Abstract
Upon their activation, CD8+ T cells in the tumor micro-environment (TME) secrete cytokines such as IFNγ, TNFα, and IL-2. While over the past years a major interest has developed in the antigenic signals that induce such cytokine release, our understanding of the cells that subsequently sense these CD8+ T-cell secreted cytokines is modest. Here, we review the current insights into the spreading behavior of CD8+ T-cell-secreted cytokines in the TME. We argue for a model in which variation in the mode of cytokine secretion, cytokine half-life, receptor-mediated clearance, cytokine binding to extracellular components, and feedback or forward loops, between different cytokines or between individual tumors, sculpts the local tissue response to natural and therapy-induced T-cell activation in human cancer.
Introduction
CD8+ cytotoxic T lymphocytes (CTLs) play a central role in immune-mediated control of cancer [1, 2]. Upon tumor cell recognition, CTLs release cytotoxic granules toward their target via the immune synapse (IS) [1]. In parallel, activated CTLs secrete cytokines, such as interferon γ (IFNγ), tumor necrosis factor α (TNFα) and interleukin-2 (IL-2), which can, on their own or jointly, modify the behavior of cells carrying the corresponding cytokine receptors. The effects of cytokine receptor signaling on the tumor micro-environment (TME) are highly diverse, and include immune cell activation, regulation of antigen presentation and immune checkpoint molecules, and, in some cases, the induction of tumor cell senescence and death [3–5]. Notably, pre-clinical and clinical studies have provided evidence for both positive and negative effects of IFNγ, TNFα and IL-2 signaling on tumor control [3–12]. One major factor that drives this differential effect is likely to be formed by context dependent differences in the outcome of cytokine receptor signaling. However, an entirely independent factor that may influence the response of tumors to cytokine secretion could be the extent to which cytokines are able to spread in the TME or, in other words, which cells and how many cells can actually sense such cytokine secretion. Here we discuss the factors that influence the spreading of CD8+ T-cell secreted cytokines within the TME and propose that variation in such factors can form a determinant of the response of individual tumors to T-cell activity.
Spatiotemporal behavior of CD8+ T-cell secreted cytokines
Cytokines can, at least in some cases, spread substantial distances in (tumor) tissue micro-environments. Indirect evidence for this notion is, for example, provided by the observation that in mouse models, the effects of IFNγ and TNFα on endothelial cells can be critical for tumor control [13] while these cells may be less likely to be directly recognized by T cells. Secondly, the observed role of cytokine receptor signaling in resistance to immune checkpoint blockade [7, 9, 10, 14] is also suggestive of a more widespread effect of T-cell secreted cytokines. For example, if the impaired IFNγ receptor (IFNγR) signaling that has been observed in αPD-1 resistant lesions would lead to tumor resistance by reducing the level of antigen presentation, this could only be expected to provide a selective advantage to cells that aren’t already being recognized by T cells. Finally, computational models have also led to a model in which cytokine diffusion gradients are invoked to explain the tumor control that is observed at low T-cell densities [15].
Direct evidence for long distance cytokine spreading comes from a small set of in vivo mouse studies that analyzed tissue effects of TNFα, IL-2, and in particular IFNγ. For example, T-cell secreted IFNγ in skin and lymphoid tissues has been shown to induce expression of IFNγ-responsive genes in large areas outside parasite or virus infected regions [16–19]. By the same token, the production of IFNγ and TNFα by intratumoral CD4+ T cells has been shown to induce senescence in tumor cells that are deficient for MHC class II, and that can thus not be directly recognized by these T cells [20]. In recent work, the spreading of CTL secreted IFNγ has been quantified in mouse tumor models. Using mosaic tumors containing both antigen-positive and antigen-negative ‘bystander’ tumor cells, it was shown that a large fraction of bystander cells does undergo productive IFNγR signaling. Such sensing of CTL-secreted IFNγ was observed for bystander cells at distances over hundreds of micrometers (>100 μm [21] >800 μm [22]) from sites of T-cell activation, as revealed by, for instance, the expression of MHC-I and PD-L1 molecules, or the induction of cell death [21, 22]. While the abovementioned data were obtained in immunogenic transplantable tumor models, and in one case lacking endogenous IFNγR-positive cells [22], qualitatively comparable conclusions can be drawn from studies that analyzed cytokine distributions in tissues such as skin and lymph nodes [16–19].
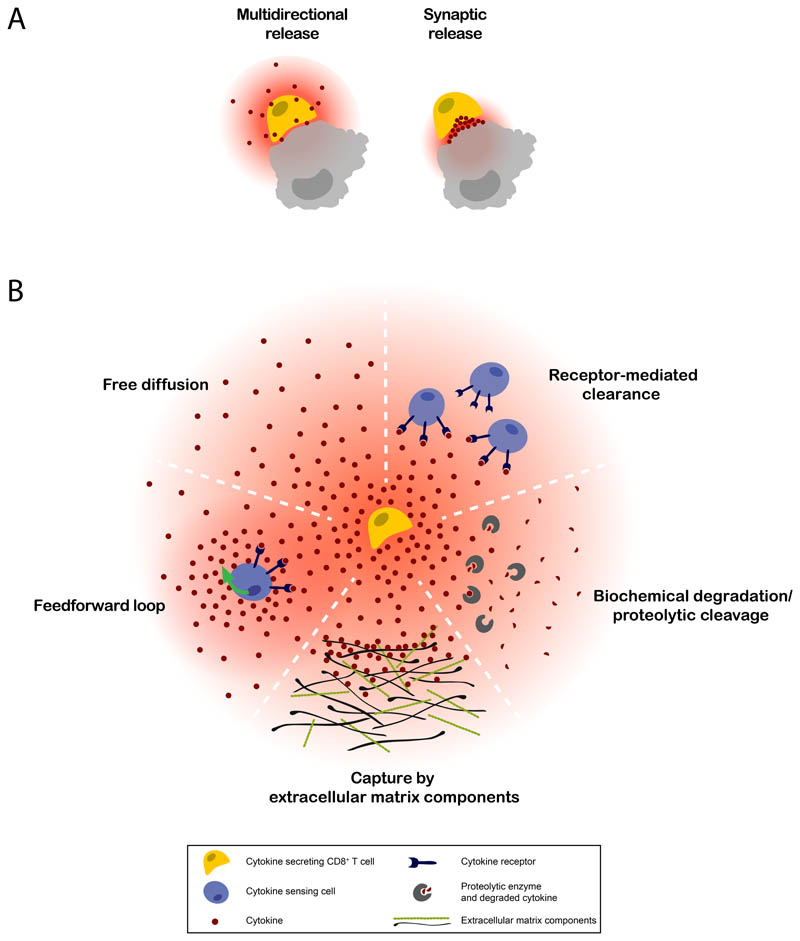
The extent to which cytokine secretion induces receptor signaling in distant cells is determined by both the degree of cytokine spreading from the producing cells, and the threshold of the receiver cells to initiate signal transduction. Here, we focus on the first of these components, distinguishing five parameters that have been proven, or are expected, to influence the spatial spreading of cytokines: the mode of cytokine secretion, cytokine half-life (including aspects such as biochemical stability and proteolytic (in)activation), receptor-mediated clearance, binding to extracellular components, and feedback/feedforward loops (Figure 1 and Table 1). Jointly, these factors determine how a pool of secreted cytokine molecules is distributed over the TME.
Figure 1. Potential factors affecting cytokine spreading in the TME.
The spatiotemporal behavior of CD8+ T cell cytokines is likely to depend on A) the mode of cytokine secretion by the producing cell, and B) environmental factors influencing receptor-mediated clearance, cytokine half-life, cytokine binding to extracellular components, and feedback/ feedforward loops. Variation between these factors is expected between tumors, due to e.g. their genetic make-up, immune infiltrate, molecular ECM structure, level of hypoxia, and nutrient availability.
Table 1. Factors shown or expected to influence the spreading behavior of CD8+ T-cell-secreted cytokines within the TME.
| Observation | References | |
|---|---|---|
| IFNγ | Synaptic release (in vitro) | [24–26] |
| TNFα | Multidirectional release (in vitro) | [25] |
| TACE-mediated cleavage of transmembrane TNFα (in vitro) | Reviewed in [38] | |
| IL-2 | Synaptic release (in vitro) | [25] |
| IFNγ | Increased half-life by HS/heparin-mediated prevention of inactivating proteolytic cleavage (in vitro) | [32, 33] |
| Activating cleavage up to ten C-terminal amino acids (facilitated by HS/heparin-binding) (in vitro) | [32, 33] | |
| TNFα | Instable trimers after membrane cleavage (in vitro) | [41] |
| IFNγ | Receptor expression modulation by internal and environmental factors such as TNFα and IL1β (in vitro) | [27, 28] |
| TNFα | TNFR1 and TNFR2 are expressed on distinct cell types and differ in their affinity to TNFα (in vitro/in vivo) | Reviewed in [42] |
| TACE-mediated cleavage of TNFRs (in vitro) | [46] | |
| IL-2 | Low, intermediate, and high affinity IL-2 receptor variants, expressed by distinct cell types (in vivo) | Reviewed in [12] |
| Receptor expression modulated by FoxP3 and TCR signaling (in vitro) | Reviewed in [12] | |
| IL-2 spreading distance is influenced by the size of the Treg pool (in vitro, suggestive in vivo) | [51] | |
| Shedding of the IL-2Rα chain mediated by tumor-cell derived metalloproteinases or as a result of T-cell activation (in vitro) | [53, 54] | |
| IFNγ | PS-mediated catch and release by tumor cells (in vitro) | [29] |
| Galectin-3 mediated containment within the TME (in vivo) | [31] | |
| HS- or heparin-binding of IFNγ (in vitro) | Reviewed in [33] | |
| Restricted spreading into tissues following heparin I.V. injection (in vivo) | [34] | |
| TNFα | Binding to biglycan, decorin, dermatan sulfate, fibronectin and laminin (in vitro) | [43–45] |
| Il-2 | Binding to galectin-3, collagen and HS/heparin (in vitro) | [31, 56, 57] |
| IFNγ | IFNγ-induced IFNγ production in antigen-stimulated CD4+ T cells (in vitro) | [35] |
| TNFα | TNFα stimulation increases sTNFR levels (in vitro) | [47] |
| Il-2 | IL-2 induced expression of IL-2R (in vitro) | [52] |
| Inhibition of IL-2 production upon IL-2R signalling (in vitro) | [55] |
IFNγ
Of all CTL derived cytokines, the spatial dynamics of IFNγ have been characterized most extensively. CD8+ T cells start secreting IFNγ molecules within minutes to hours after T-cell receptor (TCR) triggering [23]. Continued secretion appears to be highly dependent on active TCR stimulation [22, 23], and substantial spreading of IFNγ is therefore unlikely to occur because of continued production by T cells after target cell dissociation. There is compelling evidence that IFNγ secretion by CTLs is specifically directed toward the synapse that is formed with the antigen-positive target cell [24]. While it has been suggested that this directional mode of secretion would result in selective delivery of IFNγ to the target cell [25], studies demonstrating IFNγ effects on bystander cells [18, 19, 21, 22, 24, 26] argue against such a ‘target cell only’ model. It remains to be determined whether this apparent discrepancy is explained by leakiness of the synapse, unnoticed multidirectional secretion of IFNγ, or release of unconsumed IFNγ into the environment at the moment the synapse is dismantled.
The IFNγR is expressed on all nucleated cells [3], allowing binding of IFNγ to immune cells, tumor cells and stromal cells in the TME. While formal evidence is lacking, the large pool of receptors available for binding makes it plausible that receptor-mediated clearance forms an important determinant in IFNγ spreading. Notably, IFNγR receptor expression can be modulated by both internal factors and by environmental cues, such as TNFα and IL1β [27, 28], conceivably influencing the magnitude of receptor-mediated clearance and hence IFNγ spreading. Besides IFNγR-mediated clearance, there is evidence that IFNγ capture by cell membrane proteins or extracellular matrix (ECM) components present in the TME influences the spatiotemporal behavior of IFNγ. For example, recent work has indicated that the binding of IFNγ to Phosphatidyl Serine (PS), a phospholipid over-represented on the outside of viable tumor cells and dead cells, results in the capture of IFNγ molecules by tumor cells and their subsequent slow release, thereby allowing delayed receptor binding and signaling. This process of IFNγ detainment is likely dependent on the pronounced positively charged surface area of IFNγ, a property shared with cytokines such as IL-12 and IL-23, but not TNFα or IL-2 [29]. In addition, binding of IFNγ to ECM components has been shown to influence its distribution in the TME. For example galectins, (tumor-)cell secreted ECM proteins that form supramolecular complexes, have been shown to trap cytokines in the TME [30]. Specifically, recent work has demonstrated that following intratumoral IFNγ injection, galectin-3 reduces IFNγ diffusion through the tumor matrix, as read out by the fraction and location of cells expressing the IFNγ inducible CXCL9 chemokine in the presence or absence of a galectin antagonist [31]. Next to galectins, the ECM glycosaminoglycans heparin and heparan sulfate (HS) are likely to influence the spatiotemporal behaviour of IFNγ. Specifically, in vitro data has demonstrated that IFNγ binding to heparin and HS can increase IFNγ half-life by preventing inactivating proteolytic cleavage [32]. In addition, HS binding can also facilitate partial proteolytic cleavage, resulting in truncated IFNγ molecules with increased activity [33]. While the relevance of HS or heparin binding to IFNγ within tumor lesions remains to be established, heparin has been shown to restrict spreading of IFNγ into tissues following I.V. injection [34]. Finally, IFNγR signaling may in certain settings induce the expression of IFNγ. Although thus far only reported for antigen-stimulated CD4+ T cells in in vitro assays [35], such direct feedforward loops could potentially have a major impact on the spatiotemporal distribution of cytokines. For example, the observed sensing of IFNγ by tumor cells that are far removed from T-cell activation sites [21, 22] could in part be due to a self-propagating process in which cells instruct neighboring cells to also become cytokine producers. As a side note, should such T cell-based positive feedback loops exist in tumor microenvironments, this may imply that T cells that are considered ‘bystanders’ based on their antigen specificity [36] could nevertheless fulfill an important role in tumor control. Conceivably, nonlymphoid cells such as macrophages, monocytes and dendritic cells may also contribute to such an IFNγ feedforward loop, as IFNγ sensing by these cells leads to increased expression of T-bet, a transcription factor that controls IFNγ expression [35, 37].
TNFα
At present, little is known regarding the in vivo spreading of TNFα and we can therefore only provide an incomplete view, based on information obtained in in vitro assays. Following T-cell activation, translation of preformed TNFα mRNA is induced, leading to the production of a trimeric membrane protein that is subsequently transported to the cell membrane [38, 39]. In contrast to IFNγ and IL-2, TNFα is not targeted towards the IS, but distributed equally over the cell membrane, as shown by live imaging of TNFα on activated murine CD4+ T cells [25]. Membrane bound TNFα (mTNFα) can subsequently be converted to soluble TNFα (sTNFα) through the action of TNF-alpha-converting enzyme (TACE) [38]. Interestingly, both mTNFα and sTNFα can signal though TNFα receptors (TNFαRs), and it is likely that TNFα signal diffusion is to a certain degree regulated by the availability of TACE, which can be increased by T-cell activation [40]. The in vitro half-life of sTNFα is limited by its spontaneous conversion into inactive monomeric TNFα [41]. Assuming that reassembly of trimeric sTNFα is unlikely at physiological TNFα concentrations, the intrinsic instability of TNFα may be an important factor determining the extent of its spreading. Trimeric TNFα can signal through both TNFR1 and TNFR2, but with receptor triggering resulting in distinct signaling outcomes. Specifically, signaling through the ubiquitously expressed TNFR1 is generally associated with cell death, although roles in inducing inflammation and proliferation have been described as well. In contrast, signaling through TNFR2, which is primarily expressed by tumor cells and immunosuppressive cells, mostly induces pro-survival, proliferative and proinflammatory effects, and TNFR2 lacks the death-domain present in TNFR1 [38, 42]. Interestingly, while TNFR1 strongly responds to both mTNFα and sTNFα, TNFR2 is primarily activated by mTNFα [42]. Thus, if one assumes that soluble TNFα molecules may spread further within tumor micro-environments, the outcome of TNFα-induced signaling may show spatial heterogeneity. We do note though that, contrary to sTNFα, mTNFα may “travel” in the tissue environment while still bound to a migrating producing cell, thereby increasing its reach. However, data on the relevance of such postulated travel in the cell-bound state are lacking.
The role of extracellular components in the tissue distribution of TNFα remains largely unexplored. Although binding of TNFα to biglycan, decorin, dermatan sulfate [43], fibronectin [44] and laminin [45] has been shown in in vitro experiments, the potential in vivo consequences of these interactions remain unclear. One class of molecules that has been suggested to be important for the regulation of TNFα activity in vivo comprises soluble TNFα receptors (sTNFR). In vitro, both TNFR1 and TNFR2 can be shed from the membrane through TACE-mediated cleavage [46], and stimulation of cells with TNFα has been shown to increase sTNFR levels [47]. Knock-in mice that only express a mutated, non-sheddable TNFR1 show immune hyperreactivity, characterized by improved control of intracellular bacterial infections, but also severe inflammatory conditions [48]. Conceivably, TNFR shedding may influence receptor signaling in two distinct ways, on the one hand reducing the number of receptors available for signaling, and on the other hand (temporarily) sequestering extracellular TNFα, potentially extending the duration of the TNFα response. In line with these data in preclinical models, germline mutations related to TNFR1 shedding have been linked to inherited autoinflammatory syndromes in humans [49]. While the role of receptor shedding in the TME has not been well investigated, high levels of sTNFRs have been observed in cancer patients [42], providing a rationale for further study.
IL-2
In contrast to IFNγ and TNFα, which can influence the behavior of almost all cells in the TME, the activity of IL-2 is mostly restricted to T cells [12]. While it is well-established that IL-2 production is critical for CD8+ T-cell mediated tumor control [12, 50], our understanding of IL-2 spreading is limited to in vitro data and in vivo analyses of CD4+ T cells in lymphoid organs. IL-2 is rapidly secreted into the IS by activated T cells [25]. However, similar to what has been described for IFNγ, in vitro data demonstrating, and in vivo data suggestive of, IL-2 sensing by cells located many cell layers away from producing cells have been obtained [51]. The IL-2 – IL-2 receptor (IL-2R) system forms a prime example of the regulation of cytokine sensing through the controlled expression of cytokine receptor variants. The IL-2R can exist in three configurations: a low affinity receptor consisting of the IL-2Rα chain, a heterodimeric intermediate affinity IL-2R consisting of the γ and β chains, and a heterotrimeric high affinity receptor composed of the α, β and γ subunits [12]. Expression of the IL-2Rα chain, which is devoid of signaling capacity and can hence be viewed as an affinity regulator, is both regulated by cell type-specific transcription factors, such as FoxP3 in regulatory T cells (Tregs) [12], and by external signals, such as TCR triggering or IL-2R signaling [52]. Moreover, shedding of the IL-2Rα chain, as a result of T-cell activation [53] or facilitated by tumor-cell derived metalloproteinases [54], can occur, forming an additional layer of IL-2R affinity regulation. The IL-2-induced expression of IL-2Rα has elements of both a feedforward and a feedback loop. Specifically, while expression of the IL-2Rα chain (and thereby the high-affinity IL-2 receptor) increases the sensitivity of an individual cell to cytokine, capture of IL-2 by the high affinity receptor and subsequent internalization can also diminish cytokine spreading. In addition, evidence for a second feedback loop has been obtained, in which IL-2 production is inhibited by IL-2R signaling [55], conceptually also resulting in a reduced IL-2 reach within tissues. Several studies have provided evidence that IL-2 can both suppress and enhance adaptive immune responses [12], likely depending on the distinct cells that consume it. Specifically, it has been suggested that Tregs, which constitutively express the high affinity α-β-γ receptor, and effector T cells, which only express the IL-2Rα chain upon activation, compete for IL-2 depending on their relative distance to the IL-2 producing cells [52]. Consistent with the notion that receptor-mediated clearance forms a major factor in the regulation of IL-2 spreading from its site of production, artificial expansion of the Treg pool was shown to limit the distances between IL-2 producing and sensing cells [51]. Furthermore, mathematical modeling based on these data suggested that IL-2 spreading is primarily regulated through a tunable diffusion-consumption mechanism, without a significant role of factors such as directionality in cytokine secretion or cell movement [51]. It is noted that in this model, potential effects of binding of IL-2 to ECM components were not considered. However, as prior work has demonstrated binding of IL-2 to ECM collagens [56] and galectin-3 [31] in in vitro assays, and to HS and heparin in vivo [57], it is possible that ECM binding will form an additional factor influencing IL-2 sensing in the tumor micro-environment. It is plausible that IL-2R/Treg based IL-2 consumption mechanisms are also relevant to human disease, as large numbers of Tregs [58], as well as high expression of IL-2Rα by Tregs [59], have been observed in human cancers.
Conclusions and future directions
As discussed above, a number of factors in the TME can regulate the spatial distribution and sensing of cytokines within tumors. Importantly, clear heterogeneity between tumors for at least part of these variables has been observed, making it likely that cytokine spreading after T-cell activation will vary between individual tumors. For example, the amount and molecular structure of ECM components, such as galectin-3, heparin and HS molecules, varies strongly between tumors, depending on their genetic make-up, degree of hypoxia, nutrient availability, and cellular infiltrate [60–62]. Additionally, variation in cytokine consumption potential may be inferred from the widely differing Treg numbers in human tumors [58, 63]. Moreover, the levels and types of proteolytic enzymes, including TACE, have been demonstrated to differ substantially between tumors [64, 65], likely influencing the kinetics of cytokine degradation, receptor shedding or, in case of TACE, skewing signaling towards either TNFR1 or TNFR2. We note that it is conceivable that cancer treatments may also influence the degree of cytokine spreading. As one example, high levels of PS are expressed on the outer membrane of dying cells, potentially reducing the diffusion rate of IFNγ after cytotoxic therapies.
Which information would help to better understand the rules governing cytokine spreading in human cancers? An area of research that has only recently started to gain significant attention, is the relative contribution of the different mechanism that may regulate cytokine spreading and sensing [66]. An important source of information in such efforts may be the use of spatially resolved transcriptomics and proteomics, which would potentially allow one to measure the spatial relationship between cytokine-producing and sensing cells, and the effect of different parameters on this relationship. Finally, understanding how cytokine containment and spreading within local TMEs may be controlled will be necessary to optimally exploit this axis in therapeutic strategies.
Highlights.
-
-
The spatiotemporal behavior of IFNγ, TNFα, and IL-2 determines their effects in tumors
-
-
Secretion mode, half-life, clearance mechanisms, and feedback loops influence signal distribution
-
-
Tumors display variation in the factors that control cytokine spreading
Acknowledgements
The authors would like to thank M. Logtenberg, A. van der Leun, L. Kok, and F. Dijkgraaf for insightful discussions. This work was supported by ERC AdG SENSIT (grant agreement No 742259) to T.N.S.
Footnotes
Declarations of interest: none
Recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- Hoekstra ME, et al. Long-distance modulation of bystander tumor cells by CD8(+) T cell-secreted IFNgamma. Nat Cancer. 2020;1(3):291–301. doi: 10.1038/s43018-020-0036-4. [• Using a GAS based IFNγ sensing reporter and multiday intravital imaging of tumors in mice, this paper reveals sensing of CD8+ T-cell secreted IFNγ over large distances in tumor masses. The observed long-range sensing of IFNγ is also shown to modify the behavior of antigen-negative tumor cells, as demonstrated by both induction of PD-L1 expression and inhibition of tumor growth.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaut R, et al. Bystander IFN-gamma activity promotes widespread and sustained cytokine signaling altering the tumor microenvironment. Nat Cancer. 2020;1(3):302–314. doi: 10.1038/s43018-020-0038-2. [• Using intravital imaging and a reporter for STAT1 translocation, this paper demonstrates that CD8+ T-cell derived IFNγ diffuses extensively in mouse tumors to alter the tumor microenvironment in distant areas. Additionally, single-cell RNA-sequencing data from melanoma patients provide evidence that IFNγR signaling also may occur in bystander cells in human tumors.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyler-Yaniv J, et al. Catch and Release of Cytokines Mediated by Tumor Phosphatidylserine Converts Transient Exposure into Long-Lived Inflammation. Mol Cell. 2017;66(5):635–647.:e7. doi: 10.1016/j.molcel.2017.05.011. [•• Combining mathematical modeling with various experimental approaches including a mouse model of thyroid cancer, this paper demonstrates that IFNγ is captured by phosphatidylserine on the surface of viable tumor cells in vivo, followed by its slow release to drive prolonged transcription of IFNγ responsive genes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Alonso M, et al. Galectin-3 captures interferon-gamma in the tumor matrix reducing chemokine gradient production and T-cell tumor infiltration. Nat Commun. 2017;8(1):793. doi: 10.1038/s41467-017-00925-6. [•• This paper reveals that the extracellular matrix protein galectin-3 binds IFNγ and reduces its diffusion through the tumor matrix in vivo.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyler-Yaniv A, et al. A Tunable Diffusion-Consumption Mechanism of Cytokine Propagation Enables Plasticity in Cell-to-Cell Communication in the Immune System. Immunity. 2017;46(4):609–620. doi: 10.1016/j.immuni.2017.03.011. [•• Using the combination of mathematical modeling and in vitro and in vivo assays, this study demonstrates that the spatial reach of CD4+ T-cell derived IL-2 in lymph nodes is primarily governed by the local density of IL-2 consuming cells. These data suggest that IL-2 penetration in tissues is primarily regulated by a diffusion-consumption mechanism.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altan-Bonnet G, Mukherjee R. Cytokine-mediated communication: a quantitative appraisal of immune complexity. Nat Rev Immunol. 2019;19(4):205–217. doi: 10.1038/s41577-019-0131-x. [•• This review provides a comprehensive overview of recent efforts in the systems biology field to quantitatively understand cytokine-mediated communication.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LE, Troeberg L. Heparan sulfate as a regulator of inflammation and immunity. J Leukoc Biol. 2019;105(1):81–92. doi: 10.1002/JLB.3RU0618-246R. [• This review covers the role of heparin sulfate in regulation of immune responses, including its role in regulating the activity of IFNγ and IL-2 in the extracellular matrix.] [DOI] [PubMed] [Google Scholar]
References
- 1.Durgeau A, et al. Recent Advances in Targeting CD8 T-Cell Immunity for More Effective Cancer Immunotherapy. Front Immunol. 2018;9:14. doi: 10.3389/fimmu.2018.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hadrup S, Donia M, Thor Straten P. Effector CD4 and CD8 T cells and their role in the tumor microenvironment. Cancer Microenviron. 2013;6(2):123–33. doi: 10.1007/s12307-012-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castro F, et al. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front Immunol. 2018;9:847. doi: 10.3389/fimmu.2018.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montfort A, et al. The TNF Paradox in Cancer Progression and Immunotherapy. Front Immunol. 2019;10:1818. doi: 10.3389/fimmu.2019.01818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim SO, et al. Deubiquitination and Stabilization of PD-L1 by CSN5. Cancer Cell. 2016;30(6):925–939. doi: 10.1016/j.ccell.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaretsky JM, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med. 2016;375(9):819–29. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin DS, et al. Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer Discov. 2017;7(2):188–201. doi: 10.1158/2159-8290.CD-16-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sucker A, et al. Acquired IFNgamma resistance impairs anti-tumor immunity and gives rise to T-cell-resistant melanoma lesions. Nat Commun. 2017;8:15440. doi: 10.1038/ncomms15440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao J, et al. Loss of IFN-gamma Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell. 2016;167(2):397–404.:e9. doi: 10.1016/j.cell.2016.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertrand F, et al. TNFalpha blockade overcomes resistance to anti-PD-1 in experimental melanoma. Nat Commun. 2017;8(1):2256. doi: 10.1038/s41467-017-02358-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vredevoogd DW, et al. Augmenting Immunotherapy Impact by Lowering Tumor TNF Cytotoxicity Threshold. Cell. 2019;178(3):585–599.:e15. doi: 10.1016/j.cell.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Spolski R, Li P, Leonard WJ. Biology and regulation of IL-2: from molecular mechanisms to human therapy. Nat Rev Immunol. 2018;18(10):648–659. doi: 10.1038/s41577-018-0046-y. [DOI] [PubMed] [Google Scholar]
- 13.Kammertoens T, et al. Tumour ischaemia by interferon-gamma resembles physiological blood vessel regression. Nature. 2017;545(7652):98–102. doi: 10.1038/nature22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grasso CS, et al. Conserved Interferon-gamma Signaling Drives Clinical Response to Immune Checkpoint Blockade Therapy in Melanoma. Cancer Cell. 2020 doi: 10.1016/j.ccell.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck RJ, Slagter M, Beltman JB. Contact-Dependent Killing by Cytotoxic T Lymphocytes Is Insufficient for EL4 Tumor Regression In Vivo. Cancer Res. 2019;79(13):3406–3416. doi: 10.1158/0008-5472.CAN-18-3147. [DOI] [PubMed] [Google Scholar]
- 16.Ariotti S, et al. T cell memory. Skin-resident memory CD8(+) T cells trigger a state of tissue-wide pathogen alert. Science. 2014;346(6205):101–5. doi: 10.1126/science.1254803. [DOI] [PubMed] [Google Scholar]
- 17.Schenkel JM, et al. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346(6205):98–101. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perona-Wright G, Mohrs K, Mohrs M. Sustained signaling by canonical helper T cell cytokines throughout the reactive lymph node. Nat Immunol. 2010;11(6):520–6. doi: 10.1038/ni.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller AJ, et al. CD4+ T cells rely on a cytokine gradient to control intracellular pathogens beyond sites of antigen presentation. Immunity. 2012;37(1):147–57. doi: 10.1016/j.immuni.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Braumuller H, et al. T-helper-1-cell cytokines drive cancer into senescence. Nature. 2013;494(7437):361–5. doi: 10.1038/nature11824. [DOI] [PubMed] [Google Scholar]
- 21.Thibaut R, et al. Bystander IFN-gamma activity promotes widespread and sustained cytokine signaling altering the tumor microenvironment. Nat Cancer. 2020;1(3):302–314. doi: 10.1038/s43018-020-0038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoekstra ME, et al. Long-distance modulation of bystander tumor cells by CD8(+) T cell-secreted IFNgamma. Nat Cancer. 2020;1(3):291–301. doi: 10.1038/s43018-020-0036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slifka MK, Rodriguez F, Whitton JL. Rapid on/off cycling of cytokine production by virus-specific CD8+ T cells. Nature. 1999;401(6748):76–9. doi: 10.1038/43454. [DOI] [PubMed] [Google Scholar]
- 24.Sanderson NS, et al. Cytotoxic immunological synapses do not restrict the action of interferon-gamma to antigenic target cells. Proc Natl Acad Sci U S A. 2012;109(20):7835–40. doi: 10.1073/pnas.1116058109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huse M, et al. T cells use two directionally distinct pathways for cytokine secretion. Nat Immunol. 2006;7(3):247–55. doi: 10.1038/ni1304. [DOI] [PubMed] [Google Scholar]
- 26.Puntel M, et al. Identification and visualization of CD8+ T cell mediated IFN-gamma signaling in target cells during an antiviral immune response in the brain. PLoS One. 2011;6(8):e23523. doi: 10.1371/journal.pone.0023523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shirey KA, et al. Upregulation of IFN-gamma receptor expression by proinflammatory cytokines influences IDO activation in epithelial cells. J Interferon Cytokine Res. 2006;26(1):53–62. doi: 10.1089/jir.2006.26.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen C, et al. Modulation of IFN-gamma receptor 1 expression by AP-2alpha influences IFN-gamma sensitivity of cancer cells. Am J Pathol. 2012;180(2):661–71. doi: 10.1016/j.ajpath.2011.10.040. [DOI] [PubMed] [Google Scholar]
- 29.Oyler-Yaniv J, et al. Catch and Release of Cytokines Mediated by Tumor Phosphatidylserine Converts Transient Exposure into Long-Lived Inflammation. Mol Cell. 2017;66(5):635–647.:e7. doi: 10.1016/j.molcel.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon-Alonso MA, Bruger M, van der Bruggen P. Extracellular galectins as controllers of cytokines in hematological cancer. Blood. 2018;132(5):484–491. doi: 10.1182/blood-2018-04-846014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon-Alonso M, et al. Galectin-3 captures interferon-gamma in the tumor matrix reducing chemokine gradient production and T-cell tumor infiltration. Nat Commun. 2017;8(1):793. doi: 10.1038/s41467-017-00925-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saesen E, et al. Insights into the mechanism by which interferon-gamma basic amino acid clusters mediate protein binding to heparan sulfate. J Am Chem Soc. 2013;135(25):9384–90. doi: 10.1021/ja4000867. [DOI] [PubMed] [Google Scholar]
- 33.Collins LE, Troeberg L. Heparan sulfate as a regulator of inflammation and immunity. J Leukoc Biol. 2019;105(1):81–92. doi: 10.1002/JLB.3RU0618-246R. [DOI] [PubMed] [Google Scholar]
- 34.Lortat-Jacob H, Baltzer F, Grimaud JA. Heparin decreases the blood clearance of interferon-gamma and increases its activity by limiting the processing of its carboxyl-terminal sequence. J Biol Chem. 1996;271(27):16139–43. doi: 10.1074/jbc.271.27.16139. [DOI] [PubMed] [Google Scholar]
- 35.Lighvani AA, et al. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc Natl Acad Sci U S A. 2001;98(26):15137–42. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheper W, et al. Low and variable tumor reactivity of the intratumoral TCR repertoire in human cancers. Nat Med. 2019;25(1):89–94. doi: 10.1038/s41591-018-0266-5. [DOI] [PubMed] [Google Scholar]
- 37.Lugo-Villarino G, et al. T-bet is required for optimal production of IFN-gamma and antigen-specific T cell activation by dendritic cells. Proc Natl Acad Sci U S A. 2003;100(13):7749–54. doi: 10.1073/pnas.1332767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horiuchi T, et al. Transmembrane TNF-alpha: structure, function and interaction with anti-TNF agents. Rheumatology (Oxford) 2010;49(7):1215–28. doi: 10.1093/rheumatology/keq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salerno F, et al. Distinct PKC-mediated posttranscriptional events set cytokine production kinetics in CD8(+) T cells. Proc Natl Acad Sci U S A. 2017;114(36):9677–9682. doi: 10.1073/pnas.1704227114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ebsen H, et al. Differential surface expression of ADAM10 and ADAM17 on human T lymphocytes and tumor cells. PLoS One. 2013;8(10):e76853. doi: 10.1371/journal.pone.0076853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Schie KA, et al. Therapeutic TNF Inhibitors can Differentially Stabilize Trimeric TNF by Inhibiting Monomer Exchange. Sci Rep. 2016;6:32747. doi: 10.1038/srep32747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Josephs SF, et al. Unleashing endogenous TNF-alpha as a cancer immunotherapeutic. J Transl Med. 2018;16(1):242. doi: 10.1186/s12967-018-1611-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tufvesson E, Westergren-Thorsson G. Tumour necrosis factor-alpha interacts with biglycan and decorin. FEBS Lett. 2002;530(1-3):124–8. doi: 10.1016/s0014-5793(02)03439-7. [DOI] [PubMed] [Google Scholar]
- 44.Alon R, et al. TNF-alpha binds to the N-terminal domain of fibronectin and augments the beta 1-integrin-mediated adhesion of CD4+ T lymphocytes to the glycoprotein. J Immunol. 1994;152(3):1304–13. [PubMed] [Google Scholar]
- 45.Hershkoviz R, Goldkorn I, Lider O. Tumour necrosis factor-alpha interacts with laminin and functions as a pro-adhesive cytokine. Immunology. 1995;85(1):125–30. [PMC free article] [PubMed] [Google Scholar]
- 46.Deng M, et al. Shedding of the tumor necrosis factor (TNF) receptor from the surface of hepatocytes during sepsis limits inflammation through cGMP signaling. Sci Signal. 2015;8(361):ra11. doi: 10.1126/scisignal.2005548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lantz M, et al. Characterization in vitro of a human tumor necrosis factor-binding protein. A soluble form of a tumor necrosis factor receptor. J Clin Invest. 1990;86(5):1396–1402. doi: 10.1172/JCI114853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xanthoulea S, et al. Tumor necrosis factor (TNF) receptor shedding controls thresholds of innate immune activation that balance opposing TNF functions in infectious and inflammatory diseases. J Exp Med. 2004;200(3):367–76. doi: 10.1084/jem.20040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pettersson T, et al. Setting up TRAPS. Ann Med. 2012;44(2):109–18. doi: 10.3109/07853890.2010.548399. [DOI] [PubMed] [Google Scholar]
- 50.Wrangle JM, et al. IL-2 and Beyond in Cancer Immunotherapy. J Interferon Cytokine Res. 2018;38(2):45–68. doi: 10.1089/jir.2017.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oyler-Yaniv A, et al. A Tunable Diffusion-Consumption Mechanism of Cytokine Propagation Enables Plasticity in Cell-to-Cell Communication in the Immune System. Immunity. 2017;46(4):609–620. doi: 10.1016/j.immuni.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Busse D, et al. Competing feedback loops shape IL-2 signaling between helper and regulatory T lymphocytes in cellular microenvironments. Proc Natl Acad Sci U S A. 2010;107(7):3058–63. doi: 10.1073/pnas.0812851107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Damoiseaux J. The IL-2-IL-2 receptor pathway in health and disease: The role of the soluble IL-2 receptor. Clin Immunol. 2020;218:108515. doi: 10.1016/j.clim.2020.108515. [DOI] [PubMed] [Google Scholar]
- 54.Sheu BC, et al. A novel role of metalloproteinase in cancer-mediated immunosuppression. Cancer Res. 2001;61(1):237–42. [PubMed] [Google Scholar]
- 55.Villarino AV, et al. Helper T cell IL-2 production is limited by negative feedback and STAT-dependent cytokine signals. J Exp Med. 2007;204(1):65–71. doi: 10.1084/jem.20061198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Somasundaram R, et al. Collagens serve as an extracellular store of bioactive interleukin 2. J Biol Chem. 2000;275(49):38170–5. doi: 10.1074/jbc.M006616200. [DOI] [PubMed] [Google Scholar]
- 57.Wrenshall LE, Platt JL. Regulation of T cell homeostasis by heparan sulfate-bound IL-2. J Immunol. 1999;163(7):3793–800. [PubMed] [Google Scholar]
- 58.Magnuson AM, et al. Identification and validation of a tumor-infiltrating Treg transcriptional signature conserved across species and tumor types. Proc Natl Acad Sci U S A. 2018;115(45):E10672–E10681. doi: 10.1073/pnas.1810580115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun Z, et al. A next-generation tumor-targeting IL-2 preferentially promotes tumor-infiltrating CD8(+) T-cell response and effective tumor control. Nat Commun. 2019;10(1):3874. doi: 10.1038/s41467-019-11782-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nagarajan A, Malvi P, Wajapeyee N. Heparan Sulfate and Heparan Sulfate Proteoglycans in Cancer Initiation and Progression. Front Endocrinol (Lausanne) 2018;9:483. doi: 10.3389/fendo.2018.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cardoso AC, et al. Galectin-3 Determines Tumor Cell Adaptive Strategies in Stressed Tumor Microenvironments. Front Oncol. 2016;6:127. doi: 10.3389/fonc.2016.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ruvolo PP. Galectin 3 as a guardian of the tumor microenvironment. Biochim Biophys Acta. 2016;1863(3):427–437. doi: 10.1016/j.bbamcr.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 63.Bates GJ, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24(34):5373–80. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 64.Kornfeld JW, et al. Overexpression of TACE and TIMP3 mRNA in head and neck cancer: association with tumour development and progression. Br J Cancer. 2011;104(1):138–45. doi: 10.1038/sj.bjc.6606017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gobin E, et al. A pan-cancer perspective of matrix metalloproteases (MMP) gene expression profile and their diagnostic/prognostic potential. BMC Cancer. 2019;19(1):581. doi: 10.1186/s12885-019-5768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Altan-Bonnet G, Mukherjee R. Cytokine-mediated communication: a quantitative appraisal of immune complexity. Nat Rev Immunol. 2019;19(4):205–217. doi: 10.1038/s41577-019-0131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]