Abstract
The activation of the Notch4-Wnt-GDF15 axis in induced Tregs dampens their immunoregulatory function and turns them into Th2 and Th17 cytokine producers, allowing them to maintain ongoing allergic asthma.
Allergic asthma is a chronic airway disease caused by an exaggerated Th2 response, which leads to airway inflammation and bronchial hyperreactivity. Several explanations have been put forward to explain why asthma develops. One of them is the inability of regulatory T cells (Treg) induced in the lungs to dampen Th2 immunity. At the same time, the maintenance of the Th2 program developed in response to allergen exposure was shown to rely on Notch activation in Th2 cells themselves1. Interestingly, in this issue of Nature Immunology, Harb et al2 uncover a novel role for the Notch pathway in Treg biology. The induction of Notch 4 expression, followed by Wnt and Hippo pathway activation, in pulmonary induced Tregs subverts their functions to promote allergic asthma.
The role of Notch signaling pathway on T helper cell differentiations has been extensively studied, and it is now clear that the identity of the Notch receptor and its ligand dictates the fate of T cells. Since 2004, the dogma had been that Jagged 1 or Jagged 2 expression on in vitro-generated dendritic cells would favor Th2 cell differentiation3. However, a more recent study performed in vivo showed that the driving force leading to allergic asthma symptoms was the expression of Notch receptors by CD4 T cells and not Notch ligands by antigen-presenting cells4. This latter study opened several questions: which subset of CD4 T cells and which one of the four members of the Notch family (Notch1-4) are responsible for the maintenance of airway inflammation? In this issue of Nature Immunology, Harb et al2 were able to fill this knowledge gap and elegantly demonstrate that repeated allergen exposure leads to the increased expression of Notch 4 specifically in induced Treg cells, and not in Th1, Th2 or Th17 effector T cells. This Treg-specific Notch 4 expression could be further increased by co-exposure with ultrafine particles contained in air pollution. Interestingly, the authors could reproduce these effects in vitro and showed that primary Jagged1+ alveolar macrophages could drive the differentiation of Notch4hi Tregs. This process is driven mainly by IL-6/IL6R signaling, and this could be explained by the presence of a binding element for the transcription factor STAT3 (which is downstream of IL-6R) in the Notch4 promoter2.
Notch4 is an understudied member of the Notch family. It is mainly known to be expressed on endothelial cells, but not on immune cells, and it has been mainly studied in the context of cancer. Its function in inflammatory diseases still remained enigmatic. In 2018, the Chatila lab was the first to report Notch4 expression in allergen-specific T cells isolated from the lungs of mice co-exposed to allergen and ultrafine particles. The blockade of Notch4 using antibodies reduced the degree of asthma features, clearly showing a pro-inflammatory role of Notch4 in their model5. These data are however in sharp contrast with another report from Verhein et al showing that Notch4 has anti-inflammatory properties and is involved in the protection from pulmonary inflammation caused by exposure to ozone, a toxic air pollutant6. This discrepancy could be due to the fact that (i) the pathways activated by different air pollutants (ozone versus UFPs) are different and that (ii) full body Notch4-/- mice were used and not mice in which Notch4 expression would be removed specifically in T cells. The latter seems to be the most crucial point in determining how Notch4 affects the immune response. Indeed, using T cell- and Treg cell-specific deletion models, Harb et al2 uncovered that Notch4 expression in induced Tregs altered the functions of these cells and inhibited their suppressive activities, allowing Th2 immunity to develop.
How can Notch4 subvert induced Treg cell functions? To address this point, Harb et al2 investigated the Notch4-dependent pathways in Tregs by looking at the transcriptional profiles of lung Tregs lacking Notch4 expression or not. The authors found a Notch4-dependent activation of two majors pathways: Hippo and Wnt, both involved in the control cell proliferation and differentiation. An exciting finding made by the group is that the deletion in Tregs of key genes involved in both pathways prevents the establishment of BHR, but differentially affects the nature of the effector response developed. Indeed, the Hippo pathway controls the Th17 whereas Wnt controls the Th2 arm of the inflammatory response developed in allergic asthma induced by the co-exposure to allergen and UFPs. These data are supported by the fact that the Hippo pathway has been shown to regulate the balance between Tregs and Th17 cells. TAZ, a key protein of the Hippo pathway, which expression is strongly upregulated in Notch4+ Tregs2, has been described as an inhibitor of Foxp3 function and its overexpression was reported to favor the differentiation of IL-17-producing cells rather than Tregs7. To lend support to the data of Harb et al, the activation of Wnt signaling was shown to inhibit Foxp3-mediated transcriptional activity as well as Treg-mediated cell suppression8. At the same time, by promoting of GATA3 expression and repressing Th1 cytokine production, the activation of the Wnt/β-catenin pathway in Tregs may facilitate their differentiation into Th2 cells9. Induced Tregs being less stable than native Treg cells, they can easily turn into Th2-, or Th17 cytokine-producing ex-Tregs depending on the microenvironment. Interestingly, Harb et al2 went on finding that such deregulated Notch4+ Tregs not only fail to suppress T cell proliferation, but also become unable to keep Th2 cytokine production by ILC2 in check, as would Notch4lo Tregs normally do10. The propensity of Notch4+ Tregs to promote and perpetuate Th2 immunity in the lung was mediated by GDF-15, a stress-induced member of the transforming growth factor β superfamily, which function is currently unclear as this cytokine has been shown to have pro- or anti-inflammatory functions depending on context, organ and timing. Seeing GDF-15 expression in Notch4+ Treg cells is very unexpected since data from the Immgen consortium clearly show that GDF-15 is expressed at very low levels in most immune cells, except in macrophages, where this cytokine was initially identified. Another intriguing point is that, in the lung, the expression of GDF-15 was found to be mainly restricted to epithelial cells upon exposure to cigarette smoke, but the authors of this study have not formally looked at GDF-15 expression in immune cells. Interestingly, using GDF-15-/- mice, the latter study found that GDF-15 was pro-inflammatory11, which is clearly in line with the findings from Harb et al. It is therefore possible that although lung epithelial cells may represent an important source of pro-inflammatory GDF-15 following exposure to certain pollutants, the newly identified subset of Notch4+ Tregs constitutes another or a complementary way by which inflammation would be perpetuated. Performing cell-specific deletion of GDF-15 should unravel more precisely the exact contribution of structural and immune cells to pulmonary inflammation.
In summary, Harb et al2 have identified a new subset of Treg cell expressing Notch4 in response to ultrafine particles contained in air pollution. This specific Treg population is unable to properly suppress inflammation because it produces Th2 and Th17 effector cytokines instead of immunoregulatory cytokines. This shift away from immunosuppression was caused by the activation of the Wnt and the Hippo pathways, which would allow Notch4+ Tregs to produce GDF-15 and in this way, perpetuate and even exacerbate Th2 inflammation in the lung. Interestingly, Harb et al2 nicely show that this cascade of events observed in mice could be found in patients suffering from asthma. Several questions remain as to how exposure to ultrafine particles drives the preferential expression of Notch4 in Tregs and how Notch4 activates downstream pathways such as Wnt and Hippo, leading to the maintenance of Th2 immunity. The co-activation of these pathways in the same cells is intriguing since earlier studies provided evidence that the Hippo pathway could inhibit Wnt /β-catenin signaling12. However, although this may be true in developing organs, it might be different in immune cells like induced Tregs, in which Harb et al convincingly showed the complementarity of both pathways in promoting Th2 and Th17 responses2. The discovery of this new Notch4-Wnt-GDF15 axis in the control of allergic asthma in mice, along with its validation in severe asthmatic patients, certainly offers novel therapeutic perspectives aiming at restoring immune tolerance and homeostasis in the lung.
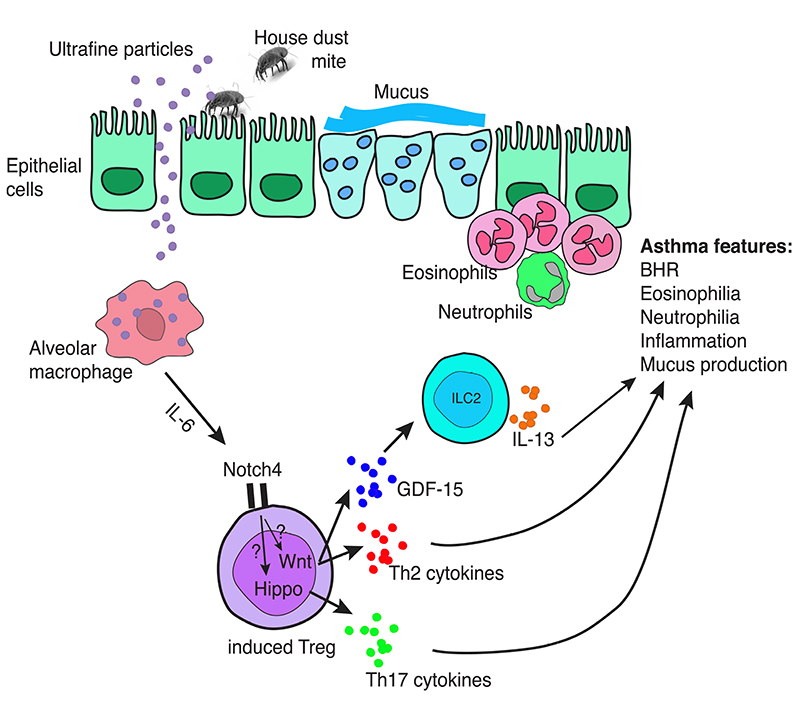
Figure 1.
Notch4+ regulatory T cells contribute to Th2 immunity to allergens and air pollutants. Following uptake of ultrafine particles, alveolar macrophages release high amounts of IL-6, which promotes Notch4 expression on induced regulatory T cells. Notch4 signaling leads to the activation of the Hippo and of the Wnt pathway. Both pathways are sufficient to shift regulatory T cells away from immunoregulation. Instead, the Wnt and Hippo pathways activate a Th2 and Th17 program in Notch4+ regulatory T cells. In addition, the Wnt pathway allows GDF-15 release by these cells, and GDF-15 triggers IL-13 release by ILC2. The cytokine milieu induced by Notch4+ regulatory T cells allows asthma feature to develop and to maintain.
References
- 1.Bailis W, Yashiro-Ohtani Y, Fang TC, Hatton RD, Weaver CT, Artis D, Pear WS. Notch simultaneously orchestrates multiple helper T cell programs independently of cytokine signals. Immunity. 2013;39(1):148–159. doi: 10.1016/j.immuni.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harb H, Stephen-Victor E, Crestani E, Benamar M, Massoud A, Cui Y, Charbonnier L, Arbag S, Baris S, Cunninham A, Levya-Castillo J, et al. A regulatory T cell Notch4-GDF15 axis licenses tissue inflammation in asthma. Natlmmunol. 2020 doi: 10.1038/s41590-020-0777-3. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117(4):515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 4.Tindemans I, Lukkes M, de Bruijn MJW, Li BWS, van Nimwegen M, Amsen D, KleinJan A, Hendriks RW. Notch signaling in T cells is essential for allergic airway inflammation, but expression of the Notch ligands Jagged 1 and Jagged 2 on dendritic cells is dispensable. J Allergy Clin Immunol. 2017;140(4):1079–1089. doi: 10.1016/j.jaci.2016.11.046. [DOI] [PubMed] [Google Scholar]
- 5.Xia M, Harb H, Saffari A, Sioutas C, Chatila TA. A Jagged 1-Notch 4 molecular switch mediates airway inflammation induced by ultrafine particles. J Allergy Clin Immunol. 2018;142(4):1243–1256.:e1217. doi: 10.1016/j.jaci.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verhein KC, McCaw Z, Gladwell W, Trivedi S, Bushel PR, Kleeberger SR. Novel Roles for Notch3 and Notch4 Receptors in Gene Expression and Susceptibility to Ozone-Induced Lung Inflammation in Mice. Environ Health Perspect. 2015;123(8):799–805. doi: 10.1289/ehp.1408852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geng J, Yu S, Zhao H, Sun X, Li X, Wang P, Xiong X, Hong L, Xie C, Gao J, Shi Y, et al. Corrigendum: The transcriptional coactivator TAZ regulates reciprocal differentiation of TH17 cells and Treg cells. Nat Immunol. 2017;18(11):1270. doi: 10.1038/ni1117-1270c. [DOI] [PubMed] [Google Scholar]
- 8.van Loosdregt J, Fleskens V, Tiemessen MM, Mokry M, van Boxtel R, Meerding J, et al. Canonical Wnt signaling negatively modulates regulatory T cell function. Immunity. 2013;39(2):298–310. doi: 10.1016/j.immuni.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Yu Q, Sharma A, Oh SY, Moon HG, Hossain MZ, Salay TM, Leeds KE, Du H, Wu B, Waterman ML, Zhu Z, et al. T cell factor 1 initiates the T helper type 2 fate by inducing the transcription factor GATA-3 and repressing interferon-gamma. Nat Immunol. 2009;10(9):992–999. doi: 10.1038/ni.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rigas D, Lewis G, Aron JL, Wang B, Banie H, Sankaranarayanan I, Galle-Treger L, Maazi H, Lo R, Freeman GJ, Sharpe AH, et al. Type 2 innate lymphoid cell suppression by regulatory T cells attenuates airway hyperreactivity and requires inducible T-cell costimulator-inducible T-cell costimulator ligand interaction. J Allergy Clin Immunol. 2017;139(5):1468–1477.:e1462. doi: 10.1016/j.jaci.2016.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verhamme FM, Seys LJM, De Smet EG, Provoost S, Janssens W, Elewaut D, Joos GF, Brusselle GG, Bracke KR. Elevated GDF-15 contributes to pulmonary inflammation upon cigarette smoke exposure. Mucosal Immunol. 2017;10(6):1400–1411. doi: 10.1038/mi.2017.3. [DOI] [PubMed] [Google Scholar]
- 12.Varelas X, Miller BW, Sopko R, Song S, Gregorieff A, Fellouse FA, Sakuma R, Pawson T, Hunziker W, McNeill H, Wrana JL, et al. The Hippo pathway regulates Wnt/beta-catenin signaling. Dev Cell. 2010;18(4):579–591. doi: 10.1016/j.devcel.2010.03.007. [DOI] [PubMed] [Google Scholar]