Abstract
Background & aims
With the introduction of DAA’s, the majority of treated chronic hepatitis C patients (CHC) achieve a viral cure. The exact mechanisms by which the virus is cleared after successful therapy, is still unknown. The aim was to assess the role of the immune system and miRNA levels in acquiring a sustained virological response after DAA treatment in CHC patients with and without prior RG-101 (antimiR-122) dosing.
Methods
In this multicenter, investigator-initiated study, 29 patients with hepatitis C virus (HCV) genotype 1 (n = 11), 3 (n = 17), or 4 (n = 1) infection were treated with sofosbuvir and daclatasvir ± ribavirin. 18 patients were previously treated with RG-101. IP-10 levels were measured by ELISA. Ex vivo HCV-specific T cell responses were quantified in IFN-γ-ELISpot assays. Plasma levels of miR-122 were measured by qPCR.
Results
All patients had an SVR12. IP-10 levels rapidly declined during treatment, but were still elevated 24 weeks after treatment as compared to healthy controls (median 53.82 and 39.4 pg/mL, p = 0.02). Functional IFN-γ HCV-specific T cell responses did not change by week 12 of follow-up (77.5 versus 125 SFU/106 PBMC, p = 0.46). At follow-up week 12, there was no difference in plasma miR-122 levels between healthy controls and patients with and without prior RG-101 dosing.
Conclusions
Our data shows that successful treatment of CHC patients with and without prior RG-101 dosing results in reduction of broad immune activation, and normalisation of miR-122 levels (EudraCT: 2014-002808-25).
Keywords: Chronic hepatitis C, DAA treatment, Immune response, miRNA-122
1. Introduction
Chronic hepatitis C virus (HCV) infection is a major public health problem affecting an estimated 170 million people worldwide(-Shepard et al., 2005). During chronic infection, plasma CXCL10 (or interferon gamma-induced protein 10, IP-10) levels are increased, reflecting a state of ongoing interferon-α signaling and prolonged immune activation(Meissner et al., 2014; Spaan et al., 2015). In addition, constant exposure to viral antigens results in so-called ‘exhausted’ virus-specific CD8+ T cells, T cells that have lost some or all of their effector functions(Bengsch et al., 2010; Klenerman and Hill, 2005). Functional virus-specific CD8+ T cells are crucial in viral clearance of acute HCV infection(Urbani et al., 2006). However, interferon-α induced clearance of chronic HCV infection is not associated with improvement of HCV-specific T cell function(Barnes et al., 2009). Current treatment regimens for chronic hepatitis C (CHC) patients include interferon-free combinations of direct acting antivirals (DAAs) which result in high sustained virological response (SVR) rates in the majority of patients. The exact mechanisms that lead to viral clearance in these patients treated with DAAs are not well known. Recent data suggests that the proliferative potential of exhausted T cells is improved after successful treatment with DAAs(Martin et al., 2014; Spaan et al., 2015). However, the extent of T cell restoration is unknown, as is the role of HCV-specific T cells in viral clearance after DAA treatment.
Inhibition of microRNA-122 (miR-122), an important host factor for replication of HCV, is an alternative therapeutic approach to clear HCV infection(Jopling et al., 2005; Lanford et al., 2010; van der Ree et al., 2017). Recently, it was shown that a single dose of RG-101, an N-acetylgalactosamine conjugated anti-miR-122 oligonucleotide, resulted in substantial viral load reduction in CHC patients, and in SVR for 76 weeks in 3 of 28 patients participating in a phase 1b study(van der Ree et al., 2017). Viral load reduction and eradication was not associated with restoration of HCV-specific T cell function(Stelma et al., 2017). Virological rebound following RG-101 dosing was associated with the emergence of resistance associated substitutions (RAS) in 5’ UTR miR-122 binding sites of the HCV genome(van der Ree et al., 2017). It is unknown if 5’ UTR C3U and C2G/C3U RAS persist and if these viruses are susceptible to DAAs in vivo. Furthermore, the effect of RG-101 and DAA treatment on plasma miR-122 levels has not been established.
The primary objective of this study was to analyse the impact of DAA treatment on immune activation and functionality of HCV-specific T cells in CHC patients with and without prior RG-101 dosing. The secondary objective was to assess treatment success, and circulating miR-122 levels prior and after DAA treatment.
2. Material and methods
2.1. Study design
In this investigator-initiated, open-label, multicenter study, we included CHC patients with genotype 1, 3, or 4 infection at two hospitals in the Netherlands; Academic Medical Center Amsterdam (AMC), and University Medical Center Groningen (UMCG). Patients with HCV genotype 1 and 4 were treated with sofosbuvir (SOF) and daclatasvir (DCV) for 12 weeks, and patients with HCV genotype 3 were treated with SOF, DCV and ribavirin (RBV) for 12 or 24 weeks (patients with cirrhosis were treated for 24 weeks) (Fig. S1). All patients were followed for 24 weeks after treatment cessation.
2.2. Patients
Twenty-nine CHC patients (n = 25 in AMC and n = 4 in UMCG) were included in this study. Of these, 18 patients had a virological rebound following RG-101 dosing (van der Ree et al., 2017), and were offered retreatment as part of this study. Only males and postmenopausal females were enrolled. Eligible patients were treatment-naïve (other than previous RG-101 dosing) or had previously had a relapse after antiviral therapy other than combination of SOF + NS5A inhibitor with or without ribavirin. Patients with coinfection (hepatitis B virus or human immunodeficiency virus infection), evidence of decompensated liver disease, or a history of HCC were excluded. Before enrolment and before any study procedure, written informed consent was obtained from all patients.
2.3. Study oversight
The study was approved by the independent ethics committee at each participating site (MEC AMC + UMCG), and was conducted in compliance with the Declaration of Helsinki, Good Clinical Practice guidelines, and local regulatory requirements. The trial was registered with EudraCT, number 2014-002808-25.
2.4. Study sampling
Plasma and heparinized peripheral blood samples were collected at various time points before (<90 days before start), during, and after treatment. PBMCs were isolated from heparinized blood using standard density gradient centrifugation and subsequently cryopreserved until the day of analysis.
2.5. Study assessments
2.5.1. Chemistry and viral assessments
Serum HCV RNA levels were measured using the Roche COBAS AmpliPrep/COBAS Taqman HCV v2.0 assay, with a reported lower limit of quantification (LLOQ) and lower limit of detection (LLOD) of 15 IU mL-1. Effective antiviral treatment was defined by undetectable HCV RNA levels 12 weeks after the cessation of antiviral therapy (SVR12). Safety assessment was based on adverse events reporting and laboratory testing of blood samples (e.g. clinical chemistry, hematology, coagulation).
2.5.2. Sequence analysis
Sequence analysis of the HCV 5’ UTR of HCV RNA was performed by 5’ rapid amplification of cDNA ends (5’ RACE System, version 2.0, Thermo Fisher Scientific, Waltham, MA, USA), followed by population-based sequencing and was done at baseline for patients with 5’UTR RAS in miR-122 binding sites at virological rebound in a previous phase 1b study(van der Ree et al., 2017).
2.5.3. Immunological assays
Plasma levels of IP-10 were measured with a DuoSet ELISA (R&D Systems, Minneapolis, MN, USA) with a lower limit of quantification of 62.5 pg mL-1. IFN-γ-ELISpot assays were performed ex vivo in duplicate at 2 × 105 PBMCs/well at the Peter Medawar Building for Pathogen Research, Oxford, UK. Thawed PBMC were rested overnight (37 °C + CO2) and were stimulated with panels of 15 mer peptides that overlapped by 11 amino acids corresponding to HCV genotypes 1a,1b, 3a, or 4a(Barnes et al., 2012; Kelly et al., 2015). The peptides were arranged into 10 pools, and each peptide was used at a final concentration of 3 μg mL-1. Internal controls were dimethyl sulfoxide (DMSO) (Sigma-Aldrich, UK) as a negative control and concanavalin A (Sigma-Aldrich, UK) as a positive control. Other antigens used were a pool of MHC class 1 restricted epitopes of influenza A, EBV and CMV (BEI Resources, Manassas, VA, USA), and a lysate of CMV infected cells (Virusys Corp, Taneytown, MD, USA). Spot forming units (SFU) were calculated per 106 PBMC and background levels (responses in matched negative control wells) were subtracted. Positive responses were defined as (i) the mean of responses to a pool minus background being greater than 48 SFU/106 PBMC and (ii) the mean of responses to a well exceeding 3 × background. Background subtracted data is shown. The background level of 48 SFU/106 PBMC per pool was determined previously in 74 healthy controls, which was the mean + 3 standard deviation (SD)(Barnes et al., 2012).
2.5.4. Plasma miR-122
Plasma levels of miR-122 were measured by RT-qPCR at baseline and throughout the study period, and were compared to plasma miR-122 levels of healthy controls (n = 10) (Supporting Methods). Plasma miR-122 levels were normalised to mean levels of miR-425-5p and miR-103a-3p using the ΔCq method (=Cq miRNA of interest – Cq reference miRNA) and are presented as the log 10 2 -ΔCq(Schmittgen and Livak, 2008). The difference in miR-122 level between baseline and follow-up week 12 was calculated with the comparative Cq-method (= 2 -(ΔCq baseline -ΔCq FU week 12)) and expressed as the fold change level(Schmittgen and Livak, 2008).
2.6. Study objectives
The primary objective of this study was to analyse the impact of DAA treatment on immune activation and functionality of HCV-specific T cells in CHC patients with and without prior RG-101 dosing. The secondary objective was to assess treatment success, and circulating miR-122 levels prior and after DAA treatment.
2.7. Data and statistical analysis
The results are presented as median with interquartile range (IQR) or frequency with percentage. All HCV RNA levels were analysed after log 10 transformation. We tested for differences between study groups (CHC patients with and without prior RG-101 dosing, and healthy controls) using the Mann-Whitney U test, Wilcoxon test, and one-way ANOVA using SPSS (IBM SPSS Statistics for Windows, Version 23.0, Armonk, NY, USA) and GraphPad Prism Software (Version 7.0, La Jolla, CA, USA). Missing data and time points after patients had drop out were excluded from the analyses.
3. Results
3.1. DAA treatment is highly effective in patients with virological rebound following RG-101 dosing
Screening began on November 25, 2014, with the last patient enrolled on December 9, 2015. Of 30 patients screened, 29 were enrolled in the study (Fig. S1). In total, 22 patients (76%) were male, and most patients were infected with HCV genotype 3 (n = 17), followed by genotype 1 (n = 11), and genotype 4 (n = 1) (Table 1). Almost all patients (27/29, 93%) had fibrosis stage F3 or higher, which was assessed by liver elastography (Fibroscan) (Table 1).
Table 1. Baseline patient characteristics.
| RG-101 (N = 18) | No RG-101 (N = 11) | Total (N = 29) | |
|---|---|---|---|
| Male | 13 (72) | 9 (82) | 22 (76) |
| Weight | 83 (70–98) | 81 (73–91) | 81 (71–93) |
| HCV RNA level | 5.79 | 6.56 | 6.18 |
| (log 10 IU/mL) | (5.16–6.50) | (5.74–6.65) | (5.42–6.59) |
| HCV genotype | |||
| 1a | 9 (50) | 1 (9) | 10 (35) |
| 1b | 1 (6) | 0 | 1 (3) |
| 3 | 7 (39) | 10 (91) | 17 (59) |
| 4 | 1 (6) | 0 | 1 (3) |
| ALT level | 62 (32–85) | 120 (79–151) | 79 (46–123) |
| Fibroscan | 11.0 | 14 (11.9–22.6) | 12.0 |
| result (kPa) | (10.3–13.9) | (10.7–14.8) | |
| Fibrosis stage | |||
| F0-F1 | 2(11) | 0 | 2 (7) |
| F1-F2 | 0 | 0 | 0 |
| F2-F3 | 0 | 0 | 0 |
| F3-F4 | 12 (67) | 7 (64) | 19 (66) |
| F4 | 4(22) | 4 (26) | 8 (28) |
Data are given as median (IQR) or as frequency (percentage). Stage of fibrosis was determined by liver elastography (Fibroscan). Fibrosis score: F0-F1 (–7 kPa), F1-F2 (7–8.8 kPa), F2-F3 (8.9–9.4 kPa), F3-F4 (9.5–14.5 kPa) and F4 (≥14.6 kPa).
Eighteen patients (62%) included in this study had viral rebound following RG-101 treatment and started SOF + DCV ± RBV treatment after a median time of 26 weeks (IQR: 19—36 weeks). Six of these patients had C3U or C2G/C3U RAS in miR-122 binding regions at time of viral rebound and/or at start of SOF + DCV ± RBV treat-ment(van der Ree et al., 2017). In two of these patients, the persistence of 5’UTR RAS could not be assessed because no extra follow-up sample was available. Of the other four patients with 5’ UTR RAS at time of viral rebound following RG-101 dosing, muta-tion(s) had disappeared in 3 patients at start of treatment (range: 7–22 weeks), whereas the C3U mutation was still present in one patient at the start of treatment (12 weeks after identification) (Fig. S2).
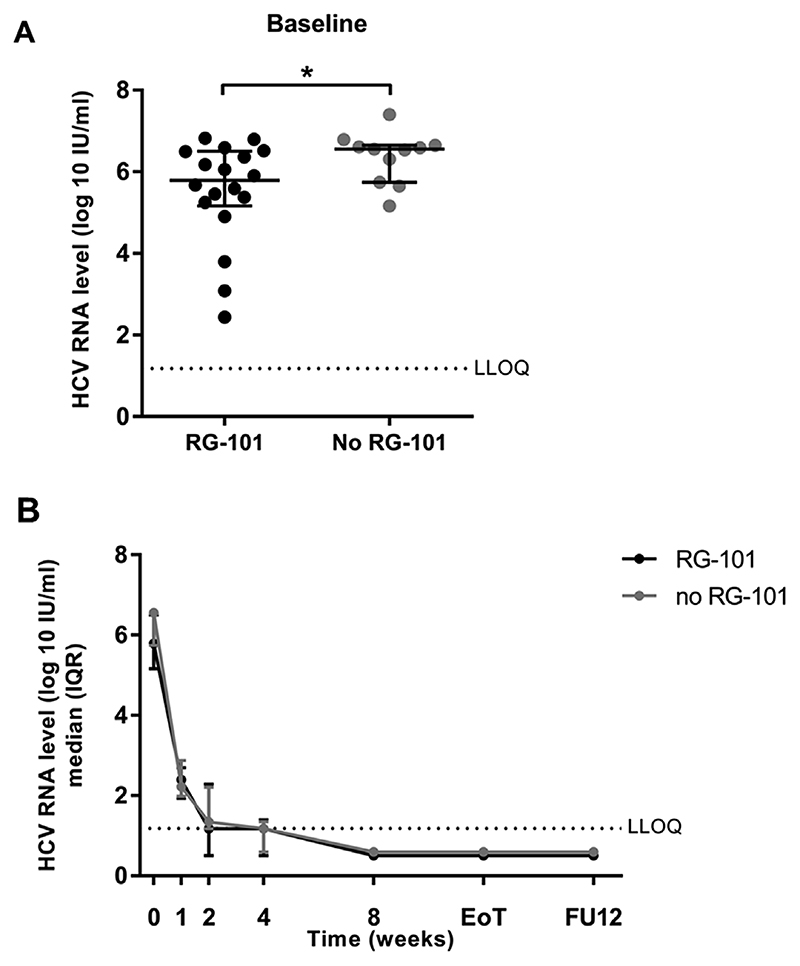
At baseline, median HCV RNA levels were lower in CHC patients who received RG-101 compared to those who did not (median: 5.79 (IQR: 5.16–6.50) log 10 IU mL-1 versus 6.56 (IQR: 5.74–6.65) log 10 IU mL-1, p = 0.043) (Fig. 1A). Median HCV RNA levels at week 1 and onwards did not differ between patients with and without prior RG-101 dosing (Fig. 1B). 26/29 (90%) patients achieved SVR12 according to treatment in the study protocol (intention to treat analysis). The remaining three patients achieved SVR12 outside the scope of this study. One HCV genotype 3 patient, fibrosis stage F3, was excluded at week 4 because of non-adherence to the study protocol and treatment was continued at the outpatient clinic resulting in SVR12. One cirrhotic HCV genotype 3 patient discontinued treatment at week 18 due to side effects (anaemia and peripheral neuropathy) and also achieved SVR12. In one patient with HCV genotype 1a, fibrosis stage F3, prior RG-101 treatment, SOF + DCV treatment was prolonged outside the scope of this study because of detectable HCV RNA levels at study week 8 and 12 (2.22 and 1.30 log 10 IU mL-1, respectively). This patient achieved SVR12 after 12 additional weeks of SOF + DCV treatment. These three patients were excluded from further analyses.
Fig. 1. HCV RNA levels of patients with and without previous RG-101 dosing.
(1A) HCV RNA level at baseline compared between patients with (black dots) and without (grey dots) previous RG-101 dosing, median and interquartile range are shown. Mann-Whitney U test was used to compare study groups. (1B) Median HCV RNA levels throughout the study period up to follow-up week 12, median and interquartile range are shown. LLOQ, lower limit of quantification. *p < 0.05.
3.2. DAA treatment is well tolerated and results in an improved Fibroscan result
None of the patients experienced a serious adverse event. 10/12 (83%) patients treated with SOF + DCV and 16/17 (94%) patients treated with SOF + DCV + RBV reported at least one adverse event (Table 2). The most frequent reported adverse events were fatigue (41%), headache (40%), insomnia (31%), nausea (21%), and dizziness (21%). The total number of adverse events was higher in patients who were treated with RBV (mean number of 6 AEs/patient) compared to those who were not (mean number of 3 AEs/patient). The dose of RBV was decreased from 1200 mg to 800 mg at week 8 in six patients due to anaemia or other side effects.
Table 2. Adverse events.
| SOF + DCV (N = 12) | SOF + DCV + RBV (N = 17) | Total (N = 29) | |
|---|---|---|---|
| Any adverse event | |||
| No. of events | 35 | 106 | 141 |
| No. of patients | 10 | 16 | 26 (90) |
| with event | |||
| Adverse events occurring in > 10% of patients | |||
| Agitation | 1 | 4 | 5 (17) |
| Anemia | 0 | 3 | 3 (10) |
| Anorexia | 1 | 2 | 3 (10) |
| Arthralgia | 2 | 1 | 3 (10) |
| Back pain | 1 | 2 | 3 (10) |
| Concentration | 3 | 1 | 4 (14) |
| impairment | |||
| Dizziness | 2 | 4 | 6 (21) |
| Dry mouth | 1 | 2 | 3 (10) |
| Dry skin | 1 | 3 | 4 (14) |
| Dyspnea | 0 | 3 | 3 (10) |
| Fatigue | 4 | 8 | 12 (41) |
| Flu like symptoms | 0 | 4 | 4 (14) |
| Headache | 3 | 8 | 11 (40) |
| Insomnia | 2 | 7 | 9(31) |
| Myalgia | 2 | 2 | 4 (14) |
| Nausea | 1 | 5 | 6 (21) |
| Skin rash | 1 | 4 | 5 (17) |
| Upper respiratory | 2 | 1 | 3 (10) |
| infection | |||
Data shown as number (%).
In 26/29 patients who completed the study, median ALT level normalised from 79 U L to 1 (IQR: 38–121) before treatment to 27 U L-1 (IQR: 19–38) 24 weeks after treatment, p < 0.0001 (Fig. S3). In 23 of these patients, a Fibroscan was performed before treatment and at follow-up week 24. A significant decline in Fibroscan result was observed after treatment from a median value of 12.0 kPa (IQR: 10.7–14.8) at baseline to a median of 8.1 kPa (IQR: 5.5–12.0) 24 weeks after treatment (p < 0.0001) (Fig. S3).
3.3. Normalisation of plasma IP-10 levels in CHC patients treated with DAAs
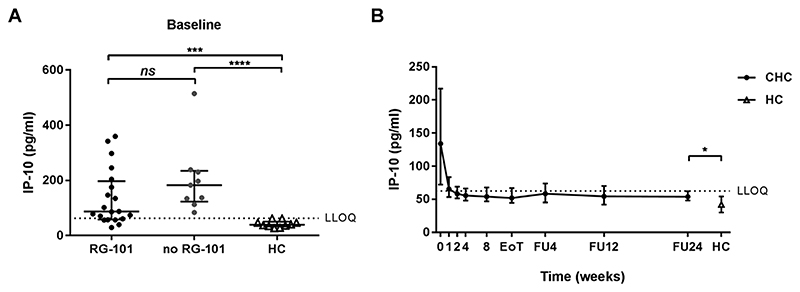
At baseline, IP-10 levels were significantly increased in CHC patients relative to healthy controls (median 123.3 pg mL-1 and 39.4 pg mL-1 respectively, p < 0.0001, Fig. 2A). No significant difference was observed in baseline IP-10 levels between CHC patients who had previously received RG-101 dosing and patients without prior RG-101 dosing (median 87.0 and 189.9 pg mL-1 respectively, p = 0.08, Fig. 2A). IP-10 levels significantly decreased upon DAA treatment. As early as 1 week after initiation of treatment, IP-10 levels had halved (from median 134.8 to 65.2 pg mL-1). At follow-up week 24, IP-10 levels in CHC patients who had cleared the virus were still increased relative to healthy controls (median 54.5 and 39.4 respectively, p = 0.02, Fig. 2B).
Fig. 2. IP-10 levels of patients with and without previous RG-101 dosing.
(2A) Baseline IP-10 levels in patients with (black dots) and without (grey dots) previous RG-101 dosing and healthy controls (HC, white triangles), median and interquartile range are shown. Mann-Whitney U test was used to compare study groups. (1B) IP-10 levels in CHC patient throughout the study period up to follow-up week 24 (FU24), median and interquartile range are shown. Mann-Whitney U test was used to compare study groups. LLOQ, lower limit of quantification. *p < 0,05; ***p < 0.001; ****p < 0,0001.
3.4. No change in HCV-specific T cell function after successful DAA treatment
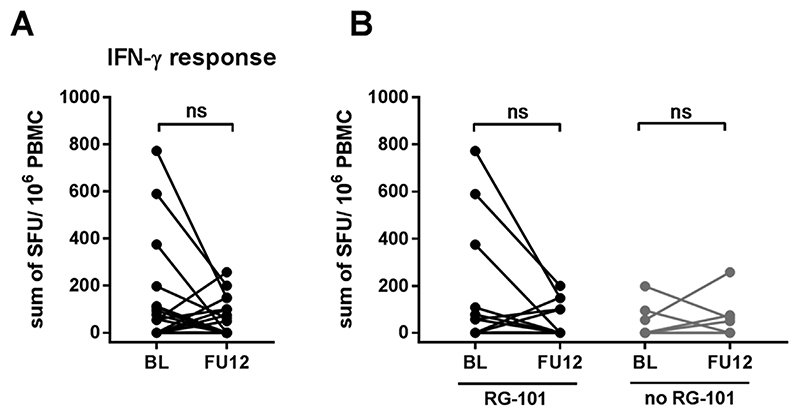
HCV-specific T cell responses at baseline were low in CHC patients (10/29 patients had a positive response to one or more HCV peptide pools in ELISpot assays). After successful treatment and clearance of HCV, there was no significant change in HCV-specific T cell responses (p = 0.09) (Fig. 3A). There were no significant changes in the IFN-γ T cell responses against structural or non-structural HCV proteins (data not shown). Furthermore, there was no difference in IFN-γ T cell response between baseline and followup in patients who were previously dosed with RG-101 (p = 0.20) nor in patients who were not previously treated with RG-101 (Fig. 3B).
Fig. 3. IFN-γ-ELISpot responses in CHC patients at baseline and follow-up week 12.
(3A) Sum of positive IFN-γ-ELISpot responses at baseline and follow-up week 12 (FU12) in CHC patients treated with SOF-DAC. Wilcoxon matched pairs test was used to compare baseline and FU12. (3B) Sum of positive IFN-γ-ELISpot responses in patients with (black dots) and without (grey dots) previous RG-101 dosing. Wilcoxon matched pairs test was used to compare baseline and FU12.
3.5. Normalisation of plasma miR-122 levels in successfully treated CHC patients
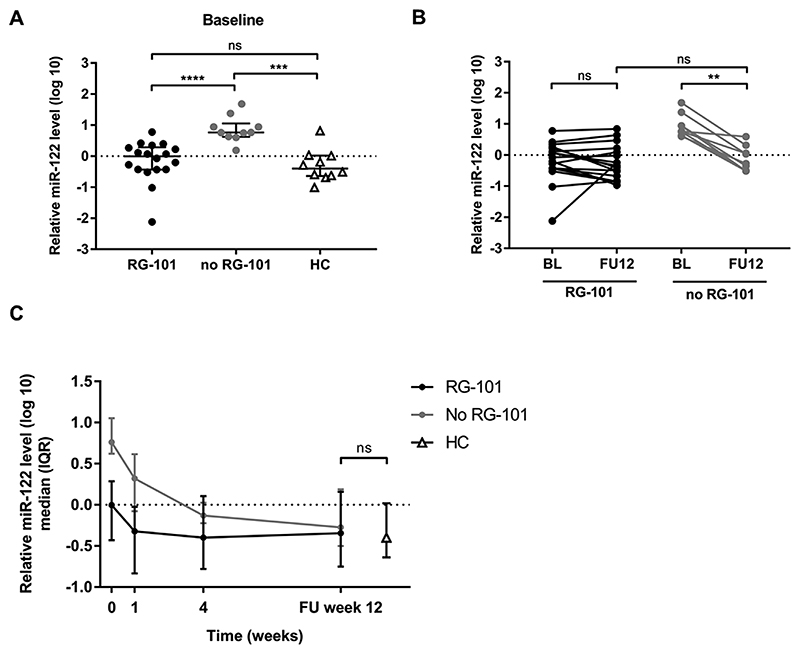
CHC patients without prior RG-101 dosing had significantly higher relative plasma miR-122 levels at baseline (median 0.761 (IQR: 0.623–1.053)) as compared to patients with prior RG-101 dosing and healthy controls (p < 0.001, and p < 0.0001, respectively) (Fig. 4A). Relative plasma miR-122 levels at baseline were not different between CHC patients previously dosed with RG-101 and healthy controls (median –0.004 (IQR: –0.431–0.286) versus –0.399 (IQR: –0.638–0.018), respectively, p = 0.175) (Fig. 4A). At follow-up week 12, plasma miR-122 levels of CHC patients without previous RG-101 dosing decreased 20-fold compared to baseline values (p = 0.008), whereas no significant difference between baseline and follow-up was observed in patients previously dosed with RG-101 (median fold decline 1.42, p = 0.287) (Fig. 4B). At follow-up week 12, there was no difference in median relative plasma miR-122 levels between healthy controls and patients with and without prior anti-miR-122 dosing (–0.399, –0.344, and –0.275, respectively, p = 0.555) (Fig. 4C).
Fig. 4. Relative plasma miR-122 levels.
(4A) Relative plasma miR-122 level at baseline in patients with (black dots) and without (grey dots) previous RG-101 dosing and healthy controls (HC, white triangles), median and interquartile range are shown. Mann-Whitney U test was used to compare study groups. (4B) Relative plasma miR-122 levels in CHC patients with (black dots) and without (grey dots) prior RG-101 dosing treated with SOF + DAC at baseline and follow-up week 12 (FU12). Wilcoxon test was used to compare baseline and FU12. Mann-Whitney U test was used to compare FU12 of different study groups. (4C) Relative plasma miR-122 levels in CHC patient throughout the study period up to FU12, as well as in healthy controls, median and interquartile range are shown. One-way ANOVA was used to compare study groups. **p < 0.01; ***: p < 0.001; ****: p < 0.0001.
4. Discussion
In CHC patients with and without prior anti-miR-122 (RG-101) treatment, DAA treatment with SOF + DCV ± RBV was highly effective. Successful antiviral treatment resulted in a reduction of immune activation as evidenced by IP-10 levels, and no increase in the magnitude of ex vivo HCV-specific T cell responses. In CHC patients without prior RG-101 dosing, circulating miR-122 levels were elevated at baseline and normalised when SVR was achieved.
This study enrolled patients with various HCV genotypes, including a substantial number of difficult-to-treat HCV genotype 3 patients with advanced fibrosis or compensated cirrhosis. Previous studies showed that 12 weeks of treatment with SOF + DCV results in SVR12 rates of only 63% in HCV genotype 3 patients with cirrhosis (Nelson et al., 2015), and that addition of RBV to the 12- or 16-week regimen increased SVR rates to 83% and 89% respectively(Leroy et al., 2016). In our study, the SVR12 rate in HCV genotype 3 patients with advanced fibrosis (treated for 12 weeks with SOF + DCV + RBV) and compensated cirrhosis (treated for 24 weeks with SOF + DCV + RBV) was 100%. Although the addition of RBV has been shown to improve SVR12 rates in a 12-week SOF + DAC regimen (Leroy et al., 2016), it is accompanied with more frequent adverse events, and the added benefit of extending the duration of this triple combination treatment beyond 12 weeks is unknown(-Leroy et al., 2016; Nelson et al., 2015; Welzel et al., 2016).
As expected, IP-10 levels were significantly increased in CHC patients as compared to healthy controls. We have previously shown that IP-10 levels decreased after dosing with RG-101(Stelma et al., 2017). However, at baseline there was no significant difference in IP-10 levels between CHC patients with and without previous RG-101 dosing. This suggests that the decline in IP-10 levels was transient after dosing with RG-101, and that IP-10 levels have increased again after rebound in HCV viral load. An increase in IP-10 levels with relapse was previously shown in CHC patients treated with DAAs (Meissner et al., 2014), and suggests reactivation of the interferon pathway. After clearance of HCV, IP-10 levels significantly decreased in all CHC patients in line with the disappearance of the virus. However, the immunological status of the patients does not fully normalise during follow-up, as IP-10 levels were still significantly increased at FU week 24 compared to healthy controls(Spaan et al., 2015).
It is unknown to what extent HCV-specific T cells play a role in the clearance of HCV upon DAA treatment. In a chimpanzee successfully treated with DAAs intrahepatic IFN-γ producing T cells were observed, but these were unable to prevent persistence upon reinfection(Callendret et al., 2014). Previous data has suggested an increase in the proliferative potential of HCV-specific T cells in humans after successful treatment with DAAs(Burchill et al., 2015; Martin et al., 2014; Spaan et al., 2015). However, the extent of the previously observed reversal of the exhausted phenotype was unknown, as no analyses of function (such as cytokine production) were performed. In patients treated with RG-101, we have shown that the function of HCV-specific T cells does not improve in patients who are long term HCV RNA negative(Stelma et al., 2017). Here we show that similarly, the function of HCV-specific T cells does not improve after successful clearance of HCV. This is in line with the recent observation of persistence of an increased proportion of regulatory T cells even when patients are successfully treated with DAAs(Langhans et al., 2016).
The majority of patients in our study were previously dosed with RG-101 in a phase 1b study. A number of these patients had RG-101 associated RAS in 5’UTR miR-122 binding sites (C2G + C3U or C3U) at time of viral rebound and/or at start of DAA treat-ment(van der Ree et al., 2017). Here we showed that DAA treatment was highly effective in CHC patients who previously received RG-101, even if 5’UTR RAS were present. This finding was consistent with a preclinical study showing that 5’UTR HCV variants were fully susceptible to DAAs(Ottosen et al., 2015). This is of special importance for testing regimens combining RG-101 and one or more DAAs. Next, we demonstrated that 5’UTR RAS spontaneously disappear within several weeks after identification, suggesting that the mutated viruses were outcompeted by the fitter wild-type variant. Taken together, we expect that the relevance of the mutations in 5’UTR miR-122 binding sites will be limited or nonexistent in future clinical practice.
Circulating miR-122 levels are higher in CHC patients as compared to healthy controls(van der Meer et al., 2013). In line with previous studies, we showed that circulating miR-122 levels decrease (by approximately 20-fold) and normalise in successfully treated CHC patients(Köberle et al., 2013; Waring et al., 2016). Moreover, we have previously shown that plasma miR-122 levels rapidly decrease by more than 1000-fold in anti-miR-122 treated CHC patients(van der Ree et al., 2016). Here we showed that plasma miR-122 levels were still decreased several months after RG-101 treatment compared to other CHC patients. However, plasma miR-122 levels in RG-101 treated patients were similar to levels of healthy controls, which is favourable from a safety perspective since miR-122 modulates the expression of a large number of hepatocyte proteins, some of which have been implicated in hep-atocarcinogenesis(Fornari et al., 2009; Gordon et al., 2000; Gramantieri et al., 2007; Lin et al., 2008; Tsai et al., 2009; Zeng et al., 2010).
In conclusion, treatment with SOF + DCV ± RBV was well tolerated and resulted in high SVR12 rates in CHC patients with and without prior RG-101 treatment. Our data suggests that there is no restoration of HCV adaptive immunity after SVR in CHC patients.
Supplementary Material
Acknowledgements
We thank Hadassa Heidsieck, Jeltje Helder and Martine Peters for their invaluable support and care in this study.
Abbreviations
- HCV
hepatitis C virus
- IP-10
interferon gamma-induced protein
- CHC
chronic hepatitis C
- DAAs
direct acting antivirals
- SVR
sustained virological response
- miR-122
microRNA-122
- RAS
resistance associated substitutions
- SOF
sofosbuvir
- DCV
daclatasvir
- RBV
ribavirin
- LLOQ
lower limit of quantification
- LLOD
lower limit of detection
- IQR
interquartile range
Footnotes
Conflict of interest
Sophie Willemse: served as a speaker, a consultant and an advisory board member for AbbVie, Bristol-Myers-Squibb, Gilead Sciences, Janssen Therapeutics and Roche.
Marc van der Valk: served on a scientific advisory board for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Johnson and Johnson, MSD and a data safety monitoring board for ViiV healthcare; Through his institution he received non-financial support by MSD.
Henk Reesink: received grants and personal fees from Roche, Bristor Myers Squibb, Gilead Sciences, Abbvie, Janssen-Cilag, MSD, PRA-international, Regulus Therapeutics and Replicor, received personal fees from Alnylam, and received a grant from Boehringer Ingelheim.
All other authors: none declared.
Financial support
This investigator initiated study was in part funded by Bristol-Myers Squibb. Paul Klenerman received financial support from Wellcome Trust (WT091663MA), the Medical Research Council (STOP HCV, MR/K01532X/1), the NIHR Biomedical Research Centre, Oxford, the Oxford Martin School, NIH (U19AI082630) and an NIHR Senior Fellowship. Eleanor Barnes is supported by the Medical Research Council UK the Oxford NIHR Biomedical Research Centre and the Oxford Martin School.
References
- Barnes E, Folgori A, Capone S, Swadling L, Aston S, Kurioka A, Meyer J, Huddart R, Smith K, Townsend R, Brown A, et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci Transl Med. 2012;4:115ra1. doi: 10.1126/scitranslmed.3003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes E, Gelderblom HC, Humphreys I, Semmo N, Reesink HW, Beld MGHM, van Lier RAW, Klenerman P. Cellular immune responses during high-dose interferon-a induction therapy for hepatitis C virus infection. J Infect Dis. 2009;199:819–828. doi: 10.1086/597072. [DOI] [PubMed] [Google Scholar]
- Bengsch B, Seigel B, Ruhl M, Timm J, Kuntz M, Blum HE, Pircher H, Thimme R. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 2010;6:e100094. doi: 10.1371/journal.ppat.1000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchill MA, Golden-Mason L, Wind-Rotolo M, Rosen HR. Memory redifferentiation and reduced lymphocyte activation in chronic HCV-infected patients receiving direct-acting antivirals. J Viral Hepat. 2015;22:983–99. doi: 10.1111/jvh.12465. [DOI] [PubMed] [Google Scholar]
- Callendret B, Eccleston HB, Hall S, Satterfield W, Capone S, Folgori A, Cortese R, Nicosia A, Walker CM. T-cell immunity and hepatitis C virus reinfection after cure of chronic hepatitis C with an interferon-free antiviral regimen in a chimpanzee. Hepatology. 2014;60:1531–1540. doi: 10.1002/hep.27278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornari F, Gramantieri L, Giovannini C, Veronese A, Ferracin M, Sabbioni S, Calin GA, Grazi GL, Croce CM, Tavolari S, Chieco P, et al. MiR-122/cyclin G1 interaction modulates p53 activity and affects doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res. 2009;69:5761–5767. doi: 10.1158/0008-5472.CAN-08-4797. [DOI] [PubMed] [Google Scholar]
- Gordon EM, Liu PX, Chen ZH, Liu L, Whitley MD, Gee C, Groshen S, Hinton DR, Beart RW, Hall FL. Inhibition of metastatic tumor growth in nude mice by portal vein infusions of matrix-targeted retroviral vectors bearing a cytocidal cyclin G1 construct. Cancer Res. 2000;60:3343–3347. [PubMed] [Google Scholar]
- Gramantieri L, Ferracin M, Fornari F, Veronese A, Sabbioni S, Liu C-G, Calin GA, Giovannini C, Ferrazzi E, Grazi GL, Croce CM, et al. Cyclin G1 is a target of miR-122a, a microRNA frequently down-regulated in human hepatocellular carcinoma. Cancer Res. 2007;67:6092–6099. doi: 10.1158/0008-5472.CAN-06-4607. [DOI] [PubMed] [Google Scholar]
- Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309:1577–158. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- Kelly C, Swadling L, Brown A, Capone S, Folgori A, Salio M, Klenerman P, Barnes E. Cross-reactivity of hepatitis C virus specific vaccine-induced T cells at immunodominant epitopes. Eur J Immunol. 2015;45:309–316. doi: 10.1002/eji.201444686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenerman P, Hill A. T cells and viral persistence: lessons from diverse infections. Nat Immunol. 2005;6:873–879. doi: 10.1038/ni1241. [DOI] [PubMed] [Google Scholar]
- Köberle V, Waidmann O, Kronenberger B, Andrei A, Susser S, Füller C, Perner D, Zeuzem S, Sarrazin C, Piiper A. Serum microRNA-122 kinetics in patients with chronic hepatitis C virus infection during antiviral therapy. J Viral Hepat. 2013;20:530–535. doi: 10.1111/jvh.12075. [DOI] [PubMed] [Google Scholar]
- Lanford RE, Hildebrandt-Eriksen ES, Petri A, Persson R, Lindow M, Munk ME, Kauppinen S, Ørum H. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327:198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhans B, Dieter Nischalke H, Krämer B, Hausen A, Dold L, van Heteren P, Hüneburg R, Nattermann J, Strassburg CP, Spengler U. Increased peripheral cd4+ regulatory t cells persist after successful direct-acting antiviral treatment of chronic hepatitis C. J Hepatol. 2016;66:888–896. doi: 10.1016/j.jhep.2016.12.019. [DOI] [PubMed] [Google Scholar]
- Leroy V, Angus P, Bronowicki J-P, Dore GJ, Hezode C, Pianko S, Pol S, Stuart K, Tse E, McPhee F, Bhore R, et al. Daclatasvir, sofosbuvir, and ribavirin for hepatitis C virus genotype 3 and advanced liver disease: a randomized phase III study (ALLY-3+) Hepatology. 2016;63:1430–1441. doi: 10.1002/hep.28473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CJ-F, Gong H-Y, Tseng H-C, Wang W-L, Wu J-L. miR-122 targets an anti-apoptotic gene, Bcl-w, in human hepatocellular carcinoma cell lines. Biochem Biophys Res Commun. 2008;375:315–320. doi: 10.1016/j.bbrc.2008.07.154. [DOI] [PubMed] [Google Scholar]
- Martin B, Hennecke N, Lohmann V, Kayser A, Neumann-Haefelin C, Kukolj G, Böcher WO, Thimme R. Restoration of HCV-specific CD8+ T cell function by interferon-free therapy. J Hepatol. 2014;61:538–543. doi: 10.1016/j.jhep.2014.05.043. [DOI] [PubMed] [Google Scholar]
- Meissner EG, Wu D, Osinusi A, Bon D, Virtaneva K, Sturdevant D, Porcella S, Wang H, Herrmann E, McHutchison J, Suffredini AF, et al. Endogenous intrahepatic IFNs and association with IFN-free HCV Treatment outcome. J Clin Investig. 2014;124:3352–3363. doi: 10.1172/JCI75938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DR, Cooper JN, Lalezari JP, Lawitz E, Pockros PJ, Gitlin N, Freilich BF, Younes ZH, Harlan W, Ghalib R, Oguchi G, et al. ALLY-3 Study Team All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61:1127–1135. doi: 10.1002/hep.27726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottosen S, Parsley TB, Yang L, Zeh K, van Doorn L-J, van der Veer E, Raney AK, Hodges MR, Patick AK. In vitro antiviral activity and pre-clinical and clinical resistance profile of miravirsen, a novel anti-hepatitis C virus therapeutic targeting the human factor miR-122. Antimicrob Agents Chemother. 2015;59:599–608. doi: 10.1128/AAC.04220-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–56. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- Spaan M, van Oord G, Kreefft K, Hou J, Hansen BE, Janssen HLa, de Knegt RJ, Boonstra A. Immunological analysis during interferon-free therapy for chronic hepatitis C virus infection reveals modulation of the natural killer cell compartment. J Infect Dis. 2015;213:216–223. doi: 10.1093/infdis/jiv391. [DOI] [PubMed] [Google Scholar]
- Stelma F, van der Ree MH, Sinnige MJ, Brown A, Swadling L, de Vree JML, Willemse SB, van der Valk M, Grint P, Neben S, Klenerman P, et al. Immune phenotype and function of NK and T cells in chronic hepatitis C patients who received a single dose of anti-miR-122, RG-101. Hepatology. 2017 doi: 10.1002/hep.29148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai W-C, Hsu PW-C, Lai T-C, Chau G-Y, Lin C-W, Chen C-M, Lin C-D, Liao Y-L, Wang J-L, Chau Y-P, Hsu M-T, et al. MicroRNA-122, a tumor suppressor microRNA that regulates intrahepatic metastasis of hepatocellular carcinoma. Hepatology. 2009;49:1571–1582. doi: 10.1002/hep.22806. [DOI] [PubMed] [Google Scholar]
- Urbani S, Amadei B, Fisicaro P, Tola D, Orlandini A, Sacchelli L, Mori C, Missale G, Ferrari C. Outcome of acute hepatitis C is related to virusspecific CD4 function and maturation of antiviral memory CD8 responses. Hepatology. 2006;44:126–139. doi: 10.1002/hep.21242. [DOI] [PubMed] [Google Scholar]
- van der Meer AJ, Farid WRR, Sonneveld MJ, de Ruiter PE, Boonstra A, van Vuuren AJ, Verheij J, Hansen BE, de Knegt RJ, van der Laan LJW, Janssen HLA. Sensitive detection of hepatocellular injury in chronic hepatitis C patients with circulating hepatocyte-derived microRNA-122. J Viral Hepat. 2013;20:158–166. doi: 10.1111/jvh.12001. [DOI] [PubMed] [Google Scholar]
- van der Ree MH, de Vree JM, Stelma F, Willemse S, van der Valk M, Rietdijk S, Molenkamp R, Schinkel J, van Nuenen AC, Beuers U, Hadi S, et al. Safety, tolerability, and antiviral effect of RG-101 in patients with chronic hepatitis C: a phase 1B, double-blind, randomised controlled trial. Lancet. 2017;6736:1–9. doi: 10.1016/S0140-6736(16)31715-9. [DOI] [PubMed] [Google Scholar]
- van der Ree MH, van der Meer AJ, van Nuenen AC, de Bruijne J, Ottosen S, Janssen HL, Kootstra NA, Reesink HW. Miravirsen dosing in chronic hepatitis C patients results in decreased microRNA-122 levels without affecting other microRNAs in plasma. Aliment Pharmacol Ther. 2016;43:102–113. doi: 10.1111/apt.13432. [DOI] [PubMed] [Google Scholar]
- Waring JF, Dumas EO, Abel S, Coakley E, Cohen DE, Davis JW, Podsadecki T, Dutta S. Serum miR-122 may serve as a biomarker for response to direct acting antivirals: effect of paritaprevir/R with dasabuvir or ombitasvir on miR-122 in HCV-infected subjects. J Viral Hepat. 2016;23:96–104. doi: 10.1111/jvh.12470. [DOI] [PubMed] [Google Scholar]
- Welzel TM, Petersen J, Herzer K, Ferenci P, Gschwantler M, Wedemeyer H, Berg T, Spengler U, Weiland O, van der Valk M, Rockstroh J, et al. Daclatasvir plus sofosbuvir, with or without ribavirin, achieved high sustained virological response rates in patients with HCV infection and advanced liver disease in a real-world cohort. Gut. 2016;65:1861–1870. doi: 10.1136/gutjnl-2016-312444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Wang R, Li D, Lin X-J, Wei Q-K, Yuan Y, Wang Q, Chen W, Zhuang S-M. A novel GSK-3 beta-C/EBP alpha-miR-122-insulin-like growth factor 1 receptor regulatory circuitry in human hepatocellular carcinoma. Hepatology. 2010;52:1702–1712. doi: 10.1002/hep.23875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.