Abstract
Background
The emergence of IL-33 as a key molecular player in the development and propagation of widespread inflammatory diseases including asthma and atopic dermatitis has established the need for effective IL-33 neutralizing biologics.
Objective
Here we describe the development and validation of a new antagonist of IL-33, termed IL-33trap, which combines the extracellular domains of the IL-33 receptor (ST2) and its co-receptor IL-1 receptor accessory protein, into a single fusion protein.
Methods
We produced and purified recombinant IL-33trap from human cells and analyzed its IL-33 binding affinity and IL-33 antagonistic activity in cultured cells and in mice. IL-33trap activity was also benchmarked with a recombinant soluble ST2 (sST2) corresponding to the naturally occurring IL-33 decoy receptor. Finally, we studied the effect of IL-33trap in the Alternaria alternata mouse model of allergic airway inflammation.
Results
In vitro, IL-33trap binds IL-33 and inhibits IL-33 activity much stronger than sST2. Furthermore, IL-33trap inhibits eosinophil infiltration, splenomegaly, and the production of signature cytokines in splenic lymphocytes and lung tissue upon IL-33 injection. Finally, administration of IL-33trap at the time of allergen challenge inhibits inflammatory responses in a preclinical mouse model of acute allergic airway inflammation.
Conclusions
IL-33trap is a novel IL-33 antagonist that outperforms the natural IL-33 decoy receptor and shows anti-inflammatory activities in a preclinical mouse model of acute allergic airway inflammation when given at the time of allergen challenge.
Keywords: IL-33, sST2, antagonist, airway inflammation, allergic asthma
Introduction
Allergic diseases such as allergic asthma are chronic inflammatory conditions that remain a serious health problem. The prevalence of asthma has been rising since the last part of the 20th century and 15% or more of the general population in many countries suffer from this disease.1 In general, treatment of asthma is well established and is dominated by inhaled corticosteroids and long-acting β2-agonists. However, about 5% of asthma patients are believed to achieve poor control of their asthma using existing treatments. Moreover, long-term treatment with corticosteroids suffers from side effects. Antibodies targeting several key molecular mediators of type 2 immunity have recently been launched or are being developed. Omalizumab, targeting IgE, has been the only biologic indicated for treatment of asthma for over a decade. However, very recently two IL-5 targeting antibodies, mepolizumab and reslizumab and an antibody targeting the IL-5 receptor (benralizumab) have been approved for the treatment of severe asthma. Also antisense molecule-based therapies that target key proteins by inactivating the corresponding RNA have shown much promise in early clinical trials. However, in vivo stability of these therapeutics, adverse side-effects and delivery routes still remain important challenges for the future.2
Asthma is a complex and heterogeneous disease, and currently available biologics are forecasted to cover only part of the patients, indicating there is still a need for other medications. Novel innovative cytokine targeting therapies with biologics on carefully selected populations of patients, personalized on the basis of their specific disease sub-phenotype (identified by discriminatory biomarkers and genetic profiling) are expected to lead to significant advances in the field of asthma treatment.3 One of the key players in allergic inflammation is interleukin-33 (IL-33), which belongs to the IL-1 superfamily of cytokines that drive the initiation of inflammatory responses.4 Next to asthma, IL-33 has also been implicated in other allergic diseases such as atopic dermatitis,4 allergic rhinitis5 and food allergy.6 IL-33 is primarily known as an alarmin that is released from epithelial barrier cells upon exposure to allergens or other stress factors,7, 8 and mainly activates innate lymphoid cells type 2 (ILC2) and Th2 lymphocyte effector cells.9, 10 The fact that IL-33 acts upstream of Th2-associated cytokines and IgE production, makes IL-33 an enticing target for biologics against type 2 immunity related diseases. Depending on the cellular context, IL-33 can also affect Th1 cells, mast cells, macrophages, dendritic cells, fibroblasts and regulatory T cells, which play key roles in various pathological conditions.4
Extracellular IL-33 coordinates an immune response by binding to a receptor complex consisting of IL-1 receptor-like 1 (IL-1RL1) (better known as ST2) and IL-1 receptor accessory protein (IL-1RAcP), followed by the initiation of several signaling pathways culminating in the activation of specific gene expression and release of pro-inflammatory cytokines.11 IL-33 activity is limited by a decoy receptor, an alternative splice variant of ST2 coding for a soluble extracellular portion of the receptor, known as soluble ST2 (sST2).12,13 Both IL-33 and ST2 have been identified as major susceptibility loci in genome-wide association studies of human asthma.14 Moreover, IL-33 is upregulated in asthma patients,15, 16 with increased levels of extracellular IL-33 being associated with asthma disease exacerbation.17 Noteworthy, previous studies indicate that lung IL-33 expression and release is resistant to steroid treatment,18, 19 suggesting that IL-33 targeting therapeutics may be particularly beneficial in a population of steroid-resistant asthmatics.
Supporting the therapeutic potential of IL-33 inhibition, several genetic (IL-33 and ST2 knockout mice) and pharmacological strategies (antibodies targeting IL-33 or ST2, or use of recombinant sST2) to interfere with IL-33/ST2 signaling have shown beneficial effects in preclinical mouse models of allergic asthma.4 Here we report the generation and characterization of a novel sST2-based biologic that exhibits greatly enhanced binding affinity to IL-33 compared to normal cellular sST2 and functions as a potent inhibitor of IL-33-induced inflammatory responses both in vitro and in vivo.
Methods
Antibodies, expression plasmids and other reagents
V450-labeled antibody to mouse CD11b, PE-labeled antibody against Siglec-F, AF700-labeled antibody to Ly6G, FITC-labeled antibodies to B220, CD8a and CD11c, BV786-labeled antibody to streptavidin and PE-CF594-labeled antibody to CD127 were purchased from BD Biosciences. PE-Cy5− or FITC-labeled antibodies to CD3 and CD19 were purchased from VWR. BV605-labeled antibody to CD90.2 was purchased from BioLegend. Biotin-labeled antibody to ST2 was purchased from MD Bioproducts. PE-Cy7-labeled antibody to CD25 was from LifeTechnologies. FITC-labeled antibodies to MHCII, NK1.1, Ter-119 and CD4, PE-labeled antibody to FoxP3, PE-Cy5-labeled antibody to CD19, APC-labeled antibodies to CD11c and CD45, APC-eF780-labeled antibodies to CD4 and CD117, fixable viability dye eFluor 506, and ELISA sets for human IL-8, mouse TNF, IL-4, IL-5, IL-6, IL-13, IL-17, IL25, IFNγ and TSLP were purchased from eBioscience. Alternaria alternata extracts were obtained from Greer Laboratories.
Plasmids have been deposited at the BCCM-GeneCorner plasmid collection (Ghent, Belgium) and accession numbers are provided for each plasmid. pNFconluc (LMBP3248), which contains NF-κB-driven luciferase, was a gift from Dr. A. Israёl (Institut Pasteur, Paris, France); pACTβgal (LMBP4341) was from Dr. J. Inoue (Institute of Medical Sciences, Tokyo, Japan). Mouse ST2 and mouse sIL-1RAcP were amplified from the mouse macrophage cell line Mf4/4 and cloned into the pEF6-myc/HisA (Invitrogen). Mouse sST2 was PCR amplified from Origene clone MC204735 and cloned into the pEF6-myc/HisA. pEF-mIL33traps were constructed as follows. A fragment of mouse sST2 without the signal sequence (amino acids 27-337), PCR amplified from pEF-msST2, together with a linker sequence of repeating Gly-Gly-Ser triplets amplified from pCLG-Duba (LMBP6610) were cloned by a 3-way ligation reaction into the pEF-msIL-1RAcP (amino acids 21-360) vector. The expression vector for mouse IL-1RAcP (pCR4-Flag-mIL-1RAcP) was kindly provided by Dr. Sophie Janssens (VIB/University of Ghent, Ghent, Belgium). pEF-BOS-hsST2 and pEF-BOS-hST2 constructs were kindly provided by Prof. Luke O’Neill (Trinity College Dublin, Ireland). Human sST2 was PCR amplified from pEF-BOS-hsST2; the human sIL1-RAcP was amplified from a human spleen cDNA library. Both fragments were cloned into the pEF6-myc/HisA vector. pEF-hIL33traps were constructed as follows. Human sST2 without the signal sequence (amino acids 19-328) together with a linker sequence of repeating Gly-Gly-Ser triplets were cloned by a 3-way ligation reaction into the pEF-hsIL-1RAcP vector. All constructs were confirmed by DNA sequencing analysis.
Production of recombinant proteins
A truncated construct of mouse IL-33 (residues 109-266), lacking the N-terminus, with an additional N-terminal hexa-histidine purification tag and a C-terminal solubilization tag20 was generated and cloned into the pETDuet-1 vector. The vector was subsequently transformed into E. coli BL21 and expression was induced with 1 mM IPTG followed by overnight incubation at 28°C. The bacterial pellet was harvested by centrifugation, resolubilized and lysed by sonication. The lysate was centrifuged and soluble IL-33 was purified from the supernatants by Immobilized Metal Affinity Chromatography (IMAC) followed by Size Exclusion Chromatography (SEC), yielding pure and homogenous IL-33 as determined by SDS-PAGE and SEC (data not shown).
Murine IL-33trap, sIL-1RAcP and sST2 were produced at the Protein Core of VIB. Briefly, suspension HEK293 FreeStyle cells in FreeStyle 293 expression medium were transiently transfected with pEF-msST2 or pEF-mlL-33trap using the polyethylenimine (PEI) method. 16h after transfection, 20 % of Ex-Cell 293 medium serum-free was added and the cells were cultured further for 5 days. The secreted recombinant proteins in the medium fractions were concentrated and diafiltrated before purification by IMAC. After a final gel-filtration over a Superdex 200 column, the purified proteins were stored in PBS at −80°C.
Affinity measurements using Isothermal Titration Calorimetry
Experiments were carried out using a VP-ITC instrument (MicroCal) at 37 °C, and data were analyzed using the Origin ITC analysis software package (recording and initial analysis), NITPIC (automated baseline corrections)21 and SEDPHAT (fitting and parameter determination).22 All protein samples were exchanged into the same buffer (20 mM HEPES, 150 mM NaCl) by size exclusion chromatography. Protein concentrations were measured spectrophotometrically at 280 nm using calculated theoretical extinction coefficients and all solutions were extensively degassed before use. Titrations were always preceded by an initial injection of 3 μl, and were carried out using 5 μl injections applied 450 s apart. The sample was stirred at a speed of 300 r.p.m. throughout. The thermal titration data were processed21 and fitted using a ‘one binding site’ model,22 and the apparent molar reaction enthalpy (ΔH°), apparent entropy (ΔS°), binding affinity (KD) and stoichiometry of binding (N) were determined. The parameters presented are averages of two (IL-33 vs. sST2) or three (IL-33 vs. IL-33trap and IL-33trap vs IL-33:sST2) experiments.
The displacement ITC was set up and performed using the same principles used in previous studies23 and extensively described by Campoy et al.24 Using the affinity measured between IL-33 and sST2 (KD,sST2) and the apparent affinity measured in the displacement ITC between IL-33 ([IL-33]T) and a mix of sST2 ([sST2]T) and IL-33trap (Kapp D,IL-33trap), the “real” affinity of IL-33 towards IL-33trap (Kreal D,IL-33trap) could be calculated using the following formula:
Note that [sST2]T should represent the total amount of active sST2 that can compete with IL-33trap for IL-33. Since the benchmark ITC between IL-33 and sST2 showed that only 60% of sST2 is active, we corrected for this deviation by adding compensating amounts of sST2. For the final calculations we used the concentration of active sST2, as 60% of the total sST2 in the cell.
IL-33 bioassay
HEK293T, RAW Blue 264.7 cells, immortalized BMDM and HMC-1 human mast cells were cultured in Dulbecco's modified Eagle's medium, supplemented with 10% fetal calf serum and 2mM L-Glutamine. HEK293T cells (human embryonic kidney cells) were a gift from Dr. Hall (Department of Biochemistry, University of Birmingham, UK). RAW Blue 264.7 cells were purchased from Invivogen, immortalized bone marrow-derived macrophages (BMDM) were a gift from Dr. Harris (Trinity College Dublin), HMC-1 cells were a gift from Dr. Lieve Coorevits (KU Leuven, Belgium). For transfection, HEK293T cells were seeded at 4 × 104 cells/well in 24-well plates and transiently transfected the next day by calcium phosphate precipitation. For IL-33 neutralization experiments, recombinant IL-33 was pre-incubated for 30 min at room temperature with inhibitors before adding to the cells. Luciferase activity was measured in the cell lysates 5h later and normalized by β-galactosidase values in order to correct for potential differences in transfection efficiency. Cytokine profiles in the cell supernatants were measured 24h later by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's protocol.
Animals and IL-33 treatment
C57BL6/J mice were purchased from Janvier. All animal experiments were approved by the Ethical Committee for Animal Experimentaion of Ghent University - Faculty of Sciences. Mice were daily i.p. injected with recombinant IL-33trap or sST2 followed 30 min later by 100 ng of recombinant mouse IL-33. Mice were sacrificed on day 6; peritoneal lavage, spleens and lungs were collected for further analysis.
Analysis of peritoneal lavage, spleens and lung homogenates
Peritoneal lavage was performed using PBS with 0.5% BSA and 2mM EDTA. Cell populations in peritoneal lavages were quantified by flow cytometry. Multiparameter analysis was performed on an LSRFortessa (BD) and data were processed using FlowJo (Tree Star). Dead cells were excluded using the Fixable Viability Dye eFluor 506 (eBioscience).
Spleens were flushed with 2 ml of PBS/0.5% BSA and passed through a 70 μm strainer (Falcon) to generate single cell suspension. Red blood cells were lysed with 1.7 ml ACK lysis buffer (Lonza) for 3 min at room temperature. Splenocytes were re-stimulated ex vivo with 20 ng/ml PMA. Cytokine profiles in the supernatants were measured 72h later by ELISA.
Lungs were homogenized with a tissue homogenizer in 320 μl of cold lysis buffer (40 mM Tris-HCl, pH 8.0, 0.275 M NaCl, 20% glycerol [vol/vol], 1 mM PMSF, 1 mM sodium orthovanadate [Na3VO4], 10 mM NaF, 1 μg/ml aprotinin, and 1 μg/ml leupeptin) using a tissue homogenizer (IKA) with the addition of 2% Igepal after homogenization. Samples were then rotated for 20 min at 4°C with agitation, followed by a centrifugation to pellet debris. Cleared lysate was quantified for protein concentration using the Bradford Bio-Rad protein assay. IL-5 concentrations in the supernatants were measured by ELISA and corrected for total protein content.
Alternaria alternata-induced asthma model
Mice were anesthetized with isoflurane and received 5 μg Alternaria alternata i.t. Seven days later, mice were challenged i.t. with 20 μg Alternaria alternata for 3 days as indicated in Figure 5C under anesthesia. IL-33 was blocked by i.t. injection of recombinant IL-33trap at the time of challenge. Mice were sacrificed 1 day after the last challenge. BAL was performed using 3x1 ml EDTA-containing PBS and analyzed using flow cytometry. For staining of ILC2 and regulatory T cells, lungs were cut into small pieces and incubated at 37°C for 30 minutes in RPMI 1640 containing Liberase TM (Roche) and DNase I (Roche). Lungs were filtered over a 70μm strainer (Falcon) and red blood cells were lysed with 1 ml ACK lysis buffer (Lonza) for 3 min at room temperature before being stained with different FACS antibodies. Lungs were snap-frozen in liquid nitrogen and kept at −80°C until further processing for real-time quantitative RT-PCR (qRT-PCR) or cytokine measurements by ELISA. RNA was obtained using the TriPure Isolation Reagent (Roche, Mannheim, Germany) and isolated according to the manufacturer's instructions. RNA was reverse transcribed with a iScript™ Advanced cDNA Synthesis Kit (Bio-Rad), and samples were analyzed by SYBR green-based qRT-PCR with a LightCycler 480 system (Roche) against reference genes (Actin, Hprt, and Sdha). Airway hyperreactivity (AHR) was analyzed 24 h after the last Alternaria alternata challenge using flexiVent invasive measurement of dynamic resistance (SCIREQ, Montreal, Quebec, Canada). Mice were anesthetized with urethane and paralyzed using d-tubocurarine, followed by mechanical ventilation through a cannula in the trachea. Increasing concentrations of methacholine (25 mg/ml - 800 mg/ml) were nebulized using Aeroneb (SCIREQ). Dynamic resistance was recorded after a standardized inhalation maneuver given every 10 s for 2 min. Baseline resistance was restored before administering the subsequent doses of methacholine.
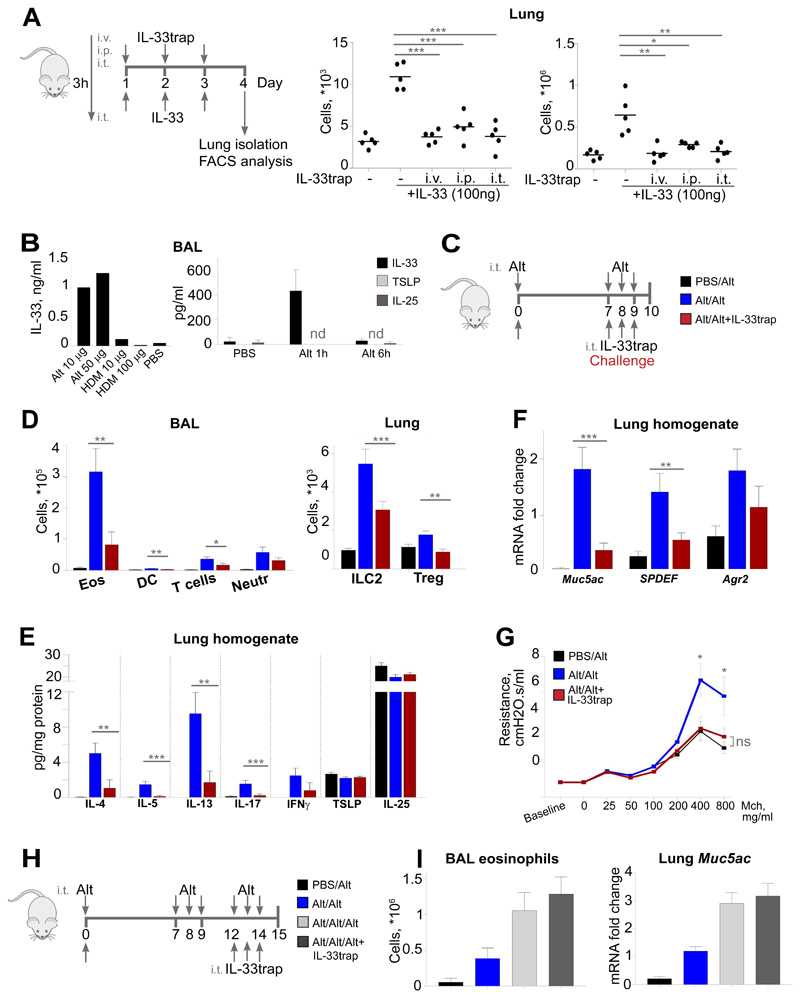
Figure 5. IL-33trap inhibits the development of Alternaria alternata-induced asthma.
(A) Experimental set up (left). C57BL/6J mice (n=5) were injected as indicated. ILC2 and eosinophils were determined in the lungs on day 4 by flow cytometry (right). (B) Mice were injected with Alternaria alternata or HDM and concentration of IL-33 in the BAL was measured by ELISA 1h later (left). Alternatively, mice were injected with 10μg Alternaria alternata and cytokine concentrations in the BAL were measured (right). (C) Experimental set up for the Alternaria alternata-induced model. (D) Airway inflammation was induced as depicted in C (n=8). Individual cell types in the BAL and lung tissue were identified by flow cytometry. (E) Cytokine concentrations in lung homogenate were measured by ELISA. (F) Gene expression in lung was measured by RT-PCR. (G) AHR was measured after exposure to increasing concentrations of metacholine. (H) Experimental set up for the established Alternaria alternata-induced asthma. (I) Airway inflammation was induced as depicted in H (n=8). Eosinophils in the BAL were identified by flow cytometry and expression of Muc5ac was measured by RT-PCR. Results are representative of two independent experiments. Error bars represent the mean ± SE. Significance levels: ns P > 0.05, **P < 0.01, and ***P < 0.001.
Statistical analyses
The results are expressed as mean ± SE. Statistical significance between groups was calculated using 1-way ANOVA, followed by Fischer’s Least Significant Difference test with GraphPad Prism software (v7.01; GraphPad Software, La Jolla, Calif). Differences between groups were considered significant when the p-value was <0.05.
Results
Design and production of recombinant IL-33trap as a monomeric high affinity IL-33 antagonist
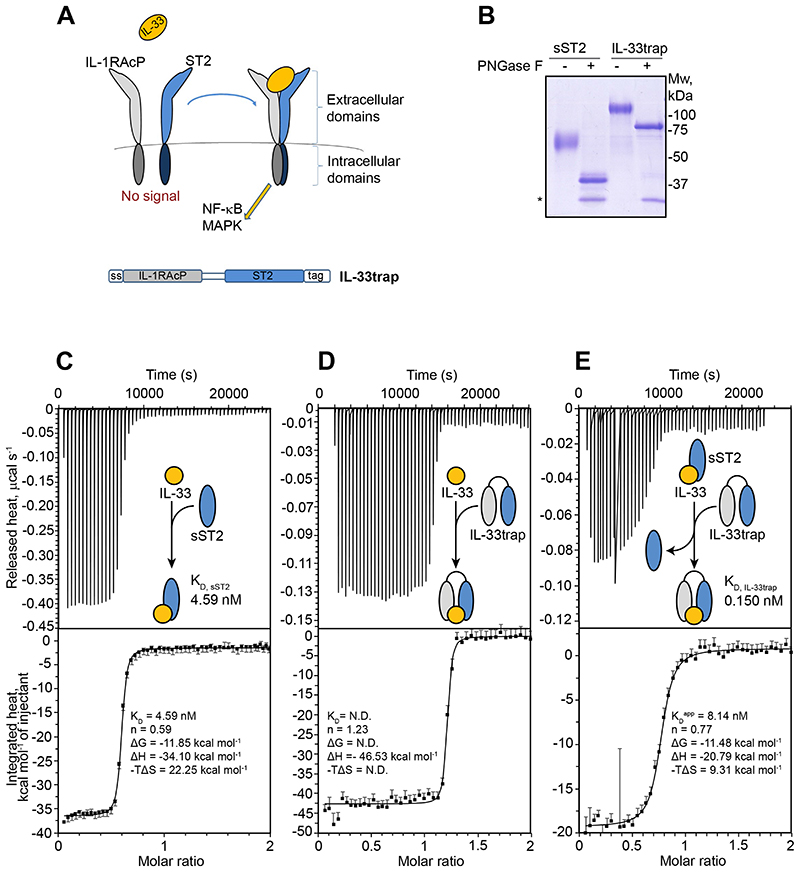
The cornerstone for the assembly of a signaling complex mediated by IL-33 is an encounter binary complex with its cognate receptor ST2, which subsequently primes recruitment of the IL-1RAcP co-receptor to establish a tripartite high affinity signaling complex11 (Figure 1A). This mechanistic principle was the basis for our strategy to engineer a recombinant fusion protein (hereafter referred to as “IL-33trap”) comprising the soluble extracellular part of mouse IL-1RAcP N-terminally fused to the soluble extracellular part of mouse ST2 via a flexible GGS (glycine-glycine-serine) linker (Figure 1A). The expression construct encodes for the IL-1RAcP signal sequence (ss) at the N-terminus, which allows protein secretion, and a myc/His tag at the C-terminus to facilitate protein purification and detection. We have opted to produce the IL-33trap in HEK293 Freestyle cells to ensure proper folding as well as correct processing and presentation of N-linked glycosylation, and purified the recombinant protein from conditioned media using affinity chromatography and size-exclusion chromatography (Figure 1B). In parallel, we also generated and purified recombinant sST2. Treatment of IL-33trap and sST2 with Peptide:N-Glycosidase F (PNGase F), an endoglycosidase that specifically removes N-linked glycans from glycoproteins, showed that sST2 and IL-33trap are both glycosylated (Figure 1B).
Figure 1. IL-33trap exhibits enhanced ligand binding affinity.
(A) Schematic representation of the IL-33 receptor complex and the IL-33trap design. The signal peptide at the N-terminus ensures secretion of the expressed protein into the medium fraction, while a carboxy terminal myc/His6 tag is used for detection and purification. (B) sST2 and IL-33trap were subjected to PNGase F treatment and analyzed by SDS-PAGE and Coomassie blue staining. Recombinant PNGase F is marked with an asterisk. (C) ITC titration of IL-33 (37.17 μM in syringe) to sST2 (3.37 μM in cell) at 37°C with Wiseman C-value of 925 (<1000). (D) ITC titration of IL-33 (14.23 μM in syringe) to IL-33trap (1.20 μM in cell) at 37°C with Wiseman C-value of 3750 (>1000). (E) Displacement ITC of IL-33trap (20.30 μM in syringe) to IL-33 (1.75 μM in cell) in competition with sST2 (3.37 μM in cell) at 37°C with Wiseman C-value of 214 (<1000). A schematic reaction is shown within the thermogram plot. Binding parameters were determined by SEDPHAT and manually averaged over two (C)) or three (D and E) experiments. In addition, manually calculated values for the Gibbs free energy (ΔG) and entropic component (-TΔS) of the reactions are displayed.
In the first instance, we sought to carry out binding studies by isothermal titration calorimetry (ITC) to determine and compare the affinity of IL-33 towards sST2 and the IL-33trap. Benchmarking of the affinity of IL-33 to sST2 revealed a moderately strong exothermic binding event and an equilibrium dissociation constant (KD) of 4.59 nM (Figure 1C) consistent with affinities determined by orthogonal methods.25 Consistent with the working mechanistic signaling model for the receptor complex mediated by IL-33, the latter only displayed a measurable affinity towards IL-1RAcP in the presence of sST2 (data not shown), confirming the expected functionality of both receptor modalities. Direct titration of IL-33 to the IL-33trap, encompassing both receptor subunits, suggested an ultrahigh affinity with a KD value in the picomolar range (Figure 1D). Given the limitations of deriving reliable KD values from such steep binding isotherms (Wiseman C-value > 1000),26 we resorted to displacement ITC.23, 27 This experiment revealed an average apparent KD of 8.14 nM, from which the actual KD of IL-33 towards the IL-33trap was calculated as 0.150 nM. Thus, the IL-33trap displays ultrahigh affinity towards IL-33 that is ~30-fold higher than the affinity of sST2 for IL-33 (Figure 1E).
IL-33trap specifically inhibits IL-33 signaling in vitro
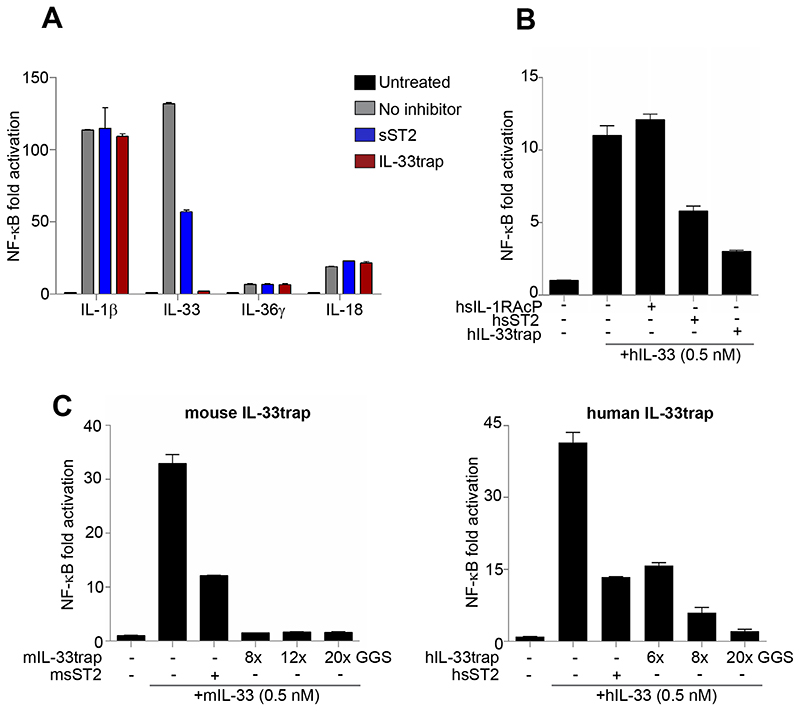
To test if the IL-33trap displays antagonistic properties, we investigated its ability to inhibit IL-33 signaling in a cellular assay. sST2 was used as a positive control. HEK293T cells were made responsive to IL-33 by transfection with ST2 and IL-1RAcP, and IL-33-induced NF-ĸB activation was followed via NF-ĸB dependent luciferase reporter gene expression as a read-out. IL-33 treatment resulted in robust NF-ĸB activation, which was inhibited by pre-incubation of IL-33 with sST2 or IL-33trap (Figure 2A). The relative inhibitory potency of sST2 and IL-33trap is analyzed in more detail below. To eliminate the possibility that our IL-33trap might interfere with IL-1β, or IL-18, or IL-36γ, all of which bind different core receptors but utilize IL-1RAcP and IL-1RAcP-related co-receptors, we established similar NF-ĸB dependent reporter assays and confirmed that the IL-33trap uniquely targets IL-33 signaling (Figure 2A). The above described experiments were all performed with mouse IL-33 and mouse IL33trap. To analyze the effect of its human counterparts, we also constructed human IL-33trap and tested its ability to inhibit NF-ĸB signaling initiated by human IL-33. Similarly to mouse IL33trap, human IL-33trap as well as human sST2 efficiently neutralized human IL-33 (Figure 2B). We also tested the impact of the GGS repeat linker length on the ability of IL-33trap to inhibit IL-33-induced NF-ĸB activity. However, mouse IL-33traps with a linker of 8- (used in further experiments),12- or 20-GGS repeats were equally effective, while a linker length of 20-GGS repeats seemed to be preferred in the case of human IL-33trap (Figure 2C).
Figure 2. IL-33trap specifically inhibits IL-33 activity.
(A) HEK293T cells were transfected with NF-ĸB reporter plasmid and the expression plasmids for the indicated receptor complexes. Cells were treated with 10 ng/ml of recombinant IL-1β, IL-33, IL-36γ or IL-18 that were pre-incubated with 500 ng/ml of IL-33trap or sST2. Luciferase activity in cell lysates was measured 5h later. (B) HEK293T cells were transfected with NF-ĸB reporter plasmid and the human IL-33 receptor complex expression plasmids. Cells were treated with recombinant human IL-33 that was pre-incubated with human IL-33trap or sST2 (note that non-equimolar concentrations were used). Luciferase activity in cell lysates was measured 5h later. (C) HEK293T cells were treated as in B. Mouse (left panel) or human (right panel) IL-33trap constructs with different linker length or sST2 were used to neutralize IL-33 as indicated. Values represent the mean of triplicates ± SE.
IL-33trap is a much more potent IL-33 inhibitor in vitro than sST2
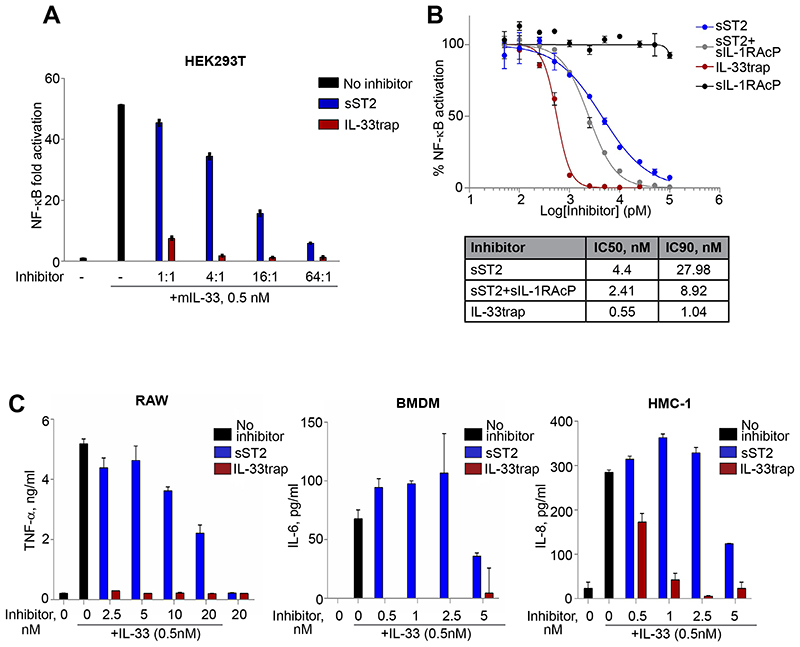
For a functional comparison, sST2 and IL-33 trap were tested at equimolar levels for their IL-33 inhibiting potential in a cell-based bio-assay. To this end, we pre-incubated recombinant IL-33 with equimolar concentrations of either sST2 or IL-33trap over a range of inhibitor-to-target ratios and assessed the residual IL-33 activity in our HEK293T cellular assay. IL-33trap exhibited dramatically enhanced ability to inhibit IL-33 at each tested concentration when compared to sST2 (Figure 3A). Strikingly, the IL-33trap was able to potently inhibit IL-33 activity already at a molar ratio of 1:1 (inhibitor to IL-33), while sST2 failed to have any significant effect at this concentration. As can be calculated from the concentration response curve, the concentration of IL-33trap that provides 90% inhibition is almost 27 times lower than that of sST2 (IC90 1.04 nM vs 27.98 nM; Figure 3B). Also the combination of sST2 and sIL-1RAcP was less potent than IL-33trap (IC90 1.04 nM vs 8.92 nM). As expected, sIL-1RAcP alone was not able to neutralize IL-33 activity (Figure 3B).
Figure 3. IL-33trap is a more potent IL-33 inhibitor than sST2 in vitro.
(A-B). HEK293T cells were transfected with NF-ĸB reporter plasmid and the IL-33 receptor complex expression plasmids. Cells were treated with recombinant IL-33 that was pre-incubated with the indicated molar excess of IL-33trap or sST2. Luciferase activity in cell lysates was measured 5h later (A). Concentration response curves, IC50 and IC90 were calculated (B). (C) RAW 264.7, immortalized BMDM and HMC-1 cells were treated with recombinant IL-33 that was pre-incubated with the indicated concentrations of IL-33trap or sST2. Cytokine concentrations in the supernatants were measured by ELISA 24h later. Values represent the mean of triplicates ± SE.
Next, to investigate the inhibitory potential of IL-33trap at endogenous receptor expression levels, we examined the response of mouse macrophages or human mast cells to IL-33 in terms of enhanced cytokine production. Consistent with the HEK293T assay, IL-33trap was a dramatically more potent blocker of IL-33 activity, as measured by IL-33-induced TNF, IL-6 or IL-8 cytokine and chemokine production from RAW 264.7 and immortalized BMDM or HMC-1 mast cells, respectively (Figure 3C). Collectively, our data shows that a novel IL-33 inhibitor, IL-33trap, exhibits significantly improved ability to neutralize IL-33 compared to sST2.
IL-33 trap inhibits IL-33 in vivo
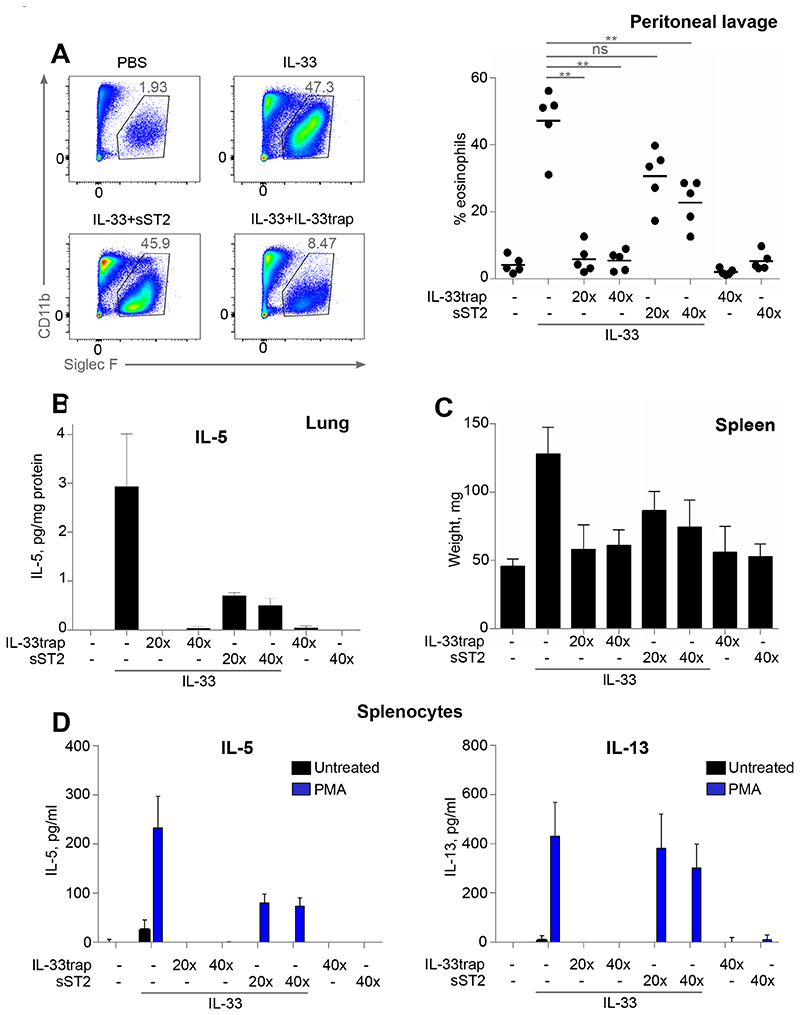
We next explored whether the observed potency of the IL-33trap in vitro could be extrapolated to a neutralization of IL-33 activity in vivo. It has been previously shown that intraperitoneal (i.p.) IL-33 injection induces increased eosinophil infiltration to the peritoneal cavity, splenomegaly and pro-inflammatory cytokine production in lungs.7, 11 Therefore, C57BL/6J mice were injected i.p. for five consecutive days with either PBS, sST2 or IL-33trap followed by IL-33 injection 30 minutes later. Consistent with previous reports, injection of IL-33 dramatically induced eosinophil infiltration, which was completely inhibited by IL-33trap already at a 20:1 ratio of inhibitor to IL-33 (Figure 4A). At the same time sST2 showed only a minimal ability to reduce eosinophilia at the same molar ratio, and could only partially reduce eosinophil infiltration at 40-fold molar excess over IL-33 (Figure 4A). Similarly, IL-5 production induced by IL-33 in the lungs and splenomegaly were completely inhibited by the IL-33trap, while sST2 was only able to partially inhibit these responses (Figure 4B and C). Finally, we isolated splenocytes and assayed pro-inflammatory cytokine production in response to in vitro PMA re-stimulation. In line with the other readouts, splenocytes derived from mice injected with IL-33 produced increased amounts of signature IL-33-induced cytokines, IL-5 and IL-13 (Figure 4D). This response was completely blunted when mice were pre-treated with IL-33trap, while sST2 had little effect. To rule out the possibility that IL-33trap or sST2 preparations have contaminants that might affect the inflammatory response, we also injected mice with inhibitors in the absence of IL-33. Neither IL-33trap nor sST2 alone induced any significant increase in the measured inflammatory responses (Figure 4A-D). Collectively, these data show that the ability of the novel IL-33trap inhibitor to neutralize IL-33 activity in vivo is superior to that of sST2.
Figure 4. IL-33trap is a more potent IL-33 inhibitor than sST2 in vivo.
C57BL/6 mice (5 mice per group) were injected i.p. for 5 consecutive days with PBS, IL-33trap or sST2 followed by i.p. injection of 100 ng IL-33 30 min later, as indicated. Mice were sacrificed 24h after the last injection. (A) Individual cell types in the peritoneal lavage were identified by flow cytometry. Representative FACS plots showing identification of Siglec F+CD11bInt eosinophils (left). Cells were pre-gated as single live CD3−CD19−Ly6G− and were SSCHi. Numbers represent eosinophils as a percentage of live cells (right). (B) IL-5 concentration in the lung homogenates was measured by ELISA and expressed per mg of protein. (C) Spleen weight at the end of the experiment for each group is shown. (D) Splenocytes were re-stimulated ex vivo with 20 ng/ml PMA. IL-5 and IL-13 concentrations in the supernatants were determined 72h later by ELISA. Results are representative of two independent experiments. Error bars represent the mean ± SE. Significance levels: ns P > 0.05, **P < 0.01, and ***P < 0.001.
IL-33trap attenuates inflammatory responses in a mouse model of acute allergic airway inflammation
The pathological role of IL-33 is most firmly established in the case of asthma, supported by a large body of experimental data.28 Therefore, we set out to investigate whether IL-33trap can reduce allergic responses in a mouse model of acute allergic airway inflammation. First, to determine the optimal route of IL-33trap delivery, we administered IL-33trap for 3 days either i.p., intravenously (i.v.) or intratracheally (i.t), followed by i.t. administration of IL-33 three hours later, after which inflammatory cell infiltration in the lungs was measured by flow cytometry. As can be seen from Figure 5A, the numbers of ILC2 or eosinophils in lungs were significantly increased in mice treated with IL-33, while pre-treatment with IL-33trap efficiently inhibited the inflammatory response, regardless of the administration route. Since local pulmonary drug delivery has significant advantages to the human patients, being less invasive than injections and minimizing the systemic side effects, we further concentrated on the i.t. route of IL-33trap administration.
The fungus Alternaria alternata is known to be a significant source of aeroallergens and an important risk factor for the development of asthma. Alternaria alternata-specific serine protease activity has been shown to cause the rapid release of IL-33 into the airways of mice, which triggers Th2 inflammation and loss of lung function.29 In contrast, IL-33 release was not detectable upon exposure to another common allergen, house-dust mite (HDM). In agreement, we also showed that intratracheal treatment with Alternaria alternata extract induced a robust release of IL-33, while HDM had no clear effect (Figure 5B). Moreover, other epithelial-derived cytokines, TSLP and IL-25, remained undetectable (Figure 5B). Alternaria alternata was therefore found to be the most appropriate mouse model to test the effect of the IL-33trap on the development of allergic asthma.
C57BL/6 mice were sensitized i.t. with a low dose of Alternaria alternata, followed by i.t. challenge with high dose of Alternaria alternata either alone or together with IL-33trap on days 7-9 post sensitization (Figure 5C). The next day, the presence of inflammatory cells in the bronchoalveolar lavage (BAL) fluid was analyzed by flow cytometry (Figure 5D, left panel). As expected, Alternaria alternata-treated mice developed signs of allergic airway inflammation, as evaluated by increased numbers of lymphocytes, dendritic cells and eosinophils in the BAL (Figure 5D). However, BAL eosinophilia and lymphocytosis, as well as increased DC numbers, were significantly inhibited by IL-33trap, showing the therapeutic potential of this inhibitor when given at the time of allergen challenge. Similarly, Alternaria alternata exposure increased ILC2, signature IL-33-responsive cells and important drivers of IL-5/IL-13 inflammatory responses, in lung tissue, which was significantly reduced in the presence of IL-33trap (Figure 5D, right panel). IL-33 administration has previously been shown to increase the number of lung regulatory T cells (Treg), which exhibited “Th2-like” characteristics and lost their inhibitory capacities, thus possibly further contributing to Th2-type inflammation.30 We similarly detected an increase in lung Treg levels upon Alternaria alternata exposure, which was prevented by IL-33trap (Figure 5D, right panel).
Furthermore, increased levels of Th2 cytokines associated with asthma, such as IL-4, IL-5 and IL-13, in the lung homogenates were also blunted by IL-33 trap (Figure 5E). Although our treatment protocol predominantly induced Th2-type responses, we also detected some increase in IL-17 and IFN-γ upon Alternaria alternata exposure, which was inhibited by IL-33trap, while the levels of epithelial-derived cytokines TSLP and IL-25 remained unchanged (Figure 5E).
IL-13 is a main driver of goblet cell metaplasia, which is characterized by increased production of MUC5AC, SPDEF and AGR2.31 In our model, Alternaria alternata challenge increased lung mRNA expression of all these genes, which was strongly reduced upon IL-33trap treatment (Figure 5F). Finally, Alternaria alternata-induced AHR to metacholine was also reduced upon IL-33trap treatment (Figure 5G).
To analyze if IL-33trap can also inhibit already established inflammation, we sensitized and challenged mice with Alternaria alternata as described above, after which we re-challenged them 3 days later with Alternaria alternata either alone or together with IL-33trap (Figure 5H). However, in this setting IL-33trap was unable to inhibit BAL eosinophilia and lung Muc5ac mRNA production (Figure 5I), suggesting that IL-33 plays a more important role at the initiation of allergic airway inflammation. Collectively, these data provide clear evidence for the ability of IL-33trap to inhibit the early development of allergic airway inflammation and subsequently suppress key pathological manifestations of asthma, including inflammation, goblet cell metaplasia and AHR.
Discussion
Recent advances in our understanding of the role of IL-33 in asthma and other allergic diseases such as atopic dermatitis has attracted much attention to IL-33 as a novel therapeutic target. A number of IL-33 targeting biologics are in clinical development (Phase 1 or 2) for asthma and other allergic diseaes. Two are monoclonal antibodies against ST2 (RG6149, formerly AMG282, Genentech; CNTO7160, GSK), and two others are monoclonal antibodies directly binding IL-33 (ANB020, AnaptysBio; REGN3500, Regeneron). However, although monoclonal antibodies are considered to be more efficient than soluble recombinant receptor molecules due to their pharmacokinetic properties, antibodies are inherently immunogenic and there is a potential for patients to develop anti-drug antibodies (ADA) over time, resulting in loss of clinical response.32, 33, 34 Of interest, a single cross-sectional study showed ADA development and lower efficacy outcomes in a higher proportion of patients with rheumatoid arthritis receiving an anti-TNF monoclonal antibody (adalimumab or infliximab) than in patients receiving a soluble dimeric TNF receptor fusion protein (etanercept).35 Since the IL-33trap sequence is based on naturally occuring receptor sequences, immunogenicity is expected to be lower than in the case of antibodies targeting IL-33 or its receptor. Therefore, our IL-33trap might be an attractive alternative to antibodies in case of ADA development. Furthermore, the absence of an Fc fragment in IL-33trap would avoid non-specific binding to Fc receptors and Fc-associated effector functions. Also, being receptor-based, IL-33trap can be expected to bind all active variants of IL-33, such as splice variants and IL-33 processing products that have been described,36, 37, 38 some of which may lack the epitope recognized by monoclonal antibodies. IL-33 has also been described to be rapidly inactivated by oxidation of critical cysteine residues.39 In this context, oxidized IL-33 will still bind most anti-IL-33 monoclonal antibodies and thus serve as a sink, reducing their efficiency. As oxidized IL-33 no longer binds ST2, such a an effect is unlikely to happen in case of IL-33trap. Similarly, the endogenous sST2 decoy receptor may serve as a sink for anti-ST2 antibodies, but not for IL-33trap. Moreover, as elevated serum sST2 levels in asthma and other inflammatory conditions are believed to exert an important regulatory role,40, 41, 42 it is better not to interfere with its normal homeostatic function.
In general, soluble receptor-based biologics have proven to be valuable alternatives for monoclonal antibodies. This is well illustrated by the TNF antagonist etanercept, the CD80/86 antagonist abatacept and the IL-1 antagonist rilonacept.43 In all cases, soluble receptors were engineered to encode an IgG Fc region to increase half-life and to permit dimerization and high affinity ligand binding. The monomeric nature and the use of a flexible linker in the design of our IL-33trap, circumventing the need for Fc fusion and production of two different recombinant proteins, offers a significant improvement in terms of manufacturing costs and possible undesired Fc-associated side effects. For future translational studies, it will however be most favorable to increase the half-life of the IL-33trap protein. The size of the IL-33trap molecule (~90 kDa for the non-glycosylated form) is above the renal filtration cut-off, but engineering FcRn recycling into the trap molecule by genetic fusion to serum albumin or serum albumin-binding moieties may be envisioned.44 Furthermore, reducing the glycosylation complexity and heterogeneity of IL-33trap might also have an impact on protein half-life in circulation due to a reduced catabolism conferred by lectin type receptors.
In conclusion, our data demonstrate that IL-33trap has great potential and can be considered a breakthrough technology for the development of new biologics against asthma and other allergic diseases. It must be mentioned that we were not able to show a protective effect of IL-33trap when given to mice with established allergic airway inflammation, as we show in figure 5 (panel H and I) that the IL-33trap has no effect on eosinophilia and Muc5a expression in the lung when analyzed in the re-challenge model. Therefore, it can be expected that also AHR and airway remodeling will not be inhibited (the opposite would not necessarily be true). These results are consistent with previously published data showing that combinatorial inhibition of IL-33, IL-25 and TSLP by monoclonal antibodies was incapable of inhibiting established chronic airway inflammation, but was effective at early stages of the disease.45 These data suggest that at later stages of disease, Th2 responses can bypass the IL-33 axis. Of interest, combined blockade of the IL-13 and IL-33 pathways was recently shown to lead to a greater inhibition of type 2 inflammation over inhibition of either pathway alone,46 supporting the idea that combinatiorial treatment approaches may yield additional efficacy over single-axis therapies alone. In this regard, also other cytokine trap proteins, such as the recently described TSLPtrap,47 may be interesting leads. The IL-33/ST2 axis has also been implicated in various other, non-Th2, diseases such as rheumatoid arthritis, colitis, multiple sclerosis, lupus, age-related macular degeneration, fibrosis, and disorders of the central nervous system,4,48 implicating that the impact of our IL-33trap technology may be much broader than allergic diseases. Given the apparent diverse roles of IL-33 in a multitude of processes, manipulation of IL-33 signaling may be highly disease dependent. Of note, as there is evidence that the IL-33/ST2 system also participates in tissue repair and (dys)regulates regulatory T cells in certain conditions,30, 49, 50 several questions and challenges remain. A better understanding of the impact of IL-33 and sST2 during disease and how IL-33/ST2 targeting could affect different organ systems will be critical for the further development of therapeutics like IL-33trap.
Clinical implications
IL-33trap may be an interesting lead towards the development of novel approaches for the treatment of inflammatory and allergic disease.
Capsule summary
IL-33 is an important therapeutic target in allergic diseases. Here we describe the generation and biological activity of a novel IL-33 antagonist, IL-33trap, which shows anti-inflammatory activities in a preclinical mouse model for acute allergic airway inflammation.
Acknowledgements
Funding sources
This work was supported by grants from the VIB, the Fund for Scientific Research Flanders (FWO), the Foundation Against Cancer, the Ghent University Concerted Research Actions (GOA), the Ghent University Industrial Research Fund (IOF). I.S.A. is a postdoctoral fellow of the FWO and holds an FWO research grant. E.V.N. is a predoctoral fellow of the FWO. S.D. is a predoctoral fellow of the Flanders Agency for Innovation and Development.
Abbreviations
- IL-33
Interleukin 33
- sST2
soluble ST2
- IgE
Immunoglobulin E
- ILC2
Innate lymphoid cells type 2
- Th2
T helper 2
- IL-1RL1
IL-1 receptor like 1
- IL-1RAcP
IL-1 receptor accessory protein
- HDM
house dust mite
- BMDM
bone marrow-derived macrophages
- i.p.
intraperitoneal
- i.t.
intratracheal
- GGS
glycine-glycine-serine
- PNGase
Peptide:N-Glycosidase F
- IMAC
immobilized metal affinity chromatography
- SEC
size exclusion chromatography
- ITC
isotermal titration calorimetry
- NF-ĸB
nuclear factor-kappa B
- TNF
tumor necrosis factor
- DC
dendritic cell
- PMA
phorbol 12-myristate 13-acetate
- TSLP
thymic stromal lymphopoietin
- IFN
interferon
- BAL
bronchoalveolar lavage
- AHR
airway hyperreactivity
- Muc5AC
Mucin 5AC
- qRT-PCR
Quantitative RT-PCR
- SPDEF
Sam-pointed domain containing Ets transcription factor
- Agr2
Anterior Gradient 2
- ADA
anti-drug antibodies
- FcRn
neonatal Fc receptor.
Footnotes
Author contributions: H.B., I.S.A., S.D., S.N.S., and R.B. contributed to the conception and design of the study. H.B., I.S.A., E.V.N., S.D., A.H., M.J.S., M.H., G.B., J.H., H.H., K.V., K.D. acquired the data. H.B., I.S.A., E.V.N., S.D., A.H., M.J.S., H.H., B.N.L., S.N.S., K.V., K.D., and R.B. analyzed and interpreted the data. H.B., I.S.A., S.N.S., and R.B. drafted the article or revised it critically for important intellectual content. All authors approved the final version of the manuscript.
Competing interests: H.B., R.B., B.N.L. and H.H. are co-inventors on a patent application (Novel IL-33 inhibitors; WO2014/090800; applicants: VIB and UGent) related to the IL-33trap used in this study, and which entered the national phase and is currently pending in Europe, Australia, Canada and the United States. All other authors declare that they have no competing interests. We thank Dr. Henry McSorley (University of Edinburgh) for advice.
References
- 1.Sears MR. Trends in the prevalence of asthma. Chest. 2014;145:219–225. doi: 10.1378/chest.13-2059. [DOI] [PubMed] [Google Scholar]
- 2.Potaczek DP, Garn H, Unger SD, Renz H. Antisense molecules: A new class of drugs. J Allergy Clin Immunol. 2016;137:1334–46. doi: 10.1016/j.jaci.2015.12.1344. [DOI] [PubMed] [Google Scholar]
- 3.Galli SJ. Toward precision medicine and health: Opportunities and challenges in allergic diseases. J Allergy Clin Immunol. 2016;137:1289–1300. doi: 10.1016/j.jaci.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun H, Afonina IS, Mueller C, Beyaert R. Dichotomous function of IL-33 in health and disease: From biology to clinical implications. Biochem Pharmacol. 2018;148:238–252. doi: 10.1016/j.bcp.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Vocca L, Di Sano C, Uasuf CG, Sala A, Riccobono L, Gangemi S, et al. IL-33/ST2 axis controls Th2/IL-31 and Th17 immune response in allergic airway diseases. Immunobiology. 2015;220:954–963. doi: 10.1016/j.imbio.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Noval Rivas M, Burton OT, Oettgen HC, Chatila T. IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function. J Allergy Clin Immunol. 2016;138:801–811.:e809. doi: 10.1016/j.jaci.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luthi AU, Cullen SP, McNeela EA, Duriez PJ, Afonina IS, Sheridan C, et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity. 2009;31:84–98. doi: 10.1016/j.immuni.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Martin SJ. Cell death and inflammation: the case for IL-1 family cytokines as the canonical DAMPs of the immune system. FEBS J. 2016;283:2599–2615. doi: 10.1111/febs.13775. [DOI] [PubMed] [Google Scholar]
- 9.Kim HY, Chang YJ, Subramanian S, Lee HH, Albacker LA, Matangkasombut P, et al. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. J Allergy Clin Immunol. 2012;129:216–227.:e211-216. doi: 10.1016/j.jaci.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halim TY, Steer CA, Matha L, Gold MJ, Martinez-Gonzalez I, McNagny KM, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–435. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Takagi T, Yanagisawa K, Tsukamoto T, Tetsuka T, Nagata S, Tominaga S. Identification of the product of the murine ST2 gene. Biochim Biophys Acta. 1993;1178:194–200. doi: 10.1016/0167-4889(93)90009-e. [DOI] [PubMed] [Google Scholar]
- 13.Felix J, Savvides SN. Mechanisms of immunomodulation by mammalian and viral decoy receptors: insights from structures. Nat Rev Immunol. 2017;17:112–129. doi: 10.1038/nri.2016.134. [DOI] [PubMed] [Google Scholar]
- 14.Grotenboer NS, Ketelaar ME, Koppelman GH, Nawijn MC. Decoding asthma: translating genetic variation in IL33 and IL1RL1 into disease pathophysiology. J Allergy Clin Immunol. 2013;131:856–865. doi: 10.1016/j.jaci.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 15.Prefontaine D, Lajoie-Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ, et al. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol. 2009;183:5094–5103. doi: 10.4049/jimmunol.0802387. [DOI] [PubMed] [Google Scholar]
- 16.Prefontaine D, Nadigel J, Chouiali F, Audusseau S, Semlali A, Chakir J, et al. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol. 2010;125:752–754. doi: 10.1016/j.jaci.2009.12.935. [DOI] [PubMed] [Google Scholar]
- 17.Jackson DJ, Makrinioti H, Rana BM, Shamji BW, Trujillo-Torralbo MB, Footitt J, et al. IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am J Respir Crit Care Med. 2014;190:1373–1382. doi: 10.1164/rccm.201406-1039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castanhinha S, Sherburn R, Walker S, Gupta A, Bossley CJ, Buckley J, et al. Pediatric severe asthma with fungal sensitization is mediated by steroid-resistant IL-33. J Allergy Clin Immunol. 2015;136:312–322.:e317. doi: 10.1016/j.jaci.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saglani S, Lui S, Ullmann N, Campbell GA, Sherburn RT, Mathie SA, et al. IL-33 promotes airway remodeling in pediatric patients with severe steroid-resistant asthma. J Allergy Clin Immunol. 2013;132:676–685.:e613. doi: 10.1016/j.jaci.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sati SP, Singh SK, Kumar N, Sharma A. Extra terminal residues have a profound effect on the folding and solubility of a Plasmodium falciparum sexual stage-specific protein over-expressed in Escherichia coli. Eur J Biochem. 2002;269:5259–5263. doi: 10.1046/j.1432-1033.2002.03237.x. [DOI] [PubMed] [Google Scholar]
- 21.Scheuermann TH, Brautigam CA. High-precision, automated integration of multiple isothermal titration calorimetric thermograms: new features of NITPIC. Methods. 2015;76:87–98. doi: 10.1016/j.ymeth.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao H, Piszczek G, Schuck P. SEDPHAT--a platform for global ITC analysis and global multi-method analysis of molecular interactions. Methods. 2015;76:137–148. doi: 10.1016/j.ymeth.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elegheert J, Bracke N, Pouliot P, Gutsche I, Shkumatov AV, Tarbouriech N, et al. Allosteric competitive inactivation of hematopoietic CSF-1 signaling by the viral decoy receptor BARF1. Nat Struct Mol Biol. 2012;19:938–947. doi: 10.1038/nsmb.2367. [DOI] [PubMed] [Google Scholar]
- 24.Velazquez-Campoy A, Ohtaka H, Nezami A, Muzammil S, Freire E. Isothermal titration calorimetry. Curr Protoc Cell Biol. 2004;23:17.8.1–17.8.24. doi: 10.1002/0471143030.cb1708s23. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Hammel M, He Y, Tainer JA, Jeng US, Zhang L, et al. Structural insights into the interaction of IL-33 with its receptors. Proc Natl Acad Sci USA. 2013;110:14918–14923. doi: 10.1073/pnas.1308651110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiseman T, Williston S, Brandts JF, Lin LN. Rapid measurement of binding constants and heats of binding using a new titration calorimeter. Anal Biochem. 1989;179:131–137. doi: 10.1016/0003-2697(89)90213-3. [DOI] [PubMed] [Google Scholar]
- 27.Velazquez-Campoy A, Freire E. Isothermal titration calorimetry to determine association constants for high-affinity ligands. Nat Protoc. 2006;1:186–191. doi: 10.1038/nprot.2006.28. [DOI] [PubMed] [Google Scholar]
- 28.Theoharides TC, Petra AI, Taracanova A, Panagiotidou S, Conti P. Targeting IL-33 in autoimmunity and inflammation. J Pharmacol Exp Ther. 2015;354:24–31. doi: 10.1124/jpet.114.222505. [DOI] [PubMed] [Google Scholar]
- 29.Snelgrove RJ, Gregory LG, Peiró T, Akthar S, Campbell GA, Walker SA, et al. Alternaria-derived serine protease activity drives IL-33-mediated asthma exacerbations. J Allergy Clin Immunol. 2014;134:583–592. doi: 10.1016/j.jaci.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen CC, Kobayashi T, Iijima K, Hsu FC, Kita H. IL-33 dysregulates regulatory T cells and impairs established immunologic tolerance in the lungs. J Allergy Clin Immunol. 2017;140:1351–1363.:e7. doi: 10.1016/j.jaci.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Godar M, Deswarte K, Vergote K, Saunders M, de Haard H, Hammad H, et al. A bispecific antibody strategy to target multiple type 2 cytokines in asthma. J Allergy Clin Immunol. 2018;142:1185–1193.:e4. doi: 10.1016/j.jaci.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartelds GM, Krieckaert CL, Nurmohamed MT, van Schouwenburg PA, Lems WF, Twisk JW, et al. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA. 2011;305:1460–1468. doi: 10.1001/jama.2011.406. [DOI] [PubMed] [Google Scholar]
- 33.van Schouwenburg PA, Rispens T, Wolbink GJ. Immunogenicity of anti-TNF biologic therapies for rheumatoid arthritis. Nat Rev Rheumatol. 2013;9:164–172. doi: 10.1038/nrrheum.2013.4. [DOI] [PubMed] [Google Scholar]
- 34.Bendtzen K, Geborek P, Svenson M, Larsson L, Kapetanovic MC, Saxne T. Individualized monitoring of drug bioavailability and immunogenicity in rheumatoid arthritis patients treated with the tumor necrosis factor alpha inhibitor infliximab. Arthritis Rheum. 2006;54:3782–3789. doi: 10.1002/art.22214. [DOI] [PubMed] [Google Scholar]
- 35.Moots RJ, Xavier RM, Mok CC, Rahman MU, Tsai WC, Al-Maini MH, et al. The impact of anti-drug antibodies on drug concentrations and clinical outcomes in rheumatoid arthritis patients treated with adalimumab, etanercept, or infliximab: Results from a multinational, real-world clinical practice, non-interventional study. PLoS One. 2017;12:e0175207. doi: 10.1371/journal.pone.0175207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gordon ED, Simpson LJ, Rios CL, Ringel L, Lachowicz-Scroggins ME, Peters MC, et al. Alternative splicing of interleukin-33 and type 2 inflammation in asthma. Proc Natl Acad Sci USA. 2016;113:8765–8770. doi: 10.1073/pnas.1601914113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong J, Bae S, Jhun H, Lee S, Choi J, Kang T, et al. Identification of constitutively active interleukin 33 (IL-33) splice variant. J Biol Chem. 2011;286:20078–20086. doi: 10.1074/jbc.M111.219089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott IC, Majithiya JB, Sanden C, Thornton P, Sanders PN, Moore T, et al. Interleukin-33 is activated by allergen- and necrosis-associated proteolytic activities to regulate its alarmin activity during epithelial damage. Sci Rep. 2018;8:3363. doi: 10.1038/s41598-018-21589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen ES, Scott IC, Majithiya JB, Rapley L, Kemp BP, England E, et al. Oxidation of the alarmin IL-33 regulates ST2-dependent inflammation. Nat Commun. 2015;6:8327. doi: 10.1038/ncomms9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diaz-Jimenez D, Nunez LE, Beltran CJ, Candia E, Suazo C, Alvarez-Lobos M, et al. Soluble ST2: a new and promising activity marker in ulcerative colitis. World J Gastroenterol. 2011;17:2181–2190. doi: 10.3748/wjg.v17.i17.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oshikawa K, Kuroiwa K, Tago K, Iwahana H, Yanagisawa K, Ohno S, et al. Elevated soluble ST2 protein levels in sera of patients with asthma with an acute exacerbation. Am J Respir Crit Care Med. 2001;164:277–281. doi: 10.1164/ajrccm.164.2.2008120. [DOI] [PubMed] [Google Scholar]
- 42.Weinberg EO, Shimpo M, Hurwitz S, Tominaga S, Rouleau JL, Lee RT. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation. 2003;107:721–726. doi: 10.1161/01.cir.0000047274.66749.fe. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt SR. Fusion-proteins as biopharmaceuticals--applications and challenges. Curr Opin Drug Discov Devel. 2009;12:284–295. [PubMed] [Google Scholar]
- 44.Kontermann RE. Strategies for extended serum half-life of protein therapeutics. Curr Opin Biotechnol. 2011;22:868–876. doi: 10.1016/j.copbio.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 45.Vannella KM, Ramalingam TR, Borthwick LA, Barron L, Hart KM, Thompson RW, et al. Combinatorial targeting of TSLP, IL-25, and IL-33 in type 2 cytokine-driven inflammation and fibrosis. Sci Transl Med. 2016;8:337ra365. doi: 10.1126/scitranslmed.aaf1938. [DOI] [PubMed] [Google Scholar]
- 46.Ramirez-Carrozzi V, Sambandam A, Zhou M, Yan D, Kang J, Wu X, et al. Combined blockade of the IL-13 and IL-33 pathways leads to a greater inhibition of type 2 inflammation over inhibition of either pathway alone. J Allergy Clin Immunol. 2017;139:705–708.:e6. doi: 10.1016/j.jaci.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 47.Verstraete K, Peelman F, Braun H, Lopez J, Van Rompaey D, Dansercoer A, et al. Structure and antagonism of the receptor complex mediated by human TSLP in allergy and asthma. Nat Commun. 2017;8:14937. doi: 10.1038/ncomms14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arshad MI, Khan HA, Noel G, Piquet-Pellorce C, Samson M. Potential Therapeutic Aspects of Alarmin Cytokine Interleukin 33 or Its Inhibitors in Various Diseases. Clin Ther. 2016;38:1000–1016.:e1001. doi: 10.1016/j.clinthera.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 49.Nascimento DC, Melo PH, Pineros AR, Ferreira RG, Colon DF, Donate PB, et al. IL-33 contributes to sepsis-induced long-term immunosuppression by expanding the regulatory T cell population. Nat Commun. 2017;8:14919. doi: 10.1038/ncomms14919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117:1538–1549. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]