Abstract
Conversion of biological feedstocks into value-added chemicals is mostly performed via microbial fermentation. An emerging alternative approach is the use of cell-free systems, consisting of purified enzymes and cofactors. Unfortunately, the in vivo and in vitro communities rarely interact, which leads to oversimplifications and exaggerations that do not permit fair comparison of the two strategies and impede synergistic interactions. Here, we provide a comprehensive account for the advantages and drawbacks associated with each strategy, and further discuss recent research efforts that aim to breach the limits of cellular and cell-free production. We also explore emerging hybrid solutions that integrate the benefits of both worlds and could expand the boundaries of biosynthesis.
Keywords: metabolic engineering, synthetic biology, pathway reconstruction, metabolic modularity, metabolic optimization
Introduction
Microbial organisms have been used for the production of food and chemicals since the dawn of civilization. Initially, this was a rather simple task. Once a microorganism that naturally converts a substrate into a product was identified and fermentation conditions were optimized, a biosynthesis process could be established [1]. However, this simplified approach is only able to support the production of a limited number of compounds – e.g., ethanol, acetone and butanol – and only from a limited range of feedstocks, mostly sugars. The emergence of genetic and metabolic engineering has dramatically expanded the limits of bioproduction but also led to new challenges [1]. It is not trivial to modify an organism to utilize a substrate it usually cannot, or produce a compound it naturally does not. To address these challenges, deep understanding of cellular metabolism and sophisticated engineering tools are required. Even though our ability to rewire microbial metabolism has vastly improved in the last years, we are still far from resolving all barriers associated with cellular bioproduction.
While the metabolic engineering field is traditionally centered on cell-based production, cell-free systems have emerged as an alternative approach. In these systems, purified enzymes and cofactors are mixed together to support the conversion of a substrate into a product [2–4]. Cell-free production originates from the so called ‘biocatalysis’ field, where a single or a few enzymes support the conversion of a commercially available compound into a specialized chemical that cannot be easily obtained by pure chemical synthesis [5,6]. With advances in this field, it has become possible to construct more complex networks, featuring many components, and capable of carrying out intricate metabolic conversions. Complete central metabolic pathways – including glycolysis [7,8], the pentose phosphate pathway [9], the TCA cycle [10], and fatty acid biosynthesis [11] – were already reconstructed in vitro from individual components. Cell-free systems were also shown to produce various chemicals such as ethanol, isobutanol [12], molecular hydrogen [9], polyhydroxybutyrate [13], and monoterpenes [8]. Even synthetic pathways that do not exist in nature, including alternative sugar fermentation [12–14], complete sugar oxidation [15,16] carbon fixation [17], and C1 assimilation [18,19] have successfully been established. In addition, major advances in cell-free protein synthesis have been made in recent years, for which we refer the reader to other reviews [20,21].
With few exceptions, the in vivo and in vitro communities do not interact much with each other. Each community tends to dismiss the other and focus on the advantages unique to its own approach. This leads to some oversimplifications and exaggerations that do not permit fair comparison of the two strategies and actually impede synergistic interactions. Here, we delve into the characteristics of engineering cellular and cell-free systems with the aim to identify the key advantages of each approach. The advantages of each approach are portrayed in Figure 1 and are exemplified in Figure 2 for the synthesis of isobutanol (as elaborated below). We further discuss the potential of combining the benefits of both strategies to resolve current limitations in bioproduction.
Figure 1. Key advantages of cellular and cell-free bioproduction.
Advantages connected to both strategies exemplify features that can be at least partially obtained in both cellular and cell-free systems.
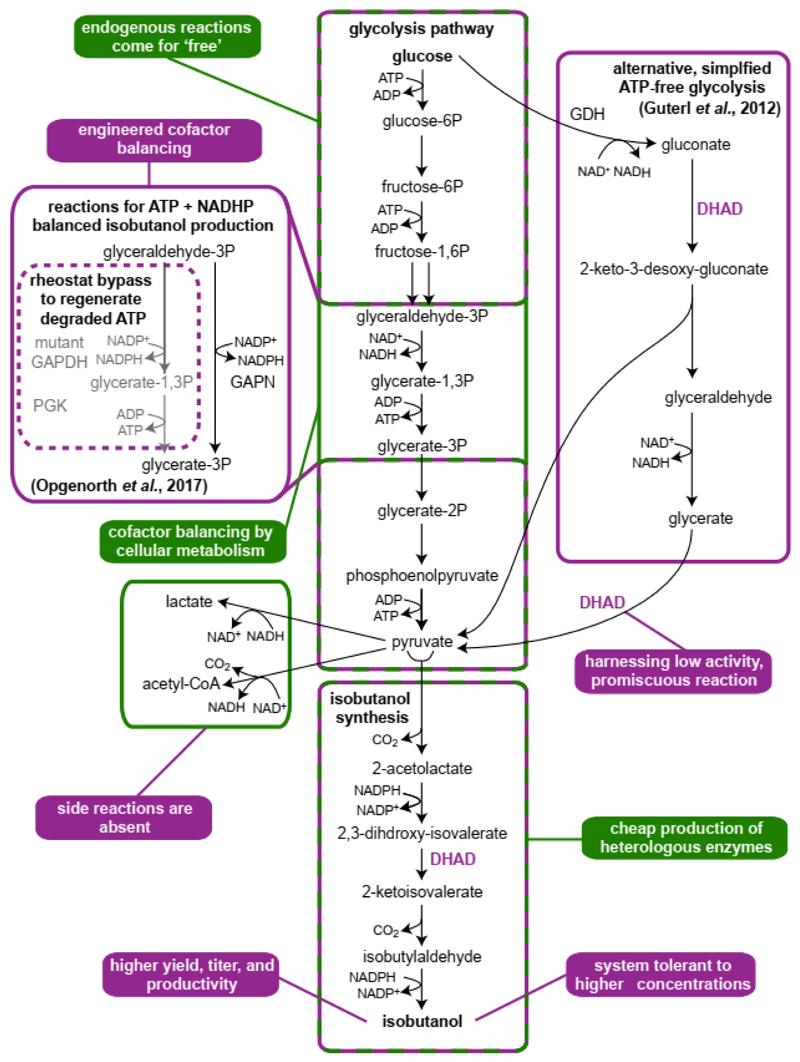
Figure 2. Isobutanol production exemplifies advantages of cellular and cell-free approaches.
Key advantages and pathways are color-marked green for cellular production and purple for cell-free production. Isobutanol production in engineered in vivo in E. coli reaches a titer of 23 g/L and 86% of the theoretical yield [78]. The first in vitro demonstration of isobutanol production was based on an ATP-independent glycolysis pathway, including the promiscuous gluconate and glycerate dehydrogenase activity of di-hydroxy-acid dehydratase (DHAD) [12]. A more recent in vitro system achieved dynamic cofactor balancing by a glycolytic ‘rheostat’ that combines a non-phosphorylating, NADPH-regenerating GapN with a mutant GapDH that can regenerate NADPH while generating ATP [31]. This design enabled dynamic balancing of cofactor consumption and regeneration during isobutanol production and supported a high isobutanol titer of 24 g/L at 91.5% theoretical yield. While isobutanol toxicity was a major limitation of cellular production, in vitro production could sustain higher titer with little adversary effects. However, the cell-free system is stable for 48 days due to enzyme inactivation, emphasizing the advantage of cellular production, in which the catalysts are produced at low cost.
The case for cellular production and how cell-free is catching up
The most prominent benefit of living cells is the low production costs of pathway components, which becomes crucial when scaling up a process for industrial application. The production of necessary enzymes and cofactors is practically free in a living cell, while a bottom-up reconstruction of an in vitro system requires the dedicated production and (often) purification of the involved components. The cost of enzymes and cofactors is still a major limitation for the economic feasibility of in vitro systems [2–4]. Yet, as the cost of enzyme production decreases and various techniques are enhancing enzyme lifetime – e.g., immobilization [22] – cell-free systems are becoming more affordable. While cofactor regeneration has become a common practice in in vitro systems [23] (see below), the initial addition of the (un-activated) cofactor still has a considerable price, which can easily surpass that of the proteins [2]. Aiming to address this challenge, cheaper and more stable cofactor analogs, as well as enzymes for their regeneration, are currently under development [24,25].
Many production pathways are imbalanced, leading to net production or consumption of ATP and/or NAD(P)H. Within the cell, these imbalances can be compensated by native metabolism [26] (e.g. the oxidative pentose phosphate pathway can be used to provide additional NADPH [27]), while an in vitro system will inevitably run into a dead-end. A cell-free set-up can address these challenges by the design and implementation of stoichiometrically-balanced, alternative pathways (e.g., ATP-balanced routes [8,12,13]) (Figure 2). However, even with balanced routes, unavoidable degradation of cofactors will occur, requiring an additional cofactor regeneration system. Fortunately, in vitro regeneration systems for redox cofactors (e.g., based on formate or phosphite) [28] and ATP (from poly-phosphate, phosphoenolpyruvate, or acetyl-phosphate) [17,29,30] have been demonstrated and optimized. Another important recent advancement is the development of strategies to dynamically control and maintain cofactor homeostasis in vitro, for example using a molecular ATP-rheostat [31] (Figure 2) and NAD(P)H purge valves that include NADH oxidase [8,13,32]. Still, even with effective regeneration systems in place, cofactor stability poses still an issue for long-time operation of in vitro systems.
An inherent advantage of cellular systems is that a substantial segment of a bioproduction pathway comes for free as an integral part of endogenous metabolism. Consider, for example, bioconversion of glucose to isobutanol (Figure 2). In most microbial hosts, the upstream part of the pathway – metabolism of glucose to pyruvate – is already established without the need of further manipulation and only the downstream segment – pyruvate conversion to isobutanol – requires dedicated engineering. In cell-free approaches, on the other hand, the entire bioconversion cascade needs to be established de novo. To alleviate this problem, in vitro systems can be designed to use alternative routes that operate with fewer components than natural pathways. For example, several studies have demonstrated the replacement of glycolysis with alternative synthetic routes that require fewer enzymes for sugar metabolism [12,33] (Figure 2). Alternatively, in vitro systems can be based on cell lysates, which provide a large part of the endogenous metabolism, as discussed below.
Cellular metabolism also offers protection of the pathway components, which is harder to achieve in cell-free systems. For example, many proteins (e.g., containing Fe-S centers), as well as cofactors (e.g., tetrahydrofolate), are sensitive to oxygen and lose activity when purified and used under aerobic conditions [34,35]. Yet, the same components are quite stable within the protective cellular environment. Furthermore, cellular chaperone systems are able to stabilize and even refold proteins, which would otherwise quickly lose structure and activity [36]. Although chaperones [37,38] and enzymes that prevent oxidative stress (e.g. catalase to degrade H2O2) [8,17] can be applied in vitro, the cellular environment seems better buffered from perturbations. On the other hand, cellular homeostasis can also have deleterious effects by limiting the operational range of metabolic pathways, as discussed in detail below.
Another inherent advantage of cellular systems is compartmentalization, the key advantage of which is ATP production via chemiosmosis, supported either by a respiratory electron transport chain or by electron bifurcation mechanisms coupled with proton-pumping (e.g., Rnf and Ech) [39]. Furthermore, compartmentalization can serve to isolate parts of the bioproduction pathway, thus limiting exposure to a reactive compound (e.g., confining small reactive aldehydes [40]) and reducing unwanted promiscuous activities of pathway enzymes (i.e., physically separating enzymes from their secondary substrates). Accordingly, achieving compartmentalization has become a key goal in cell-free efforts. Recent studies have demonstrated the reconstruction of the chemiosmosis apparatus within artificial membranes for the production of ATP [41,42]. Others reported the in vitro engineering of lipid vesicles [43], protein microcompartments [44,45], and other forms of compartmentalization and scaffolding [46].
Finally, within cellular systems it is possible to directly couple the activity of a synthetic pathway with microbial growth. This is especially true for the engineering of new routes for substrate utilization, but can occasionally also be achieved for production pathways [47,48]. Such coupling can be highly beneficial as it opens the door for adaptive laboratory evolution to optimize the newly introduced activities [49–52]. Yet, if pathway activity cannot be coupled to growth, evolution becomes a foe rather than a friend: the introduction of a synthetic pathway will lower microbial fitness and impose a selective pressure to silence its activity [53]. In these cases, in vitro reconstructed pathways provide higher stability.
The case for cell-free production and how cellular systems compare to it
There are aspects in which a cell-free approach clearly outperforms living microbes. Primary among these is the ability to precisely control the composition - i.e. concentrations of enzymes and cofactors - of an in vitro system. This enables the use of computational tools to accurately simulate pathway kinetics, thus supporting model-driven characterization and optimization of pathway activity [8,9,31,54]. Such precise control of system composition is very difficult to achieve within the cellular environment, thus rendering computational models less useful.
Another major advantage is that cell-free systems can support higher yields and productivities than microbial cells [55,56]. This is attributed to several factors, including complete conversion of substrate to products without loss to biomass, better use of available volume (the entire reactor volume rather than the limited cell volume), and the lack of membrane barriers. This latter aspect is especially important as cellular bio-catalysis is often limited by substrate uptake and product excretion [3]. Purifying intracellular products (e.g. polyhydroxybutyrate) from the cell can present another major hurdle [3]. On the other hand, excretion of soluble products by microorganisms allows for simpler downstream processing, as their separation from the cells is easier than from a mixture of enzymes, cofactors and metabolites (as in a cell-free system).
Another key advantage of cell-free systems is that unwanted or even deleterious metabolic interactions are reduced to a minimum. In vivo pathway implementation almost always leads to an overlap between the newly introduced pathway and the host endogenous metabolic network, which are likely to share at least a few metabolites. This overlap can severely disrupt cellular metabolism and its regulation by establishing new metabolic connections between metabolites and pathways [1,57]. Equally important, such overlap could divert flux from the synthetic pathway towards other metabolic routes and thus repress its activity. Accordingly, one of the main advantages of in vitro pathway reconstruction relates to the insulation of the synthetic route from other metabolic processes.
Still, cell-free systems, just like living cells, can suffer from deleterious side reactions that direct flux towards non-productive routes. While enzymes unrelated to pathway activity are indeed absent, the enzymes of the pathway itself can catalyze competing reactions. An example of this was encountered during the in vitro establishment of the CETCH cycle, a synthetic carbon-fixing pathway, where methylmalyl-CoA lyase – catalyzing an essential pathway reaction – was found to generate the dead-end metabolite malyl-CoA [17]. This necessitated the addition of an acyl-CoA hydrolase enzyme as a metabolic repair tool. The more complex a synthetic pathway is, the higher is the probability that such metabolic repair mechanisms will be needed. Yet, these side reactions are easier to detect and circumvent in in vitro systems when compared to in vivo approaches.
Cell-free production is generally more tolerant towards inhibition imposed by pathway substrates, metabolites, and products [3,8,12]. This is mainly since in vitro systems do not contain many of the cellular components that are especially sensitive to these compounds. For example, higher tolerance to an alcohol product can be directly ascribed to the lack of membranes [3,12]. Yet, as discussed above, the cellular environment is especially suitable to neutralize inhibitory byproducts that might dangerously accumulate in in vitro systems.
Another benefit of cell-free systems is the broad range of metabolite concentrations they can tolerate. In the cellular environment, metabolite concentrations are restricted due to (i) thermodynamics and kinetics, e.g. when concentrations of metabolites must be kept at especially low or high concentration to enable cellular conversions unrelated to the synthetic pathway [58]; (ii) physicochemical properties of metabolites, e.g., when reactivity (e.g., small aldehydes) or hydrophobicity (e.g., nucleic acids) of pathway metabolites results in low cellular concentrations [59]; and (iii) endogenous regulation, e.g., when the concentration of a metabolite has a strong effect on many other metabolic pathways and enzymes [1]; NAD(P)(H), ATP and flux-signaling metabolites such as fructose 1,6-bisphosphate [60] are prime examples of such regulatory metabolites. As in vitro systems are free from such constraints, their metabolites can reach concentrations which would be unrealistic in vivo, thus supporting otherwise infeasible pathways. For example, the synthetic formolase pathway, which was demonstrated in vitro to condense three formaldehyde molecules into dihydroxyacetone [19], is hard to realize in vivo: to operate this pathway, a high concentration of formaldehyde is required (in the mM range), which is highly challenging due to the reactivity and toxicity of this small aldehyde.
Cell-free systems can operate across a wider range of conditions (e.g. pH, ionic strength, temperature, or light) to allow for metabolic transformations that are not possible in cellular systems. For example, at neutral pH, the reduction potential of carboxylic acids to aldehydes is much lower than that of the primary cellular electron carriers (e.g., NAD(P)), requiring activation of the acid (via ATP-consuming kinases or CoA ligases) before it can be reduced. Yet, the reduction potential changes with pH and, for carboxylic acids, it sharply increases with a decreasing pH. Specifically, at pH<5 carboxylic acids have sufficiently high reduction potential to hypothetically enable their direct reduction by NAD(P)H without costly activation [61]. Hence, working at low pH could support higher pathway efficiency, due to reduced ATP costs.
No living organism is known to have such an acidic intracellular environment, while such a working pH could be potentially realized in a cell-free system. Still, confirming activity and stability of enzymes, cofactors, and metabolites under these conditions is challenging. For example, while CoA is quite stable at pH 2-4 [62], NADH and NADPH are unstable under similar conditions [63] and would need to be replaced by tolerant cofactors such as ferredoxins [64]. Several synthetic variants of NAD were synthesized and shown to be accepted by oxidoreductrase enzymes [24,25]; some of these might also be stable under conditions which NAD cannot tolerate [65].
Furthermore, non-physiological, high ionic strength can enhance bioproduction, as was demonstrated for the in vitro production of amorpha-3,11-diene, where high monovalent ion concentrations enhanced the activity of the key enzyme amorpha-3,11-diene synthase [66]. Cell-free systems can also operate at higher temperatures than most living organisms, supporting increased biocatalytic rates [67,68]. A specific advantage of high temperature in vitro systems is that the thermophilic enzymes can be obtained from heterologous expression in a mesophilic host (e.g. E. coli) without the need for expensive purification: heating the lysate denatures competing native, mesophilic enzymes [67,68].
Within in vitro systems, promiscuous activities of enzymes, which would otherwise be negligible, can be put into productive use due to the absence of competing natural substrates. A good example is the use of dihydroxy-acid dehydratase for the dehydration of the non-natural substrates glycerate and gluconate within a synthetic sugar fermentation pathway [12] (Figure 2). This reaction is unlikely to proceed at a sufficient rate in vivo, where multiple competing di-hydroxy-acid metabolites are available, but within an insulated system it can become a key pathway component. Another example are oxidoreductase enzymes that accept both NAD and NADP; while the cofactor utilization of such enzymes cannot be easily controlled within a cellular environment, a cell-free system lacking one of these cofactors would ensure high activity with the other.
Another potential advantage of cell-free systems is that they allow for fast, modular assembly and testing of pathways. Multiple homologues for each pathway enzyme can be tested without the need to reengineer organisms, allowing for rapid screening and iterative optimization. The CETCH carbon fixation cycle highlights these benefits as it was assembled by combining enzymes from nine different organisms from all domains of life, including three engineered enzymes [17]. The construction of this complex pathway was facilitated by in vitro testing and optimization of its parts using simple analytical read-out methods to find bottlenecks and unwanted side-reactions.
However, modular engineering is not really unique to in vitro systems. Recent developments in in vivo metabolic engineering also include pathway modularization and easy read-outs of module activity based on growth. Multiple studies demonstrate the division of pathways into metabolic modules, the activity of which is selected for in dedicated gene deletion strains whose growth depends on module flux [49,69–71]. This approach provides a simple readout of module activity – no growth, poor growth, or sufficient growth – that can be directly applied to modify and optimize module performance.
Combining the strengths of cell-free and cellular systems
As mentioned above, the major drawbacks of cell-free systems relate to the need of enzyme synthesis, supply of cofactors, and costly scale-up for industrial production. On the other hand, cellular systems suffer from limitations in control of expression levels and reaction conditions, as well as from deleterious overlaps with native metabolism. These drawbacks hamper rapid prototyping of pathways and enzyme variants. It could therefore be beneficial for synthetic pathways to be designed, tested and optimized first in cell-free systems and subsequently transplanted into living cells for further optimization via laboratory evolution and eventually up-scaling. Such workflow was demonstrated in the establishment of a synthetic non-oxidative glycolytic pathway [52] and was further used to improve farnasene production in E. coli [72].
A hybrid cellular / cell-free system could incorporate the advantages of both approaches. For example, a lysate of E. coli expressing only three heterologous enzymes enabled the production of 2,3-butanediol from glucose at near theoretical yield and high titer (>80 g/L) and productivity (11 g/L/h) [73]. Other studies used several E. coli strains, each expressing a different pathway enzyme, which were lysed to provide crude cell extracts [74,75]. Combinatorial assembly of the lysates allowed accurate control of the concentration of pathway enzymes and further enabled fast screening of many enzyme and pathway variants. The availability of the natural glycolytic enzymes and essential cofactors (e.g., CoA) in the lysates, further supported by cofactor balancing via the cellular machinery, dramatically reduced the need for external supplements. Such hybrid approach might therefore be highly efficient for future bioproduction.
Another promising way for integration involves the division of a metabolic process between cellular and cell-free systems, where each pathway segment is implemented using the system that can best support its activity. For example, cell-free enzymatic degradation of cellulose to glucose is commonly coupled – sometimes in the same reactor – with microbial glucose fermentation to produce ethanol [76,77].
Conclusions
It is not surprising that our detailed analysis did not result in a clear verdict of which system is superior. As we have shown, there are many overlapping aspects that need to be considered when deciding which approach is expected to be most suitable for a given need. Given the high costs that are still associated with producing the components of a cell-free system, it is likely that, in most cases, cellular bioproduction provides the better alternative. However, this situation may soon change with the advance of the in vitro field and development of new approaches to decrease protein and cofactor costs and extend their lifetime. It is quite plausible that in a short time we will actually face a real dilemma of which system to employ. In this case, it is important to approach the decision without prejudice and with an open mind. In some cases, as we discussed here, an optimal solution might be found is combining cellular and cell-free systems, paving the way for numerous new production possibilities.
Highlights.
Bioproduction is currently predominantly supported by living microorganisms
Cell-free systems provide an emerging alternative for bioproduction
Cellular and cell-free systems have distinct advantages and drawbacks
Combining cellular and cell-free systems could have substantial benefits
Acknowledgements
The authors thank Charlie Cotton for critical reading of the manuscript. This work is supported by funding from the Max Planck Society, by the German Ministry of Education and Research Grant FormatPlant (part of BioEconomy 2030, Plant Breeding Research for the Bioeconomy) and the European Research Council Grant ERC 637675 (“SYBORG”). NJC is supported by The Netherlands Organisation for Scientific Research (NWO) through a Rubicon Grant (Project 019.163LW.035).
References
- 1.Nielsen J, Keasling JD. Engineering Cellular Metabolism. Cell. 2016;164:1185–1197. doi: 10.1016/j.cell.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Rollin JA, Tam TK, Zhang YHP. New biotechnology paradigm: Cell-free biosystems for biomanufacturing. Green Chem. 2013;15:1708–1719. [Google Scholar]
- 3.Dudley QM, Karim AS, Jewett MC. Cell-free metabolic engineering: Biomanufacturing beyond the cell. Biotechnol J. 2015;10:69–82. doi: 10.1002/biot.201400330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilding KM, Schinn SM, Long EA, Bundy BC. The emerging impact of cell-free chemical biosynthesis. Curr Opin Biotechnol. 2018;53:115–121. doi: 10.1016/j.copbio.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 5.Sheldon RA, Woodley JM. Role of Biocatalysis in Sustainable Chemistry. Chem Rev. 2018;118:801–838. doi: 10.1021/acs.chemrev.7b00203. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez S, Demain AL. Enzymes and bioconversions of industrial, pharmaceutical, and biotechnological significance. Org Process Res Dev. 2011;15:224–230. [Google Scholar]
- 7.Krutsakorn B, Honda K, Ye X, Imagawa T, Bei X, Okano K, Ohtake H. In vitro production of n-butanol from glucose. Metab Eng. 2013;20:84–91. doi: 10.1016/j.ymben.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Korman TP, Opgenorth PH, Bowie JU. A synthetic biochemistry platform for cell free production of monoterpenes from glucose. Nat Commun. 2017;8:1–8. doi: 10.1038/ncomms15526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rollin JA, Martin del Campo J, Myung S, Sun F, You C, Bakovic A, Castro R, Chandrayan SK, Wu C-H, Adams MWW, et al. High-yield hydrogen production from biomass by in vitro metabolic engineering: Mixed sugars coutilization and kinetic modeling. Proc Natl Acad Sci. 2015;112:4964–4969. doi: 10.1073/pnas.1417719112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sokic-Lazic D, Minteer SD. Pyruvate/Air Enzymatic Biofuel Cell Capable of Complete Oxidation. Electrochem Solid-State Lett. 2009;12:F26. [Google Scholar]
- 11.Yu X, Liu T, Zhu F, Khosla C. In vitro reconstitution and steady-state analysis of the fatty acid synthase from Escherichia coli . Proc Natl Acad Sci. 2011;108:18643–18648. doi: 10.1073/pnas.1110852108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guterl JK, Garbe D, Carsten J, Steffler F, Sommer B, Reiße S, Philipp A, Haack M, Rühmann B, Koltermann A, et al. Cell-free metabolic engineering: Production of chemicals by minimized reaction cascades. ChemSusChem. 2012;5:2165–2172. doi: 10.1002/cssc.201200365. [DOI] [PubMed] [Google Scholar]
- 13.Opgenorth PH, Korman TP, Bowie JU. A synthetic biochemistry module for production of bio-based chemicals from glucose. Nat Chem Biol. 2016;12:393–395. doi: 10.1038/nchembio.2062. [DOI] [PubMed] [Google Scholar]
- 14.Bogorad IW, Lin T-S, Liao JC. Synthetic non-oxidative glycolysis enables complete carbon conservation. Nature. 2013;502:693–697. doi: 10.1038/nature12575. [DOI] [PubMed] [Google Scholar]
- 15.Xu S, Minteer SD. Enzymatic biofuel cell for oxidation of glucose to CO2 . ACS Catal. 2012;2:91–94. [Google Scholar]
- 16.Zhu Z, Zhang YHP. In vitro metabolic engineering of bioelectricity generation by the complete oxidation of glucose. Metab Eng. 2017;39:110–116. doi: 10.1016/j.ymben.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Schwander T, Schada von Borzyskowski L, Burgener S, Cortina NS, Erb TJ. A synthetic pathway for the fixation of carbon dioxide in vitro . Science. 2016;354:900–904. doi: 10.1126/science.aah5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bogorad IW, Chen C, Theisen MK, Wu T, Schlenz AR, Lam AT. Building carbon - carbon bonds using a biocatalytic methanol condensation cycle. Proc Natl Acad Sci U S A. 2014;111:15928–15933. doi: 10.1073/pnas.1413470111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegel JB, Smith AL, Poust S, Wargacki AJ, Bar-Even A, Louw C, Shen BW, Eiben CB, Tran HM, Noor E, et al. Computational protein design enables a novel one-carbon assimilation pathway. Proc Natl Acad Sci. 2015;112:3704–3709. doi: 10.1073/pnas.1500545112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoborg JA, Jewett MC. Synthetic Biology: Parts, Devices and Applications. Wiley-VCH; 2018. Cell-free protein synthesis: An emerging technology for understanding, harnessing, and expanding the capabilities of biological systems; pp. 309–330. [Google Scholar]
- 21.Zemella A, Thoring L, Hoffmeister C, Kubick S. Cell-Free Protein Synthesis: Pros and Cons of Prokaryotic and Eukaryotic Systems. ChemBioChem. 2015;16:2420–2431. doi: 10.1002/cbic.201500340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grimaldi J, Collins CH, Belfort G. Toward cell-free biofuel production: Stable immobilization of oligomeric enzymes. Biotechnol Prog. 2014;30:324–331. doi: 10.1002/btpr.1876. [DOI] [PubMed] [Google Scholar]
- 23.Zhao H, Van Der Donk WA. Regeneration of cofactors for use in biocatalysis. Curr Opin Biotechnol. 2003;14:583–589. doi: 10.1016/j.copbio.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Nowak C, Beer B, Pick A, Roth T, Lommes P. A water-forming NADH oxidase from Lactobacillus pentosus suitable for the regeneration of synthetic biomimetic cofactors. Front Microbiol. 2015;6:957. doi: 10.3389/fmicb.2015.00957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nowak C, Pick A, Lommes P, Sieber V. Enzymatic Reduction of Nicotinamide Biomimetic Cofactors Using an Engineered Glucose Dehydrogenase: Providing a Regeneration System for Artificial Cofactors. ACS Catal. 2017;7:5202–5208. [Google Scholar]
- 26.Chen Z, Liu D. Toward glycerol biorefinery: metabolic engineering for the production of biofuels and chemicals from glycerol. Biotechnol Biofuels. 2016;9 doi: 10.1186/s13068-016-0625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aslan S, Noor E, Bar-Even A. Holistic bioengineering: rewiring central metabolism for enhanced bioproduction. Biochem J. 2017;474:3935–3950. doi: 10.1042/BCJ20170377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Der Donk WA, Zhao H. Recent developments in pyridine nucleotide regeneration. Curr Opin Biotechnol. 2003;14:421–426. doi: 10.1016/s0958-1669(03)00094-6. [DOI] [PubMed] [Google Scholar]
- 29.Mordhorst S, Siegrist J, Müller M, Richter M, Andexer JN. Catalytic Alkylation Using a Cyclic S-Adenosylmethionine Regeneration System. Angew Chemie - Int Ed. 2017;56:4037–4041. doi: 10.1002/anie.201611038. [DOI] [PubMed] [Google Scholar]
- 30.Alissandratos A, Caron K, Loan TD, Hennessy JE, Easton CJ. ATP Recycling with Cell Lysate for Enzyme-Catalyzed Chemical Synthesis, Protein Expression and PCR. ACS Chem Biol. 2016;11:3289–3293. doi: 10.1021/acschembio.6b00838. [DOI] [PubMed] [Google Scholar]
- 31.Opgenorth PH, Korman TP, Iancu L, Bowie JU. A molecular rheostat maintains ATP levels to drive a synthetic biochemistry system. Nat Chem Biol. 2017;13:938–942. doi: 10.1038/nchembio.2418. [DOI] [PubMed] [Google Scholar]
- 32.Opgenorth PH, Korman TP, Bowie JU. A synthetic biochemistry molecular purge valve module that maintains redox balance. Nat Commun. 2014;5 doi: 10.1038/ncomms5113. [DOI] [PubMed] [Google Scholar]
- 33.Xu S, Minteer SD. Enzymatic biofuel cell for oxidation of glucose to CO2. ACS Catal. 2012;2:91–94. [Google Scholar]
- 34.Reed LS, Archer MC. Oxidation of Tetrahydrofolic Acid by Air. J Agric Food Chem. 1980;28:801–805. [Google Scholar]
- 35.van Vugt-Lussenburg BMA, van der Weel L, Hagen WR, Hagedoorn PL. Biochemical Similarities and Differences between the Catalytic [4Fe-4S] Cluster Containing Fumarases FumA and FumB from Escherichia coli . PLoS One. 2013;8 doi: 10.1371/journal.pone.0055549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hartl FU. Unfolding the chaperone story. Mol Biol Cell. 2018;28:2919–2923. doi: 10.1091/mbc.E17-07-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhat JY, MiliciC G, Thieulin-Pardo G, Bracher A, Maxwell A, Ciniawsky S, Mueller-Cajar O, Engen JR, Hartl FU, Wendler P, et al. Mechanism of Enzyme Repair by the AAA+ Chaperone Rubisco Activase. Mol Cell. 2017;67:744–756.:e6. doi: 10.1016/j.molcel.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Niwa T, Kanamori T, Ueda T, Taguchi H. Global analysis of chaperone effects using a reconstituted cell-free translation system. Proc Natl Acad Sci. 2012;109:8937–8942. doi: 10.1073/pnas.1201380109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schuchmann K, Müller V. Autotrophy at the thermodynamic limit of life: a model for energy conservation in acetogenic bacteria. Nat Rev Microbiol. 2014;12:809–821. doi: 10.1038/nrmicro3365. [DOI] [PubMed] [Google Scholar]
- 40.Kerfeld Ca, Erbilgin O. Bacterial microcompartments and the modular construction of microbial metabolism. Trends Microbiol. 2015;23:22–34. doi: 10.1016/j.tim.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Otrin L, Marušič N, Bednarz C, Vidaković-Koch T, Lieberwirth I, Landfester K, Sundmacher K. Toward Artificial Mitochondrion: Mimicking Oxidative Phosphorylation in Polymer and Hybrid Membranes. Nano Lett. 2017;17:6816–6821. doi: 10.1021/acs.nanolett.7b03093. [DOI] [PubMed] [Google Scholar]
- 42.Lee KY, Park SJ, Lee KA, Kim SH, Kim H, Meroz Y, Mahadevan L, Jung KH, Ahn TK, Parker KK, et al. Photosynthetic artificial organelles sustain and control ATP-dependent reactions in a protocellular system. Nat Biotechnol. 2018;36:530–535. doi: 10.1038/nbt.4140. [DOI] [PubMed] [Google Scholar]
- 43.Elani Y, Law RV, Ces O. Vesicle-based artificial cells as chemical microreactors with spatially segregated reaction pathways. Nat Commun. 2014;5 doi: 10.1038/ncomms6305. [DOI] [PubMed] [Google Scholar]
- 44.Frey R, Mantri S, Rocca M, Hilvert D. Bottom-up Construction of a Primordial Carboxysome Mimic. J Am Chem Soc. 2016;138:10072–10075. doi: 10.1021/jacs.6b04744. [DOI] [PubMed] [Google Scholar]
- 45.Hagen A, Sutter M, Sloan N, Kerfeld CA. Programmed loading and rapid purification of engineered bacterial microcompartment shells. Nat Commun. 2018;9 doi: 10.1038/s41467-018-05162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rabe KS, Müller J, Skoupi M, Niemeyer CM. Cascades in Compartments: En Route to Machine-Assisted Biotechnology. Angew Chemie - Int Ed. 2017;56:13574–13589. doi: 10.1002/anie.201703806. [DOI] [PubMed] [Google Scholar]
- 47.Du W, Jongbloets JA, Van Boxtel C, Pineda Hernández H, Lips D, Oliver BG, Hellingwerf KJ, Branco Dos Santos F. Alignment of microbial fitness with engineered product formation: Obligatory coupling between acetate production and photoautotrophic growth. Biotechnol Biofuels. 2018;11 doi: 10.1186/s13068-018-1037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klamt S, Mahadevan R. On the feasibility of growth-coupled product synthesis in microbial strains. Metab Eng. 2015;30:166–178. doi: 10.1016/j.ymben.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 49.Bouzon M, Perret A, Loreau O, Delmas V, Perchat N, Weissenbach J, Taran F, Marliè Re P. A Synthetic Alternative to Canonical One-Carbon Metabolism. 2017;6:1520–1533. doi: 10.1021/acssynbio.7b00029. [DOI] [PubMed] [Google Scholar]
- 50.Döring V, Darii E, Yishai O, Bar-Even A, Bouzon M. Implementation of a reductive route of one-carbon assimilation in Escherichia coli through directed evolution. ACS Synth Biol. 2018;7:2029–2036. doi: 10.1021/acssynbio.8b00167. [DOI] [PubMed] [Google Scholar]
- 51.Meyer F, Keller P, Hartl J, Gröninger OG, Kiefer P, Vorholt JA. Methanol-essential growth of Escherichia coli . Nat Commun. 2018;9 doi: 10.1038/s41467-018-03937-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin PP, Jaeger AJ, Wu T-Y, Xu SC, Lee AS, Gao F, Chen P-W, Liao JC. Construction and evolution of an Escherichia coli strain relying on nonoxidative glycolysis for sugar catabolism. Proc Natl Acad Sci. 2018;115:3538–3546. doi: 10.1073/pnas.1802191115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Renda BA, Hammerling MJ, Barrick JE. Engineering reduced evolutionary potential for synthetic biology. Mol Biosyst. 2014;10:1668–1678. doi: 10.1039/c3mb70606k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hold C, Billerbeck S, Panke S. Forward design of a complex enzyme cascade reaction. Nat Commun. 2016;7 doi: 10.1038/ncomms12971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang YHP. Production of biofuels and biochemicals by in vitro synthetic biosystems: Opportunities and challenges. Biotechnol Adv. 2015;33:1467–1483. doi: 10.1016/j.biotechadv.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 56.Beer B, Pick A, Sieber V. In vitro metabolic engineering for the production of α-ketoglutarate. Metab Eng. 2017;40:5–13. doi: 10.1016/j.ymben.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 57.Bar-Even A. Formate assimilation: The metabolic architecture of natural and synthetic pathways. Biochemistry. 2016;55:3851–3863. doi: 10.1021/acs.biochem.6b00495. [DOI] [PubMed] [Google Scholar]
- 58.Noor E, Bar-Even A, Flamholz A, Reznik E, Liebermeister W, Milo R. Pathway thermodynamics highlights kinetic obstacles in central metabolism. PLoS Comput Biol. 2014;10 doi: 10.1371/journal.pcbi.1003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bar-Even A, Noor E, Flamholz A, Buescher JM, Milo R. Hydrophobicity and charge shape cellular metabolite concentrations. PLoS Comput Biol. 2011;7 doi: 10.1371/journal.pcbi.1002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kochanowski K, Volkmer B, Gerosa L, Haverkorn van Rijsewijk BR, Schmidt A, Heinemann M. Functioning of a metabolic flux sensor in Escherichia coli . Proc Natl Acad Sci. 2013;110:1130–1135. doi: 10.1073/pnas.1202582110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bar-Even A, Flamholz A, Noor E, Milo R. Thermodynamic constraints shape the structure of carbon fixation pathways. Biochim Biophys Acta. 2012;1817:1646–1659. doi: 10.1016/j.bbabio.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 62.Buyske DA, Handschumacher RE, Schilling ED, Strong FM. The Stability of Coenzyme A. J Am Chem Soc. 1954;76:3575–3577. [Google Scholar]
- 63.Lilius EM, Multanen VM, Toivonen V. Quantitative extraction and estimation of intracellular nicotinamide nucleotides of Escherichia coli . Anal Biochem. 1979;99:22–27. doi: 10.1016/0003-2697(79)90039-3. [DOI] [PubMed] [Google Scholar]
- 64.Siebers B, Schönheit P. Unusual pathways and enzymes of central carbohydrate metabolism in Archaea. Curr Opin Microbiol. 2005;8:695–705. doi: 10.1016/j.mib.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 65.Lo HC, Fish RH. Biomimetic NAD+ models for tandem cofactor regeneration, horse liver alcohol dehydrogenase recognition of 1,4-NADH derivatives, and chiral synthesis. Angew Chemie Int Ed. 2002;41:478–481. doi: 10.1002/1521-3773(20020201)41:3<478::aid-anie478>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 66.Chen X, Zhang C, Zou R, Zhou K, Stephanopoulos G, Too HP. Statistical experimental design guided optimization of a one-pot biphasic multienzyme total synthesis of amorpha-4,11-diene. PLoS One. 2013;8 doi: 10.1371/journal.pone.0079650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Honda K, Kimura K, Ninh PH, Taniguchi H, Okano K, Ohtake H. In vitro bioconversion of chitin to pyruvate with thermophilic enzymes. J Biosci Bioeng. 2017;124:296–301. doi: 10.1016/j.jbiosc.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 68.Taniguchi H, Okano K, Honda K. Modules for in vitro metabolic engineering: Pathway assembly for bio-based production of value-added chemicals. Synths Syst Biotechnol. 2017;2:65–74. doi: 10.1016/j.synbio.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wenk S, Yishai O, Lindner SN. An Engineering Approach for Rewiring Microbial Metabolism. Methods Enzymol. 2018;608:329–367. doi: 10.1016/bs.mie.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 70.Yishai O, Bouzon M, Döring V, Bar-Even A. In vivo assimilation of one-carbon via a synthetic reductive glycine pathway in Escherichia coli . ACS Synth Biol. 2018;7:2023–2028. doi: 10.1021/acssynbio.8b00131. [DOI] [PubMed] [Google Scholar]
- 71.He H, Muth CE, Lindner SN, Bar-even A. Ribulose monophosphate shunt provides nearly all biomass and energy required for growth of E. coli . ACS Synth Biol. 2018;7:1601–1611. doi: 10.1021/acssynbio.8b00093. [DOI] [PubMed] [Google Scholar]
- 72.Zhu F, Zhong X, Hu M, Lu L, Deng Z, Liu T. In vitro reconstitution of mevalonate pathway and targeted engineering of farnesene overproduction in Escherichia coli . Biotechnol Bioeng. 2014;111:1396–1405. doi: 10.1002/bit.25198. [DOI] [PubMed] [Google Scholar]
- 73.Kay JE, Jewett MC. Lysate of engineered Escherichia coli supports high-level conversion of glucose to 2,3-butanediol. Metab Eng. 2015;32:133–142. doi: 10.1016/j.ymben.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 74.Karim AS, Jewett MC. A cell-free framework for rapid biosynthetic pathway prototyping and enzyme discovery. Metab Eng. 2016;36:116–126. doi: 10.1016/j.ymben.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 75.Dudley QM, Anderson KC, Jewett MC. Cell-Free Mixing of Escherichia coli Crude Extracts to Prototype and Rationally Engineer High-Titer Mevalonate Synthesis. ACS Synth Biol. 2016;5:1578–1588. doi: 10.1021/acssynbio.6b00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.You C, Chen H, Myung S, Sathitsuksanoh N, Ma H, Zhang X-Z, Li J, Zhang Y-HP. Enzymatic transformation of nonfood biomass to starch. Proc Natl Acad Sci. 2013;110:7182–7187. doi: 10.1073/pnas.1302420110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liao JC, Mi L, Pontrelli S, Luo S. Fuelling the future: microbial engineering for the production of sustainable biofuels. Nat Rev Microbiol. 2016;14:288–304. doi: 10.1038/nrmicro.2016.32. [DOI] [PubMed] [Google Scholar]
- 78.Atsumi S, Hanai T, Liao JC. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature. 2008;451:86–89. doi: 10.1038/nature06450. [DOI] [PubMed] [Google Scholar]
Highlighted references
- ●●.Guterl JK, Garbe D, Carsten J, Steffler F, Sommer B, Reiβe S, Philipp A, Haack M, Rühmann B, Koltermann A, et al. Cell-free metabolic engineering: Production of chemicals by minimized reaction cascades. ChemSusChem. 2012;5:2165–2172. doi: 10.1002/cssc.201200365. [ In vitro demonstration of a synthetic, short, ATP-independent fermentation route for ethanol and isobutanol production. The route includes thermophilic enzymes and relies on promiscuous activity of glycerate dehydratase to react with gluconate] [DOI] [PubMed] [Google Scholar]
- ●.Korman TP, Opgenorth PH, Bowie JU. A synthetic biochemistry platform for cell free production of monoterpenes from glucose. Nat Commun. 2017;8:1–8. doi: 10.1038/ncomms15526. [Cell-free efficient conversion of glucose into monoterpenes at titers an order of magnitude above cellular toxicity levels. The system harbors 27 enzymes and includes a NAD(P)H purge valve consisting of both NADPH and NADH forming glyceraldehyde-3-phosphate dehydrogenase and NADH oxidase to dynamically regulate NAD(P)H concentrations] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ●.Schwander T, Schada von Borzyskowski L, Burgener S, Cortina NS, Erb TJ. A synthetic pathway for the fixation of carbon dioxide in vitro . Science (80-) 2016;354:900–904. doi: 10.1126/science.aah5237. [ In vitro demonstration of the synthetic CETCH cycle for CO2 fixation, consisting of 17 enzymes from 9 different organisms from all domains of life, including a polyphosphate-driven ATP regeneration system, H2O2 detoxification and a proofreading enzyme to correct the formation of a dead-end metabolite] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ●●.Dudley QM, Anderson KC, Jewett MC. Cell-Free Mixing of Escherichia coli Crude Extracts to Prototype and Rationally Engineer High-Titer Mevalonate Synthesis. ACS Synth Biol. 2016;5:1578–1588. doi: 10.1021/acssynbio.6b00154. [First demonstration of mixing lysates from strains expressing individual heterologous pathway enzymes, which allowed for testing different enzyme variants and concentrations, while the glycolytic enzymes as well as cofactors and their regeneration system were available ‘for free’ in the lysate] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ●●.Opgenorth PH, Korman TP, Iancu L, Bowie JU. A molecular rheostat maintains ATP levels to drive a synthetic biochemistry system. Nat Chem Biol. 2017;13:938–942. doi: 10.1038/nchembio.2418. [Efficient cell-free system for the production of isobutanol at concentrations unprecendented in cellular and cell-free systems, supported by two complementary glyceraldehyde phosphate dehydrogenases, ATP-generating and non-ATP-generating, to dynamically balance ATP concentrations] [DOI] [PubMed] [Google Scholar]
- ●.Lee KY, Park SJ, Lee KA, Kim SH, Kim H, Meroz Y, Mahadevan L, Jung KH, Ahn TK, Parker KK, et al. Photosynthetic artificial organelles sustain and control ATP-dependent reactions in a protocellular system. Nat Biotechnol. 2018;36:530–535. doi: 10.1038/nbt.4140. [ In vitro reconstitution of two membrane-integrated photosystems and ATP synthase in small lipid vessicles encapsulated in a giant vessicle; the light-controlled ATP regeneration drives CO2 fixation by pyruvate carboxylase and actin polymerization] [DOI] [PubMed] [Google Scholar]
- ●.Meyer F, Keller P, Hartl J, Gröninger OG, Kiefer P, Vorholt JA. Methanol-essential growth of Escherichia coli. Nat Commun. 2018;9 doi: 10.1038/s41467-018-03937-y. [ In vivo engineering of the ribulose monophosphate pathway by coupling growth to methanol consumption in an E. coli selection strain, which, after laboratory evolution, led to partial growth on methanol] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ●.Yishai O, Bouzon M, Döring V, Bar-Even A. In vivo assimilation of one-carbon via a synthetic reductive glycine pathway in Escherichia coli. ACS Synth Biol. 2018;7:2023–2028. doi: 10.1021/acssynbio.8b00131. [Cell-based engineering of a synthetic, highly efficient formate assimilation pathway by testing and optimization in different pathway modules in dedicated selection strains. The synthetic route includes the reversed activity of the native glycine cleavage system, catalyzing glycine synthesis from CO2 and methylene-THF] [DOI] [PubMed] [Google Scholar]
- ●.Lin PP, Jaeger AJ, Wu T-Y, Xu SC, Lee AS, Gao F, Chen P-W, Liao JC. Construction and evolution of an Escherichia coli strain relying on nonoxidative glycolysis for sugar catabolism. Proc Natl Acad Sci. 2018;115:3538–3546. doi: 10.1073/pnas.1802191115. [Combination of rational engineering and laboratory evolution to generate a novel glycolysis pathway converting glucose to three acetyl-CoA instead of two, which was demonstrated previously in vitro and now completely replacing native glycolysis in vivo ] [DOI] [PMC free article] [PubMed] [Google Scholar]