Abstract
Objectives
Potassium and magnesium are frequently administered after cardiac surgery to reduce the risk of atrial fibrillation (AF). The evidence for this practice is unclear. This study was designed to evaluate the relationship between serum potassium and magnesium levels and AF after cardiac surgery.
Design
Observational cohort study.
Setting
A cardiac intensive care unit in the United Kingdom.
Participants
Patients undergoing cardiac surgery between January 2013 and November 2017.
Interventions
None.
Measurements and Main Results
Cardiac rhythm was assessed using continuous electrocardiogram (ECG) monitoring in 3,068 patients on the cardiac intensive care unit. Associations between serum potassium and magnesium concentrations extracted from hospital databases and postoperative AF were assessed using univariable and multivariable analyses. The association between electrolyte supplementation therapy and AF was also analyzed.
AF developed within 72 hours of cardiac surgery in 545 (17.8%) of the 3,068 patients. After adjusting for logistic EuroSCORE, surgery type, cardiopulmonary bypass time and age, mean serum potassium concentration <4.5 mmol/L was associated with an increased risk of AF (odds ratio [OR] 1.43 (95% confidence interval (CI): 1.17-1.75), p < 0.001). Mean magnesium concentration <1.0 mmol/L was not associated with an increased risk of AF (OR 0.89, 0.71-1.13, p = 0.342), but the administration of magnesium was associated with increased risk of developing AF (OR 1.61, 1.33-1.96, p < 0.001).
Conclusions
Maintaining a serum potassium concentration ≥4.5 mmol/L after cardiac surgery may reduce the incidence of postoperative AF. Magnesium supplementation was associated with an increased risk of postoperative AF. Prospective randomized trials are required to clarify these associations.
Keywords: Atrial Fibrillation, electrolytes, cardiac surgery, critical care
POSTOPERATIVE ATRIAL fibrillation or flutter (AF) occurs in 18% to 50% of patients undergoing cardiac surgery, depending on the type of surgery being performed,1–4 and is associated with prolonged stay in hospital,5 increased healthcare costs,6 and increased short-7 and long-term mortality risk.8 The plasma concentrations of potassium and magnesium are thought to be important factors in the development of AF,9 and the administration of potassium supplementation therapy to prevent postoperative AF is a common practice that is based on cardiac myocyte electrophysiology.10 Magnesium supplementation is also frequently used in the prevention and treatment of AF. It is administered with the aims of increasing the response to potassium supplementation11 and lowering the risk of AF directly.9,12 However, there is a lack of strong evidence to support electrolyte supplementation as a means of preventing AF,13 and studies that have investigated predictors of postoperative AF have failed to identify postoperative electrolyte concentrations or supplementation therapy as risk factors.14–17
Analyzing data from 1,800 patients, Lancaster et al. reported that patients who experienced AF after cardiac surgery had higher concentrations of magnesium (0.96 v 0.89 mmol/L) and potassium (4.30 v 4.21 mmol/L) around the time of onset of AF than those who did not.18 Although after multivariable regression analyses, only the association between magnesium and postoperative AF remained statistically significant. In a randomized control trial of 910 cardiac surgery patients, Hoekstra et al. failed to identify a benefit in terms of a reduction in AF rates from potassium administration with the target of achieving postoperative serum potassium concentrations ≥4.5 mmol/L.19
The lack of clear evidence led to the omission of potassium and magnesium supplementation from the evidence-based guidelines recently published by the Society of Cardiovascular Anesthesiologists/European Association of Cardiothoracic Anaesthesiology on the management of AF after cardiac surgery.20 However, despite the lack of supportive evidence, in a study of current practice in critical care, over three-quarters of respondents stated that their primary treatment for new onset AF would include supplementing electrolytes to high normal levels.21
The objective of this study was to compare potassium and magnesium concentrations and rates of electrolyte supplementation in those who did and did not develop AF after cardiac surgery. The null hypothesis was that after controlling for confounders there would be no difference in the concentrations of electrolytes or rates of electrolyte supplementation in those who did and did not experience AF.
Methods
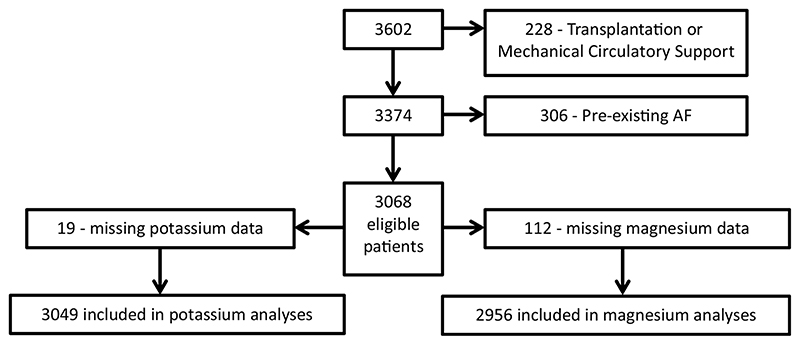
Data from patients admitted to the cardiac intensive care unit (CICU) at Wythenhawe Hosptial, Manchester following cardiac surgery between January 2013 and November 2017 (limited to the first 72 hours after surgery) were collected and analyzed. Patients undergoing transplantation or requiring mechanical circulatory support and those diagnosed with atrial fibrillation or atrial flutter preoperatively were excluded. Any patients for whom no postoperative serum potassium and magnesium levels were available were also excluded (Fig 1).
Fig 1. study flowchart.
Hourly cardiac rhythm assessments were performed by the treating clinicians through examination of the continuous ECG traces displayed by the Draeger Infinity bedside monitors (Draeger, Lübeck, Germany). All hourly assessments together with all potassium concentrations measured by the Gem5000 (Instrumentation Laboratory, Massachusetts, USA) point-of-care blood gas analyzers were extracted from the electronic patient record (EPR). All administrations of intravenous potassium or magnesium supplementation therapy were also extracted from the EPR along with the date and time of administration. Preoperative magnesium and potassium concentrations for each patient and postoperative magnesium levels over the first 72 hours of the patients’ CICU stay were extracted from the hospital’s pathology laboratory database. All laboratory analyses were performed using the Architect C1600 (Abbott, Maidenhead, UK) analyzers used for routine biochemical assays in this institution. All data were collected as part of routine clinical care.
The primary question was whether potassium concentrations <4.5 mmol/L or magnesium concentrations <1.0 mmol/L were associated with postoperative AF. The threshold value for potassium (4.5 mmol/L) was chosen as this had been the threshold used in previous studies19,21,22 and is also the target potassium concentration in the institution where this study was conducted. The magnesium threshold of 1.0 mmol/L was chosen based on the institution’s treatment protocol. The target concentrations in this study’s institution have been chosen in line with the practice of the majority of respondents in Chean’s study21 with the aim of reducing the incidence of postoperative cardiac arrhythmias. For those who developed AF, the mean of all potassium concentrations recorded in the 12 hours immediately prior to onset of AF was calculated. For those who did not develop AF, the overall mean potassium concentration was calculated. Similar mean magnesium concentration values were calculated, but as magnesium concentrations were routinely measured daily, the mean magnesium concentration for those who developed AF was calculated over 24 rather than 12 hours.
Previous studies compared electrolyte concentrations recorded around the onset of AF for those who developed AF with concentrations recorded for non-AF patients around the median onset time of AF in the AF group.18 This study used mean electrolyte concentrations during the CICU admission limited to 72 hours for non-AF patients instead.
A sensitivity analysis was also performed to ensure that the overall classification of each non-AF patient’s mean electrolyte concentrations was not skewed by a single extreme value. For the non-AF group, CICU stays were divided into portions of 12 hours for potassium concentrations and 24 hours for magnesium concentrations. The mean value of each electrolyte concentration during each portion was calculated and classified using the same thresholds (4.5 mmol/L for potassium and 1.0 mmol/L for magnesium). For the sensitivity analysis, patients were assigned to the group into which their mean electrolyte concentrations fell during the majority of their discrete blocks. Analyses were then repeated using the revised classifications. The reclassification was not performed for patients who developed AF; for these patients, the mean electrolyte concentrations in the time period directly before onset of AF (12 hours for potassium, 24 hours for magnesium) were utilized as per the primary analyses.
Further analyses compared the distributions of the concentrations of these electrolytes in those who did and did not develop AF. To test whether lower target thresholds might be useful, the incidences of potassium concentrations <3.5 mmol/L and magnesium concentrations <0.7 mmol/L for those who developed AF were compared with those of patients who did not. To test whether change relative to preoperative concentration (rather than absolute value) was relevant, the difference between pre- and postoperative potassium concentrations (Δ [K+]) was compared between those who did and did not develop AF. Preoperative values analyzed were the latest preoperative value recorded in the laboratory database, and these samples were typically taken during the preoperative visit or the night before surgery. For those who developed AF, Δ [K+] was defined as the difference between a patient’s mean potassium concentration during the 12 hours before the onset of AF and their preoperative potassium concentration. For those who did not develop AF, Δ [K+] was defined as the difference between the patient’s mean postoperative potassium concentration and their preoperative potassium concentration. Preoperative magnesium concentrations were not assessed as they were not routinely measured.
Finally, the amount of electrolyte supplementation therapy administered before the onset of AF for those who developed AF was compared with that administered to those who did not develop AF. To allow a fair comparison, for those who did not develop AF, only doses administered before the median time of onset of AF in the AF group were counted. As a further sensitivity analysis, the number of doses of electrolyte supplementation therapy given per 24 hours on CICU was compared for the entire length of stay for patients who did and did not develop AF.
Data collection, cleaning, and storage were performed according to the ethical and research and development approvals for the Vascular Governance NorthWest database. As the data utilized in this study were routinely collected and pseudonymized, specific patient consent and ethical approval for analyses presented in this manuscript were not required as per the recommendations of the ethics committees.
Statistical Analyses
Univariable analyses of differences between proportions were performed using chi-square tests except in cases of sparse data for which analyses were performed using the Fisher exact test. Electrolyte concentrations were compared using the Student t test because they were found to be normally distributed. Univariable comparisons of the number of doses of electrolyte supplementation therapy administered were made using the Wilcoxon rank sum test because these variables were not normally distributed.
The associations between postoperative AF and potassium concentrations <4.5 mmol/L and between postoperative AF and magnesium concentrations <1.0 mmo/L were assessed using multivariable logistic regression analyses adjusting for type of surgery, cardiopulmonary bypass (CPB) time, preoperative comorbidity and urgency (using the logistic EuroSCORE23), and age. The comparisons were repeated for the sensitivity analyses using the revised classifications described above. Similar multivariable models were also used to assess whether electrolyte supplementation therapy was associated with AF. All analyses were conducted using R (R foundation for statistical computing).24
Results
During the study period, 3,602 cardiac surgery patients were admitted to CICU. There were 228 patients excluded because they underwent cardiac transplantation or required mechanical circulatory support, and 306 were excluded because they were in AF before admission to CICU. A total of 3,068 eligible patients (mean [standard deviation (SD)] age was 66.1 [11.0] years) were identified of whom 19 were excluded from the potassium analyses owing to a lack of postoperative potassium values within the specified time period (within the 12 hours preceding AF for those who developed AF or during the CICU stay for those who did not). Similarly, 112 were excluded from the magnesium analyses owing to an absence of postoperative magnesium concentrations within the specified time period. Preoperative potassium values were missing from a further 45 patients so the Δ K+ analyses included only 3,004 patients.
The most common procedure performed was isolated coronary artery bypass graft (CABG), which was performed for 1,867 (60.9%) patients, and the median length of CICU stay was 46.3 (25.2-69.2) hours. Detailed characteristics of the patients are shown in Table 1.
Table 1. Patient Characteristics.
| Characteristic | AF (n = 545) | No AF (n = 2523) | p value |
|---|---|---|---|
| Age, mean (SD), y | 70.1 (9.9) | 65.3 (11.0) | < 0.01 |
| Female, n (%) | 152 (27.9) | 680 (27.0) | 0.69 |
| Weight, mean (SD), kg | 82.5 (15.9) | 81.9 (16.2) | 0.39 |
| Median logistic EuroSCORE (interquartile range) |
5.3 (2.9-9.7) | 3.1 (1.7-6.2) | < 0.01 |
| Operation, n (%) | |||
| CABG | 268 (49.2) | 1599 (63.4) | < 0.01 |
| Valve | 127 (23.3) | 454 (18.0) | 0.01 |
| CABG + valve | 102(18.7) | 308 (12.2) | < 0.01 |
| Aortic | 43 (7.9) | 121 (4.8) | < 0.01 |
| Other—minor | 5 (0.9) | 41 (1.6) | 0.30 |
| Urgency, n (%) | |||
| Elective | 333 (61.1) | 1399 (55.4) | 0.02 |
| Urgent | 198 (36.3) | 1080 (42.8) | 0.01 |
| Emergency | 13 (2.4) | 36 (1.4) | 0.15 |
| Salvage | 1 (0.2) | 8 (0.3) | 0.58 |
| Median CPB time, (interquartile range), min |
107.0(83.0-138.5) | 100.0 (80.0-126.0) | < 0.01 |
Abbreviations: AF, atrial fibrillation; CABG, coronary artery bypass graft; CPB, cardiopulmonary bypass; SD, standard deviation.
A total of 545 patients (17.8%) developed AF during the first 72 hours of their CICU admission. The median (interquartile range [IQR]) time to onset of AF was 39.0 (29.2-51.0) hours. The median (IQR) time from last potassium concentration measurement to onset of AF was 2.0 (1.0-3.0) hours. The median (IQR) time from last recorded magnesium concentration to onset of AF was 12.0 (4.5-18.0) hours. The variables derived from the electrolyte concentrations analyzed in this study are detailed in Tables 2 and 3.
Table 2. Number of Patients With Electrolyte Concentrations Above the Thresholds Among Those Who Did and Did Not Develop AF.
| Electrolyte Group | AF, n (%) | No AF, n (%) | p value |
|---|---|---|---|
| Mean [K+] <4.5 mmol/L, n (%) | 274 (51.6) | 1057 (42.0) | <0.001 |
| Mean [K+] ≥4.5 mmol/L, n (%) | 257 (48.4) | 1461 (58.0) | <0.001 |
| Mean [Mg2+] <1.0 mmol/L, n (%) | 145 (30.3) | 973 (39.3) | <0.001 |
| Mean [Mg2+]≥1.0 mmol/L, n (%) | 333 (69.7) | 1505 (60.7) | <0.001 |
Due to missing [K+] and [Mg2+] data, 3,049 patients were included in potassium analyses and 2,956 were included in magnesium analyses as described in Fig 1. For those who developed AF the mean [K+] was calculated over the 12 hours before onset of AF and the mean [Mg2+] was calculated over the 24 hours before onset of AF. For those who didn’t suffer AF the mean values were calculated over the first 72 hours of the CICU stay. Abbreviation: AF, atrial fibrillation; CICU, Cardiac Intensive Care Unit
Table 3. Comparisons of Electrolyte Concentrations for Those Who Did and Did Not Develop AF.
| AF Group | No AF Group | p value | ||
|---|---|---|---|---|
| Derivative | Mean (SD) Concentration mmol/L | Mean (SD) Concentration mmol/L | Derivative | |
| Preoperative [K+] | 4.29 (0.41) | 4.32 (0.40) | Preoperative [K+] | 0.13 |
| Mean [K+] in the 12 h before onset of AF | 4.50 (0.35) | 4.58 (0.26) | Mean [K+] in first 72 hours | <0.001 |
| 3.96 (0.36) | Minimum [K+] in first 72 hours | <0.001* | ||
| Δ[K+] | 0.21 (0.48) | 0.26 (0.41) | Δ[K+] | 0.05 |
| Mean [Mg2+] in the 24 h before onset of AF | 1.09 (0.26) | 1.05 (0.21) | Mean [Mg2+] in first 72 hours | <0.001 |
| 0.95 (0.18) | Minimum [Mg2+] in first 72 hours | <0.001^ | ||
For those who developed AF, Δ [K+] = mean [K+] in the 12 hours before onset of AF — preoperative [K+]. For those who did not develop AF, Δ [K+] = mean [K+] in the 72 hours after surgery — preoperative [K+].
Abbreviations: AF, atrial fibrillation.
Compared with Mean [K+] in the 12 h before onset of AF.
Compared with Mean [Mg2+] in the 24 h before onset of AF.
Primary Analyses of Electrolyte Concentrations
As seen in Table 2, in the 12 hours preceding onset of AF, 274 patients (51.6%) experienced a mean potassium concentration <4.5 mmol/L. Of those who did not develop AF, 1,057 (42.0%) experienced a mean potassium concentration < 4.5 mmol/L during the first 72 hours of their CICU stay (p < 0.001). On multivariable analysis, potassium concentration <4.5 mmol/L (OR 1.43, 95% CI: 1.17-1.75), increased logistic EuroSCORE (OR 1.03, 95% CI: 1.01-1.04), valve surgery (OR 1.38, 95% CI: 1.10-1.73), and age (OR 1.03 95% CI: 1.02-1.05) were associated with the development of AF (p ≤ 0.005 for all). Details of all multivariable analyses including the sensitivity analyses are included in the Appendix.
For the sensitivity analysis, of those who did not develop AF and experienced an overall mean potassium concentration of ≥4.5 mmol/L, 125 (8.6%) experienced 12 hourly mean potassium concentrations <4.5 mmol/L for the majority of their 12-hour blocks. Of those who did not develop AF and experienced an overall mean potassium concentration of <;4.5 mmol/L, 33 (3.1%) experienced 12 hourly mean potassium concentrations ≥4.5 mmol/L for the majority of their 12-hour blocks. When logistic regression analyses were repeated using the reclassified potassium concentrations, potassium concentration ≥4.5 mmol/L (OR 1.26, 95% CI: 1.03-1.54, p = 0.025), increased logistic EuroSCORE (OR 1.03, 95% CI: 1.01-1.04, p < 0.001), valve surgery (OR 1.38, 95% CI: 1.10-1.72, p = 0.006), and age (OR 1.03 95% CI: 1.02-1.05, p < 0.001) were associated with the development of AF.
Only 145 (30.3%) patients who developed AF experienced a magnesium concentration <1.0 mmol/L in the 24 hours prior to onset of AF compared with 973 (39.3%) of those who did not develop AF (p < 0.001). After multivariable adjustment, magnesium concentration < 1.0 mmol/L (OR 0.89, 95% CI: 0.71-1.13) was not found to be associated with an increase in the risk of developing of AF (p = 0.342).
Thirty-seven (2.5%) of those who did not develop AF and experienced an overall mean magnesium concentration of >1.0 mmol/L experienced 24 hourly mean magnesium concentrations <1.0 mmol/L for the majority of their 24-hour blocks. Twenty-seven (2.8%) of those who did not develop AF and experienced an overall mean magnesium concentration of ≥ 1.0 mmol/L experienced 24 hourly mean magnesium concentrations >1.0 mmol/L for the majority of their 24-hour block. When the logistic regression analyses were repeated using the reclassified magnesium concentrations, magnesium concentration ≥1.0 mmol/L was not associated with the development of AF (OR 0.88, 95% CI: 0.69-1.11, p = 0.270).
Secondary Analyses of Electrolyte Concentrations
Of those who developed AF, 31 (5.8%) experienced a potassium concentration <3.5 mmol/L prior to the onset of the arrhythmia compared with 149 (5.9%) of those who did not develop AF (p = 1.0). A magnesium concentration <0.7 mmol/L was observed in one (0.2%) patient who went on to develop AF and in 20 (0.8%) patients who did not (p = 0.23).
Univariable comparisons between the mean potassium concentration recorded in the 12 hours before the onset of AF and potassium concentrations recorded in those who did not develop AF are shown in Table 3. Minimum potassium concentrations for those who did not develop AF occurred relatively early in the postoperative period; the median time from CICU admission to the minimum potassium concentration recorded was 17.8 hours (IQR 2.0-32.8).
As shown in Table 3, there was no statistically significant difference between preoperative potassium concentrations of those who did and did not go on to develop postoperative AF. Those who developed AF exhibited a smaller rise in mean potassium concentration relative to the preoperative value (Δ [K+]) than those who did not. However, for both groups, the mean postoperative potassium concentration was greater than the preoperative value.
Univariable comparisons between the mean magnesium concentration recorded in the 24 hours before the onset of AF and magnesium concentrations recorded in those who did not develop AF are also shown in Table 3. The minimum magnesium concentration recorded in those who did not develop AF was observed slightly later than for potassium with a median time from ICU admission of 34.8 hours (IQR 14.8-40.4).
Electrolyte Supplementation Therapy
Potassium supplementation therapy was administered to 2551 (83.1%) patients and magnesium to 1240 (40.4%) patients. The median (IQR) number of doses of potassium (20 mmol IV) and magnesium supplementation therapy (20 mmol IV) administered during the first 72 hours of ICU admission were 3 (1-5) and 0 (0-1), respectively.
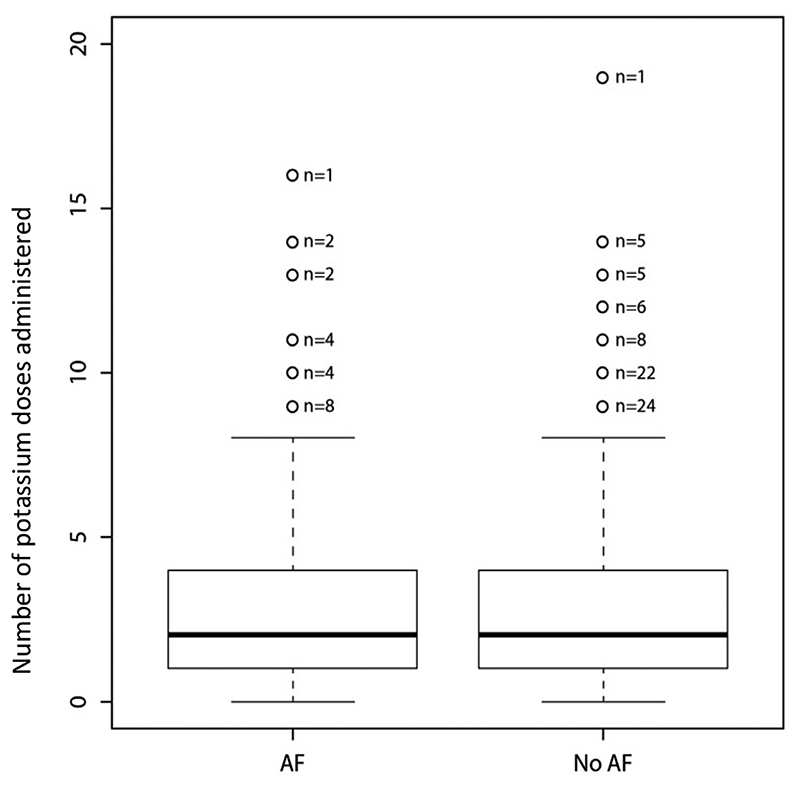
As seen in Figure 2, before the onset of the arrhythmia, patients who developed AF received a similar number of potassium doses to those who did not (median 2.0 v 2.0, p = 0.70). Of those who developed AF, 139 (25.5%) received magnesium compared with 470 (18.6%) who did not (p < 0.001). These findings were confirmed on multivariable regression analyses controlling for logistic EuroSCORE, CPB time, valve surgery and age which identified no association between potassium administration and AF (OR 1.02, 95% CI: 0.98-1.06, p = 0.352). However, AF was more likely in those who received a higher number of doses of magnesium supplementation therapy (OR 1.61, 95% CI: 1.33-1.96, p < 0.001). Full details of these multivariable analyses are included in the appendix.
Fig 2. Boxplots illustrating the administration of potassium replacement therapy to those who did and did not develop AF.
When analyzing the amount of electrolyte supplementation therapy administered through the entire stay on CICU, patients who experienced AF received fewer doses of potassium per 24 hours (median [IQR] 1.01 [0.49-1.80]) than those who did not experience AF (1.29 [0.47-2.57]) but more doses of magnesium per 24 hours (median 0.25 [0-0.43] v 0 [0-0.32], p < 0.001 for both).
Discussion
This study demonstrated that a potassium concentration below 4.5 mmol/L was associated with an increase in the risk of AF, whereas the impact of a magnesium concentration below 1.0 mmol/L was the opposite of that expected. During the first 72 hours of CICU admission, the group who did not develop AF experienced minimum potassium and magnesium concentrations, which were, on average, lower than those recorded in the 12 hours before AF onset in those who developed the arrhythmia. The median times at which the minimum potassium and magnesium concentrations were recorded were both earlier than the median AF onset time for the AF group. Despite experiencing lower electrolyte concentrations at times when AF was most likely to develop, such patients remained in sinus rhythm. It is therefore clear that electrolyte concentrations alone do not adequately explain the risk of AF and that models aiming to identify those at risk of AF are unlikely to rely heavily on the analysis of electrolyte concentrations. However, electrolyte optimization and in particular maintaining a potassium concentration above 4.5 mmol/L may prevent AF developing in some patients.
Investigating the potential impact of high-normal target electrolyte thresholds, such as the potassium thresholds of 4.5 mmol/L described by Auer22 and Hoekstra,19 was the primary objective of this study. Auer et al. retrospectively examined data from 253 patients in the Study of Prevention of Postoperative Atrial Fibrillation (SPPAF) randomized control trial. They found higher rates of AF for patients with potassium concentrations <3.9 mmol/L compared with those who had potassium concentrations >4.4 mmol/L (50.7% v 32.9%, p < 0.05). Hoekstra et al.’s study failed to identify a benefit of maintaining higher potassium concentrations, possibly because the mean plasma concentration achieved in their higher target concentration group (4.33 mmol/L) was only 0.11 mmol/L higher than the mean concentration achieved in their lower target concentration group (4.22 mmol/L).19 A larger, multicenter, non-inferiority, randomized controlled trial investigating the effects of supplementing serum potassium levels to ≥3.6 mmol/L versus ≥4.5 mmol/L is scheduled to start recruitment shortly13 with the aim of providing the definitive answer to the question, “Does potassium supplementation to maintain high-normal serum levels prevent AF?”
The association between greater magnesium supplementation doses (together with higher magnesium concentrations) and increased risk of AF supports similar findings from Lancaster et al.18 Although it may be that magnesium was supplemented more aggressively when clinicians suspected an increased risk of AF, the prophylactic administration of magnesium to patients with normal magnesium concentrations should be questioned. In their retrospective analysis of data from cardiac surgery patients, Lancaster et al. compared potassium concentrations (for 1688 patients) and magnesium concentrations (for 925 patients) between groups who did and did not develop postoperative AF. On univariable analysis, Lancaster’s study found that around the time of onset of AF, those who developed AF had higher mean potassium and magnesium concentrations than those who did not. However, the association between potassium concentrations and AF was not confirmed during multivariable logistic regression analysis. This study also found that those who experienced AF received were more likely to have received magnesium supplementation therapy than those who did not. However, that study did not identify a difference between rates of administration of supplementation replacement therapy in those who did and did not experience AF postoperatively.
Existing models, which were designed to predict AF after cardiac surgery, are largely based on preoperative patient characteristics.14–17 In this study, preoperative risk factors known to be associated with the risk of AF, such as renal impairment, COPD, and heart failure, were adjusted for via their inclusion in the logistic EuroSCORE calculation. While preoperative risk factors are clearly important, only postoperative risk factors can be modified once the patient has undergone surgery, and as such these risk factors are more important in clinical care on the CICU. Where postoperative risk factors are analyzed by existing risk scores, the postoperative variables included are mainly based on the administration of medications or therapies which have since been studied in more detail.17 Consequently, some of the medications included as variables, such as beta blockers, are now widely administered after cardiac surgery as part of treatment protocols.
Limitations
In order to maximize the number of potassium concentration measurements included in the study, we used point-of-care measurements from the CICU blood gas analyzers. This achieved a median time interval between the latest potassium concentration and the onset of AF of only 2.0 hours. Concerns have been raised previously about the accuracy of electrolyte concentration measurements made by point-of-care analyzers at extremes of expected ranges. However, their accuracy has been demonstrated repeatedly to meet internationally recognized calibration targets.25–27 As all postoperative potassium readings were measured using the same instrument, they can probably be relied upon to the extent required by this study design.
The retrospective nature of this study necessarily limited analyses to the data available from the institution’s EPR, which only includes medications administered intravenously. Since the cardiac surgery protocol includes the routine administration of beta blockade daily using a pre-printed prescription chart unless actively omitted by treating physicians, it is unlikely that the prescription of beta blockers influenced these results. Although oral electrolyte supplementation data were not available, intravenous supplementation is almost universal after cardiac surgery in patients on this CICU. The retrospective design also precluded the strict standardization of patient management. In particular, while this institution’s protocol only aimed to keep magnesium concentrations ≥1.0 mmol/L, it is likely that unless a patient was experiencing hypermagne-saemia, additional magnesium would be administered to any patient identified as being at increased risk of developing AF.
The large number of patients in the study allowed the inclusion of preoperative logistic EuroSCORE, CPB time, surgery type, and age into the logistic regression models used to adjust for potential confounders. Unfortunately, high quality data on other possible confounders were not available.
The mean electrolyte concentrations recorded during a short period immediately before onset of arrhythmia were analyzed for those who developed AF because this was thought to best represent the levels of electrolytes present at that time. Since no event regarding arrhythmia onset was available in the control group, comparisons were made with mean and minimum values recorded in those who did not develop AF over the first 72 hours of their admission. In their retrospective study, Lancaster et al. used the electrolyte concentrations for non-AF patients recorded closest to the mean onset time of AF in the AF group (50.9 hours) as the potassium concentration for the no-AF group.18 The authors believe comparison with electrolyte concentrations measured throughout the first 72 hours of the CICU episode for those who did not develop AF is more useful than comparison with a point value taken at around 50 hours into the ICU episode. To ensure the overall mean concentration wasn’t skewed by one extreme reading, the sensitivity analyses, in which the authors assigned the patients to groups based on the mean electrolyte concentrations for each discrete portion of their admission, were performed. The similarity of the results observed in the primary analyses and the associated sensitivity analyses indicate that inappropriate classification of electrolyte concentrations is unlikely to have affected these findings.
The frequency of postoperative AF identified in this study is at the lower end of the ranges reported in the literature.1–3,16 This is because the study only included data from the patients’ CICU admissions as frequent electrolyte concentrations and heart rhythm classification data were available only in this setting. The CICU stays were truncated at 72 hours in order to avoid comparing electrolyte concentrations from the immediate postoperative period, where onset of AF was most likely, with concentrations measured much later in prolonged CICU stays of patients who did not develop AF. As the study did not examine data from more than 72 hours after cardiac surgery, the application of its findings to the management of electrolytes later in the postoperative period should be undertaken with caution. The postoperative treatment protocols used in this study, including routine beta blocker prophylaxis and supplementation of potassium to a target concentration of 4.5 mmol/L (which resulted in an overall mean potassium concentration for all patients of >4.5 mmol/L during the first postoperative 72 hours) may have favorably impacted the frequency of AF. The low incidence of AF and the high-normal overall mean potassium concentrations in the study may limit transferability to settings in which AF rates are higher and potassium concentrations are not supplemented to the same targets. However, it is possible that differences in potassium concentrations between groups who did and did not develop AF may have been larger had the high target concentration not been in place.
Conclusion
This observational study provides supporting evidence that maintenance of potassium concentrations above 4.5 mmol/L in the early postoperative period may reduce the risk of developing AF after cardiac surgery. Supplementation of magnesium was unexpectedly associated with increased risk of developing AF. Management of postoperative electrolyte concentrations and, in particular, the practice of magnesium supplementation in the absence of magnesium deficiency should be investigated further in prospective randomized studies.
Supplementary Material
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
References
- 1.LaPar DJ, Speir AM, Crosby IK, et al. Postoperative atrial fibrillation significantly increases mortality, hospital readmission, and hospital costs. Ann Thorac Surg. 2014;98:527–33. doi: 10.1016/j.athoracsur.2014.03.039. discussion 533. [DOI] [PubMed] [Google Scholar]
- 2.Tran DTT, Perry JJ, Dupuis J-Y, et al. Predicting new-onset postoperative atrial fibrillation in cardiac surgery patients. J Cardiothorac Vasc Anesth. 2015;29:1117–26. doi: 10.1053/j.jvca.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Hosokawa K, Nakajima Y, Umenai T, et al. Predictors of atrial fibrillation after off-pump coronary artery bypass graft surgery. Br J Anaesth. 2007;98:575–80. doi: 10.1093/bja/aem067. [DOI] [PubMed] [Google Scholar]
- 4.D’Agostino RS, Jacobs JP, Badhwar V, et al. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2018 update on outcomes and quality. Ann Thorac Surg. 2018;105:15–23. doi: 10.1016/j.athoracsur.2017.10.035. [DOI] [PubMed] [Google Scholar]
- 5.Todorov H, Janssen I, Honndorf S, et al. Clinical significance and risk factors for new onset and recurring atrial fibrillation following cardiac surgery—A retrospective data analysis. BMC Anesthesiol. 2017;17:163. doi: 10.1186/s12871-017-0455-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaporciyan AA, Correa AM, Rice DC, et al. Risk factors associated with atrial fibrillation after noncardiac thoracic surgery: Analysis of 2588 patients. J Thorac Cardiovasc Surg. 2004;127:779–86. doi: 10.1016/j.jtcvs.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Attaran S, Shaw M, Bond L, et al. Atrial fibrillation postcardiac surgery: A common but a morbid complication. Interact Cardiovasc Thorac Surg. 2011;12:772–7. doi: 10.1510/icvts.2010.243782. [DOI] [PubMed] [Google Scholar]
- 8.Tulla H, Hippelainen M, Turpeinen A, et al. New-onset atrial fibrillation at discharge in patients after coronary artery bypass surgery: short-and longterm morbidity and mortality. Eur J Cardiothorac Surg. 2015;48:747–52. doi: 10.1093/ejcts/ezu526. [DOI] [PubMed] [Google Scholar]
- 9.Raiten JM, Ghadimi K, Augoustides JGT, et al. Atrial fibrillation after cardiac surgery: Clinical update on mechanisms and prophylactic strategies. J Cardiothorac Vasc Anesth. 2015;29:806–16. doi: 10.1053/j.jvca.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Bharucha DB, Kowey PR. Management and prevention of atrial fibrillation after cardiovascular surgery. Am J Cardiol. 2000;85:20D–4D. doi: 10.1016/s0002-9149(00)00903-6. [DOI] [PubMed] [Google Scholar]
- 11.Whang R, Whang DD, Ryan MP. Refractory potassium repletion. A consequence of magnesium deficiency. Arch Intern Med. 1992;152:40–5. [PubMed] [Google Scholar]
- 12.Miller S, Crystal E, Garfinkle M, et al. Effects of magnesium on atrial fibrillation after cardiac surgery: a meta-analysis. Heart. 2005;91:618–23. doi: 10.1136/hrt.2004.033811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell NG, Allen E, Sanders J, et al. The impact of maintaining serum potassium >/=3.6 mEq/L vs >/=4.5 mEq/L on the incidence of new-onset atrial fibrillation in the first 120 hours after isolated elective coronary artery bypass grafting—Study protocol for a randomised feasibility trial for the proposed Tight K randomized non-inferiority trial. Trials. 2017;18:618. doi: 10.1186/s13063-017-2349-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cameron MJ, Tran DTT, Abboud J, et al. Prospective external validation of three preoperative risk scores for prediction of new onset atrial fibrillation after cardiac surgery. Anesth Analg. 2018;126:33–8. doi: 10.1213/ANE.0000000000002112. [DOI] [PubMed] [Google Scholar]
- 15.Mariscalco G, Biancari F, Zanobini M, et al. Bedside tool for predicting the risk of postoperative atrial fibrillation after cardiac surgery: The POAF score. J Am Heart Assoc. 2014;3:e000752. doi: 10.1161/JAHA.113.000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferreira AF, F AS, Moreira R, et al. Postoperative atrial fibrillation after coronary artery bypass grafting surgery. Rev Port Cir Cardiotorac Vasc. 2017;24:129. [PubMed] [Google Scholar]
- 17.Mathew JP, Fontes ML, Tudor IC, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720–9. doi: 10.1001/jama.291.14.1720. [DOI] [PubMed] [Google Scholar]
- 18.Lancaster TS, Schill MR, Greenberg JW, et al. Potassium and magnesium supplementation do not protect against atrial fibrillation after cardiac operation: A time-matched analysis. Ann Thorac Surg. 2016;102:1181–8. doi: 10.1016/j.athoracsur.2016.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoekstra M, Hessels L, Rienstra M, et al. Computer-guided normal-low versus normal-high potassium control after cardiac surgery: No impact on atrial fibrillation or atrial flutter. Am Heart J. 2016;172:45–52. doi: 10.1016/j.ahj.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 20.O’Brien B, Burrage PS, Ngai JY, et al. Society of Cardiovascular Anes-thesiologists/European Association of Cardiothoracic Anaesthetists practice advisory for the management of perioperative atrial fibrillation in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2019;33:12–26. doi: 10.1053/j.jvca.2018.09.039. [DOI] [PubMed] [Google Scholar]
- 21.Chean CS, McAuley D, Gordon A, et al. Current practice in the management of new-onset atrial fibrillation in critically ill patients: A UK-wide survey. Peer J. 2017:8. doi: 10.7717/peerj.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Auer J, Weber T, Berent R, et al. Serum potassium level and risk of postoperative atrial fibrillation in patients undergoing cardiac surgery. J Am Coll Cardiol. 2004;44:938–9. doi: 10.1016/j.jacc.2004.05.035. author reply 939. [DOI] [PubMed] [Google Scholar]
- 23.Roques F, Michel P, Goldstone AR, et al. The logistic EuroSCORE. Eur Heart J. 2003;24:881–2. doi: 10.1016/s0195-668x(02)00799-6. [DOI] [PubMed] [Google Scholar]
- 24.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Accessed 06 December 2019]. Available at: https://www.R-project.org. [Google Scholar]
- 25.Jain A, Subhan I. Joshi M Comparison of the point-of-care blood gas analyzer versus the laboratory auto-analyzer for the measurement of electrolytes. Int J Emerg Med. 2009;2:117–20. doi: 10.1007/s12245-009-0091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allardet-Servent J, Lebsir M, Dubroca C, et al. Point-of-care versus central laboratory measurements of hemoglobin, hematocrit, glucose, bicarbonate and electrolytes: A prospective observational study in critically ill patients. Plos One. 2017;12:e0169593. doi: 10.1371/journal.pone.0169593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jose RJ, Preller J. Near-patient testing of potassium levels using arterial blood gas analyzers: can we trust these results? Emerg Med J. 2008;25:510–3. doi: 10.1136/emj.2007.053322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.