Abstract
Background
The muscarinic receptor agonist xanomeline has antipsychotic properties and is devoid of dopamine receptor–blocking activity but causes cholinergic adverse events. Trospium is a peripherally restricted muscarinic receptor antagonist that reduces peripheral cholinergic effects of xanomeline. The efficacy and safety of combined xanomeline and trospium in patients with schizophrenia are unknown.
Methods
In this double-blind, phase 2 trial, we randomly assigned patients with schizophrenia in a 1:1 ratio to receive twice-daily xanomeline–trospium (increased to a maximum of 125 mg of xanomeline and 30 mg of trospium per dose) or placebo for 5 weeks. The primary end point was the change from baseline to week 5 in the total score on the Positive and Negative Syndrome Scale (PANSS; range, 30 to 210, with higher scores indicating more severe symptoms of schizophrenia). Secondary end points were the change in the PANSS positive symptom subscore, the score on the Clinical Global Impression–Severity (CGI-S) scale (range, 1 to 7, with higher scores indicating greater severity of illness), the change in the PANSS negative symptom subscore, the change in the PANSS Marder negative symptom subscore, and the percentage of patients with a response according to a CGI-S score of 1 or 2.
Results
A total of 182 patients were enrolled, with 90 assigned to receive xanomeline–trospium and 92 to receive placebo. The PANSS total score at baseline was 97.7 in the xanomeline–trospium group and 96.6 in the placebo group. The change from baseline to week 5 was −17.4 points with xanomeline–trospium and −5.9 points with placebo (least-squares mean difference, −11.6 points; 95% confidence interval, −16.1 to −7.1; P<0.001). The results for the secondary end points were significantly better in the xanomeline–trospium group than in the placebo group, with the exception of the percentage of patients with a CGI-S response. The most common adverse events in the xanomeline–trospium group were constipation, nausea, dry mouth, dyspepsia, and vomiting. The incidences of somnolence, weight gain, restlessness, and extrapyramidal symptoms were similar in the two groups.
Conclusions
In a 5-week trial, xanomeline–trospium resulted in a greater decrease in the PANSS total score than placebo but was associated with cholinergic and anticholinergic adverse events. Larger and longer trials are required to determine the efficacy and safety of xanomeline–trospium in patients with schizophrenia. (Funded by Karuna Therapeutics and the Wellcome Trust; ClinicalTrials.gov number, NCT03697252.)
ANTIPSYCHOTIC DRUGS ARE ASSOCIATED with adverse events such as extrapyramidal symptoms, sedation, weight gain, metabolic disturbances, and hyperprolactinemia that contribute to poor medication adherence and relapses of psychosis.1,2 Moreover, 20 to 33% of patients do not have a response to conventional treatment, and others have residual psychotic symptoms.3 Many patients with schizophrenia have poor functional status and quality of life despite lifelong treatment with current antipsychotic agents.4,5
Although antipsychotics that are approved for schizophrenia work primarily by antagonizing D2 dopamine receptors,6 evidence suggests that the muscarinic cholinergic system is also involved in the pathophysiology of schizophrenia.7–11 Xanomeline is an oral muscarinic cholinergic receptor agonist that is devoid of direct effects on dopamine receptors12 and that preferentially stimulates M1 and M4 muscarinic cholinergic receptors,13 which have been implicated in the pathophysiology of schizophrenia.14 In a trial involving patients with Alzheimer’s disease and in a small exploratory trial involving patients with schizophrenia, xanomeline led to greater decreases in some psychotic symptoms than did placebo.15,16 However, there were dose-dependent cholinergic adverse events of nausea, vomiting, diarrhea, sweating, and hypersalivation mediated by stimulation of peripheral muscarinic cholinergic receptors.
Trospium chloride is an oral pan-muscarinic receptor antagonist that is approved for the treatment of overactive bladder in the United States and the European Union.17 Its highly polar tertiary amine structure prevents it from reaching detectable levels in the cerebrospinal fluid and should obviate adverse central nervous system effects.18 In a phase 1 trial involving healthy volunteers, the incidence of cholinergic adverse events was approximately 50% lower when trospium was added to xanomeline than when xanomeline alone was used19; these findings suggest a potential way to stimulate brain muscarinic receptors with therapeutic antipsychotic doses of xanomeline while limiting these adverse events.20 The current phase 2 trial assessed the efficacy and safety of the combination oral agent xanomeline–trospium in patients with acute exacerbations of schizophrenia.
Methods
Trial Oversight
The sponsor, Karuna Therapeutics, designed the trial, provided the trial drug and placebo, and analyzed the data. The qualification process for end-point raters was overseen by Signant Health, and the overall trial was overseen by Syneos Health. The trial protocol and consent form were approved by a central institutional review board (Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). Written informed consent was obtained from all the patients before any procedures or interventions were performed. Patients were counseled about the trial before giving written informed consent to participate in the trial. The informed-consent form is provided in the protocol, available at NEJM.org.
All the authors vouch for the adherence of the trial to the protocol, the completeness and accuracy of the data, and the reporting of adverse events. The first, third, and last authors contributed to the trial design; all the authors interpreted the data, and the last two authors wrote the manuscript. All the authors approved the manuscript before submission. Confidentiality agreements exist between the authors and Karuna Therapeutics.
Patient Population
Patients were 18 to 60 years of age and had a primary diagnosis of schizophrenia according to the criteria in the Diagnostic and Statistical Manual of Mental Disorders, fifth edition.21 Site investigators (listed in the Supplementary Appendix) and certified staff who were trained on the protocol by a sponsor-funded contract research organization (Signant Health) established the diagnoses by a comprehensive psychiatric evaluation using the Mini-International Neuropsychiatric Interview22 and supported by other sources, including medical records and informant interviews.
Patients were required to have a baseline Positive and Negative Syndrome Scale (PANSS) total score of 80 points or more (range, 30 to 210, with higher scores indicating more severe symptoms of schizophrenia), with a score of at least 5 on one positive symptom item or at least 4 on two positive symptom items.23 The scale contains 30 items (including seven positive symptom items and seven negative symptom items), each scored from 1 to 7; higher scores indicate more severe symptoms. Positive symptoms include delusions, hallucinations, and conceptual disorganization. Negative symptoms include restricted emotional expression, paucity of speech, and diminished interest, social drive, and activity.
Patients were also required to have a score on the Clinical Global Impression–Severity (CGI-S) scale of 4 or higher (range, 1 to 7, with higher scores indicating greater severity of illness).24 Patients had to be having an acute exacerbation or relapse of psychosis requiring hospitalization with an onset within 2 months before screening and had to be free of antipsychotic medication for at least 2 weeks before the baseline assessment. These patients were either admitted for treatment of their disorder before the inception of the trial or were admitted for trial treatment.
Key exclusion criteria were a history of treatment resistance to antipsychotic medications, defined as no response to two adequate courses of pharmacotherapy, and a decrease in the PANSS total score by more than 20% between screening and baseline. A full list of inclusion and exclusion criteria is provided in the Methods section of the Supplementary Appendix.
Trial Design and Procedures
This was a phase 2, randomized, double-blind, placebo-controlled, 5-week inpatient trial that enrolled patients at 12 sites in the United States between September 2018 and August 2019. Eligible patients were randomly assigned in a 1:1 ratio to receive twice-daily oral xanomeline–trospium or placebo according to a computer-generated randomization schedule created by Syneos Health, with the use of a site-based block size of four; the random assignments were concealed from the patients, trial personnel, and investigators. The sponsor, contract research organization, data analysts, personnel at the laboratories, and clinical site team including investigators were unaware of the trial group assignments.
The dosing schedule was flexible, starting with 50 mg of xanomeline and 20 mg of trospium twice daily and increased to a maximum of 125 mg of xanomeline and 30 mg of trospium twice daily, with the option to return to 100 mg of xanomeline and 20 mg of trospium twice daily if the maximum dose caused unacceptable adverse events as judged by the investigator’s clinical assessment. (See the Methods section in the Supplementary Appendix for further details of the flexible dosing schedule.) The xanomeline dosing regimen was selected on the basis of previous trials showing antipsychotic efficacy at 225 mg per day15,16 and studies showing that a combination of xanomeline at 200 mg per day with trospium achieved similar or higher xanomeline exposures as compared with those in xanomeline monotherapy trials and was associated with lower incidences of cholinergic adverse events than xanomeline alone.19,20
End Points
The primary end point was the change from baseline to week 5 in the PANSS total score.23 Secondary end points were the change in the PANSS positive symptom subscore, the score on the CGI-S scale,24 the change in the PANSS negative symptom subscore, the change in the PANSS Marder negative symptom subscore,25 and the percentage of patients with a response according to a CGI-S score of 1 (“normal”) or 2 (“borderline ill”). The PANSS positive symptom subscore, negative symptom subscore, and Marder negative symptom subscore each range from 7 to 49, with higher scores indicating more severe symptoms.
Safety
Safety was assessed through adverse-event monitoring and by measuring weight and vital signs, clinical laboratory values, electrocardiographic variables, and suicidal ideation, according to the score on the Columbia Suicide Severity Rating Scale.26 The Simpson–Angus Scale27 was used to measure drug-related extrapyramidal symptoms (range, 0 to 40, with higher scores indicating greater drug-induced parkinsonian symptoms), and the Barnes Akathisia Rating Scale28 was used to assess akathisia (range, 0 to 14, with higher scores indicating greater symptoms of akathisia). Adverse events that occurred during the treatment period were defined as those that started or worsened from the time of the first dose of xanomeline–trospium or placebo (visit 1 at day 2) to the time of discharge (visit 9 at day 35).
Statistical Analysis
Assuming a difference in the PANSS total score of 9 points in the change from baseline between xanomeline–trospium and placebo and a standard deviation of 18, we calculated that a sample size of 180 (90 patients who could be evaluated per group) would provide 91% power to detect a difference between the trial groups with a twosided alpha level of 0.05. Efficacy analyses were performed in the modified intention-to-treat population, which included all the patients who had undergone randomization, had received at least one dose of xanomeline–trospium or placebo, and had received a PANSS assessment at baseline and at least once after baseline. The primary efficacy end point was analyzed by means of a mixed model for repeated measures for the difference between groups in the leastsquares mean change in the PANSS total score from baseline to week 5.
If the results for the primary efficacy end point differed significantly between the xanomeline–trospium group and the placebo group at a two-sided alpha level of 0.05, then secondary efficacy end points were analyzed at week 5 with the use of hierarchical hypothesis tests in a fixed-sequence procedure in the following order: the least-squares mean change from baseline in the PANSS positive symptom subscale, the score on the CGI-S scale,24 the least-squares mean change from baseline in the PANSS negative symptom subscale, the least-squares mean change from baseline in the PANSS Marder negative symptom subscore,25 and the percentage of patients with a response according to a CGI-S score of 1 or 2. There was no imputation of missing data for primary or secondary end points. The statistical analysis plan is available with the protocol at NEJM.org.
Sensitivity analyses were conducted to evaluate the assumptions used in the primary analysis, including those related to the handling of missing data (Table S2). We determined the Cohen’s d statistic29 for the primary efficacy end point, using the absolute between-group difference in the unadjusted mean change in PANSS score from baseline to week 5, divided by the pooled standard deviation of the between-group difference. The prespecified CGI-S ordinal categorical analysis comparing the score on the CGI-S scale between the xanomeline–trospium and placebo groups at week 5 (unadjusted for baseline values) was analyzed with the Mann–Whitney–Wilcoxon test. In the prespecified analysis of response according to a CGI-S score of 1 or 2 at week 5, the percentage of patients with a response was compared between the xanomeline–trospium and placebo groups with the use of the Cochran-Mantel-Haenszel test stratified according to the baseline CGI-S score. A longitudinal cumulative logit model predicting the ordinal value of CGI-S at each visit, adjusted for the baseline CGI-S value, visit, trial group, and visit-by-group interaction, was also applied to test for a treatment effect of xanomeline–trospium against placebo and confirmed the assumption of proportional odds for all but one time point included in the model (week 1) (Table S3). A post hoc analysis of CGI-S was conducted with a mixed model for repeated measures (Fig. S1). In the last two analyses, the 95% confidence intervals for the between-group difference were not adjusted for multiplicity, so these analyses should not be used to draw conclusions regarding efficacy.
The safety population included all the patients who had undergone randomization and had received at least one dose of xanomeline–trospium or placebo. Adverse events that emerged during the trial period were rated for severity and summarized according to trial group. All data regarding safety and adverse events were summarized descriptively according to trial group and time point.
Results
Patients
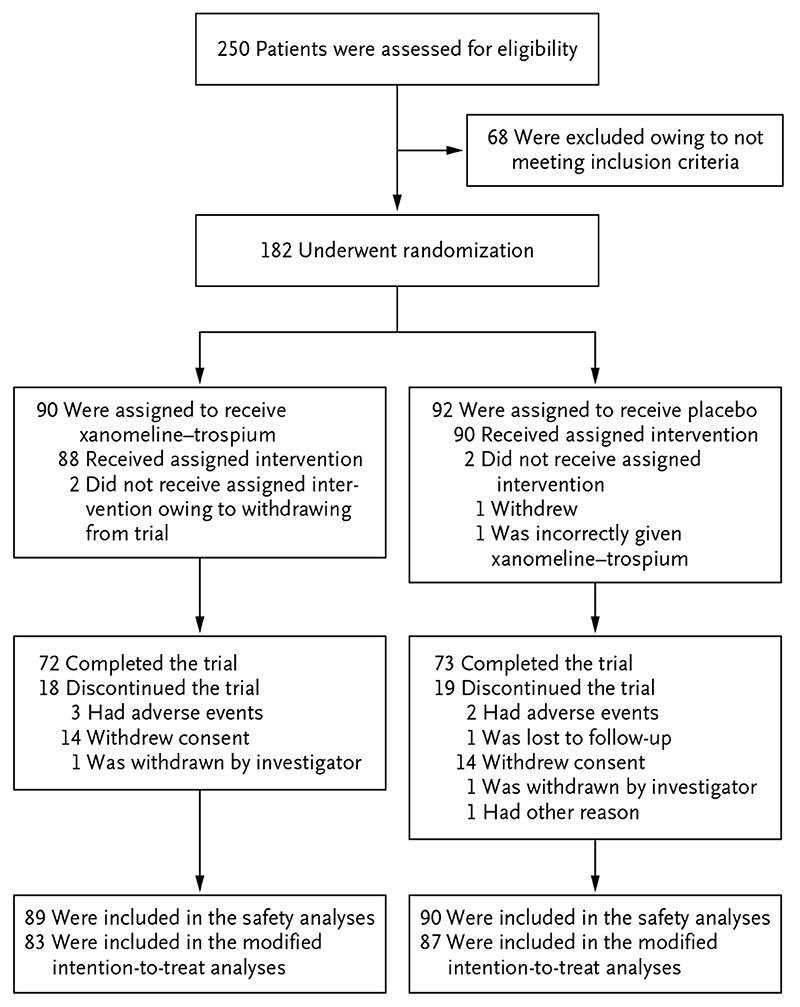
A total of 250 patients were screened, and 182 who met the enrollment criteria were randomly assigned to receive xanomeline–trospium (90 patients, 83 of whom were included in the modified intention-to-treat analysis) or placebo (92 patients, 87 of whom were included in the modified intention-to-treat analysis). The number of patients who completed the trial was 72 in the xanomeline–trospium group and 73 in the placebo group. A total of 179 patients received at least one dose of xanomeline–trospium or placebo and were included in the safety population (Fig. 1). The demographic and clinical characteristics of the patients at baseline were similar in the two trial groups (Table 1). Mean PANSS total scores at baseline were 97.7 points in the xanomeline–trospium group and 96.6 points in the placebo group.
Figure 1. Assessment, Randomization, and Analysis.
The safety population included all the patients who had undergone randomization and had received at least one dose of xanomeline–trospium or placebo. The modified intention-to-treat population included all the patients who had undergone randomization, had received at least one dose of xanomeline–trospium or placebo, and had received a Positive and Negative Syndrome Scale assessment at baseline and at least once after baseline. One patient who was assigned to receive placebo was incorrectly given xanomeline–trospium and was therefore included in the safety analyses for the xanomeline–trospium group.
Table 1. Characteristics of the Patients at Baseline (Intention-to-Treat Population).* .
| Characteristic | Xanomeline–Trospium (N = 90) |
Placebo (N = 92) |
|---|---|---|
| Age — yr | 43.4±10.1 | 41.6±10.1 |
| Male sex — no. (%) | 72 (80) | 68 (74) |
| Race — no. (%)† | ||
| Black | 67 (74) | 70 (76) |
| White | 20 (22) | 17 (18) |
| Other | 3 (3) | 5 (5) |
| Non-Hispanic or non-Latino ethnic group — no. (%)† | 71 (79) | 79 (86) |
| Body-mass index‡ | 28.1±5.0 | 29.6±5.4 |
| PANSS score§ | ||
| Total | 97.7±9.7 | 96.6±8.3 |
| Positive symptom subscore | 26.4±3.4 | 26.3±3.2 |
| Negative symptom subscore | 22.6±4.4 | 22.8±4.6 |
| Marder negative symptom subscore | 22.3±4.7 | 22.3±5.0 |
| Score on the CGI-S scale¶ | 5.0±0.6 | 4.9±0.6 |
Plus-minus values are means ±SD. The intention-to-treat population included all the patients who had undergone randomization. Percentages may not total 100 because of rounding.
Race and Hispanic or Latino ethnic group were reported by the patients.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
The total score on the Positive and Negative Syndrome Scale (PANSS) ranges from 30 to 210, and the PANSS positive symptom subscore, negative symptom subscore, and Marder negative symptom subscore each range from 7 to 49; higher scores indicate more severe symptoms of schizophrenia.
Scores on the Clinical Global Impression–Severity (CGI-S) scale range from 1 to 7; higher scores indicate greater severity of illness.
The percentages of patients reaching the highest dose of the active drug or placebo were 91% in the xanomeline–trospium group and 97% in the placebo group; 4% of the patients in the xanomeline–trospium group and 1% of those in the placebo group had a single, per-protocol reduction to a lower dose due to excessive cholinergic or anticholinergic events.
Efficacy
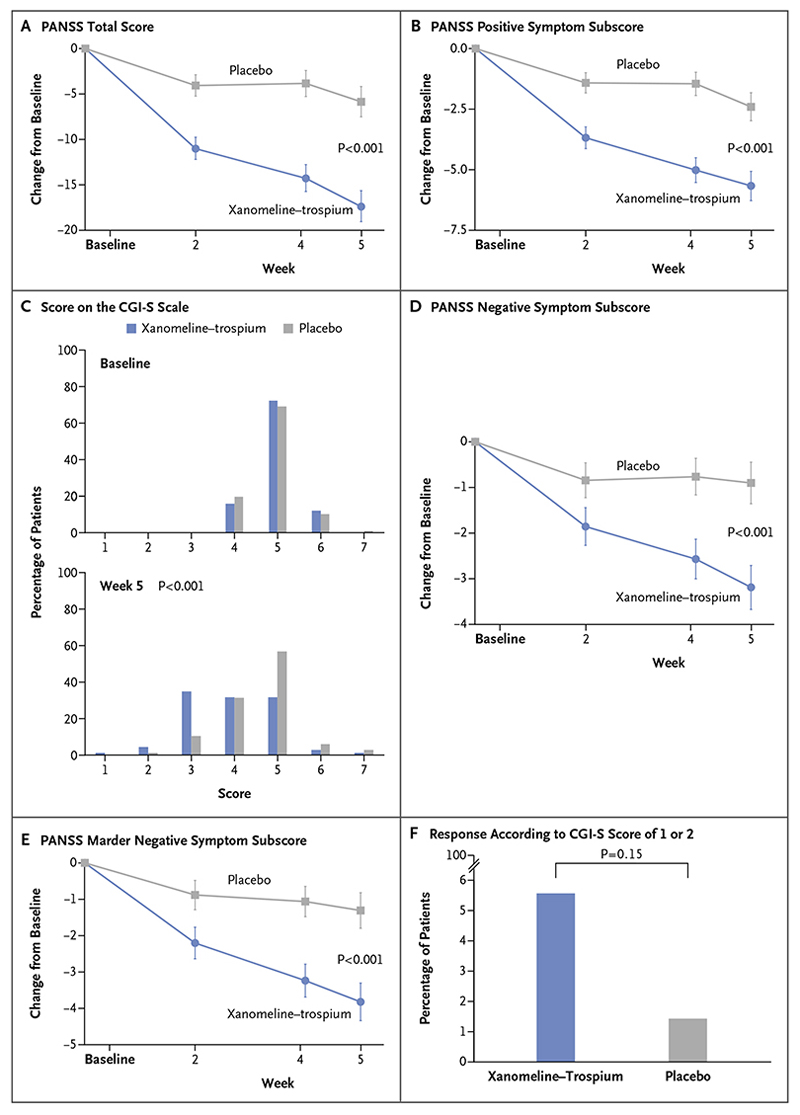
The change in the PANSS total score from baseline to week 5 was −17.4 points in the xanomeline–trospium group and −5.9 points in the placebo group (least-squares mean difference, −11.6 points; 95% confidence interval [CI], −16.1 to −7.1; P<0.001) (Table 2 and Fig. 2A). The results of sensitivity analyses of the change in the PANSS total score from baseline to week 5 were in the same direction as those of the primary analysis (Table S2).
Table 2. Efficacy Measures (Modified Intention-to-Treat Population).* .
| Efficacy Measure† | Xanomeline–Trospium (N = 83) |
Placebo (N = 87) |
Difference (95% CI) |
P Value |
|---|---|---|---|---|
| Primary end point | ||||
| Least-squares mean change from baseline in PANSS total score | −17.4±1.8 | −5.9±1.7 | −11.6 (−16.1 to −7.1)‡ | <0.001‡ |
| Secondary end points § | ||||
| Least-squares mean change from baseline in PANSS positive symptom subscore | −5.6±0.6 | −2.4±0.6 | −3.2 (−4.8 to −1.7) | <0.001 |
| Score on the CGI-S scale — % | <0.001 | |||
| 1: normal | 1 | 0 | 1 | |
| 2: borderline ill | 4 | 1 | 3 | |
| 3: mildly ill | 32 | 10 | 22 | |
| 4: moderately ill | 29 | 29 | 0 | |
| 5: markedly ill | 29 | 52 | -23 | |
| 6: severely ill | 3 | 6 | -3 | |
| 7: extremely ill | 1 | 3 | -1 | |
| Least-squares mean change from baseline in PANSS negative symptom subscore | −3.2±0.5 | −0.9±0.5 | −2.3 (−3.5 to −1.1) | <0.001 |
| Least-squares mean change from baseline in PANSS Marder negative symptom subscore | −3.9±0.5 | −1.3±0.5 | −2.5 (−3.9 to −1.2) | <0.001 |
| Response according to CGI-S score of 1 or 2 — % | 6 | 1 | 4 (-3 to 12) | 0.15 |
Plus-minus values are means ±SE. The modified intention-to-treat population included all the patients who had undergone randomization, had received at least one dose of xanomeline–trospium, and had received a PANSS assessment at baseline and at least once after baseline.
For continuous end points (PANSS total score and all PANSS subscores), the difference in least-squares mean change from baseline between the xanomeline–trospium group and the placebo group at week 5 was estimated with the use of mixed models for repeated measurements. For the score on the CGI-S scale, the difference between the xanomeline–trospium and placebo groups at week 5 was evaluated with the Mann–Whitney–Wilcoxon test. For the analysis of response according to a CGI-S score of 1 or 2, the difference between the xanomeline–trospium and placebo groups at week 5 was evaluated with the Cochran-Mantel-Haenszel test. There was no imputation of missing data for the primary end point or secondary end points.
The effect size (0.75) was calculated as the absolute value of the difference in unadjusted mean change in score from baseline at week 5 between the xanomeline–trospium group and the placebo group, divided by the pooled standard deviation of the between-group difference.
Secondary end points are presented in the hierarchical order in which they were tested in the statistical analysis plan.
Figure 2 (facing page). Efficacy End Points.
The primary end point was the change from baseline to week 5 in the total score on the Positive and Negative Syndrome Scale (PANSS; range, 30 to 210, with higher scores indicating more severe symptoms of schizophrenia) (Panel A). The prespecified secondary efficacy end points are presented in the hierarchical order in which they were tested in the statistical analysis plan: the change in the PANSS positive symptom subscore (Panel B), the score on the Clinical Global Impression–Severity (CGI-S) scale (Panel C), the change in the PANSS negative symptom subscore (Panel D), the change in the PANSS Marder negative symptom subscore (Panel E), and the percentage of patients with a response according to a CGI-S score of 1 or 2 (Panel F). CGI-S scores range from 1 to 7, with higher scores indicating greater severity of illness. The PANSS positive symptom subscore, negative symptom subscore, and Marder negative symptom subscore each range from 7 to 49, with higher scores indicating greater severity of symptoms. All efficacy analyses were conducted in the modified intention-to-treat population. Changes in efficacy in continuous variable outcome measures (Panels A, B, D, and E) were evaluated with a mixed model for repeated measures, and differences in categorical variables were evaluated with nonparametric statistical tests (Mann–Whitney–Wilcoxon test, Panel C; Cochran–Mantel–Haenszel test, Panel F).
With respect to secondary outcomes, the change from baseline to week 5 in the PANSS positive symptom subscore was −5.6 points in the xanomeline–trospium group and −2.4 points in the placebo group (least-squares mean difference, −3.2 points; 95% CI, −4.8 to −1.7; P<0.001); the categorical distribution of scores on the CGI-S scale favored xanomeline–trospium as compared with placebo (P<0.001); the change from baseline in the PANSS negative symptom subscore was −3.2 points and −0.9 points, respectively (least-squares mean difference, −2.3 points; 95% CI, −3.5 to −1.1; P<0.001); the change from baseline in the PANSS Marder negative symptom subscore was −3.9 points and −1.3 points, respectively (least-squares mean difference, −2.5 points; 95% CI, −3.9 to −1.2; P<0.001); and the percentage of patients with a response according to a CGI-S score of 1 or 2 did not differ significantly between the two groups (Table 2 and Fig. 2B through 2F).
Safety
The percentage of patients who discontinued the active drug or placebo was 20% in the xanome-line-trospium group and 21% in the placebo group, and the percentage of patients in whom adverse events occurred during the treatment period was 54% and 43%, respectively. The most common adverse events in the xanomeline–trospium group were constipation (17%), nausea (17%), dry mouth (9%), dyspepsia (9%), and vomiting (9%) (Table 3). None of these adverse events resulted in the discontinuation of xanome-line-trospium, and all were rated by site investigators as mild or moderate in severity. The incidences of nausea, vomiting, and dry mouth in the xanomeline–trospium group were highest early in the trial (11%, 6%, and 7%, respectively, at week 1) and lower at the end of the trial (3%, 1%, and 1%, respectively, at week 5) (Fig. S2). Constipation was also reported early in the trial, but the incidence remained constant through week 5. The incidences of other adverse events were similar in the xanomeline–trospium and placebo groups (Table 3).
Table 3. Adverse Events and Safety during the Treatment Period (Safety Population).* .
| Variable | Xanomeline–Trospium (N = 89) |
Placebo (N = 90) |
|---|---|---|
| Any adverse event — no (%) | 48 (54) | 39 (43) |
| Serious adverse event — no. (%)† | 1 (1) | 0 |
| Severe adverse event — no. (%)‡ | 1 (1) | 1 (1) |
| Adverse event leading to discontinuation of the active drug or placebo — no. (%) | 2 (2) | 2 (2) |
| Adverse events occurring in ≥2% of the patients in the xanomeline–trospium group — no. (%) | ||
| Constipation | 15 (17) | 3 (3) |
| Nausea | 15 (17) | 4 (4) |
| Dry mouth | 8 (9) | 1 (1) |
| Dyspepsia | 8 (9) | 4 (4) |
| Vomiting | 8 (9) | 4 (4) |
| Headache | 6 (7) | 5 (6) |
| Somnolence | 5 (6) | 4 (4) |
| Akathisia | 3 (3) | 0 |
| Dizziness | 3 (3) | 3 (3) |
| Increased weight | 3 (3) | 4 (4) |
| Tachycardia | 3 (3) | 2 (2) |
| Sedation | 2 (2) | 2 (2) |
| Diarrhea | 2 (2) | 4 (4) |
| Increased γ-glutamyltransferase level | 2 (2) | 0 |
| Agitation | 2 (2) | 1 (1) |
| Insomnia | 2 (2) | 2 (2) |
| Decreased appetite | 2 (2) | 0 |
| Hyperhidrosis | 2 (2) | 1 (1) |
| Mean change from baseline in body weight at wk 5 — kg | 1.5±2.8 | 1.1±3.5 |
| Mean change from baseline in score on Simpson–Angus Scale at wk 5§ | −0.1±0.7 | −0.1±0.8 |
| Mean change from baseline in score on Barnes Akathisia Rating Scale at wk 5¶ | −0.1±1.0 | 0.0±0.7 |
The safety population included all the patients who had undergone randomization and had received at least one dose of xanomeline–trospium or placebo. Adverse events that occurred during the treatment period were defined as those that started or worsened from the time of the first dose of xanomeline–trospium or placebo (visit 1 at day 2) to the time of discharge (visit 9 at day 35). Plus–minus values are means ±SD.
A serious adverse event was defined as any adverse event that resulted in death, was immediately life-threatening, led to inpatient hospitalization or prolongation of hospitalization, or caused persistent or clinically significant disability or incapacity.
A severe adverse event was defined as any event that was incapacitating or caused an inability to perform normal activities of daily living.
Scores on the Simpson–Angus Scale range from 0 to 40; higher scores indicate greater severity of drug-induced parkinsonian symptoms.
Scores on the Barnes Akathisia Rating Scale range from 0 to 14; higher scores indicate greater symptoms of akathisia.
An increase in weight from baseline to week 5 was reported in 3% of the patients in the xanomeline–trospium group and in 4% of those in the placebo group, and the mean (±SD) change in weight from baseline to week 5 was 1.5±2.8 kg and 1.1±3.5 kg, respectively (Table 3). The percentage of patients who had an increase of more than 7% in weight from baseline to week 5 was 2% in the xanomeline–trospium group and 6% in the placebo group, and the mean change in the body-mass index (the weight in kilograms divided by the square of the height in meters) from baseline to week 5 was 0.5±1.0 and 0.4±1.2, respectively (Table S4).
In the xanomeline–trospium group, 3% of the patients reported symptoms that were classified as akathisia by site investigators during the trial; all of these symptoms resolved during the trial without changes in the dose. The mean change from baseline to week 5 in the score on the Simpson–Angus Scale for extrapyramidal symptoms was −0.1±0.7 points in the xanomeline–trospium group and −0.1±0.8 points in the placebo group, and the mean change in the score on the Barnes Akathisia Rating Scale was −0.1±1.0 points and 0.0±0.7 points, respectively (Table 3). Elevation in the γ-glutamyltransferase concentration that was at least twice the upper limit of the normal range occurred in two patients in the xanomeline–trospium group and one in the placebo group (Table S5). The between-group difference in the change from baseline in the supine heart rate peaked at day 8 (mean change, 6.9±13.1 beats per minute [range, -18 to 43] in the xanomeline–trospium group and 1.4±10.2 beats per minute [range, -22 to 27] in the placebo group) and decreased through day 28 (mean change, 5.9±13.9 beats per minute [range, -18 to 51] in the xanomeline–trospium group and 1.5±9.6 beats per minute [range, -26 to 23] in the placebo group) (Fig. S3). Changes in blood pressure and the corrected QT interval were similar in the two trial groups (Tables S6 and S7). No patients in either group had syncope, corrected QT intervals calculated with Fridericia’s formula of more than 450 msec, or increases in corrected QT intervals calculated with Fridericia’s formula of more than 60 msec.
Discussion
In this trial involving acutely psychotic patients with schizophrenia, treatment with the M1- and M4-selective muscarinic receptor agonist xanomeline in combination with trospium was associated with greater decreases in positive and negative symptoms than was placebo over a period of 5 weeks. Xanomeline–trospium was associated with significant benefits over placebo with respect to the PANSS positive and negative symptom subscores as well as categorical CGI-S scores and the PANSS Marder negative symptom subscore at week 5, but there was no significant between-group difference in the percentage of patients with global illness severity ratings of “normal” or “borderline ill” at week 5.
Cholinergic or anticholinergic adverse events were more frequent in the xanomeline–trospium group than in the placebo group; in the xanomeline–trospium group, these adverse events began soon after the initiation of treatment. However, the percentage of patients who discontinued either xanomeline–trospium or placebo was similar in the two groups, and the number of discontinuations due to adverse events that occurred during the treatment period was equal in the two groups. The incidences of nausea, vomiting, and dry mouth decreased over the course of the trial, but the incidence of constipation remained constant throughout the trial. The resting heart rate increased more in the xanomeline–trospium group than in the placebo group. There were no syncopal events, and the incidences of weight gain, somnolence, restlessness, and extrapyramidal symptoms were similar in the two groups. Two patients in the xanomeline–trospium group and one in the placebo group had elevations in hepatic aminotransferase levels. Scores on the scales that measure extrapyramidal features were similar in the two groups.
This trial had limitations. It was conducted over a period of only 5 weeks and could not address the durability of effect of the active drug in a lifelong illness. The trial was conducted in inpatient units, which mitigated nonadherence to xanomeline–trospium or placebo, use of unapproved medications, and substance abuse. The smaller placebo response in this trial (e.g., leastsquares mean change from baseline in the PANSS total score at week 5, 5.9 points) relative to other recent trials of antipsychotic drugs in schizophrenia1,2 may relate to trial-design features, such as the inclusion of only two groups and 12 sites.30,31 Finally, our trial participants were predominantly Black owing to the mostly urban locations of the clinical trial sites in our United States–based trial, where the majority of available patients with schizophrenia are Black. As such, future trials are required to understand whether these results can be extended to more diverse populations.
A combination of the muscarinic receptor agonist xanomeline and the anticholinergic agent trospium resulted in greater reductions than placebo in the degree of psychosis according to the scores on several scales. Treatment with xanomeline–trospium resulted in cholinergic and anticholinergic adverse events but was not associated with a higher incidence of extrapyramidal symptoms or weight gain than placebo. Longer and larger trials are required to establish the efficacy and safety of xanomeline–trospium in the treatment of schizophrenia.
Supplementary Material
Acknowledgments
We thank all the patients, investigators, and staff for participation in the trial; members of the Karuna Therapeutics Clinical Operations Team for planning and managing the trial; Korie Handwerger, Ph.D., of Karuna Therapeutics Scientific Communications for critical review of an earlier version of the manuscript; and Sarah Kavanagh, M.P.H., of Kavanagh Statistical Consulting for biostatistics support.
Footnotes
Supported by Karuna Therapeutics and the Wellcome Trust (award 208970/Z/17/Z).
Dr. Brannan reports being employed by and owning stock in Karuna Therapeutics; Ms. Sawchak, being employed by and owning stock options in Karuna Therapeutics; Dr. Miller, being employed by and owning stock in Karuna Therapeutics and holding patent US10369143 on muscarinic combinations, licensed to Karuna Therapeutics; Dr. Lieberman, serving on an advisory board for Karuna Therapeutics and Intra-Cellular Therapies; Dr. Paul, serving as a board member for and owning stock in Alnylam Pharmaceuticals, Sage Therapeutics, and Voyager Therapeutics, being employed by and owning stock and stock options in Karuna Therapeutics, and owning stock options in BioXcel Therapeutics; and Dr. Breier, receiving consulting fees from BioXcel Therapeutics, receiving consulting fees and advisory board fees from and owning stock and stock options in Karuna Therapeutics, and receiving consulting fees and advisory board fees from and owning stock options in Perception Neuroscience. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Contributor Information
Jeffrey A. Lieberman, Columbia University Vagelos College of Physicians and Surgeons, New York
Steven M. Paul, Karuna Therapeutics, Boston
Alan Breier, Indiana University School of Medicine, Indianapolis
References
- 1.Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multiepisode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394:939–51. doi: 10.1016/S0140-6736(19)31135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951–62. doi: 10.1016/S0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]
- 3.Conley RR, Kelly DL. Management of treatment resistance in schizophrenia. Biol Psychiatry. 2001;50:898–911. doi: 10.1016/s0006-3223(01)01271-9. [DOI] [PubMed] [Google Scholar]
- 4.Marder SR, Cannon TD. Schizophrenia. N Engl J Med. 2019;381:1753–61. doi: 10.1056/NEJMra1808803. [DOI] [PubMed] [Google Scholar]
- 5.Lieberman JA, First MB. Psychotic disorders. N Engl J Med. 2018;379:270–80. doi: 10.1056/NEJMra1801490. [DOI] [PubMed] [Google Scholar]
- 6.Carlsson A. The current status of the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1988;1:179–86. doi: 10.1016/0893-133x(88)90012-7. [DOI] [PubMed] [Google Scholar]
- 7.Raedler TJ, Bymaster FP, Tandon R, Copolov D, Dean B. Towards a muscarinic hypothesis of schizophrenia. Mol Psychiatry. 2007;12:232–46. doi: 10.1038/sj.mp.4001924. [DOI] [PubMed] [Google Scholar]
- 8.Ghoshal A, Rook JM, Dickerson JW, et al. Potentiation of M1 muscarinic receptor reverses plasticity deficits and negative and cognitive symptoms in a schizophrenia mouse model. Neuropsychopharmacology. 2016;41:598–610. doi: 10.1038/npp.2015.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raedler TJ, Knable MB, Jones DW, et al. In vivo determination of muscarinic acetylcholine receptor availability in schizophrenia. Am J Psychiatry. 2003;160:118–27. doi: 10.1176/appi.ajp.160.1.118. [DOI] [PubMed] [Google Scholar]
- 10.Crook JM, Dean B, Pavey G, Copolov D. The binding of [3H]AF-DX 384 is reduced in the caudate-putamen of subjects with schizophrenia. Life Sci. 1999;64:1761–71. doi: 10.1016/s0024-3205(99)00114-9. [DOI] [PubMed] [Google Scholar]
- 11.Felder CC, Bymaster FP, Ward J, DeLapp N. Therapeutic opportunities for muscarinic receptors in the central nervous system. J Med Chem. 2000;43:4333–53. doi: 10.1021/jm990607u. [DOI] [PubMed] [Google Scholar]
- 12.Shannon HE, Bymaster FP, Calligaro DO, et al. Xanomeline: a novel muscarinic receptor agonist with functional selectivity for M1 receptors. J Pharmacol Exp Ther. 1994;269:271–81. [PubMed] [Google Scholar]
- 13.Thorn CA, Moon J, Bourbonais CA, et al. Striatal, hippocampal, and cortical networks are differentially responsive to the M4- and M1-muscarinic acetylcholine receptor mediated effects of xanomeline. ACS Chem Neurosci. 2019;10:3910. doi: 10.1021/acschemneuro.9b00335. [DOI] [PubMed] [Google Scholar]
- 14.Erskine D, Taylor J-P, Bakker G, Brown AJH, Tasker T, Nathan PJ. Cholinergic muscarinic M1 and M4 receptors as therapeutic targets for cognitive, behavioural, and psychological symptoms in psychiatric and neurological disorders. Drug Discov Today. 2019;24:2307–14. doi: 10.1016/j.drudis.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Bodick NC, Offen WW, Levey AI, et al. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch Neurol. 1997;54:465–73. doi: 10.1001/archneur.1997.00550160091022. [DOI] [PubMed] [Google Scholar]
- 16.Shekhar A, Potter WZ, Lightfoot J, et al. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry. 2008;165:1033–9. doi: 10.1176/appi.ajp.2008.06091591. [DOI] [PubMed] [Google Scholar]
- 17.Sanctura (trospium chloride) (package insert) Lexington, MA: Indevus Pharmaceuticals; 2012. [Google Scholar]
- 18.Staskin D, Kay G, Tannenbaum C, et al. Trospium chloride has no effect on memory testing and is assay undetectable in the central nervous system of older patients with overactive bladder. Int J Clin Pract. 2010;64:1294–300. doi: 10.1111/j.1742-1241.2010.02433.x. [DOI] [PubMed] [Google Scholar]
- 19.Kavoussi R, Miller AC, Brannan SK, Breier A. Results of a double-blind, placebo-controlled, tolerability study of KarXT, a novel combination targeting muscarinic acetylcholine receptors using xanomeline with trospium chloride to mitigate cholinergic side effects; Poster presented at American Society of Clinical Psychopharmacology Annual Meeting; Miami Beach, FL. May 29–June 2, 2017. [Google Scholar]
- 20.Brannan SK, Miller AC, Paul SM, Breier A. KarXT, a combination of the M1/M4 cholinergic receptor agonist xanomeline and trospium for the treatment of psychosis and cognitive impairment in schizophrenia: phase I studies; Presented at poster session I of the American College of Neuropsychopharmacology 57th Annual Meeting; Hollywood, FL. December 9–13, 2018. [Google Scholar]
- 21.DSM-V. 5th ed. Washington, DC: American Psychiatric Association; 2013. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- 22.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–57. [PubMed] [Google Scholar]
- 23.Kay SR, Opler LA, Lindenmayer JP. The Positive and Negative Syndrome Scale (PANSS): rationale and standardisation. Br J Psychiatry Suppl. 1989;7:59–67. [PubMed] [Google Scholar]
- 24.Haro JM, Kamath SA, Ochoa S, et al. The Clinical Global Impression-Schizophrenia scale: a simple instrument to measure the diversity of symptoms present in schizophrenia. Acta Psychiatr Scand Suppl. 2003;416:16–23. doi: 10.1034/j.1600-0447.107.s416.5.x. [DOI] [PubMed] [Google Scholar]
- 25.Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry. 1997;58:538–46. doi: 10.4088/jcp.v58n1205. [DOI] [PubMed] [Google Scholar]
- 26.Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266–77. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–9. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 28.Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–6. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- 29.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: L. Erlbaum; 1988. [Google Scholar]
- 30.Rutherford BR, Pott E, Tandler JM, Wall MM, Roose SP, Lieberman JA. Placebo response in antipsychotic clinical trials: a meta-analysis. JAMA Psychiatry. 2014;71:1409–21. doi: 10.1001/jamapsychiatry.2014.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agid O, Siu CO, Potkin SG, et al. Meta-regression analysis of placebo response in antipsychotic trials, 1970-2010. Am J Psychiatry. 2013;170:1335–44. doi: 10.1176/appi.ajp.2013.12030315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.