Abstract
Background and Purpose
The mechanisms underlying motor recovery after stroke are not fully understood. Several studies used functional MRI longitudinally to relate brain activity changes with performance gains of the upper limb after therapy, but research into training-induced recovery of lower limb function has been relatively neglected thus far.
Methods
We investigated functional reorganization after 4 weeks of treadmill training with partial body weight support in 18 chronic patients (mean age, 59.9±13.5 years) with mild to moderate paresis (Motricity Index affected leg: 77.7± 10.5; range, 9 to 99) and gait impairment (Functional Ambulation Category: 4.4±0.6; range, 3 to 5) due to a single subcortical ischemic stroke using repeated 3.0-T functional MRI and an ankle-dorsiflexion paradigm.
Results
Walking endurance improved after training (2-minute timed walking distance: 121.5±39.0 versus pre: 105.1 ±38.1 m; P=0.0001). For active movement of the paretic foot versus rest, greater walking endurance correlated with increased brain activity in the bilateral primary sensorimotor cortices, the cingulate motor areas, and the caudate nuclei bilaterally and in the thalamus of the affected hemisphere.
Conclusions
Despite the strong subcortical contributions to gait control, rehabilitation-associated walking improvements are associated with cortical activation changes. This is similar to findings in upper limb rehabilitation with some differences in the involved cortical areas. We observed bihemispheric activation increases with greater recovery both in cortical and subcortical regions with movement of the paretic foot. However, although the dorsal premotor cortex appears to play an important role in recovery of hand movements, evidence for the involvement of this region in lower extremity recovery was not found.
Keywords: fMRI, motor recovery, physiotherapy, plasticity, treadmill training
The underlying mechanisms for recovery of function after stroke are not well understood. A better insight into the biological mechanisms underlying functional recovery cannot be expected on the basis of clinical measures only. This limits development of new approaches for enhancing recovery. However, there is some promise that functional MRI (fMRI) can address these problems.1,2
fMRI studies show increased activation of the contralesional primary sensorimotor cortex (SMC) with movement of the impaired limb in the early period after stroke.3,4 When assessed in a cross-sectional manner, subsequent recovery of motor function is associated with a reduction in contralesional and an increase in ipsilesional activity of the SMC.3,5–7 This suggests a trend toward normalization of activation patterns in moderately impaired patients who recover well. Greater ipsilesional SMC activity in the early period after a stroke may be associated with better recovery.8,9 Several groups have attempted to test this hypothesis directly with longitudinal studies.10–18 These observations highlight the potential to use brain activity during well-defined simple motor tasks as markers that can be related to clinical outcomes after stroke. More generally, understanding of the brain functional correlates of recovery could allow better triage of patients for more intensive rehabilitation, better selection of targeted therapies, and more efficient evaluation of outcomes.
fMRI studies of the effects of upper limb training on brain activity already have been reported.16,19 However, study of fMRI changes associated with training-induced recovery of leg function has been largely neglected.20,21 Although this reflects the greater technical challenges to study lower limb movements with fMRI,22 it is surprising, because restoration of locomotion is considered a primary goal by people experiencing stroke23 and both clinically effective interventions like treadmill training with partial body weight support24 and fMRI paradigms to study key components of gait have become available.22 Fundamental differences between the neural control of hand movements and the control of the more automated and bilateral movements involved in walking preclude direct extension of conclusions from studies of upper limb recovery.25,26 The recently observed correlation between fMRI activation increases in subcortical brain regions elicited by knee movements and recovery of the paretic lower limb after treadmill training by Luft and coworkers strongly supports this notion.20
We wanted to investigate functional reorganization subsequent to treadmill training24 in patients with stroke with gait impairment using fMRI and a paradigm involving more distal active and passive lower limb movements (ie, ankle dorsiflexion).22 Accounts of the cross-sectional fMRI findings from this cohort at baseline27 and of the behavioral training data28 already have been reported. Only patients with subcortical ischemic lesions that did not affect the cortex were included. We tested in the chronic phase after stroke to minimize the potential for changes in motor deficits and related brain activity patterns unrelated to the training. We hypothesized that recovery of leg function after a 4-week period of treadmill training with partial body weight support would be associated with specific changes in brain activation.
Subjects and Methods
Patients
Inclusion Criteria
Patients had residual gait impairment attributable to a single MRI-visible subcortical ischemic stroke, which had occurred 6 months or more before recruitment for the study27 and affected either the posterior limb of the internal capsule (n=11) or efferent corticospinal tracts within the corona radiata (n=7) with the cortex left morphologically spared (for a synopsis of lesion topography, see also Dawes et al).28 Patients had to score ≥3 on the Functional Ambulatory Capacity rating scale.29 Residual gait impairment had also to be confirmed by an abnormal 10-m walk time with age-adjusted thresholds.30 Furthermore, patients had to be able to dorsiflex the ankle by a minimum of 10° to allow use of the fMRI paradigm. The study was approved by the Central Research Ethics Committee.
Exclusion Criteria
Patients were excluded from participation for the following reasons: cognitive impairment (Mini-Mental State Examination score of <27), extensive leukoaraiosis (confluent white matter lesions according to the Fazekas scale),31 other clinically significant causes for reduced mobility (eg, disabling arthritis, musculoskeletal or cardiorespiratory disease), current rehabilitation or previous rehabilitation within 4 months before inclusion, any contraindications for MRI, and somatosensory or proprioceptive abnormalities apparent on a standardized neurological examination, including detailed somatosensory testing.
Patient Characteristics
Demographics and clinical characteristics of the patient group are presented in Table 1. Twelve patients had right-sided hemiparesis and 6 patients had left-sided hemiparesis. Sixteen patients were right-handed, one patient was left-handed, and one ambidextrous.32 In their history, 12 had used a walking stick and 6 patients had used an ankle-foot orthosis. At the beginning of and constantly throughout the study, 6 patients used a walking stick, 6 used a tripod, and one used an ankle-foot orthosis (more than one response allowed).
Table 1. Characteristics of the Patients With Stroke (8 females, 10 males).
| Mean±1 SD | Median (range) | |
|---|---|---|
| Age, years | 59.8±13.5 | 63.0 (32-74) |
| Interval to stroke, months | 37.3±36.8 | 21.0(6-144) |
| Functional Ambulation Category | 4.4±0.6 | 4.0 (3-5) |
| Days spent in inpatient rehabilitation | 67.1 ±60.9 | 62.0(0-180) |
| Rivermead Mobility Index | 12.8±1.9 | 13(8-15) |
| Modified Barthel Index | 18.6±1.6 | 19(15-20) |
| Motricity Index of the affected arm | 71.3±23.9 | 76 (9-99) |
| Motricity Index of the affected leg | 77.7±10.5 | 77(58-91) |
Outcome Scores
Motor loss was measured using the Motricity Index (Tables 1 and 2).33 Mobility was measured using the Rivermead Mobility Index,34 the 10-m timed walk, and the 2-minute walking distance (both were assessed twice after 5-minute periods of rest and the average score was used). Walking aids were allowed for testing (identical for pre- and posttraining sessions), but not for training. General dependence was measured using the Barthel Index.35
Table 2. Mobility Data Before and After Treadmill Training.
| Before Treadmill Training | After Treadmill Training | ||||
|---|---|---|---|---|---|
| Measure | Mean±1 SD | Median (IQR) | Mean±1 SD | Median (IQR) | P * |
| 10-m timed walk, seconds | 15.9±20.3 | 9.4(8.6-13.7) | 12.1 ±9.6 | 8.5 (7.9-11.7) | 0.001 |
| 2-minute walk, meters | 103.6±38.1 | 114.6 (78.9-140.4) | 119.7±39.0 | 134.5(97.1-152.1) | 0.0001 |
Paired nonparametric Wilcoxon-test.
IQR indicates interquartile range.
Gait Training
Individuals followed a gait training rehabilitation protocol based on that of Sullivan et al.24 Patients walked on a motorized treadmill with partial body weight support for 4 5-minute periods 3 times a week for 4 weeks (12 training sessions). For further details, see Dawes et al.28
Magnetic Resonance Imaging
Data acquisition was performed on a 3.0-T Varian INOVA MRI system (Siemens, Erlangen, Germany) using a multislice gradientecho echoplanar image sequence (TR=3000 ms, TE=30 ms, 24×6-mm axial slices, voxel dimensions 4×4×6 mm, field of view 256×256, matrix 64×64, spin angle 90°). Care was taken to cover all critical brain regions, including the vertex and the cerebellum.
Functional runs were acquired on 3 occasions using identical scanning parameters and the same paradigm (pre1: first session on inclusion; pre2: second session after 4 weeks of normal activities with start of the rehabilitation program 1 day thereafter; post: third session after 4 weeks of training).
Conventional T2-weighted scans and a high-resolution T1-weighted structural image also were acquired for each subject at baseline to allow functional image registration for precise localization of activations and to assess the topography of structural brain damage caused by the infarcts.
Motor Testing
The paradigm was based on that used previously in our laboratory.22,27 Unilateral foot movements were made in a purpose-built wooden apparatus. An fMRI “block” design was used with 2 conditions: active ankle dorsiflexion paced by a visual cue and passive movement of the ankle by the experimenter by 30°. Active and passive movement periods (blocks) of 30 seconds alternated with interspersed periods of absolute rest (21 seconds). Each experimental session included 5 active movement blocks and 4 passive movement blocks. The total scanning time for unilateral movement of one foot was approximately 7.5 minutes.
To achieve a similar level of effortfulness for each patient, before scanning, a self-paced comfortable rate of movement in the apparatus (based on the self-selected walking speed) was determined for each subject’s foot through full voluntary dorsi- and plantarflexion (mean rates for dorsiflexion 1127±364 ms [range, 800 to 1800 ms] and for plantarflexion 1236±364 ms [range, 850 to 1200 ms]). The visual cue for movement during scanning was set at this rate. This rate was kept constant across all 3 scanning sessions. Performance was monitored by goniometer tracings. The paradigm was performed with pseudorandom selection of the right or left leg. Further details have been reported elsewhere.27
Data Analysis
Functional imaging analysis was carried out using FEAT (FMRI Expert Analysis Tool; Version 5.63, part of FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl).
The following prestatistical processing was applied: motion correction using MCFLIRT; nonbrain removal using BET; spatial smoothing using a Gaussian kernel of 5 mm full-width halfmaximum; global (volumetric) multiplicative mean intensity normalization; and high-pass temporal filtering (Gaussian-weighted least squares straight line fitting, with sigma=50.0s). Time-series statistical analysis was carried out using FILM with local autocorrelation correction. Registration to high-resolution and/or standard images was carried out using FLIRT. Higher-level analysis was done using FLAME (FMRIB’s Local Analysis of Mixed Effects). Z (Gaussianised T/F) statistic images were thresholded using clusters determined by Z >2.3 and a (corrected) cluster significance threshold of P=0.05.
In a first-level analysis, the effects of the active and passive movement blocks versus rest were determined for each subject, session, and limb (paretic or nonparetic). No subject needed to be excluded due to excessive head motion (>3 mm in any direction as assessed from displacement in the head images by FSL). The mean absolute displacement in patients was 0.17±0.07 mm for movement of the hemiparetic side compared with 0.14±0.09 mm for control subjects (P>0.4). To further minimize the impact of differences, motion parameters were included as a covariate of no interest in the general linear model. Functional and structural images of patients with right hemispheric strokes were flipped right to left so that the image of the left hemisphere represented the lesioned hemisphere.
Second-level (fixed effects) analyses for each subject were run to calculate the differences between activation patterns from the 2 sessions (pre1 versus pre2, average of pre1 and pre2, post versus average of pre1 and pre2).
Then, the following group level (mixed effects) analyses were run to combine first- or second-level analyses from individual patients: (1) average movement-related activity at baseline (pre1); (2) average change (increase or decrease) in activity over time before training begins (pre1 versus pre2); (3) average change (increase or decrease) in activity post-versus pretraining (where pretraining is the average of pre1 and pre2); and (4) parametric variation between behavioral outcome score (change in 2-minute walk distance, ie, walking endurance) and pre-versus posttherapy change in activation. All these analyses were run for both active and passive movement and both with and without inclusion of age and walking speed as covariates.
Functional regions of interest, selected from activation clusters defined by Analysis 4, were applied to the second-level analyses for each individual to compute estimates of the median signal change from baseline to follow-up within the regions of interest for the active movement conditions of the paretic foot versus rest using FEATQUERY (part of FSL).
For representation, activation clusters were overlaid on the group mean normalized high-resolution brain image. All images are shown in radiological convention in which the left side of the image is the right side of the brain. The anatomic atlases of Duvernoy36 and Schmahmann37 were used to localize functional activation. Motor areas were designated as proposed by Picard and Strick.38
Statistics
The Statistical Package of Social Sciences (Version 14.0.1; SPSS Inc, Chicago, Ill) was used to test categorical variables by Pearson’s X2 test and continuous variables by Student t test or the Mann-Whitney U test, where appropriate. Bivariate correlations were tested using Spearman’s Rho nonparametric test in the absence of normal distribution. The level of significance was set at 0.05.
Results
Effect of Treadmill Training on Gait
Patients had mild to moderate residual motor deficits due to their stroke (Table 1). Performance gains in walking speed (10-m walk) and endurance (2-minute timed walk) were observed after the 4-week training intervention (Table 2; for details, see Dawes et al28).
Effect of Therapy as Assessed by fMRI
Movement-Related Brain Activation Before Therapy
Active movement (versus rest) at baseline was associated with activation in the primary sensorimotor (SMC) and secondary somatosensory cortices (primarily contralateral to foot movement), the supplementary (SMA) and cingulate motor areas and the ventral premotor cortices, and in the vermis and lobules IV, V, and VI of the cerebellum ipsilateral to foot movement both with movement of the paretic and of the nonparetic foot.27 As described in our earlier report, the extent of activation (particularly in the SMC and SMA of the unlesioned hemisphere) increased with disability.27
Testing the stability of the activation patterns at baseline, we found no areas of significant increase or decrease of activation between the 2 preintervention scans (separated by 4 weeks without study-specific intervention) either for the paretic or the nonparetic foot (data not shown).
Therapy-Related Changes in Brain Activation With Movement of the Paretic Foot
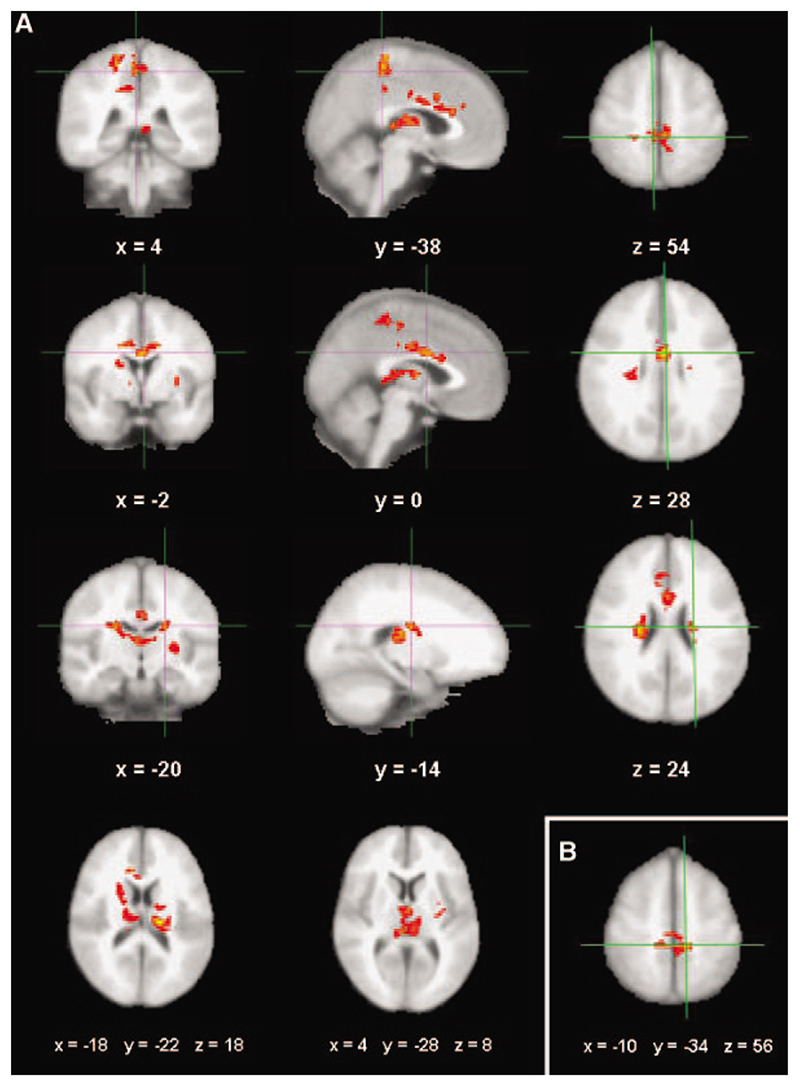
At a group level, there were no areas with significant increase or decrease of activation after treadmill training associated with active or passive movement of the paretic foot versus rest. However, relative brain activity with active movement of the paretic foot versus rest showed a positive correlation between signal change in cortical and subcortical motor areas and the increase in walking endurance after the intervention (Figure 1A). Greater walking endurance was associated with increased brain activity in the SMC, the paracentral lobules, the cingulate motor area, and the caudate nuclei bilaterally and in the lateral thalamus of the affected hemisphere (Figure 1; Table 3).
Figure 1.
Mixed effects z-statistics image at the group level showing areas where signal change from baseline to follow-up with movement of the (right) paretic foot versus rest correlated with performance gains after training. A, For active movement. Significant voxels were found in the SMC (I row), the cingulate motor area (II row), and the caudate nucleus (III row) in both the lesioned and unlesioned hemispheres (coronal, sagittal, axial sections) as well as in both thalami (IV row, far left and middle image in axial orientation). B, For passive movement. Significant voxels were identified in the SMC bilaterally in areas that show partial overlap with those defined by the contrasts with active movement. (All results are from clusterbased mixed effects analyses; z >2.3, corrected P=0.05; crosshairs at local maxima specified by MNI coordinates; images shown in radiological convention; left hemisphere=lesioned by the strokes).
Table 3.
Coordinates (in MNI standard space) and Activation Significance (Z statistics) of Local Maxima of Clusters With a Significant Correlation Between Signal Change With Active Movement of the Paretic Foot Post-versus Pretraining and Performance Gains (defined by absolute change in the 2-minute timed walk; cluster-based mixed effects group analysis, z>2.3, P corrected P=0.05; baseline 2-minute timed walk as covariate of no interest)
| MNI Coordinates of Maximum Z-Score, mm |
|||||
|---|---|---|---|---|---|
| Region(s) | Side | Maximum Z-Score |
X | Y | Z |
| Paracentral lobule | R | 4.24 | 4 | −38 | 54 |
| (SMC) | L | 3.85 | −4 | −34 | 56 |
| R | 3.82 | 4 | −34 | 66 | |
| R | 3.81 | 4 | −36 | 60 | |
| Cingulate gyrus | L | 4.31 | −2 | 0 | 28 |
| R | 4.19 | 10 | 24 | 20 | |
| R | 3.84 | 10 | 30 | 18 | |
| L | 3.75 | −14 | 2 | 34 | |
| R | 3.59 | 2 | 14 | 22 | |
| Caudate nucleus | L | 4.07 | −20 | −14 | 24 |
| R | 3.74 | 24 | −16 | 24 | |
| Lateral dorsal thalamic | L | 4.05 | −18 | −22 | 18 |
| nucleus | L | 3.90 | −8 | −20 | 12 |
| Mediodorsal thalamic nucleus | R | 3.48 | 4 | −28 | 8 |
| Precentral gyrus | R | 3.74 | 20 | −38 | 60 |
| R | 3.49 | 16 | −40 | 64 | |
R indicates right; L, left.
A similar contrast for passive movement of the paretic foot versus rest also showed a significant correlation between performance gains and increased bilateral SMC activation (z-max 4.12; peak activation coordinates; see Figure 1B) yet in comparatively smaller clusters. No significant correlations for other cortical or subcortical regions were found with this contrast.
Adding age or walking speed at baseline as regressors in the general linear model did not change the activation maps for training-related changes with either the active or the passive movement contrasts. The differences in brain activation before and after training also could not be explained by differences in self-paced movement rates (set at baseline) independently; no significant activation was found in a contrast testing directly for parametric variation with foot movement rates (data not shown). A parametric contrast of brain activation based on performance in the 2-minute timed walk at baseline also did not reveal significant activation.
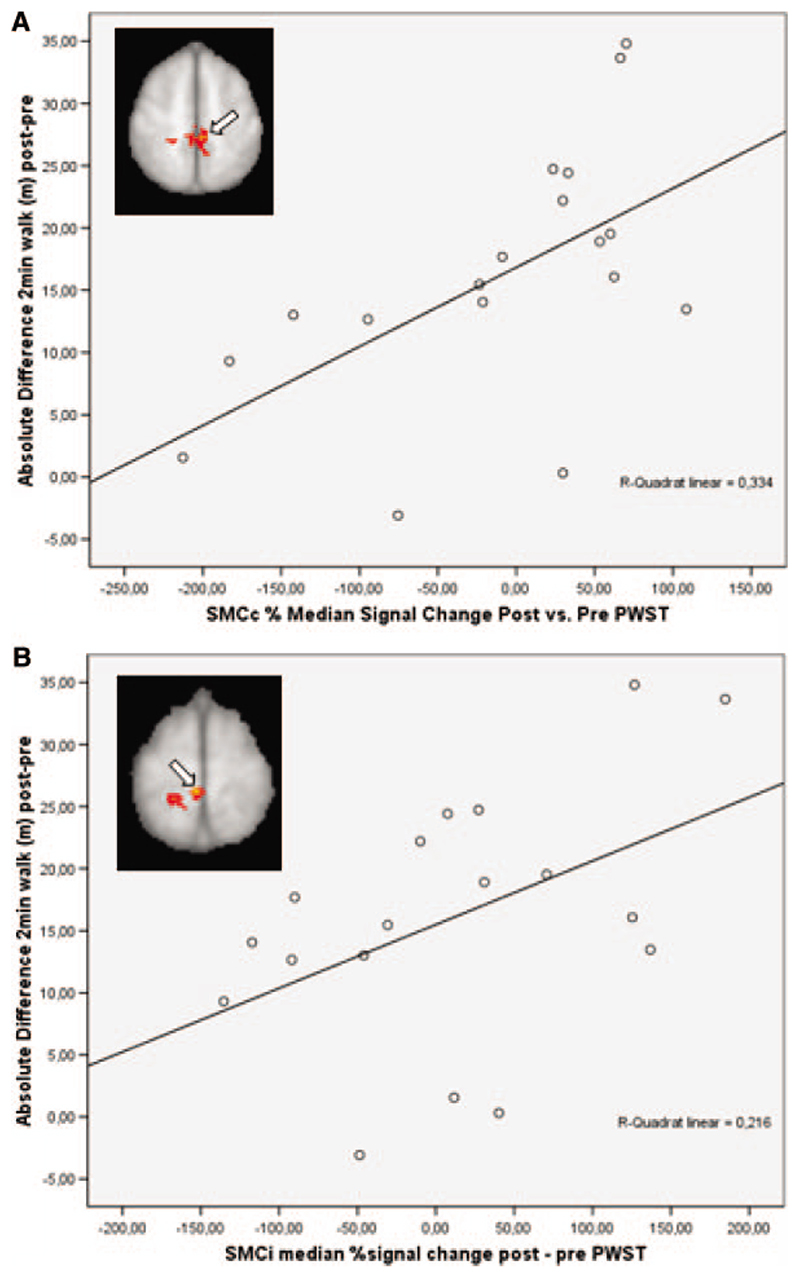
To further define the correlation between activation changes with active movement of the paretic foot and functional gains after therapy and also to check for possible outliers, we performed region of interest analyses (see “Methods”). These confirmed a correlation between signal change in the SMC bilaterally and an increase in walking endurance (r=0.589, P=0.005 for the contralateral and r=0.461, P=0.027 for the ipsilateral SMC cluster, respectively; Figure 2).
Figure 2.
Region of interest analyses. Scatterplots with fitted linear regression curves demonstrating the significant correlation between the median signal change from baseline to follow-up with movement of the paretic foot versus rest in the primary SMC and the absolute increase in walking endurance (A, SMCc cluster in the lesioned left hemisphere marked by the arrowhead; (B) SMCi cluster in the unlesioned right hemisphere, marked by the arrowhead).
Therapy-Related Brain Activation Changes With Movement of the Unaffected Foot
No significant changes in brain activation were found for the analogous contrasts with movement of the unaffected limb versus rest.
Discussion
Our observations provide objective, neurophysiological correlates of the performance gains due to treadmill training in patients with lower limb paresis after chronic, subcortical ischemic stroke. These occur as increases in activity in cortical and subcortical brain regions, which extensive previous functional-anatomic studies have associated with aspects of motor control of the lower limb.25,26,39 The pattern of changes also is functional-anatomically distinct from that previously reported with hand movements after recovery or rehabilitation directed to the upper limb.16,19,40,41
Whereas brain activity was strongly lateralized in previous studies of rehabilitation-associated recovery of the hand movements,16,19,41 here we observed bilateral activation increases with greater recovery, particularly in the SMC. Furthermore, and in contrast to the apparently central role for the premotor cortex in hand movement recovery, we did not identify premotor activation and instead found increased activation in midline cortical regions (SMA, pre-SMA, cingulate motor area) with improvements of walking endurance after the treadmill training. This emphasizes the greater bihemispheric control of lower limb movement generally.22,26,42
Nonetheless, as we reported previously,27 at baseline, increased activation (particularly in the SMC and SMA) in the unlesioned hemisphere was associated with greater paresis. In arguments analogous to those offered to explain the reduced lateralization of brain activation with increasing impairment of hand movements after stroke,3,7 there are contrasting hypotheses to explain this. One hypothesis is that the loss of normal interhemispheric inhibition of movement-related brain activation impairs performance by reducing the selectivity of motor unit activation.43,44 Alternatively, the correlation between increased activation in the unlesioned hemisphere and greater functional impairments cross-sectionally could reflect greater adaptive compensation with the functionally more severe lesions.
Our longitudinal analysis shows a correlation between improved walking after rehabilitative training and increased SMC activation not only in the lesioned, but also in the unlesioned hemisphere. Increasing walking endurance also correlated with increased bilateral SMA and basal ganglia activity. These observations do not support the hypothesis that activations in the contralesional hemisphere are necessarily maladaptive and therefore suggest a role for bihemi-spheric recruitment in functional recovery of gait. Gait training-induced changes in corticomotor excitability in the motor maps of the tibialis anterior in both hemispheres after stroke appear to support this notion.45
Our findings elicited by ankle movements appear to fit into the model of an altered brain circuit linked to functional improvement after treadmill training proposed by Luft et al on the basis of their observations with a more proximal knee movement fMRI paradigm.20 They defined a network consisting of the cerebellum and a midbrain locomotor region near the red nucleus. Because this region receives neural signals from the basal ganglia and cortex, which in turn have been identified as key regions in our study, this could indicate an increased activation of a cortico-basalganglia-midbrain-cerebellar pathway, finally resulting in activation of the spinal locomotor pattern generators. Although we thus confirm Luft et al’s finding of a strong subcortical contribution to training-induced recovery of the lower limb function after stroke, we also extend these findings by illustrating that a cortical modulation appears to be critically important in improving such a complex behavior like gait.
Previous studies of hand movement in patients with arm paresis suggest that brain activity changes associated with “spontaneous” recovery (ie, in the absence of specific interventions) and those with training for rehabilitation may be different. Ward and colleagues used an isometric dynamic hand grip task to relate fMRI and behavioral changes over the “natural history” of recovery poststroke. They observed task-related decreases in brain activation as a function of recovery in the SMC, premotor and prefrontal cortices, SMA, cingulate motor area, and basal ganglia, whereas recovery-related increases were variable and only seen in 50% of the patients.40
Indeed, the intervention is important in the brain response observed, as evidenced by Johansen-Berg et al’s study, who studied training-induced recovery of arm function using a simple hand flexion/extension task. They reported a correlation between activation increases in premotor and secondary somatosensory cortices contralateral to the paretic hand and cerebellar activation bilaterally and improved hand function after modified constraint-induced therapy.16 A different pattern yet again was found using a different training approach (6 weeks of bilateral arm training with rhythmic auditory cueing) and repeated fMRI during elbow movements by Luft and coworkers who reported an association between greater improvement of arm function and activation increases in pre- and postcentral contralesional gyri and the ipsilesional cerebellum.19
The data therefore currently are too limited for any confident conclusions regarding details of the functional anatomy but is consistent with the hypothesis that the “natural history” of recovery after stroke is associated with decreased activation in brain motor control regions, whereas increases in activation in specific regions within the broader control network accompany performance gains after rehabilitation poststroke. We found increases in activation with improved gains after training, consistent with this.
Normal motor learning in healthy subjects is associated with increased activation in cortical and subcortical regions involved in an extensive network for motor control.46 In our interpretations, we do not assume that the functional cerebral changes elicited by the ankle dorsiflexion paradigm reflect those associated with such a complex behavior as gait, but they are likely to bear some relation. This inference is supported by evidence from combined near-infrared spectroscopy and fMRI studies showing that foot extension flexion movements generate a brain activation pattern similar to that associated with walking.42 Balance, muscle coordination, and other joint movements are essential for walking, but integrating these components together with a kinematic approach into an fMRI experiment is a major challenge that bears the risk of introducing extra variability through motion artifacts, therapy-associated changes, and disability-related behavioral performance differences. We therefore chose to stick with a simpler paradigm, but future studies will have to show how to improve on our approach.47
There are limitations to the interpretation of our results. First, the study population was small but highly selected. We included only patients with subcortical stroke to avoid confounds from damaged cortex. Furthermore, we studied a group with a rather high level of functioning, but the data obtained to date indicate that patients with mild to moderate deficits benefit most from repetitive task-oriented practice.48 Our findings therefore cannot be regarded as representative of the entire spectrum of stroke-related disability. We also adjusted the fMRI task individually at baseline to achieve a similar effort across patients and then kept movement rates and the range of motion constant at subsequent sessions, so task difficulty may have decreased over time in patients with functional gains and subtle changes in movement kinematics might have occurred. However, the range of motion and the rates of movement were controlled by goniometer recordings and recovery-related bilateral SMC signal increases were also noted with the fixed, passive task (ie, in the absence of volitional drive) so a major effect of this potential confound appears unlikely. Also, a parametric contrast of signal change with actual movement rates in the scanner was negative, attesting to the fact that the small variations in this parameter did not significantly affect the patterns of activation.
Inclusion of a control intervention for comparison might have further increased the value of our contribution. Moreover, investigations into the potential effects of aerobic treadmill training on neural activity patterns in healthy control subjects with normal stable gait would have provided important information on the effect of improved cardiovascular fitness alone on brain function as evidenced by fMRI. This would have helped to clarify whether part of the imaging changes observed in the stroke group could have been explained by more general hemodynamic or metabolic effects. Such studies will need to be done in appropriately large cohorts.49
Finally, as previously suggested,48 future fMRI intervention studies could benefit from specifically tailoring the intensity of the training by finding a definite clinical plateau in the degree of individual improvement. This could minimize the risk of delivering a low training intensity for rather high-level functioning subjects, thus potentially improving the fMRI-clinical associations and ultimately reduce the variability between studies observed in the past. Also, subsequent studies might want to test the clinically important question of a predictive value of baseline fMRI in regard to training response. However, this might necessitate testing even larger patient groups, because overall changes in brain activation in our cohort were relatively small. Also, a time point to demonstrate retention of effects would certainly have been useful.
In conclusion, we investigated functional reorganization in brain activation after treadmill training in patients with gait impairment due to a single subcortical ischemic stroke. We observed bihemispheric activation increases with greater recovery both in cortical and subcortical regions with movement of the paretic foot. A careful recent study also emphasized strong subcortical contributions to gait control.20 However, although Luft and coworkers studied brain activity changes after treadmill training with the more proximal knee movements, we studied distal ankle movements as a closer analog to hand movements both by means of an active and passive paradigm in a more homogenous patient cohort on a 3.0-T magnet. Using this approach, rehabilitation-associated walking improvements were associated also with cortical activation changes as with the recovery of hand movements, although the cortical network with foot movement is distinct. The 2 studies therefore have to be regarded as complementary. Based on our findings, we emphasize that, despite subcortical contributions, the cortex contributes to training-induced recovery of lower limb function in chronic stroke.
Acknowledgments
We thank all study participants and the personnel onsite at FMRIB and the OCE for their enthusiasm and help with this project.
Sources of Funding
This work has been supported by the Austrian Science Fund (FWF; C.E. and S.R., grant numbers J2373-B02 and P15158, respectively); the UK Medical Research Council (P.M.M.); and the Wellcome Trust (H.J.-B.).
Footnotes
Disclosures
P.M.M. became an employee of GlaxoSmithKline after completion of the experimental phase of this project.
Contributor Information
Christian Enzinger, Department of Neurology, Medical University of Graz, Graz, Austria; Centre for Functional MRI of the Brain, John Radcliffe Hospital, University of Oxford, Oxford, UK; Oxford Centre for Enablement, Oxford, UK; Section of Neuroradiology, Department of Radiology, Medical University of Graz, Graz, Austria.
Helen Dawes, Movement Science Group, School of Biological and Molecular Sciences, Oxford Brookes University, Oxford, UK; Oxford Centre for Enablement, Oxford, UK.
Heidi Johansen-Berg, Centre for Functional MRI of the Brain, John Radcliffe Hospital, University of Oxford, Oxford, UK.
Derick Wade, Oxford Centre for Enablement, Oxford, UK.
Marko Bogdanovic, Centre for Functional MRI of the Brain, John Radcliffe Hospital, University of Oxford, Oxford, UK; Department of Clinical Neurology, Radcliffe Infirmary, University of Oxford, Oxford, UK.
Jonathan Collett, Movement Science Group, School of Biological and Molecular Sciences, Oxford Brookes University, Oxford, UK; Oxford Centre for Enablement, Oxford, UK.
Paul M. Matthews, Centre for Functional MRI of the Brain, John Radcliffe Hospital, University of Oxford, Oxford, UK; Department of Clinical Neurology, Radcliffe Infirmary, University of Oxford, Oxford, UK.
References
- 1.Baron JC, Cohen LG, Cramer SC, Dobkin BH, Johansen-Berg H, Loubinoux I, Marshall RS, Ward NS. Neuroimaging in stroke recovery: a position paper from the first international workshop on neuroimaging and stroke recovery. Cerebrovasc Dis. 2004;18:260–267. doi: 10.1159/000080293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodics T, Cohen LG, Cramer SC. Functional imaging of intervention effects in stroke motor rehabilitation. Arch Phys Med Rehabil. 2006;87:S36–42. doi: 10.1016/j.apmr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Ward NS, Brown MM, Thompson AJ, Frackowiak RSJ. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain. 2003;126:1430–1448. doi: 10.1093/brain/awg145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward NS, Brown MM, Thompson AJ, Frackowiak RS. The influence of time after stroke on brain activations during a motor task. Ann Neurol. 2004;55:829–834. doi: 10.1002/ana.20099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carey LM, Abbott DF, Egan GF, O’Keefe GJ, Jackson GD, Bernhardt J, Donnan GA. Evolution of brain activation with good and poor motor recovery after stroke. Neurorehabil Neural Repair. 2006;20:24–41. doi: 10.1177/1545968305283053. [DOI] [PubMed] [Google Scholar]
- 6.Carey LM, Abbott DF, Egan GF, Bernhardt J, Donnan GA. Motor impairment and recovery in the upper limb after stroke: behavioral and neuroanatomical correlates. Stroke. 2005;36:625–629. doi: 10.1161/01.STR.0000155720.47711.83. [DOI] [PubMed] [Google Scholar]
- 7.Calautti C, Naccarato M, Jones PS, Sharma N, Day DD, Carpenter AT, Bullmore ET, Warburton EA, Baron J. The relationship between motor deficit and hemisphere activation balance after stroke: a 3T fMRI study. Neuroimage. 2007;1:322–331. doi: 10.1016/j.neuroimage.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 8.Cramer SC, Parrish TB, Levy RM, Stebbins GT, Ruland SD, Lowry DW, Trouard TP, Squire SW, Weinand ME, Savage CR, Wilkinson SB, et al. Predicting functional gains in a stroke trial. Stroke. 2007;38:2108–2114. doi: 10.1161/STROKEAHA.107.485631. [DOI] [PubMed] [Google Scholar]
- 9.Loubinoux I, Dechaumont-Palacin S, Castel-Lacanal E, De Boissezon X, Marque P, Pariente J, Albucher J-F, Berry I, Chollet F. Prognostic value of fMRI in recovery of hand function in subcortical stroke patients. Cereb Cortex. 2007;17:2980–2987. doi: 10.1093/cercor/bhm023. [DOI] [PubMed] [Google Scholar]
- 10.Calautti C, Leroy F, Guincestre JY, Baron JC. Dynamics of motor network overactivation after striatocapsular stroke: a longitudinal pet study using a fixed-performance paradigm. Stroke. 2001;32:2534–2542. doi: 10.1161/hs1101.097401. [DOI] [PubMed] [Google Scholar]
- 11.Wittenberg GF, Bastian AJ, Dromerick AW, Thach WT, Powers WJ. Mirror movements complicate interpretation of cerebral activation changes during recovery from subcortical infarction. Neurorehabil Neural Repair. 2000;14:213–221. doi: 10.1177/154596830001400307. [DOI] [PubMed] [Google Scholar]
- 12.Gerloff C, Bushara K, Sailer A, Wassermann EM, Chen R, Matsuoka T, Waldvogel D, Wittenberg GF, Ishii K, Cohen LG, Hallett M. Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain. 2006;129:791–808. doi: 10.1093/brain/awh713. [DOI] [PubMed] [Google Scholar]
- 13.Jaillard A, Martin CD, Garambois K, Lebas JF, Hommel M. Vicarious function within the human primary motor cortex? A longitudinal fMRI stroke study. Brain. 2005;128:1122–1138. doi: 10.1093/brain/awh456. [DOI] [PubMed] [Google Scholar]
- 14.Tombari D, Loubinoux I, Pariente J, Gerdelat A, Albucher JF, Tardy J, Cassol E, Chollet F. A longitudinal fMRI study: in recovering and then in clinically stable sub-cortical stroke patients. Neuroimage. 2004;23:827–839. doi: 10.1016/j.neuroimage.2004.07.058. [DOI] [PubMed] [Google Scholar]
- 15.Schaechter JD, Kraft E, Hilliard TS, Dijkhuizen RM, Benner T, Finklestein SP, Rosen BR, Cramer SC. Motor recovery and cortical reorganization after constraint-induced movement therapy in stroke patients: a preliminary study. Neurorehabil Neural Repair. 2002;16:326–338. doi: 10.1177/154596830201600403. [DOI] [PubMed] [Google Scholar]
- 16.Johansen-Berg H, Dawes H, Guy C, Smith SM, Wade DT, Matthews PM. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain. 2002;125:2731–2742. doi: 10.1093/brain/awf282. [DOI] [PubMed] [Google Scholar]
- 17.Dong Y, Winstein CJ, Albistegui-DuBois R, Dobkin BH. Evolution of fMRI activation in the perilesional primary motor cortex and cerebellum with rehabilitation training-related motor gains after stroke: a pilot study. Neurorehabil Neural Repair. 2007;21:412–428. doi: 10.1177/1545968306298598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.You SH, Jang SH, Kim Y-H, Hallett M, Ahn SH, Kwon Y-H, Kim JH, Lee MY. Virtual reality-induced cortical reorganization and associated locomotor recovery in chronic stroke: an experimenter-blind randomized study. Stroke. 2005;36:1166–1171. doi: 10.1161/01.STR.0000162715.43417.91. [DOI] [PubMed] [Google Scholar]
- 19.Luft AR, McCombe-Waller S, Whitall J, Forrester LW, Macko R, Sorkin JD, Schulz JB, Goldberg AP, Hanley DF. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. JAMA. 2004;20:1853–1861. doi: 10.1001/jama.292.15.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luft AR, Macko RF, Forrester LW, Villagra F, Ivey F, Sorkin JD, Whitall J, McCombe-Waller S, Katzel L, Goldberg AP, Hanley DF. Treadmill exercise activates subcortical neural networks and improves walking after stroke: a randomized controlled trial. Stroke. 2008;39:3341–3350. doi: 10.1161/STROKEAHA.108.527531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobkin BH, Firestine A, West M, Saremi K, Woods R. Ankle dorsiflexion as an fMRI paradigm to assay motor control for walking during rehabilitation. Neuroimage. 2004;23:370–381. doi: 10.1016/j.neuroimage.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahyoun C, Floyer-Lea A, Johansen-Berg H, Matthews PM. Towards an understanding of gait control: brain activation during the anticipation, preparation and execution of foot movements. Neuroimage. 2004;21:568. doi: 10.1016/j.neuroimage.2003.09.065. [DOI] [PubMed] [Google Scholar]
- 23.Lord SE, McPherson K, McNaughton HK, Rochester L, Weatherall M. Community ambulation after stroke: how important and obtainable is it and what measures appear predictive? Arch Phys MedRehabil. 2004;85:234. doi: 10.1016/j.apmr.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan KJ, Knowlton BJ, Dobkin BH. Step training with body weight support: effect of treadmill speed and practice paradigms on poststroke locomotor recovery. Arch Phys Med Rehabil. 2002;83:683–691. doi: 10.1053/apmr.2002.32488. [DOI] [PubMed] [Google Scholar]
- 25.Capaday C. The special nature of human walking and its neural control. Trends Neurosci. 2002;25:370–376. doi: 10.1016/s0166-2236(02)02173-2. [DOI] [PubMed] [Google Scholar]
- 26.Orlovsky G. Neuronal Control of Locomotion: From Mollusc to Man. Oxford University Press; Oxford: 2000. [Google Scholar]
- 27.Enzinger C, Johansen-Berg H, Dawes H, Bogdanovic M, Collett J, Guy C, Ropele S, Kischka U, Wade D, Fazekas F, Matthews PM. Functional MRI correlates of lower limb function in stroke victims with gait impairment. Stroke. 2008;39:1507–1513. doi: 10.1161/STROKEAHA.107.501999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dawes H, Enzinger C, Johansen-Berg H, Bogdanovic M, Guy C, Collett J, Izadi H, Stagg C, Wade D, Matthews PM. Walking performance and its recovery in chronic stroke in relation to extent of lesion overlap with the descending motor tract. Exp Brain Res. 2007;186:325–333. doi: 10.1007/s00221-007-1237-0. [DOI] [PubMed] [Google Scholar]
- 29.Reed JW, Harvey JC. Rehabilitating the chronically ill; a method for evaluating the functional capacity of ambulatory patients. Geriatrics. 1964;19:87–103. [PubMed] [Google Scholar]
- 30.Wade DT, Wood VA, Heller A, Maggs J, Langton Hewer R. Walking after stroke. Measurement and recovery over the first 3 months. Scand J Rehabil Med. 1987;19:25–30. [PubMed] [Google Scholar]
- 31.Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, Radner H, Lechner H. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- 32.Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 33.Gibson L, MacLennan WJ, Gray C, Pentland B. Evaluation of a comprehensive assessment battery for stroke patients. Int J Rehabil Res. 1991;14:93–100. doi: 10.1097/00004356-199106000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Collen FM, Wade DT, Robb GF, Bradshaw CM. The Rivermead Mobility Index: a further development of the Rivermead Motor Assessment. Int Disabil Stud. 1991;13:50–54. doi: 10.3109/03790799109166684. [DOI] [PubMed] [Google Scholar]
- 35.Wade DT, Langton Hewer R. Stroke: associations with age, sex, and side of weakness. Arch Phys Med Rehabil. 1986;67:540–545. [PubMed] [Google Scholar]
- 36.Duvernoy H. The Human Brain Surface, Three-Dimensional Sectional Anatomy With MRI, and Blood Supply. Springer-Verlag; New York: 1999. [Google Scholar]
- 37.Schmahmann JD, Doyon J, Toga AW, Petrides M, Evans AC. MRI Atlas of the Human Cerebellum. Academic Press; San Diego: 2000. [DOI] [PubMed] [Google Scholar]
- 38.Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- 39.MacKay-Lyons M. Central pattern generation of locomotion: a review of the evidence. Phys Ther. 2002;82:69–83. doi: 10.1093/ptj/82.1.69. [DOI] [PubMed] [Google Scholar]
- 40.Ward NS, Brown MM, Thompson AJ, Frackowiak RSJ. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003;126:2476–2496. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dong Y, Dobkin BH, Cen SY, Wu AD, Winstein CJ. Motor cortex activation during treatment may predict therapeutic gains in paretic hand function after stroke. Stroke. 2006;37:1552–1555. doi: 10.1161/01.STR.0000221281.69373.4e. [DOI] [PubMed] [Google Scholar]
- 42.Miyai I, Tanabe HC, Sase I, Eda H, Oda I, Konishi I, Tsunazawa Y, Suzuki T, Yanagida T, Kubota K. Cortical mapping of gait in humans: a near-infrared spectroscopic topography study. Neuroimage. 2001;14:1186. doi: 10.1006/nimg.2001.0905. [DOI] [PubMed] [Google Scholar]
- 43.Manson SC, Wegner C, Filippi M, Barkhof F, Beckmann C, Ciccarelli O, De Stefano N, Enzinger C, Fazekas F, Agosta F, Gass A, et al. Impairment of movement-associated brain deactivation in multiple sclerosis: further evidence for a functional pathology of interhemispheric neuronal inhibition. Exp Brain Res. 2008;187:25–31. doi: 10.1007/s00221-008-1276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manson SC, Palace J, Frank JA, Matthews PM. Loss of interhemispheric inhibition in patients with multiple sclerosis is related to corpus callosum atrophy. Exp Brain Res. 2006;174:728–733. doi: 10.1007/s00221-006-0517-4. [DOI] [PubMed] [Google Scholar]
- 45.Yen CL, Wang RY, Liao KK, Huang CC, Yang YR. Gait training induced change in corticomotor excitability in patients with chronic stroke. Neurorehabil Neural Repair. 2008;22:22–30. doi: 10.1177/1545968307301875. [DOI] [PubMed] [Google Scholar]
- 46.Floyer-Lea A, Matthews PM. Distinguishable brain activation networks for short- and long-term motor skill learning. J Neurophysiol. 2005;94:512–518. doi: 10.1152/jn.00717.2004. [DOI] [PubMed] [Google Scholar]
- 47.MacIntosh BJ, Mraz R, Baker N, Tam F, Staines WR, Graham SJ. Optimizing the experimental design for ankle dorsiflexion fMRI. Neuroimage. 2004;22:1619. doi: 10.1016/j.neuroimage.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 48.Dobkin B. Training and exercise to drive poststroke recovery. Nat Clin Pract Neurol. 2008;4:76–85. doi: 10.1038/ncpneuro0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Bode S, Mathern GW, Bookheimer S, Dobkin B. Locomotor training remodels fMRI sensorimotor cortical activations in children after cerebral hemispherectomy. Neurorehabil Neural Repair. 2007;21:497–508. doi: 10.1177/1545968307299523. [DOI] [PMC free article] [PubMed] [Google Scholar]