Abstract
Background
Sialic acid-binding immunoglobulin-like lectin (Siglec)-8 is expressed on mast cells and eosinophils, but information about Siglec-8 expression and function in the lung is limited. A humanized antibody, AK002, targeting Siglec-8 is undergoing development for treatment of diseases associated with mast cell and eosinophil-driven inflammation.
Objective
To characterize Siglec-8 expression in the airway in asthma and determine if antibodies that target Siglec-8 (S8mAbs) can decrease airway eosinophils in asthma or inhibit lung mast cell activation.
Methods
Gene expression profiling and flow cytometry were used to characterize Siglec-8 expression in sputum cells from stable asthma. An antibody-dependent cellular cytotoxicity (ADCC) assay was used to determine if an S8mAb can decrease eosinophils in sputum from asthma patients ex vivo. A mast cell activation assay was used to determine if an S8mAb can inhibit mast cell activation in human lung tissue ex vivo.
Results
Gene expression for Siglec-8 is increased in sputum cells in asthma and correlates with gene expression for eosinophils and mast cells. Gene expression for Siglec-8 is inversely and significantly correlated with measures of airflow obstruction in asthma patients. Siglec-8 is prominently expressed on the surface of eosinophils and mast cells in sputum. S8mAbs decrease eosinophils in sputum from patients with asthma and inhibit FcεR1-activated mast cells in lung tissues.
Conclusions and clinical relevance
Siglec-8 is highly expressed on eosinophils and mast cells in asthmatic sputum and targeting Siglec-8 with an antibody is a plausible strategy to decrease sputum eosinophils and inhibit lung mast cells in asthma.
Introduction
Siglec-8 is member of the sialic acid binding immunoglobulin-like lectin (Siglec) family of immune receptors, and it is expressed on blood and tissue eosinophils, mast cells and (weakly) on basophils [1, 2]. Cross-linking of Siglec-8 with a monoclonal antibody (mAb) on blood eosinophils can mediate apoptosis via a caspase dependent pathway [3, 4], and activation of eosinophils with pro-inflammatory cytokines such as IL-5 further sensitizes eosinophils to Siglec-8-mediated apoptosis [5, 6]. This has particular relevance to asthma as eosinophils may be predisposed to apoptosis with anti-Siglec-8 mAbs due to elevated levels of pro-inflammatory cytokines. Engagement of Siglec-8 on mast cells does not cause apoptosis but induces broad inhibition of these cells [7, 8].
The specific expression of Siglec-8 on eosinophils and mast cells has led to the development of AK002, a humanized, non-fucosylated IgG1 monoclonal antibody that targets Siglec-8 for therapeutic purposes in eosinophilic and mast cell-driven diseases [9]. AK002 was developed with the idea that binding to Siglec-8 on eosinophils and mast cells would trigger antibody-dependent cellular cytotoxicity (ADCC) against blood eosinophils in the presence of NK cells, induce apoptosis of tissue eosinophils, and inhibit mast cells. Indeed, AK002 effectively depleted blood eosinophils in a phase I clinical trial and has demonstrated clinical activity in multiple mast cell and eosinophil-driven diseases 9,[10]. Recent studies have shown that bronchoalveolar lavage (BAL) eosinophils collected after airway allergen challenge in patients with mild asthma have high Siglec-8 expression [11] and that Siglec-8 is expressed on eosinophils and mast cells from dissociated lung tissue [8]. Other studies of eosinophils, performed in limited numbers of human lung tissue samples or in mice show that eosinophils can be depleted by an anti-Siglec-8 mAb [8, 12].
To further understand the role of Siglec-8 in asthma and the utility of targeting eosinophils and mast cells with an anti-Siglec-8 mAb in the human airway, we set out here to characterize Siglec-8 gene expression in sputum cells from a large cohort of asthma patients and healthy controls. We also explored whether an anti-Siglec-8 mAb can deplete eosinophils in sputum from patients with asthma and inhibit mast cells in human lung tissue.
Methods
Human Subjects
Induced sputum was collected from 74 asthmatic subjects and 37 healthy subjects (Table 1). The diagnosis of chronic stable asthma was defined as a prior physician diagnosis of asthma, and airway hyperreponsiveness (defined as a PC20 methacholine of less than 8.0mg/ml while not taking steroids or less than 16mg/ml while taking steroids) or reversible airflow obstruction (postbronchodilator increase in FEV1 of ≥12%) in subjects whose FEV1 percent predicted was less that 60%. For studies of sputum cell gene expression, RNA from induced sputum cells was available in the UCSF Airway Tissue Bank (ATB), a UCSF repository of biospecimens from patients with airway disease. The ATB uses common characterization procedures so that biospecimens are banked from subjects with standardized characterization data. For FACS-based studies which required freshly collected induced sputum, we designed a specific study protocol with an initial study visit for characterization purposes and additional study visits for sputum induction and collection of induced sputum for FACS and antibody depletion experiments. Venous blood was collected in parallel with induced sputum to allow for blood cell controls for the sputum cell experiments. The ATB procedures and study protocol were reviewed and approved by the UCSF Institutional Review Board. Fresh human lung tissue was procured and provided by the NCI Cooperative Human Tissue Network (CHTN) from subjects with no previous history of chronic lung disease, as approved by the Vanderbilt University Institutional Review Board (IRB# 031078 and 010294).
Table 1. Subject characteristics.
| qPCR study | FACS study | Depletion Study | ||
|---|---|---|---|---|
| Asthma | Asthma | Asthma | ||
| N | 74 | 7 | 8 | |
| Age, yr | 40.4 ± 16 | 48.4 ± 16.9 | 49.3 ± 16.4 | |
| Female, n (%) | 46 (62.2) | 5 (71.4) | 5 (62.5) | |
| BMI, kg/m2 | 29.8 ± 7.9 | 29.8 ± 5.6 | 30.1 ± 8.1 | |
| FEV1, (L) | 2.6 ± 1.0 | 2.3 ± 1.1 | 2.2 ± 0.9 | |
| FEV1, (% predicted) | 73 ± 25.4 | 70.5 ± 15.7 | 68.4 ± 16.4 | |
| PC20 mg/ml | 1.5 ± 1.9 | 1.0 ± 1.1 | 0.8 ± 0.9 | |
| On ICS, n (%) | 43 (58.1) | 2 (33.3) | 5 (71.4) | |
| Peripheral blood eosinophils, (109/ L) | 0.3 ± 0.2 | 0.4 ± 0.2 | 0.9 ± 1.1 | |
| Smoking History | ||||
| Never, n (%) | 48 (65.8) | 5 (71.4) | 7 (87.5) | |
| Former, n (%) | 25 (34.2) | 2 (28.6) | 1 (12.5) | |
Sputum collection
Induced sputum was collected and processed according to previously published protocols [13]. Briefly, the subjects inhale a nebulized 3% solution of hypertonic saline through a mouthpiece for 12 minutes, pausing every two minutes to spit saliva into a saliva cup and sputum into a sputum cup. Saliva was discarded and sputum was processed using a standard protocol. Induced sputum was mixed in a 1:1 g/mL ratio with 10% sputolysin (MilliporeSigma, Burlington, MA) and incubated at 37°C in a shaking water bath for 15 minutes. At 5, 10 and 15 minute intervals, the sputum was mixed using a serological pipette. A 1mL aliquot of the mixture was used for cell counting and determination of cell differentials. Sputum with greater than 80% squamous cells were excluded from our studies as poor quality samples.
RNA extraction and qPCR
Dissociated sputum was centrifuged at 800g for 10 minutes at 4°C. The sputum cell pellet was resuspended in 1mL of Saliva Protect reagent (Qiagen, Germantown, MD) and stored at -80°C until RNA extraction. Induced sputum was collected from a further six subjects for each of flow cytometry staining and ADCC mediated killing assays (Table 1). The sputum was processed as described below in the respective sections. Sputum RNA was extracted using RNeasy kits (Qiagen, Germantown, MD) according to our previously published protocols [14]. RNA was assessed for quality using an Agilent Bioanalyzer (Santa Clara, CA) that assigns a metric called the RNA Integrity Number (RIN) where 1 is poor quality and 10 is excellent. RNA with a RIN of less than 5 were excluded from the study. Taqman gene expression profiling on sputum cell RNA was performed according to our previous protocols [14]. All gene expression data were in the linear portion of the amplification curve. Samples that could not generate signal after 40 cycles were assigned a value equal to the minimum expression for that gene in other samples for analysis. Gene expression data was compared and normalized against the geometric mean of four housekeeping genes (GAPDH, YWHAZ, PSMB2 and PPIA). Primer and probe details are shown in Table S1.
Eosinophil peroxidase ELISA
For quantification of eosinophil peroxidase, induced sputum supernatant was assayed using a Human EPO ELISA kit (Diagnostics Development, Sweden). Samples were incubated in wells coated with anti-human EPO mouse monoclonal antibodies. After washing, a biotinylated anti-human EPO mouse monoclonal antibody was added to the wells and incubated for 60 minutes at room temperature. After further washing, streptavidin conjugated with horseradish peroxidase (HRP) was added to the wells and incubated for 30 minutes at room temperature. More washes were performed, then the HRP substrate, TMB (3,3’,5,5’-Tetramethylbenzidine), was added to the wells and allowed to incubate for 15 minutes. A sulfuric acid solution (2 N H2SO3) was added to each well to terminate the enzyme reaction and to stabilize the color development. The optical density of each well was measured at 450nm using a microplate reader. The concentration of EPO was calculated from the dose response standard curve, using a 4 parameter logistic curve fit.
Anti-Siglec-8 monoclonal antibodies
The S8mAb used in these studies refers to a non-fucosylated chimeric 2E2 IgG1 (c2E2 IgG1 KIF) mAb comprised of murine variable regions specific for Siglec-8 attached to non-fucosylated human k and IgG1 constant regions, as previously described [15]. Pre-clinical-grade fully human IgG1 antibodies were used as an isotype control (Eureka Therapeutics, Emeryville, Calif).
Flow cytometry staining
Dissociated sample was passed through a 70μm cell strainer prior to cell counting. The sample was centrifuged at 800g for 10 minutes at 4°C. The supernatant was discarded and the cells were resuspended in PBS + 0.1% BSA + 2mM EDTA. Sputum samples were treated with Fc Block at 1:100 (BD Biosciences, San Jose, CA) for five minutes before being stained with a cocktail of antibodies (Table S2) for 30 minutes on ice. FMO controls contained the antibody panel with the exception of anti-Siglec-8. Cells were washed twice in PBS + 0.1% BSA + 2mM EDTA and if secondary reagents were required, they were added for 30 minutes at 4°C. Cells were washed twice in PBS + 0.1% BSA + 2mM EDTA prior to analysis on a Becton Dickenson LSRII. Dextran gradient purified leukocytes were used as compensation controls, and the cells were stained as described above using the antibody panel shown in Table S3. For human lung tissue, approximately 0.1−0.5x106 cells/well were incubated in 96-well plates with Human Fc Block (BD Biosciences, San Jose, CA) for 10 minutes at 4°C followed by staining with conjugated antibodies for 10 minutes at 4°C. Cells were then washed with FACS buffer (0.1% bovine serum albumin [BSA] in PBS), resuspended in fixative (1% paraformaldehyde in PBS) and analyzed on a NovoCyte flow cytometer (Acea Biosciences, San Diego, CA). Mast cell were identified in lung tissue as CD45+ 7AAD- CD117+ FcεRI+.
Peripheral blood leukocyte processing
20 mL of blood was collected by venipuncture into EDTA containing vacutainers (BD Biosciences, San Jose, CA). The blood was diluted 2:1 with PBS and mixed with a 3% solution of dextran (500,000MW, Alfa Aesar, Tewksbury, MA) before being allowed to sediment at 37°C. The white cells were removed in the top layer and the bottom layer containing red blood cells was discarded. Cells were washed with PBS + 0.1% BSA + 2mM EDTA prior to use. Cells were stored in liquid nitrogen in RPMI + 40% FBS + 10% DMSO.
Blood eosinophil killing assays
Human blood eosinophil apoptosis and ADCC was measured as previously described [8, 15]. Briefly human eosinophils were purified and then seeded in 96-well flat-bottom tissue culture plates at 20,000 eosinophils/well. S8mAb or isotype control antibody (Allakos, Inc.) were added at 10 μg/mL in the presence of 50 ng/mL IL-3 or overnight activated human NK cells (effector : target 10:1 ratio). Cells were cultured overnight at 37°C in 5% CO2. Following overnight incubation, cells were stained with 7AAD and Annexin V-PE (BD Biosciences) for 30 min at room temperature in the dark. The labeled eosinophils were analyzed by flow cytometry, and percentage of dead eosinophils was determined.
Sputum ADCC assay
Dissociated sputum was centrifuged at 800g for 10 minutes at 4°C, and the cell pellet was resuspended in ADCC media (RPMI + 10% FCS + 1% P/S, 1% amphotericin B, 0.1% gentamycin, 1% Nystatin and 1μM Econazole). Cells were passed through a 70μm cell strainer and plated into a 12 well cell culture plate. Purified NK cells that had been activated in culture overnight in RPMI + 10% FCS + 1% P/S + 5ng/μl rIL-2 were added to the wells at an effector:target ratio of 1:1 NK: sputum cell (assuming approximate ratio of 10:1 effector:target NK: Siglec-8 bearing cell). Finally, S8mAb or isotype matched control antibody (Allakos, Redwood City, CA) were added to the wells at a final concentration of 10μg/ml. Cells were cultured for 24 hours in the presence of antibodies. 50,000 cells were removed to make cytospin slides and determination of eosinophil %.
Human lung tissue and mast cell activation assay
Fresh human lung tissue was procured and provided by the NCI Cooperative Human Tissue Network (CHTN) from subjects with no previous history of chronic lung disease. The tissue was enzymatically and mechanically dissociated using the gentleMACs™ Dissociator (Miltenyi Biotec), according to manufacturer’s protocol. Tissue was minced into 2 mm pieces and incubated at 37°C for one hour in digestion solution containing proprietary enzymes. Before, during, and after incubation, tissue was mechanically disrupted and run through a 70-micron filter to obtain single cells. Cells were then treated with RBC lysing buffer, washed in PBS and resuspended in RPMI 1640+10% Low IgG FBS. Immediately after digestion, cell viability was examined using flow cytometry. Only single-cell suspensions from dissociated tissue that had at least 70% viability were used in subsequent experiments. To activate mast cells, approximately 0.1-0.5 x 106 cells were plated/well in 96-well U-bottom plates with the indicated concentration of anti-FcεRI (CRA-1, BioLegend, San Diego, CA) for 30 minutes at 37°C followed by analysis of CD63 MFI on mast cells by flow cytometry. The mast cell inhibitory activity of anti-Siglec-8 was determined by incubating either 5 μg/mL S8mAb or isotype matched control antibody (Allakos, Inc) simultaneously with anti-FcεRI for 30 minutes at 37°C followed by flow cytometry analysis as described above. ΔDCD63 MFI was calculated by subtracting the unstimulated CD63 MFI from the MFI of CRA-1 stimulated cells.
Statistical analysis
Data analyses were performed GraphPad Prism version seven (GraphPad, San Diego, CA). ANOVA was used for three-group comparisons followed by pairwise analyses with the Tukey multiple comparisons test when appropriate. Two group comparisons were analyzed using the Students t-test or for non-parametric analyses, a ranked Mann-Whitney test.
Results
Siglec-8 gene expression in airway sputum cells
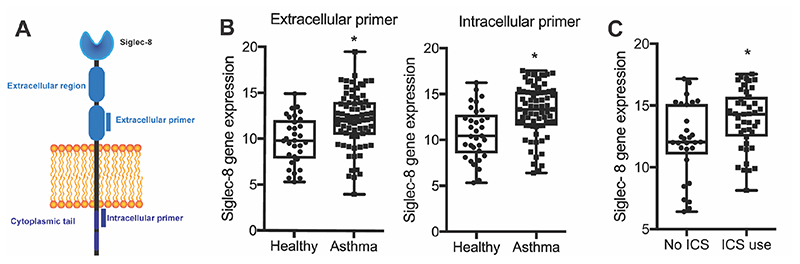
Siglec-8 has been shown to be expressed as a long form isoform with a cytoplasmic tail containing the typical Siglec tyrosine-based inhibitory motif (ITIM) and ITIM-like motif and a short form isoform that lacks the cytoplasmic tail [16]. Accordingly, we designed two different primer sets, one in the extracellular domain to measure all forms of Siglec-8 and one in the intracellular domain to measure the isoform with signaling motifs in the cytoplasmic tail (Fig 1A). We found similar patterns of gene expression for both primer sets in sputum cells from asthma subjects and healthy controls, suggesting that Siglec-8 long isoform expression is representative of overall Siglec-8 expression (Fig 1B). Based on these data, we focused subsequent analyses on data generated for the long Siglec-8 isoform containing the signaling motifs. We found that Siglec-8 gene expression in sputum cells from patients with asthma is significantly higher than in healthy controls (Fig 1B), including in a subset of asthma subjects taking inhaled corticosteroids (ICS) (Fig 1C).
Figure 1. Siglec-8 gene expression is increased in asthma sputum cells.
A) Schematic showing location of extracellular and intracellular primer sets. B) Quantification of Siglec-8 gene expression by qPCR showed that Siglec-8 is significantly upregulated in asthma sputum cells with both primer sets. C) Siglec-8 is significantly upregulated in sputum cells from asthmatic subjects taking ICS.
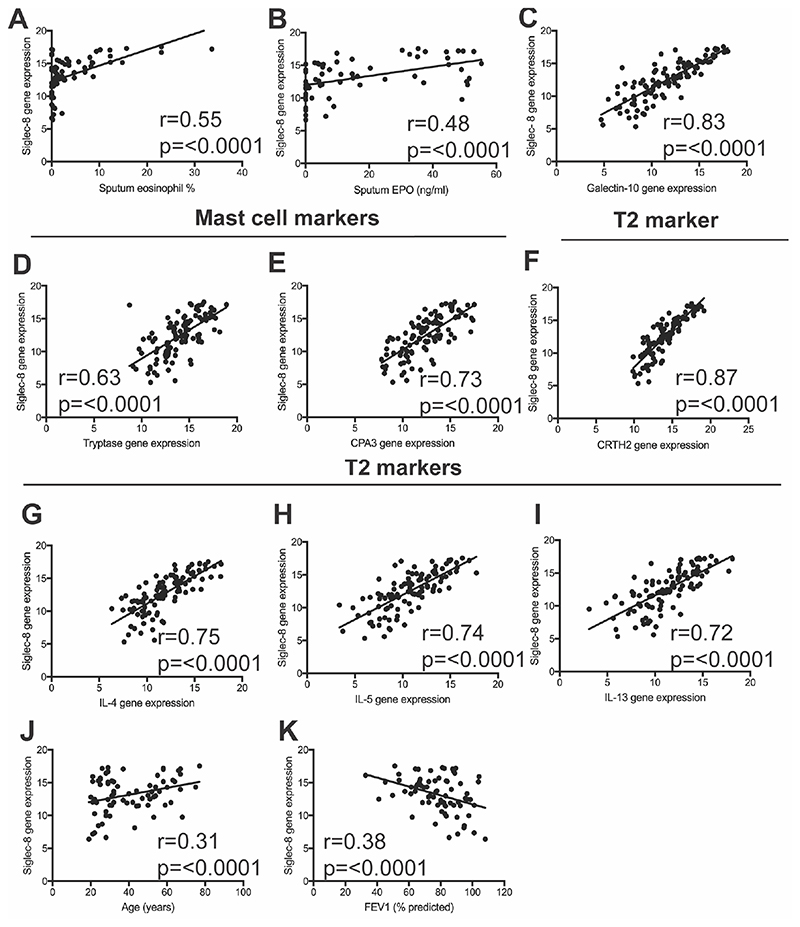
We next examined the relationship between Siglec-8 gene expression and sputum eosinophils. We found that the expression of Siglec-8 in sputum cells from asthma subjects strongly correlated with the sputum eosinophil percentage (Fig 2A) and eosinophil peroxidase (EPO) in sputum supernatants (Fig 2B). Siglec-8 was also strongly correlated with the expression of galectin-10 (an eosinophil related gene)(Fig 2C), tryptase and carboxypeptidase A3 (CPA3) (mast cell genes)(Fig 2D, 2E), and chemoattractant receptor-homologous molecule expressed on receptor on TH2 cells (CRTH2) (Fig 2F), IL-4, IL-5 and IL-13 (genes related to type 2 inflammation)(Fig 2G-I). These data support that eosinophils and mast cells are the only cell sources of Siglec-8 expression in sputum from asthma subjects and that Siglec-8 gene expression correlates with features of type 2-high asthma.
Figure 2. Siglec-8 gene expression correlates with eosinophils, mast cells, and lung function.
A) Siglec-8 gene expression in asthma sputum cells is strongly correlated with sputum eosinophil percentage and B) EPO; C) Galectin-10; D-E) tryptase, CPA3; T2 related genes F-I) CRTH2, IL-4, IL-5 and IL-13 and J) age. K) Siglec-8 gene expression in asthma sputum cells inversely correlates with FEV 1 (% predicted).
To determine how Siglec-8 gene expression in sputum cells relates to age and asthma severity, we examined relationships between Siglec-8 expression and age and measures of airflow. We found that Siglec-8 expression increases significantly with age (Fig 2J) and is inversely correlated with FEV1 (Fig 2K ).
Siglec-8 expression on airway cells by flow cytometry
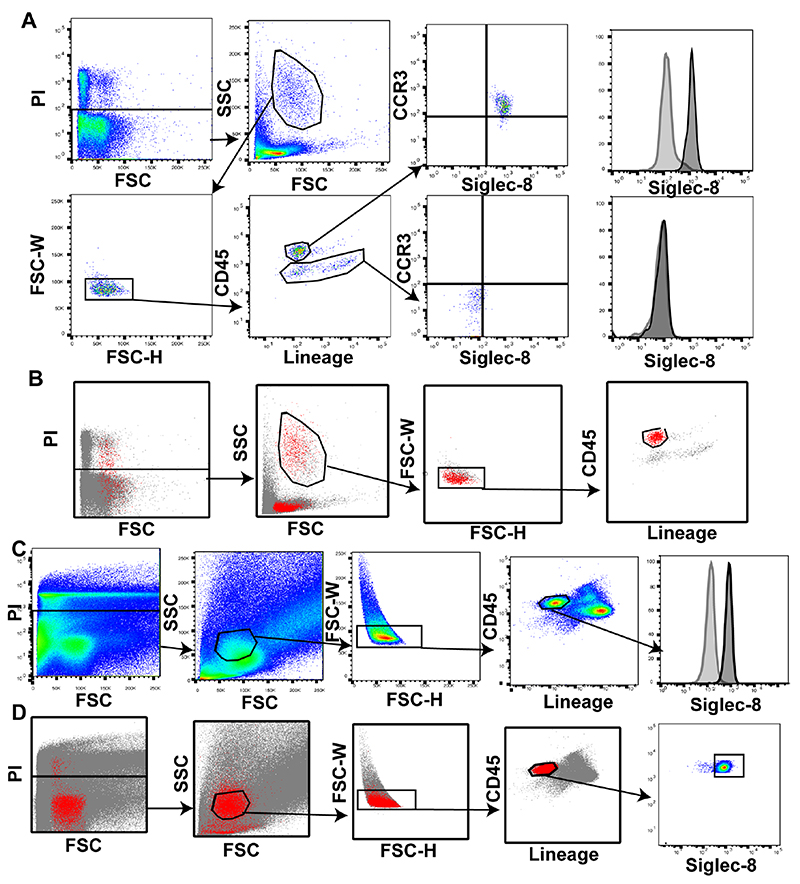
Building on our gene expression data, we set out to examine expression of Siglec-8 on eosinophils, mast cells and basophils in sputum. Although immunostaining blood cells with anti-CCR3 works well to stain eosinophils, we found that immunostaining sputum cells with anti-CCR3 does not (data not shown). Eosinophils are lineage negative cells with high side scatter and high expression of CD45 [17], so we gated sputum cells on live/dead, size, and singlets in order to identify eosinophils as CD45hi and lineagelo. To verify that sputum cells within this gate were eosinophils, we validated the approach in peripheral blood cells. We found that blood eosinophils that were CD45hi/lineagelo showed high expression of both CCR3 and Siglec-8 (Fig 3A). Backgating analyses based on Siglec-8 positive cells showed that Siglec-8 expressing cells were localized in this gate (Fig 3B). Applying this gating strategy to sputum cells showed that airway eosinophils strongly express Siglec-8 (Fig 3C). Backgating Siglec-8 expressing cells in sputum showed these cells localize to the CD45hi/lineagelo gate (Fig 3D).
Figure 3. Siglec-8 is expressed on the majority of sputum eosinophils.
A) Gating strategy to identify eosinophils as CD45hi, lineagelo in sputum cells. B) Backgating of Siglec-8 positive cells in peripheral blood. C) Airway eosinophils strongly express Siglec-8 compared to FMO control. D) Backgating Siglec-8 positive cells in sputum to validate gating strategy. In each panel Siglec-8 is shown in the black outline with an FMO control in the grey outline.
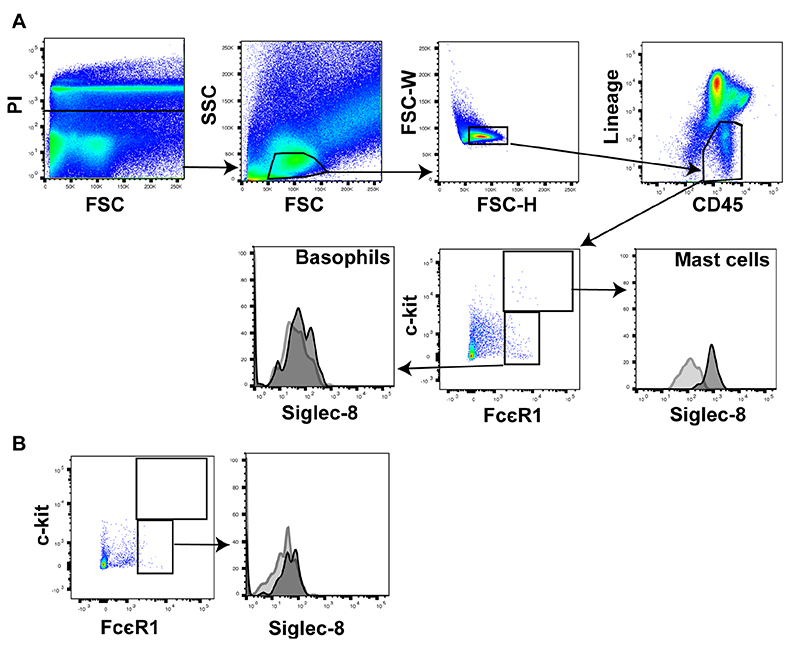
To identify mast cell and basophils in sputum, we gated on a population of CD45hi, lineagelo cells and defined mast cells as c-kithi, FcεR1hi and basophils as FcεR1hi c-kit-. In agreement with previously published studies in blood cells [2], we found that Siglec-8 was strongly expressed on airway mast cells (Fig 4A) while expression of Siglec-8 on airway basophils was very weak (Fig 4A) and similar to the weak expression seen on peripheral blood basophils (Fig 4B).
Figure 4. Siglec-8 is strongly expressed on sputum mast cells but very weakly on sputum basophils.
A) Sputum mast cells express high levels of Siglec-8 in comparison to the very low but detectable levels of Siglec-8 on airway basophils. B) Peripheral blood basophils show a similar level of Siglec-8 staining as airway basophils. In each panel Siglec-8 is shown in the black outline with an FMO control in the grey outline.
ADCC mediated depletion of asthmatic sputum eosinophils
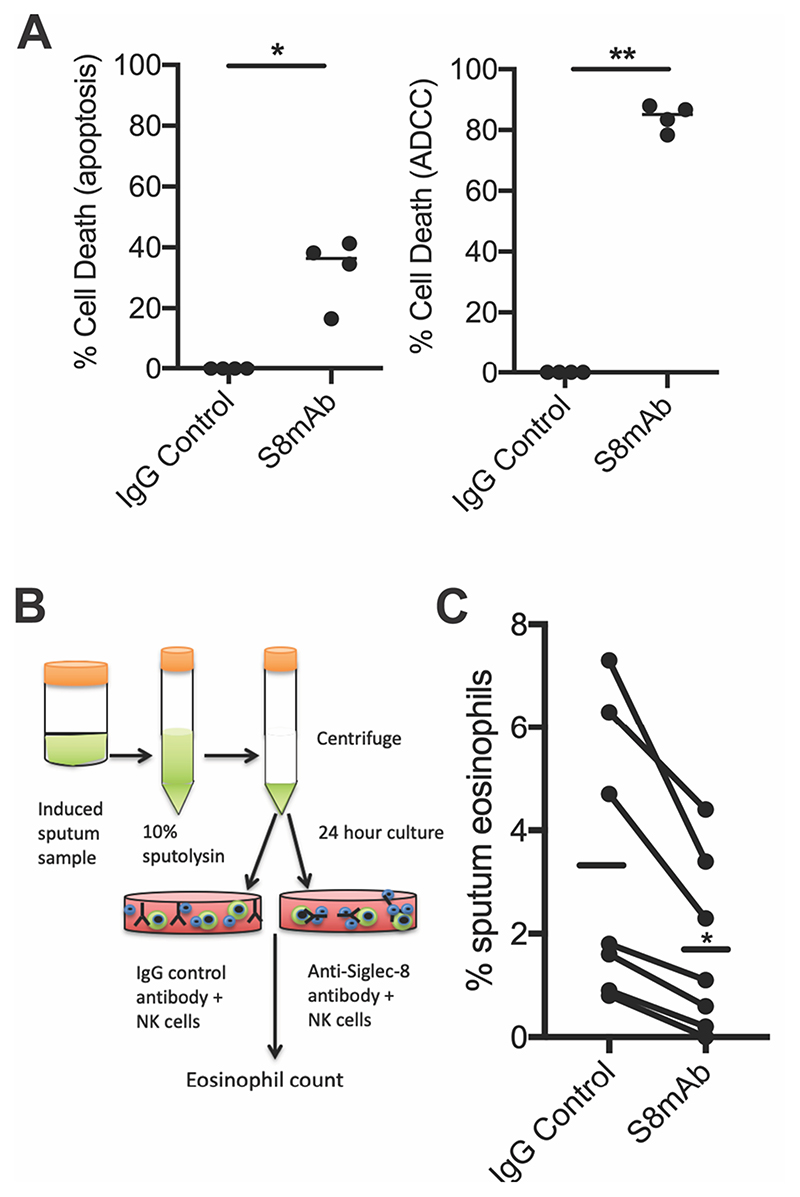
Previous studies have demonstrated that S8mAb treatment induces apoptosis of cytokine-primed eosinophils and ADCC of blood eosinophils in the presence of NK cells [15]. Eosinophils are relatively rare in sputum therefore in order to explore if an anti-Siglec-8 antibody could deplete eosinophils in sputum, we first tested if S8mAb-mediated apoptosis or ADCC was the more potent mechanism at reducing eosinophils ex vivo. Using healthy donor peripheral blood eosinophils, we found a much higher rate of eosinophil death in the presence of NK cells (Figure 5A) suggesting ADCC is the more potent mechanism of eosinophil reduction. Next we developed an ex vivo ADCC sputum cell assay using asthmatic subjects, summarized in Fig 5B. After 24 hours in culture, dead cells are removed using magnetic bead selection and the remaining cells are enumerated on cytospin slide preparations. In each of six sputum samples tested (independent donors), we found that the percentage of eosinophils in sputum treated with the S8mAb was significantly lower than when it was treated with the isotype control antibody (Fig 5C).
Figure 5. ADCC mediated depletion of eosinophils.
A) Cytokine-primed eosinophils from peripheral blood show lower rates of cell death when treated with S8mAb alone (apoptosis) compared to ADCC in the presence of NK cells. B) Schematic showing experimental strategy to isolate cells from sputum and culture in the presence of activated NK cells and S8mab or control antibody. C) Treatment of sputum cells with S8mAb and NK cells results in a significant decrease in sputum eosinophils.
Inhibition of IgE-mediated mast cell activation in human lung tissue
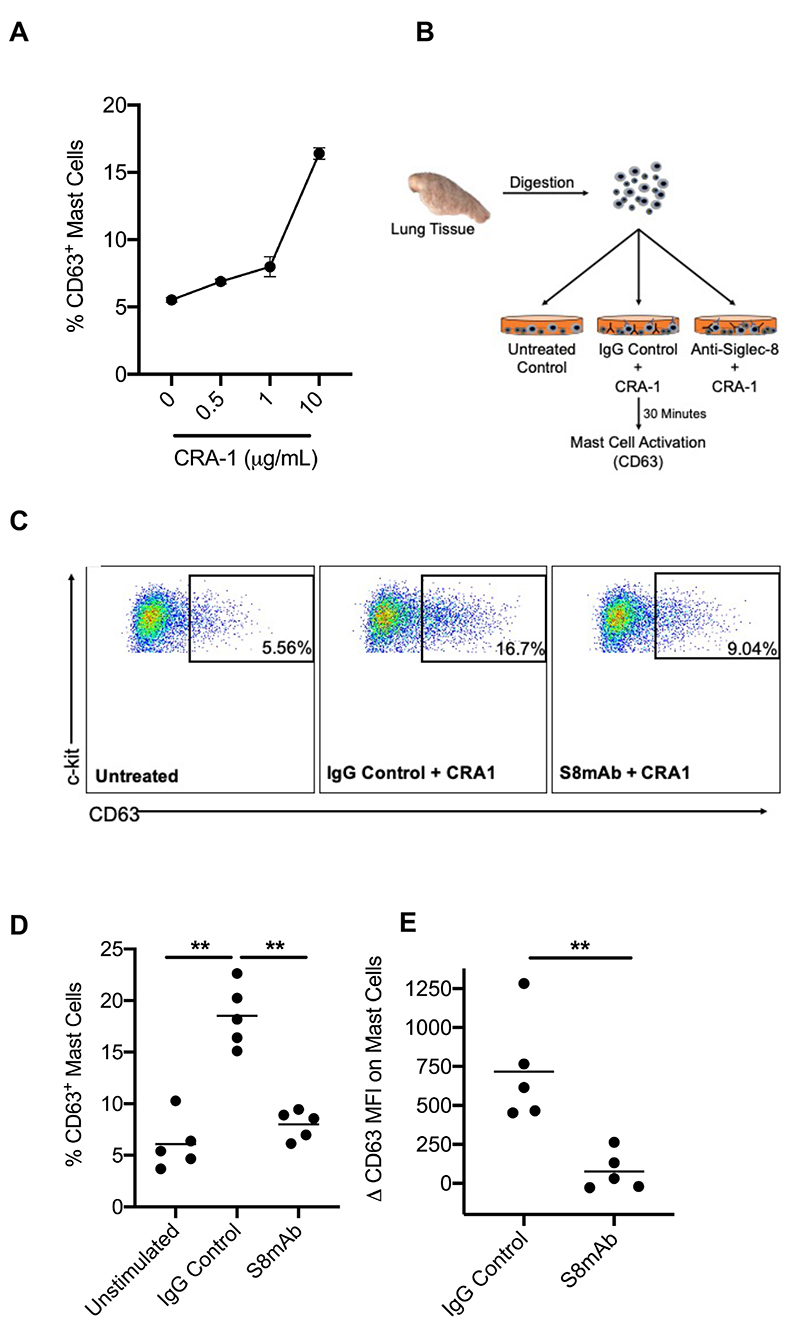
Crosslinking of Siglec-8 with an antibody induces broad inhibition of mast cell activity in vitro and in vivo [7, 8, 12]. Since mast cells in sputum are rare, we isolated mast cells from fresh human lung tissue in order to test the activity of an anti-Siglec-8 antibody. Human lung mast cells were identified by as CD45hi, viable 7AADlo, c-kithi, FcεR1hi (Supplemental Fig. S1) and robustly expressed Siglec-8 as previously published [12]. Human lung tissue mast cells were activated through the FcεR1 by an anti-FcεR1 antibody (CRA-1) which has been shown to induce mast cell degranulation in vitro [7]. To evaluate if human lung mast cells could be activated ex vivo, we titrated CRA-1 and analyzed the degranulation marker CD63 on lung mast cells. CRA-1 activated lung mast cells in a dose dependent manner, as evidenced by upregulation of CD63 (Fig. 6A). Next, we examined CRA-1 mediated mast cell activation in the presence of S8mAb or isotype control mAb (Fig. 6B). In each of the five human lung tissues tested (independent donors), S8mAb significantly decreased CRA-1 mediated CD63 upregulation compared to isotype control antibody treated mast cells (Fig. 6C-E). The reduction in CD63 expression was not associated with a decrease in mast cell number after S8mAb treatment (data not shown).
Figure 6. Anti-Siglec-8 inhibits IgE-mediated human lung mast cell activation.
A) The percentage of CD63+ mast cells after incubation with anti-FcεRI antibody (CRA-1). B) Schematic showing ex vivo mast cell activation assay. C) Representative dot plots of the percentage of CD63+ mast cells D) The percentage of CD63+ mast cells or E) ΔCD63 MFI on mast cells unstimulated or activated with CRA-1 in the presence of an isotype control or S8mAb.
Discussion
In this study we characterized the expression of Siglec-8 in induced sputum cells from patients with chronic stable asthma. We found that Siglec-8 gene expression correlates closely with airway gene expression for eosinophils, mast cells and type 2 cytokines and that the surface expression is high on eosinophils and mast cells. Using ex vivo assays in sputum and human lung tissue, we demonstrate for the first time, that an anti Siglec-8 antibody can evoke ADCC activity to decrease asthmatic sputum eosinophils and inhibit IgE-mediated mast cell activation in human lung tissue.
In initial experiments, we examined Siglec-8 gene expression in sputum cells from patients with chronic stable asthma and a group of healthy control subjects. Siglec-8 gene expression was increased in asthma and this increase was evident even in patients who were being treated with inhaled corticosteroids. We anticipated that Siglec-8 expression would be associated with biomarkers of eosinophils and mast cells in sputum and this proved correct.
Siglec-8 correlated closely with eosinophil percentage and even more strongly with multiple gene expression markers of eosinophil and mast cells. Eosinophils and mast cells are key cells in type 2 immune responses, and we also found that Siglec-8 expression correlated very strongly with gene expression for prostaglandin D2 receptor 2 (DP2 or CRTH2) and type 2 cytokines. Taken together, these gene expression data support that Siglec-8 gene expression in asthma sputum cells is a feature of the type 2-high subtype of asthma. Type 2-high asthma is characterized by older age and more severe airflow obstruction than the type 2-low endotype [18, 19] ; consistent with this, we report that Siglec-8 expression is associated with older age and with measures of airflow obstruction in asthma.
Our flow cytometry data for Siglec-8 expression on airway eosinophils and mast cells from asthma sputum confirm and extend prior published data. Siglec-8 has been identified in studies of peripheral blood eosinophils [1–3] and in BAL eosinophils after allergen challenge [11] but had not previously been investigated in chronic stable asthma. Studies in mast cells are not as numerous, but experiments using cord blood [2] and dissociated tissues from human lung and skin [8] have consistently found that Siglec-8 is expressed on mast cells. Less consistent has been Siglec-8 expression on basophils and we show here that Siglec-8 expression on basophils is weak both in the blood and sputum compartments.
AK002 is a humanized non-fucosylated IgG1 antibody directed against Siglec-8 that has shown enhanced NK cell-mediated ADCC activity in against blood eosinophils and apoptosis of tissue eosinophils. Eosinophils from type 2-high asthmatics could have a higher sensitivity to treatment with AK002 due to priming with IL-5 which increases sensitivity to apoptosis [6]. The studies here were done using a non-fucosylated chimeric anti-Siglec-8 antibody that is an analog to AK002. We first tested whether treatment with a S8mAb could decrease sputum eosinophils. We found that S8mAb treatment caused significant depletion of eosinophils in induced sputum from multiple donors when the sputum cells were cultured overnight in the presence of NK cells. Thus, we have demonstrated that anti-Siglec-8 antibodies can mediate ADCC activity against airway eosinophils raising the possibility that anti-Siglec-8 antibodies such as AK002 might have clinical utility in asthma.
In contrast to the ADCC and apoptosis activity seen with eosinophils, Siglec-8 antibody crosslinking does not induce apoptosis or kill mast cells [7, 8]. Instead, Siglec-8 mAbs inhibit mast cell activation both in vitro [7] and in vivo, including in a humanized IgE-mediated systemic anaphylaxis model and an allergic eosinophilic gastroenteritis model [8, 12]. To our knowledge, S8mAb-mediated inhibition of mast cells has not been demonstrated in primary human lung mast cells. We found that S8mAb treatment inhibits activation and degranulation of human lung mast cells. The decrease in mast cell activation after S8mAb treatment was not associated with a reduction in mast cell number, consistent with the lack of killing seen after S8mAb crosslinking on mast cells.
In summary, we report that Siglec-8 is robustly expressed in sputum eosinophils and mast cells in asthma and is associated with type 2 immune profiles in these samples. Targeting Siglec-8 with an anti-Siglec-8 antibody, such as AK002 is a plausible strategy to decrease sputum eosinophils and inhibit mast cells in asthma.
Supplementary Material
Acknowledgements
This work was supported by NHLBI grant HL107201. Allakos Inc, provided Siglec-8 reagents but did not provide funding.
References
- 1.Floyd H, Ni J, Cornish AL, et al. Siglec-8. A novel eosinophil-specific member of the immunoglobulin superfamily. J Biol Chem. 2000 Jan 14;275(2):861–6. doi: 10.1074/jbc.275.2.861. Epub 2000/01/08. [DOI] [PubMed] [Google Scholar]
- 2.Kikly KK, Bochner BS, Freeman SD, et al. Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells, and basophils. J Allergy Clin Immunol. 2000 Jun;105(6 Pt 1):1093–100. doi: 10.1067/mai.2000.107127. [DOI] [PubMed] [Google Scholar]
- 3.Nutku E, Aizawa H, Hudson SA, et al. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood. 2003 Jun 15;101(12):5014–20. doi: 10.1182/blood-2002-10-3058. [DOI] [PubMed] [Google Scholar]
- 4.Nutku E, Hudson SA, Bochner BS. Mechanism of Siglec-8-induced human eosinophil apoptosis: role of caspases and mitochondrial injury. Biochem Biophys Res Commun. 2005 Oct 28;336(3):918–24. doi: 10.1016/j.bbrc.2005.08.202. [DOI] [PubMed] [Google Scholar]
- 5.Carroll DJ, O’Sullivan JA, Nix DB, et al. Sialic acid-binding immunoglobulin-like lectin 8 (Siglec-8) is an activating receptor mediating beta2-integrin-dependent function in human eosinophils. J Allergy Clin Immunol. 2017 Sep 6; doi: 10.1016/j.jaci.2017.08.013. Epub 2017/09/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nutku-Bilir E, Hudson SA, Bochner BS. Interleukin-5 priming of human eosinophils alters siglec-8 mediated apoptosis pathways. Am J Respir Cell Mol Biol. 2008 Jan;38(1):121–4. doi: 10.1165/rcmb.2007-0154OC. Epub 2007/08/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yokoi H, Choi OH, Hubbard W, et al. Inhibition of FcepsilonRI-dependent mediator release and calcium flux from human mast cells by sialic acid-binding immunoglobulin-like lectin 8 engagement. J Allergy Clin Immunol. 2008 Feb;121(2):499–505.:e1. doi: 10.1016/j.jaci.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Youngblood BA, Brock EC, Leung J, et al. AK002, a Humanized Sialic Acid-Binding Immunoglobulin-Like Lectin-8 Antibody that Induces Antibody-Dependent Cell-Mediated Cytotoxicity against Human Eosinophils and Inhibits Mast Cell-Mediated Anaphylaxis in Mice. Int Arch Allergy Immunol. 2019 Aug 9;:1–12. doi: 10.1159/000501637. Epub 2019/08/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Sullivan JA, Chang AT, Youngblood BA, et al. Eosinophil and mast cell Siglecs: From biology to drug target. J Leukoc Biol. 2020 Jan 22; doi: 10.1002/jlb.2mr0120-352rr. Epub 2020/01/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rasmussen HS, Chang AT, Tomasevic N, et al. A randomized, double-blind, placebo controlled, ascending dose phase 1 study of AK002, a novel Siglec-8 selective monoclonal antibody, in healthy subjects (abstract) Journal of Allergy and Clinical Immunology. 2018;141:AB403 [Google Scholar]
- 11.Johansson MW, Kelly EA, Nguyen CL, et al. Characterization of Siglec-8 Expression on Lavage Cells after Segmental Lung Allergen Challenge. Int Arch Allergy Immunol. 2018;177(1):16–28. doi: 10.1159/000488951. Epub 2018/06/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Youngblood BA, Brock EC, Leung J, et al. Siglec-8 antibody reduces eosinophils and mast cells in a transgenic mouse model of eosinophilic gastroenteritis. JCI Insight. 2019 Oct 3;4(19) doi: 10.1172/jci.insight.126219. Epub 2019/08/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Innes AL, Carrington SD, Thornton DJ, et al. Ex vivo sputum analysis reveals impairment of protease-dependent mucus degradation by plasma proteins in acute asthma. Am J Respir Crit Care Med. 2009 Aug 1;180(3):203–10. doi: 10.1164/rccm.200807-1056OC. Epub 2009/05/09, 200807-1056OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters MC, Mekonnen ZK, Yuan S, et al. Measures of gene expression in sputum cells can identify TH2-high and TH2-low subtypes of asthma. J Allergy Clin Immunol. 2014 Feb;133(2):388–94. doi: 10.1016/j.jaci.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Legrand F, Cao Y, Wechsler JB, et al. Sialic acid-binding immunoglobulin-like lectin (Siglec) 8 in patients with eosinophilic disorders: Receptor expression and targeting using chimeric antibodies. J Allergy Clin Immunol. 2019 Jun;143(6):2227–37.:e10. doi: 10.1016/j.jaci.2018.10.066. Epub 2018/12/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aizawa H, Plitt J, Bochner BS. Human eosinophils express two Siglec-8 splice variants. J Allergy Clin Immunol. 2002 Jan;109(1):176. doi: 10.1067/mai.2002.120550. [DOI] [PubMed] [Google Scholar]
- 17.Gorczyca W, Sun ZY, Cronin W, et al. Immunophenotypic pattern of myeloid populations by flow cytometry analysis. Methods Cell Biol. 2011;103:221–66. doi: 10.1016/B978-0-12-385493-3.00010-3. Epub 2011/07/05. [DOI] [PubMed] [Google Scholar]
- 18.Peters MC, Kerr S, Dunican EM, et al. Refractory airway type 2 inflammation in a large subgroup of asthmatic patients treated with inhaled corticosteroids. J Allergy Clin Immunol. 2019 Jan;143(1):104–13.:e14. doi: 10.1016/j.jaci.2017.12.1009. Epub 2018/03/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters MC, Ringel L, Dyjack N, et al. A Transcriptomic Method to Determine Airway Immune Dysfunction in T2-High and T2-Low Asthma. Am J Respir Crit Care Med. 2019 Feb 15;199(4):465–77. doi: 10.1164/rccm.201807-1291OC. Epub 2018/10/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.