Abstract
Context
Cardiometabolic profiles of different body composition phenotypes are poorly characterized in young children, where it is well-established that high adiposity is unfavorable, but the role of lean mass is unclear.
Objective
We hypothesized that higher lean mass attenuates cardiometabolic risk in children with high fat mass.
Design, Setting, Participants
In 6-year-old children (n=377) from the Growing Up in Singapore Towards healthy Outcomes (GUSTO) prospective birth cohort, whole-body composition was measured by quantitative magnetic resonance, a novel validated technology. Based on fat mass index (FMI) and lean mass index (LMI), 4 body composition phenotypes were derived: low FMI-low LMI (LF-LL), low FMI-high LMI (LF-HL), high FMI-low LMI (HF-LL), high FMI-high LMI (HF-HL).
Main Outcome Measures
BMI z-score, fasting plasma glucose, insulin resistance, metabolic syndrome risk score, fatty liver index, and blood pressure
Results
Compared to the LF-HL group, children in both high FMI groups had increased BMI z-score (HF-HL: 1.43units 95% CI [1.11,1.76]; HF-LL: 0.61units [0.25,0.96]) and metabolic syndrome risk score (HF-HL: 1.64 [0.77,2.50]; HF-LL: 1.28 [0.34,2.21]). The HF-HL group also had increased fatty liver index (1.15 [0.54,1.77]). Girls in HF-HL group had lower fasting plasma glucose (-0.29mmol/L [-0.55,-0.04]) and diastolic blood pressure (-3.22mmHg [-6.03,-0.41]) than girls in the HF-LL group. No similar associations were observed in boys.
Conclusions
In a multi-ethnic Asian cohort, lean mass seemed to protect against some cardiometabolic risk markers linked with adiposity, but only in girls. Fat mass index seemed more important than lean mass index in relation to cardiometabolic profiles of young children.
Keywords: Body composition, lean, adiposity, cardiometabolic, metabolic syndrome
Introduction
An adverse cardiometabolic risk profile and adiposity in childhood are becoming increasingly prevalent and are linked to poorer cardiometabolic health later in life(1,2). Traditionally, BMI cut-offs of “overweight” and “obesity” have been used to identify high-risk children. However, BMI might not be a good indicator of adiposity as it does not distinguish between fat, muscle, or bone. Further, BMI depends on factors including age, sex, ethnicity, and maturation stage(3,4). Disentangling the fat and lean components of total body mass and studying different body composition phenotypes might provide additional insights on childhood cardiometabolic risk due to the differing roles of lean and fat mass in health and disease.
Whole-body lean mass includes skeletal muscle, which is the largest insulin-sensitive tissue in the body responsible for insulin-mediated glucose disposal(5). A progressive loss of skeletal muscle with aging(6), known as sarcopenia, is linked to the metabolic syndrome(7,8). However, the role of skeletal muscle or lean mass on child cardiometabolic profile is unclear. Lean mass has been reported to be relatively protective of vascular structure and function in children(9) and lower appendicular skeletal muscle mass relative to total body fat is linked to metabolic syndrome risk in overweight children(10). In contrast, increased lean mass index (LMI) has also been linked to decreased high-density lipoprotein and increased cardiometabolic risk factors, independent of fat mass index (FMI), in children and adolescents(11,12). Compared to lean mass, the role of fat mass is more well-established. Higher FMI, calculated using fat mass divided by height squared, is linked to adverse cardiometabolic profiles in adults and children(9,12–17). However, it is unknown if lean mass attenuates the adverse effect of fat mass on cardiometabolic health, especially in young children where the role of lean mass is unclear.
As adverse cardiometabolic profiles tend to track from early childhood to adulthood(1,2), early risk stratification is important. However, the cardiometabolic profile of different body composition phenotypes are not well characterized in young children. Previous studies usually involved older children aged 8 to 19 years(9–12), probably due to measurement of body composition by dual X-ray absorptiometry, where radiation exposure might be a concern(18,19).
We aimed to characterize the cardiometabolic profile of distinct body composition phenotypes in young children aged 6 years by measuring whole-body lean and fat mass using a novel, validated quantitative nuclear magnetic resonance technology(20). We hypothesized that high fat mass is associated with adverse cardiometabolic profiles while high lean mass may attenuate the adverse cardiometabolic profile in children with high fat mass. Sex-specific interactions will also be investigated as sex differences in body composition and cardiometabolic markers can be observed even before puberty(21).
Materials and methods
Study population
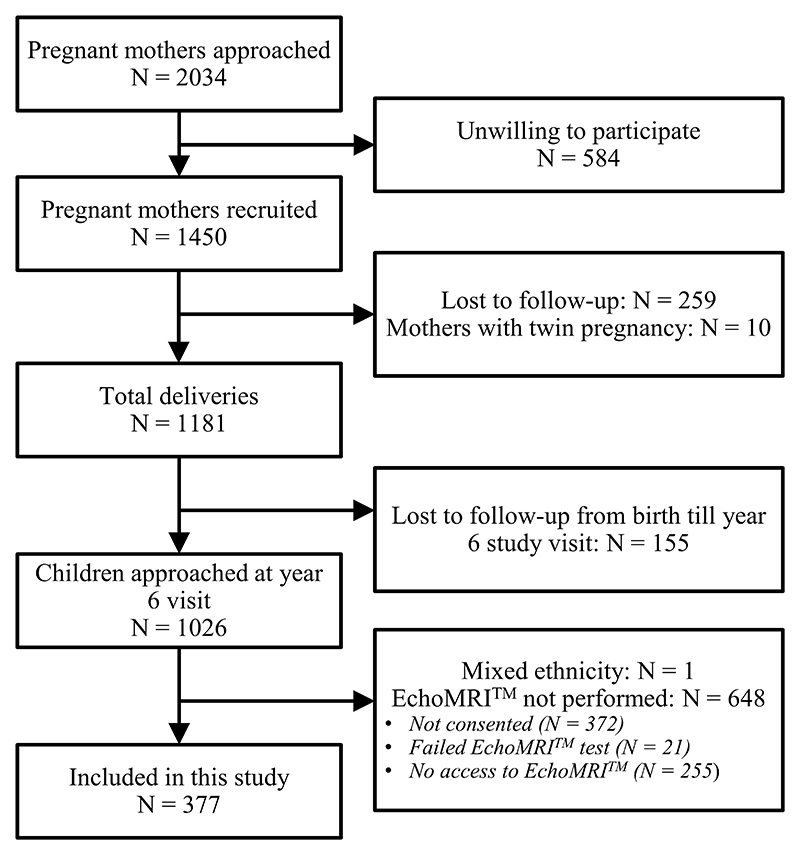
Children were from the Growing Up in Singapore Towards healthy Outcomes (GUSTO) prospective birth cohort study recruited from two major public hospitals in Singapore – the KK Women’s and Children’s Hospital and the National University Hospital – between 2009 and 2010(22). Pregnant women in their first trimester were eligible if they were Singapore citizens or permanent residents aged 18 years and above, planned to deliver in KK Women’s and Children’s Hospital or National University Hospital, live in Singapore for the next 5 years, willing to donate birth tissues at delivery, not receiving chemotherapy, not on psychotropic drugs, and not having type 1 diabetes. At a clinical follow-up visit at 6 years of age out of 1026 children approached for body composition analysis, 377 children were included (Figure 1). The main reasons for not being included were no access to the body composition machine (n=255), parental concerns (n=372) and other reasons (n=22) including machine related issues. Ethic approvals were granted from the National Healthcare Group Domain Specific Review Board and SingHealth Centralized Institutional Review Board. The children filled an assent form to document their understanding and participation in the study while their parents gave written informed consent.
Figure 1. Study flow chart showing children who participated in this study.
Body composition
Whole-body lean mass and fat mass were measured by EchoMRI™ Adolescent Humans Body Composition Analyzer (EchoMRI Corporation, Singapore)(20). EchoMRI™ uses quantitative nuclear magnetic resonance technology based on stimulation of hydrogen nuclei in a magnetic field by radio frequency pulses. Relaxation of the hydrogen nuclei generates radio signals that are different in fat and lean mass, enabling differentiation of these tissues. Therefore, lean mass measured by EchoMRI™ excludes fat, bone minerals, and substances which do not contribute to the nuclear magnetic resonance signal, such as hair and nails. Quality control measures for EchoMRI™ were performed daily according to the manufacturer’s recommendations, including calibration of the machine with 8 bottles of canola oil. Children were measured in light clothing, in the supine position, and instructed to minimize their movement, although slight motions are generally well-tolerated. FMI and LMI were calculated using fat mass or lean mass (kg) divided by height (m) squared and dichotomized into “high” and “low” groups based on cohort- and sex-specific medians to derive four distinct body composition groups: low FMI-low LMI (LF-LL), low FMI-high LMI (LF-HL), high FMI-low LMI (HF-LL) and high FMI-high LMI (HF-HL).
Cardiometabolic markers at age 6 years
Cardiometabolic markers were measured in subsets of children who attended the study visit and gave consent for the various measures, described below.
Standing height (SECA213 stadiometer), weight (SECA803 Weighing Scale) and abdominal circumference (measuring tape) were measured using standardized protocols(22). Sex and age standardized z-scores of BMI (z-BMI) and height (z-height) were calculated using World Health Organization growth standards(23).
After an overnight fast, venous blood was drawn to measure fasting plasma glucose (Abbott Architect c8000 analyzer at KK Women’s and Children’s Hospital and Beckman AU5800 analyzer at National University Hospital) as well as serum insulin (Beckman DXL800 analyzer, Beckman Coulter), high density lipoprotein cholesterol (Beckman AU5800 analyzer, Beckman Coulter), triglycerides, and gamma-glutamyl-transferase (Beckman AU5800 analyzer, Beckman Coulter). Homeostasis model assessment of insulin resistance was calculated using the following formula(24): [fasting insulin (mU/L) * fasting glucose (mmol/L)] / 22.5.
Peripheral systolic blood pressure and diastolic blood pressure were measured (Dinamap CARESCAPE V100, GE Healthcare, Milwaukee, WI) from the right upper arm by trained research coordinators using a standardized protocol(25).
A pediatric metabolic syndrome risk score was calculated based on a previously published equation(26). First, cohort-specific sex-standardized abdominal circumference, systolic blood pressure, diastolic blood pressure, homeostasis model assessment of insulin resistance, triglycerides, and high density lipoprotein cholesterol z-scores were derived. Then, metabolic syndrome risk score was calculated by summing the z-scores of four components of cardiometabolic risk: 1) abdominal circumference, 2) mean z-scores of systolic and diastolic blood pressure, 3) homeostasis model assessment of insulin resistance and 4) mean z-scores of triglycerides and high density lipoprotein cholesterol (z-score of high density lipoprotein cholesterol multiplied with −1, due to its inverse association with metabolic risk). Fatty liver index was calculated based on a published equation using triglycerides, gamma-glutamyl-transferase, and abdominal circumference(27). It is an index to estimate non-alcoholic fatty liver disease with modest efficacy compared to magnetic resonance spectroscopy which is expensive and not routinely accessible.
Covariates
Ethnicity, age, household income, and self-reported pre-pregnancy weight were obtained through interviewer-administered questionnaires. A 75g 2h-oral glucose tolerance test was performed to measure gestational (26-28 weeks) fasting plasma glucose and 2-hour plasma glucose [Advia 2400 Chemistry system (Siemens Medical Solutions Diagnostics, Deerfield, IL, USA) and Beckman LX20 Pro analyser (Beckman Coulter, USA)]. Gestational diabetes was diagnosed according to the 1999 World Health Organization criteria (fasting plasma glucose ≥7.0mmol/L or 2-hour plasma glucose ≥7.8mmol/l). Infant’s birthweight and sex were obtained from medical records. Gestational age was derived based on first trimester ultrasound scans. Cohort-specific birthweight percentiles, adjusted for sex and gestational age, were calculated(28) to identify small-for-gestational-age (birthweight <10th centile), large-forgestational-age (birthweight >90th centile), and appropriate-for-gestational-age infants(28).
Statistical analyses
We analyzed differences in baseline sociodemographic and clinical characteristics between the body composition groups using one-way ANOVA for continuous variables and chi-square test for categorical variables. Multiple linear regression was performed to analyze associations between the body composition groups and cardiometabolic markers at age 6 years, adjusted for ethnicity, sex, household income, maternal age, pre-pregnancy body mass index, gestational diabetes, prematurity, and size at birth, to reduce confounding bias by these potential confounders.
Differences in cardiometabolic markers for the body composition groups of primary interest, HF-HL and HF-LL, together with LF-LL, were compared against the LF-HL group, which was used as the reference group because it was hypothesized to be the healthy group among the four body composition groups. Differences in cardiometabolic markers between the two body composition groups with high FMI (HF-HL vs. HF-LL) were compared to determine whether the HF-HL group was metabolically favorable compared to HF-LL. By including a multiplicative interaction term, significant interactions were found between sex and body composition groups with high FMI (HF-HL, HF-LL) on two outcomes: fasting plasma glucose and diastolic blood pressure. Sex-stratified analyses were presented for these outcomes. All analyses were performed using the Stata16.0 software (StataCorp LP, TX). Two-sided P values <0.05 were considered statistically significant.
Results
Population characteristics
Table 1 shows the characteristics of the 377 children who participated in this study grouped by body composition groups: LF-HL (21%), LF-LL (29%), HF-HL (29%), HF-LL (21%). There were 202 (53.6%) Chinese, 113 (30.0%) Malay, and 62 (16.4%) Indian children.
Table 1. Characteristics of study participants by body composition groups.
| All (n=377), mean (SD) or n (%) |
Low FMI- High LMI (n=79), mean (SD) or n (%) |
Low FMI- Low LMI (n=109), mean (SD) or n (%) |
High FMI- High LMI (n=110), mean (SD) or n (%) |
High FMI- Low LMI (n=79), mean (SD) or n (%) |
p | |
|---|---|---|---|---|---|---|
| Parental characteristics | ||||||
| Ethnicity | 0.009 | |||||
| Chinese | 202 (53.6%) |
49 (62.0%) | 61 (56.0%) | 48 (43.6%) | 44 (55.7%) | |
| Malay | 113 (30.0%) |
25 (31.6%) | 29 (26.6%) | 43 (39.1%) | 16 (20.3%) | |
| Indian | 62 (16.4%) | 5 (6.3%) | 19 (17.4%) | 19 (17.3%) | 19 (24.1%) | |
| Monthly household income | 0.03 | |||||
| High (≥ S$6000) | 73 (20.9%) | 21 (28.8%) | 22 (22.0%) | 19 (18.4%) | 11 (15.1%) | |
| Mid(S$4000 – 5999) | 91 (26.1%) | 16 (21.9%) | 26 (26.0%) | 20 (19.4%) | 29 (39.7%) | |
| Low (< S$4000) | 185 (53.0%) |
36 (49.3%) | 52 (52.0%) | 64 (62.1%) | 33 (45.2%) | |
| Maternal age at delivery (yr) | 31.1 (5.3) | 31.5 (4.8) | 30.5 (5.4) | 30.4 (5.4) | 32.4 (5.4) | 0.03 |
| Pre-pregnancy BMI (kg/m2) |
22.64 (4.29) |
22.23 (4.05) |
21.60 (3.59) |
24.01 (4.71) |
22.75 (4.44) |
<0.001 |
| Gestational fasting | 0.15 | |||||
| plasma glucose (mmol/L) | 4.39 (0.47) | 4.36 (0.34) | 4.32 (0.40) | 4.45 (0.57) | 4.44 (0.51) | |
| Gestational 2-hour | 0.10 | |||||
| plasma glucose (mmol/L) | 6.36 (1.36) | 6.14 (1.44) | 6.29 (1.23) | 6.38 (1.30) | 6.67 (1.52) | |
| Gestational diabetes | 0.02 | |||||
| No | 310 (85.6%) |
68 (88.3%) | 93 (87.7%) | 94 (89.5%) | 55 (74.3%) | |
| Yes | 52 (14.4%) | 9 (11.7%) | 13 (12.3%) | 11 (10.5%) | 19 (25.7%) | |
| Child characteristics | ||||||
| Sex | 0.80 | |||||
| Girl | 188(49.9%) | 37 (46.8%) | 57 (52.3%) | 57 (51.8%) | 37 (46.8%) | |
| Boy | 189 (50.1%) |
42 (53.2%) | 52 (47.7%) | 53 (48.2%) | 42 (53.2%) | |
| Prematurity | 0.03 | |||||
| Term | 351(93.1%) | 76 (96.2%) | 95 (87.2%) | 104(94.5%) | 76 (96.2%) | |
| Preterm | 26 (6.9%) | 3 (3.8%) | 14 (12.8%) | 6 (5.5%) | 3 (3.8%) | |
| Birthweight (kg) | 3.08 (0.43) | 3.13 (0.35) | 2.94 (0.50) | 3.18 (0.44) | 3.10 (0.35) | <0.001 |
| Size at birth | 0.02 | |||||
| Appropriate-for-gestational-age | 269 (71.4%) |
59 (74.7%) | 70 (64.2%) | 79 (71.8%) | 61 (77.2%) | |
| Small-for-gestational-age | 44 (11.7%) | 7 (8.9%) | 23 (21.1%) | 7 (6.4%) | 7 (8.9%) | |
| Large-for-gestational-age | 64 (17.0%) | 13 (16.5%) | 16 (14.7%) | 24 (21.8%) | 11 (13.9%) | |
| Adiposity markers at 6 years | ||||||
| z-BMI | -0.01 (1.32) | -0.35(0.56) | -1.12 (0.76) | 1.15 (1.27) | 0.27 (1.12) | <0.001 |
| z-height | -0.09 (1.01) | -0 27(0.93) | -0.38 (0.96) | 0.16 (0.97) | 0.14 (1.06) | <0.001 |
| Fat mass (kg) | 4.38 (2.52) | 2.99 (0.52) | 2.87 (0.70) | 6.24 (3.33) | 5.25 (1.88) | <0.001 |
| FMI (kg/m2) | 3.23 (1.64) | 2.28 (0.40) | 2.21 (0.49) | 4.49 (2.08) | 3.82 (1.20) | <0.001 |
| Lean mass (kg) | 12.46 (1.89) | 13.08 (1.30) | 10.99 (1.35) | 13.85 (1.72) | 11.93 (1.55) | <0.001 |
| LMI (kg/m2) | 9.33 (1.00) | 9.95 (0.53) | 8.47 (0.75) | 10.15(0.69) | 8.76 (0.62) | <0.001 |
| Metabolic markers at 6 years | ||||||
| Fasting plasma glucose (mmol/L) | 4.53 (0.38) | 4.53 (0.44) | 4.51 (0.40) | 4.50 (0.36) | 4.59 (0.32) | 0.50 |
| Fasting insulin (pmol/L) | 30.62 (16.25) | 27.43 (13.54) | 26.58 (14.11) | 33.56 (15.77) | 35.00 (19.83) | 0.01 |
| Homeostasis model assessment of insulin resistance (units) | 0.90 (0.50) | 0.81 (0.44) | 0.77 (0.43) | 0.98 (0.48) | 1.04 (0.61) | 0.01 |
| Metabolic syndrome risk score | -0.08 (2.22) | -0.95 (1.47) | -0.97 (1.74) | 0.67 (2.17) | 0.90 (2.66) | <0.001 |
| Fatty liver index | 1.07 (1.43) | 0.53 (0.18) | 0.49 (0.20) | 1.72 (2.00) | 1.39 (1.57) | <0.001 |
| Cardiovascular markers at 6 years | ||||||
| Systolic blood pressure (mmHg) | 101.06 (8.18) | 100.55 (8.16) | 99.30 (7.93) | 102.84 (8.03) | 101.50 (8.35) | 0.01 |
| Diastolic blood pressure (mmHg) | 59.62 (5.48) | 58.71 (5.07) | 59.17 (5.48) | 60.08 (5.58) | 60.50 (5.61) | 0.13 |
Abbreviations: FMI – fat mass index; LMI – lean mass index
Children in the 4 body composition phenotype groups had differing sociodemographic characteristics. Compared to all children included in this study, the LF-HL group had a higher proportion of children of Chinese ethnicity and who came from high income households. The HF-HL group included a higher proportion of children of Malay ethnicity and who came from low income households, while the HF-LL group included a higher proportion of children of Indian ethnicity and who had older mothers (Table 1). Children in the 4 body composition phenotype groups also had differing maternal prenatal and perinatal characteristics. Children in the LF-LL group had mothers with the lowest mean pre-pregnancy BMI, gestational fasting plasma glucose, and had the highest prevalence of preterm and small-for-gestational-age infants. In contrast, children in the HF-HL group had mothers with the highest mean pre-pregnancy BMI, gestational fasting plasma glucose, and had the highest prevalence of large-for-gestational-age infants. Mothers of children in the HF-LL group had the highest prevalence of gestational diabetes.
Cardiometabolic profile of body composition phenotypes
Adjusting for confounders, both high FMI groups (HF-HL and HF-LL) had several elevated cardiometabolic markers compared to the LF-HL reference group. Higher z-BMI was observed in the HF-HL group, β (95% CI), 1.43units (1.11, 1.76) and HF-LL group, 0.61units (0.25, 0.96) (Table 2). Higher metabolic syndrome risk score was also observed in the HF-HL group, 1.64 (0.77, 2.50) and HF-LL group, 1.28 (0.34, 2.21). In addition, the HF-HL group had higher fatty liver index, 1.15 (0.54, 1.77). Comparing the low FMI groups (LF-LL vs. LF-HL), other than having lower z-BMI, -0.72units (-1.05, -0.40) the LF-LL group did not differ significantly in any other cardiometabolic risk markers compared to the LF-HL reference group.
Table 2. Associations of body composition groups with adiposity and cardiometabolic markers in 6-year-old children.
| Low FMI-High LMI | Low FMI-Low LMI | High FMI-High LMI | High FMI-Low LMI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | β (95% CI) | N | β (95% CI) | P | N | β (95% CI) | P | N | β (95% CI) | P | |
| Adiposity markers | |||||||||||
| z-BMI | 66 | Ref. | 92 | -0.72 (-1.05, - 0.40) | <0.001 | 85 | 1.43 (1.11,1.76) | <0.001 | 62 | 0.61 (0.25,0.96) | <0.001 |
| z-height | 66 | Ref. | 92 | -0.16 (-0.47,0.14) | 0.29 | 85 | 0.27 (-0.04, 0.58) | 0.08 | 62 | 0.15 (-0.19, 0.48) | 0.39 |
| Metabolic markers | |||||||||||
| Fasting plasma glucose (mmol/L) | 46 | Ref. | 67 | -0.04 (-0.19, 0.11) | 0.59 | 61 | -0.06 (-0.21, 0.08) | 0.39 | 50 | 0.02 (-0.13, 0.18) | 0.76 |
| Fasting insulin (pmol/L) | 40 | Ref. | 56 | -2.23 (-8.77, 4.31) | 0.50 | 51 | 6.23 (-0.39, 12.85) | 0.07 | 40 | 5.24 (-1.88, 12.36) | 0.15 |
| Homeostasis model assessment of insulin resistance (units) | 40 | Ref. | 56 | -0.08 (-0.28,0.13) | 0.47 | 51 | 0.18 (-0.03,0.38) | 0.10 | 40 | 0.15 (-0.07,0.38) | 0.18 |
| Metabolic syndrome risk score | 38 | Ref. | 55 | -0.09 (-0.94,0.77) | 0.84 | 50 | 1.64 (0.77, 2.50) | <0.001 | 39 | 1.28 (0.34, 2.21) | 0.008 |
| Fatty liver index | 31 | Ref. | 47 | -0.05 (-0.66, 0.56) | 0.86 | 43 | 1.15 (0.54, 1.77) | <0.001 | 37 | 0.51 (-0.14, 1.16) | 0.12 |
| Cardiovascular markers | |||||||||||
| Systolic blood pressure (mmHg) | 65 | Ref. | 90 | -0.45 (-3.01, 2.10) | 0.73 | 84 | 0.89 (-1.70, 3.48) | 0.50 | 61 | -0.93 (-3.75, 1.90) | 0.52 |
| Diastolic blood pressure (mmHg) | 65 | Ref. | 90 | 0.67 (-1.11, 2.45) | 0.46 | 84 | 0.94 (-0.86, 2.74) | 0.31 | 61 | 1.79 (-0.17, 3.76) | 0.07 |
Abbreviations: FMI – fat mass index; LMI – lean mass index
Models were adjusted for ethnicity, sex, household income, maternal age, pre-pregnancy body mass index, gestational diabetes, prematurity, and size at birth. Coefficients (β) shown are adjusted differences in adiposity and cardiometabolic markers between each body composition group and the Low FMI-High LMI reference group.
Lean mass in children with high adiposity
Comparing the high FMI body composition groups which were of interest (HF-HL vs. HF-LL), other than having higher z-BMI, 0.89units (0.45,1.32), the HF-HL group did not differ significantly in any other cardiometabolic risk markers compared to the HF-LL group (Table 3). There were significant interactions between sex and the two body composition groups (HF-HL and HF-LL) on fasting plasma glucose and diastolic blood pressure, P=0.006 and 0.03, respectively (Table 4). Compared to the HF-LL group, girls from the HF-HL group had lower fasting plasma glucose, -0.29 mmol/L (-0.55, -0.04) and lower diastolic blood pressure, -3.22mmHg (-6.03, -0.41). These associations were not significant in boys.
Table 3. Differences in adiposity and cardiometabolic markers between the high fat mass index body composition groups in 6-year-old children.
| High FMI-High LMI (vs. High FMI-Low LMI) | ||
|---|---|---|
| β (95% CI) | P | |
| Adiposity markers | ||
| z-BMI | 0.89 (0.45,1.32) | <0.001 |
| z-height | 0.15 (-0.50, 0.21) | 0.42 |
| Metabolic markers | ||
| Fasting plasma glucose (mmol/L) | -0.12 (-0.26, 0.02) | 0.09 |
| Fasting insulin (pmol/L) | 0.05 (-1.19,1.30) | 0.93 |
| Homeostasis model assessment of insulin resistance (units) | 0.00 (-0.27,0.26) | 0.98 |
| Metabolic syndrome risk score | 0.20 (-0.91,1.31) | 0.72 |
| Fatty liver index | 0.69 (-0.23,1.61) | 0.14 |
| Cardiovascular markers | ||
| Systolic blood pressure (mmHg) | 1.47 (-1.38,4.31) | 0.31 |
| Diastolic blood pressure (mmHg) | -0.98 (-2.94,0.98) | 0.33 |
Abbreviations: FMI – fat mass index; LMI – lean mass index
Models were adjusted for ethnicity, sex, household income, maternal age, pre-pregnancy body mass index, gestational diabetes, prematurity, and size at birth.
Table 4. Differences in cardiometabolic markers between the high fat mass index body composition groups, stratified by sex.
| Fasting plasma glucose (mmol/L) | Diastolic blood pressure (mmHg) | |||
|---|---|---|---|---|
| β (95%CI) | P | β (95%CI) | P | |
| Girls | ||||
| High FMI-Low LMI | Ref. | Ref. | ||
| High FMI-High LMI | -0.29 (-0.55, -0.04) | 0.03 | -3.22 (-6.03, -0.41) | 0.03 |
| Boys | ||||
| High FMI-Low LMI | Ref. | Ref. | ||
| High FMI-High LMI | 0.06 (-0.12, 0.24) | 0.49 | 1.65 (-1.59, 4.88) | 0.31 |
| P for interaction1 | 0.006 | 0.03 | ||
Abbreviations: FMI – fat mass index; LMI – lean mass index
Models were adjusted for ethnicity, household income, maternal age, pre-pregnancy body mass index, gestational diabetes, prematurity, and size at birth.
Discussion
In the present study, 6-year-old children in the “high FMI” body composition phenotype groups had more adverse cardiometabolic profiles compared with those with low FMI. Among children with high FMI, having high LMI seems to slightly attenuate the adverse cardiometabolic profile, but only in girls. The potentially sex-specific protective role of lean mass on cardiometabolic profile of girls with high adiposity needs to be confirmed in larger studies and other populations. In contrast, among children with low FMI, high LMI was not linked to significantly different cardiometabolic profiles, suggesting that the potentially protective effect of LMI might not be detectable in children with low adiposity. Our study suggests that in young children, the protective role of high LMI is neither strong nor consistent. Therefore, it might be important to focus on all children with high FMI for early risk stratification, monitoring, and potential interventions.
Consistent with the literature on older children and adults, we observed strong associations between high FMI groups and adverse cardiometabolic risk markers in young children. By estimating fat mass and fat-free mass based on a combination of bioelectrical impedance and body measurements, a longitudinal study which followed up children aged 8, 11, and 14 years for up to 4 years found that the association between BMI and adverse blood lipid levels was mainly attributable to FMI, rather than the fat-free mass index(29). Pooled analysis of two adult twin cohorts, which measured body composition by dual X-ray absorptiometry and bioelectrical impedance, respectively, similarly found that FMI was more strongly associated with cardiometabolic profile than fat-free mass index(30). We also observed that children from the high FMI groups were more likely to be from minority ethnicities, from low income households, and to have mothers with higher pre-pregnancy BMI and gestational glycemia. Hence, FMI seems to be a good marker of cardiometabolic risk in young children and curbing excessive child adiposity through diet(31,32) or exercise(33), especially focusing on families with socioeconomic disadvantage or mothers with prenatal risk factors, might be vital for optimizing cardiometabolic health.
Several studies in children, adolescents, and adults have reported associations between increased lean mass, skeletal muscle mass, or muscle to fat ratio, with more favorable cardiometabolic profiles. In Korean children and adolescents aged 10–18 years, increased appendicular skeletal muscle to body fat ratio was associated with lower cardiometabolic risk(10) while in Chilean adolescents aged 16 to 17 years, low lean mass was associated with higher cardiometabolic risk(34). In 17 280 Korean adults (mean age: 48.1±8.2 years), transitioning from a low fat-high muscle phenotype to any of the low muscle phenotypes over the 5 year follow-up period was associated with increased type 2 diabetes risk(35). Hence, we hypothesized that increased lean mass might attenuate adverse cardiometabolic profiles in children with high adiposity.
Our findings suggest potential sex-specific protective effects of high LMI on cardiometabolic markers in girls with high FMI. Among girls with high FMI, high LMI was associated with slight reductions in fasting plasma glucose and diastolic blood pressure, without significant changes in any other cardiometabolic risk markers investigated. Sex-specific associations between body composition and cardiometabolic risk have been reported in other studies(10). One explanation might be that the protective effects of LMI are greater and more easily detected in people at higher cardiometabolic risk, such as older adults(35), or children with higher adiposity(10) such as girls in our cohort who had higher mean FMI than boys. Another explanation is there might be sex-specific differences in body fat partitioning where boys in our cohort might concurrently have high lean mass and high intramyocellular lipids, which is associated with skeletal muscle insulin resistance and attenuates the protective effect of lean mass(36). However, our imaging technique using quantitative magnetic resonance measures total body fat and will therefore not provide information on specific body fat partitioning such as intramyocellular fat.
Overall, in young children, the protective effect of LMI seems to be weak and sex-specific. In pooled analyses, we found that children in the HF-HL group had similarly elevated cardiometabolic risk markers as children in the HF-LL group, consistent with a cross-sectional study in 14 807 Korean adults aged 18-65 years, which suggests no significant protective role of lean mass among people with high adiposity(37). A few studies even reported detrimental, instead of protective, effects of LMI or fat-free mass index on cardiometabolic risk. In adults aged 50–70 years, higher fat-free mass index was independently associated with metabolic syndrome risk after adjusting for fat mass(13) while in adolescents aged 12-20 years, LMI was positively associated with elevated cardiometabolic risk markers, even after adjustment for FMI(11). The biological mechanisms for the contrasting associations between lean mass and cardiometabolic markers are poorly understood and further studies are required.
This study has several strengths and limitations. First, it involves the use of a novel, validated quantitative nuclear magnetic resonance technology (EchoMRI™), with several advantages over other methods of measuring body composition such as bioelectrical impedance analysis, air displacement plethysmography, or dual X-ray absorptiometry. Unlike bioelectrical impedance analysis and air displacement plethysmography, EchoMRI™ is more tolerant to movement, not influenced by body hydration, and not based on assumptions from derived body density models or density of fat-free mass(20). Unlike dual X-ray absorptiometry, EchoMRI™ does not involve radiation and is suitable for use in young children. Second, in our deeply-phenotyped cohort, we measured a comprehensive panel of cardiometabolic risk markers, which included blood glucose and lipids, a holistic pediatric metabolic syndrome score, and fatty liver index, to capture any early subclinical changes in cardiometabolic profile. Third, we prospectively collected a range of socio-demographic factors, maternal comorbidities, and perinatal factors which reduced recall bias and enabled us to further understand the associations independent of these potential confounders. Limitations of our study include the cross-sectional design which prevents us from making causal inferences of the effects of different FMI and LMI composition on cardiometabolic markers. The sample size for each body composition group is relatively small, especially for sex-specific analyses. We did not investigate tissue-specific distribution of fats such as intramyocellular lipids and liver fat, which might also contribute to the observed cardiometabolic profile(38). Caution must be taken when trying to generalize findings from our multi-ethnic Asian cohort to other populations.
Conclusions
From the four body composition phenotypes characterized, young children with high FMI had elevated cardiometabolic risk markers, regardless of level of LMI. Hence, preventing excessive accumulation of fat rather than optimizing lean mass might be vital to curb the early development of cardiometabolic risk. Further studies are needed to confirm the potentially sex-specific protective role of LMI in children with high FMI, and to understand the evolving role of lean mass over the life course.
Acknowledgements
We thank all GUSTO participants as well as the GUSTO study group, which includes Allan Sheppard, Amutha Chinnadurai, Anne Eng Neo Goh, Anne Rifkin-Graboi, Anqi Qiu, Arijit Biswas, Bee Wah Lee, Birit F.P. Broekman, Boon Long Quah, Borys Shuter, Chai Kiat Chng, Cheryl Ngo, Choon Looi Bong, Christiani Jeyakumar Henry, Claudia Chi, Cornelia Yin Ing Chee, Yam Thiam Daniel Goh, Doris Fok, E Shyong Tai, Elaine Tham, Elaine Quah Phaik Ling, Evelyn Chung Ning Law, Evelyn Xiu Ling Loo, Falk Mueller-Riemenschneider, George Seow Heong Yeo, Helen Chen, Heng Hao Tan, Hugo P S van Bever, Iliana Magiati, Inez Bik Yun Wong, Ivy Yee-Man Lau, Izzuddin Bin Mohd Aris, Jeevesh Kapur, Jenny L. Richmond, Jerry Kok Yen Chan, Joanna D. Holbrook, Joanne Yoong, Joao N. Ferreira., Jonathan Tze Liang Choo, Jonathan Y. Bernard, Joshua J. Gooley, Kenneth Kwek, Krishnamoorthy Niduvaje, Kuan Jin Lee, Leher Singh, Lieng Hsi Ling, Lin Lin Su, Lourdes Mary Daniel, Lynette P Shek, Mark Hanson, Mary Rauff, Mei Chien Chua, Melvin Khee-Shing Leow, Michael Meaney, Ngee Lek, Oon Hoe Teoh, P. C. Wong, Paulin Tay Straughan, Pratibha Agarwal, Queenie Ling Jun Li, Rob M. van Dam, Salome A. Rebello, Seang-Mei Saw, See Ling Loy, Seng Bin Ang, Shang Chee Chong, Sharon Ng, Shirong Cai, Shu-E Soh, Sok Bee Lim, Stella Tsotsi, Chin-Ying Stephen Hsu, Sue Anne Toh, Swee Chye Quek, Victor Samuel Rajadurai, Walter Stunkel, Wayne Cutfield, Wee Meng Han, Wei Wei Pang, Yin Bun Cheung, and Yiong Huak Chan
Funding/Support
This work was supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Programme and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC), Singapore [NMRC/TCR/004-NUS/2008, NMRC/TCR/012-NUHS/2014]. Additional funding is provided by the Singapore Institute for Clinical Sciences, Agency for Science Technology and Research, Singapore.
Footnotes
Disclosure Statement:
YSC, KMG, and SYC are part of an academic consortium that has received research funding from companies selling nutritional products. KMG and SYC has received reimbursement for speaking at conferences sponsored by companies selling nutritional products. KMG is supported by the UK Medical Research Council (MC_UU_12011/4), the National Institute for Health Research (NIHR Senior Investigator (NF-SI-0515-10042), NIHR Southampton 1000DaysPlus Global Nutrition Research Group (17/63/154) and NIHR Southampton Biomedical Research Centre (IS-BRC-1215-20004)), the European Union (Erasmus+ Programme Early Nutrition eAcademy Southeast Asia-573651-EPP-1-2016-1-DE-EPPKA2-CBHE-JP and ImpENSA 598488-EPP-1-2018-1-DEEPPKA2-CBHE-JP) and the British Heart Foundation (RG/15/17/3174). All other authors have nothing to disclose.
P-value of interaction term between two high fat mass index body composition groups and sex
Data availability statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1.Camhi SM, Katzmarzyk PT. Tracking of cardiometabolic risk factor clustering from childhood to adulthood. International Journal of Pediatric Obesity. 2010;5(2):122–129. doi: 10.3109/17477160903111763. [DOI] [PubMed] [Google Scholar]
- 2.Salbe AD, Weyer C, Lindsay RS, Ravussin E, Tataranni PA. Assessing Risk Factors for Obesity Between Childhood and Adolescence: I.Birth Weight, Childhood Adiposity, Parental Obesity, Insulin, and Leptin. Pediatrics. 2002;110(2):299–306. doi: 10.1542/peds.110.2.299. [DOI] [PubMed] [Google Scholar]
- 3.Daniels SR, Khoury PR, Morrison JA. The Utility of Body Mass Index as a Measure of Body Fatness in Children and Adolescents: Differences by Race and Gender. Pediatrics. 1997;99(6):804–807. doi: 10.1542/peds.99.6.804. [DOI] [PubMed] [Google Scholar]
- 4.Demerath EW, Schubert CM, Maynard LM, Sun SS, Chumlea WC, Pickoff A, Czerwinski SA, Towne B, Siervogel RM. Do Changes in Body Mass Index Percentile Reflect Changes in Body Composition in Children? Data From the Fels Longitudinal Study. Pediatrics. 2006;117(3):e487–e495. doi: 10.1542/peds.2005-0572. [DOI] [PubMed] [Google Scholar]
- 5.Stump CS, Henriksen EJ, Wei Y, Sowers JR. The metabolic syndrome: role of skeletalmuscle metabolism. Ann Med. 2006;38(6):389–402. doi: 10.1080/07853890600888413. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5):990S–991S. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 7.Moon S-S. Low skeletal muscle mass is associated with insulin resistance, diabetes, and metabolic syndrome in the Korean population: the Korea National Health and Nutrition Examination Survey(KNHANES)2009-2010. Endocr J. 2014;61(1):61–70. doi: 10.1507/endocrj.ej13-0244. [DOI] [PubMed] [Google Scholar]
- 8.Lee J, Hong Y, Shin HJ, Lee W. Associations of Sarcopenia and Sarcopenic Obesity With Metabolic Syndrome Considering Both Muscle Mass and Muscle Strength. J Prev Med Public Health. 2016;49(1):35–44. doi: 10.3961/jpmph.15.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sletner L, Mahon P, Crozier SR, Inskip HM, Godfrey KM, Chiesa S, Bhowruth DJ, Charakida M, Deanfield J, Cooper C, et al. Childhood fat and lean mass: differing relations to vascular structure and function at age 8-9-years. Arterioscler Thromb Vasc Biol. 2018;38(10):2528–2537. doi: 10.1161/ATVBAHA.118.311455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim K, Hong S, Kim EY. Reference Values of Skeletal Muscle Mass for Korean Children and Adolescents Using Data from the Korean National Health and Nutrition Examination Survey 2009-2011. PLoS One. 2016;11(4) doi: 10.1371/journal.pone.0153383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber DR, Leonard MB, Shults J, Zemel BS. A Comparison of Fat and Lean Body Mass Index to BMI for the Identification of Metabolic Syndrome in Children and Adolescents. J Clin Endocrinol Metab. 2014;99(9):3208–3216. doi: 10.1210/jc.2014-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duran I, Martakis K, Schafmeyer L, Jackels M, Rehberg M, Schoenau E. Inverse Association of High-Density Lipoprote in Cholesterol Concentration with Muscle Mass in Children. Childhood Obesity. 2019;15(7):476–484. doi: 10.1089/chi.2019.0122. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Rennie KL, Gu W, Li H, Yu Z, Lin X. Independent associations of body-sizeadjusted fat mass and fat-free mass with the metabolic syndrome in Chinese. Annals of Human Biology. 2009;36(1):110–121. doi: 10.1080/03014460802585079. [DOI] [PubMed] [Google Scholar]
- 14.Ramírez-Vélez R, Correa-Bautista JE, Sanders-Tordecilla A, Ojeda-Pardo ML, Cobo-Mejía EA, Castellanos-Vega RDP, García-Hermoso A, González-Jiménez E, Schmidt-RioValle J, González-Ruíz K. Percentage of Body Fat and Fat Mass Index as a Screening Tool for Metabolic Syndrome Prediction in Colombian University Students. Nutrients. 2017;9(9):1009. doi: 10.3390/nu9091009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu P, Ma F, Lou H, Liu Y. The utility of fat mass index vs. body mass index and percentage of body fat in the screening of metabolic syndrome. BMC Public Health. 2013;13(1):629. doi: 10.1186/1471-2458-13-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JY, Oh S, Chang MR, Cho YG, Park KH, Paek YJ, Yoo SH, Cho JJ, Caterson ID, Song HJ. Comparability and utility of body composition measurement vs. anthropometric measurement for assessing obesity related health risks in Korean men. International Journal of Clinical Practice. 2013;67(1):73–80. doi: 10.1111/ijcp.12038. [DOI] [PubMed] [Google Scholar]
- 17.Pasdar Y, Hamzeh B, Najafi F, Darbandi M. Optimal cutoff values of fat mass index, body fat percentage and visceral fat area for identifying metabolic syndrome in the Kurdish population: Results from an Iranian RaNCD cohort study. Mediterranean Journal of Nutrition and Metabolism. 2019;12(4):397–409. [Google Scholar]
- 18.Baur LA. Body composition measurement in normal children: ethical and methodological limitations. Asia Pacific Journal of Clinical Nutrition. 1995;4(1):35–38. [PubMed] [Google Scholar]
- 19.Wasserman H, O’Donnell JM, Gordon CM. Use of dual energy X-ray absorptiometry in pediatric patients. Bone. 2017;104:84–90. doi: 10.1016/j.bone.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L-W, Tint M-T, Fortier MV, Aris IM, Shek LP-C, Tan KH, Rajadurai VS, Gluckman PD, Chong Y-S, Godfrey KM, et al. Body composition measurement in young children using quantitative magnetic resonance: a comparison with air displacement plethysmography. PediatrObes. 2018;13(6):365–373. doi: 10.1111/ijpo.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayyavoo A, Derraik JGB, Hofman PL, Biggs J, Cutfield WS. Metabolic, cardiovascular and anthropometric differences between prepubertal girls and boys. Clin Endocrinol (Oxf) 2014;81(2):238–243. doi: 10.1111/cen.12436. [DOI] [PubMed] [Google Scholar]
- 22.Soh S-E, Tint MT, Gluckman PD, Godfrey KM, Rifkin-Graboi A, Chan YH, Stünkel W, Holbrook JD, Kwek K, Chong Y-S, et al. Cohort Profile: Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. Int J Epidemiol. 2014;43(5):1401–1409. doi: 10.1093/ije/dyt125. [DOI] [PubMed] [Google Scholar]
- 23.WHO Multicentre Growth Reference Study Group. WHO child growth standards: length/height for age, weight-for-age, weight-for-length, weight-for-height and bodymass index-for-age, methods and development. Geneva: World Health Organization; 2006. [Accessed November 19, 2018]. Available at: http://www.who.int/childgrowth/standards/technical_report/en/ [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 25.Aris IM, Bernard JY, Chen L-W, Tint MT, Lim WY, Soh SE, Saw S-M, Shek LP-C, Godfrey KM, Gluckman PD, et al. Postnatal height and adiposity gain, childhood blood pressure and prehypertension risk in an Asian birth cohort. International Journal of Obesity. 2017;41(7):1011–1017. doi: 10.1038/ijo.2017.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahrens W, Moreno LA, Mårild S, Molnár D, Siani A, De Henauw S, Böhmann J, Günther K, Hadjigeorgiou C, Iacoviello L, et al. Metabolic syndrome in young children: definitions and results of the IDEFICS study. International Journal of Obesity. 2014;38(S2):S4–S14. doi: 10.1038/ijo.2014.130. [DOI] [PubMed] [Google Scholar]
- 27.Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mikolajczyk RT, Zhang J, Betran AP, Souza JP, Mori R, Gülmezoglu AM, Merialdi M. A global reference for fetal-weight and birthweight percentiles. The Lancet. 2011;377(9780):1855–1861. doi: 10.1016/S0140-6736(11)60364-4. [DOI] [PubMed] [Google Scholar]
- 29.Dai S, Eissa MA, Steffen LM, Fulton JE, Harrist RB, Labarthe DR. Associations of BMI and its fat-free and fat components with blood lipids in children: Project Heart Beat! Clin Lipidol. 2011;6(2):235–244. doi: 10.2217/clp.11.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jukarainen S, Holst R, Dalgård C, Piirilä P, Lundbom J, Hakkarainen A, Lundbom N, Rissanen A, Kaprio J, Kyvik KO, Sørensen TIA, Pietiläinen KH. Cardiorespiratory Fitness and Adiposity as Determinants of Metabolic Health—Pooled Analysis of Two Twin Cohorts. J Clin Endocrinol Metab. 2017;102(5):1520–1528. doi: 10.1210/jc.2016-3435. [DOI] [PubMed] [Google Scholar]
- 31.Papadaki A, Linardakis M, Larsen TM, van Baak MA, Lindroos AK, Pfeiffer AFH, Martinez JA, Handjieva-Darlenska T, Kunesová M, et al. The Effect of Protein and Glycemic Index on Children’s Body Composition: The DiO Genes Randomized Study. Pediatrics. 2010;126(5):e1143–e1152. doi: 10.1542/peds.2009-3633. [DOI] [PubMed] [Google Scholar]
- 32.Gillman MW, Rifas-Shiman SL, Fernandez-Barres S, Kleinman K, Taveras EM, Oken E. Beverage Intake During Pregnancy and Childhood Adiposity. Pediatrics. 2017;140(2) doi: 10.1542/peds.2017-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson MS, Figueroa-Colon R, Herd SL, Fields DA, Sun M, Hunter GR, Goran MI. Aerobic Fitness, Not Energy Expenditure, Influences Subsequent Increase in Adiposity in Black and White Children. Pediatrics. 2000;106(4):e50–e50. doi: 10.1542/peds.106.4.e50. [DOI] [PubMed] [Google Scholar]
- 34.Burrows R, Correa‐Burrows P, Reyes M, Blanco E, Albala C, Gahagan S. Low muscle mass is associated with cardiometabolic risk regardless of nutritional status in adolescents: A cross-sectional study in a Chilean birth cohort. Pediatric Diabetes. 2017;18(8):895–902. doi: 10.1111/pedi.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim H-K, Lee MJ, Kim E-H, Bae S-J, Choe J, Kim C-H, Park J-Y. Longitudinal Changes of Body Composition Phenotypes and Their Association with Incident Type 2 Diabetes Mellitus duringa 5-Year Follow-up in Koreans. Diabetes Metab J. 2019;43(5):627–639. doi: 10.4093/dmj.2018.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brumbaugh DE, Crume TL, Nadeau K, Scherzinger A, Dabelea D. Intramyocellular Lipid Is Associated with Visceral Adiposity, Markers of Insulin Resistance, and Cardiovascular Risk in Prepubertal Children: The EPOCH Study. J Clin Endocrinol Metab. 2012;97(7):E1099–E1105. doi: 10.1210/jc.2011-3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim K, Park SM. Association of muscle mass and fat mass with insulin resistance and the prevalence of metabolic syndrome in Korean adults: a cross-sectional study. Scientific Reports. 2018;8(1):2703. doi: 10.1038/s41598-018-21168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larson-Meyer DE, Newcomer BR, Ravussin E, Volaufova J, Bennett B, Chalew S, Cefalu WT, Sothern M. Intrahepatic and intramyocellular lipids are determinants of insulin resistance inprepubertal children. Diabetologia. 2011;54(4):869–875. doi: 10.1007/s00125-010-2022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.