Abstract
Background
In pre-clinical models, behavioral training early after stroke produces larger gains compared with delayed training. The effects are thought to be mediated by increased and widespread reorganization of synaptic connections in the brain. It is viewed as a period of spontaneous biological recovery during which synaptic plasticity is increased.
Objective
To look for evidence of a similar change in synaptic plasticity in the human brain in the weeks and months after ischemic stroke.
Methods
We used continuous theta burst stimulation (cTBS) to activate synapses repeatedly in the motor cortex. This initiates early stages of synaptic plasticity that temporarily reduces cortical excitability and motor evoked potential (MEP) amplitude. Thus, the greater the effect of cTBS on the MEP, the greater the inferred level of synaptic plasticity. Data were collected from separate cohorts (Australia and UK). In each cohort, serial measurements were made in the weeks to months following stroke. Data were obtained for the ipsilesional motor cortex in 31 stroke survivors (Australia, 66.6±17.8 years) over 12 months and the contralesional motor cortex in 29 stroke survivors (UK, 68.2±9.8 years) over 6 months.
Results
Depression of cortical excitability by cTBS was most prominent shortly after stroke in the contralesional hemisphere and diminished over subsequent sessions (p=0.030). cTBS response did not differ across the 12 month follow-up period in the ipsilesional hemisphere (p=0.903).
Conclusions
Our results provide the first neurophysiological evidence consistent with a period of enhanced synaptic plasticity in the human brain after stroke. Behavioral training given during this period may be especially effective in supporting post-stroke recovery.
Keywords: Stroke, Plasticity, Recovery, Motor Cortex, Non-Invasive Brain Stimulation, Transcranial Magnetic Stimulation
Introduction
Stroke is a leading global cause of disability.1 Although mortality and age-adjusted incidence rates have decreased, the annual crude incidence of stroke, number of stroke survivors and burden of disease have increased over the past two decades.2 Those who survive stroke often require extensive therapy to support recovery. In humans, the majority of motor recovery happens early after stroke and appears to plateau by 3-6 months.3–7 This recovery involves reorganization of motor output in surviving neural structures through a process known as plasticity.
Pre-clinical studies provide evidence that ischemic events give rise to a spontaneous period of increased neural plasticity, leading to heightened responsiveness to training.8,9 A time-limited, spontaneously occurring period of upregulation in gene and protein expression to support neuronal growth, synaptogenesis, proliferation of dendritic spines, reduced peri-neuronal nets and enhanced brain excitability with changes in the excitation-inhibition balance have been observed within days of experimental stroke in the peri-infarct and contralesional cortex, suggesting widespread changes in both hemispheres.10–21 These changes appear to support behavioral recovery. Rats exposed to enriched environments within 5 days of ischemia had greater skilled forelimb recovery and enhanced dendritic growth compared to those where therapy was delayed until 30 days post-stroke.15 Similarly, in monkeys, initiation of skill training within days of an infarct preserved the cortical hand territory which was thought to play an important role in motor recovery, while the absence of training substantially decreased size of the representations.22,23
At present, there is no direct evidence at a neural level of a period of enhanced synaptic plasticity in human stroke patients. Behavioral data indicates that the effectiveness of rehabilitative therapy diminishes over time.3,24 Furthermore, there is some evidence that early initiation of rehabilitation is associated with better stroke outcomes.25 This would be compatible with a period of increased plasticity soon after stroke although it is difficult to disentangle other potential contributing factors such as stroke severity, medical complications, variations in service delivery and therapy dosage.
The aim of the present study was to measure synaptic plasticity at a neural level in human stroke survivors to test whether there is an early period of enhanced synaptic plasticity. We used continuous theta burst (cTBS) transcranial magnetic stimulation to repetitively activate synaptic connections in the motor cortex and engage early processes of synaptic plasticity via activation of NMDA receptors.26 The effect is a temporary decrease in cortical excitability (long term depression, LTD-like) that can be quantified as a reduction in the amplitude of the MEP evoked by single pulse transcranial magnetic stimulation (TMS). Note that an LTD-like brain stimulation protocol was selected primarily for safety reasons since seizure risk is elevated in acute stroke and could be exacerbated by facilitatory stimulation. Moreover, behavioral benefits and plastic changes during learning, such as recovery following stroke, involve not only strengthening of synaptic connections but also weakening of inappropriate synapses.27 Importantly, in this instance cTBS was not being used as a potential treatment, but as a way of assessing the level of synaptic plasticity within the motor cortex. We expected that if there was an increase in synaptic plasticity in the early weeks after stroke, cTBS would reduce excitability to a greater extent soon after stroke, with the magnitude of this response decreasing over subsequent sessions. Sub-analyses were conducted to investigate possible associations between cTBS response with: 1) stroke severity and upper limb recovery as we hypothesized that greater plasticity of the motor cortex might positively correlate with upper limb recovery; and 2) lesion location as we hypothesized ipsilesional neural responses may be reduced in people with cortical stroke.28,29
Methods
Protocol
This study presents two separate cohorts that investigated plasticity of the ipsilesional and contralesional motor cortex, respectively. Plasticity was defined as the depression of corticospinal excitability produced by a short period of cTBS applied to the motor cortex. Separate cohorts were required to test both hemispheres as interhemispheric interactions would confound the cTBS response if we were to test both hemispheres in each participant. All testing took place in either the Neuromotor Plasticity and Development TMS laboratory located at the University of Adelaide, Australia or the TMS Laboratories in the Sobell Department at the National Hospital for Neurology and Neurosurgery, London, UK. The study was approved by 1) the Central Adelaide Local Health Network Human Research Ethics Committee; and 2) the Joint Ethics Committee of the Institute of Neurology, UCL and National Hospital for Neurology and Neurosurgery, UCL Hospitals NHS Foundation Trust. All participants provided written informed consent in accordance with the Declaration of Helsinki.
The Adelaide laboratory tested the ipsilesional motor cortex and the London laboratory tested the contralesional motor cortex. These datasets will be referred to as the ipsilesional data and contralesional data respectively. Ipsilesional data were obtained at eight time points (1 week, 2 weeks, 3 weeks, 4 weeks, 8 weeks, 12 weeks, 26 weeks and 52 weeks post-stroke). Contralesional data were obtained at four time points (2 weeks, 4 weeks, 6 weeks and 26 weeks post-stroke). Selection of these time points reflects a pragmatic approach at each site, with participants required to return to the neurophysiological laboratory for each experimental session. Note that a variety of reasons (transport, personal, treatment timetable, etc) prevented all patients from being tested on precisely each intended time point, but sessions were conducted as close as reasonably possible to each time point. Experimental sessions were scheduled for a similar time of day to control for physiological diurnal variation that can modify brain stimulation responses.30 Stroke survivors sat in a comfortable armchair and were asked to keep their eyes open, their hand relaxed and their legs uncrossed during testing.
Participants
Participants were recruited from three stroke units across the two countries; the Royal Adelaide Hospital in Australia, the Hyper-Acute Stroke Unit at University College Hospital in the United Kingdom and the Acute Brain Injury Unit at the National Hospital for Neurology & Neurosurgery in the United Kingdom (Table 1 provides individual stroke severity and lesion characteristics data). At each site, all participants meeting inclusion criteria between September 2014 and April 2017 were invited to participate. All participants received standard care through their respective stroke unit during this study. Potential participants were included if they were >18 years of age, had experienced a first-ever ischemic stroke confirmed on imaging with upper limb motor impairment, were medically stable and had a recordable motor evoked potential (MEP; >50 μV) with single-pulse TMS. Those with a history of other neurological disease, recent craniotomy or other acute neurosurgical intervention, inability to provide informed consent, any concurrent medication known to modify seizure threshold or contraindications for TMS such as metallic implants in the skull, history of seizures or implanted permanent pacemaker were excluded.31
Table 1. Individual participant stroke severity and lesion characteristics.
ACA, anterior cerebral artery; MCA, middle cerebral artery; NIHSS, National Institutes of Health Stroke Scale; PCA, posterior cerebral artery; PICA, posterior inferior cerebellar artery.
| Ipsilesional Data | Contralesional Data | ||||||
|---|---|---|---|---|---|---|---|
| ID | NIHSS | CVA Territory | Cortical/Subcortical | ID | NIHSS | CVA Territory | Cortical/Subcortical |
| 1 | 7 | Left MCA | Cortical | 1 | 2 | Right MCA | Cortical |
| 2 | 2 | Right MCA | Subcortical | 2 | 8 | Left MCA | Cortical & Subcortical |
| 3 | 1 | Right MCA | Cortical & Subcortical | 3 | 2 | Left Border Zone | Cortical & Subcortical |
| 4 | 14 | Left ACA/MCA | Cortical | 4 | 2 | Left MCA | Subcortical |
| 5 | 16 | Right ACA | Cortical | 5 | 4 | Right MCA | Cortical & Subcortical |
| 6 | 3 | Right MCA | Cortical | 6 | 4 | Left MCA | Cortical & Subcortical |
| 7 | 13 | Right MCA | Cortical | 7 | 1 | Right MCA | Subcortical |
| 8 | 4 | Left MCA | Cortical & Subcortical | 8 | 5 | Right MCA | Cortical & Subcortical |
| 9 | 3 | Right MCA | Subcortical | 9 | 5 | Right MCA | Cortical & Subcortical |
| 10 | 13 | Right MCA | Cortical & Subcortical | 10 | 2 | Left PCA | Subcortical |
| 11 | 13 | Right ACA/MCA | Subcortical | 11 | 7 | Left MCA | Cortical & Subcortical |
| 12 | 6 | Left MCA | Cortical | 12 | 3 | Left MCA | Subcortical |
| 13 | 4 | Left MCA | Subcortical | 13 | 4 | Left PCA | Subcortical |
| 14 | 5 | Left MCA | Cortical & Subcortical | 14 | 3 | Right MCA | Subcortical |
| 15 | 13 | Right MCA | Cortical | 15 | 4 | Left MCA | Subcortical |
| 16 | 11 | Left MCA | Cortical & Subcortical | 16 | 5 | Left MCA | Cortical & Subcortical |
| 17 | 3 | Right MCA | Subcortical | 17 | 1 | Left PCA | Subcortical |
| 18 | 4 | Left MCA | Cortical & Subcortical | 18 | 1 | Left MCA | Subcortical |
| 19 | 17 | Right MCA | Subcortical | 19 | 3 | Right MCA | Cortical |
| 20 | 2 | Right MCA | Cortical | 20 | 2 | Left MCA | Cortical |
| 21 | 4 | Right MCA | Cortical | 21 | 1 | Right MCA | Subcortical |
| 22 | 3 | Left MCA | Cortical | 22 | 4 | Left MCA | Subcortical |
| 23 | 5 | Right PCA | Cortical | 23 | 9 | Left MCA | Cortical |
| 24 | 6 | Left MCA | Cortical | 24 | 3 | Left MCA | Subcortical |
| 25 | 3 | Left MCA | Subcortical | 25 | 7 | Right MCA | Cortical |
| 26 | 2 | Right MCA | Cortical | 26 | 6 | Right MCA | Subcortical |
| 27 | 3 | Left MCA | Cortical | 27 | 3 | Right MCA | Subcortical |
| 28 | 4 | Right PICA | Subcortical | 28 | 12 | Left MCA | Subcortical |
| 29 | 4 | Right ACA/MCA | Subcortical | 29 | 1 | Left MCA | Subcortical |
| 30 | 14 | Right MCA | Cortical & Subcortical | ||||
| 31 | 7 | Right MCA | Subcortical | ||||
Stroke severity and upper limb recovery
The National Institutes of Health Stroke Scale (NIHSS) was performed by a trained physician on acute hospital admission. Participants were scored from 0–42 across a series of domains based on clinical examination findings, with 0 indicating no clinical signs of stroke and 42 indicating a severe stroke. The action research arm test (ARAT) and Fugl-Meyer Upper Extremity (FM-UE) quantified upper limb recovery at each neurophysiological test session for the ipsilesional and contralesional datasets respectively. The use of different upper limb scales reflects local clinical standard care at each data collection site. Both the ARAT and FM-UE are valid and reliable assessments of motor function that are sensitive to change and recommended for measurement of upper limb motor recovery.32 The ARAT consists of 19 items grouped into four subscales of grasp, grip, pinch and gross movement, with each item scored from 0 to 3. Higher scores are indicative of greater arm activity, with total scores ranging from 0 to 57. The FM-UE consists of 33 items scored on a three-point ordinal scale (0 to 2). Total possible scores range from 0 to 66, with higher scores indicating reduced upper limb impairment.
Stroke diagnosis
Recent stroke diagnosis was confirmed by an experienced neuroradiologist using either magnetic resonance imaging (MRI) or computed tomography (CT). MRI was acquired on either a Siemens Trio 3T scanner, GE Genesis Signa 1.5T scanner or Siemens Avanto 1.5T scanner. Imaging sequences included T1-weighted, axial T2-weight, fluid-attenuated inversion recovery, and diffusion-weighted imaging as part of each participants routine stroke work-up. CT was acquired on a Siemens SOMATOM Definition AS scanner and included non-contrast CT brain and CT angiography routine stroke sequences.
Electromyography
Surface electromyography (EMG) recorded MEPs from the first dorsal interosseous (FDI) muscle of the paretic (ipsilesional data) or non-paretic (contralesional data) hand using Ag/AgCL electrodes (Ambu, Ballerup, Denmark) in a belly-tendon montage (Figure 1). Skin overlying the FDI was prepared by cleaning with alcohol and lightly abrading with NuPrep paste. A ground strap was placed on the wrist. Signals were sampled at 5kHz (CED 1401, Cambridge Electronic Design, Cambridge, UK), amplified 1000x (CED 1902, Cambridge Electronic Design, Cambridge, UK or Digitimer D360, Welwyn Garden City, Herts, UK), band-pass filtered (20-1000Hz) and stored for offline analysis (Signal software, Cambridge Electronic Design, Cambridge).
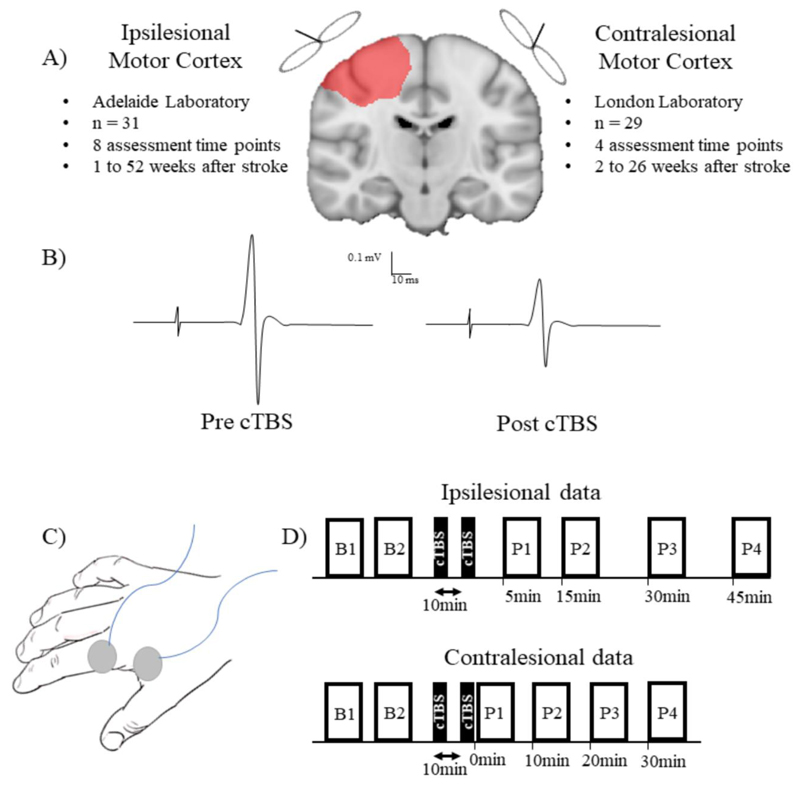
Figure 1. Experimental paradigm.
A) Continuous theta burst stimulation (cTBS) response of the ipsilesional motor cortex was assessed in 31 people with stroke over 8 experimental sessions. For the contralesional motor cortex, cTBS response was assessed in 29 people with stroke over 4 experimental sessions. B) Response to cTBS was quantified as a change in MEP amplitude from baseline (left) to post stimulation (right). Pharmacological studies indicate cTBS produces a long-term depression-like response, therefore leading to a decrease in MEP amplitude 26. The decrease of MEP amplitude was used as a measure of plasticity. C) MEPs were recorded using surface EMG from the first dorsal interosseous muscle of the paretic hand (ipsilesional data) or non-paretic hand (contralesional data). D) Experimental paradigm at each session for the ipsilesional data (top) and contralesional data (bottom). B1 and B2 refer to blocks of baseline MEPs. P1, P2, P3 and P4 refer to blocks of MEPs recorded after continuous theta burst stimulation. Note that the differences in post cTBS timepoints for MEP collection reflects standard practice for neurophysiological experiments at each data collection site. This does not influence the analysis of cTBS responses as the modelling accounts for all time points in each participant
Transcranial magnetic stimulation
Single pulse TMS was delivered using a Magstim 2002 stimulator (Magstim Co., Whitland, Dyfeld, UK) and figure-of-eight 70mm internal diameter Alpha coil (Magstim Co., Whitland, Dyfeld, UK). Stimulation was applied to either the ipsilesional motor cortex (ipsilesional data) or contralesional motor cortex (contralesional data; Figure 1). The coil was held tangentially to the scalp with the handle positioned 45° posterolateral to induce a posterior-anterior current across the hand motor cortex. The optimal coil position for evoking MEPs in the paretic (ipsilesional data) or non-paretic (contralesional data) FDI muscle at rest was located and marked on the scalp using a water-soluble felt tip marker (ipsilesional data) with coil position consistently monitored throughout experimental procedures, or fixed using Brainsight™ neuronavigation system (contralesional data; Rogue Resolutions Inc., Cardiff, UK). Neuronavigation was guided using surface landmarks and electrophysiological feedback in the form of EMG and without the incorporation of neuroanatomical imaging. Participants wore custom glasses for the duration of testing with a reflective Subject Tracker attached on the side opposite to that receiving stimulation. A second reflector was attached to the TMS coil using a Brainsight™ TMS Coil Tracker Fixation Adaptor. TMS Coil Tracker, Subject Tracker (reflective glasses) and anatomical landmarks were calibrated in 3D space using an NDI Polaris Vicra™ optical infrared position sensor (NDI Medical, Waterloo, Ontario, Canada) fixed to the ceiling. Brainsight™ software provided continuous real-time feedback on coil position and orientation. MEPs collected whilst the coil was more than 1 mm or 3° off target in any plane were discarded and repeated. Resting motor threshold (RMT) was defined as the minimum stimulus intensity required to evoke an MEP in the relaxed FDI with a peak-to-peak amplitude larger than 50μV in at least 5 out of 10 trials. Corticospinal excitability was quantified by recording MEPs and measuring peak-to-peak amplitudes (ipsilesional data, stimulus intensity equal to 120% RMT; contralesional data, stimulus intensity to evoke a 1mV MEP). Baseline corticospinal excitability was determined by recording two blocks of 20 MEPs, separated by a short rest interval (~2 min). Blocks of 20 MEPs were selected to provide high within- and between-session reliability of mean MEP amplitude.33 Following cTBS, blocks of 20 MEPs were recorded at multiple time intervals (ipsilesional data, 5 min, 15 min, 30 min, 45 min post cTBS; contralesional data, 0 min, 10 min, 20 min, 30 min post cTBS; Figure 1). Difference in the post cTBS timepoints for MEP collection between the ipsilesional and contralesional datasets reflects standard practice for neurophysiological experiments at each data collection site. These experimental differences do not influence the statistical analysis of cTBS responses as the modelling accounts for all time points in each participant. Single TMS pulses were delivered at 0.2Hz ± 10%. For each trial, EMG in a 200 ms pre-stimulus window was visually inspected at high gain to ensure the FDI was at rest. Trials contaminated with pre-stimulus muscle activity were removed. Peak-to-peak amplitude of MEPs were quantified and averaged for each time point. cTBS response was quantified as a change in MEP amplitude from baseline to post stimulation (Figure 1).
Continuous theta burst stimulation
A Magstim Rapid stimulator connected to an air-cooled figure-of-eight coil (Magstim Company, Dyfed, UK) applied cTBS with a biphasic pulse waveform to the optimal scalp position for evoking responses in the FDI. The cTBS protocol consisted of 600 pulses delivered in triplets at 50Hz, repeated at 5Hz for a total of 40 s.34 In healthy adults, there is evidence of good between-session reliability of cTBS.35,36 A paired cTBS paradigm with a 10 min interval between cTBS trains was applied as it has been reported to induce a greater magnitude and more consistent plasticity response.37,38 Between cTBS trains, participants were asked to relax and refrain from muscle contraction of the upper limb. The intensity of stimulation was set to 70% RMT, with RMT assessed prior to cTBS application using the rTMS coil.
Statistical analysis
Participant demographics and clinical characteristics were compared between the ipsilesional and contralesional datasets with independent t-tests (age and admission NIHSS) or Pearson’s chi-squared tests (sex, lesioned hemisphere, recombinant tissue plasminogen activator treatment). Changes in response to cTBS across stages of stroke recovery were assessed using linear modelling in R and R Studio using the lme4 and dplyr packages.39–41 Taking this statistical approach allowed for more complete and accurate analysis of a longitudinal patient dataset with some missing data than would be afforded with a repeated measures ANOVA. It also allowed us to use the actual MEP amplitude at each cTBS time point (including baseline) in each individual. Separate models were run for the ipsilesional and contralesional data. Each model was constructed to have MEP amplitude as the dependent variable, predicted with time pre- and post-cTBS (TIMEPOINT) and timing of the session relative to the date of the infarct (SESSION).
Therefore, TIMEPOINT, SESSION, and the associated TIMEPOINTxSESSION interaction were included in the model as fixed effects. Random effects ensured that each individual had a unique intercept and slope across timepoints for each session. To understand whether the response to ipsilesional cTBS was influenced by lesion location, we ran a similar linear model to that just described on the ipsilesional data including lesion location as a fixed effect (TIMEPOINTxSESSIONxLESION LOCATION). Linear modelling also assessed changes in RMT over the course of stroke recovery. We first assessed whether these variables were best modelled with a fixed slope random intercepts or a random slopes and intercepts approach, before moving forward with the model that produced the lowest Akaike Information Criterion (AIC) or prevented overfitting of the data. In these models, PARTICIPANT was always included as random effect, and we assessed whether SESSION should also be included as a random effect, indicating that each individual has both a unique starting MEP amplitude and a unique slope in change in MEP amplitudes across sessions. Based on the AIC when comparing these models, a fixed effect of SESSION was used. Assumptions of normality and homoscedasticity of the residuals for each model were assessed visually using quantile-quantile normal plots and fitted-versus residual-value plots. To confirm participants with missing data points were not overtly responsible for the study findings, linear modelling for cTBS response was repeated with only participants who had complete datasets. As an exploratory analysis to provide indication of the duration of a critical period of enhanced plasticity, the change in mean MEP amplitudes from baseline to post cTBS at each session were compared with paired t-tests. If a critical period of enhanced cTBS response was identified, the change in cTBS response in that hemisphere and at that time point were correlated with upper limb recovery scores, controlling for baseline assessment of arm function. cTBS response was calculated as the mean post cTBS MEP amplitude normalized to baseline MEP amplitude. Finally, stroke severity (admission NIHSS) and baseline upper limb outcomes were correlated with plasticity responses at each session. For all statistical tests, the level of significance was set at p≤0.05.
Results
Participant demographics and clinical characteristics
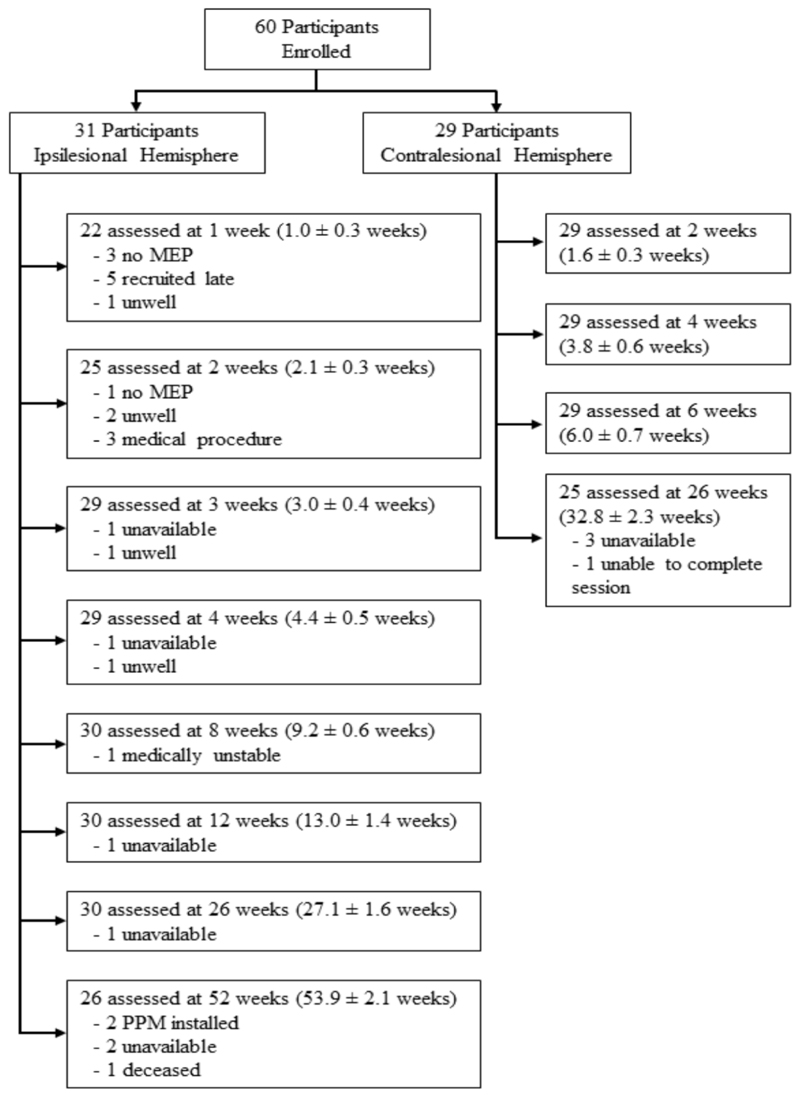
A total of 333 experimental sessions were conducted across the two data collection sites with no significant adverse events (Figure 2). There were no differences in age, sex, lesioned hemisphere side or recombinant tissue plasminogen activator treatment between participants in the ipsilesional and contralesional dataset (Table 2). NIHSS scores were in the range of mild to moderate severity; the contralesional group was slightly less severe than the ipsilesional group. Baseline ARAT and FM-UE for the ipsilesional and contralesional datasets respectively are reported in table 2 with both cohorts exhibiting moderate to good levels of upper limb performance on average. The ARAT and FM-UE scores at each session are available in Supplementary Table 1.
Figure 2. Flow of participants through experimental procedures.
The mean ± SD time post-stroke for each session is reported.
MEP, motor evoked potential; PPM, permanent pacemaker
Table 2. Participant demographics and clinical characteristics.
Note, statistical values compare data between the ipsilesional dataset and contralesional dataset. Statistically significant differences are shown in bold.
ARAT, action research arm test; FM-UE, Fugl Meyer Upper Extremity; NIHSS, National Institutes of Health Stroke Scale; rtPA, recombinant tissue plasminogen activator.
| Ipsilesional | Contralesional | Group | Statistics | |
|---|---|---|---|---|
| Age (mean ± SD) | 66.6 ± 17.8 | 68.2 ± 9.8 | 67.4 ± 14.4 | t (58) = 0.44, p = 0.66 |
| Years | (range 26-93) | (range 46-82) | (range 26-93) | |
| Sex | χ2 = 0.43, p = 0.59 | |||
| Male (n) | 20 | 21 | 41 | |
| Female (n) | 11 | 8 | 19 | |
| Lesioned hemisphere | χ2 = 1.72, p = 0.21 | |||
| Right (n) | 17 | 11 | 28 | |
| Left (n) | 14 | 18 | 32 | |
| rtPA treated | ||||
| yes, n (%) | 10 (32%) | 8 (27%) | 18 (30%) | χ2 = 0.16, p = 0.78 |
| NIHSS (mean ± SD) | 6.7 ± 4.9 | 3.9 ± 2.7 | 5.4 ± 4.2 | t(58) = 2.75, p = 0.008 |
| (range 1-17) | (range 1-12) | (range 1-17) | ||
| ARAT at baseline | 48.0 ± 14.0 | - | - | - |
| (mean ± SD) | (range 8-57) | |||
| FM-UE at baseline | - | 61.8 ± 2.9 | - | - |
| (mean ± SD) | (range 56-66) | |||
Neurophysiological data
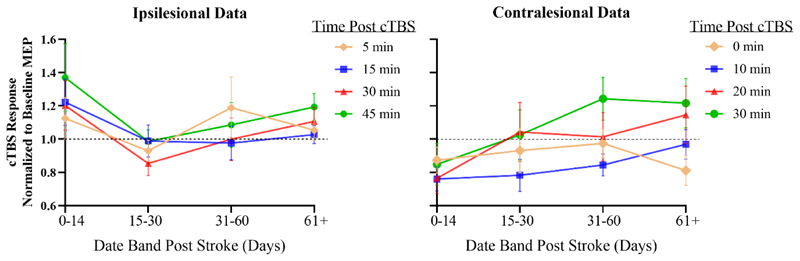
For a variety of personal and treatment-related reasons, patients did not always return for follow-up on precisely the planned dates or may have missed a session. However, sessions were conducted as close as possible to the intended date (see Figure 2) and the statistical analysis accommodates any missing data points. However, in order to give a simple visual impression of the data we grouped the follow-up assessments into 4 periods: 0-14 days; 15-30 days; 31-60 days; and ≥61 days. This allows us to present the data from both sites in a similar format (Figure 3). In the contralesional data, depression of corticospinal excitability after cTBS was greatest shortly after stroke compared with later time points whereas there is no clear trend in the ipsilesional data. Additional figures presenting the cTBS response for each test session (Supplementary Figure 1) and the linear modelling of the TIMEPOINTxSESSION cTBS response (Supplementary Figure 2) are available in the supplementary material.
Figure 3. Continuous theta burst stimulation response for the ipsilesional hemisphere (left) and contralesional hemisphere (right).
Amplitudes of motor evoked potentials have been normalized to baseline. Error bars are standard deviation.
Note, continuous theta burst stimulation is thought to induce a suppression of cortical excitability. Therefore, a larger decrease in motor evoked potential amplitude provides indication of greater plasticity. Data points below the dashed black line indicate motor evoked potential suppression.
cTBS, continuous theta burst stimulation; MEP, motor evoked potential.
For the statistical model examining cTBS response in ipsilesional hemisphere (ipsilesional data, Table 3), there was a significant negative effect of SESSION, indicating that MEP amplitude decreased over time (β=-0.045, p=0.016). There was no significant main effect of TIMEPOINT (p=0.805), and no interaction between TIMEPOINT and SESSION (p=0.903). Thus, cTBS response appeared to remain approximately constant over the study. Follow-up analysis examining the influence of lesion location confirmed a significant effect of SESSION (β=-0.088, p=0.048), with no interaction between TIMEPOINT and SESSION (p=0.187) and no interaction between TIMEPOINT and SESSION and LESION LOCATION (p=0.173). In the contralesional hemisphere (contralesional data, Table 3) there was an interaction between TIMEPOINT and SESSION that influenced MEP amplitudes (β=0.029, p=0.030). There was a decrease in MEP amplitudes in response to cTBS at the first session (2 weeks post-stroke), but this response to stimulation was reduced at subsequent sessions. This observation was confirmed by comparing the change in mean MEP amplitude from baseline to post cTBS at each session that found a significant decrease in MEP amplitude at 2 weeks (t(28)=2.16, p=0.039), but not 4 (p=0.152), 6 (p=0.852) or 26 (p=0.557) weeks post-stroke. Thus, in the contralesional hemisphere, cTBS response was maximum early after stroke and declined over subsequent weeks. RMT and baseline MEP amplitudes for each session and hemisphere are reported in Supplementary Table 2. There were no significant predictors in any of the models examining ipsilesional or contralesional RMT (all p≥0.336, Supplementary Table 3).
Table 3. Fixed effects of motor evoked potential response to continuous theta burst stimulation in the ipsilesional hemisphere (top) and contralesional hemisphere (bottom).
| Model Parameter | Beta Estimate | Standard Error | Confidence Interval | p-value |
|---|---|---|---|---|
| Ipsilesional Data | ||||
| Intercept | 0.919 | 0.095 | 0.732 to 1.106 | <2e-16* |
| Timepoint | -0.005 | 0.021 | -0.042 to 0.041 | 0.805 |
| Session | -0.045 | 0.019 | -0.082 to -0.008 | 0.016* |
| Timepoint:Session | 0.0005 | 0.004 | -0.007 to 0.008 | 0.903 |
| Contralesional Data | ||||
| Intercept | 0.877 | 0.097 | 0.685 to 1.069 | 5.11e-15* |
| Timepoint | -0.077 | 0.036 | -0.148 to -0.006 | 0.034* |
| Session | -0.005 | 0.036 | -0.076 to 0.066 | 0.879 |
| Timepoint:Session | 0.029 | 0.013 | 0.003 to 0.055 | 0.030* |
indicates statistically significant at p ≤ 0.05
These results did not appear to be influenced by those participants with missing data. Re-analysis of cTBS response with only participants who had complete datasets found that there were no significant predictors of MEP amplitude for ipsilesional data. As in the model with all participants, analysis of cTBS response in the contralesional hemisphere found an interaction between TIMEPOINT and SESSION that influenced MEP amplitudes (β=0.03, SE=0.01, 95% CI 0.0004 – 0.053, p=0.050). This relationship was similar, such that there was a decrease in MEP amplitudes in response to cTBS at the first session, but this response to stimulation was reduced at subsequent sessions.
Response to cTBS as a predictor of upper limb recovery
The depression of corticospinal excitability by cTBS was strongest at 2 weeks post-stroke in the contralesional hemisphere. For the contralesional data, upper limb recovery measured with the FM-UE at each time point is reported in Supplementary Table 1. The partial correlations between cTBS response at 2 weeks post-stroke and FM-UE at 4 weeks (rho=0.265, p=0.16), 6 weeks (rho=0.108, p=0.58) or 26 weeks (rho=0.135, p=0.50) were not significant when controlling for baseline FM-UE.
Stroke severity and response to cTBS
NIHSS scores on acute hospital admission were not associated with cTBS response for any session for the ipsilesional (all p>0.16) or contralesional data (all p>0.32). Similarly, upper limb behavior at baseline was not associated with cTBS response for any session for the ipsilesional (all p>0.15) or contralesional data (all p>0.45).
Discussion
This multisite, longitudinal study quantified neural plasticity in the ipsilesional and contralesional human motor cortex over several months following stroke. Our measure of plasticity was the transient reduction in corticospinal excitability produced by cTBS. This was strongest around 2 weeks after stroke in the contralesional motor cortex, dissipating across subsequent sessions. Although we have no measures of pre-stroke cTBS response in our participants, the finding is consistent with the hypothesis that plasticity of the contralesional motor cortex is enhanced early after stroke. In the ipsilesional hemisphere there was no change in response to cTBS over time. As we note below, this could have been due to networks within the ipsilesional hemisphere not being as responsive to cTBS and/or the effect of injury on the response to TMS in that hemisphere.
Assessment of plasticity following stroke in humans
Corticospinal excitability is suppressed for about 30 min following cTBS. Since the effect is blocked by NMDA receptor antagonists, it seems likely that it is due to short-term, LTD-like changes in the efficacy of synaptic connections.26,34 Recordings of corticospinal volleys evoked by single TMS pulses during the period of reduced excitability suggest that the likely site of action is within the cerebral cortex. These show that cTBS reduces the excitability of circuits generating I1 wave input to corticospinal neurons and imply that cTBS influences intrinsic circuits of the motor cortex.42 The reduced I-wave input decreases MEP amplitude following cTBS and provides some indication of the capacity to transiently change synaptic strength in the motor cortex.
Since the responses to theta burst stimulation appear to be relatively consistent in healthy adults,35,36,43 the fact that it changes over time in the contralesional cortex after stroke suggests that an initial high level of neural plasticity at around 2-4 weeks declines over the next 6 months. This is not dissimilar to animal models, where widespread increased plasticity within days to weeks after injury have been reported, and which declines over time.10–19 These changes within the contralesional cortex could contribute directly to recovery. For example, in humans, both hemispheres are recruited during execution or learning of unilateral motor tasks,44–46 suggesting both motor cortices may work cooperatively during motor learning.47 In people with stroke, increased activation of the contralesional cortex on imaging is reported during paretic upper limb movement,48,49 while suppression of contralesional activity has been shown to impair motor performance.50
Contrary to our hypothesis, we did not observe any change in neural plasticity over time in the ipsilesional motor cortex. While pre-clinical models have reported plasticity in both hemispheres after stroke, often the most robust effects are observed in ipsilesional hemisphere.11–13,17,18,20,21 In humans, reorganization within the ipsilesional hemisphere appears more prominent in mild to moderate stroke severity,51,52 similar to patients within this study. It is therefore surprising that we did not see a change in plasticity within this hemisphere. This result may be explained by considering the role of long-term potentiation (LTP) and LTD synaptic plasticity. While both potentiation and depression of surviving neural circuits contribute to neural repair after stroke, it is possible there is a shift in plasticity to favor LTP in the ipsilesional hemisphere. In support, pre-clinical models have shown that reducing excessive GABA mediated hypoactivity in the peri-infarct zone after stroke promotes recovery through cellular excitability changes and enhanced LTP.20,53,54 Other studies have shown upregulation of growth factors, sprouting and proliferation of dendritic spines enable recovery, with potentiation of neural circuits likely to be of paramount importance in the ipsilesional hemisphere.10–21 In humans, cortical excitability of the ipsilesional hemisphere is reduced early after stroke and recovery appears related to the increase in excitability,55 likely mediated through LTP processes. As a result, it is possible that synaptic plasticity within the ipsilesional hemisphere is biased toward promoting LTP, rather than LTD, perhaps explaining our inability to observe a change in the cTBS response over time. This proposed shift toward LTP in the ipsilesional hemisphere is unlikely to be similar in magnitude for the contralesional hemisphere where hyperexcitability is observed after stroke, along with the reduction in excitability correlating with behavior in mild to moderate stroke.56 While testing both LTP and LTD plasticity in all patients would help decipher these neural mechanisms in each hemisphere, it is not possible to do so without confounding physiological responses. In addition, as noted earlier, LTP protocols could theoretically impose greater seizure risk after stroke.
There may be other explanations for our inability to observe a change in plasticity over time in the ipsilesional hemisphere. In a large majority of patients in this group, the stroke damaged cortical structures. Cortical lesions result in substantial neural loss, which influences the interaction of non-invasive brain stimulation with cortical circuits and reduces their capacity to be modified by stimulation. In support, studies have shown that although repetitive TMS applied to the ipsilesional motor cortex can modify corticospinal excitability and motor performance in people with subcortical stroke, it is less effective for cortical stroke.28,29 Furthermore, the hemodynamic response to brain stimulation differs between cortical and subcortical lesions with an increase in blood flow velocity after stimulation in subcortical stroke, whilst this was less prominent in cortical stroke.57 We conclude that lack of change in neural plasticity in ipsilesional cortex could be the result of either a bias toward LTP and/or cortical damage produced by the stroke.
Relation of neural plasticity to stroke recovery
There is evidence to support the relationship between synaptic plasticity and motor learning ability in human studies.58,59 However, in the present study we did not observe an association between plasticity responses and upper limb recovery. This may be due to ceiling effects of the Fugl-Meyer as participants scored an average of ~62 points on admission to the study (maximal score 66) leaving minimal opportunity to quantify recovery. Patients with much lower scores tended to not meet inclusion criteria. Furthermore, given the relatively mild impairment of participants in this study, it could be that any plasticity change induced by the lesion were also mild, reducing opportunity to observe an association with stroke recovery. It may be that in more severe stroke, increased neural damage and greater opportunity for recovery before reaching a ceiling could promote a stronger or prolonged plasticity response. In support, reduced inhibition, a mechanism of plasticity, is more persistent in severe stroke.60 It is possible that with a more pronounced increase in plasticity and magnitude of behavioral improvement there may be greater opportunity to observe a relationship between plasticity and recovery.
Clinical implications
A period of enhanced plasticity early following stroke suggests a therapeutic window may exist that could enable greater recovery from impairment. In support, most human studies suggest earlier rehabilitation is beneficial.25,61–64 However, we refer readers to other work suggesting very early initiation of rehabilitation could be detrimental.65 Perhaps of greatest concern, delays in initiating rehabilitation could result in patients receiving limited, or no therapy, within this period of increased plasticity. Although early rehabilitation is recommended in many stroke guidelines, evidence indicates less than eight minutes of daily therapy is dedicated to upper limb rehabilitation within the first 4 weeks of stroke,66 a period that overlaps with a window of enhanced plasticity. Therapy delivered early after stroke is likely critical to maximize recovery as it would engage a period of heightened plasticity.
Future directions
A novel, but clinically important future direction would be to identify techniques to prolong, or even re-open, a period of increased plasticity. This may enhance the capacity for behavioral restoration and reduce persistent disability following stroke. There is some evidence from pre-clinical studies that re-opening a period of enhanced plasticity is beneficial for behavioral recovery. In rodents that had experienced a stroke with incomplete recovery, a second, subsequent stroke was shown to re-open a window of enhanced plasticity that enabled full recovery from the initial ischemic event.9 While this is not a feasible treatment in humans, there are several promising therapies such as pharmacological and cellular therapies, non-invasive brain stimulation and cardiovascular exercise that may be capable of prolonging or re-opening a period of increased plasticity.26,67,68 These therapies could represent an exciting opportunity for greater recovery after stroke.
Limitations
There are several limitations to this study that should be acknowledged. First, there were slight differences in neurophysiological methodology at the two experimental sites. It could be that differences in plasticity between hemispheres is partially underpinned by the disparity in methodology between sites. However, we suggest this is unlikely as both sites delivered identical cTBS paradigms with change in MEP amplitude as an outcome to test for a critical period of enhanced LTD-like plasticity. In addition, statistical modelling accounted for differences in timing of MEPs recorded after cTBS and both hemispheres were analyzed separately. It is therefore highly unlikely that minor differences in methodology between sites is an explanation for different plasticity responses between hemisphere. Second, although neuronavigation equipment was not available at one site (ipsilesional data), previous literature emphasizes both navigated and non-navigated TMS to the motor cortex are similar in terms of variability and reproducibility of MEPs.69 Nevertheless, small variations in coil placement can influence MEP measurements.70 Therefore, it is not inconceivable that the non-navigated approach for the ipsilesional data could have confounded plasticity measurements. Third, several datapoints were missing, predominantly for the ipsilesional hemisphere reflecting practicalities and challenges of testing acutely unwell stroke survivors within a complex medical setting. That this was more common for the ipsilesional dataset may reflect the greater number of experimental sessions, starting earlier after stroke and finishing at 12 months. Different analytical approaches of including or excluding participants with missing data led to the same overall result, providing some level of confidence our results were not driven by those participants who had missing datapoints. Finally, we acknowledge that in assessing plasticity of the motor cortex, our results are specific to cTBS which is thought to resemble an LTD-like synaptic plasticity response.26 It is possible that the temporal characteristics of other plasticity mechanisms may differ to that reported here.
Conclusion
In conclusion, our results provide the first physiological evidence to demonstrate a period of enhanced neural plasticity following stroke in humans. Our study provides neurophysiological support for an intense, front-end loaded approach to post-stroke rehabilitation. Therapy delivered within the first few weeks post-stroke could coincide with a critical period of enhanced plasticity and be especially effective.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Professor Nick Ward for valuable comments on the manuscript and Dr Richard Perry, Renuka Erande, Caroline Hogan and the North Thames LCRN for support with participant recruitment.
Funding
This study was supported by the National Health and Medical Research Council (NHMRC) of Australia (1058639), The Stroke Association (UK; TSA 2014-04) and the Medical Research Council (UK; grant MR/K01384X/1).
BH is supported by a research fellowship from the National Health and Medical Research Council of Australia (1125054). KEB is supported by the Natural Science and Engineering Council of Canada. AMV is supported by an Australian Research Council Discovery Early Career Researcher Award (DE190100694).
Footnotes
Competing Interests
None of the authors have potential conflicts of interest to be disclosed
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update a report from the American heart association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. The Lancet. 2014;383:245–55. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prabhakaran S, Zarahn E, Riley C, et al. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil Neural Repair. 2008;22:64–71. doi: 10.1177/1545968307305302. [DOI] [PubMed] [Google Scholar]
- 4.Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Stroke. Neurologic and functional recovery the Copenhagen Stroke Study. Phys Med Rehabil Clin N Am. 1999;10:887–906. [PubMed] [Google Scholar]
- 5.Hankey GJ, Spiesser J, Hakimi Z, et al. Rate, degree, and predictors of recovery from disability following ischemic stroke. Neurology. 2007;68:1583–7. doi: 10.1212/01.wnl.0000260967.77422.97. [DOI] [PubMed] [Google Scholar]
- 6.Duncan PW, Goldstein LB, Matchar D, Divine GW, Feussner J. Measurement of motor recovery after stroke. Outcome assessment and sample size requirements. Stroke. 1992;23:1084–9. doi: 10.1161/01.str.23.8.1084. [DOI] [PubMed] [Google Scholar]
- 7.Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011;377:1693–702. doi: 10.1016/S0140-6736(11)60325-5. [DOI] [PubMed] [Google Scholar]
- 8.Centonze D, Rossi S, Tortiglione A, et al. Synaptic plasticity during recovery from permanent occlusion of the middle cerebral artery. Neurobiol Dis. 2007;27:44–53. doi: 10.1016/j.nbd.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Zeiler SR, Hubbard R, Gibson EM, et al. Paradoxical Motor Recovery From a First Stroke After Induction of a Second Stroke: Reopening a Postischemic Sensitive Period. Neurorehabil Neural Repair. 2016;30:794–800. doi: 10.1177/1545968315624783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takatsuru Y, Fukumoto D, Yoshitomo M, et al. Neuronal Circuit Remodeling in the Contralateral Cortical Hemisphere during Functional Recovery from Cerebral Infarction. J Neurosci. 2009;29:10081–6. doi: 10.1523/JNEUROSCI.1638-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol. 2006;59:735–42. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- 12.Carmichael ST, Archibeque I, Luke L, et al. Growth-associated gene expression after stroke: Evidence for a growth-promoting region in peri-infarct cortex. Exp Neurol. 2005;193:291–311. doi: 10.1016/j.expneurol.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Keyvani K, Witte OW, Paulus W. Gene expression profiling in perilesional and contralateral areas after ischemia in rat brain. J Cereb Blood Flow Metab. 2002;22:153–60. doi: 10.1097/00004647-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Biernaskie J, Corbett D. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. J Neurosci. 2001;21:5272–80. doi: 10.1523/JNEUROSCI.21-14-05272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biernaskie J, Chernenko G, Corbett D. Efficacy of Rehabilitative Experience Declines with Time after Focal Ischemic Brain Injury. J Neurosci. 2004;24:1245–54. doi: 10.1523/JNEUROSCI.3834-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zai L, Ferrari C, Subbaiah S, et al. Inosine alters gene expression and axonal projections in neurons contralateral to a cortical infarct and improves skilled use of the impaired limb. J Neurosci. 2009;29:8187–97. doi: 10.1523/JNEUROSCI.0414-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carmichael ST. Gene expression changes after focal stroke, traumatic brain and spinal cord injuries. Curr Opin Neurol. 2003;16:699–704. doi: 10.1097/01.wco.0000102621.38669.77. [DOI] [PubMed] [Google Scholar]
- 18.Carmichael ST, Chesselet M-F. Synchronous Neuronal Activity Is a Signal for Axonal Sprouting after Cortical Lesions in the Adult. J Neurosci. 2002;22:6062–70. doi: 10.1523/JNEUROSCI.22-14-06062.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones TA, Schallert T. Use-dependent growth of pyramidal neurons after neocortical damage. J Neurosci. 1994;14:2140–52. doi: 10.1523/JNEUROSCI.14-04-02140.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clarkson AN, Huang BS, MacIsaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468:305. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarkson AN, Overman JJ, Zhong S, et al. AMPA receptor-induced local brain-derived neurotrophic factor signaling mediates motor recovery after stroke. J Neurosci. 2011;31:3766–75. doi: 10.1523/JNEUROSCI.5780-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272:1791–4. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- 23.Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. 1996;75:2144–9. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- 24.van Kordelaar J, van Wegen E, Kwakkel G. Impact of Time on Quality of Motor Control of the Paretic Upper Limb After Stroke. Arch Phys Med Rehabil. 2014;95:338–44. doi: 10.1016/j.apmr.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Horn SD, DeJong G, Smout RJ, et al. Stroke Rehabilitation Patients, Practice, and Outcomes: Is Earlier and More Aggressive Therapy Better? Arch Phys Med Rehabil. 2005;86:101–14. doi: 10.1016/j.apmr.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 26.Huang Y-Z, Chen R-S, Rothwell JC, Wen H-Y. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol. 2007;118:1028–32. doi: 10.1016/j.clinph.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 27.Sanes JN. Neocortical mechanisms in motor learning. Curr Opin Neurobiol. 2003;13:225–31. doi: 10.1016/s0959-4388(03)00046-1. [DOI] [PubMed] [Google Scholar]
- 28.Ameli M, Grefkes C, Kemper F, et al. Differential effects of high-frequency repetitive transcranial magnetic stimulation over ipsilesional primary motor cortex in cortical and subcortical middle cerebral artery stroke. Ann Neurol. 2009;66:298–309. doi: 10.1002/ana.21725. [DOI] [PubMed] [Google Scholar]
- 29.Emara T, El Nahas N, Elkader HA, Ashour S, El Etrebi A. MRI can Predict the Response to Therapeutic Repetitive Transcranial Magnetic Stimulation (rTMS) in Stroke Patients. J Vasc Interv Neurol. 2009;2:163–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Sale MV, Ridding MC, Nordstrom MA. Cortisol inhibits neuroplasticity induction in human motor cortex. J Neurosci. 2008;28:8285–93. doi: 10.1523/JNEUROSCI.1963-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Screening questionnaire before TMS: An update. Clin Neurophysiol. 2011;122:1686. doi: 10.1016/j.clinph.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 32.Kwakkel G, Lannin NA, Borschmann K, et al. Standardized Measurement of Sensorimotor Recovery in Stroke Trials: Consensus-Based Core Recommendations from the Stroke Recovery and Rehabilitation Roundtable. Neurorehabil Neural Repair. 2017;31:784–92. doi: 10.1177/1545968317732662. [DOI] [PubMed] [Google Scholar]
- 33.Goldsworthy MR, Hordacre B, Ridding MC. Minimum number of trials required for within- and between-session reliability of TMS measures of corticospinal excitability. Neuroscience. 2016;320:205–9. doi: 10.1016/j.neuroscience.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–6. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 35.Vallence AM, Goldsworthy MR, Hodyl NA, et al. Inter- and intra-subject variability of motor cortex plasticity following continuous theta-burst stimulation. Neuroscience. 2015;304:266–78. doi: 10.1016/j.neuroscience.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 36.Vernet M, Bashir S, Yoo W-K, et al. Reproducibility of the effects of theta burst stimulation on motor cortical plasticity in healthy participants. Clin Neurophysiol. 2014;125:320–6. doi: 10.1016/j.clinph.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldsworthy MR, Pitcher JB, Ridding MC. Neuroplastic modulation of inhibitory motor cortical networks by spaced theta burst stimulation protocols. Brain Stimul. 2013;6:340–5. doi: 10.1016/j.brs.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Goldsworthy MR, Pitcher JB, Ridding MC. The application of spaced theta burst protocols induces long-lasting neuroplastic changes in the human motor cortex. Eur J Neurosci. 2012;35:125–34. doi: 10.1111/j.1460-9568.2011.07924.x. [DOI] [PubMed] [Google Scholar]
- 39.Wickham H, Francois R, Henry L, Müller K. dplyr: A grammar of data manipulation. R package version 04. 2015:3. [Google Scholar]
- 40.Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw. 2015;67:48. [Google Scholar]
- 41.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011; 2019. URL https://www.R-project.org. [Google Scholar]
- 42.Di Lazzaro V, Pilato F, Saturno E, et al. Theta-burst repetitive transcranial magnetic stimulation suppresses specific excitatory circuits in the human motor cortex. J Physiol (Lond) 2005;565:945–50. doi: 10.1113/jphysiol.2005.087288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hinder MR, Goss EL, Fujiyama H, et al. Inter- and intra-individual variability following intermittent theta burst stimulation: Implications for rehabilitation and recovery. Brain Stimul. 2014;7:365–71. doi: 10.1016/j.brs.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 44.Cabibel V, Hordacre B, Perrey S. Implication of the ipsilateral motor network in unilateral voluntary muscle contraction: the cross-activation phenomenon. J Neurophysiol. 2020;123:2090–8. doi: 10.1152/jn.00064.2020. [DOI] [PubMed] [Google Scholar]
- 45.Derosière G, Alexandre F, Bourdillon N, et al. Similar scaling of contralateral and ipsilateral cortical responses during graded unimanual force generation. Neuroimage. 2014;85(1):471–7. doi: 10.1016/j.neuroimage.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Muellbacher W, Facchini S, Boroojerdi B, Hallett M. Changes in motor cortex excitability during ipsilateral hand muscle activation in humans. Clin Neurophysiol. 2000;111:344–9. doi: 10.1016/s1388-2457(99)00243-6. [DOI] [PubMed] [Google Scholar]
- 47.Waters S, Wiestler T, Diedrichsen J. Cooperation not competition: bihemispheric tDCS and fMRI show role for ipsilateral hemisphere in motor learning. J Neurosci. 2017;37:7500–12. doi: 10.1523/JNEUROSCI.3414-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cramer SC, Nelles G, Benson RR, et al. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997;28:2518–27. doi: 10.1161/01.str.28.12.2518. [DOI] [PubMed] [Google Scholar]
- 49.Cuadrado ML, Egido JA, González-Gutiérrez JL, Varela-de-Seijas E. Bihemispheric Contribution to Motor Recovery after Stroke: A Longitudinal Study with Transcranial Doppler Ultrasonography. Cerebrovasc Dis. 1999;9:337–44. doi: 10.1159/000016009. [DOI] [PubMed] [Google Scholar]
- 50.Johansen-Berg H, Rushworth MFS, Bogdanovic MD, et al. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002;99:14518–23. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ward NS, Newton JM, Swayne OB, et al. The relationship between brain activity and peak grip force is modulated by corticospinal system integrity after subcortical stroke. Eur J Neurosci. 2007;25:1865–73. doi: 10.1111/j.1460-9568.2007.05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ward NS, Newton JM, Swayne OBC, et al. Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain. 2006;129:809–19. doi: 10.1093/brain/awl002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hess G, Aizenman CD, Donoghue JP. Conditions for the induction of long-term potentiation in layer II/III horizontal connections of the rat motor cortex. J Neurophysiol. 1996;75:1765–78. doi: 10.1152/jn.1996.75.5.1765. [DOI] [PubMed] [Google Scholar]
- 54.Collinson N, Kuenzi FM, Jarolimek W, et al. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the α5 subunit of the GABAAReceptor. 2002;22:5572–80. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McDonnell MN, Stinear CM. TMS measures of motor cortex function after stroke: A meta-analysis. Brain Stimul. 2017;10:721–34. doi: 10.1016/j.brs.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 56.Buetefisch CM. Role of the Contralesional Hemisphere in Post-Stroke Recovery of Upper Extremity Motor Function. 2015:6. doi: 10.3389/fneur.2015.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khaleel SH, Bayoumy IM, El-Nabil LM, Moustafa RR. Differential Hemodynamic Response to Repetitive Transcranial Magnetic Stimulation in Acute Stroke Patients with Cortical versus Subcortical Infarcts. Eur Neurol. 2010;63:337–42. doi: 10.1159/000302708. [DOI] [PubMed] [Google Scholar]
- 58.Teo JTH, Swayne OBC, Cheeran B, Greenwood RJ, Rothwell JC. Human theta burst stimulation enhances subsequent motor learning and increases performance variability. Cereb Cortex. 2011;21:1627–38. doi: 10.1093/cercor/bhq231. [DOI] [PubMed] [Google Scholar]
- 59.Lopez-Alonso V, Cheeran B, Fernandez-del-Olmo M. Relationship Between Non-invasive Brain Stimulation-induced Plasticity and Capacity for Motor Learning. Brain Stimul. 2015;8:1209–19. doi: 10.1016/j.brs.2015.07.042. [DOI] [PubMed] [Google Scholar]
- 60.Manganotti P, Acler M, Zanette GP, Smania N, Fiaschi A. Motor Cortical Disinhibition During Early and Late Recovery After Stroke. Neurorehabil Neural Repair. 2007;22:396–403. doi: 10.1177/1545968307313505. [DOI] [PubMed] [Google Scholar]
- 61.Maulden SA, Gassaway J, Horn SD, Smout RJ, DeJong G. Timing of initiation of rehabilitation after stroke. Arch Phys Med Rehabil. 2005;86:S34–S40. doi: 10.1016/j.apmr.2005.08.119. [DOI] [PubMed] [Google Scholar]
- 62.Kwakkel G, Winters C, van Wegen EE, et al. Effects of Unilateral Upper Limb Training in Two Distinct Prognostic Groups Early After Stroke: The EXPLICIT-Stroke Randomized Clinical Trial. Neurorehabil Neural Repair. 2016;30:804–16. doi: 10.1177/1545968315624784. [DOI] [PubMed] [Google Scholar]
- 63.Liu N, Cadilhac DA, Andrew NE, et al. Randomized Controlled Trial of Early Rehabilitation After Intracerebral Hemorrhage Stroke. Stroke. 2014;45:3502–7. doi: 10.1161/STROKEAHA.114.005661. [DOI] [PubMed] [Google Scholar]
- 64.Momosaki R, Yasunaga H, Kakuda W, et al. Very Early versus Delayed Rehabilitation for Acute Ischemic Stroke Patients with Intravenous Recombinant Tissue Plasminogen Activator: A Nationwide Retrospective Cohort Study. Cerebrovasc Dis. 2016;42:41–8. doi: 10.1159/000444720. [DOI] [PubMed] [Google Scholar]
- 65.Bernhardt J, Langhorne P, Lindley RI, et al. Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): a randomised controlled trial. The Lancet. 2015;386:46–55. doi: 10.1016/S0140-6736(15)60690-0. [DOI] [PubMed] [Google Scholar]
- 66.Serrada I, McDonnell MN, Hillier SL. What is current practice for upper limb rehabilitation in the acute hospital setting following stroke? A systematic review. NeuroRehabilitation. 2016;39:431–8. doi: 10.3233/NRE-161374. [DOI] [PubMed] [Google Scholar]
- 67.Mang CS, Snow NJ, Campbell KL, Ross CJ, Boyd LA. A single bout of high-intensity aerobic exercise facilitates response to paired associative stimulation and promotes sequence-specific implicit motor learning. J Appl Physiol. 2014;117:1325–36. doi: 10.1152/japplphysiol.00498.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cramer S. Drugs to Enhance Motor Recovery After Stroke. Stroke. 2015;46:2998–3005. doi: 10.1161/STROKEAHA.115.007433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jung NH, Delvendahl I, Kuhnke NG, et al. Navigated transcranial magnetic stimulation does not decrease the variability of motor-evoked potentials. Brain Stimul. 2010;3:87–94. doi: 10.1016/j.brs.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 70.Schmidt S, Bathe-Peters R, Fleischmann R, et al. Nonphysiological factors in navigated TMS studies; confounding covariates and valid intracortical estimates. 2015;36:40–9. doi: 10.1002/hbm.22611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.