Capsule summary
This mouse study demonstrates that repetitive inhalation of a single major house dust mite (HDM) allergen prevents HDM-induced allergic asthma development through suppressing the function of lung dendritic cells, thus providing an alternative to classical allergen-specific immunotherapy.
Keywords: Allergic asthma, house dust mite/HDM, Der p 2/Derp2, allergen-specific immunotherapy/AIT, inhalation, pulmonary, dendritic cell, type 2 conventional dendritic cell/cDC2, GM-CSF
To the Editor,
The prevalence of allergic diseases is increasing, urging new ways of prevention. Allergen immunotherapy (AIT) is currently the only clinical intervention that can alter the natural course of allergy and can offer long-term clinical benefit. Although AIT is traditionally used as treatment in patients with established disease, the National Institute of Health Immune Tolerance Network (ITN) has proposed that prophylactic AIT could also be used as primary prevention for new sensitizations and allergic disease, in high risk children born to atopic parents and with a personal history of atopic dermatitis and/or food allergen sensitization in early life1. AIT involves the repeated administration of allergen extracts, leading to the induction of an ill-defined state of systemic allergen-specific immunological tolerance that is associated with decreased symptom scores, particularly in allergic rhinitis patients, but less so in asthmatics2. While current AIT involves the subcutaneous or sublingual administration of crude allergen extracts, regimens based on natural routes of mucosal allergen administration and defined single allergens might improve success rate. Several studies have suggested that the natural route of allergen exposure (e.g. ingestion of peanut allergen in infants, bee stings in bee keepers, inhalation of high doses of cat allergens in pet owners) can be very successful in preventing the onset of clinical allergies3–5. The exposure to immunodominant allergens in these studies, such as Arah6 peanut allergen in breast milk, phospholipase A2 in bee venom, and Feld1 cat allergen in house dust, suggests that the same allergens that can cause disease are also best at inducing tolerance via the natural route of exposure.
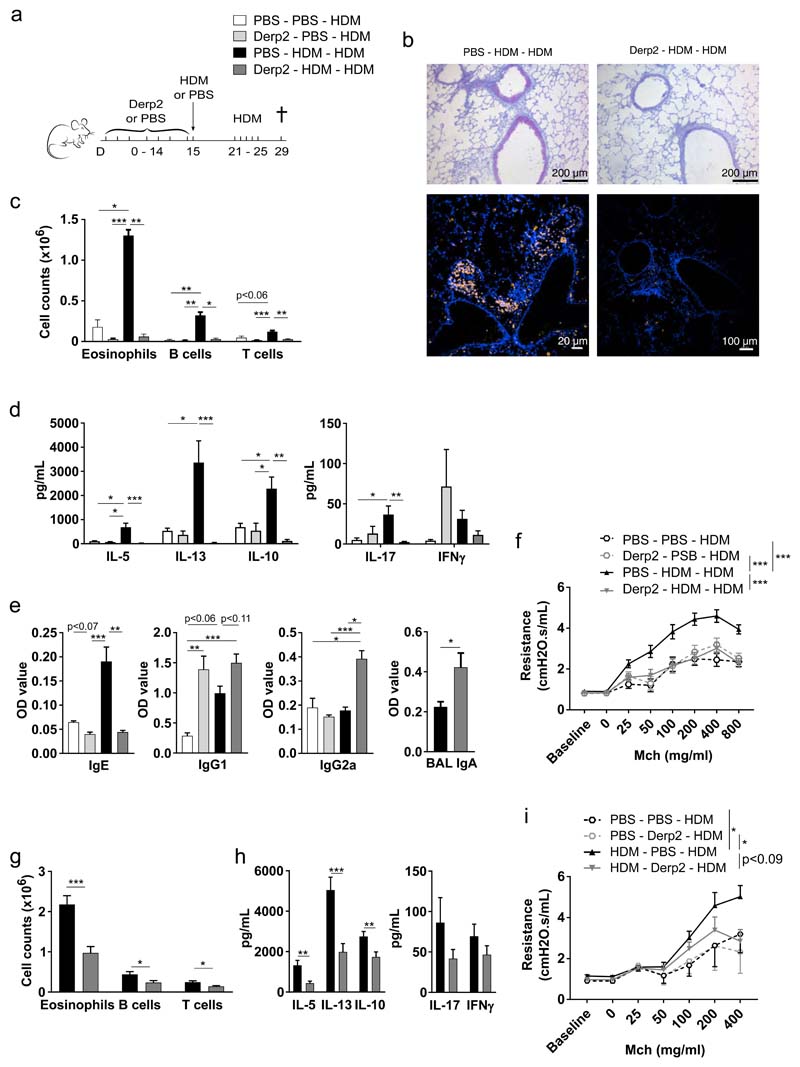
To improve the success rate of prophylactic AIT in asthma, we hypothesized that inhalation of Derp2, a major immunodominant house dust mite (HDM) allergen, would prevent the onset of HDM sensitization and HDM-induced asthma. Recombinant Derp2 was produced in the yeast Pichia pastoris, and was given as prophylactic AIT intranasally to naive C57Bl/6J mice every other day for a period of 15 days, prior to intratracheal (i.t.) sensitization to the full HDM extract, and HDM extract airway challenges (Fig 1a). In mice treated with sham PBS AIT, HDM sensitization and challenges induced robust asthma features, including airway and tissue eosinophilia (Fig 1b–c), goblet cell metaplasia (Fig 1b), T and B cell influx in the bronchoalveolar space (BAL) (Fig 1c), T helper 2 (Th2) cytokine production by lung-draining lymph node (LDLN) cells (Fig 1d), immunoglobulin (Ig)E synthesis (Fig 1e) and bronchial hyperreactivity (BHR) to methacholine (Fig 1f). These allergic asthma features were almost completely abolished in mice that received active Derp2 AIT (Fig 1b–f). To address if intranasal Derp2 AIT would also be effective in a more therapeutic secondary prevention setting, mice were first sensitized to HDM extract, and after one week treated for 17 days with Derp2 AIT, followed by HDM airway challenges. In this setting too, Derp2 AIT prevented development of the salient features of asthma, among which eosinophilia, Th2 cytokine production in LDLNs, and BHR (p<0.09) (Fig 1g–i).
Figure 1. Prophylactic Derp2 inhalation prevents HDM-induced allergic asthma development.
(a) Experimental setup for Fig 1b–f. (b) Mucus production (purple, upper panel), and eosinophils (yellow, lower panel) in lungs. (c) Immune cells in bronchoalveolar lavage (BAL) fluids. (d) Cytokine production by HDM-restimulated lung-draining lymph node (LDLN) cells. (e) Immunoglobulins (Igs) in serum (IgE, IgG1, IgG2c), or BAL (IgA). (f) Airway resistance in response to methacholine (Mch). (g-i) Secondary prevention setting. (g) Immune cells in BAL. (h) Cytokine production by HDM-restimulated LDLN cells. (i) Airway resistance in response to Mch. Results are representative of 1 (b), 2 (e), 4 (d) or 6 (c), or pooled from 2 (f, i) or 3 (g, h) independent experiments, with n = 4-7 (b-f) or 3-6 (g-i) mice/group for single experiments. Data are shown as means ± SEM, or in (f) and (i) as predictions of means ± SEM obtained from repeated measurement analysis using residual maximum likelihood (REML). *p<0.05, **p<0.01, ***p<0.001. OD: optical density at 650 nm/850 nm.
We next sought for the immunological mechanism(s) of prophylactic AIT. In the active Derp2 AIT group, we observed increased concentrations of HDM-specific IgG2c and IgG1 in serum, and of HDM-specific IgA in BAL fluid (Fig 1e). In humans, successful AIT is often accompanied by increased titers of IgG1, IgG4 and/or IgA6. However, despite the increase in several Igs, our prophylactic Derp2 AIT did not require B cells, since the effects of AIT on lung eosinophilia and Th2 cytokine production were preserved in Mb1 Cre x Rosa26-Lox-Stop-LoxDTA mice genetically lacking B cells (Fig E1).
The generation of adaptive type 2 immunity to HDM depends on allergen presentation by type 2 conventional dendritic cells (cDC2s), which bridge innate and adaptive immunity. The capacity of lungs DCs to take up HDM allergen in the lungs and transport it to the LDLNs was not reduced by prophylactic Derp2 AIT (Fig E2a), and there were only small effects of Derp2 AIT on the expression of the co-stimulatory molecules CD80 and CD86 on cDC1s and cDC2s (Fig E2b). To study the impact of Derp2 AIT on functional allergen presentation by DCs, we used TCR-transgenic 1-DER mice in which all CD4+ T cells react to an immunodominant peptide of Derp17. CD4+ 1-DER T cells were transferred to mice previously treated with Derp2 or sham PBS AIT, and their active division induced by HDM extract inhalation. 4, 7 and 10 days after HDM inhalation, the LDLNs of sham treated mice contained highly proliferating 1-DER T cells, yet proliferation was strongly reduced in mice receiving Derp2 AIT (Fig. 2a–b). Derp2 AIT also suppressed IL-5, IL-13, and IL-10 secretion (Fig. 2c), and Gata3 mRNA expression (Fig E2c) by LDLN cells, and reduced the total and effector CD44+CD62L- 1-DER T cell numbers in lung tissue (Fig 2d–e). Similar effects of Derp2 AIT on 1-DER T cell activity were observed when i.t. Derp1 was administered instead of HDM to trigger 1-DER T cell responses (data not shown). In support of a role for Derp2 AIT-induced suppression of cDC2 functions, we found that the adoptive transfer of in vivo HDM-primed cDC2, obtained from LDLNs of mice that had never been exposed to Derp2 AIT, was sufficient to break the tolerant state induced by prophylactic Derp2 AIT (Fig. 2f). Furthermore, vice versa, LDLN cDC2s from mice undergoing prophylactic Derp2 AIT and then exposed to a single HDM extract inhalation, were less efficient in priming type 2 immunity upon adoptive transfer to naive hosts (Fig E2d). In another set of experiments, cDCs were sorted from the LDLNs of sham or Derp2 AIT mice that were primed with a single Derp1 protein inhalation, and were co-cultured with 1-DER T cells ex vivo. cDC2s from the Derp2 AIT group induced less 1-DER T cell proliferation than cDC2s from the sham AIT group (Fig 2g). T cell proliferation induced by cDC1s was not influenced by Derp2 AIT (data not shown).
Figure 2. Prophylactic Derp2 inhalation suppresses cDC2-mediated Th2 responses by blocking GM-CSF release.
(a-e) Derp2 or sham PBS mice received CFSE-labeled 1-DER T cells intravenously (iv) and HDM intratracheally (i.t.), and were analyzed 4, 7 or 10 d later. (a) Cell division profiles, and (b) proliferation parameters, of tissue-resident (CD45iv injected (iv) -) 1-DER T cells in lung-draining lymph nodes (LDLNs). (c) Cytokine production by HDM-restimulated LDLN cells. (d) Number and (e) phenotype of tissue-resident lung 1-DER T cells. (f) Treatment as in Fig 1a, with 2 groups of mice i.t. sensitized with donor HDM-primed cDC2s instead of HDM. Immune cells in bronchoalveolar lavage (BAL) fluids. (g) V450-labeled 1-DER T cells co-cultured with LDLN cDC2s of Derp2 or sham PBS mice i.t. instilled with Derp1. Cell division profiles, expansion indexes, and counts of 1-DER T cells. (h) Cytokines and chemokines in lung tissue of Derp2 or sham PBS mice 2 h after HDM instillation. (i) Treatment as in Fig 1a, with 1 group of mice i.t. sensitized with HDM + GM-CSF. BAL immune cells. (j) Derp2 or sham PBS mice received CFSE-labeled 1-DER T cells iv and Derp1 with or without GM-CSF i.t.. Proliferation parameters of LDLN 1-DER T cells. Results are representative of 2 (f-h, j) or 3 (a-e), or pooled from 2 (i) independent experiments, with n = 4-8 (a-f, h-j) mice/group or 2-3 (g) replicates/group for single experiments. Shown as means ± SEM (or ± SD in (g)). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
We finally addressed the mechanism of the reduced cDC2-mediated antigen presentation in mice treated with prophylactic Derp2 AIT. In response to HDM inhalation, cDC2s are activated to perform this function through the epithelial release of DC-instructing cytokines8. We therefore measured whether prophylaxis with Derp2 affected the release of pro-allergic cytokines and chemokines following a first inhalation of whole HDM allergen extract. In the sham AIT group, a single dose of i.t. instilled HDM extract led to an increased production of MCP-1, KC, IL-1α, and GM-CSF, in lungs, compared to i.t. instilled PBS (Fig 2h). Prophylactic Derp2 AIT only significantly reduced the production of GM-CSF, a cytokine that has been shown to break inhalation tolerance by activating DCs9. To study the functional importance of reduced GM-CSF release in the tolerance mediated by Derp2, mice treated with sham or Derp2 AIT and sensitized and challenged with HDM extract, were supplemented i.t with recombinant GM-CSF at the time of sensitization. Strikingly, Derp2 AIT mice that received GM-CSF at the time of HDM sensitization were no longer protected from allergy development, as assessed by their development of a robust BAL eosinophilia (Fig 2i). We found comparable results in a secondary prevention setting (Fig E3a). Similarly, the effect of prophylactic Derp2 AIT on 1-DER T cell division was also rescued by GM-CSF supplementation (Fig 2j and Fig E3b). In conclusion, our findings in mice show that prophylactic exposure to the single immunodominant HDM allergen Derp2 via the airways offers an alternative way to prevent respiratory allergy to the complex allergen HDM, by suppressing GM-CSF-driven activation of lung cDC2s.
Footnotes
Disclosure of potential conflict of interest: The authors declare no conflicts of interest.
Contributor Information
Haspeslagh Eline, Email: eline.haspeslagh@irc.vib-ugent.be.
Vanheerswynghels Manon, Email: manon.vanheerswynghels@ugent.be.
Deswarte Kim, Email: kim.deswarte@ugent.be.
Justine van Moorleghem, Email: justine.vanmoorleghem@IRC.vib-ugent.be.
Bart N Lambrecht, Email: bart.lambrecht@ugent.be.
Hammad Hamida, Email: Hamida.Hammad@UGent.be.
References
- 1.Holt PG, Sly PD, Sampson HA, Robinson P, Loh R, Lowenstein H, et al. Prophylactic use of sublingual allergen immunotherapy in high-risk children: A pilot study. J Allergy Clin Immunol. 2013;132(4):991–4. doi: 10.1016/j.jaci.2013.04.049. [DOI] [PubMed] [Google Scholar]
- 2.Dhami S, Kakourou A, Asamoah F, Agache I, Lau S, Jutel M, et al. Allergen immunotherapy for allergic asthma: A systematic review and meta-analysis. Allergy. 2017;72(12):1825–48. doi: 10.1111/all.13208. [DOI] [PubMed] [Google Scholar]
- 3.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized Trial of Peanut Consumption in Infants at Risk for Peanut Allergy. N Engl J Med. 2015;372(9):803–13. doi: 10.1056/NEJMoa1414850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeal H, Draper A, Harris J, Taylor AN, Cullinan P, Jones M. Modified Th2 responses at high-dose exposures to allergen: Using an occupational model. Am J Respir Crit Care Med. 2006;174(1):21–5. doi: 10.1164/rccm.200506-964OC. [DOI] [PubMed] [Google Scholar]
- 5.Cullinan P, MacNeill SJ, Harris JM, Moffat S, White C, Mills P, et al. Early allergen exposure, skin prick responses, and atopic wheeze at age 5 in English children: A cohort study. Thorax. 2004;59(10):855–61. doi: 10.1136/thx.2003.019877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James LK, Shamji MH, Walker SM, Wilson DR, Wachholz PA, Francis JN, et al. Long-term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. J Allergy Clin Immunol. 2011;127(2):509–16.:e5. doi: 10.1016/j.jaci.2010.12.1080. [DOI] [PubMed] [Google Scholar]
- 7.Coquet JM, Schuijs MJ, Smyth MJ, Deswarte K, Beyaert R, Braun H, et al. Interleukin-21-Producing CD4+ T Cells Promote Type 2 Immunity to House Dust Mites. Immunity. 2015;43(2):318–30. doi: 10.1016/j.immuni.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15(4):410–6. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swirski FK, Sajic D, Robbins CS, Gajewska BU, Jordana M, Stampfli MR. Chronic Exposure to Innocuous Antigen in Sensitized Mice Leads to Suppressed Airway Eosinophilia That Is Reversed by Granulocyte Macrophage Colony-Stimulating Factor. J Immunol. 2002;169(7):3499–506. doi: 10.4049/jimmunol.169.7.3499. [DOI] [PubMed] [Google Scholar]