Abstract
Multidrug-resistant Plasmodium falciparum malaria is a severe public health problem on the Thailand–Myanmar border. Many villagers buy packets of 4–5 mixed medicines (“yaa chud”) from shops without medical assessment as their first-line malaria treatment. In 2000–2001 a local researcher purchased 50 yaa chud from 44 shops around Mae Sot, Thailand and Myawaddy, Myanmar (Burma), for his wife who was said to be pregnant with fever and drowsiness. The tablets/capsules were provisionally identified by appearance and active ingredients determined in a subset by using mass and atomic spectrometry. The most frequently detected active ingredients were acetaminophen (22%), chlorpheniramine (13.4%), chloroquine (12.6%), tetracycline/doxycycline (11.4%), and quinine (5.1%). Only seven bags contained potentially curative medicine for malaria. A total of 82% of the bags contained medicines contraindicated in pregnancy. Inappropriate, ineffective antimalarial drugs on the Thailand–Myanmar border are likely to increase malaria morbidity, mortality and health costs and engender the emergence and spread of antimalarial drug resistance.
Introduction
Multidrug-resistant Plasmodium falciparum malaria is a severe public health problem within Myanmar (Burma) and on the Thailand-Myanmar border.1–3 Chloroquine and pyri-methamine-sulfadoxine are no longer effective and, if used alone, quinine and mefloquine monotherapies have relatively low efficacy.1 There is clear evidence that artemisinin derivative combination therapy (ACT) is superior1,3–5 and the current nationally recommended standard of care for the treatment of uncomplicated P. falciparum malaria in non-pregnant patients is artesunate plus mefloquine for three days. Severe P. falciparum malaria in non-pregnant women is treated with parenteral artesunate,6 usually in combination with a tetracycline for seven days. These drugs are available from government clinics, and especially for the large population of refugees from Myanmar (Burma), from non-governmental organizations (NGOs). However, many Thai villagers and Burmese migrant workers still buy small packets of drugs over-the-counter from vendors, grocery shops, and small pharmacies as their first line of treatment of fever.
These small plastic bags, which contain 4-5 tablets or capsules, are called “yaa chud” in Thai, or literally “combination medicine,” and are sold to patients or their families without prescription or medical assessment (Supplementary Figure 1, available online at www.ajtmh.org).7–9 Similar small combination packs of medicines are sold by shops and pharmacies in many areas of Southeast Asia for the treatment of fevers and, more specifically, for malaria.10 In western Thailand, these combinations are prepared by putting the tablets and capsules, obtained from pharmaceutical wholesalers, in small plastic bags that are sold individually. They are often the first line of care for patients with malaria and fever. Some shops and mobile injection doctors also dispense and administer injections of drugs and saline infusions.11 Foster estimated that approximately half of total worldwide antimalarial distribution was through informal outlets and private sellers.12 However, there are no firm data on how often patients with malaria take yaa chud in Southeast Asia.
There are many potential problems with the unregulated distribution of drugs in this way because their use will reduce the effectiveness of antimalarial therapy.13–15 The medicines are unlabelled, in varied combinations, and without expiration dates or instructions on how to take the drugs. They are prepared by staff untrained in pharmacy who are almost certainly unaware of drug interactions and contraindications. There is evidence that many yaa chud medicines are composed of inappropriate and/or unnecessary drugs.7–9 Anecdotally, shop owners in Thailand state that the bags of yaa chud are composed of drugs to reduce fever, such as paracetamol, antibiotics, such as ampicillin, and antimalarial drugs such as quinine and chloroquine. Shopkeepers may sell a sick customer only one plastic bag, which will be insufficient to cure any of the diseases that the patient is likely to have, with the possible exception of scrub typhus.16
Yaa chud may contain drugs contraindicated in pregnancy or childhood and may give rise to unexpected adverse effects. The tablets and capsules within the bags may be genuine, counterfeit, substandard, or degraded but without evidence of their chemical identity or packaging, this is extremely difficult to determine. They may also have important wider implications for public health because sub-therapeutic doses of anti-infective drugs may select for the emergence and spread of organisms resistant to the drugs administered.4,17,18 This finding is especially important for malaria because sub-therapeutic doses will select for the survival of P. falciparum parasites resistant to the antimalarial drug.4,5 The use of yaa chud may have contributed to the high prevalence of chloroquine and pyrimethamine-sulfadoxine-resistant malaria in the region, and if currently used drugs such as artesunate, tetracyclines, and mefloquine are included in yaa chud, this may facilitate the spread of resistance to these vital drugs.1,19
Three major reasons for patients having subtherapeutic plasma drug concentrations are reduced adherence to optimum regimens,20 regimens of inadequate dose and/or duration,21 and poor quality drugs containing either low concentrations of the active ingredient(s) or low bioavailability.22 There is very limited field evidence comparing the relative importance of these three factors on treatment failure and the spread of antimalarial resistance because it is very difficult to tease apart the affects of the misuse of anti-infective drugs by health workers, patient adherence, and poor quality drugs. The identification of the pharmaceuticals in these unlabelled drugs is thus of great importance. Color dye tests8 and thin-layer chromatography10 (TLC) have been used to identify the drugs in yaa chud. To identify the active ingredients in tablets and capsules in yaa chud from the Thailand-Myanmar border and therefore to what malaria patients may be exposed, a local researcher bought samples that were provisionally identified by their appearance and a subset were assayed using mass and atomic spectrometry.23,24 The collector also asked the seller for details of dose, possible side effects, and what the buyer should do if the fever persisted. The provisional identities, experimentally determined active ingredients, and collection details were correlated and we present data on the composition of yaa chud and discuss their public health importance.
Methods
Sample collection
Between July 2000 and January 2001 a Thai-, Burmese-, and Karen-speaking researcher, dressed as a Burmese migrant worker, visited all the “kong cham” shops that could be found in 16 villages astride the Thailand-Myanmar border in Mae Sot District, Tak Province, Thailand and adjacent Myawaddy District, Myanmar (Burma). Kong cham shops sell dry foods, sweets, vegetables, cooking utensils, and drugs, and occasionally meat, fish, alcohol, petrol, clothes, and shoes. This mystery shopper said his “wife was pregnant, has bad fever, and is drowsy, could I have some medicine please?” The area is endemic for multidrugresistant P. falciparum malaria and the first diagnosis to consider in such a clinical scenario would be malaria.1–3 We chose a fictional pregnant patient as she would be especially vulnerable to malaria and treatment is not straightforward.25 If the shopper was offered a packet of yaa chud, he asked where the drugs came from, how many his wife should take and when, will it work for malaria, and might it harm her or the baby. The responses of the outlet seller were recorded on a standardized questionnaire after the shopper left the outlet. All samples were inspected, described, and reviewed by local pharmacists (Mae Sot Hospital) and Faculty of Pharmacy, Mahidol University, Bangkok prior to chemical analysis.
Sample analysis
Chemical analysis was performed using multiple complementary techniques including liquid chromatography-mass spectrometry (LC-MS),26,27 direct analysis in real time mass spectrometry (DART-MS),28 and atomic absorption spectroscopy (AAS), a method capable of detecting inorganic components in samples. All samples were kept refrigerated (4°C) until analysis.
Liquid chromatography-mass spectrometry
Drug standards (Sigma-Aldrich, St. Louis, MO) and yaa chud samples were first crushed with a mortar and pestle and approximately 30 mg of sample was suspended in 1 mL of methanol (Sigma-Aldrich) and extracted on a rotary shaker for approximately 2 hours. After extraction, suspensions were filtered through 0.45-μm polytetrafluoroethylene membrane filters (Pall Corporation, Ann Arbor, MI). The extracts were kept refrigerated until analysis with a liquid chromatographic system equipped with a Zorbax Extend-C18 column (1100 LC System; Agilent, Santa Clara, CA) interfaced to a time-of-flight mass spectrometer (JMS-100TLC, AccuTOF™ MS; JEOL, Peabody, MA) by an electrospray ionization source operated in positive ion mode.29 The LC and MS settings were optimized to provide high sensitivity for a wide range of drugs as previously described.24 Sample extracts were diluted with methanol (Sigma-Aldrich) or 50:50 acetonitrile:water (v/v) as needed.
Direct analysis in real time mass spectrometry
This technique does not require sample preparation and thus samples were analyzed as provided. A commercial DART ion source (IonSense, Saugus, MA) was coupled to the AccuTOF mass spectrometer. Uncoated tablets were held with a pair of metal tweezers in front of the DART ion source for an average time of 20 seconds. Coated tablets were first broken in half and the interior of the sample was analyzed by DART. Capsules were opened and those with granular contents were analyzed by holding the granule(s) in front of the DART source. The contents of capsules containing powders were packed into the open end of a melting point capillary for DART analysis. Experimental settings for this technique have been described elsewhere.23
Because of the packaging of the yaa chud loose in bags, cross-contamination between tablets was common and problematic for DART-MS analysis because contaminant particles embedded on the sample surface produced signals corresponding to active ingredients from other tablets in the package. The active ingredients were thus assigned on the basis of the common peaks observed for the external tablet surface and the internal surface of the broken tablet.
Data analysis
Mass spectral data analysis was performed with the built-in mass spectrometer software (MassCenter version 1.3.4m; JEOL). Identification of sample ingredients was performed by importing the spectral peak list into Excel® (Microsoft, Auburn, WA) and using a system of macros to search for matches against an in-house library of protonated molecules derived from the Model List of Essential Drugs published by the World Health Organization.30 A positive match was indicated when the difference between the experimental and theoretical m/z values was less than 5 mmu. If discrepancies in the active ingredients identified using LC-MS and DART-MS were observed, the analyses were repeated by continuous infusion electrospray ionization-MS to provide final confirmation of the identity. Data on drug collection and identity were analyzed by using Stata 10 software (Stata Corporation, College Station, TX).
Atomic absorption spectroscopy
Samples that were provisionally identified as containing either mineral supplements or antacids (3/132 or 2%) were analyzed using AAS. Atomic spectrometry enables the determination of total metal contents in different kind of samples (organic, inorganic, and biological). For solids, it usually requires a previous step of dissolution. In this case, the samples (approximately 100 mg) were digested in closed polyfluorotetraethylene (PFTE®) vessels with 5 mL of 70% (w/w) nitric acid using a microwave digestion system (MDS 2000; CEM Co., Matthews, NC). The digestion conditions were automatically controlled by the microwave unit through a standard five-step heating program. Once the cycle was completed, the PFTE vessels were cooled down to room temperature and the final clear solution was adjusted to a volume of 10 mL with distilled water. Once dissolved, metals concentrations were determined by atomic absorption spectrometry using an air/acetylene flame as atomic source. A 6700 atomic absorption spectrometer (Shi-madzu, Kyoto, Japan) equipped with Hamamatzu (Kyoyo, Japan) single hollow cathode lamps of copper, iron, manganese, zinc, calcium, and magnesium was used for the measurements. The standard programs (Shimadzu) provided were used throughout the experiments. The metals concentrations in the samples were obtained by the working curve method. Suitable dilutions of the dissolved samples were performed to fit the working linear ranges of the different metals being studied.
Results
Yaa chud collection
The collector purchased 50 yaa chud bags and two Burmese traditional medicines (one labeled as “Tha ma”) from 44 shops (43 kong cham and one traditional medicine shop) (Supplementary Figure 1). A total of 31 (70%) shops were in Thailand and 13 were in Myanmar. An additional 20 kong cham shops visited said that they did not have yaa chud. The 50 yaa chud bags contained 254 tablets and capsules with a median (range) of 5 (1-8) tablets or capsules per plastic bag (Table 1). The median (range) retail price per bag and per tablet/capsule were 5 (4-12) and 1 (0.7-5.0) baht, respectively (5 baht was worth approximately 0.12 and 0.16 U.S. dollars in 2000 and 2007, respectively).
Table 1. Composition of 50 bags of “yaa chud” containing 254 tablets and capsules collected at different locations on the Thailand-Myanmar border* .
| Drug | No. (%) of physically distinct tablets and capsules analyzed chemically (n = 100) | No. (%) of tablets and capsules (n = 254) | No. (%) of inferred tablets and capsules (n = 154) | No. (%) yaa chud-containing drugs (n = 50) | FDA pregnancy categoryf and potential negative consequences (adverse effect) |
|---|---|---|---|---|---|
| Paracetamol | 18 (18) | 56 (22.0) | 38 (24.7) | 39 (78) | |
| Chlorpheniramine | 12 (12) | 34 (13.4) | 22 (14.3) | 30 (60) | Unexpected drowsiness and anti-muscarinic |
| Chloroquine | 7(7) | 32 (12.6) | 25 (16.2) | 29 (58) | Not efficacious against Plasmodium falciparum malaria |
| Tetracycline/doxycycline | 12 (12) | 29 (11.4) | 17 (11.0) | 26 (52) | (D) Efficacious against P. falciparum malaria in combination with quinine or artemisinin derivative. Upper GI irritation |
| Quinine | 2 (2) | 13 (5.1) | 11 (9.1) | 11 (22) | Remains effective if given for 7 days in combination with tetracycline. Tinnitus, hypoglycemia |
| Vitamin B6 | 3(3) | 12 (4.7) | 9 (5.8) | 12 (24) | – |
| Chlorpheniramine and paracetamol | 2 (2) | 12 (4.7) | 10 (6.5) | 8 (16) | Unexpected drowsiness, anti-muscarinic |
| No active ingredient detected | 11 (11) | 11 (4.3) | – | 0 | – |
| Acetylsalicylic acid | 5(5) | 11 (4.3) | 6(3.9) | 8 (16) | (D) Acidosis |
| Metamizole | 5(5) | 10(3.9) | 5 (3.2) | 10 (20) | (D) Agranulocystosis |
| Vitamins B3 and C | 2 (2) | 5 (2.0) | 3 (1.9) | 4 (8) | |
| Ampicillin | 2 (2) | 5 (2.0) | 3 (1.9) | 4 (8) | Rash |
| Antacid 1 | 1 (1) | 3 (1.2) | 2 (1.3) | 3 (6) | Negative interaction with doxycycline absorption |
| Paracetamol and sulfamethoxazole | 1 (1) | 3 (1.2) | 2 (1.3) | 3 (6) | (C) Neonatal hemolysis, methemoglobinemia |
| Dexamethasone | 2 (2) | 2 (0.8) | 0 | 2 (4) | Immunosuppression |
| Indomethacin | 2 (2) | 2 (0.8) | 0 | 2 (4) | (D) Gastric irritation |
| Primaquine | 1 (1) | 2 (0.8) | 1 (0.7) | 2 (4) | (C) Hemolysis in G6PD deficiency |
| Thiamin | 2 (2) | 2 (0.8) | 0 | 2 (4) | |
| Pyrimethamine | 1 (1) | 1 (0.4) | 0 | 1(2) | |
| Chloroquine and paracetamol | 1 (1) | 1 (0.4) | 0 | 1 (2) | Not efficacious against P. falciparum malaria |
| Alprenolol | 1 (1) | 1 (0.4) | 0 | 1 (2) | (C) Bradycardia |
| Tolperisone | 1 (1) | 1 (0.4) | 0 | 1 (2) | Contraindicated in pregnancy |
| Amitriptyline | 1 (1) | 1 (0.4) | 0 | 1 (2) | Anti-muscarinic |
| Prednisolone | 1 (1) | 1 (0.4) | 0 | 1 (2) | Immunosuppression |
| Prazepam | 1 (1) | 1 (0.4) | 0 | 1 (2) | Habit forming |
| Vitamins B3, B6, and B7 | 1 (1) | 1 (0.4) | 0 | 1 (2) | |
| Diclofenac | 1 (1) | 1 (0.4) | 0 | 1 (2) | (D) Gastric irritation |
| Antacid 2 | 1 (1) | 1 (0.4) | 0 | 1 (2) | Negative interaction with doxycycline absorption |
FDA = Food and Drug Administration; GI = gastrointestinal; Antacid 1 = copper, zinc, magnesium, iron, calcium, and manganese; G6PD = glucose-6-phosphate dehydrogenase; Antacid 2 = magnesium, iron, and calcium.
FDA pregnancy categories are shown in parentheses. D = evidence of human risk, but clinical benefits may outweigh risks;32 C = animal studies show toxicity, human data are insufficient, but clinical benefit may exceed risks.
Shopkeepers and their advice on the use of yaa chud
Of the 44 shopkeepers, 27 were female, 16 were male, and one was a transsexual. Seventeen of the sellers were Thai, 12 were Karen, 11 were plains Burmese, 1 was Hmong, 2 were Chinese-Burmese, and 1 was Mon. Fifteen (34%) yaa chud were made by the sellers themselves. Of the remaining 29 sellers, 23 said that they bought them in shops in Mae Sot town.
Of 43 shopkeepers for whom it was recorded, 21 (49%) did not spontaneously offer health advice for the 49 packets collected at these locations. Shopkeepers usually recommended taking the packets singly (96%). Of the two remaining packets that came with offered or requested verbal dosage information, one was recommended to be taken two packets at a time and the other was recommended to be taken three packets at a time. Of 43 packets with verbal dosage interval information, the interval between packets was recommended to be 24 hours in 15 (35%), 8 hours in 14 (33%), 12 hours in 13 (30%), and 4 hours in 1 (2%). None of the shopkeepers said that one yaa chud packet was insufficient or gave any advice as to how long the packets should be taken.
Shopkeepers said that 45 (90%) of yaa chud samples would work for malaria, 4 (8%) said that they would not work, and 1 (2%) did not know. Of 48 packets with available information, shopkeepers said that for 23 (48%) packets it would not matter if the buyer’s wife was pregnant, that it would matter for 19 (40%) packets, and did not know for 6 (12%) packets. Of 43 packets with available information, shopkeepers said that 32 (74%) yaa chud would not harm her, that 7 (16%) would cause harm, and that 4 (9%) may or may not cause harm.
Identification of yaa chud contents
An attempt was made to identify the tablets and capsules from knowledge as to what was locally available, but this proved difficult and chemical identification was necessary.
A subset of 100 physically distinct tablets/capsules (39%), representing all taxa of medicine with different shape, printed codes, and color present in the collection were analyzed using DART-MS, LC-MS and/or AAS. An active ingredient was identified in 89% of the subset of physically distinct preparations (Table 1). The data from these 100 chemically identified medicines were extrapolated to identical tablets/capsules (by shape, codes, and color) in the rest of the collection, which were not analyzed chemically. The most frequent active ingredients identified were paracetamol (acetaminophen) (22%), chlorpheniramine (13.4%), chloroquine (12.6%), tet-racycline/doxycycline (11.4%), and quinine (5.1%) (Figure 1). Tetracycline could not be distinguished from doxycycline because of their identical elemental compositions. A few samples (10) contained two or more active ingredients each: chlorpheniramine in combination with paracetamol (2 samples), vitamin B3 and vitamin C (2), vitamin B3, vitamin B6, and vitamin B7 (1), chloroquine plus paracetamol (1), sulfamethoxazole and paracetamol (1), and two different types of antacids (3).
Figure 1. Major active ingredients identified in the “yaa chud” survey (n = 254). Active ingredients identified in less than 5% of total samples are listed as Other.
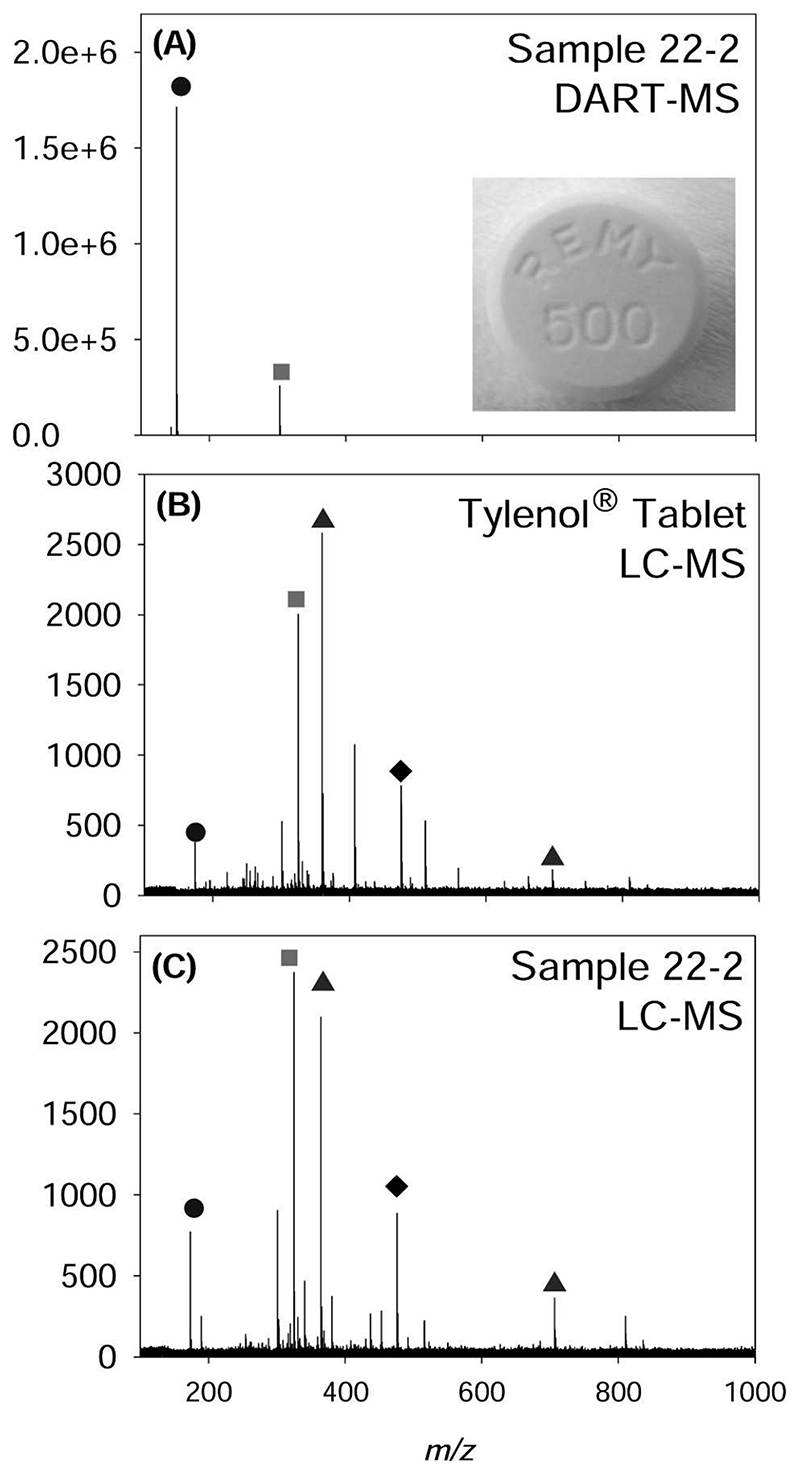
Figure 2A shows a typical DART-MS spectrum of a sample identified by its shape and color to contain paracetamol (acetaminophen) and protonated monomer and dimer peaks corresponding to that active ingredient. The identification of paracetamol as the active ingredient in this sample was verified by LC-MS. Figure 2B shows the LC-MS mass spectrum of an acetaminophen (Tylenol; McNeil-PPC Inc., Fort Washington, PA) tablet along with that of the sample previously analyzed by DART-MS (Figure 3C). The LC-MS spectrum shows sodiated monomer, dimer, and trimer peaks formed by the adduction of sodium to the neutral paracetamol molecules and the presence of lactose/sucrose as an excipient. Throughout the survey, there was good agreement between the DART-MS and LC-MS results, which provided an additional level of confidence about the identities of the active ingredient in any given sample. In 11 of the physically distinct tablets or capsules, no signals were detected in the mass and atomic spectra that could be assigned to any common active ingredient. Sporadic, low-intensity signals caused by impurities and partially extracted excipients were observed, but their assignment to known chemical substances was outside the scope of this study. A database of color photographs of the tablets, mass spectral peak lists and compounds identified is available as Supplementary Material (http://web.chemistry.gatech.edu/l/%7Efernandez/Fernandez_Website/Yaa%20chud%202007.pdf).
Figure 2.
A, Direct analysis in real time mass spectrometry (DART-MS) of a sample visually identified as paracetamol (code 22-2). B, Liquid chromatography-mass spectrometry (LC-MS) of a paracetamol-containing tablet (Tylenol®). C, LC-MS of sample 22-2. The peaks observed correspond to monomer adduct ions (⬤ = [M + Na]+ for LC-MS, [M + H]+ for DART-MS); dimer adduct ions (◆ = [2M + Na]+ for LC-MS, [2M + H]+ for DART-MS), trimer adduct ions [3M + Na]+ (◆), and excipients (▴). The presence of dimer and trimer adducts of the neutral molecule is a common feature of mass spectra obtained by electrospray ionization methods used in LC-MS.29
Figure 3.
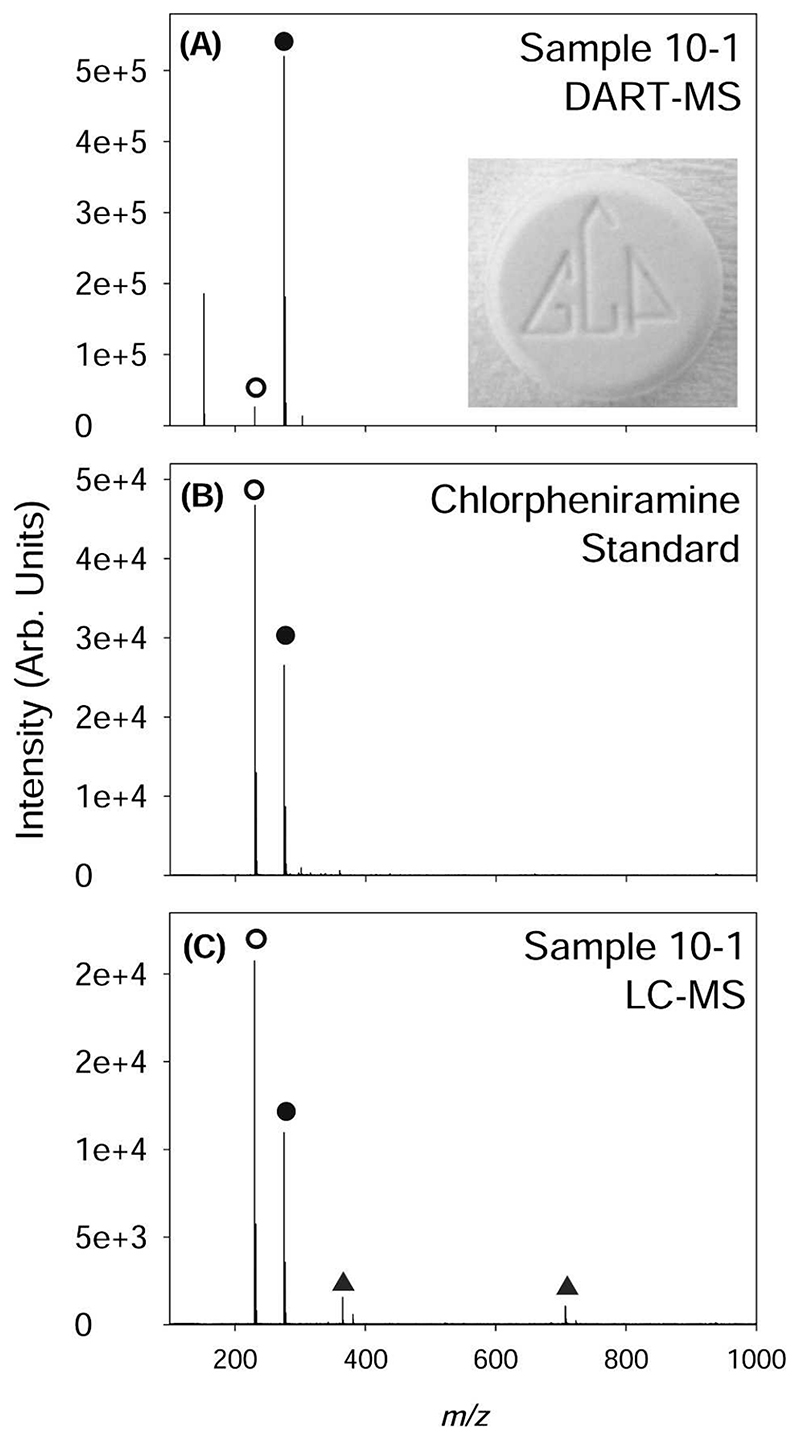
A, Direct analysis in real time mass spectrometry spectrum of a sample (code 10-1) that had been visually identified as containing diazepam. The peaks observed correspond to [M + H]+ (⬤) and a major fragmentation product [M-NHC2H6 + H]+ (⦿). Liquid chromatography-mass spectrometry (LC-MS) of a chlorpheniramine standard. C, LC-MS mass spectrum of sample 10-1 also showing signals corresponding to excipients (▴).
Of 132 yaa chud capsules and/or tablets that had a provisional identity on the basis of physical appearance and a confirmed or inferred identity from chemical analysis, 85 (64%) were identified correctly by visual inspection. In addition to the active ingredients proven to be present, the pharmacists wrongly suspected that yaa chud samples contained diazepam, phenylbutazone, chloramphenicol, co-trimoxazole, ergotamine tartrate, ambroxol, vitamin A and E compound, and phenylpropapanolamine and belladonna alkaloids. For example, Figure 3 shows the chemical analysis results of a sample that had been visually identified as diazepam. The MS analysis of this sample showed that this sample contained chlorpheniramine and not diazepam, as can be concluded by comparison of the mass spectrum for a chlorpheniramine standard with the sample mass spectra obtained by both LC-MS and DART-MS.
The four packets that the shopkeepers said would not work for malaria did not contain chloroquine or quinine. Only 7 bags (14%) contained medicine that could have potentially cured P. falciparum malaria if taken by a 50-kg adult (assuming that the tablets were quinine, 300 mg/bag, and tetracycline, 250 mg/bag) at a dose of two bags every 8 hours for 7 days, although the tetracycline dose would be higher than the recommended 4 mg/kg of body weight every 6 hours.31 No shopkeeper offered such a regimen. The remainder of yaa chud bought had no potential curative effect for malaria. All but one of the yaa chud contained medicines (paracetamol, nonsteroidal anti-inflammatory drugs [NSAIDs]), which may have alleviated some symptoms (fever, headache) but would have caused harm by delaying access to curative antimalarial drugs, which are available for free through local Thai hospitals or NGOs. Using the U.S. Food and Drug Administration (FDA) pregnancy categories32 and the British National Formulary33 guidelines, 7 (26%) of 27 of the drugs in the yaa chud were contraindicated or had an FDA Category of C or D (tetracycline, primaquine, NSAIDs, alprenolol, tolperi-sone), which indicated that an adverse effect to the drug was observed in pregnant animal or human studies. These were contained in 41 (82%) of 50 of the yaa chud. Comparing the seller’s statement as to whether the yaa chud was safe in pregnancy and what pharmaceuticals were found in their product, of 22 (48%) of 46 yaa chud that were said to be safe in pregnancy, 17 (77%) contained contraindicated drugs.
Discussion
This study suggests that inappropriate antimalarial drugs are available on the Thailand-Myanmar border and that in addition to not being efficacious, the use of these drugs may be harmful to pregnant patients and their fetuses. Even if the yaa chud contained efficacious antimalarial drugs, they are unlikely to be fully effective if taken as recommended by the sellers because a seven-day treatment course is needed to cure P. falciparum malaria with quinine-tetracycline in this area.1 However, because the number of patients with malaria who take yaa chud is unknown, drug use surveys of the consumption of yaa chud will be required to estimate their public health impact. Because the use of these packets is illegal in Thailand, and the Ministry of Public Health of the Thai government has introduced publicity campaigns to reduce consumption (Supplementary Figure 2, available online at www.ajtmh.org), it will be difficult to obtain honest estimates of their use. Shopkeepers were reluctant to explain what was in the packets and they would only sell to apparently local people.
The sample subset with no active ingredients present might be composed of degraded or counterfeit drugs. Interestingly, of a total of 11 samples without any detectable active ingre-dient, 7 (64%) could also not be given a provisional identity by the group of experts that examined the yaa chud samples, which suggested that these tablet/capsules might be counterfeit. Conversely, 36 (82%) of 44 samples that could not be visually identified, but did undergo chemical analysis, contained a detectable active ingredient. Visual inspection of tablets and capsules is not sufficient to decide the identity of an active ingredient with certainty.
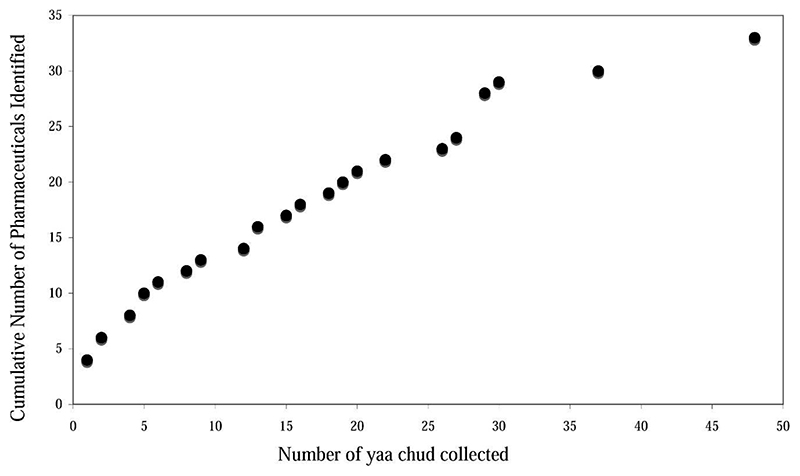
Limitations of the study included that we could not be sure that all kong cham shops were sampled; we sampled all that we could find. We assummed that visually identical samples had the same active ingredient composition and dissolution properties were not investigated. We did not measure the quantity of active ingredient in each tablet/capsule, and we do not know whether these inappropriate medicines contain counterfeit, substandard, or degraded drugs.22,34 Although the sample size was relatively small, inspection of a graph of the cumulative number of distinct chemical identities found with increasing yaa chud sample size suggests that few more additional active ingredients would be found if the sample size had been larger (Figure 4). An additional limitation is that the yaa chud were collected in 2000 and 2001 and malaria treatment has changed locally since then and considerable effort has been expended in Tak province to improve the use of ACT therapy.3 Further work to determine whether artemisinin derivatives and mefloquine are now used in yaa chud and how often yaa chud is taken by malaria patients is needed.
Figure 4. Cumulative number of packets of “yaa chud” collected versus number of new pharmaceuticals identified per new packet.
These results are similar to (pre-ACT) findings from the Thailand-Cambodia border, where 87-94% of those interviewed had used yaa chud. The yaa chud contained 3-5 drugs, most commonly pyrimethamine-sulfadoxine, quinine, pyri-methamine-dapsone, paracetamol, dipyrone, metamizole, tetracyclines, vitamin B1-12, ferrous sulfate, dexamethasone, prednisolone, aspirin, and chlorpheniramine.9 On the Thailand-Myanmar border, 76% of patients with a malarial-like illness (again pre-ACT) bought yaa chud as their first therapy.8 Despite 95% of patients asking for antimalarial drugs, only 65% of yaa chud sampled contained antimalarial drugs (chloroquine, quinacrine, primaquine, amodiaquine, pyri-methamine-sulfadoxine), 74% contained analgesics (aspirin, paracetamol), 21% contained antibiotics (chloramphenicol, tetracyclines, sulfonamides), 22% contained vitamins (B complex), 8% contained steroids (prednisolone, dexamethasone), and 8% contained tranquilizers (diazepam, chlordiazepoxide) and antihistamines (chlorpheniramine, cyproheptadine).
Plasai and Spielman examined whether the frequent subcurative self-administration of yaa chud by gem miners on the Thailand-Cambodia border explained the spread of mefloquine resistance.10 The samples that they collected included 23 packets of tablets containing chloroquine, primaquine, sul-famethoxazole-trimethoprim, tetracycline, prochlorperazine, chlorpheniramine, dimenhydrinate, paracetamol, aspirin, dipyrone, sulfadiazine, dexamethasone, and vitamin B, and 8 ampules sold for malaria as supplements contained quinine, chloroquine, calcium gluconate, metamizole and vitamin B. No mefloquine or oral quinine were found in the yaa chud in 1993, which suggested that yaa chud was not important in the spread of mefloquine-resistant P. falciparum malaria, but could have facilitated the spread of chloroquine and sulfadox-ine-pyrimethamine resistance.
Mainland Southeast Asia has had great importance in the spread of drug-resistant malaria and preliminary reports of clinically important artemisinin resistance occurs on the Thailand-Cambodia border are of considerable concern.35,36 If the consumption of yaa chud containing inadequate antima-larial therapy or with inadequate dosing leads to treatment failure, gametocytemia is likely to be higher and prolonged, risking the enhanced spread of resistance parasites to others in the community and beyond.37,38 Malaria is perceived as the major cause of treatable fever in these areas. However, with the possible exception of scrub typhus,16 the yaa chud in the dose provided would not have cured the patient of any infectious disease.
Much, if not most, of anti-infective drug use in Asia is unregulated39,40 and yaa chud-like packets of medicines are sold to impoverished villagers in Laos (Mayxay M, unpublished data), Cambodia,41 Myanmar, and Thailand but such practices have not yet been documented in Africa (Amin A, Barnes K, Goodman C, unpublished data). However, some sellers offer bowls of exposed tablets and capsules among which customers pick and choose15,41 and sell tablets, but apparently of only one type, in plastic bags (Figure 1 in the report by Patterson and others42). It will be important to ensure that new antimalarial drugs, especially the artemisinin derivatives, are not sold in these ways.15 It seems extremely unlikely that the packets sold between 2000 and 2001 had any beneficial effect for the patients and probably caused harm both to patients and to public health. Drug regulatory action and patient education are required to reduce the trade in yaa chud and similar inappropriate pharmacy practices. However, only 20% of World Health Organization member states are estimated to have well-developed drug regulation and 30% have either no drug regulation or a capacity that hardly functions.43 The lack of financial and human resources available to many drug regulatory authorities often makes action against inappropriate and poor quality medicines impossible.
Acknowledgments
We thank Yongyuth Losuppakarn (Mae Sot Hospital) and Kamolrat Silamut for assistance; the students of the Faculty of Pharmacy, Mahidol University (Bangkok) for help in provisionally identifying the yaa chud ingredients; Catherine Goodman, Elizabeth Ashley, Rose McGready, Shunmay Yeung, and Francois Nosten for helpful comments on the manuscript; and Amin Abdina-sir, Karen Barnes, and Mayfong Mayxay for advice
Footnotes
Note: Supplementary Figure 1 (Picture of yaa chud samples collected) and Supplementary Figure 2 (Poster of the Royal Government of Thailand warning of the dangers of yaa chud) appear online at www.ajtmh.org.
Financial support: The collection of samples and part of the chemical analysis were supported by the Wellcome Trust of Great Britain as part of the Wellcome Trust-Mahidol University-Oxford Tropical Medicine Research Programme. Facundo M. Fernández was supported by a National Science Foundation CAREER grant for DART-MS analysis. Christina Y. Hampton was supported by a Molecular Biophysics Training Program from the Georgia Institute of Technology.
Contributor Information
Paul N. Newton, Wellcome Trust-Mahosot Hospital-Oxford Tropical Medicine Research Collaboration, Mahosot Hospital, Vientiane, Lao People’s Democratic Republic, Tel/Fax: 856-21-242-168 and Centre for Clinical Vaccinology and Tropical Medicine, University of Oxford, Churchill Hospital, Oxford, OX3 7LJ, United Kingdom
Christina Y. Hampton, Email: christina.hampton@gatech.edu, School of Chemistry and Biochemistry, Georgia Institute of Technology, Atlanta, GA 30332, Tel: 404-385-4432, Fax: 404-385-6447.
Krystyn Alter-Hall, School of Chemistry and Biochemistry, Georgia Institute of Technology, Atlanta, GA 30332, Tel: 404-385-4432, Fax: 404-385-6447.
Thanongsak Teerwarakulpana, Email: pyspk@mahidol.ac.th., Mae Sot, Tak Province, Thailand. Sompol Prakongpan, Faculty of Pharmacy, Mahidol University, Bangkok, Thailand
Ronnatrai Ruangveerayuth, Email: ronnatrai@yahoo.com., Mae Sot Hospital, Mae Sot, Tak Province, Thailand
Nicholas J. White, Faculty of Tropical Medicine, Mahidol University, 420/6 Rajvithi Road, Bangkok 10400, Thailand, Tel: 66-2-203-633, Fax: 662-203-6334 and Centre for Clinical Vaccinology and Tropical Medicine, University of Oxford, Churchill Hospital, Oxford, OX3 7LJ, United Kingdom.
Nicholas P. J. Day, Faculty of Tropical Medicine, Mahidol University, 420/6 Rajvithi Road, Bangkok 10400, Thailand, Tel: 66-2-203-633, Fax: 662-203-6334 and Centre for Clinical Vaccinology and Tropical Medicine, University of Oxford, Churchill Hospital, Oxford, OX3 7LJ, United Kingdom.
Mabel B. Tudino, Email: tudino@q1.fcen.uba.ar., Departamento de Química Inorgánica, Analítica y Química Física/Instituto de Quimica de los Materiales, Medio Ambiente y Energia, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Ciudad Universitaria, 1428, Buenos Aires, Argentina, Tel: 54-114576-3360, Fax: 54-11-4576-3341
Natalia Mancuso, Departamento de Química Inorgánica, Analítica y Química Física/Instituto de Quimica de los Materiales, Medio Ambiente y Energia, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Ciudad Universitaria, 1428, Buenos Aires, Argentina, Tel: 54-114576-3360, Fax: 54-11-4576-3341.
Facundo M. Fernández, School of Chemistry and Biochemistry, Georgia Institute of Technology, Atlanta, GA 30332, Tel: 404-385-4432, Fax: 404-385-6447
References
- 1.Nosten F, van Vugt M, Price R, Luxemburger C, Thway KL, Brockman A, McGready R, ter Kuile F, Looareesuwan S, White NJ. Effects of artesunate-mefloquine combination on incidence of Plasmodium falciparum malaria and mefloquine resistance in western Thailand: a prospective study. Lancet. 2000;356:297–302. doi: 10.1016/s0140-6736(00)02505-8. [DOI] [PubMed] [Google Scholar]
- 2.Price RN, Nosten F. Drug resistant falciparum malaria: clinical consequences and strategies for prevention. Drug Resist Updat. 2001;4:187–196. doi: 10.1054/drup.2001.0195. [DOI] [PubMed] [Google Scholar]
- 3.Carrara VI, Sirilak S, Thonglairuam J, Rojanawatsirivet C, Proux S, Gilbos V, Brockman A, Ashley EA, McGready R, Krud-sood S, Leemingsawat S, et al. Deployment of early diagnosis and mefloquine-artesunate treatment of falciparum malaria in Thailand: the Tak Malaria Initiative. PLoS Med. 2006;3:e183. doi: 10.1371/journal.pmed.0030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White NJ. Antimalarial drug resistance and combination chemotherapy. Philos Trans R Soc Lond B Biol Sci. 1999;354:739–749. doi: 10.1098/rstb.1999.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White NJ. Antimalarial drug resistance. J Clin Invest. 2004;113:1084–1092. doi: 10.1172/JCI21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dondorp A, Nosten F, Stepniewska K, Day N, White N. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2005;366:717–725. doi: 10.1016/S0140-6736(05)67176-0. [DOI] [PubMed] [Google Scholar]
- 7.Jaidee S, Limpananon J, Wittayanartpaisal S. Drug utilization of yaa-chud in Thailand. Thai J Pharm Sci. 1980;5:219–220. [Google Scholar]
- 8.Wiwat C, Silapa-archa W, Temsirilerkhul R, Chiowatana J. Rural self-medication in malaria infection. Thai J Pharm Sci. 1982;7:14–29. [Google Scholar]
- 9.Kamolratanakul P, Dhanamun B, Thaithong S. Human behavior in relation to selection of malaria treatment. Southeast Asian J Trop Med Public Health. 1992;23:189–194. [PubMed] [Google Scholar]
- 10.Plasai V, Spielman A. Mefloquine insusceptibility of malaria in Thailand not promoted by nonregulated drug-use. Acta Trop. 1996;60:281–289. doi: 10.1016/0001-706x(95)00133-y. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham CE. Thai “injection doctors”; antibiotic mediators. Soc Sci Med. 1970;4:1–24. doi: 10.1016/0037-7856(70)90056-9. [DOI] [PubMed] [Google Scholar]
- 12.Foster SD. Pricing, distribution, and use of antimalarial drugs. Bull World Health Organ. 1991;69:349–363. [PMC free article] [PubMed] [Google Scholar]
- 13.Amin AA, Hughes DA, Marsh V, Abuya TO, Kokwaro GO, Winstanley PA, Ochola SA, Snow RW. The difference between effectiveness and efficacy of antimalarial drugs in Kenya. Trop Med Int Health. 2004;9:967–974. doi: 10.1111/j.1365-3156.2004.01291.x. [DOI] [PubMed] [Google Scholar]
- 14.Basco LK. Molecular epidemiology of malaria in CamerooN XIX. Quality of antimalarial drugs used for selfmedication. Am J Trop Med Hyg. 2004;70:245–250. [PubMed] [Google Scholar]
- 15.Goodman C, Brieger W, Unwin A, Mills A, Meek S, Greer G. Medicine sellers and malaria treatment in sub-Saharan Africa: what do they do and how can their practice be improved? Am J Trop Med Hyg. 2007;77:203–218. [PMC free article] [PubMed] [Google Scholar]
- 16.Brown GW, Saunders JP, Singh S, Huxsoll DL, Shirai A. Single dose doxycycline therapy for scrub typhus. Trans R Soc Trop Med Hyg. 1978;72:412–416. doi: 10.1016/0035-9203(78)90138-4. [DOI] [PubMed] [Google Scholar]
- 17.Taylor RB, Shakoor O, Behrens RH. Drug quality, a contributor to drug-resistance. Lancet. 1995;346:122. doi: 10.1016/s0140-6736(95)92145-1. [DOI] [PubMed] [Google Scholar]
- 18.Okeke IN, Lamikanra A, Edelman R. Socioeconomic and behavioral factors leading to acquired bacterial resistance to antibiotics in developing countries. Emerg Infect Dis. 1999;5:18–27. doi: 10.3201/eid0501.990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noranate N, Durand R, Tall A, Marrama L, Spiegel A, Sokhna C, Pradines B, Cojean S, Guillotte M, Bischoff E, Ekala MT, et al. Rapid dissemination of Plasmodium falciparum drug resistance despite strictly controlled antimalarial use. PLoS ONE. 2007;2:e139. doi: 10.1371/journal.pone.0000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeung S, White NJ. How do patients use antimalarial drugs? A review of the evidence. Trop Med Int Health. 2005;10:121–138. doi: 10.1111/j.1365-3156.2004.01364.x. [DOI] [PubMed] [Google Scholar]
- 21.Kachur SP, Black C, Abdulla S, Goodman C. Putting the genie back in the bottle? Availability and presentation of oral artemisinin compounds at retail pharmacies in urban Dar-es-Salaam. Malar J. 2006;5:25. doi: 10.1186/1475-2875-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newton PN, Green MD, Fernandez FM, Day NP, White NJ. Counterfeit anti-infective drugs. Lancet Infect Dis. 2006;6:602–613. doi: 10.1016/S1473-3099(06)70581-3. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez FM, Cody RB, Green MD, Hampton CY, McGready R, Sengaloundeth S, White NJ, Newton PN. Characterization of solid counterfeit drug samples by desorption electrospray ionization and direct-analysis-in-real-time coupled to time-of-flight mass spectrometry. ChemMedChem. 2006;1:702–705. doi: 10.1002/cmdc.200600041. [DOI] [PubMed] [Google Scholar]
- 24.Hall KA, Newton PN, Green MD, De Veij M, Vandenabeele P, Pizzanelli D, Mayxay M, Dondorp A, Fernandez FM. Characterization of counterfeit artesunate antimalarial tablets from southeast Asia. Am J Trop Med Hyg. 2006;75:804–811. [PubMed] [Google Scholar]
- 25.Ward SA, Sevene EJ, Hastings IM, Nosten F, McGready R. Antimalarial drugs and pregnancy: safety, pharmacokinetics, and pharmacovigilance. Lancet Infect Dis. 2007;7:136–144. doi: 10.1016/S1473-3099(07)70025-7. [DOI] [PubMed] [Google Scholar]
- 26.Lee MS, Kerns EH. LC/MS applications in drug development. Mass Spectrom Rev. 1999;18:187–279. doi: 10.1002/(SICI)1098-2787(1999)18:3/4<187::AID-MAS2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 27.Tomer KB. Separations combined with mass spectrometry. Chem Rev. 2001;101:297–328. doi: 10.1021/cr990091m. [DOI] [PubMed] [Google Scholar]
- 28.Cody R, Laramee J, Durst H. Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal Chem. 2005;77:2297–2302. doi: 10.1021/ac050162j. [DOI] [PubMed] [Google Scholar]
- 29.Gaskell S. Electrospray: principles and practice. J Mass Spectrom. 1997;32:677–688. [Google Scholar]
- 30.WHO Model List of Essential Medicines. World Health Organization; Geneva: 2008. [Google Scholar]
- 31.World Health Organization. WHO Guidelines for the Treatment of Malaria. World Health Organization; Geneva: 2006. [Google Scholar]
- 32.Beers MH, Porter RS, Jones TV, Kaplan JL, Berwits M. The Merck Manual of Diagnosis and Therapy. Merck Research Laboratories; Whitehouse Station, NJ: 2006. [Google Scholar]
- 33.Anonymous. British National Formulary. BMJ Publishing Group and RPS Publishing; London: 2007. [Google Scholar]
- 34.Ballereau F, Prazuck T, Schrive I, Lafleuriel MT, Rozec D, Fisch A, Lafaix C. Stability of essential drugs in the field: results of a study conducted over a two-year period in Burkina Faso. Am J Trop Med Hyg. 1997;57:31–36. doi: 10.4269/ajtmh.1997.57.31. [DOI] [PubMed] [Google Scholar]
- 35.Roper C, Pearce R, Nair S, Sharp B, Nosten F, Anderson T. Intercontinental spread of pyrimethamine-resistant malaria. Science. 2004;305:1124. doi: 10.1126/science.1098876. [DOI] [PubMed] [Google Scholar]
- 36.AnoN. Resistance to artemisinin derivatives along the Thai-Cambodian border. Wkly Epidemiol Rec. 2007;82:360. [PubMed] [Google Scholar]
- 37.Bousema JT, Gouagna LC, Meutstege AM, Okech BE, Akim NI, Githure JI, Beier JC, Sauerwein RW. Treatment failure of pyrimethamine-sulphadoxine and induction of Plasmodium falciparum gametocytaemia in children in western Kenya. Trop Med Int Health. 2003;8:427–430. doi: 10.1046/j.1365-3156.2003.01047.x. [DOI] [PubMed] [Google Scholar]
- 38.Price R, Nosten F, Simpson JA, Luxemburger C, Phaipun L, ter Kuile F, van Vugt M, Chongsuphajaisiddhi T, White NJ. Risk factors for gametocyte carriage in uncomplicated falciparum malaria. Am J Trop Med Hyg. 1999;60:1019–1023. doi: 10.4269/ajtmh.1999.60.1019. [DOI] [PubMed] [Google Scholar]
- 39.Tomson G, Sterky G. Self-prescribing by way of pharmacies in 3 Asian developing-countries. Lancet. 1986;2:620–622. doi: 10.1016/s0140-6736(86)92438-4. [DOI] [PubMed] [Google Scholar]
- 40.Wondemagegnehu E. Counterfeit and Substandard Drugs in Myanmar and Vietnam. World Health Organization; Geneva: 1999. WHO Report WHO/EDM/QSM/993. [Google Scholar]
- 41.Lon CT, Tsuyuoka R, Phanouvong S, Nivanna N, Socheat D, Sokhan C, Blum N, Christophel EM, Smine A. Counterfeit and substandard antimalarial drugs in Cambodia. Trans R Soc Trop Med Hyg. 2006;100:1019–1024. doi: 10.1016/j.trstmh.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Patterson AE, Winch PJ, Gilroy KE, Doumbia S. Local terminology for medicines to treat fever in Bougouni District, Mali: implications for the introduction and evaluation of malaria treatment policies. Trop Med Int Health. 2006;11:1613–1624. doi: 10.1111/j.1365-3156.2006.01713.x. [DOI] [PubMed] [Google Scholar]
- 43.Ratanowijitrasin S, Wondemagegnehu E. Effective drug regulation: a multicountry study. Essent Drugs Monit. 2003;32:2–4. [Google Scholar]