Abstract
Inflammation is a physiological response to aggression of pathogenic agents aimed at eliminating the aggressor agent and promoting healing. Excessive inflammation, however, may contribute to tissue damage and an alteration of arterial structure and function. Increased arterial stiffness is a well-recognized cardiovascular risk factor independent of blood pressure levels and an intermediate endpoint for cardiovascular events. In the present review we discuss immune mediated mechanisms by which inflammation can influence arterial physiology and lead to vascular dysfunction such as atherosclerosis and arterial stiffening. We also show that acute inflammation predisposes the vasculature to arterial dysfunction and stiffening, and alteration of endothelial function and that chronic inflammatory diseases such as rheumatoid arthritis, inflammatory bowel disease and psoriasis are accompanied by profound arterial dysfunction which is proportional to the severity of inflammation. Current findings suggest that treatment of inflammation by targeted drugs leads to regression of arterial dysfunction. There is hope that these treatments will improve outcomes for patients.
Condensed abstract
Inflammation is a physiological response to aggression of pathogenic agents aimed at eliminating the aggressor agent and promoting healing. Excessive inflammation, however, may contribute to tissue damage and an alteration of arterial structure and function. Increased arterial stiffness is a well-recognized cardiovascular risk factor independent of blood pressure levels. In the present review we discuss (i) mechanisms by which inflammation can influence arterial physiology and lead to vascular dysfunction such as atherosclerosis and arterial stiffening, (ii) clinical models of vascular inflammation, and (iii) current evidence on anti-inflammatory therapy and regression of arterial dysfunction.
Keywords: Arterial stiffness, inflammation, pulse wave velocity, large arteries, cardiovascular disease.
Introduction
Inflammation is a ubiquitous, integrated and complex response to insults by pathogens, immunologically-mediated stimuli, irritants or chemicals. Inflammation, however, may also contribute to tissue damage if excessive or chronic. The vascular system is key to the inflammatory response since most components of the inflammatory response transit through the blood and vessels. In the vascular system, acute and chronic inflammation lead to endothelial dysfunction and arterial remodelling, which underlie many cardiovascular diseases. Vascular inflammation is a common response to injury and involves many cell types (immune cells, vascular smooth muscle cells (VSMC), perivascular adipocytes and fibroblasts), numerous mediators (cytokines, chemokines and reactive oxygen species (ROS)), multiple receptors (toll-like receptors, receptor for advanced glycation end-products, tumour necrosis factor (TNF)α receptor-associated factors, NOD-like receptors, transforming growth factor-β-(TGF-β) activated kinase 1, cytokine and chemokine receptors) and complex pro-inflammatory signalling pathways (e.g., nuclear factor κB, mitogen-activated protein kinases, canonical wingless-related integration site (WNT)/β-catenin and Signal transducer and activator of transcription 3, STAT3). Prolonged inflammation causes DNA damage, first step to vascular injury. At the core of many of these processes is an increased production of ROS, especially superoxide anions (O2 -) and hydrogen peroxide (H2O2) and activation of injurious redox-sensitive signalling pathways [1]. In the present review, we propose a general overview of the basic mechanisms by which inflammation can alter arterial physiology and lead to vascular complications such as atherosclerosis and arterial stiffening. We further aim to demonstrate the bidirectional association between inflammation and vascular diseases, i.e. vascular consequences of primarily inflammatory diseases, and systemic disease caused primarily by inflammatory vascular disease. Finally, we review the epidemiological evidence of the association between low-grade chronic inflammation and vascular diseases and we will also expose to what extent ant-iinflammatory drugs can reverse the effects of inflammation on large vessels.
Biological basis and redox biology of inflammation in arterial disease
The inflammatory response in arteries is triggered by humoral, physical and mechanical factors including vasoactive hormones (angiotensin II (AII), endothelin-1, aldosterone), mechanical factors (vascular stretch, pressure), ischaemic insults (ischemia-reperfusion, hypoxia), metabolic factors (hyperglycaemia, oxidized low density lipoproteins, LDL), cytokines and chemokines [2], as well as by clonal haematopoiesis of indeterminate potential and epigenetic dysregulation [3]. The autonomic nervous system has also been implicated in the acute phases of inflammation through the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis since increased catecholamine levels and glucocorticoids may stimulate innate immune cells, such as neutrophils, macrophages and lymphocytes [4–6]. These factors play a role in vascular damage associated with cardiovascular diseases.
Innate immunity in arterial disease
One of the earliest events in the inflammatory response is the activation of the innate immunity [7,8]. Endothelial adhesion molecules interact with glycoproteins on the neutrophil surface promoting interactions with the vessel wall [9]. E-selectin facilitates neutrophil tethering, rolling and adhesion through binding with P-selectin glycoprotein ligand 1, CD44 and E-selectin ligand I [2,10]. This interaction triggers integrin activation through tyrosine kinases, which induces platelet adhesion, further contributing to endothelial injury and dysfunction [10]. Following neutrophil adhesion, the cytoskeleton undergoes reorganization to establish cell polarity, which facilitates transmigration of cells into the vascular media, where resident macrophages are present. Activated neutrophils may also migrate to the perivascular adventitial tissue. Within the vascular wall and perivascular tissue, neutrophils and macrophages further drive inflammation causing vascular damage. Infiltration of innate and adaptive immune cells in perivascular fat, kidneys and myocardium is found to different degrees in experimental and human hypertension [8,11,12]. Innate immune cells sense pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) from injured tissue via toll-like receptors (TLRs) [13]. Proinflammatory macrophages and dendritic cells (DCs) release interleukin (IL)-1β, IL-6, IL-12, IL-23, TNFα and ROS; anti-inflammatory macrophages and DCs produce IL-10. Natural Killer (NK) cells produce pro- or anti-inflammatory cytokines (interferon γ (IFNγ) or IL-10). Myeloid-derived suppressor cells are increased in blood, spleen and kidneys of AII-infused mice. Their depletion induces an exaggerated BP elevation to AII.
Adaptive immunity in arterial disease
Adaptive immunity is involved in response to combined stimulation by antigen, co-stimulators, and specific cytokines [8]. Naïve CD4+ Th cells differentiate into T helper (Th)1, Th2, Th17 effector (all proinflammatory), or Treg cells (anti-inflammatory). Th1 cells produce IFNγ, IL-2 and TNFα, Th2 cells produce IL-4, IL-5, IL-9 and IL-13; Th17 cells secrete IL-17, IL-21 and IL-22. Upon activation with MHC I-restricted antigens, naïve CD8+ T cells mature into cytotoxic (Tc) cells that secrete IFNγ and TNFα, perforin and granzyme B. Perforin creates pores within the target cell membranes, through which granzymes enter cells and induce apoptosis. T-regulatory (Treg) cells express the IL-2 receptor α-subunit (CD25), and the transcription factor forkhead box P3 (Foxp3). The suppressive actions of Treg cells are mediated by cell-cell contact mechanisms and/or via the release of ant-iinflammatory IL-10, IL-35 and TGF-β [14].
Immunological memory plays a role in hypertension. Central memory T (TCM) cells are found in lymphoid organs, whereas effector memory T (TEM) cells recirculate between lymphoid tissues, blood and peripheral organs and produce proinflammatory IFNγ and IL-17A. AII infusion caused TEM cell accumulation in the aorta, kidney and lymph nodes of humanized mice [15]. Hypertensive patients have increased frequency of circulating senescent (CD28–CD57+) CD8+, CD8+ T cells expressing IFγ, TNFα, perforin and granzyme B, CD4+ Th1 and Th17 cells [16,17]. A subpopulation of CD4+ TEM cells that expresses choline acetyltransferase (ChAT) is activated via β2-adrenergic receptors and releases acetylcholine, which binds to α7 nicotinic acetylcholine receptors on macrophages and suppresses lipopolysaccharide-induced TNFα release [18]. BP is higher in mice lacking ChAT in CD4+ T cells than in control mice, and infusion of Jurkat T cells overexpressing ChAT decreases BP [19].
Decreased immune Treg (CD4+CD25+ T cells) activity could counteract hypertension and cardiovascular injury. Adoptive transfer of Treg reduced AII- or aldosterone/salt-induced BP elevation, together with vascular and cardiac injury [20–23]. AII decreased Treg function via the binding of complement components C3a and C5a to their cognate receptor, which decreases Foxp3 expression [24]. Finally, there is an inverse correlation between circulating CD4+CD25HighCD127Low Treg and media/lumen ratio of subcutaneous resistance arteries and retinal arterioles in human essential hypertension [25].
γδ T cells represent ~0.5-10% of circulating lymphocytes in humans. They belong to CD4-CD8- populations, tend to reside in non-lymphoid tissues and have a faster response after activation and after a recall response to antigens than MHC-restricted T cells. They produce IL-17 in response to the pro-inflammatory cytokines IL-1β and IL-23 like Th17 cells. AII caused an increase in number and activation of γδ T cells [26]. Deficiency in γδ T cells prevented AII-induced hypertension, endothelial dysfunction and spleen and mesenteric artery perivascular adipose tissue CD4+ and CD8+ T cell activation [26].
The vascular inflammatory cascade
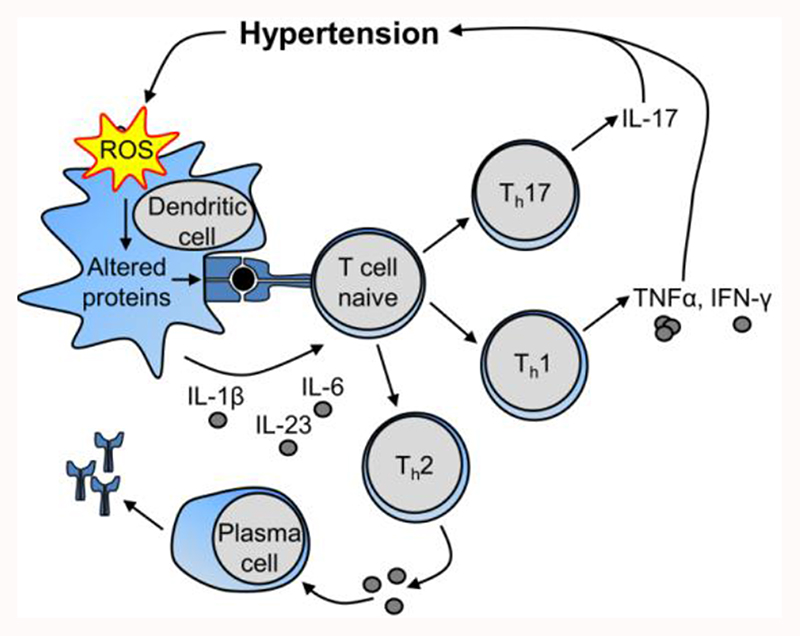
How and when the immune system is activated in hypertension remains unclear. We propose the following cascade (Figure 1). Initial blood pressure (BP) elevation and/or pro-hypertensive stimuli could induce cardiovascular injury leading to development of DAMPs or of neoantigens. In a hypertensive mice models, angiotensin II causes ROS generation in DCs that could result in production of γ-ketoaldehydes or isoketals, which react with protein lysine residues of injured tissues to form isoketal protein adducts that function as neoantigens [27]. These activate DCs, and stimulate release of IL-6, IL-1β@ and IL-23, which in turn stimulate innate immunity via toll-like receptors on proinflammatory macrophages, DCs, and NK cells, as well as innate-like γδ T cells. The latter may prime both innate and adaptive immune cells. Innate immune cells and γδ T lymphocytes contribute to inflammation, both directly or by activation of adaptive immunity, inducing proinflammatory cytokines like IL-17 and IFNγ, and autoantibody production leading to vascular and kidney injury, thus contributing to progressive BP elevation. TEM cells generated during this process accumulate in lymphoid organs including the bone marrow. Upon a second hit such as high-salt intake, TEM cells are activated and contribute to hypertension. Throughout, anti-inflammatory cells such as Treg, myeloid-derived suppressor cell and M2 macrophages may limit this response and help fine-tune vascular and kidney inflammation [28].
Figure 1. Proposed mechanisms by which the immune system is activated in hypertension. IL, interleukin; ROS, reactive oxygen species; Th, T helper cell; TNFα, tumor necrosis factor-alpha.
Reactive oxygen species, oxidative stress and vascular inflammation
The generation of ROS is central to the effects of pro-inflammatory cells because, in phagocytic cells, it is responsible for host-defence responses. In the vascular system, VSMCs, endothelial cells, fibroblasts, perivascular adipocytes, macrophages and immune cells produce ROS [29]. The major source of ROS in vascular cells is nicotinamide adenine dinucleotide phosphate oxidase (Nox), although other enzymatic sources may also contribute, such as xanthine oxidase, mitochondrial electron transport chain, uncoupled endothelial nitric oxide (NO) synthases, cyclooxygenase, lipoxygenase and cytochrome P450 oxidases [29]. Of the seven Nox isoforms identified, Nox1,2,4 and 5 are present in human arteries [30]. Nox1,2 and 5 produce O2 - while Nox4 activation leads to increased H2O2 production. Vascular Nox activity is increased in cardiovascular disease leading to dysregulated ROS production, oxidative stress, activation of pro-inflammatory transcription factors and activation of redox-sensitive signalling pathways [29,30]. Increased endothelial cell O2 - limits bioavailability of nitric oxide and also increases formation of ONOO-, which is highly unstable causing vascular inflammation, fibrosis, hypertrophy and endothelial dysfunction. Oxidative stress is counterbalanced by numerous antioxidant systems, including superoxide dismutase, catalase, peroxidases, glutathione, thioredoxin and nuclear factor erythroid 2 (Nrf-2), the master regulator of antioxidant genes [31]. In cardiovascular disease, many of these antioxidant systems are downregulated, thereby further contributing to excessive ROS accumulation.
In addition to the inactivation of NO, ROS influence vascular cell function by altering protein activity through post-translational oxidative modifications [29,30]. In vascular cells, proteins that are redox-sensitive include receptors, kinases, phosphatases, transcription factors, ion channels, cytoskeletal proteins and matrix metalloproteases (MMPs), all of which play a role in regulating endothelial and VSMC function. In addition, ROS stimulate production of vasoactive agents, such as AII, endothelin-1 and cyclooxygenase-2 and they influence intracellular calcium concentration, important in regulating vasoconstriction. In the endothelium, H2O2 acts as a vasodilator and is considered as an endothelium-derived relaxing factor, acting through activation of protein kinase G alpha (PKG1α) [30]. ROS in return stimulate activation of transcription factors and pro-inflammatory genes, chemokine and cytokine production and recruitment and activation of inflammatory and immune cells in a positive feedback for vascular inflammation.
Mitochondrial ROS production and DNA damage are another important sources of oxidative stress in cardiovascular disease [32]. Physiologically, mitochondrial ROS act as signalling molecules and modulate vasomotion and myogenic tone (i.e. the interaction between vasomotor tone and mechanical stimuli). In several pathological conditions, mitochondria produce excessive ROS that activate proinflammatory pathways in the vascular endothelial cells and result in vasoconstriction, inflammation and thrombosis [32]. Moreover, mitochondrial ROS, through the triggering of NOD-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome formation and activation, lead to IL-1β activation [33].
IL-1β inflammation pathway
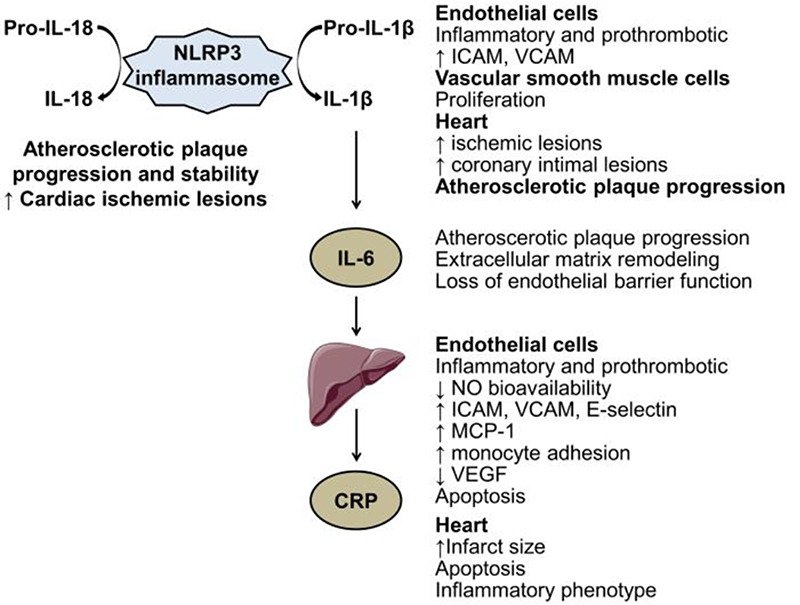
Dissecting the inflammatory cascade shows a tight link between its components; the upstream cytokine IL-1β affects IL6 levels that in turn control the secretion of a downstream mediator C-reactive protein (CRP) (Figure 2). IL-1β and IL-6 inhibition is now an important target for some inflammatory diseases (see infra). In the plasma of patients with myocardial infarction, an IL-1β peak preceded the IL-6 peak, strengthening the upstream position of IL-1β [34]. In vitro, IL-1β strongly stimulates IL-6 synthesis by various types of cells including vascular cells. IL-1β induces a proinflammatory phenotype of endothelial cells and increases the expression of adhesion molecules such as intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule-1. IL-1β also alters cardiomyocyte functions. Chronic administration of IL-1β induced coronary intimal lesions and vasospastic responses in pigs while IL-1 receptor antagonist infusion decreased neointima area [35].
Figure 2.
Biological effects of inflammatory biomarkers on the cardiovascular system. CRP, C-reactive protein; ICAM, intercellular adhesion molecule; IL, interleukin; MCP, monocyte chemoattractant protein; NO, nitric oxide; VCAM, vascular cell adhesion molecule; VEGF, vascular endothelial growth factor.
IL-6 is a soluble cytokine with pleiotropic effect on inflammation and immune responses. The synthesis of IL-6 is driven, among others, by toll like receptors activation, TNFα and IL-1 signalling activation. In patients with myocardial infarction, direct measurements of IL-6 concentration at the site of plaque rupture and in the general circulation indicate a local production of IL-6 [36]. In addition to its position in the inflammatory cascade, IL-6 has been involved in the loss of endothelial barrier function [37], extracellular matrix remodelling and atherosclerotic plaque progression in mice [38].
IL-18 and IL-1β are major proinflammatory cytokines with pleiotropic activities. These cytokines are synthesized as inactive precursors requiring cleavage by caspase-1, within the complex NLRP3 inflammasome, to become biologically active molecules in inflammatory cells and also in endothelial and vascular smooth muscle cells. IL-18, produced by endothelial cells, cardiomyocytes, and by infiltrating macrophages [39], is expressed in human failing myocardium and in human carotid atherosclerotic plaques in presence of plaque instability [40]. Experimentally, IL-18 administration in apoE-/- mice increased plaque size and progression, and altered plaque stability by decreasing intimal collagen content and plaque thickness [39].
CRP is synthetized by hepatocytes in response to IL-6 exposure. CRP deposition or expression in atherosclerotic lesions of human coronary arteries and in human infarcted heart tissues raises the question of a direct pathogenic role of CRP in cardiovascular diseases. Recombinant CRP administration has been associated with prothrombotic and proinflammatory effects in vitro in endothelial cells [41], in cardiomyocytes, and in vivo in human healthy subjects [42]. Despite data from transgenic mice overexpressing CRP could suggest an implication of CRP in thrombosis after arterial injury and in cardiac remodelling in the context of myocardial infarction [41], epidemiological and Mendelian randomization studies refute the causal role of CRP in the risk of cardiovascular events (see below).
Box 1.
Chronic low-grade sub-clinical inflammation plays an important role in vascular dysfunction and damage in cardiovascular diseases. The inflammatory response is mediated in large part through ROS. Many pro- or anti-inflammatory immune cells and cytokines are involved in the inflammatory response and mechanisms of hypertension.
Inflammation and atherosclerosis
Atherosclerotic plaques develop in predisposed areas of the arterial tree where blood flow is either slow or oscillatory and endothelium displays increased susceptibility to activation, as well as greater permeability to LDL and remnant cholesterol, favouring subendothelial retention. One of the triggering events in the initiation of atherosclerotic process is the oxidation of LDL (although other targets are involved such as high density lipoproteins, HDL), the consequent release of bioactive (phospho)lipids in the arterial intima and production of proinflammatory and chemotactic factors for lymphocytes and monocytes [43]. Infiltrated monocytes differentiate into macrophages or DCs, that internalize oxidized LDL (oxLDL) and become foam cells. Surprisingly, early uptake of cholesterol by macrophages suppresses inflammatory responses, and promotes a reparative M2 phenotype [44]. Yet, continued cholesterol accumulation leads to predominant M1 populations. Recruited immune cells accumulate under hypoxic conditions and undergo apoptosis and necrosis, leading to the formation of the necrotic core and contributing to destabilisation of plaque. Advanced, rupture-prone lesions that are associated with clinical events are rich in M and T cells exhibit large necrotic cores with thinned fibrous caps, rarefaction of SMCs and reduced collagen content. Finally, inflammation can hamper the protective effects of HDL on endothelium. In presence of inflammation, HDL fails to prevent LDL oxidation, exhibits reduced reverse cholesterol transport function and induces endothelial dysfunction [45].
Innate immunity in atherosclerosis
The chronic inflammatory disease of atherosclerosis is promoted by both innate and adaptive immunity [7]. The innate response is instigated by the activation of vascular cells and monocytes/macrophages. Subsequently, an adaptive immune response develops against an array of potential antigens presented to effector T lymphocytes by antigen-presenting cells. In the early stages of atherosclerosis, proinflammatory cytokines can alter endothelial functions. TNFα, for example, increases cytosolic Ca2+ and regulates myosin light chain kinase and RhoA [46], which disrupts endothelial junctions [47], leading to loss of barrier function and facilitation of leukocyte transmigration. Cytokines also induce the expression of chemokines and adhesion molecules in endothelial cells, favouring the recruitment, adhesion and migration of lymphocytes and monocytes into the inflammatory vessel wall. Once in the intima, leukocytes can be permanently activated by locally generated cytokines, which can accelerate the transformation of macrophages into foam cells by stimulating the expression of scavenger receptors and enhancing cell-mediated oxidation. Cholesterol crystals act as metabolic triggers of the NLRP3 inflammasome, which promotes the maturation of IL-1β and IL-18 [48]. At an advanced stage of the disease, proinflammatory cytokines (IL-1β, TNFα and IFNγ) destabilize atherosclerotic plaques by promoting cell apoptosis and matrix degradation. Macrophage apoptosis results in the formation of cell debris with toxic components, which contributes to the enlargement of the lipid core; plaque SMC apoptosis leads to thinning of the fibrous cap, favouring its rupture. Proinflammatory cytokines significantly affect the expression of MMPs and their inhibitors, TIMPs, inducing substantial remodeling of many components of the extracellular matrix of the arterial wall. For example, IFNγ inhibits collagen synthesis whereas IL-1 and TNFα induce a broad range of MMPs in vascular cells, including MMP- 1, -3, -8, and -9. Finally, the antithrombotic properties of endothelial cells are deeply altered by cytokines, in addition to profoundly altered shear stress conditions. Proinflammatory cytokines also modify the fibrinolytic properties of endothelial cells, precipitating thrombus formation and promoting the development of acute ischemic syndromes.
Adaptive immunity in atherosclerosis
T cell responses are initiated when specific molecular epitopes on antigens, including oxLDL and heat shock proteins, are presented by antigen-presenting cells and recognized by T cell antigen receptors. DCs are the main cell type responsible for the activation of naïve T cells and play a crucial role in triggering adaptive immunity. In atherosclerotic plaques, DCs co-localize with T cells, suggesting that they are involved in T cell activation within the plaque. However, sensitization of naïve T cells most likely occurs in the regional lymph nodes [7]. A number of experimental studies have clearly shown a critical pathogenic role for the Th1 response, associated with the production of IFNγ [7].
Atherosclerosis is also associated with B cells activation. IgG antibody production by B2 cells requires T cell co-stimulation, whereas innate production of natural IgM antibodies by B1 cells does not require co-activation of T cells. Both IgG and IgM antibodies against oxLDL have been described. Studies in mouse models provided evidence for a proatherogenic role of B2 cells [49]. Natural Treg cells are detected in much lower amounts in atherosclerotic plaques than in other chronically inflamed tissues, suggesting an impairment of local tolerance against potential antigens in atherosclerotic lesions [50]. Treg cells exert a protective role in atherosclerosis and are reduced in patients with acute coronary syndromes.
From acute to chronic inflammation in atherosclerosis
Physiologically, most inflammatory processes are self-limiting and controlled by endogenous pro-resolutions pathways. Whether acute inflammation is needed or not to induce chronic inflammation is unknown. The general view is that defective acute inflammation resolution, caused by the imbalance between proinflammatory and specialized pro-resolving mediators (SPMs) such as thromboxane A2/prostacyclin ratio, proinflammatory leukotrienes/lipoxins, leads to the defective efferocytosis and chronic inflammatory state observed in atherosclerosis [51]. In early and stable plaques there is an high SPMs/proinflammatory mediator ratio. High SPMs levels reduce lesion necrosis, enhance efferocytosis and have a protective role in several factors that exacerbate atherosclerosis. In advanced/vulnerable plaques, levels of proinflammatory mediators increase and the imbalance between SPMs and proinflammatory mediators lead to plaque progression.
Box 2.
The atherosclerotic process is promoted by both innate and adaptive immunity.
Low-grade inflammation and cardiovascular diseases
Although inflammation is a key factor in vascular disease, most of the time it remains low-grade and subclinical. Clinical studies performed in several groups of patients have shown consistent associations between inflammatory molecules and subclinical markers of arterial diseases such as endothelial dysfunction and arterial stiffness. In this section we will discuss on the evidences originated from event data.
Association between inflammatory markers and cardiovascular diseases (CVD)
Subjects with high baseline levels of CRP (independently of previous CVD) have an increased risk of developing future myocardial infarction independently of established risk factors. Several meta-analyses on individual data have now reported an association between single measurements of CRP or IL-6 and incident CVD [52,53]. Moreover, circulating CRP and/or IL-6 significantly improve CVD risk prediction beyond the effect of traditional risk factors, especially for those at intermediate risk.
Studies based on repeated assessments of CRP provide contrasted results. In the ARIC study, subjects with sustained elevated CRP (≥3mg/dL) or with increased CRP (from <3mg/dL to ≥3mg/dL) over 6 years had a significantly increased risk of coronary heart disease (CHD) and mortality in the subsequent 14 years of follow-up compared with subjects having a constant low/moderate CRP level (<3mg/dL) [54]. Interestingly, the risk of CHD did not decrease in subjects with decreased CRP compared to those with constantly low/moderate CRP. In the Whitehall II study, while the concentrations of CRP over 15 years prior to fatal CHD were systematically higher as compared to controls, the slope of the CRP trajectories between the two groups did not differ [55]. Given these evidences, the question of the causality between CRP and incident CVD has been raised. In addition to biological plausibility, Mendelian randomisation studies suggest that the association between IL-6 Receptor (IL-6R) pathway but not CRP and CHD could be causal [56–58]. In particular, a single nucleotide polymorphisms in the gene IL-6R rs7529229 was used to evaluate the efficacy and safety of IL-6R inhibition for primary prevention of coronary heart disease. The IL-6R rs7529229 SNP was associated with a decreased odds of CHD events [58]. This Mendelian randomisation approach is consistent with the cardiovascular protection induced by IL-6R blockade from infusion of Tocilizumab in patients with rheumatoid arthritis in randomised trials [59] and provides a much higher level of evidence on causal pathways than observational associations. This makes IL6 appear as a potential therapeutic target while CRP is more a marker of inflammation. These Mendelian randomization studies have also limitations; among them, they do not take into account interactions between the genome and environment (epigenetics), they might be confounded by the presence of other interacting genes [60], and only explain a very small risk (here 5%).
IL-1ß inflammation pathway as a target for CVD prevention?
Canakinumab is a specific antibody blocking the IL-1ß receptor and thus the inflammation pathway. CANTOS is the first randomized controlled trial that tested whether canakinumab anti IL-1ß could reduce the risk of recurrent CVD events in patients with prior myocardial infarction on optimal secondary prevention [61]. CANTOS demonstrated a 14-15% reduction in the combined primary end-point (nonfatal myocardial infarction, any nonfatal stroke, or cardiovascular death) in the canakinumab 150-300 mg groups. Interestingly, the treatment did not modify the risk of diabetes. The median concentration of CRP, but not of lipids, decreased significantly between 26% and 41% at 48 months according to the Canakinumab dose when compared to baseline value, with similar effects on IL-6 level. This indicates that this treatment may specifically impact the inflammation pathway in atherosclerosis with beneficial effect. The CIRT trial [62], tested whether low-dose methotrexate, a non-specific modulator of inflammation as compared to placebo reduced major vascular events among a group of post-myocardial infarction patients with either diabetes or metabolic syndrome. This trial was based on observational evidence of reduced cardiovascular events among patients with rheumatoid arthritis and psoriatic arthritis on treatment with methotrexate and on the ability of methotrexate to reduce TNFα, IL-6 and CRP levels. The CIRT trial failed to demonstrate a benefit with low-dose methotrexate compared to placebo. The CANTOS trial is the first proof of concept that targeting inflammation through IL leads to improvement of cardiovascular outcome while CRP is a marker of the efficacy of the anti-inflammatory treatment.
Box 3.
At the population level, IL-6 is the inflammatory biomarker demonstrating the most robust and independent association with incident CVD events. More data are needed to confirm that targeting inflammation pathways prevents recurrent CVD events.
Inflammation, salt, microbiota and arterial stiffness
The action of salt as a promotor of hypertension is often related to an increased stimulation of the Renin-Angiotensin-System and associated with volume expansion. Surfacing evidence attributes a vascular inflammation promotion effect for salt through IL-17 stimulation. Salt promotes dedifferentiation of CD4+ cells into Th17 cells, responsible for the production of IL-17, which in turn plays a role in the control of renal tubular sodium transport channels. This salt-induced differentiation of immune cells promotes a pro-inflammatory phenotype, ultimately leading to increased BP, target organ damage and increased arterial stiffness. In a recent meta-analysis, salt consumption reduction was associated with a decrease in pulse wave velocity (PWV), independently of BP [63].
The gut microbiome is a new dimension in cardiovascular research, with a mechanistic role being attributed to its capacity to interfere in the control of inflammation, glucose tolerance, insulin sensitivity and oxidative stress [64,65]. Recent evidence suggests a reverse association between the composition and diversity of gut microbiome and arterial stiffness in women [66]. Moreover, short-chain fatty acids, a product of gut microbial metabolism, reduce arterial stiffness and blood pressure in mice [67]. Factors such as dietary intake and promotion of biodiversity in the microbiotic component of the gut can interfere in the induction of a systemic inflammatory effect, depending on the ability of certain gut bacterial species to express pro-inflammatory metabolites [65,68]. In this regards, the gut-derived metabolite trimethylamine N-oxide has a role in the development of oxidative stress, vascular inflammation and dysfunction [65,69]. Gut microbiome may influence arterial stiffness through the metabolism of polyphenols and trimethylamine N-oxide [69], however, data from Pluznick on a mice model do not validate this concept since stiffness increase was only dependent on blood pressure changes [67]. These findings are particularly relevant considering that several strategies are proposed to modify the gut’s microbiota composition.
Box 4.
Increased salt intake promotes the emergence of pro-inflammatory phenotypes. Models analysing the gut microbiome should be explored to uncover new pathways leading to reduction of inflammation-induced arterial stiffness and BP.
Inflammation and vascular aging
Physiologically, the wall of large arteries lose elasticity over time. As consequence, arterial stiffening reduce the reservoir function of the conduct arteries and, through the increase of systolic and pulse pressure, has an impact on cardiac function, microcirculation and end organ functions. Inflammation can lead to an accelerated vascular aging through the alteration of the arterial wall properties and the increase of arterial stiffness [1,70]. A widely accepted mechanism by which vascular stiffness increases by aging and inflammation involves remodeling and content changes in the extracellular matrix (i.e., elastin and collagen). Current findings suggest that also the intrinsic stiffening of vascular cells and endothelial mechanisms may also contribute to the vascular aging [71,72].
Arterial inflammation and mechanical stress
The arteries are stretched under pulsatile conditions because of blood pressure and beating of the heart [73]. Stretch and resulting stresses act as major trophic factors and induce the activation of important signaling pathways [74]. Mechanical stress is also the main determinant of plaque rupture. The interaction between stress and stretch also defines arterial stiffness. The Moens-Korteweg equation shows that, conceptually,
| (1) |
with E the incremental Young’s modulus (a measure of material stiffness), h the wall thickness, r the inner wall radius, and ρ the blood mass density. E·h defines the structural stiffness of the wall. It follows that a PWV increase can be due to 1) an increase in E, 2) an increase in h (through hypertrophy and/or hyperplasia), and/or 3) a decrease in r.
Contrary to the global variable PWV, VSMCs sense and maintain local tensile (circumferential) stress (σ):
| (2) |
Physiologically, in order to maintain σ, in response to a pressure (P) increase a VSMC will show an adaptive response and produce matrix (↑h) to restore σ to its homeostatic value. To maintain σ, if P changes by a given factor, h should change by the same factor (given a constant r) [75].
In parallel, endothelial cells sense shear stress. Mean wall shear stress is approximately proportional to blood viscosity and volume flow rate, while inversely proportional to the 3th power of radius. Any change in radius will have consequences in term of exposure of the endothelium to high/low shear stress. Concentric remodeling (↑h and ↓r) will increase wall shear stress with subsequent secretion of endothelium derived factors which will dilate (and thin) the artery. Several factors (e.g., hypertension and aging) eventually lead to a loss of this dilatory mechanism (“endothelial dysfunction”). In other words, the structure of large (and small) vessels depends on the dynamic balance between tensile stress and wall shear stress, determined by changes in wall structure. The relation between shear stress and atherosclerosis has been extensively studied [76]. Atherosclerotic lesions tend to develop in low shear/bidirectional/turbulent zones, particularly the inner curvature and bifurcations, explaining aggravating of lesions downstream of atherosclerotic stenosis. Furthermore, arterial stresses and stretches are pulsatile, significantly influencing the VSMC’s biological response to these stresses/stretches [77].
Inflammation leads to mechanical over-adaptation
Hypertension lead to media thickening by matrix deposition and/or VSMC hypertrophy. AII infusion in mice leads to a BP increase, but also has an inflammatory effect causing overa-daptation: two weeks of AII infusion led to a mean blood pressure increase from 106 to 144 mmHg (a factor 1.36), but caused thoracic aortic wall thickness to increase from 39 to 102 μm (a factor 2.62) [78]. This in turn led to a decrease in σ and distensibility. Circumferential material stiffness (similar to E) did not change whereas structural stiffness (E·h) did increase due to the wall thickness increase, causing a sharp decrease in distensibility and a concomitant increase in PWV. The observed increase in wall thickness was partially due to medial hypertrophy (1.7-fold increase in medial cross-sectional area), but much more due to adventitial thickening (4.7-fold increase in adventitial area) caused by collagen deposition. Additionally, AII infusion leads to IL-6 production as well as macrophage recruitment and wall thickening, all mainly in the adventitia [79].
Inflammatory over-adaptation is regionally different along the aorta
In experimental models, AII infusion revealed regionally different effects along the aorta: proximal ascending thoracic aorta is prone to aneurysm development, whereas the suprarenal abdominal aorta is prone to dissection [79,80]. In all regions, with AII infusion, σ increased with BP during the first 7 days. After that, remodeling caused σ to decrease. In the infrarenal aorta, σ returned to pre-infusion values (adaptation), whereas in the other segments of the aorta, σ dropped below baseline (over-adaptation). The observed over-adaptation and adventitial thickening directly correlated with the presence of adventitial CD45+ cells, indicating the key role of inflammation in mechanical over-adaptation. In the thoracic aortic regions, the presence of CD3+ cells tracked that of CD45+ cells, indicating involvement of T cells. Previous studies have shown a direct role of T cells in hypertension and in adventitial thickening of the descending thoracic aorta: in male recombination activating gene 1 knock-out mice (which do not produce mature B or T cells), AII infusion did not lead to excessive adventitial thickening and led to a blunted hypertensive response [81]. Arterial thickening (as well as the hypertensive response) was restored upon adoptive transfer of T cells (but not B cells).
Box 5.
Arterial inflammation co-occurring with hypertension may cause arterial stiffening through an over-adaptative VSMC response.
Acute inflammation and arterial function
While infection is not synonymous of inflammation, the majority of data on the effect of the latter on arterial function have been derived from infection/vaccination studies. Data are limited, but interestingly they are not limited to cross-sectional associations but extend to mechanistic studies that suggest cause-and-effect relationships between acute inflammation and endothelial dysfunction [86,87] whereas the link between acute inflammation and arterial stiffening is doubtful [88,89], likely because the mechanisms involved in stiffening have longer time constants. The short duration and reversibility of inflammation does not affect negatively prognosis in infection/vaccination models [84].
Prophylaxis-reversal of arterial damage caused by acute inflammation
The acute impairment of endothelium-dependent dilatation (both conduit arteries and resistance vessels), the increase of arterial stiffness and the reduction of wave reflection (augmentation index, Aix) returned to normal 32 hours after vaccination [87,89]. Based on the inflammation/vaccine model, aspirin and statins have shown a protective effect on endothelial function and aortic stiffness [89,90], whereas the impact of exercise on endothelial function and arterial stiffness/wave reflection indices is doubtful [88,91,92].
Mechanisms of acute inflammation-induced vascular dysfunction
We have several clues about the mechanisms involved in the stiffening caused by acute inflammation. Adhesion molecules and tight junctions increasing the links between VSMC and the extracellular matrix, together with intrinsic VSMC stiffness can change in the short term. Inflammatory markers/mediators, such as IL-6, as well as MMP-9, were reversibly increased 8 hours after vaccination, whereas CRP levels sloped upwards 32 hours post-vaccination [89]. Similar responses of IL-6 to the Salmonella Typhi vaccine have been repeatedly shown [87,89,90]. However, while pre-treatment with oral aspirin abrogated the effect on aortic stiffness, IL-6 still increased [89], suggesting that other cytokines, such as IL-1, could be involved. The cytokine response was neither prevented by pretreatment with statins in hypercholesterolaemic patients, while the endothelial function deterioration was abrogated [90]. On the other hand, only in the placebo arm and not in the statin pretreatment arm, a reduction of NO metabolites and total antioxidant capacity was observed [90], implying involvement of NO bioavailability and antioxidant status.
In the salmonella vaccine model, asymmetric dimethylarginine, an endogenous L-arginine analogue that interferes with L-arginine for NO production, increased in healthy subjects, in whom a reduction in flow mediated dilatation (FMD) was observed, while it did not in coronary artery disease patients in whom FMD reduction was marginal [93]. Reduction of FMD in vaccinated healthy subjects is correlated with an increase of tetrahydrobiopterin, a cofactor necessary for the production of NO and a possible defence mechanism against inflammatory challenges.
Box 6.
In infection/vaccination studies, acute inflammation leads to endothelial dysfunction.
Low-grade chronic inflammation and vascular diseases
Transplant arteriosclerosis
The immune response to endothelial and VSMCs contributes to acute and chronic graft failure of transplanted solid organs. T and B cells of the adaptive immune system, activated by graft-derived antigens, lead to the development of transplant arteriosclerosis, a vascular condition characterized by intimal hyperplasia, alteration of extracellular matrix composition, vasodilatory dysfunction, lipid deposition, and intraplaque haemorrhage [94]. Transplant arteriosclerosis is mediated by cytokines that stimulate the migration and activation of T cells into allograft arteries and promote VSMCs proliferation and endothelial dysfunction (i.e., IFNγ, IL-1, IL-17 and TNFα), and antibodies that facilitate immune cell transmigration into the arterial wall and amplify T cell responses to allograft arteries through the up-regulation of cell adhesion molecules and von Willebrand factor and the increase of the antigen-presenting capabilities of the endothelium.
Periodontitis inflammation and cardiovascular diseases
Periodontitis is a highly prevalent and multifactorial chronic low-grade inflammatory condition associated with dysbiotic plaque biofilms and destruction of the tooth-supporting apparatus. Patients with periodontitis have more coronary events and stroke compared to non-periodontal disease subjects [95,96]. Chronic low-grade inflammation can explain the increased CV risk reported in these patients through the development of endothelial dysfunction, impaired arterial stiffness and greater carotid intima-media thickness (IMT) [97,98].
Several mechanisms have been proposed to explain the link between periodontitis, vascular dysfunction and CV events. Periodontal pathogens can enter the bloodstream through the abundant gingival vasculature surrounding the teeth and activate the host inflammatory response. Constant bacteraemia has been shown in everyday life events such as chewing or tooth brushing. However, the intensity and the frequency of the bacteraemia is higher in periodontitis patients than in control. Different pathophysiological mechanisms have been suggested. The direct model deals with the invasion of the vascular endothelium by pathogenic bacteria such as Porphyromonas gingivalis. This interpretation is supported by an increased production of innate immune markers such as ICAM family proteins, pro-inflammatory cytokines and chemokines, leading to an immunological switch of endothelial cells from a normal anti-thrombotic to a pro-thrombotic state. Periodontal pathogens have been identified in atheromatous plaques [99], can directly invade endothelial cells leading to vascular inflammation [100], and trigger an innate [101] or auto-immune response [102]. Animal studies indicate that infection with periodontal pathogens, Porphyromonas gingivalis in particular, can support the formation of atheromatous plaques. The indirect model is based on the upregulation of inflammatory cascades involving TNFα, IL-1, IFs, IL-8, monocyte chemoattractant protein-1, and CRP, which are elevated in periodontitis patients. Further, there is evidence of a neutrophil hyperresponsiveness in periodontitis patients leading to an increased production/activity of ROS than in healthy controls [103]. Finally, recent studies indicates common genetic locus associated with coronary artery disease and periodontitis.
Taken together, the above data strongly suggest that periodontitis is an independent contributor to systemic inflammation, and may serve as a model to explore the biological relationship between low-grade chronic inflammation and vascular diseases.
Periodontitis is a treatable disease. The treatment is based on the reduction of the periodontal bacterial load. Intervention trials suggest that periodontal therapy improves endothelial function and intima media thickness (IMT) and reduces TNFα and CRP [104–106]. A significant decrease in fibrinogen levels and platelet activation have been also reported following periodontal therapy. Similarly, the hyper-reactivity of the peripheral blood neutrophils by periodontal bacteria is also reduced by lowering the bacterial load through periodontal therapy.
Box 7.
Transplant arteriosclerosis is mediated by cytokines and antibodies that lead to VSMCs proliferation and endothelial dysfunction. There is a large body of evidence that links periodontitis to cardiovascular diseases. Periodontal therapy reduces chronic inflammation and improves vascular function.
Chronic severe inflammation and arterial function
The risk of cardiovascular events is higher among subjects with chronic severe inflammation, such as those with inflammatory bowel disease (IBD), rheumatoid arthritis (RA), systemic lupus erythematosus (SLE) and systemic sclerosis (SSc). However, only part of the excess in cardiovascular risk reported in these patients can be explained by classical cardiovascular risk factors, suggesting that additional mechanisms are involved.
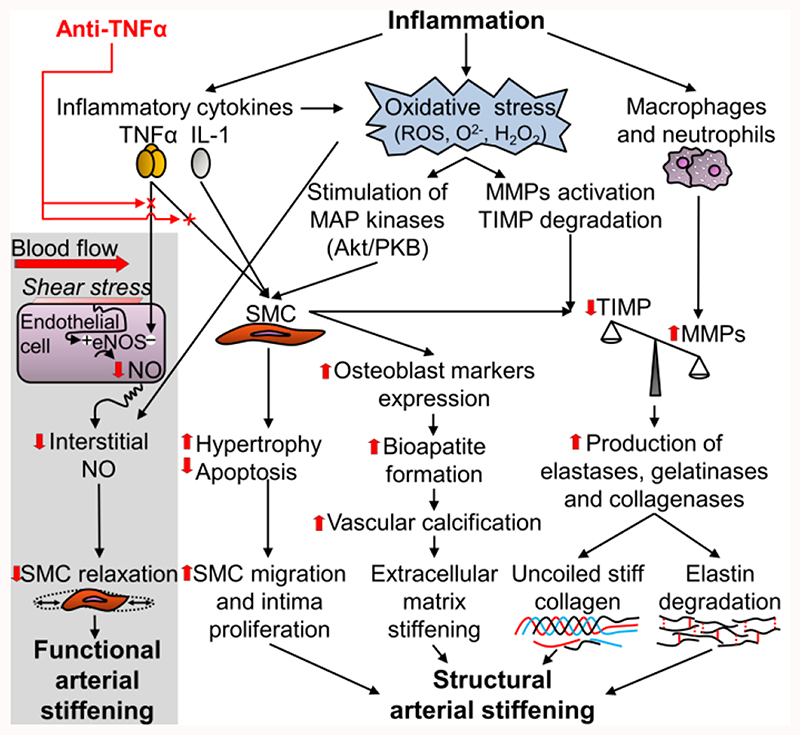
Recently, it has been reported in several meta-analyses of cross-sectional studies that aortic PWV and AIx are significantly increased in patients with IBD [107–109], RA [110], SLE and SSc [111,112]. Few cross-sectional studies suggest that muscular artery stiffness is increased in these patients [110,113–116]. In patients with severe chronic inflammation, also other alterations of the arterial wall (reduced FMD and increased carotid IMT) are reported [109,112,117–120]. Taken together, current data suggest that chronic severe inflammation can lead to functional and structural arterial stiffening (Table 1). Moreover, as suggested by interventional longitudinal studies performed in these patients (see above), the increased arterial stiffness is, at least in part, related to TNFα processes (Figure 3).
Table 1. Available evidence on arterial damage in subjects with chronic severe inflammation [96–108].
| Disease | Endothelial dysfunction |
Accelerated atheromatosis |
Accelerated arterial stiffening |
|
|---|---|---|---|---|
| Elastic arteries (aorta) |
Muscular arteries (brachial artery) |
|||
| IBD | +++ [109] | +++ [109] | +++ [108] | +[113,114] |
| RA | +++ [117] | +++ [118] | +++ [110] | + [110] |
| SLE | +++ [120] | +++ [119] | +++ [111] | + [115] |
| SSc | +++ [112] | +++ [112] | +++ [112] | + [116] |
IBD, inflammatory bowel disease; RA. Rheumatoid arthritis; SLE, systemic lupus erythematosus; SSc, systemic sclerosis;
data derived from few single centre cohorts;
evidence based on meta-analyses.
Figure 3.
Potential mechanisms by which inflammation could induce functional and structural arterial stiffening and how this process could be reverted by anti-TNFα drugs. eNOS, endothelial nitric oxide synthase; H2O2, hydrogen peroxide; IL-1, interleukin-1; MMPs, matrix metalloproteinases; NO, nitric oxide; O2-, superoxide; ROS, reactive oxygen species; SMC, smooth muscle cell; TIMP, tissue inhibitor of matrix metalloproteinases; TNFα: tumour necrosis factor alpha. Adapted from Zanoli L et al. [150].
Several markers of inflammation are associated with alterations of arterial structure and function in patients with chronic severe inflammation. In a multicentre longitudinal study, aortic PWV was increased in IBD patients with active disease and was reduced in those in remission during a follow up of 4 years [121]. Disease duration is associated negatively with FMD in patients with SLE [122], positively with IMT in patients with RA and SLE [123,124], and positively with carotid-femoral PWV in patients with IBD and SLE [108,111]. Patients with a longer disease duration are exposed to a significantly higher amount of inflammation than patients with short disease duration. Other factors associated with alterations of arterial structure and function in patients with chronic severe inflammation are erythrocyte sedimentation rate [124], CRP [110], scores of disease activity [110,124], and leucocyte count [108].
Box 8.
Chronic severe inflammation is associated with increased artery stiffness. Both functional and structural arterial stiffening processes can be involved in these patients.
Primary systemic vasculitides and arterial structure and function
Arteries can be the principal target of inflammation during primary systemic (non-infectious) vasculitides (PSV). The mechanisms involved in the pathogenesis of vascular wall inflammation include endothelial dysfunction, autoantibody-mediated or -associated vascular damage, immune complex formation and complement activation [128]. The main vascular consequences involve a loss of vessel wall integrity and/or lumen compromise of the microcirculation, as well as, thrombosis and aneurysm rupture of the macrocirculation. As a result, there is an increased incidence of cardiovascular events (coronary artery disease, stroke, aneurysm rupture) and mortality in most PSV [129,130]. In each of them, intense inflammation can promote atherosclerosis and arteriosclerosis even in unaffected vessels. The arterial disease observed even in PSV patients free of overt PSV-related complications include increased IMT and arterial stiffness (Table 2) [131]. Taken together, functional and structural abnormalities of the macrocirculation can be observed in all types of PSV, independently of the size of vessel involved in the primary inflammatory process of the vasculitis. Whether similar systemic lesions exist in the microcirculation (e.g., retina) of all patients with PSV is poorly investigated. The potential mechanisms underlying this association are reported in Figure 4.
Table 2. Available evidence on arterial damage in primary systemic vasculitides (PSV) (table modified by Argyropoulou OD et al. [131]).
| Disease | Accelerated atheromatosis |
Accelerated arterial stiffening |
|---|---|---|
| Large vessel PSV | ||
| Takayasu arteritis | ++ | + |
| Giant cell arteritis | ++ | + |
| Medium vessel PSV | ||
| Polyarteritis nodosa | ° | ° |
| Kawasaki disease | ++ | ++ |
| Small vessel PSV | ||
| ANCA-associated* | ++ | + |
| IgA (Henoch Schonlein) | ° | ° |
| Cryoglobulinemia | ° | ° |
| Variable size PSV | ||
| Behcet’s disease | +++ | +++ |
| Cogan’s syndrome | ° | ° |
ANCA, anti-neutrophil cytoplasmic antibodies; PSV, primary systemic vasculitides; °, lack or scarce data;
data derived from few single center cohorts;
large number of evidence derived from multiple single center cohorts;
evidence based on meta-analysis;
the evidence concern granulomatosis with polyangiitis vasculitis (previously known as Wegener’s).
Figure 4. Potential mechanisms leading to the acceleration of atheromatosis and arteriosclerosis in primary systemic vasculitidies (PSV). Adapted from Argyropoulou OD et al.[131].
The clinical implications of accelerated atheromatosis and arteriosclerosis in PSV are poorly studied. It is likely that vascular pathologies in PSV patients contribute significantly to the increased incidence of cardiovascular morbidity and mortality. It has been also suggested that the reversibility of functional properties might be an useful marker of remission or activity in PSV [131].
Box 9.
An acceleration of arteriosclerosis and atheromatosis have been reported in PSV due to the response to local vessel wall inflammation. The underlying causes of these systemic macrovascular effects and their potential clinical implications should be better investigated.
The effect of anti-inflammatory therapies on arterial stiffness in patients with chronic inflammation
Many studies have investigated the effect of anti-inflammatory drugs on arterial stiffness in a variety of chronic inflammatory conditions [121,132–142]. Classical non-steroid and steroid anti-inflammatory drugs have not shown protective effects against arterial stiffening, likely because of their mode of action leading to an adverse cardiovascular risk profile (inhibition of prostaglandin secretion, mineral-corticoid action of glucocorticoids leading to an increase in blood pressure). Recently, anti-inflammatory drugs have been developed which target more specific pathways. In a recent meta-analysis performed in patients with RA, aortic PWV and AIx improved following anti-TNFα therapy independently of age and clinical response to treatment [132]. Similarly, in 55 patients with inflammatory arthritis, a 1-year treatment with anti-TNFα therapy, but not placebo, led to a slowing of aortic stiffness and carotid IMT progression [133]. Contrary to anti-TNFα agents, the effect of IL-6R antagonist tocilizumab on aortic PWV is not clear in patients with RA [134,135].
In psoriasis patients, global longitudinal strain, left-ventricular twisting and coronary flow reserve were improved after 4 months of treatment with anti-IL-12/23 agent (ustekinumab), anti-TNFα treatment (etanercept), or cyclosporine with the greatest improvements seen in the ustekinumab group [136]. Interestingly, PWV and AIx were only improved by ustekinumab. This could potentially be explained by the fact that IL-12 signalling plays an integral part in the pathogenesis of psoriasis and hence inhibition of IL-12 would have a greater benefit in the vasculature in this particular patient cohort. In another study performed in 29 patients with moderate to severe psoriasis, 6 month-treatment with anti-TNFα monoclonal antibody adalimumab led to an improvement of FMD and a reduction of PWV [137].
In a recent multicentre longitudinal study [121], PWV increased during 4 years of follow-up in IBD patients treated with salicylates and was reduced in those treated with anti-TNFα therapy. The effect of anti-TNFα therapy was more evident in patients with a recent (<4 years) diagnosis of IBD [138]. These data are in accordance with two meta-analyses [139,140] and suggest that a better and early control of inflammation can help to slow down aortic stiffening over time together with improving the disease.
In patients with ankylosing spondylitis, despite significant improvement in markers of disease activity and inflammation, anti-TNFα therapy did not reduce arterial stiffness [141]. These somewhat unexpected results could potentially be due to a low baseline PWV in these relatively young subjects.
Finally, it has been recently shown that inhibiting IL-1B by canakinumab [61] led to reduction in cardiovascular event rate in patients with atherosclerosis (see above for details). The effect of this class of drugs on arterial stiffness (and/or IMT or endothelial function) is not reported so far, however only weak correlations between arterial stiffness and IL-1B were found in cohort studies [142].
The mechanisms by which anti-inflammatory therapies lead to a reduction of arterial stiffness (Figure 3) derive from what we know through animal models. Potentially, treatment with anti-inflammatory drugs (i.e., anti-TNFα), leading to reduced release of cytokines, could lead to beneficial changes in the arterial wall composition via improvement of endothelial function, reducing direct vascular inflammation and hence reducing the number of inflammatory cells present within the aortic wall, or via changes in the arterial wall properties, such as inhibition of smooth muscle cell proliferation, changes in glycosaminoglycan content of the extracellular matrix, reduction of calcification and inhibition of elastin degrading matrix metalloproteinase synthesis [143]. What is missing here is the time constant of these phenomena, compared with the very long time constant of arterial remodelling [144]. Despite the crucial role of oxidation in the pathophysiology of vascular lesions due to inflammation, attempts at enhancing endogenous antioxidant substances or supplementation with antioxidants have not shown positive effects on cardiovascular events despite demonstrated short term effects [145].
Together with the potential benefits on vascular dysfunction and cardiovascular events, also the potential side effects of drugs affecting the immune processes should be considered. In this regards, several immunosuppressive drugs, including anti-TNFα, anti-IL-12/23, anti-IL-1B and anti-IL-6R, reducing the activity of the immune system may lead to an increased risk for opportunistic infections; most of these drugs (with the exception of anti-IL-1B) have been also associated with an increased risk for cancer. Moreover, the IL-6R antagonist tocilizumab lead also to a reduction of lipoprotein(a), increase in LDL and reduction in HDL, as well as modification of inflammatory and thrombotic markers. Therefore, the net effect of cardiovascular benefits and/or risks of tocilizumab remains unclear.
Box 10.
Numerous anti-inflammatory treatments have been shown to be beneficial in reducing arterial stiffness in patients with chronic inflammatory conditions. The mechanism by which these drugs lead to a reduction of arterial stiffness may include: improvement of endothelial function, beneficial changes in the arterial wall components, inhibition of matrix metalloproteinases and reduction of arterial wall inflammation.
Conclusion
Inflammatory processes profoundly disrupt arterial physiology and promote both arteriosclerosis and atherosclerosis. The inflammatory process induces most of its adverse effects through oxidative stress and production of ROS. With aging and progression of vascular disease, production of ROS increases and protection mechanisms are altered. Innate and adaptive immunity plays a prominent role in the pathophysiology of hypertension and mediates at least part of the adverse effect of renin-angiotensin system activation on arteries and CV system. Inflammation may result from unsuccessful adaptation of the human body to its environment, and particularly to the microbiota, both in gut and in the mouth. The dietary changes (sodium load and protein intake) on microbiota may induce systemic low-grade inflammation and promote vascular damage. Atherosclerosis is now considered as a predominantly inflammatory disease involving innate and adaptive immunity. Most of the inflammatory cascades are activated in atherosclerosis primarily for clear oxidized LDL from the arterial wall. A positive feedback links excessive transfer of cholesterol via LDL, insufficient clearance of ox-LDL and focal deposition of cholesterol with subsequent inflammation and immune activation. Major cytokines are elevated in atherosclerosis and arteriosclerosis. Great effort has been made toward identifying key cytokines as therapeutic targets, with IL-1β being the most promising. Certain components of the inflammatory response may have cytotoxic effects, such as CRP. The role of mechanical factors by themselves has been largely overlooked. Mechanical factors such as pressure, heart rate and blood flow are strong determinants of the trophic response of the arterial wall, and promote transfer of macromolecules and cells to the wall. On the other hand, inflammatory remodelling directly alters these factors. As such, they may (partially) explain the relation between microvascular and macrovascular aspects of arterial lesions.
Epidemiological and clinical evidence of the link between inflammation, either low grade, acute, chronic, focal or systemic, and arterial lesions is overwhelming. We have only presented some aspects of the many links, several others could have been included in this review. Considering that aortic PWV is a vascular biomarker and a CV risk factor [146,147], increased aortic stiffness could be used to pinpoint subjects with low grade or severe inflammation who are at increased cardiovascular risk. We have already reported that low-grade and severe inflammation lead to vascular disease. Therefore, considering that the risk of CVD in subjects with chronic inflammation is increased, their management should not be limited to control the inflammatory manifestations of disease, but rather, in a preventative and proactive action, also aimed at identifying subjects with a higher risk of developing CVD, such as those with hypertension and increased aortic stiffness. Currently, there are no specific indications for the management of the CVD risk in patients with inflammation. Therefore, these subjects should be treated according to the grade of arterial hypertension and the presence of further risk factors.
Further studies could be needed to test whether inflammation can be implemented in algorithms for CVD risk prediction. Many links, however, are still indirect and need to be further validated. One of the methodological difficulty is that proxies are used both for inflammation and cardiovascular lesions. Biological biomarkers only represent a snapshot of selected inflammatory markers. When linking inflammatory biomarkers with cardiovascular events, one should keep in mind that those remain proxies for complex and pleiotropic inflammatory processes, that are highly variable between and within persons. Similarly, biomarkers of arterial lesions such as endothelial dysfunction or arterial stiffening are less variable in time, but are influenced by many factors, such as blood pressure, aging and neuroendocrine status, which have been shown to influence the effect of acute inflammation on the vasculature. Even evidence from interventional trials do not provide a definitive proof of a causal relationship between inflammation and arterial lesions. For instance, microorganisms are known to cause severe arterial lesions (streptococcus, treponema pallidum, mycotic aneurysms, tuberculosis, etc.). However, the mechanisms by which infection can lead to arterial lesions are complex, as for periodontitis (immunologic, circulating microorganisms?). Whether less severe infections and bacteraemia can lead to arterial lesions (microorganisms can be present in the arterial wall) remains an open question. Finally, fluoroquinolones increase the risk of aortic dissection and rupture by activating MMP expression in fibrous tissues [148]. Other anti-inflammatory drugs such as anti-IL-6 tocilizumab have an unfavourable metabolic profile that could counteract their positive anti-inflammatory effects. This further illustrates the complex relationship between inflammation, infection and some anti-infectious agents.
A clear benefit is added when the evaluation of CVD risk is done through an objective measurement of intermediate end points associated to arterial function, such as arterial stiffness (PWV) and others (FMD, carotid IMT). An added and very important argument for this strategy is that patients may have their manifestations of inflammation controlled, maintaining a persistent low-grade inflammatory activity (with no clinical indication for any added medical intervention), that will keep fuelling vascular structural modifications. This can be followed and evaluated with consecutive measures of arterial function indexes (namely PWV), identifying patients that have increasing cardiovascular risk despite apparently controlled inflammation [149].
Funding
Luca Zanoli was supported by 2016/2018 Department Research Plan of University of Catania, Department of Clinical and Experimental Medicine (Project #A). Rhian M Touyz was supported by grants from the British Heart Foundation (CH/4/29762, RE/13/5/30177). The Antoine Caillon, Pierre Paradis and Ernesto L. Schiffrin work was supported by Canadian Institutes of Health Research (CIHR) grants 102606 and 123465, CIHR First Pilot Foundation Grant 143348, a Canada Research Chair (CRC) on Hypertension and Vascular Research by the CRC Government of Canada/CIHR Program, and by the Canada Fund for Innovation (all to ELS). Bart Spronck was supported by grants from the Netherlands Organisation for Scientific Research (Rubicon 452172006) and from the European Union’s Horizon 2020 research and innovation program (No 793805).
List of abbreviations
- AII
Angiotensin 2
- AIX
augmentation index
- BP
Blood pressure
- ChAT
choline acetyltransferase
- CHD
coronary heart disease
- CRP
mediator C-reactive protein
- CVD
cardiovascular diseases
- DAMPS
Damage-associated molecular patterns
- DC
dendritic cells
- FMD
flow mediated dilatation
- Foxp3
forkhead box P3
- HDL
high density lipoprotein
- IBD
inflammatory bowel disease
- ICAM
intercellular adhesion molecule
- IF
interferon
- IL
Interleukin
- IMT
intima media thickness
- LDL
Low density lipoproteins
- MMP
Matrix metalloproteinases
- NLRP3
NOD-like receptor family, pyrin domain containing 3
- NO
nitric oxide
- NOX
adenine dinucleotide phosphate oxidase
- Nrf-2
Nuclear factor erythroid 2-related factor 2
- PAMPS
pathogen-associated molecular patterns
- PKG1a
protein kinase G alpha
- PSV
primary systemic vasculitides
- PWV
pulse wave velocity
- RA
rhumatoid arthritis
- RhoA
Ras homolog family member A
- ROS
Radical oxygen species
- SLE
systemic lupus erythematosus
- SPMs
specialized pro-resolving mediators
- SS
systemic sclerosis
- STAT3
Signal transducer and activator of transcription 3
- TCM
Central memory cells
- TEM
effector memory cells
- TGF
transforming growth factor
- TLR
toll like receptor
- TNF
tumour necrosis factor
- VSMC
vascular smooth muscle cells
- WNT
wingless-related integration site
Footnotes
Conflict of Interest
None declared.
References
- 1.Zanoli L, Rastelli S, Inserra G, Castellino P. Arterial structure and function in inflammatory bowel disease. World J Gastroenterol. 2015;21(40):11304–11311. doi: 10.3748/wjg.v21.i40.11304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma A, Tate M, Mathew G, Vince JE, Ritchie RH, de Haan JB. Oxidative Stress and NLRP3-Inflammasome Activity as Significant Drivers of Diabetic Cardiovascular Complications: Therapeutic Implications. Front Physiol. 2018;9:114. doi: 10.3389/fphys.2018.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med. 2017;377(2):111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanashiro A, Hiroki CH, da Fonseca DM, Birbrair A, Ferreira RG, Bassi GS, et al. The role of neutrophils in neuro-immune modulation. Pharmacol Res. 2020;151:104580. doi: 10.1016/j.phrs.2019.104580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips RJ, Powley TL. Macrophages associated with the intrinsic and extrinsic autonomic innervation of the rat gastrointestinal tract. Auton Neurosci. 2012;169(1):12–27. doi: 10.1016/j.autneu.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vida G, Peña G, Kanashiro A, Thompson-Bonilla Mdel R, Palange D, Deitch EA, et al. β2-Adrenoreceptors of regulatory lymphocytes are essential for vagal neuromodulation of the innate immune system. FASEB J. 2011;25(12):4476–4485. doi: 10.1096/fj.11-191007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ait-Oufella H, Sage AP, Mallat Z, Tedgui A. Adaptive (T and B cells) immunity and control by dendritic cells in atherosclerosis. Circ Res. 2014;114(10):1640–1660. doi: 10.1161/CIRCRESAHA.114.302761. [DOI] [PubMed] [Google Scholar]
- 8.Caillon A, Paradis P, Schiffrin EL. Role of immune cells in hypertension. Br J Pharmacol. 2019;176(12):1818–1828. doi: 10.1111/bph.14427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alhussien MN, Dang AK. Potential roles of neutrophils in maintaining the health and productivity of dairy cows during various physiological and physiopathological conditions: a review. Immunol Res. 2019 Feb;67(1):21–38. doi: 10.1007/s12026-019-9064-5. [DOI] [PubMed] [Google Scholar]
- 10.Pircher J, Engelmann B, Massberg S, Schulz C. Platelet-Neutrophil Crosstalk in Atherothrombosis. Thromb Haemost. 2019;119(8):1274–1282. doi: 10.1055/s-0039-1692983. [DOI] [PubMed] [Google Scholar]
- 11.Mikolajczyk TP, Guzik TJ. Adaptive Immunity in Hypertension. Curr Hypertens Rep. 2019;21(9):68. doi: 10.1007/s11906-019-0971-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res. 2015;116(6):1022–1033. doi: 10.1161/CIRCRESAHA.116.303697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mann DL. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circ Res. 2011;108(9):1133–11345. doi: 10.1161/CIRCRESAHA.110.226936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiffrin EL. Immune mechanisms in hypertension and vascular injury. Clin Sci (Lond) 2014;126(4):267–274. doi: 10.1042/CS20130407. [DOI] [PubMed] [Google Scholar]
- 15.Okusa MD, Rosin DL, Tracey KJ. Targeting neural reflex circuits in immunity to treat kidney disease. Nat Rev Nephrol. 2017;13:669–680. doi: 10.1038/nrneph.2017.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Youn JC, Yu HT, Lim BJ, Koh MJ, Lee J, Chang DY, et al. Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension. 2013;62:126–133. doi: 10.1161/HYPERTENSIONAHA.113.00689. [DOI] [PubMed] [Google Scholar]
- 17.Ji Q, Cheng G, Ma N, Huang Y, Lin Y, Zhou Q, et al. Circulating Th1, Th2, and Th17 Levels in Hypertensive Patients. Dis Markers. 2017;2017:7146290. doi: 10.1155/2017/7146290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, Reardon C, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olofsson PS, Steinberg BE, Sobbi R, Cox MA, Ahmed MN, Oswald M, et al. Blood pressure regulation by CD4(+) lymphocytes expressing choline acetyltransferase. Nat Biotechnol. 2016;34(10):1066–1071. doi: 10.1038/nbt.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, et al. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension. 2011;57:469–476. doi: 10.1161/HYPERTENSIONAHA.110.162941. [DOI] [PubMed] [Google Scholar]
- 21.Kasal DA, Barhoumi T, Li MW, Yamamoto N, Zdanovich E, Rehman A, et al. T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension. 2012;59:324–330. doi: 10.1161/HYPERTENSIONAHA.111.181123. [DOI] [PubMed] [Google Scholar]
- 22.Kvakan H, Kleinewietfeld M, Qadri F, Park JK, Fischer R, Schwarz I, et al. Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation. 2009;119:2904–2912. doi: 10.1161/CIRCULATIONAHA.108.832782. [DOI] [PubMed] [Google Scholar]
- 23.Matrougui K, Abd Elmageed Z, Kassan M, Choi S, Nair D, Gonzalez-Villalobos RA, et al. Natural regulatory T cells control coronary arteriolar endothelial dysfunction in hypertensive mice. Amer J Pathol. 2011;178:434–441. doi: 10.1016/j.ajpath.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen XH, Ruan CC, Ge Q, Ma Y, Xu JZ, Zhang ZB, et al. Deficiency of Complement C3a and C5a Receptors Prevents Angiotensin II-Induced Hypertension via Regulatory T Cells. Circ Res. 2018;122(7):970–983. doi: 10.1161/CIRCRESAHA.117.312153. [DOI] [PubMed] [Google Scholar]
- 25.De Ciuceis C, Rossini C, Airo P, Scarsi M, Tincani A, Tiberio GA, et al. Relationship Between Different Subpopulations of Circulating CD4+ T-lymphocytes and Microvascular Structural Alterations in Humans. Am J Hypertens. 2017;30(1):51–60. doi: 10.1093/ajh/hpw102. [DOI] [PubMed] [Google Scholar]
- 26.Caillon A, Mian MOR, Fraulob-Aquino JC, Huo KG, Barhoumi T, Ouerd S, et al. γδ T Cells Mediate Angiotensin II-Induced Hypertension and Vascular Injury. Circulation. 2017;135(22):2155–2162. doi: 10.1161/CIRCULATIONAHA.116.027058. [DOI] [PubMed] [Google Scholar]
- 27.Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, et al. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. 2014;124:4642–4656. doi: 10.1172/JCI74084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen T, Cao Q, Wang Y, Harris DCH. M2 Macrophages in Kidney Disease: Biology, Therapies, and Perspectives. Kidney Int. 2019 Apr;95(4):760–773. doi: 10.1016/j.kint.2018.10.041. 2019. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen Dinh Cat A, Montezano AC, Burger D, Touyz RM. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid Redox Signal. 2013;19(10):1110–1120. doi: 10.1089/ars.2012.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montezano AC, Tsiropoulou S, Dulak-Lis M, Harvey A, Camargo Lde L, Touyz RM. Redox signaling, Nox5 and vascular remodeling in hypertension. Curr Opin Nephrol Hypertens. 2015;24(5):425–433. doi: 10.1097/MNH.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramprasath T, Vasudevan V, Sasikumar S, Puhari SS, Saso L, Selvam GS. Regression of oxidative stress by targeting eNOS and Nrf2/ARE signaling: a guided drug target for cardiovascular diseases. Curr Top Med Chem. 2015;15(9):857–871. doi: 10.2174/1568026615666150220114417. [DOI] [PubMed] [Google Scholar]
- 32.Widlansky ME, Gutterman DD. Regulation of endothelial function by mitochondrial reactive oxygen species. Antioxid Redox Signal. 2011;15(6):1517–1530. doi: 10.1089/ars.2010.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abais JM, Xia M, Zhang Y, Boini KM, Li PL. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal. 2015;22(13):1111–1129. doi: 10.1089/ars.2014.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guillén I, Blanes M, Gómez-Lechón MJ, Castell JV. Cytokine signaling during myocardial infarction: sequential appearance of IL-1 beta and IL-6. Am J Physiol. 1995;269(2 Pt 2):R229–R235. doi: 10.1152/ajpregu.1995.269.2.R229. [DOI] [PubMed] [Google Scholar]
- 35.Libby P. Interleukin-1 Beta as a Target for Atherosclerosis Therapy: Biological Basis of CANTOS and Beyond. J Am Coll Cardiol. 2017;70(18):2278–2289. doi: 10.1016/j.jacc.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maier W, Altwegg LA, Corti R, Gay S, Hersberger M, Maly FE, et al. Inflammatory markers at the site of ruptured plaque in acute myocardial infarction: locally increased interleukin-6 and serum amyloid A but decreased C-reactive protein. Circulation. 2005;111(11):1355–1361. doi: 10.1161/01.CIR.0000158479.58589.0A. [DOI] [PubMed] [Google Scholar]
- 37.Alsaffar H, Martino N, Garrett JP, Adam AP. Interleukin-6 promotes a sustained loss of endothelial barrier function via Janus kinase-mediated STAT3 phosphorylation and de novo protein synthesis. Am J Physiol Cell Physiol. 2018;314(5):C589–C602. doi: 10.1152/ajpcell.00235.2017. [DOI] [PubMed] [Google Scholar]
- 38.Huber SA, Sakkinen P, Conze D, Hardin N, Tracy R. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1999;19(10):2364–2367. doi: 10.1161/01.atv.19.10.2364. [DOI] [PubMed] [Google Scholar]
- 39.O’Brien LC, Mezzaroma E, Van Tassell BW, Marchetti C, Carbone S, Abbate A, et al. Interleukin-18 as a therapeutic target in acute myocardial infarction and heart failure. Mol Med. 2014;20:221–229. doi: 10.2119/molmed.2014.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mallat Z, Corbaz A, Scoazec A, Besnard S, Lesèche G, Chvatchko Y, Tedgui A. Expression of interleukin-18 in human atherosclerotic plaques and relation to plaque instability. Circulation. 2001;104(14):1598–1603. doi: 10.1161/hc3901.096721. [DOI] [PubMed] [Google Scholar]
- 41.Badimon L, Peña E, Arderiu G, Padró T, Slevin M, Vilahur G, et al. C-Reactive Protein in Atherothrombosis and Angiogenesis. Front Immunol. 2018;9:430. doi: 10.3389/fimmu.2018.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bisoendial RJ, Kastelein JJ, Levels JH, Zwaginga JJ, van den Bogaard B, Reitsma PH, et al. Activation of inflammation and coagulation after infusion of C-reactive protein in humans. Circ Res. 2005;96(7):714–716. doi: 10.1161/01.RES.0000163015.67711.AB. [DOI] [PubMed] [Google Scholar]
- 43.Tedgui A, Mallat Z. Cytokines in atherosclerosis: Pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 44.Spann NJ, Garmire LX, McDonald JG, Myers DS, Milne SB, Shibata N, et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–152. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Papageorgiou N, Zacharia E, Androulakis E, Briasoulis A, Charakida M, Tousoulis D. HDL as a prognostic biomarker for coronary atherosclerosis: the role of inflammation. Expert Opin Ther Targets. 2016;20(8):907–921. doi: 10.1517/14728222.2016.1152264. [DOI] [PubMed] [Google Scholar]
- 46.Chen M, Ma L, Hall JE, Liu X, Ying Z. Dual regulation of tumor necrosis factor-α on myosin light chain phosphorylation in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2015;308(5):H398–406. doi: 10.1152/ajpheart.00691.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen Q, Rigor RR, Pivetti CD, Wu MH, Yuan SY. Myosin light chain kinase in microvascular endothelial barrier function. Cardiovasc Res. 2010;87(2):272–280. doi: 10.1093/cvr/cvq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. Nlrp3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ait-Oufella H, Herbin O, Bouaziz JD, Binder CJ, Uyttenhove C, Laurans L, et al. B cell depletion reduces the development of atherosclerosis in mice. J Exp Med. 2010;207:1579–1587. doi: 10.1084/jem.20100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Foks AC, Lichtman AH, Kuiper J. Treating atherosclerosis with regulatory T cells. Arterioscler Thromb Vasc Biol. 2015;35(2):280–287. doi: 10.1161/ATVBAHA.114.303568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fredman G, Tabas I. Boosting Inflammation Resolution in Atherosclerosis: The Next Frontier for Therapy. Am J Pathol. 2017;187(6):1211–1221. doi: 10.1016/j.ajpath.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350(14):1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 53.Kaptoge S, Seshasai SR, Gao P, Freitag DF, Butterworth AS, Borglykke A, et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J. 2014;35(9):578–589. doi: 10.1093/eurheartj/eht367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parrinello CM, Lutsey PL, Ballantyne CM, Folsom AR, Pankow JS, Selvin E. Six-year change in high-sensitivity C-reactive protein and risk of diabetes, cardiovascular disease, and mortality. Am Heart J. 2015;170(2):380–389. doi: 10.1016/j.ahj.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tabák AG, Kivimäki M, Brunner EJ, Lowe GD, Jokela M, Akbaraly TN, et al. Changes in C-reactive protein levels before type 2 diabetes and cardiovascular death: the Whitehall II study. Eur J Endocrinol. 2010;163(1):89–95. doi: 10.1530/EJE-10-0277. [DOI] [PubMed] [Google Scholar]
- 56.IL6R Genetics Consortium Emerging Risk Factors Collaboration. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet. 2012;379(9822):1205–1213. doi: 10.1016/S0140-6736(11)61931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.C Reactive Protein Coronary Heart Disease Genetics Collaboration (CCGC) Association between C reactive protein and coronary heart disease: mendelian randomisation analysis based on individual participant data. BMJ. 2011;342:d548. doi: 10.1136/bmj.d548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium. Swerdlow DI, Holmes MV, Kuchenbaecker KB, Engmann JE, Shah T, et al. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet. 2012;379(9822):1214–24. doi: 10.1016/S0140-6736(12)60110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singh S, Fumery M, Singh AG, Singh N, Prokop LJ, Dulai PS, et al. Comparative Risk of Cardiovascular Events with Biologic and Synthetic Disease-Modifying Anti-Rheumatic Drugs in Patients with Rheumatoid Arthritis: A Systematic Review and Meta-analysis. Arthritis Care Res (Hoboken) 2019 Mar 15; doi: 10.1002/acr.23875. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ogbuanu IU, Zhang H, Karmaus W. Can we apply the Mendelian randomization methodology without considering epigenetic effects? Emerg Themes Epidemiol. 2009;6:3. doi: 10.1186/1742-7622-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377(12):1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 62.Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, et al. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N Engl J Med. 2019;380(8):752–762. doi: 10.1056/NEJMoa1809798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.D’Elia L, Galletti F, La Fata E, Sabino P, Strazzullo P. Effect of dietary sodium restriction on arterial stiffness: systematic review and meta-analysis of the randomized controlled trials. J Hypertens. 2018;36(4):734–743. doi: 10.1097/HJH.0000000000001604. [DOI] [PubMed] [Google Scholar]
- 64.Grigg JB, Sonnenberg GF. Host-microbiota interactions shape local and systemic inflammatory diseases. J Immunol. 2017;198:564–571. doi: 10.4049/jimmunol.1601621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brunt VE, Gioscia-Ryan RA, Richey JJ, Zigler MC, Cuevas LM, Gonzalez A, et al. Suppression of the gut microbiome ameliorates age-related arterial dysfunction and oxidative stress in mice. J Physiol. 2019;597(9):2361–2378. doi: 10.1113/JP277336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Menni C, Lin C, Cecelja M, Mangino M, Matey-Hernandez ML, Keehn L, et al. Gut microbial diversity is associated with lower arterial stiffness in women. Eur Heart J. 2018;39(25):2390–2397. doi: 10.1093/eurheartj/ehy226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Natarajan N, Hori D, Flavahan S, Steppan J, Flavahan NA, Berkowitz DE, et al. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol Genomics. 2016;48(11):826–834. doi: 10.1152/physiolgenomics.00089.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 69.De Bruyne T, Steenput B, Roth L, De Meyer GRY, Santos CND, Valentová K, et al. Dietary Polyphenols Targeting Arterial Stiffness: Interplay of Contributing Mechanisms and Gut Microbiome-Related Metabolism. Nutrients. 2019;11(3) doi: 10.3390/nu11030578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zanoli L, Boutouyrie P, Fatuzzo P, Granata A, Lentini P, Oztürk K, et al. Inflammation and Aortic Stiffness: An Individual Participant Data Meta-Analysis in Patients With Inflammatory Bowel Disease. J Am Heart Assoc. 2017;6(10) doi: 10.1161/JAHA.117.007003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sehgel NL, Vatner SF, Meininger GA. “Smooth Muscle Cell Stiffness Syndrome”-Revisiting the Structural Basis of Arterial Stiffness. Front Physiol. 2015;6(335) doi: 10.3389/fphys.2015.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yin H, Pickering JG. Cellular Senescence and Vascular Disease: Novel Routes to Better Understanding and Therapy. Can J Cardiol. 2016;32(5):612–623. doi: 10.1016/j.cjca.2016.02.051. [DOI] [PubMed] [Google Scholar]
- 73.Lacolley P, Regnault V, Segers P, Laurent S. Vascular Smooth Muscle Cells and Arterial Stiffening: Relevance in Development, Aging, and Disease. Physiol Rev. 2017;97(4):1555–1617. doi: 10.1152/physrev.00003.2017. [DOI] [PubMed] [Google Scholar]
- 74.Lehoux S, Castier Y, Tedgui A. Molecular mechanisms of the vascular responses to haemodynamic forces. J Intern Med. 2006;259(4):381–392. doi: 10.1111/j.1365-2796.2006.01624.x. [DOI] [PubMed] [Google Scholar]
- 75.Humphrey JD. Mechanisms of arterial remodeling in hypertension: coupled roles of wall shear and intramural stress. Hypertension. 2008;52(2):195–200. doi: 10.1161/HYPERTENSIONAHA.107.103440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91(1):327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lacolley P, Regnault V, Segers P, Laurent S. Vascular Smooth Muscle Cells and Arterial Stiffening: Relevance in Development, Aging, and Disease. Physiol Rev. 2017;97(4):1555–1617. doi: 10.1152/physrev.00003.2017. [DOI] [PubMed] [Google Scholar]
- 78.Bersi MR, Bellini C, Wu J, Montaniel KR, Harrison DG, Humphrey JD. Excessive adventitial remodeling leads to early aortic maladaptation in angiotensin-induced hypertension. Hypertension. 2016;67(5):890–896. doi: 10.1161/HYPERTENSIONAHA.115.06262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tieu BC, Lee C, Sun H, LeJeune W, Recinos A, Ju X, et al. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 2009;119(12):3637–3651. doi: 10.1172/JCI38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bersi MR, Khosravi R, Wujciak AJ, Harrison DG, Humphrey JD. Differential cell-matrix mechanoadaptations and inflammation drive regional propensities to aortic fibrosis, aneurysm or dissection in hypertension. J R Soc Interface. 2017;14(136):20170327. doi: 10.1098/rsif.2017.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu J, Thabet SR, Kirabo A, Trott DW, Saleh MA, Xiao L, et al. Inflammation and Mechanical Stretch Promote Aortic Stiffening in Hypertension Through Activation of p38 MAP Kinase. Circ Res. 2014;114(4):616–625. doi: 10.1161/CIRCRESAHA.114.302157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kwong JC, Schwartz KL, Campitelli MA, Chung H, Crowcroft NS, Karnauchow T, et al. Acute Myocardial Infarction after Laboratory-Confirmed Influenza Infection. N Engl J Med. 2018;378(4):345–353. doi: 10.1056/NEJMoa1702090. [DOI] [PubMed] [Google Scholar]
- 83.Vlachopoulos C, Xaplanteris P, Aboyans V, Brodmann M, Cífková R, Cosentino F, et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis. 2015;241(2):507–532. doi: 10.1016/j.atherosclerosis.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 84.Udell JA, Zawi R, Bhatt DL, Keshtkar-Jahromi M, Gaughran F, Phrommintikul A, et al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA. 2013;310(16):1711–1720. doi: 10.1001/jama.2013.279206. [DOI] [PubMed] [Google Scholar]
- 85.Vaudo G, Marchesi S, Siepi D, Brozzetti M, Lombardini R, Pirro M, et al. Human endothelial impairment in sepsis. Atherosclerosis. 2008;197(2):747–752. doi: 10.1016/j.atherosclerosis.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 86.Charakida M, Donald AE, Terese M, Leary S, Halcox JP, Ness A, et al. Endothelial dysfunction in childhood infection. Circulation. 2005;111(13):1660–1665. doi: 10.1161/01.CIR.0000160365.18879.1C. [DOI] [PubMed] [Google Scholar]
- 87.Hingorani AD, Cross J, Kharbanda RK, Mullen M, Bhagat K, Taylor M, et al. Acute systemic inflammation impairs endothelium-dependent dilatation in humans. Circulation. 2000;102:994–999. doi: 10.1161/01.cir.102.9.994. [DOI] [PubMed] [Google Scholar]
- 88.Ranadive SM, Kappus RM, Cook MD, Yan H, Lane AD, Woods JA, et al. Effect of acute moderate exercise on induced inflammation and arterial function in older adults. Exp Physiol. 2014;99(4):729–739. doi: 10.1113/expphysiol.2013.077636. [DOI] [PubMed] [Google Scholar]
- 89.Vlachopoulos C, Dima I, Aznaouridis K, Vasiliadou C, Ioakeimidis N, Aggeli C, et al. Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation. 2005;112(14):2193–2200. doi: 10.1161/CIRCULATIONAHA.105.535435. [DOI] [PubMed] [Google Scholar]
- 90.Vlachopoulos C, Aznaouridis K, Dagre A, Vasiliadou C, Masoura C, Stefanadi E, et al. Protective effect of atorvastatin on acute systemic inflammation-induced endothelial dysfunction in hypercholesterolaemic subjects. Eur Heart J. 2007;28(17):2102–2109. doi: 10.1093/eurheartj/ehm247. [DOI] [PubMed] [Google Scholar]
- 91.Lane-Cordova AD, Phillips SA, Baynard T, Woods JA, Motl RW, Fernhall B. Effects of ageing and physical activity on blood pressure and endothelial function during acute inflammation. Exp Physiol. 2016;101(7):962–971. doi: 10.1113/EP085551. [DOI] [PubMed] [Google Scholar]
- 92.Schroeder EC, Lane-Cordova AD, Ranadive SM, Baynard T, Fernhall B. Influence of fitness and age on the endothelial response to acute inflammation. Exp Physiol. 2018;103(6):924–931. doi: 10.1113/EP086922. [DOI] [PubMed] [Google Scholar]
- 93.Antoniades C, Demosthenous M, Tousoulis D, Antonopoulos AS, Vlachopoulos C, Toutouza M, et al. Role of asymmetrical dimethylarginine in inflammation-induced endothelial dysfunction in human atherosclerosis. Hypertension. 2011;58(1):93–98. doi: 10.1161/HYPERTENSIONAHA.110.168245. [DOI] [PubMed] [Google Scholar]
- 94.von Rossum A, Laher I, Choy JC. Immune-mediated vascular injury and dysfunction in transplant arteriosclerosis. Front Immunol. 2015;5:684. doi: 10.3389/fimmu.2014.00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fagundes NCF, Almeida APCPSC, Vilhena KFB, Magno MB, Maia LC, Lima RR. Periodontitis As A Risk Factor For Stroke: A Systematic Review And Meta-Analysis. Vasc Health Risk Manag. 2019;15:519–532. doi: 10.2147/VHRM.S204097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rydén L, Buhlin K, Ekstrand E, de Faire U, Gustafsson A, Holmer J, et al. Periodontitis Increases the Risk of a First Myocardial Infarction: A Report From the PAROKRANK Study. Circulation. 2016;133(6):576–583. doi: 10.1161/CIRCULATIONAHA.115.020324. [DOI] [PubMed] [Google Scholar]
- 97.Orlandi M, Suvan J, Petrie A, Donos N, Masi S, Hingorani A, et al. Association between periodontal disease and its treatment, flow-mediated dilatation and carotid intima-media thickness: a systematic review and meta-analysis. Atherosclerosis. 2014;236(1):39–46. doi: 10.1016/j.atherosclerosis.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 98.Schmitt A, Carra MC, Boutouyrie P, Bouchard P. Periodontitis and arterial stiffness: a systematic review and meta-analysis. J Clin Periodontol. 2015;42(11):977–987. doi: 10.1111/jcpe.12467. [DOI] [PubMed] [Google Scholar]
- 99.Figuero E, Sanchez-Beltran M, Cuesta-Frechoso S, Tejerina JM, del Castro JA, Gutiérrez JM, et al. Detection of periodontal bacteria in atheromatous plaque by nested polymerase chain reaction. J Periodontol. 2011;82(10):1469–1477. doi: 10.1902/jop.2011.100719. [DOI] [PubMed] [Google Scholar]
- 100.Reyes L, Herrera D, Kozarov E, Roldan S, Progulske-Fox A. Periodontal bacterial invasion and infection: contribution to atherosclerotic pathology. J Clin Periodontol. 2013;40(14):S30–S50. doi: 10.1111/jcpe.12079. [DOI] [PubMed] [Google Scholar]
- 101.Rosenfeld ME, Campbell LA. Pathogens and atherosclerosis: update on the potential contribution of multiple infectious organisms to the pathogenesis of atherosclerosis. Thromb Haemost. 2011;106:858–867. doi: 10.1160/TH11-06-0392. [DOI] [PubMed] [Google Scholar]
- 102.Buhlin K, Hultin M, Norderyd O, Persson L, Pockley AG, Rabe P, et al. Risk factors for atherosclerosis in cases with severe periodontitis. J Clin Periodontol. 2009;36:541–549. doi: 10.1111/j.1600-051X.2009.01430.x. [DOI] [PubMed] [Google Scholar]
- 103.Range H, Labreuche J, Louedec L, Rondeau P, Planesse C, Sebbag U, et al. Periodontal bacteria in human carotid atherothrombosis as a potential trigger for neutrophil activation. Atherosclerosis. 2014;236:448–455. doi: 10.1016/j.atherosclerosis.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 104.Tonetti MS, D’Aiuto F, Nibali L, Donald A, Storry C, Parkar M, et al. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356(9):911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- 105.D’Aiuto F, Gkranias N, Bhowruth D, Khan T, Orlandi M, Suvan J, et al. Systemic effects of periodontitis treatment in patients with type 2 diabetes: a 12 month, single-centre, investigator-masked, randomised trial. Lancet Diabetes Endocrinol. 2018;6(12):954–965. doi: 10.1016/S2213-8587(18)30038-X. [DOI] [PubMed] [Google Scholar]
- 106.Kapellas K, Maple-Brown LJ, Jamieson LM, Do LG, O’Dea K, Brown A, et al. Effect of periodontal therapy on arterial structure and function among aboriginal australians: a randomized, controlled trial. Hypertension. 2014;64(4):702–708. doi: 10.1161/HYPERTENSIONAHA.114.03359. [DOI] [PubMed] [Google Scholar]
- 107.Zanoli L, Granata A, Lentini P, Gaudio A, Castellino P. Augmentation index is increased in patients with inflammatory bowel disease, a meta-analysis. Eur J Intern Med. 2017;39:e31–e32. doi: 10.1016/j.ejim.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 108.Zanoli L, Boutouyrie P, Fatuzzo P, Granata A, Lentini P, Oztürk K, et al. Inflammation and aortic stiffness: an individual participant data meta-analysis in patients with inflammatory bowel disease. J Am Heart Assoc. 2017;6:e007003. doi: 10.1161/JAHA.117.007003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wu GC, Leng RX, Lu Q, Fan YG, Wang DG, Ye DQ. Subclinical Atherosclerosis in Patients With Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis. Angiology. 2017;68(5):447–461. doi: 10.1177/0003319716652031. [DOI] [PubMed] [Google Scholar]
- 110.Ambrosino P, Tasso M, Lupoli R, Di Minno A, Baldassarre D, Tremoli E, et al. Non-invasive assessment of arterial stiffness in patients with rheumatoid arthritis: a systematic review and metaanalysis of literature studies. Ann Med. 2015;47(6):457–467. doi: 10.3109/07853890.2015.1068950. [DOI] [PubMed] [Google Scholar]
- 111.Wang P, Mao YM, Zhao CN, Liu LN, Li XM, Li XP, et al. Increased Pulse Wave Velocity in Systemic Lupus Erythematosus: A Meta-Analysis. Angiology. 2018;69(3):228–235. doi: 10.1177/0003319717715964. [DOI] [PubMed] [Google Scholar]
- 112.Meiszterics Z, Tímár O, Gaszner B, Faludi R, Kehl D, Czirják L, et al. Early morphologic and functional changes of atherosclerosis in systemic sclerosis-a systematic review and meta-analysis. Rheumatology (Oxford) 2016;55(12):2119–2130. doi: 10.1093/rheumatology/kew236. [DOI] [PubMed] [Google Scholar]
- 113.Zanoli L, Cannavò M, Rastelli S, Di Pino L, Monte I, Di Gangi M, et al. Arterial stiffness is increased in patients with inflammatory bowel disease. J Hypertens. 2012;30(9):1775–1781. doi: 10.1097/HJH.0b013e3283568abd. [DOI] [PubMed] [Google Scholar]
- 114.Zanoli L, Lentini P, Boutouyrie P, Fatuzzo P, Granata A, Corrao S, et al. Pulse wave velocity differs between ulcerative colitis and chronic kidney disease. Eur J Intern Med. 2018;47:36–42. doi: 10.1016/j.ejim.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 115.Cypiene A, Kovaite M, Venalis A, Dadoniene J, Rugiene R, Petrulioniene Z, et al. Arterial wall dysfunction in systemic lupus erythematosus. Lupus. 2009;18(6):522–529. doi: 10.1177/0961203308099625. [DOI] [PubMed] [Google Scholar]
- 116.Cypiene A, Laucevicius A, Venalis A, Dadoniene J, Ryliskyte L, Petrulioniene Z, et al. The impact of systemic sclerosis on arterial wall stiffness parameters and endothelial function. Clin Rheumatol. 2008;27(12):1517–1522. doi: 10.1007/s10067-008-0958-1. [DOI] [PubMed] [Google Scholar]
- 117.Di Minno MN, Ambrosino P, Lupoli R, Di Minno A, Tasso M, Peluso R, et al. Clinical assessment of endothelial function in patients with rheumatoid arthritis: A meta-analysis of literature studies. Eur J Intern Med. 2015;26(10):835–842. doi: 10.1016/j.ejim.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 118.Wang P, Guan SY, Xu SZ, Li HM, Leng RX, Li XP, et al. Increased carotid intima-media thickness in rheumatoid arthritis: an update meta-analysis. Clin Rheumatol. 2016;35(2):315–323. doi: 10.1007/s10067-015-3130-8. [DOI] [PubMed] [Google Scholar]
- 119.Wu GC, Liu HR, Leng RX, Li XP, Li XM, Pan HF, et al. Subclinical atherosclerosis in patients with systemic lupus erythematosus: A systemic review and meta-analysis. Autoimmun Rev. 2016;15(1):22–37. doi: 10.1016/j.autrev.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 120.Wang DG, Tang XW, Fan Y, Leng RX, Ni J, Deng SM, et al. Decreased flow-mediated dilatation in patients with systemic lupus erythematosus: a meta-analysis. Inflammation. 2014;37(6):2067–75. doi: 10.1007/s10753-014-9940-z. [DOI] [PubMed] [Google Scholar]
- 121.Zanoli L, Ozturk K, Cappello C, Inserra G, Geraci G, Tuttolomondo A, et al. Inflammation and aortic pulse wave velocity. A multicentre longitudinal study in patients with inflammatory bowel disease. J Am Heart Assoc. 2019;8(3):e010942. doi: 10.1161/JAHA.118.010942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang DG, Tang XW, Fan Y, Leng RX, Ni J, Deng SM, et al. Decreased flow-mediated dilatation in patients with systemic lupus erythematosus: a meta-analysis. Inflammation. 2014;37(6):2067–2075. doi: 10.1007/s10753-014-9940-z. [DOI] [PubMed] [Google Scholar]
- 123.Wang P, Guan SY, Xu SZ, Li HM, Leng RX, Li XP, et al. Increased carotid intima-media thickness in rheumatoid arthritis: an update meta-analysis. Clin Rheumatol. 2016;35(2):315–323. doi: 10.1007/s10067-015-3130-8. [DOI] [PubMed] [Google Scholar]
- 124.Wu GC, Liu HR, Leng RX, Li XP, Li XM, Pan HF, et al. Subclinical atherosclerosis in patients with systemic lupus erythematosus: A systemic review and meta-analysis. Autoimmun Rev. 2016;15(1):22–37. doi: 10.1016/j.autrev.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 125.Hemkens LG, Bucher HC. HIV infection and cardiovascular disease. Eur Heart J. 2014;35:1373–1381. doi: 10.1093/eurheartj/eht528. [DOI] [PubMed] [Google Scholar]
- 126.Sun D, Wu Y, Yuan Y, Wang Y, Liu W, Yang J. Is the atherosclerotic process accentuated under conditions of HIV infection, antiretroviral therapy, and protease inhibitor exposure? Metaanalysis of the markers of arterial structure and function. Atherosclerosis. 2015;242(1):109–116. doi: 10.1016/j.atherosclerosis.2015.06.059. [DOI] [PubMed] [Google Scholar]
- 127.Hanna DB, Post WS, Deal JA, Hodis HN, Jacobson LP, Mack WJ, et al. HIV Infection Is Associated With Progression of Subclinical Carotid Atherosclerosis. Clin Infect Dis. 2015;61(4):640–650. doi: 10.1093/cid/civ325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 129.Eleftheriadis E, Eleftheriadis N. Coronary Artery Diseases. Vol. 13. INNTECH; 2012. Coronary Artery Disease and Systemic Vasculitis: Case report and Review; pp. 281–300. [Google Scholar]
- 130.Misra DP, Shenoy SN. Cardiac involvement in primary systemic vasculitis and potential drug therapies to reduce cardiovascular risk. Rheumatol Int. 2017;37(1):151–167. doi: 10.1007/s00296-016-3435-1. [DOI] [PubMed] [Google Scholar]
- 131.Argyropoulou OD, Protogerou AD, Sfikakis PP. Accelerated atheromatosis and arteriosclerosis in primary systemic vasculitides: current evidence and future perspectives. Curr Opin Rheumatol. 2018;30(1):36–43. doi: 10.1097/BOR.0000000000000453. [DOI] [PubMed] [Google Scholar]
- 132.Vlachopoulos C, Gravos A, Georgiopoulos G, Terentes-Printzios D, Ioakeimidis N, Vassilopoulos D, et al. The effect of TNF-a antagonists on aortic stiffness and wave reflections: a meta-analysis. Clin Rheumatol. 2018;37(2):515–526. doi: 10.1007/s10067-017-3657-y. [DOI] [PubMed] [Google Scholar]
- 133.Angel K, Provan SA, Fagerhol MK, Mowinckel P, Kvien TK, Atar D. Effect of 1-year anti-TNF-α therapy on aortic stiffness, carotid atherosclerosis, and calprotectin in inflammatory arthropathies: a controlled study. Am J Hypertens. 2012;25(6):644–650. doi: 10.1038/ajh.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McInnes IB, Thompson L, Giles JT, Bathon JM, Salmon JE, Beaulieu AD, et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann Rheum Dis. 2015;74(4):694–702. doi: 10.1136/annrheumdis-2013-204345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kume K, Amano K, Yamada S, Hatta K, Ohta H, Kuwaba N. Tocilizumab monotherapy reduces arterial stiffness as effectively as etanercept or adalimumab monotherapy in rheumatoid arthritis: an open-label randomized controlled trial. J Rheumatol. 2011;38(10):2169–71. doi: 10.3899/jrheum.110340. [DOI] [PubMed] [Google Scholar]
- 136.Ikonomidis I, Papadavid E, Makavos G, Andreadou I, Varoudi M, Gravanis K, et al. Lowering Interleukin-12 Activity Improves Myocardial and Vascular Function Compared With Tumor Necrosis Factor-a Antagonism or Cyclosporine in Psoriasis. Circ Cardiovasc Imaging. 2017;10(9) doi: 10.1161/CIRCIMAGING.117.006283. [DOI] [PubMed] [Google Scholar]
- 137.Pina T, Corrales A, Lopez-Mejias R, Armesto S, Gonzalez-Lopez MA, Gómez-Acebo I, et al. Anti-tumor necrosis factor-alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: A 6-month prospective study. J Dermatol. 2016;43(11):1267–1272. doi: 10.1111/1346-8138.13398. [DOI] [PubMed] [Google Scholar]
- 138.Zanoli L, Inserra G, Cappello M, Oztürk K, Castellino P. Anti-tumour necrosis factor therapy within 4 years from diagnosis of inflammatory bowel disease reduces aortic stiffness. A multicentre longitudinal study. J Am Coll Cardiol. 2019;73(8):981–982. doi: 10.1016/j.jacc.2018.12.032. [DOI] [PubMed] [Google Scholar]
- 139.Zanoli L, Boutouyrie P, Lentini P, Rastelli S, Castellino P. Maintenance therapy with salicylates is associated with aortic stiffening in patients with inflammatory bowel disease. J Hypertens. 2017;35(4):898–899. doi: 10.1097/HJH.0000000000001235. [DOI] [PubMed] [Google Scholar]
- 140.Zanoli L, Rastelli S, Granata A, Inserra G, Empana JP, Boutouyrie P, et al. Arterial stiffness in inflammatory bowel disease: a systematic review and meta-analysis. J Hypertens. 2016;34(5):822–829. doi: 10.1097/HJH.0000000000000867. [DOI] [PubMed] [Google Scholar]
- 141.Capkin E, Karkucak M, Kiris A, Durmus I, Karaman K, Karaca A, et al. Anti-TNF-α therapy may not improve arterial stiffness in patients with AS: a 24-week follow-up. Rheumatology (Oxford) 2012;51(5):910–914. doi: 10.1093/rheumatology/ker434. [DOI] [PubMed] [Google Scholar]
- 142.Peyster E, Chen J, Feldman HI, Go AS, Gupta J, Mitra N, et al. Inflammation and Arterial Stiffness in Chronic Kidney Disease: Findings From the CRIC Study. Am J Hypertens. 2017;30(4):400–408. doi: 10.1093/ajh/hpw164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mäki-Petäjä KM, Wilkinson IB. Anti-Inflammatory Drugs and Statins for Arterial Stiffness Reduction. Curr Pharm Des. 2009;15(3):290–303. doi: 10.2174/138161209787354221. [DOI] [PubMed] [Google Scholar]
- 144.Ait-Oufella H, Collin C, Bozec E, Laloux B, Ong KT, Dufouil C, et al. Long-term reduction in aortic stiffness: a 5.3-year follow-up in routine clinical practice. J Hypertens. 2010;28(11):2336–2341. doi: 10.1097/HJH.0b013e32833da2b2. [DOI] [PubMed] [Google Scholar]
- 145.Plantinga Y, Ghiadoni L, Magagna A, Giannarelli C, Franzoni F, Taddei S, et al. Supplementation with vitamins C and E improves arterial stiffness and endothelial function in essential hypertensive patients. Am J Hypertens. 2007;20(4):392–397. doi: 10.1016/j.amjhyper.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 146.Vlachopoulos C, Xaplanteris P, Aboyans V, Brodmann M, Cífková R, Cosentino F, et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis. 2015;241(2):507–532. doi: 10.1016/j.atherosclerosis.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 147.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36(10):1953–2041. doi: 10.1097/HJH.0000000000001940. [DOI] [PubMed] [Google Scholar]
- 148.Noman AT, Qazi AH, Alqasrawi M, Ayinde H, Tleyjeh IM, Lindower P, et al. Fluoroquinolones and the risk of aortopathy: A systematic review and meta-analysis. Int J Cardiol. 2019;274:299–302. doi: 10.1016/j.ijcard.2018.09.067. [DOI] [PubMed] [Google Scholar]
- 149.Nilsson PM, Boutouyrie P, Laurent S. Vascular aging: A tale of EVA and ADAM in cardiovascular risk assessment and prevention. Hypertension. 2009;54(1):3–10. doi: 10.1161/HYPERTENSIONAHA.109.129114. [DOI] [PubMed] [Google Scholar]
- 150.Zanoli L, Rastelli S, Inserra G, Castellino P. Arterial structure and function in inflammatory bowel disease. World J Gastroenterol. 2015;21(40):11304–11311. doi: 10.3748/wjg.v21.i40.11304. [DOI] [PMC free article] [PubMed] [Google Scholar]