridged 1,3-diazepanes. Angew Chem Int Ed. 2020;59:4121–4130. doi: 10.1002/anie.201914390. [DOI] [PubMed] [Google Scholar]
- 13.Preindl J, Chakrabarty S, Waser J. Dearomatization of electron poor six-membered N-heterocycles through [3 + 2] annulation with aminocyclopropanes. Chem Sci. 2017;8:7112–7118. doi: 10.1039/c7sc03197a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiong Q, Dong S, Chen Y, Liu X, Feng X. Asymmetric synthesis of tetrazole and dihydroisoquinoline derivatives by isocyanide-based multicomponent reactions. Nat Commun. 2019;10:2116. doi: 10.1038/s41467-019-09904-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bird CW. The relationship of classical and magnetic criteria of aromaticity. Tetrahedron. 1996;52:9945–9952. [Google Scholar]
- 16.Strieth-Kalthoff F, James MJ, Teders M, Pitzer L, Glorius F. Energy transfer catalysis mediated by visible light: principles, applications, directions. Chem Soc Rev. 2018;47:7190–7202. doi: 10.1039/c8cs00054a. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Q-Q, Zou Y-Q, Lu L-Q, Xiao W-J. Visible-light-induced organic photochemical reactions through energy-transfer pathways. Angew Chem Int Ed. 2019;58:1586–1604. doi: 10.1002/anie.201803102. [DOI] [PubMed] [Google Scholar]
- 18.Strieth-Kalthoff F, Glorius F. Triplet energy transfer photocatalysis: unlocking the next level. Chem. 2020;6:1888–1903. [Google Scholar]
- 19.Strieth-Kalthoff F, Henkel C, Teders M, Kahnt A, Knolle W, Gómez-Suárez A, Dirian K, Alex W, Bergander K, Daniliuc CG, Abel B, et al. Discovery of unforeseen energy-transfer-based transformations using a combined screening approach. Chem. 2019;5:2183–2194. [Google Scholar]
- 20.James MJ, Schwarz JL, Strieth-Kalthoff F, Wibbeling B, Glorius F. Dearomative cascade photocatalysis: divergent synthesis through catalyst selective energy transfer. J Am Chem Soc. 2018;140:8624–8628. doi: 10.1021/jacs.8b03302. [DOI] [PubMed] [Google Scholar]
- 21.Zhu M, Zheng C, Zhang X, You S-L. Synthesis of cyclobutane-fused angular tetracyclic spiroindolines via visible-light-promoted intramolecular dearomatization of indole derivatives. J Am Chem Soc. 2019;141:2636–2644. doi: 10.1021/jacs.8b12965. [DOI] [PubMed] [Google Scholar]
- 22.Ma J, Strieth-Kalthoff F, Dalton T, Freitag M, Schwarz JL, Bergander K, Daniliuc C, Glorius F. Direct dearomatization of pyridines via an energy-transfer-catalyzed intramolecular [4+2] cycloaddition. Chem. 2019;5:2854–2864. [Google Scholar]
- 23.Zhu M, Huang X-L, Xu H, Zhang X, Zheng C, You S-L. Visible-light-mediated synthesis of cyclobutene-fused indolizidines and related structural analogs. CCS Chem. 2020:652–664. [Google Scholar]
- 24.Oderinde MS, Mao E, Ramirez A, Pawluczyk J, Jorge C, Cornelius LAM, Kempson J, Vetrichelvan M, Pitchai M, Gupta A, Gupta AK, et al. Synthesis of cyclobutane-fused tetracyclic scaffolds via visible-light photocatalysis for building molecular complexity. J Am Chem Soc. 2020;142:3094–3103. doi: 10.1021/jacs.9b12129. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Yi D, Zhang M, Wei J, Lu J, Yang L, Wang J, Hao N, Pan X, Zhang S, Wei S, et al. Photocatalytic intramolecular [2 + 2] cycloaddition of indole derivatives via energy transfer: a method for late-stage skeletal transformation. ACS Catal. 2020;10:10149–10156. [Google Scholar]
- 26.Rolka AB, Koenig B. Dearomative cycloadditions utilizing an organic photosensitizer: an alternative to iridium catalysis. Org Lett. 2020;22:5035–5040. doi: 10.1021/acs.orglett.0c01622. [DOI] [PubMed] [Google Scholar]
- 27.Zhu M, Zhang X, Zheng C, You S-L. Visible-light-induced dearomatization via [2+ 2] cycloaddition or 1, 5-hydrogen atom transfer: divergent reaction pathways of transient diradicals. ACS Catal. 2020;10:12618–12626. [Google Scholar]
- 28.Miura T, Funakoshi Y, Murakami M. Intramolecular dearomatizing [3 + 2] annulation of α-imino carbenoids with aryl rings furnishing 3,4-fused indole skeletons. J Am Chem Soc. 2014;136:2272–2275. doi: 10.1021/ja412663a. [DOI] [PubMed] [Google Scholar]
- 29.Trost BM, Ehmke V, O’Keefe BM, Bringley DA. Palladium-catalyzed dearomative trimethylenemethane cycloaddition reactions. J Am Chem Soc. 2014;136:8213–8216. doi: 10.1021/ja5044825. [DOI] [PubMed] [Google Scholar]
- 30.Pham HV, Karns AS, Vanderwal CD, Houk KN. Computational and experimental investigations of the formal dyotropic rearrangements of Himbert arene/allene cycloadducts. J Am Chem Soc. 2015;137:6956–6964. doi: 10.1021/jacs.5b03718. [DOI] [PubMed] [Google Scholar]
- 31.Shen L, Zhao K, Doitomi K, Ganguly R, Li Y-X, Shen Z-L, Hirao H, Loh T-P. Lewis acid-catalyzed selective [2 + 2]-cycloaddition and dearomatizing cascade reaction of aryl alkynes with acrylates. J Am Chem Soc. 2017;139:13570–13578. doi: 10.1021/jacs.7b07997. [DOI] [PubMed] [Google Scholar]
- 32.Inami T, Takahashi T, Kurahashi T, Matsubara S. Nickel-catalyzed [5+2] cycloaddition of 10π-electron aromatic benzothiophenes with alkynes to form thermally metastable 12π-electron nonaromatic benzothiepines. J Am Chem Soc. 2019;141:12541–12544. doi: 10.1021/jacs.9b07948. [DOI] [PubMed] [Google Scholar]
- 33.Lee J, Ko D, Park H, Yoo EJ. Direct cyclopropanation of activated N-heteroarenes via site- and stereoselective dearomative reactions. Chem Sci. 2020;11:1672–1676. doi: 10.1039/c9sc06369b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colomer I, Chamberlain AER, Haughey MB, Donohoe TJ. Hexafluoroisopropanol as a highly versatile solvent. Nat Rev Chem. 2017;1:0088. [Google Scholar]
- 35.Pozhydaiev V, Power M, Gandon V, Moran J, Lebœuf D. Exploiting hexafluoroisopropanol (HFIP) in Lewis and Brønsted acid-catalyzed reactions. Chem Commun. 2020;56:11548–11564. doi: 10.1039/d0cc05194b. [DOI] [PubMed] [Google Scholar]
- 36.Sherbrook EM, Jung H, Cho D, Baik M-H, Yoon TP. Brønsted acid catalysis of photosensitized cycloadditions. Chem Sci. 2020;11:856–861. doi: 10.1039/c9sc04822g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blum TR, Miller ZD, Bates DM, Guzei IA, Yoon TP. Enantioselective photochemistry through Lewis acid–catalyzed triplet energy transfer. Science. 2016;354:1391–1395. doi: 10.1126/science.aai8228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang X, Meggers E. Asymmetric photocatalysis with bis-cyclometalated rhodium complexes. Acc Chem Res. 2019;52:833–847. doi: 10.1021/acs.accounts.9b00028. [DOI] [PubMed] [Google Scholar]
- 39.Chénard E, Sutrisno A, Zhu L, Assary RS, Kowalski JA, Barton JL, Bertke JA, Gray DL, Brushett FR, Curtiss LA, Moore JS. Synthesis of Pyridine– and Pyrazine–BF3 Complexes and Their Characterization in Solution and Solid State. J Phy Chem C. 2016;120:8461–8471. [Google Scholar]
- 40.Pitzer L, Schäfers F, Glorius F. Rapid Assessment of the Reaction-Condition-Based Sensitivity of Chemical Transformations. Angew Chem Int Ed. 2019;58:8572–8576. doi: 10.1002/anie.201901935. [DOI] [PubMed] [Google Scholar]
- 41.Saha SL, Roche VF, Pendola K, Kearley M, Lei L, Romstedt KJ, Herdman M, Shams G, Kaisare V, Feller DR. Synthesis and in vitro platelet aggregation and TP receptor binding studies on bicyclic 5,8-ethanooctahydroisoquinolines and 5,8-ethanotetrahydroisoquinolines. Biorg Med Chem. 2002;10:2779–2793. doi: 10.1016/s0968-0896(02)00101-3. [DOI] [PubMed] [Google Scholar]
- 42.Lovering F, Bikker J, Humblet C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J Med Chem. 2009;52:6752–6756. doi: 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- 43.Mykhailiuk PK. Saturated bioisosteres of benzene: where to go next? Org Biomol Chem. 2019;17:2839–2849. doi: 10.1039/c8ob02812e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Characterization of new compounds and detailed reaction optimization and mechanistic studies are provided in the supplementary material; crystallographic data are available free of charge under CCDC reference numbers 2041010 (intermediate I’), 2060268 (71·MeI), 2041012 (75), 2041013 (81) and 2041014 (89·HCl).