Abstract
Dendritic cells (DCs) are critical for the activation of naïve CD4+ T cells and are considered professional antigen presenting cells (APCs), as are macrophages and B cells. It has recently emerged that several innate type 2 immune cells such as basophils, mast cells, eosinophils and innate type 2 lymphocytes (ILC2) can also behave as APCs. Through surface expression or transfer of peptide-loaded MHC-II, expression of costimulatory and co-inhibitory molecules, as well as secretion of polarizing cytokines, these innate cells can extensively communicate with effector and regulatory CD4+ T cells. An exciting new concept is that the complementary tasks of these “amateur” APCs contributes to shaping and regulating adaptive immunity to allergens and helminths, often in collaboration with professional APCs.
Keywords: Antigen-presenting cells, Antigen presentation, Basophils, Mast cells, Innate lymphoid cells, Type-2 Immunity
Importance of antigen presentation in type 2 immunity
In all mammals, cellular type 2 immunity is characterized by the tissue influx of eosinophils, mast cells (MCs), basophils and alternatively activated macrophages (AAMs), as well as by the remodelling of tissues with increased mucus-producing cells, increased contractility of smooth muscle cells, and ultimately fibrosis. This type of inflammation has been selected through evolution in defence to helminths, and stings or bites from snakes, insects and ticks. (Mukai et al., 2016; Palm et al., 2012). Excessive and misdirected type 2 inflammation to harmless environmental food and inhaled allergens is also typically seen in allergic diseases including asthma, rhinitis, atopic dermatitis, and eosinophilic oesophagitis, which are clearly on the rise in industrialized countries (Lambrecht and Hammad, 2017; Paul, 2010). Although innate immunity plays a predominant role in type 2 inflammation, adaptive Th2 CD4+ T cells play a crucial role in enforcing this type of response, through production of the type 2 cytokines IL-4, IL-5, IL-9 and IL-13. Therefore, understanding the presentation of antigens and subsequent CD4+ T cell activation both at the naïve and memory T cell stage has important implications. It would not only allow us to better understand why and how allergies are increasing and often become chronic, but also identify ways to prevent allergic diseases, or allow better design of vaccines for long term protection against helminth infection. A professional antigen presenting cell (APC) has the ability to capture and process foreign antigens, and to deliver the three signals for T cell activation (peptide MHC-TCR, costimulation and polarizing cytokines). Additionally, for induction of primary immune responses the professional APC should be able to migrate to the draining lymph nodes, to efficiently activate those few recirculating naïve T cell clones that express ‘useful’ cognate antigen receptors (Kambayashi and Laufer, 2014). Although dendritic cells (DCs), macrophages and B cells are well known as professional APCs, other cells bearing MHC class II molecules could harbour antigen-presenting functions in type 2 immune responses; including basophils, mast cells, eosinophils, and type 2 innate lymphoid cells (ILC2). Due to poor antigen processing capacity in naïve hosts and tissue residency, it is unlikely that these cells have major capacities to prime naïve T cells. In the context of chronic type 2 inflammation however these cells often become activated, armed with IgE molecules and might become very apt in presenting antigens to effector and long term memory CD4+ T cells in tissues and thus contribute to chronicity, remodelling and resolution of inflammation. Here, we review the experimental data available on the APC function of the various MHCII expressing cells in type 2 immunity.
How do APCs induce type 2 immunity?
Studies in mice have shown that type 2 stimuli such as chitin, helminths or allergens often first elicit a chemokine and cytokine response in barrier epithelial cells, within minutes after exposure. Chemokines such as eotaxin, CCL17 and CCL22 subsequently rapidly recruit eosinophils and ILC2s into the barrier tissue, while the stress-induced epithelial cytokines thymic stromal lymphopoietin (TSLP), interleukin-25 (IL-25) and IL-33 can activate IL-5 and IL-13 production in the recruited ILC2s, thus boosting eosinopoiesis and goblet cell metaplasia (Hammad et al., 2009; Van Dyken et al., 2014; Van Dyken et al., 2016; Zaph et al., 2007). Concurrent with this innate response taking place in exposed tissues, APCs will also respond to the epithelial cytokines, will take up antigens and subsequently migrate to lymph nodes or spleen to induce T cell activation and differentiation of naïve CD4+ T cells (Lambrecht and Hammad, 2012). The precise molecular interactions that lead to Th2 polarization are still incompletely understood, despite years of intense research. DC activation in a type 2 environment is however not like direct pattern recognition receptor mediated activation, and does not elicit IL-12 production, one of the main cytokines driving the alternative Th1 fate. In mice and humans, some type 2 stimuli or endogenous type 2 mediators such as excretory-secretory (ES) products of helminths, prostanoids and cytokines even actively suppress IL-12 production in DCs in vitro and in vivo (Everts et al., 2012; Ito et al., 2005; Massacand et al., 2009; Traidl-Hoffmann et al., 2005). Alternatively, the epithelial cytokines IL-1α, IL-33, TSLP and granulocyte-macrophage colony stimulating factor (GM-CSF) are responsible for the Th2 programming of DCs, by suppressing IL-12 production and inducing expression of OX40L and Notch receptor ligands on DCs (de Kleer et al., 2016; Willart et al., 2012). Although these surface molecules can promote Th2 polarization, blockade in vivo of any of these pathways doesn’t necessarily affect Th2 polarization in all models studied (Chu et al., 2013; Tu et al., 2005). At the T cell level, Th2 differentiation starts with induction of a TCR driven activation of GATA3 transcription factor, and induction of STAT5 phosphorylation, triggered by IL-2, or TSLP (Zhang et al., 1997). Further commitment to the Th2 lineage, is greatly enhanced by STAT-6 phosphorylation in response to IL-4Rα triggering by the polarizing cytokine IL-4 (Shimoda et al., 1996). Since this cytokine is not made by DCs themselves, a lot of effort has been placed in trying to elucidate the cellular source of the initial burst of IL-4 production. Innate basophils, eosinophils and ILC2s have all been proposed to provide this early source of IL-4 in trans, and the fact that these cells can be found in the T cell area early in an immune response makes this a possible scenario (Ben-Sasson et al., 1990; Hammad et al., 2010; Sokol et al., 2008; van Rijt et al., 2003). Antigens that lead to low dose MHCII-peptide display or low affinity ligands for TCR triggering, also tend to favour Th2 polarization. The potential of basophils and eosinophils to simultaneously produce IL-4 and to display low dose peptide-MHCII led some investigators to suggest that type 2 immune responses are preferentially and even exclusively induced by these non-professional APCs in vitro and in vivo(Perrigoue et al., 2009; Sokol et al., 2008; Sokol et al., 2009).
Immune responses to allergens and helminths also lead to Bcl6-dependent IL-4 producing CD4+ T follicular helper cells (TFH) that stay in the lymphoid organs (Dell’Aringa and Reinhardt, 2018; Kobayashi et al., 2017; Krishnaswamy et al., 2017; Liang et al., 2012; Reinhardt et al., 2009). Antigen experienced B cells will, under the influence of IL-4 producing TFH, undergo immunoglobulin (Ig) class switching to produce the type 2 immunoglobulins IgE and IgG1. The production of antigen-specific IgE and IgG1 (in mice) are hallmarks of the humoral type 2 immune response (Talay et al., 2012). In type 2 immune responses, antigen-specific IgE and IgG1 arms tissue MCs and circulating basophils so that these can degranulate immediately upon renewed encounter with the antigen and crosslinking of prebound antibodies by the antigen, thus causing an immediate hypersensitivity reaction (Galli and Tsai, 2012; Yamanishi et al., 2017).
APCs in ongoing type 2 immunity
After a priming and proliferation phase in the draining lymph nodes, Th2 effector cells migrate back to the exposed tissues, to enforce ongoing innate immunity (Coquet et al., 2015; Harris et al., 2002; Shinkai et al., 2002; Van Dyken et al., 2016). Given the nature of the type of antigens that induce it (e.g. environmental ubiquitous allergens and tissue dwelling parasites that evade immune recognition), type 2 immunity often becomes chronic and localized to tissues, since the antigen is persistently present (Li et al., 2016a(Li et al., 2016a). Some of the Th2 cells will also adopt a phenotype of long lived tissue resident TRM cells, which can be restimulated months later and produce immediate cytokines in the periphery (Hondowicz et al., 2016; Lloyd and Harker, 2016). For immediate CD4+ effector T cell reactivation (van Rijt et al., 2005), as well as for setting up a niche for long term survival of CD4+ T cells, interactions with MHCII-positive APCs are needed, although the precise nature of these MHCII expressing cells has not been elucidated. Given the fact that effector and memory T cells are less dependent on costimulatory molecules, are kept alive by low level TCR signalling, and reside in tissues rather than lymph nodes, any non-professional non-migratory APC that expresses MHCII might suffice.
Another major change that occurs in the antigen experienced host is the persistence of antigen-specific immunoglobulins, that can significantly alter the activation state of professional and non-professional APCs by binding to various activating or inhibitory Fc receptors (Guilliams et al., 2014). Antigen-specific IgE and IgG1 can bind to low and high affinity Fc receptors on mast cells, basophils, eosinophils, macrophages and DCs, and can be used to much more efficiently capture allergens for presentation to tissue dwelling effector or resident memory T cells.
Professional antigen-presenting cells for type 2 immunity
Studies in mice have shown that DCs are crucial for induction of type 2 immunity in naïve hosts. In particular, IRF4-expressing CD11b+ CD172+ type 2 conventional DCs (cDC2s) are necessary and sufficient to drive Th2 polarization in the skin, gut, and lung (Connor et al., 2017; Deckers et al., 2017b; Gao et al., 2013; Hammad et al., 2010; Krishnaswamy et al., 2017; Kumamoto et al., 2013; Phythian-Adams et al., 2010; Plantinga et al., 2013; Tussiwand et al., 2015; Williams et al., 2013). Type 2 immunity induced by cDC2s is heavily instructed by barrier epithelial cells. By their release of Th2 instructive cytokines including TSLP, GM-CSF, IL-33, IL-1 and IL-25, and via induction of a type I interferon response, the barrier epithelial cells actively determine whether DCs respond or tolerate antigenic challenges and what type of immune response is induced (Connor et al., 2017; de Kleer et al., 2016; Deckers et al., 2017a; Hammad and Lambrecht, 2015; Lambrecht and Hammad, 2014). Age also determines the Th2 potential of cDC2. In the neonatal period, mouse cDC2s express OX40L and produce little polarizing IL-12, which may help explain the bias towards type 2 immunity in this early period of life (de Kleer et al., 2016). The other type of conventional DCs, the IRF8-expressing cDC1 as well as plasmacytoid DCs seem to control tolerance to allergens and actively suppress Th2 responses, sometimes via induction of regulatory T cells (Tregs) (de Heer et al., 2004; Everts et al., 2016; Khare et al., 2013; Kuipers and Lambrecht, 2004; Lombardi et al., 2012; Semmrich et al., 2012)). In models of HDM driven asthma and immunity to the helminth H. polygyrus, generation of TFH responses in the lung draining lymph nodes depend on IRF4-expressing cDC2 that also express CXCR5 an migrate to T-B cell border of lymph nodes (Krishnaswamy et al., 2017; Leon et al., 2012). In models of N. brasiliensis helminth infection and immunization with the Th2 adjuvant alum, IL-4+ TFH development was also shown to depend on Notch ligand expression by conventional DCs and T cell intrinsic Notch signalling, whereas Th2 development did not (Dell’Aringa and Reinhardt, 2018). A big question in the field is whether subsets of cDC2s exist that would preferentially induce TFH over effector Th2 immune responses. Recent data also suggest that the potential of cDC2s to induce pure Th2 immunity or a mixed Th2/Th17 type of response is tightly regulated by cell intrinsic mechanisms, including fine tuning of TLR signalling pathways and metabolic programming (Sinclair et al., 2017; Vroman et al., 2017). Understanding induction of such mixed Th2/Th17 responses is important, since this profile is often seen in steroid-resistant difficult-to-treat allergic diseases.
B cells can induce Th2 and TFH responses in vitro and elicit help from CD4+ T cells in an MHC-II dependent manner, but using T cell antigen receptor (TCR) transgenic 1-DER mice that react to the type 2 antigen Der p 1 of the house dust mite Dermatophagoides pteronyssinus, little evidence for B cells was found in driving primary Th2 differentiation in vivo (Dullaers et al., 2016). However, there are some recent data to suggest that antigen presentation by B cells in the HDM model controls the rate of formation of T resident memory cells versus IL-4 producing TFH cells, since these are mutually exclusive cell fates for antitehn-reactive naïve T cells (Ballesteros-Tato et al., 2016; Hondowicz et al., 2016). Thus, mice lacking B cells had less TFH cells and an increase in TRM cells, explaining why B cell deficient mice had increased airway inflammation when challenged with HDM inhalation long after the priming period (Hondowicz et al., 2016). However, further studies are warranted to better understand B-cell induction of Th2 cells in vivo.
Macrophages capture large amounts of type 2 allergens and become M2 polarized in type 2 immune responses such as helminth infection and asthma in mouse and man (Girodet et al., 2016; Reece et al., 2006). In the lungs, clodronate based depletion systems have shown that alveolar macrophages can actively suppress DC and T cell activation, while interstitial macrophages can promote tolerance to inhaled ovalbumin antigen by inducing CD4+ Foxp3+ Tregs in a partially IL-10 dependent manner (Bedoret et al., 2009; Bilyk and Holt, 1993; Sabatel et al., 2017; Soroosh et al., 2013). Monocyte derived DCs (moDCs) are found in sites of type 2 inflammation and share characteristics of cDC2s (they can process and present antigens and are MHCIIhiCD11bhiCD11chi) as well as those of macrophages (they express CD64, are poorly migratory, and labelled by MafB lineage tracing) (Plantinga et al., 2013; Wu et al., 2016). Given their poor migratory capacity, it is unlikely that lung macrophages or moDCs contribute strongly to priming Th2 responses in naïve hosts. We have proposed that high dose HDM allergen exposure can lead to migratory moDCs that can promote allergen-specific Th2 responses when adoptively transferred to naïve hosts (Plantinga et al., 2013), but the moDCs used were sorted based on expression of the marker MAR1, which could have led to co-purification of a small population of cDC2s.
In ongoing and established type 2 inflammation, models of conditional depletion of CD11chi cells using diphtheria toxin in Cd11c-DTR transgenic mice have shown that cDCs and/or moDCs are crucially needed for the effector Th2 response irrespective of effects on Th2 priming, and even in mice that have very chronic type 2 inflammation in the lungs (van Rijt et al., 2005; Van Rijt et al., 2004; van Rijt et al., 2011). Deletion of CD11b+ moDCs during ongoing type 2 immune responses in the lungs of Cd11b-DTR mice can abolish Th2 cytokine production and allergic eosinophilic airway inflammation (Medoff et al., 2009). Monocyte derived DCs produce the chemokines that can attract Th2 cells back to the lungs, and provide MHCII and constimulatory molecules for incoming effector cells to increase their cytokine production within the lungs (Harris et al., 2002; Medoff et al., 2009; Van Rijt et al., 2004). Given the almost complete absence of Th2 priming or recall responses in mice devoid of DCs, these experiments have certainly thought us that DCs are professional APCs that are crucial during every phase of the adaptive type 2 immune response.
Basophils as APCs
In humans and mice, basophils are a scarce population of circulating blood leukocytes, that play non-redundant roles in Th2 immunity by secreting various inflammatory mediators such as histamine, platelet activating factor, proteoglycans and venom degrading proteases. A key activating event for basophils (and mast cells) is the cross-linking of the high-affinity IgE receptor (FcεRIα) by allergens or venoms (Schwartz et al., 2016; Yamanishi et al., 2017). In order to address the APC function of basophils, depleting MAR-1 (directed against FcεRIα) or Ba103 (directed against CD200R3) monoclonal antibodies, have been used in different mouse in vivo allergen-driven models of inflammatory disease, driven by antigens such as papain (Sokol et al., 2009), ovalbumin (Yoshimoto et al., 2009), or Trichuris muris (Perrigoue et al., 2009). These studies reported basophils to be both necessary and sufficient to induce Th2 immunity, presenting them as APCs and a potent source of early polarizing IL-4 (Perrigoue et al., 2009; Sokol et al., 2009; Yoshimoto et al., 2009). The idea of a type 2 inducing APC that can simultaneously produce polarizing IL-4 and present antigen was very attractive since it offered a long sought-after model that resembled the model of Th1 immunity, where the DC simultaneously presents antigen and produces Th1 polarizing IL-12. However, later studies have shown that the antibodies used to deplete basophils can also target bona fide DCs, and thus the effects of basophil depletion on APC function could be explained by concomitant depletion of DCs (Hammad et al., 2010). Moreover, ex vivo isolated basophils from HDM-challenged mice were only mildly able to present and process antigen, compared to FcεRIα + DCs, and gene expression analysis revealed that there was little if any expression of proteins involved in antigen processing, making it unlikely they would be truly strong APCs (Hammad et al., 2010). However, in some mouse models, DCs alone were unable to skew T cells to a Th2 phenotype ex vivo and required additional help of basophils (Allenspach et al., 2008). This idea of collaboration between basophils and DCs was also recapitulated in a model of subcutaneous papain injection in the mouse, were the generation of reactive oxygen species and the subsequent activation of DCs was shown to promote basophil recruitment into the draining lymph node. Subsequently, basophils and DCs collaborated to promote Th2 polarization (Tang et al., 2010). In a model of spontaneous development of type 2 immunity by skin-specific overexpression of TSLP in mice, dermal DCs induced IL-3 production by recently activated unpolarised CD4 T cells in an OX40L-dependent manner, which led to the recruitment of IL-4 producing basophils and the subsequent polarization of Th2 T cells, suggesting that the collaboration between basophils and DCs requires a responding cognate T cell, but not necessarily pin-pointing the basophil as the presenting cell type (Leyva-Castillo et al., 2013). Basophils have also been proposed to enhance antibody production in a model of bacterial infection in mice and particularly boost memory B cell responses via direct effects on B cells and indirect enhancement of T cell help for B cells (Denzel et al., 2008). However, whether basophils can boost TFH differentiation, and if this would occur in type 2 immune responses remains to be shown.
Recently, genetic models to deplete basophils have become available, and these allowed for a more definitive assessment whether basophils are really required as APCs to generate adaptive Th2 immunity. Th2 cell polarization was found to be unaffected in genetically basophil-depleted Mcpt8Cre conditional knockout mice during primary infection with different helminths (Ohnmacht et al., 2010; Schwartz et al., 2014). Sensitization and challenge of Mcpt8Cre mice with Ovalbumin (OVA)-Alum and papain-OVA also showed normal expansion of Th2 responses, while genetically DC-depleted animals showed severely impaired responses (Kim et al., 2013b; Ohnmacht et al., 2010). Genetic depletion of basophils using Basoph8 x Rosa-DTA mice followed by footpad immunization with the parasite Schistosoma mansoni, also resulted in normal Th2 priming (Sullivan et al., 2011). Not only do these experiments question the role of the basophil as an APC for type 2 immunity, they even cast some doubts on the dogma that the basophil is an important bystander provider of the crucial polarizing IL-4 cytokine during initiation of type 2 immunity (Kim et al., 2013b; Sokol et al., 2008).
Recently however, the idea of the basophil as an APC has been reignited. Although it was shown that basophils in mice and humans express little MHCII on their surface (Hammad et al., 2010), bone-marrow cultured murine basophils generated in vitro using IL-3 and GM-CSF, have been reported to substantially upregulate MHC-II on their surface, although these cells still showed little transcription of the corresponding MHC-II gene, and these cultures also lead to expansion of MHCII-positive DCs (Yamanishi et al., 2017). More in-depth cell biological analysis using co-culture experiments of wild type DCs and Mhc2-deficient basophils revealed transfer of MHC-II molecules from DCs to basophils in a contact-dependent manner (Yamanishi et al., 2017). In vivo basophils were also found to display low levels of MHC-II on their cell surface, possibly acquired from DCs through trogocytosis (Miyake et al., 2017; Yoshimoto et al., 2009). Even in the absence of antigen processing, the cell surface display of peptide-loaded MHC-II in these basophils was able to stimulate T cells (Miyake et al., 2017). Together, these data provide evidence that although basophils may not be directly involved in primary antigen presentation, they can modulate type-2 immune responses in a variety of ways, although the mechanisms by which this happens and the different outcomes need further in-depth study.
Mast cells as APCs
In contrast to basophils, mast cells (MCs) are considered to be more like tissue-resident cells, conveniently located in mucosal tissues were they are in close contact with the external environment. Murine and human MCs possess many APC characteristics, but the physiological importance of this feature in vivo is still debated (Galli and Gaudenzio, 2018). In mouse models of experimental autoimmune encephalomyelitis (Gregory et al., 2005) and Leishmania major (Dudeck et al., 2011; Maurer et al., 2006) infection, MC-deficient mice have been shown to have defective CD4+ T cell responses. However, the antigen presenting capacity is very context dependent (Elieh Ali Komi and Grauwet, 2018; Galli et al., 2005; Kambayashi et al., 2009; Kambayashi and Laufer, 2014). For instance, freshly isolated mouse peritoneal cavity MCs and human skin MCs lacked MHC-II expression, although treatment with IL-4 and IFNγ or ligation of Notch receptors could activate MHC-II expression after in-vitro culture (Frandji et al., 1996; Frandji et al., 1995; Galli and Gaudenzio, 2018; Gaudenzio et al., 2009; Lotfi-Emran et al., 2018; Nakano et al., 2012). MCs can also fine-tune human CD4+ T cell cytokine profiles via the induction of IFNγ and IL-22 production from circulating memory CD4+ T cells (Gaudenzio et al., 2013; Lotfi-Emran et al., 2018). After Toll like receptor TLR4-dependent activation, as well as after undergoing an FcƐRI-mediated degranulation process, human and mouse MCs upregulate other molecules associated with antigen presentation, such as the invariant chain (Ii) and the peptide exchanger HLA-DM, and thus become proficient at inducing autologous memory T cell activation (Kambayashi et al., 2009; Lotfi-Emran et al., 2018). Together these data provide the notion that MHC-II expression on MCs may be regulated either by cell maturation in the tissue, or by the induction of MHC-II at specific anatomical locations via the expression of endogenous ligands. Since these cells our however poorly migratory to lymph nodes, and can poorly activate naïve T cells due to minor cos-stimulatory molecule expression, it is unlikely that they serve as APCs in the primary immune response.
Just like basophils cooperate with DCs during the antigen presentation process, MCs and DCs join forces in type 2 immunity, by forming immunological synapses with each other. These dynamic interactions facilitate transfer of endosomal contents, including the transfer of antigen and membrane of activated MCs to DCs, resulting in the processing and presentation of transferred antigens and ultimately the activation of T cell responses (Carroll-Portillo et al., 2015; Kambayashi et al., 2008). MCs certainly differ from professional APCs in being able to use different mast cell granule proteolytic pathways to process antigen, and it would be favourable for DCs to exploit these additional pathways to generate peptide ligands for MHC, as the peptides generated might lead to diversification of the TCR repertoire (Lotfi-Emran et al., 2018). In a model of dinitrofluorobenzene skin injection in mice, DCs demonstrate a scanning behaviour on stationary MCs, triggered by mast cell degranulation (Dudeck et al., 2017). Prior depletion of skin DCs in these experiments led to reduced MHCII expression on mast cells, suggesting the transfer of MHCII from DC to MC, just like it occurs through trogocytosis between basophils and DCs, and to reduced antigen presentation to CD4 T cells. Moreover, long-term cultures of bone marrow-derived MCs in resting conditions showed that while MCs did not express MHC-II on their surface, they accumulated these in secretory vesicles (Kambayashi et al., 2009; Raposo et al., 1997). Following IgE crosslinking, MCs have been reported to secrete MHCII+ exosomes that also express OX40L and promote development of Th2 responses, presumably after being taken up by DCs (Li et al., 2016b; Skokos et al., 2003). Finally, MCs can also indirectly help shape the nature of the adaptive immune response by promoting the migration of DCs to draining nodes in mice (Galli and Gaudenzio, 2018; Suto et al., 2006). In this regard MC-deficient mice might demonstrate defects in antigen presentation, that are not intrinsic to mast cells.
Together, it cannot be excluded that some MC populations may present antigen and activate naïve CD4+ T cells in vivo, although given their sessile nature it seems more likely that they preferentially activate and expand antigen-experienced T cells and Tregs in tissues (Kambayashi et al., 2009).
Eosinophils as APCs
Eosinophils constitute a primitive myeloid cell population historically viewed as effector cells in the defence against extracellular parasites (Rosenberg et al., 2013). Recent work, however, has extended our views on the role of eosinophils in homeostasis and in immunity. Indeed, under homeostatic conditions, mouse eosinophils can exert a variety of homeostatic functions, including control of metabolism, growth and involution of organs, and immunoregulation through suppression of DC activation (Jacobsen et al., 2012; Mesnil et al., 2016; Travers and Rothenberg, 2015; Weller and Spencer, 2017). It has been well established that during inflammation, eosinophils respond to signals provided by T cells and ILC2s (such as IL-5, GM-CSF and chemokines produced during allergic inflammation). Through different modes of secretion as well as eosinophil extracellular DNA trap formation, eosinophils release granules containing toxic cationic proteins and inflammatory mediators that can in turn, activate DCs and stromal cells (Rosenberg et al., 2013). Eosinophils also produce chemokines crucial for CD4+ Th2 cell recruitment (MacKenzie et al., 2001), and produce Th2 polarizing cytokines such as IL-4 (Gessner et al., 2005; Sabin et al., 1996).
In models of allergy, helminth infection and IL-5 transgenic mice, eosinophils, can express MHC-II and the co-stimulatory molecules CD80 and CD86, and can even migrate to the draining nodes of the lungs and peritoneum in a CCR7 and leukotriene C4 dependent manner (Wang et al., 2016; Wang et al., 2007). Whereas mouse intestinal and lymph node eosinophils constitutively express MHC class II (Shi et al., 2000; van Rijt et al., 2003; Xenakis et al., 2018), eosinophils in other microenvironments, such as in the blood or peritoneal cavity do not (Wang et al., 2007). It is known that eosinophils can also process antigens and stimulate primed CD4+ T cells in an antigen-dependent manner, resulting in T cell proliferation and cytokine production. Such eosinophils have been predominantly found in the T cell zones of the draining lymph node and to form clusters with antigen-specific T cells in mice, suggesting that they present antigen to naïve T cells, and at the same time provide polarizing IL-4 (Gessner et al., 2005; Wang et al., 2007). However, due to the low number of eosinophils in the draining lymph nodes, it is unclear if eosinophils in vivo are able to compete with professional APCs (van Rijt et al., 2003; Wang et al., 2007). Studies using MHC-II-deficient eosinophils have confirmed that the observed activation of T cells and the subsequent increased cytokine production, were MHC class II dependent (Padigel et al., 2007). Experiments performed in strains of eosinophil-deficient mice have suggested that while eosinophils can augment allergic inflammation by regulating CCL17 and CCL22 production and through their interactions with DCs (Jacobsen et al., 2008; Jacobsen et al., 2011; Spencer et al., 2009), they may play minor roles as antigen presenting cells (Jacobsen et al., 2014).
In summary, the APC potential of eosinophils seems minor compared with professional DCs, and highly context dependent. However, additional experiments investigating the precise role of MHC-II on eosinophils in vivo are eagerly awaited, using new tools to target gene expression in eosinophils, e.g. employing the eosinophil specific eosinophil peroxidase (Epx)-Cre mouse to delete MHCII expression. An APC function might be a selective characteristic of particular subsets of immunoregulatory eosinophils, involved in the homeostatic functions of these cells in the lungs, gut or adipose tissue (Mesnil et al., 2016).
Innate lymphoid cells as APCs
Recent evidence in models of gut helminth infection, lung allergy, and microbiome colonization models have implicated ILCs as central regulators of innate immunity, inflammation and repair at mucosal sites (Artis and Spits, 2015; Hepworth et al., 2013; Monticelli et al., 2016; Wang et al., 2017). In addition, all subsets of ILCs have been shown to also be present in adult human and mouse LNs and spleen, suggesting that these cells might migrate to T cell rich regions (Mackley et al., 2015).
Several groups have demonstrated that some mouse ILCs in neonatal LN, adult LN, spleen, and the intestine express MHC-II and can present antigen to CD4+ T cells (Hepworth et al., 2013; Mebius et al., 1997; Oliphant et al., 2014; von Burg et al., 2015). Genome-wide transcriptional profiling of RORγT+ ILC3s has shown that these cells not only express MHCII structural components, but also express antigen processing machinery components, and can migrate to the T cell area of gut draining lymph nodes in a CCR7 dependent manner (Hepworth et al., 2013; Mackley et al., 2015). However, costimulatory molecules were minimally expressed by ILC3s and these cells were unable to induce CD4+ T cell proliferation in vitro (Hepworth et al., 2013). One striking observation however was that cell-specific deletion of MHCII in RORγT+ ILC3s lead to the dysregulation of commensal bacteria-specific CD4+ T cells and the loss of intestinal barrier integrity in mice, suggesting that ILCs mainly acted as APCs for an immunoregulatory response (Hepworth et al., 2013).
Although the concept of ILC3s as non-professional APCs is gaining momentum, the evidence supporting an APC function of ILC2s is scarce. Since their discovery in the gut during helminth infections, ILC2s have also been found at other sites that harbour type-2 immune responses such as the allergic lung and nose, and metabolically healthy fat tissues that also harbour Th2 cells and Treg cells (Halim et al., 2016; Moro et al., 2010; Neill et al., 2010; Price et al., 2010). ILC2s have been reported to maintain a dialogue with CD4+ T cells via expression of MHC-II (Hepworth et al., 2013; Mirchandani et al., 2014; Neill et al., 2010), aiding Th2 cell differentiation and function in allergic airway inflammation as well as after helminthic infection in mice (Halim et al., 2014; Mirchandani et al., 2014; Oliphant et al., 2014). Furthermore, in vitro and in vivo experiments using OVA and the model antigen papain have shown that MHC-II+ ILC2s can induce Th2 cell differentiation in vitro from naïve CD4+ T cells, in an OX40L and IL-4 dependent manner (Drake et al., 2014). In a model of Nippostrongylus brasiliensis infection and expulsion, mice lacking MHCII in ILC2s had defective expansion of CD4 Th2 cells, and the helminth failed to be expulsed from the gut (Drake et al., 2014; Oliphant et al., 2014). However, another group recently reported, using various mouse models of Th2-driven immunity (e.g. Nippostrongylus brasiliensis-infection and HDM induction) that priming of CD4+ T cells in the lymph node as well as CD4+ T cell terminal differentiation into Th2 cells, was independent of ILC2s, but rather, seemed to rely on the exposure of CD4+ T cells to epithelial derived cytokines such as IL-33, IL-25, and TSLP in the local tissue microenvironment (Van Dyken et al., 2016). One aspect that needs further investigation is the expression of co-inhibitory receptor ligand PD-L1 by ILC2s. Although this is generally seen as a negative signal, interaction with the PD-1 receptor could exert an unexpected immunostimulatory effect on PD1+ CD4+ Th2 cells, deemed to be an important effector subset in type 2 immune responses (Coquet et al., 2015; Schwartz et al., 2017).
Like basophils and mast cells, ILC2s seem to also collaborate with DCs to regulate antigen presentation. ILC2s are a predominant source of OX40L, and ILC2-specific loss of OX40L, has been shown to lead to deficient induction of Th2 priming to allergens and helminths in mice, leading to failure to clear helminths (Halim et al., 2018). Additionally, adoptive transfer of ILC2s can potentiate Th2 responses in recipient mice, including an IL-13-driven enhanced production of Th2 selective chemokine CCL17 by cDC2s relative to controls, and an increased migration of DCs to the draining lymph node T cell area (Halim et al., 2016; Kim et al., 2013a; Neill et al., 2010).
Collectively, these data highlight the concept that ILCs can modulate Th2 responses, but additional work is needed to determine for instance, the in vivo antigen presenting capabilities of ILC2s, as well as MHC-II expression in these cells can modulate their intrinsic ability to either inhibit or enhance adaptive immune responses.
Concluding Remarks
In this review we have discussed professional APCs that can drive and maintain Th2 immunity to allergens and helminths. To make a clear difference between the accepted term “professional APC”, we suggest to use the term “amateur” APC to describe non-conventional cells bearing MHC class II proteins that can modulate immune responses initiated by DCs, rather than driving primary antigen presentation to naïve cells. As the immune response is programmed by the timed expression and engagement of sets of costimulatory molecules, the production of cytokines, as well as antigen processing and migration, it is unlikely that many amateur APCs can replace cDC2s as master inducers of type 2 adaptive immunity. Nonetheless, antigen presentation by “amateur” APCs may be the mechanism by which these cells are able to maintain or modulate ongoing immune responses or to induce peripheral tolerance. The requirement of different “amateur” APCs is likely to be context dependent. Basophils, mast cells, and eosinophils are mostly implicated in perpetuating Th2 inflammation, and they might do so by directly stimulating effector or tissue resident memory CD4+ T cells, in some cases only when these cells are armed with IgE on their surface. For some of these APCs, the expression or acquisition of MHC-II molecules could be used as a localization or survival signal for Th2 cells, such that they exert their function in close proximity of CD4+ T cells, and eliciting help from the latter. As more becomes known about the role of non-professional APCs, and we understand better the heterogeneity of cells in general, the contribution to adaptive immune responses in both mice and human tissues must be carefully considered. Further studies addressing the roles of these cells as antigen presenters are necessary, aiming to validate their role not only in the initiation but also in the maintenance of chronic immune responses. Unravelling these complexities and exploiting some of the tolerance inducing capacity of non-professional APCs could lead to disease modifying strategies in type 2 inflammatory diseases. Now that more genetic tools are available to selectively knock out any gene of interest in basically all of the cells of the immune system, it will not be too long before we have definitive answers as to the relative contribution of MHC-II antigen presentation by various type 2 immune cells compared with professional APCs.
Another giant leap will be to translate these findings to human studies. Recently however, a lot of new biologicals targeting key pathways in type 2 immunity such as anti-IgE, anti-IL5, anti-IL-5R, and anti-IL4Ra have become available for the treatment of severe asthma and chronic atopic dermatitis. Other drugs targeting IL-33, TSLP and GM-CSF are in advanced phases of drug testing for these indications. Since we are now profoundly interfering with type 2 immunity in patients, we need therefore urgently better understand how type 2 immunity is regulated in humans. Although these biologicals are mainly targeted at the various effector pathways of type 2 immunity (such as eosinophils, smooth muscle contraction, mucus production) we hope that this review has highlighted that they might also interfere with the antigen presenting functions of amateur and professional APCs, and in this way have a long lasting disease-modifying effect.
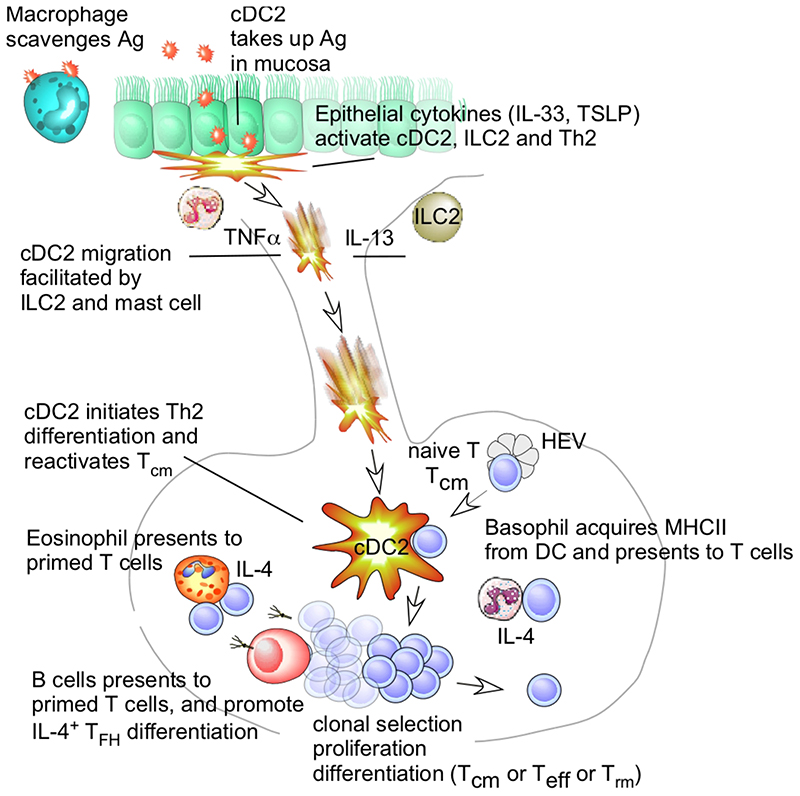
Figure 1.
Allergen Presentation Leading to a Primary T helper Type 2 (Th2) Response in Mice and Humans. When inhaled allergens (Ag) reach the deeper parts of the lungs upon first antigen encounter (left), they can be taken up by conventional dendritic cells (cDCs) probing the mucosa, or by alveolar macrophages scavenging the allergen for destruction. Allergens are often good at eliciting a type 2 innate immune responses in barrier epithelial cells producing interleukin (IL)-33 and thymic stromal lymphopoietin (TSLP). These cytokines instruct cDC2s to become inducers of Th2 polarization (often suppressing IL-12 and inducing OX40L, not depicted). The same epithelial cytokines also boost the innate functions of type 2 innate lymphocytes (ILC2) and resident Th2 cells [in the absence of cognate MHC-T cell receptor (TCR) interactions], boosting DC migration via secretion of IL-13; mast cells (MCs) boost DC migration via the secretion of TNFα. cDC2s migrate via the afferent lymphatics to the draining nodes and interact with naïve T cells that extravasate from high endothelial venules (HEV). cDC2s induce clonal selection, arrest, and proliferation of antigen-reactive T cells. Some of these primed T cells also interact with B cells by presenting their cognate antigen, eliciting help via B cell immunoglobulin E (IgE) class switching, and promoting their differentiation into IL-4-producing T follicular helper cells (TFH). The precise source of polarizing IL-4 for Th2 differentiation is a matter of intense debate. It could derive from basophils, acquiring MHCII from DCs via trogocytosis, and interacting with T cells via peptide-MHCII complexes, while also releasing IL-4. Eosinophils also have an accessible IL4 locus, and can also interact with primed T cells in draining lymph nodes. It is unclear whether these eosinophils might communicate with naïve T cells or with recirculating T central memory (TCM) cells. The source of IL-4 might also be the naïve T cells themselves, producing IL-4 when DCs generate IL-12 and express surface molecules, such as OX40-L and Notch ligands.
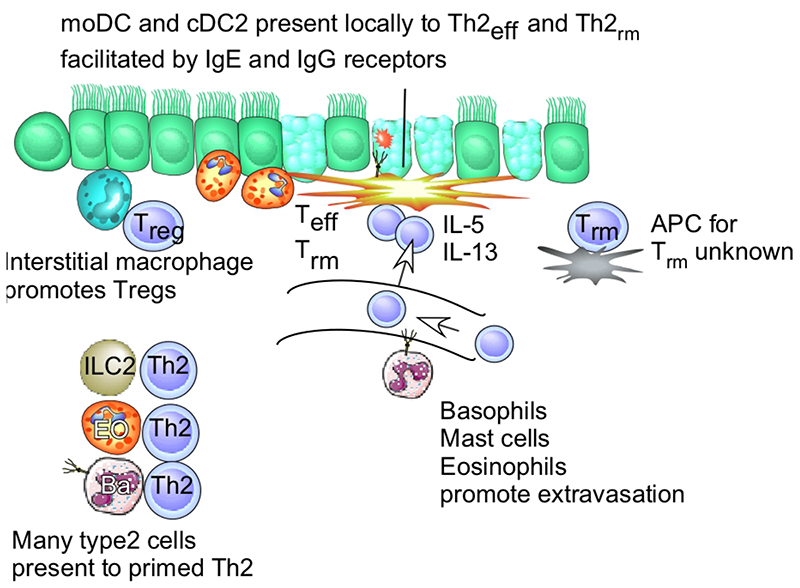
Figure 2.
Antigen Presentation during Ongoing T Helper Type 2 (Th2) Immunity in Mice and Humans. Upon repeated or ongoing contact with allergens, T cells that have been primed during the primary phase of the immune response now extravasate into tissues, facilitated by signals from basophils (BA) and other innate immune cells priming the vessel wall via interleukin (IL)-4 and/or IL-13. Tissue-resident memory T cells (TRM) are sessile in tissues. These TEFF cells are restimulated primarily by CD11b+ CD172+ type 2 conventional dendritic cells (cDC2s) and monocyte-derived DCs (moDCs) harboring a non-migratory behavior (e.g., similar to macrophages). The precise nature of the antigen-presenting cell (APC) that presents antigen to TRM cells remains unknown. At this stage of the response, moDCs are also armed with Fc receptors for immunoglobulin (Ig)-E and IgG so that allergens are better recognized. The interstitial macrophages still act to suppress the response, either by scavenging the allergen or by boosting the formation of regulatory T cells (Tregs). Accumulating evidence suggests that, in sites of ongoing type 2 immunity, many type 2 innate immune cells, such as innate type 2 lymphocytes (ILC2s), basophils, and eosinophils (EO), express MHCII, potentially leading to direct antigen presentation to Th2 cells. This could elicit help from Th2 cells for innate functions or the process might help Th2 cells to survive or modulate their function. As an eventual outcome, Th2 effector cells and innate immune cells produce massive amounts of IL-4, IL-5, and IL-13, controlling many of the features of allergic airway inflammation, such as goblet cell metaplasia, tissue eosinophilia, and bronchial hyper-reactivity.
References
- Allenspach EJ, Lemos MP, Porrett PM, Turka LA, Laufer TM. Migratory and lymphoid-resident dendritic cells cooperate to efficiently prime naive CD4 T cells. Immunity. 2008;29:795–806. doi: 10.1016/j.immuni.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- Ballesteros-Tato A, Randall TD, Lund FE, Spolski R, Leonard WJ, Leon B. T Follicular Helper Cell Plasticity Shapes Pathogenic T Helper 2 Cell-Mediated Immunity to Inhaled House Dust Mite. Immunity. 2016;44:259–273. doi: 10.1016/j.immuni.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedoret D, Wallemacq H, Marichal T, Desmet C, Quesada Calvo F, Henry E, Closset R, Dewals B, Thielen C, Gustin P, et al. Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice. J Clin Invest. 2009;119:3723–3738. doi: 10.1172/JCI39717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sasson SZ, Le Gros G, Conrad DH, Finkelman FD, Paul WE. Cross-linking Fc receptors stimulate splenic non-B, non-T cells to secrete interleukin 4 and other lymphokines. ProcNatlAcadSciUSA. 1990;87:1421–1425. doi: 10.1073/pnas.87.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilyk N, Holt PG. Inhibition of the immunosuppressive activity of resident pulmonary alveolar macrophages by granulocyte/macrophage colony-stimulating factor. J Exp Med. 1993;177:1773–1777. doi: 10.1084/jem.177.6.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll-Portillo A, Cannon JL, te Riet J, Holmes A, Kawakami Y, Kawakami T, Cambi A, Lidke DS. Mast cells and dendritic cells form synapses that facilitate antigen transfer for T cell activation. J Cell Biol. 2015;210:851–864. doi: 10.1083/jcb.201412074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DK, Llop-Guevara A, Walker TD, Flader K, Goncharova S, Boudreau JE, Moore CL, Seunghyun In T, Waserman S, Coyle AJ, et al. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol. 2013;131:187–200.:e181-188. doi: 10.1016/j.jaci.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Connor LM, Tang SC, Cognard E, Ochiai S, Hilligan KL, Old SI, Pellefigues C, White RF, Patel D, Smith AA, et al. Th2 responses are primed by skin dendritic cells with distinct transcriptional profiles. J Exp Med. 2017;214:125–142. doi: 10.1084/jem.20160470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquet JM, Schuijs MJ, Smyth MJ, Deswarte K, Beyaert R, Braun H, Boon L, Karlsson Hedestam GB, Nutt SL, Hammad H, Lambrecht BN. Interleukin-21-Producing CD4(+) T Cells Promote Type 2 Immunity to House Dust Mites. Immunity. 2015;43:318–330. doi: 10.1016/j.immuni.2015.07.015. [DOI] [PubMed] [Google Scholar]
- de Heer HJ, Hammad H, Soullie T, Hijdra D, Vos N, Willart MA, Hoogsteden HC, Lambrecht BN. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200:89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kleer IM, Kool M, de Bruijn MJ, Willart M, van Moorleghem J, Schuijs MJ, Plantinga M, Beyaert R, Hams E, Fallon PG, et al. Perinatal Activation of the Interleukin-33 Pathway Promotes Type 2 Immunity in the Developing Lung. Immunity. 2016;45:1285–1298. doi: 10.1016/j.immuni.2016.10.031. [DOI] [PubMed] [Google Scholar]
- Deckers J, De Bosscher K, Lambrecht BN, Hammad H. Interplay between barrier epithelial cells and dendritic cells in allergic sensitization through the lung and the skin. Immunol Rev. 2017a;278:131–144. doi: 10.1111/imr.12542. [DOI] [PubMed] [Google Scholar]
- Deckers J, Sichien D, Plantinga M, Van Moorleghem J, Vanheerswynghels M, Hoste E, Malissen B, Dombrowicz D, Guilliams M, De Bosscher K, et al. Epicutaneous sensitization to house dust mite allergen requires interferon regulatory factor 4-dependent dermal dendritic cells. J Allergy Clin Immunol. 2017b doi: 10.1016/j.jaci.2016.1012.1970. in press. [DOI] [PubMed] [Google Scholar]
- Dell’Aringa M, Reinhardt RL. Notch signaling represents an important checkpoint between follicular T-helper and canonical T-helper 2 cell fate. Mucosal Immunol. 2018;11:1079–1091. doi: 10.1038/s41385-018-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzel A, Maus UA, Rodriguez Gomez M, Moll C, Niedermeier M, Winter C, Maus R, Hollingshead S, Briles DE, Kunz-Schughart LA, et al. Basophils enhance immunological memory responses. Nat Immunol. 2008;9:733–742. doi: 10.1038/ni.1621. [DOI] [PubMed] [Google Scholar]
- Drake LY, Iijima K, Kita H. Group 2 innate lymphoid cells and CD4+ T cells cooperate to mediate type 2 immune response in mice. Allergy. 2014;69:1300–1307. doi: 10.1111/all.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudeck A, Suender CA, Kostka SL, von Stebut E, Maurer M. Mast cells promote Th1 and Th17 responses by modulating dendritic cell maturation and function. Eur J Immunol. 2011;41:1883–1893. doi: 10.1002/eji.201040994. [DOI] [PubMed] [Google Scholar]
- Dudeck J, Medyukhina A, Frobel J, Svensson CM, Kotrba J, Gerlach M, Gradtke AC, Schroder B, Speier S, Figge MT, Dudeck A. Mast cells acquire MHCII from dendritic cells during skin inflammation. J Exp Med. 2017;214:3791–3811. doi: 10.1084/jem.20160783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dullaers M, Schuijs MJ, Willart M, Fierens K, Van Moorleghem J, Hammad H, Lambrecht BN. House dust mite-driven asthma and allergen-specific T cells depend on B cells when the amount of inhaled allergen is limiting. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.09.020. [DOI] [PubMed] [Google Scholar]
- Elieh Ali Komi D, Grauwet K. Role of Mast Cells in Regulation of T Cell Responses in Experimental and Clinical Settings. Clin Rev Allergy Immunol. 2018;54:432–445. doi: 10.1007/s12016-017-8646-z. [DOI] [PubMed] [Google Scholar]
- Everts B, Hussaarts L, Driessen NN, Meevissen MH, Schramm G, van der Ham AJ, van der Hoeven B, Scholzen T, Burgdorf S, Mohrs M, et al. Schistosome-derived omega-1 drives Th2 polarization by suppressing protein synthesis following internalization by the mannose receptor. J Exp Med. 2012;209:1753–1767.:S1751. doi: 10.1084/jem.20111381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts B, Tussiwand R, Dreesen L, Fairfax KC, Huang SC, Smith AM, O’Neill CM, Lam WY, Edelson BT, Urban JF, Jr, et al. Migratory CD103+ dendritic cells suppress helminth-driven type 2 immunity through constitutive expression of IL-12. J Exp Med. 2016;213:35–51. doi: 10.1084/jem.20150235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandji P, Tkaczyk C, Oskeritzian C, David B, Desaymard C, Mecheri S. Exogenous and endogenous antigens are differentially presented by mast cells to CD4(+) T lymphocytes. Eur J Immunol. 1996;26:2517–2528. doi: 10.1002/eji.1830261036. [DOI] [PubMed] [Google Scholar]
- Frandji P, Tkaczyk C, Oskeritzian C, Lapeyre J, Peronet R, David B, Guillet JG, Mecheri S. Presentation of soluble antigens by mast cells: upregulation by interleukin-4 and granulocyte/macrophage colony-stimulating factor and downregulation by interferon-gamma. Cell Immunol. 1995;163:37–46. doi: 10.1006/cimm.1995.1096. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Gaudenzio N. Human mast cells as antigen-presenting cells: When is this role important in vivo? J Allergy Clin Immunol. 2018;141:92–93. doi: 10.1016/j.jaci.2017.05.029. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Nish SA, Jiang R, Hou L, Licona-Limon P, Weinstein JS, Zhao H, Medzhitov R. Control of T helper 2 responses by transcription factor IRF4-dependent dendritic cells. Immunity. 2013;39:722–732. doi: 10.1016/j.immuni.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudenzio N, Espagnolle N, Mars LT, Liblau R, Valitutti S, Espinosa E. Cell-cell cooperation at the T helper cell/mast cell immunological synapse. Blood. 2009;114:4979–4988. doi: 10.1182/blood-2009-02-202648. [DOI] [PubMed] [Google Scholar]
- Gaudenzio N, Laurent C, Valitutti S, Espinosa E. Human mast cells drive memory CD4+ T cells toward an inflammatory IL-22+ phenotype. J Allergy Clin Immunol. 2013;131:1400–1407.:e1411. doi: 10.1016/j.jaci.2013.01.029. [DOI] [PubMed] [Google Scholar]
- Gessner A, Mohrs K, Mohrs M. Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. J Immunol. 2005;174:1063–1072. doi: 10.4049/jimmunol.174.2.1063. [DOI] [PubMed] [Google Scholar]
- Girodet PO, Nguyen D, Mancini JD, Hundal M, Zhou X, Israel E, Cernadas M. Alternative Macrophage Activation Is Increased in Asthma. Am J Respir Cell Mol Biol. 2016;55:467–475. doi: 10.1165/rcmb.2015-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory GD, Robbie-Ryan M, Secor VH, Sabatino JJ, Jr, Brown MA. Mast cells are required for optimal autoreactive T cell responses in a murine model of multiple sclerosis. Eur J Immunol. 2005;35:3478–3486. doi: 10.1002/eji.200535271. [DOI] [PubMed] [Google Scholar]
- Guilliams M, Bruhns P, Saeys Y, Hammad H, Lambrecht BN. The function of Fcgamma receptors in dendritic cells and macrophages. Nat Rev Immunol. 2014;14:94–108. doi: 10.1038/nri3582. [DOI] [PubMed] [Google Scholar]
- Halim TY, Hwang YY, Scanlon ST, Zaghouani H, Garbi N, Fallon PG, McKenzie AN. Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses. Nat Immunol. 2016;17:57–64. doi: 10.1038/ni.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halim TY, Steer CA, Matha L, Gold MJ, Martinez-Gonzalez I, McNagny KM, McKenzie AN, Takei F. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–435. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halim TYF, Rana BMJ, Walker JA, Kerscher B, Knolle MD, Jolin HE, Serrao EM, Haim-Vilmovsky L, Teichmann SA, Rodewald HR, et al. Tissue-Restricted Adaptive Type 2 Immunity Is Orchestrated by Expression of the Costimulatory Molecule OX40L on Group 2 Innate Lymphoid Cells. Immunity. 2018;48:1195–1207.:e1196. doi: 10.1016/j.immuni.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad H, Lambrecht BN. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity. 2015;43:29–40. doi: 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, Muskens F, Lambrecht BN. Inflammatory dendritic cells--not basophils--are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010;207:2097–2111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NL, Watt V, Ronchese F, Le Gros G. Differential T cell function and fate in lymph node and nonlymphoid tissues. J Exp Med. 2002;195:317–326. doi: 10.1084/jem.20011558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepworth MR, Monticelli LA, Fung TC, Ziegler CG, Grunberg S, Sinha R, Mantegazza AR, Ma HL, Crawford A, Angelosanto JM, et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013;498:113–117. doi: 10.1038/nature12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondowicz BD, An D, Schenkel JM, Kim KS, Steach HR, Krishnamurty AT, Keitany GJ, Garza EN, Fraser KA, Moon JJ, et al. Interleukin-2-Dependent Allergen-Specific Tissue-Resident Memory Cells Drive Asthma. Immunity. 2016;44:155–166. doi: 10.1016/j.immuni.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen EA, Helmers RA, Lee JJ, Lee NA. The expanding role(s) of eosinophils in health and disease. Blood. 2012;120:3882–3890. doi: 10.1182/blood-2012-06-330845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen EA, Lesuer WE, Willetts L, Zellner KR, Mazzolini K, Antonios N, Beck B, Protheroe C, Ochkur SI, Colbert D, et al. Eosinophil activities modulate the immune/inflammatory character of allergic respiratory responses in mice. Allergy. 2014;69:315–327. doi: 10.1111/all.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, Lee NA, Lee JJ. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J Exp Med. 2008;205:699–710. doi: 10.1084/jem.20071840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen EA, Zellner KR, Colbert D, Lee NA, Lee JJ. Eosinophils regulate dendritic cells and Th2 pulmonary immune responses following allergen provocation. J Immunol. 2011;187:6059–6068. doi: 10.4049/jimmunol.1102299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambayashi T, Allenspach EJ, Chang JT, Zou T, Shoag JE, Reiner SL, Caton AJ, Koretzky GA. Inducible MHC class II expression by mast cells supports effector and regulatory T cell activation. J Immunol. 2009;182:4686–4695. doi: 10.4049/jimmunol.0803180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambayashi T, Baranski JD, Baker RG, Zou T, Allenspach EJ, Shoag JE, Jones PL, Koretzky GA. Indirect involvement of allergen-captured mast cells in antigen presentation. Blood. 2008;111:1489–1496. doi: 10.1182/blood-2007-07-102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambayashi T, Laufer TM. Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell? Nat Rev Immunol. 2014;14:719–730. doi: 10.1038/nri3754. [DOI] [PubMed] [Google Scholar]
- Khare A, Krishnamoorthy N, Oriss TB, Fei M, Ray P, Ray A. Cutting Edge: Inhaled Antigen Upregulates Retinaldehyde Dehydrogenase in Lung CD103+ but Not Plasmacytoid Dendritic Cells To Induce Foxp3 De Novo in CD4+ T Cells and Promote Airway Tolerance. J Immunol. 2013;191:25–29. doi: 10.4049/jimmunol.1300193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BS, Wojno ED, Artis D. Innate lymphoid cells and allergic inflammation. Curr Opin Immunol. 2013a;25:738–744. doi: 10.1016/j.coi.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Karasuyama H, Lopez AF, Ouyang W, Li X, Le Gros G, Min B. IL-4 Derived from Non-T Cells Induces Basophil- and IL-3-independent Th2 Immune Responses. Immune Netw. 2013b;13:249–256. doi: 10.4110/in.2013.13.6.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Iijima K, Dent AL, Kita H. Follicular helper T cells mediate IgE antibody response to airborne allergens. J Allergy Clin Immunol. 2017;139:300–313.:e307. doi: 10.1016/j.jaci.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaswamy JK, Gowthaman U, Zhang B, Mattsson J, Szeponik L, Liu D, Wu R, White T, Calabro S, Xu L, et al. Migratory CD11b(+) conventional dendritic cells induce T follicular helper cell-dependent antibody responses. Sci Immunol. 2017;2 doi: 10.1126/sciimmunol.aam9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers H, Lambrecht BN. The interplay of dendritic cells, Th2 cells and regulatory T cells in asthma. Curr Opin Immunol. 2004;16:702–708. doi: 10.1016/j.coi.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Kumamoto Y, Linehan M, Weinstein JS, Laidlaw BJ, Craft JE, Iwasaki A. CD301b+ dermal dendritic cells drive T helper 2 cell-mediated immunity. Immunity. 2013;39:733–743. doi: 10.1016/j.immuni.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht BN, Hammad H. Lung dendritic cells in respiratory viral infection and asthma: from protection to immunopathology. Annu Rev Immunol. 2012;30:243–270. doi: 10.1146/annurev-immunol-020711-075021. [DOI] [PubMed] [Google Scholar]
- Lambrecht BN, Hammad H. Allergens and the airway epithelium response: gateway to allergic sensitization. J Allergy Clin Immunol. 2014;134:499–507. doi: 10.1016/j.jaci.2014.06.036. [DOI] [PubMed] [Google Scholar]
- Lambrecht BN, Hammad H. The immunology of the allergy epidemic and the hygiene hypothesis. Nat Immunol. 2017;18:1076–1083. doi: 10.1038/ni.3829. [DOI] [PubMed] [Google Scholar]
- Leon B, Ballesteros-Tato A, Browning JL, Dunn R, Randall TD, Lund FE. Regulation of T(H)2 development by CXCR5+ dendritic cells and lymphotoxin-expressing B cells. Nat Immunol. 2012;13:681–690. doi: 10.1038/ni.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyva-Castillo JM, Hener P, Michea P, Karasuyama H, Chan S, Soumelis V, Li M. Skin thymic stromal lymphopoietin initiates Th2 responses through an orchestrated immune cascade. Nat Commun. 2013;4:2847. doi: 10.1038/ncomms3847. [DOI] [PubMed] [Google Scholar]
- Li BW, de Bruijn MJ, Tindemans I, Lukkes M, KleinJan A, Hoogsteden HC, Hendriks RW. T cells are necessary for ILC2 activation in house dust mite-induced allergic airway inflammation in mice. Eur J Immunol. 2016a;46:1392–1403. doi: 10.1002/eji.201546119. [DOI] [PubMed] [Google Scholar]
- Li F, Wang Y, Lin L, Wang J, Xiao H, Li J, Peng X, Dai H, Li L. Mast Cell-Derived Exosomes Promote Th2 Cell Differentiation via OX40L-OX40 Ligation. J Immunol Res. 2016b;2016:3623898. doi: 10.1155/2016/3623898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang HE, Reinhardt RL, Bando JK, Sullivan BM, Ho IC, Locksley RM. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol. 2012;13:58–66. doi: 10.1038/ni.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd CM, Harker JA. Location, Location, Location: Localized Memory Cells Take Residence in the Allergic Lung. Immunity. 2016;44:13–15. doi: 10.1016/j.immuni.2015.12.012. [DOI] [PubMed] [Google Scholar]
- Lombardi V, Speak AO, Kerzerho J, Szely N, Akbari O. CD8alpha(+)beta(-) and CD8alpha(+)beta(+) plasmacytoid dendritic cells induce Foxp3(+) regulatory T cells and prevent the induction of airway hyper-reactivity. Mucosal Immunol. 2012 doi: 10.1038/mi.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotfi-Emran S, Ward BR, Le QT, Pozez AL, Manjili MH, Woodfolk JA, Schwartz LB. Human mast cells present antigen to autologous CD4(+) T cells. J Allergy Clin Immunol. 2018;141:311–321.:e310. doi: 10.1016/j.jaci.2017.02.048. [DOI] [PubMed] [Google Scholar]
- MacKenzie JR, Mattes J, Dent LA, Foster PS. Eosinophils promote allergic disease of the lung by regulating CD4(+) Th2 lymphocyte function. J Immunol. 2001;167:3146–3155. doi: 10.4049/jimmunol.167.6.3146. [DOI] [PubMed] [Google Scholar]
- Mackley EC, Houston S, Marriott CL, Halford EE, Lucas B, Cerovic V, Filbey KJ, Maizels RM, Hepworth MR, Sonnenberg GF, et al. CCR7-dependent trafficking of RORgamma(+) ILCs creates a unique microenvironment within mucosal draining lymph nodes. Nat Commun. 2015;6:5862. doi: 10.1038/ncomms6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massacand JC, Stettler RC, Meier R, Humphreys NE, Grencis RK, Marsland BJ, Harris NL. Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function. Proc Natl Acad Sci U S A. 2009;106:13968–13973. doi: 10.1073/pnas.0906367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer M, Lopez Kostka S, Siebenhaar F, Moelle K, Metz M, Knop J, von Stebut E. Skin mast cells control T cell-dependent host defense in Leishmania major infections. FASEB J. 2006;20:2460–2467. doi: 10.1096/fj.06-5860com. [DOI] [PubMed] [Google Scholar]
- Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3- LTb+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- Medoff BD, Seung E, Hong S, Thomas SY, Sandall BP, Duffield JS, Kuperman DA, Erle DJ, Luster AD. CD11b+ myeloid cells are the key mediators of Th2 cell homing into the airway in allergic inflammation. J Immunol. 2009;182:623–635. doi: 10.4049/jimmunol.182.1.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesnil C, Raulier S, Paulissen G, Xiao X, Birrell MA, Pirottin D, Janss T, Starkl P, Ramery E, Henket M, et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest. 2016;126:3279–3295. doi: 10.1172/JCI85664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirchandani AS, Besnard AG, Yip E, Scott C, Bain CC, Cerovic V, Salmond RJ, Liew FY. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J Immunol. 2014;192:2442–2448. doi: 10.4049/jimmunol.1300974. [DOI] [PubMed] [Google Scholar]
- Miyake K, Shiozawa N, Nagao T, Yoshikawa S, Yamanishi Y, Karasuyama H. Trogocytosis of peptide-MHC class II complexes from dendritic cells confers antigen-presenting ability on basophils. Proc Natl Acad Sci U S A. 2017;114:1111–1116. doi: 10.1073/pnas.1615973114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticelli LA, Buck MD, Flamar AL, Saenz SA, Tait Wojno ED, Yudanin NA, Osborne LC, Hepworth MR, Tran SV, Rodewald HR, et al. Arginase 1 is an innate lymphoid-cell-intrinsic metabolic checkpoint controlling type 2 inflammation. Nat Immunol. 2016;17:656–665. doi: 10.1038/ni.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- Mukai K, Tsai M, Starkl P, Marichal T, Galli SJ. IgE and mast cells in host defense against parasites and venoms. Semin Immunopathol. 2016;38:581–603. doi: 10.1007/s00281-016-0565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano H, Free ME, Whitehead GS, Maruoka S, Wilson RH, Nakano K, Cook DN. Pulmonary CD103(+) dendritic cells prime Th2 responses to inhaled allergens. Mucosal Immunol. 2012;5:53–65. doi: 10.1038/mi.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity. 2010;33:364–374. doi: 10.1016/j.immuni.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Oliphant CJ, Hwang YY, Walker JA, Salimi M, Wong SH, Brewer JM, Englezakis A, Barlow JL, Hams E, Scanlon ST, et al. MHCII-Mediated Dialog between Group 2 Innate Lymphoid Cells and CD4(+) T Cells Potentiates Type 2 Immunity and Promotes Parasitic Helminth Expulsion. Immunity. 2014;41:283–295. doi: 10.1016/j.immuni.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padigel UM, Hess JA, Lee JJ, Lok JB, Nolan TJ, Schad GA, Abraham D. Eosinophils act as antigen-presenting cells to induce immunity to Strongyloides stercoralis in mice. J Infect Dis. 2007;196:1844–1851. doi: 10.1086/522968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature. 2012;484:465–472. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul WE. What determines Th2 differentiation, in vitro and in vivo? Immunol Cell Biol. 2010;88:236–239. doi: 10.1038/icb.2010.2. [DOI] [PubMed] [Google Scholar]
- Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, Nair MG, Du Y, Zaph C, van Rooijen N, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phythian-Adams AT, Cook PC, Lundie RJ, Jones LH, Smith KA, Barr TA, Hochweller K, Anderton SM, Hammerling GJ, Maizels RM, MacDonald AS. CD11c depletion severely disrupts Th2 induction and development in vivo. J Exp Med. 2010;207:2089–2096. doi: 10.1084/jem.20100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, Vanhoutte L, Neyt K, Killeen N, Malissen B, et al. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity. 2013;38:322–335. doi: 10.1016/j.immuni.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Tenza D, Mecheri S, Peronet R, Bonnerot C, Desaymard C. Accumulation of major histocompatibility complex class II molecules in mast cell secretory granules and their release upon degranulation. Mol Biol Cell. 1997;8:2631–2645. doi: 10.1091/mbc.8.12.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece JJ, Siracusa MC, Scott AL. Innate immune responses to lung-stage helminth infection induce alternatively activated alveolar macrophages. Infect Immun. 2006;74:4970–4981. doi: 10.1128/IAI.00687-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13:9–22. doi: 10.1038/nri3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatel C, Radermecker C, Fievez L, Paulissen G, Chakarov S, Fernandes C, Olivier S, Toussaint M, Pirottin D, Xiao X, et al. Exposure to Bacterial CpG DNA Protects from Airway Allergic Inflammation by Expanding Regulatory Lung Interstitial Macrophages. Immunity. 2017;46:457–473. doi: 10.1016/j.immuni.2017.02.016. [DOI] [PubMed] [Google Scholar]
- Sabin EA, Kopf MA, Pearce EJ. Schistosoma mansoni egg-induced early IL-4 production is dependent upon IL-5 and eosinophils. J Exp Med. 1996;184:1871–1878. doi: 10.1084/jem.184.5.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C, Eberle JU, Voehringer D. Basophils in inflammation. Eur J Pharmacol. 2016;778:90–95. doi: 10.1016/j.ejphar.2015.04.049. [DOI] [PubMed] [Google Scholar]
- Schwartz C, Khan AR, Floudas A, Saunders SP, Hams E, Rodewald HR, McKenzie ANJ, Fallon PG. ILC2s regulate adaptive Th2 cell functions via PD-L1 checkpoint control. J Exp Med. 2017;214:2507–2521. doi: 10.1084/jem.20170051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C, Oeser K, Prazeres da Costa C, Layland LE, Voehringer D. T cell-derived IL-4/IL-13 protects mice against fatal Schistosoma mansoni infection independently of basophils. J Immunol. 2014;193:3590–3599. doi: 10.4049/jimmunol.1401155. [DOI] [PubMed] [Google Scholar]
- Semmrich M, Plantinga M, Svensson-Frej M, Uronen-Hansson H, Gustafsson T, Mowat AM, Yrlid U, Lambrecht BN, Agace WW. Directed antigen targeting in vivo identifies a role for CD103+ dendritic cells in both tolerogenic and immunogenic T-cell responses. Mucosal Immunol. 2012;5:150–160. doi: 10.1038/mi.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi HZ, Humbles A, Gerard C, Jin Z, Weller PF. Lymph node trafficking and antigen presentation by endobronchial eosinophils. J Clin Invest. 2000;105:945–953. doi: 10.1172/JCI8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda K, Van Deursen JM, Sangster MY, Sarawar SR, Carson RT, Tripp RA, Chu C, Quelle FW, Nosaka T, Vignali DA, et al. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- Shinkai K, Mohrs M, Locksley RM. Helper T cells regulate type-2 innate immunity in vivo. Nature. 2002;420:825–829. doi: 10.1038/nature01202. [DOI] [PubMed] [Google Scholar]
- Sinclair C, Bommakanti G, Gardinassi L, Loebbermann J, Johnson MJ, Hakimpour P, Hagan T, Benitez L, Todor A, Machiah D, et al. mTOR regulates metabolic adaptation of APCs in the lung and controls the outcome of allergic inflammation. Science. 2017;357:1014–1021. doi: 10.1126/science.aaj2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skokos D, Botros HG, Demeure C, Morin J, Peronet R, Birkenmeier G, Boudaly S, Mecheri S. Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. J Immunol. 2003;170:3037–3045. doi: 10.4049/jimmunol.170.6.3037. [DOI] [PubMed] [Google Scholar]
- Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soroosh P, Doherty TA, Duan W, Mehta AK, Choi H, Adams YF, Mikulski Z, Khorram N, Rosenthal P, Broide DH, Croft M. Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J Exp Med. 2013;210:775–788. doi: 10.1084/jem.20121849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer LA, Szela CT, Perez SA, Kirchhoffer CL, Neves JS, Radke AL, Weller PF. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J Leukoc Biol. 2009;85:117–123. doi: 10.1189/jlb.0108058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan BM, Liang HE, Bando JK, Wu D, Cheng LE, McKerrow JK, Allen CD, Locksley RM. Genetic analysis of basophil function in vivo. Nat Immunol. 2011;12:527–535. doi: 10.1038/ni.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto H, Nakae S, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cell-associated TNF promotes dendritic cell migration. J Immunol. 2006;176:4102–4112. doi: 10.4049/jimmunol.176.7.4102. [DOI] [PubMed] [Google Scholar]
- Talay O, Yan D, Brightbill HD, Straney EE, Zhou M, Ladi E, Lee WP, Egen JG, Austin CD, Xu M, Wu LC. IgE(+) memory B cells and plasma cells generated through a germinal-center pathway. Nat Immunol. 2012;13:396–404. doi: 10.1038/ni.2256. [DOI] [PubMed] [Google Scholar]
- Tang H, Cao W, Kasturi SP, Ravindran R, Nakaya HI, Kundu K, Murthy N, Kepler TB, Malissen B, Pulendran B. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat Immunol. 2010;11:608–617. doi: 10.1038/ni.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traidl-Hoffmann C, Mariani V, Hochrein H, Karg K, Wagner H, Ring J, Mueller MJ, Jakob T, Behrendt H. Pollen-associated phytoprostanes inhibit dendritic cell interleukin-12 production and augment T helper type 2 cell polarization. J Exp Med. 2005;201:627–636. doi: 10.1084/jem.20041065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers J, Rothenberg ME. Eosinophils in mucosal immune responses. Mucosal Immunol. 2015;8:464–475. doi: 10.1038/mi.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu L, Fang TC, Artis D, Shestova O, Pross SE, Maillard I, Pear WS. Notch signaling is an important regulator of type 2 immunity. J Exp Med. 2005;202:1037–1042. doi: 10.1084/jem.20050923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tussiwand R, Everts B, Grajales-Reyes GE, Kretzer NM, Iwata A, Bagaitkar J, Wu X, Wong RW, Murphy TL, Pearce EJ, Murphy KM. KLF4 expression in conventional dendritic cells is required for T helper 2 responses. Immunity. 2015 doi: 10.1016/j.immuni.2015.04.017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyken SJ, Mohapatra A, Nussbaum JC, Molofsky AB, Thornton EE, Ziegler SF, McKenzie AN, Krummel MF, Liang HE, Locksley RM. Chitin activates parallel immune modules that direct distinct inflammatory responses via innate lymphoid type 2 and gammadelta T cells. Immunity. 2014;40:414–424. doi: 10.1016/j.immuni.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyken SJ, Nussbaum JC, Lee J, Molofsky AB, Liang HE, Pollack JL, Gate RE, Haliburton GE, Ye CJ, Marson A, et al. A tissue checkpoint regulates type 2 immunity. Nat Immunol. 2016;17:1381–1387. doi: 10.1038/ni.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijt LS, Jung S, Kleinjan A, Vos N, Willart M, Duez C, Hoogsteden HC, Lambrecht BN. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med. 2005;201:981–991. doi: 10.1084/jem.20042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijt LS, Vos N, Hijdra D, de Vries VC, Hoogsteden HC, Lambrecht BN. Airway eosinophils accumulate in the mediastinal lymph nodes but lack antigen-presenting potential for naive T cells. J Immunol. 2003;171:3372–3378. doi: 10.4049/jimmunol.171.7.3372. [DOI] [PubMed] [Google Scholar]
- Van Rijt LS, Vos N, Willart M, Kleinjan A, Coyle AJ, Hoogsteden HC, Lambrecht BN. Essential role of dendritic cell CD80/CD86 costimulation in the induction, but not reactivation, of TH2 effector responses in a mouse model of asthma. J Allergy Clin Immunol. 2004;114:166–173. doi: 10.1016/j.jaci.2004.03.044. [DOI] [PubMed] [Google Scholar]
- van Rijt LS, Vos N, Willart M, Muskens F, Tak PP, van der Horst C, Hoogsteden HC, Lambrecht BN. Persistent activation of dendritic cells after resolution of allergic airway inflammation breaks tolerance to inhaled allergens in mice. Am J Respir Crit Care Med. 2011;184:303–311. doi: 10.1164/rccm.201101-0019OC. [DOI] [PubMed] [Google Scholar]
- von Burg N, Turchinovich G, Finke D. Maintenance of Immune Homeostasis through ILC/T Cell Interactions. Front Immunol. 2015;6:416. doi: 10.3389/fimmu.2015.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vroman H, Bergen IM, van Hulst JAC, van Nimwegen M, van Uden D, Schuijs MJ, Pillai SY, van Loo G, Hammad H, Lambrecht BN, et al. TNF-alpha-induced protein 3 levels in lung dendritic cells instruct TH2 or TH17 cell differentiation in patients with eosinophilic or neutrophilic asthma. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.08.012. [DOI] [PubMed] [Google Scholar]
- Wang HB, Akuthota P, Kanaoka Y, Weller PF. Airway eosinophil migration into lymph nodes in mice depends on leukotriene C4. Allergy. 2016 doi: 10.1111/all.13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HB, Ghiran I, Matthaei K, Weller PF. Airway eosinophils: allergic inflammation recruited professional antigen-presenting cells. J Immunol. 2007;179:7585–7592. doi: 10.4049/jimmunol.179.11.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HC, Qian L, Zhao Y, Mengarelli J, Adrianto I, Montgomery CG, Urban JF, Jr, Fung KM, Sun XH. Downregulation of E Protein Activity Augments an ILC2 Differentiation Program in the Thymus. J Immunol. 2017;198:3149–3156. doi: 10.4049/jimmunol.1602009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller PF, Spencer LA. Functions of tissue-resident eosinophils. Nat Rev Immunol. 2017 doi: 10.1038/nri.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willart MA, Deswarte K, Pouliot P, Braun H, Beyaert R, Lambrecht BN, Hammad H. Interleukin-1alpha controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33. J Exp Med. 2012;209:1505–1517. doi: 10.1084/jem.20112691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JW, Tjota MY, Clay BS, Vander Lugt B, Bandukwala HS, Hrusch CL, Decker DC, Blaine KM, Fixsen BR, Singh H, et al. Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation. Nat Commun. 2013;4:2990. doi: 10.1038/ncomms3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Briseno CG, Durai V, Albring JC, Haldar M, Bagadia P, Kim KW, Randolph GJ, Murphy TL, Murphy KM. Mafb lineage tracing to distinguish macrophages from other immune lineages reveals dual identity of Langerhans cells. J Exp Med. 2016;213:2553–2565. doi: 10.1084/jem.20160600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xenakis JJ, Howard ED, Smith KM, Olbrich CL, Huang Y, Anketell D, Maldonado S, Cornwell EW, Spencer LA. Resident intestinal eosinophils constitutively express antigen presentation markers and include two phenotypically distinct subsets of eosinophils. Immunology. 2018;154:298–308. doi: 10.1111/imm.12885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanishi Y, Miyake K, Iki M, Tsutsui H, Karasuyama H. Recent advances in understanding basophil-mediated Th2 immune responses. Immunol Rev. 2017;278:237–245. doi: 10.1111/imr.12548. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, Fujimori Y, Nakanishi K. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009;10:706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- Zaph C, Troy AE, Taylor BC, Berman-Booty LD, Guild KJ, Du Y, Yost EA, Gruber AD, May MJ, Greten FR, et al. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- Zhang DH, Cohn L, Ray P, Bottomly K, Ray A. Transcription factor GATA-3 is differentially expressed in murine Th1 and Th2 cells and controls Th2-specific expression of the interleukin-5 gene. J Biol Chem. 1997;272:21597–21603. doi: 10.1074/jbc.272.34.21597. [DOI] [PubMed] [Google Scholar]