Abstract
Background
There is considerable interest in positive allosteric modulators (PAMs) of the α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) subtype of ionotropic glutamate receptors as therapeutic agents for a range of cognitive and mood disorders. However, the challenge is to increase AMPA receptor (AMPAR) function sufficient to enhance cognitive function but not to the extent that there are mechanism-related proconvulsant or convulsant side effects. In this present study we report the pre-clinical pharmacology data for MDI-222, an AMPAR PAM which enhances cognition but has a much reduced side-effect (i.e., convulsant) liability relative to other molecules of this mechanism.
Methods
The pharmacological effects of MDI-222 were characterised in in vitro and in vivo preclinical electrophysiology, efficacy (cognition), side-effect (proconvulsant/convulsant), tolerability and toxicity assays.
Results
We demonstrate that MDI-222 is an AMPAR PAM since it enhanced AMPAR function in vitro at human (hGluA1-4) and rat (rGluA2) homomeric receptors, and potentiated heterooligomeric AMPARs in rat neurons. MDI-222 enhanced electrically-evoked AMPAR-mediated synaptic transmission in the anaesthetised rat at 10 mg/kg (i.v.) and did not significantly lower the seizure threshold in the pro-convulsant MEST at any dose tested up to a maximum of 30 mg/kg (p.o.). MDI-222 reversed a delay-induced deficit in novel object recognition (NOR) in rats with an MED of 0.3 mg/kg (p.o.) following acute administration which was reduced to 0.1 mg/kg following sub-chronic administration; improved passive avoidance performance in scopolamine-impaired rats with an MED of 10 mg/kg p.o. On the other hand, MDI-222 was not proconvulsant in the maximal electroshock threshold test (MEST) resulting in a therapeutic window between plasma concentrations that enhanced cognitive performance and those associated with mechanism-related side effects of ≥1000-fold. Unfortunately, despite the excellent preclinical profile of this compound, further development had to be halted due to non-mechanism related issues.
Conclusions
We conclude that MDI-222 is an AMPAR PAM which enhances cognitive performance in rats and has a significantly improved safety profile in preclinical species.
Keywords: AMPA receptor, positive allosteric modulator, GluA, patch-clamp, cognition, novel object recognition
There is growing evidence to implicate a dysfunction in glutamate neurotransmission, and the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) subtype of ionotropic glutamate receptors (iGluRs) in particular, in the pathophysiology of a variety of CNS disorders (Chang et al., 2012; Lee et al., 2016; Dauvermann et al., 2017). More specifically, AMPA receptors (AMPARs) play a critical role in synaptic function since their activation by glutamate results in a depolarisation of the postsynaptic membrane which relieves the voltage-dependent Mg2+-blockade of N-methyl-D-aspartate receptors (NMDARs; Huganir and Nicoll, 2013). In addition, AMPARs are trafficked into and out of the synapse in a use-dependent manner, underscoring the importance of these receptors in synaptic plasticity (Henley and Wilkinson, 2016).
Given their importance in the physiology and pathology of synapses, it is of little surprise that AMPARs have attracted considerable attention as potential therapeutic targets (Ward et al., 2010, 2015; Partin et al., 2015; Reuillon et al., 2016). For example, a reduction in the activity of AMPARs by non-competitive antagonists such as talampanel (GYKI 537773, LY300164) and perampanel are effective in a variety of preclinical anticonvulsant models, with the latter being approved as an antiepileptic drug under the brand name Fycompa (Tsai et al., 2018). On the other hand, the well-established role of AMPAR in synaptic plasticity and the nootropic actions of the weak AMPA-potentiating effects of aniracetam (Ito et al., 1990; Tang et al., 1991) has resulted in the increase in AMPAR function being pursued as a very attractive approach to improving cognitive performance in a variety of disorders (Chang et al., 2012). In this regard, a wide range of AMPAR positive allosteric modulators (PAMs) have been described of which several from Cortex Pharmaceuticals (CX516, CX614, CX691, also known as ORG24448 and Farampator, CX717, CX1739) and individual molecules from Eli Lilly (LY451395), Organon (ORG26576), Pfizer (PF-04958242), Servier (S18986 and S47445), GSK (GSK729327) and Takeda (TAK-137 and TAK-653) have entered clinical studies. However, none have yet achieved regulatory approval with the development of these various compounds being halted for a variety of molecule- or mechanism-specific properties such as short half-life, insufficient potency, poor tolerability or inability to dose high enough to achieve sufficient exposure (Buccafusco, 2009; Reuillon et al., 2016; Purcell et al., 2018).
One of the primary concerns with modulating AMPAR function is the potential for mechanism-related proconvulsant or convulsant liability. Hence, in the same way that negative allosteric modulators of AMPARs such as Peramapanel are anticonvulsant, then so potentiating AMPARs can promote excitotoxicity and/or seizures. This is of particular concern for the so-called Class I (or high-impact) AMPAR PAMs, which alter the kinetics of AMPAR-mediated receptor deactivation and desensitization to enhance and prolong synaptic currents (Aria and Kessler, 2007). On the other hand, Class II (low-impact) AMPAR PAMs only affect deactivation and therefore have a much reduced effect on synaptic currents compared to Class I AMPAR PAMs (Aria and Kessler, 2007).
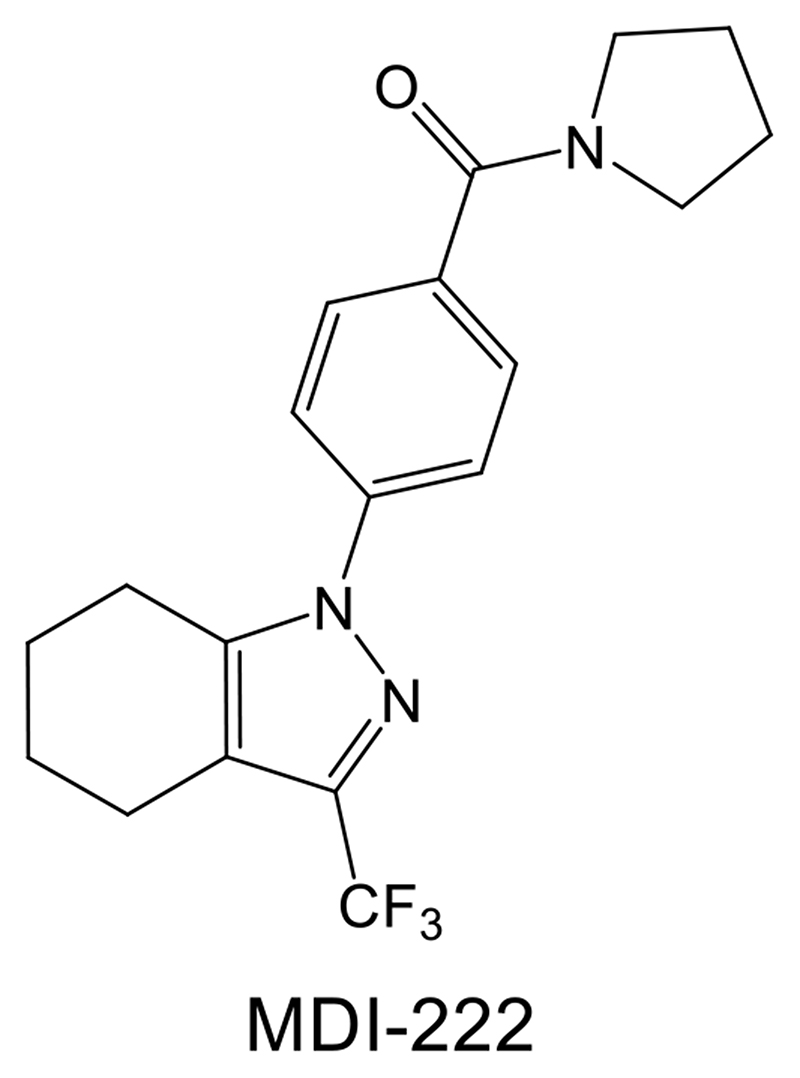
In a previous paper, we described the properties of UoS12258, a Class I AMPAR PAM which progressed into clinical studies. In single-dose (0.25-1.75 mg) studies in man this compound had a long half-life (c.130 hr) and clinical development was halted due to the risk of drug accumulation beyond the limits established by the preclinical convulsant liability in rats, dogs and monkeys (Ward et al., 2017). In the present paper, we describe the properties of MDI-222 (Ward et al., 2011), a back-up compound with a pyrazole chemotype distinct from that of the lead sulphonamide compound UoS12258 (Figure 1). Hence, MDI-222 is a Class II AMPAR PAM (Ward et al., 2011) that enhances cognition but has a much reduced convulsant liability relative to its predecessor. Unfortunately, the preclinical development of this compound was halted due to issues unrelated to the AMPAR PAM mechanism. Nevertheless, this compound serves as an example of an AMPAR PAM with a much improved safety profile relative to others within this class.
Figure 1. Structure of the AMPAR PAM MDI-222 (1-[4-(1-pyrrolidinylcarbonyl) phenyl]-3-(trifluoromethyl)-4,5,6,7-tetrahydro-1H-indazole; Compound 9a in Ward et al., 2011).
Materials and methods
The biological assay methods are described in more detail elsewhere (Ward et al., 2017). All animal studies were ethically reviewed and carried out in accordance with Animals (Scientific Procedures) Act 1986 and the GSK Policy on the Care, Welfare and Treatment of Animals.
Compound
MDI-222 (Figure 1) has a molecular weight of 363 and polar surface area of 38 Å2 and the preparation and preliminary characterization of this compound has been described previously (Ward et al., 2011). The compound has generally good brain penetration, with the ratio of brain: plasma drug concentrations in the region of 1.5 and has binding to rat, dog, monkey and human plasma protein and rat brain protein of ≥99%. The respective plasma half-life and oral bioavailability values were 1.4h and 54% in rat and 1.3 h and 18% in dog.
Intracellular Ca2+ influx assay
Homomeric human GluA1 flip isoform (hGluA1i), hGluA3i and hGluA4i and rat GluA2i AMPAR were all transiently transfected into HEK-293 cells using appropriate DNA constructs (all Q-unedited in TM2 region to allow Ca2+ permeability) and a lipofectamine 2000 system whereas hGluA2i were stably expressed in HEK293 cells. Cells were seeded into 384-well poly-D-lysine-coated plates) black with a clear bottom; Greiner) at a density of seeding 7500cell/well 2 days prior to assay.
Heteromeric hGluN1A/GluN2B NMDARs were produced by transfection of HEK-293 cells with a mixture of hGluN1A-BacMam and hGluN2B-BacMam expression vectors. Cells were incubated with 500 μM of the non-competitive NMDAR antagonist ketamine hydrochloride after which the BacMam vectors were washed away and cells were plated onto poly-D-lysine coated black/clear plates at a density of 20,000 cells/well, again in the presence of 500 μM ketamine, and incubated for 24 h prior to the assay. Homomeric hGluK1 kainate receptors were produced by the transient transfection of HEK-293 cells with human GRIK1 (Q/unedited) using the lipofectamine method. Human GluK2 receptors were stably expressed in HEK cells. Cells were seeded into 384-well plates at a density of 7,500 cells/well 24 h prior to assay.
The general method for intracellular Ca2+ measurements was that on the day of the assay, cells were washed 3-times with assay buffer (in mM; 20 HEPES, 145 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 5.5 glucose, pH 7.3 with NaOH) after which 20 μL was added to each well. Twenty-μL of Fluo-4AM were then added to each well (final assay concentration = 2 μM; Molecular Probes) and plates were incubated for 60 min in the dark. Cell plates were then washed 3-times to remove unloaded Fluo-4 and 30 μL of assay buffer were added to each well, compound added in a 1:3 serial dilution using a dual 10 μL compound + 10 μL agonist (glutamate, final assay concentration = 100 μM) addition protocol. Fluorescence intensity was measured using a Fluorescent Imaging Plate Reader (FLIPR) instrument and LY404187 was included in each plate as a reference compound.
Recombinant receptor electrophysiology
hGluA2 cells were plated onto poly-D-lysine coated microscope slide coverslips (BD Biosciences) at a density of 50-75,000 cells/mL and used 1-2 days later. Whole-cell currents were recorded using the perforated patch-clamp technique with an EPC9 or EPC10 patch-clamp system and the Pulse program (HEKA). External and internal recording solutions comprised (in mM), 140 NaCl, 2 KCl, 1 MgCl2, 2 CaCl2, 10 glucose and 12 HEPES, pH 7.35 and 150 CsCl, 10 EGTA and 10 HEPES, pH 7.3, respectively. Patch pipettes had resistances of 2-5 MΩ and solution exchange was achieved by using the RSC160 (Biologic) fast perfusion system to produce a rapid application of glutamate. Cells were clamped at -60 mV and the current induced by a 2 s application of agonist was recorded and agonist application was repeated every 30 s. in the absence or presence of test compound. Compound responses were related to the current produced by 1 mM alone.
Native receptor electrophysiology
Embryonic rat brains were harvested following sacrifice of the pregnant female by CO2 inhalation. The dissected hippocampi were placed into ice-cold medium comprised of: Hank’s Balanced Salt Solution (HBSS; Ca2+-and Mg2+-free); pyruvate, 1 mM; penicillin, 100 mg/mL; streptomycin, 100 mg/mL; HEPES, 10 mM; NaHCO3, 0.035% and tissue was then trypsinised for 30 min at 37 °C in trypsin/EDTA diluted (final concentration 0.05%) in HBSS with sodium pyruvate (Ca2+-and Mg2+-free). Tissue pieces were physically dissociated and neurons were plated onto poly-D-lysine coated coverslips in plating medium (Neurobasal Medium + 1 mM sodium pyruvate; penicillin, 100 mg/mL; streptomycin, 100 mg/mL; B27 supplement 1x; L-glutamine, 1 mM) and cells were used for recordings after 5 to 13 days in culture.
Whole-cell voltage-clamp recordings from rat cultured hippocampal neurons were made using standard methods and an Axopatch 200B amplifier and pClamp 8.0 software (Axon Instruments) with cells being held at -70 mV throughout. Solution exchange was achieved using a fast-step 2 tube perfusion system (Biologic RSC 160). Inward currents induced by AMPA application were complex in that their magnitude and duration depended on three different mechanisms: (1) opening of the AMPA receptor channel measured in terms of peak amplitude, (2) desensitization of AMPA receptors measured as the degree of relaxation of the inward current from its initial peak amplitude to a reduced steady state plateau level in the continued presence of AMPA and (3) deactivation of AMPA receptors measured as the rate at which the inward current decayed back to baseline following termination of the AMPA application. For each of these parameters, the effects of MDI-222 were compared to the effects of AMPA alone.
In vivo electrophysiology
Surgery was performed on adult male Lister hooded rats (240-340 g) under isoflurane anaesthesia and involved cannulating the jugular vein using Portex tubing such that the cannula projected externally from the animal. Each rat was then placed in a stereotaxic frame with body temperature being maintained using a thermostatically-controlled heated blanket and rectal probe. A midline incision was made to expose the dorsal aspect of the skull and a burr hole made to allow implantation of a concentric bipolar stimulating electrode (World Precision Instruments™) into the medial perforant pathway (from Lambda: AP 0 mm, ML 4.0-4.2 mm, DV ~2.5-2.8 mm). A small craniotomy was made and the dura reflected to allow a tungsten recording electrode (0.1 MΩ, World Precision Instruments™) to be implanted into the ipsilateral dentate gyrus (from Bregma: AP -3.6 mm, ML 2.0-2.55 mm, DV ~2.5-3.0 mm).
Recordings were made with reference to a silver ground electrode placed under the skin margin and potentials were amplified and filtered using a Neurolog™ extracellular AC recording system (gain 200; filters 0.1 Hz to 3 kHz). A linear constant stimulus isolator driven by Spike2 software and a CED pulse shaper was used to deliver electrical stimuli (<50% maximum; duration 90-200 μs) through the concentric bipolar stimulating electrode at either 0.1 or 0.03 Hz. Stable field excitatory postsynaptic potentials (fEPSPs) and superimposed population spikes were recorded in response to each stimulus with a paired-pulse stimulation paradigm being used to verify electrode placement. Once stable responses were established, a baseline set of responses was recorded for a 20 min period after which vehicle (99% PEG200/1% DMSO doses at 1 mL/kg) or MDI-222 were administered via the jugular vein cannula and recordings made for a 20 min period. The study was then terminated and blood and brain samples were collected and frozen at -80oC prior to subsequent analysis of drug concentrations using liquid chromatography/tandem mass spectrometry (LC-MS/MS) using a Quattro Premier (Micromass) mass spectrometer with positive-ion electrospray ionisation.
Novel object recognition
Rats were habituated to Perspex test arenas (42 cm length x 21 cm wide x 20 cm high) for 60 min (am) and 3 min (pm) on two consecutive days prior to testing. Rats were sham dosed with vehicle prior to both 1 h habituation sessions. On the first test day, rats were placed in a test arena for a 3 min habituation period and then presented with 2 identical objects (T1, 3 min). 24 h later rats were placed back in the test arena and presented one of the previous (familiar) objects and a novel object (T2, 3 min). Objects used were similar sized black plastic tower and pyramid shapes (approximately 6.5 cm high x 6 cm wide). Objects were cleaned with 70% ethanol between animals to remove any odour trace. The novel object and novel object side (left or right) was randomised equally across all treatment groups. The time spent exploring the objects during T1 and T2 was recorded by an observer blind to the novel object.
Rats (n=12/group) received MDI-222 or vehicle (1% methylcellulose) by oral gavage (p.o.) unless stated otherwise. For the acute study MDI-222 was administered at 0.1, 0.3, 1 and 3 mg/kg p.o. 30 min prior to testing in both T1 and T2 sessions. For the sub-chronic study MDI-222 was administered at 0.03, 0.1, 0.3 and 1 mg/kg p.o. once daily for 7 days and then 30 min prior to T1 and T2 sessions on days 8 and 9 respectively. Immediately after T2 behavioural testing, rats (n = 3 for all treatment groups) were culled and blood and brains taken for pharmacokinetic analysis as described previously.
The key parameters derived from the NOR assay are the T1 and T2 times, which are the total time spent exploring both objects during Trial 1 and Trial 2, respectively. The D1 index is the time spent exploring the novel object during Trial 2 minus the time spent exploring the familiar object during Trial 2 whereas the D2 index is the D1 index/total exploration time during Trial 2 (T2). Of these different parameters, the D2 index is used as the primary indicator of an ability to “remember” the objects experienced during Trial 1. Hence, a larger D2 index in drug-treated relative to vehicle-treated animals represents an increased ability to “remember” the objects experienced during Trial 1.
Passive avoidance
The passive avoidance task (PA) records the ability of animals to remember an aversive stimulus. Performance is impaired by post-training treatment with the muscarinic antagonist, scopolamine. Male Wistar rats were pre-treated with MDI-222 30 min prior to training (3, 10 and 30 mg/kg, p.o. in 25% labrasol, n = 6). Scopolamine (1 mg/kg, i.p.) was administered 6 h post training. Task recall was assessed 24 h post training in the absence of drug.
Maximal electroshock threshold (MEST) test
Half an hour before testing, male Lister Hooded rats (118-145 g; n=12/group) received i.p. injections of either saline or the known pro-convulsant, picrotoxin (2 mg/kg i.p.), or p.o. doses of either vehicle (1% methylcellulose) or 3, 10 or 30 mg/kg MDI-222. During the testing phase, the induction of a tonic hind limb extensor seizure was assessed following a 0.1 second shock administered via corneal electrodes according to the ‘up and down’ method (Kimball et al., 1957). A separate (satellite) group of animals (n=2/3 per dose) were dosed and blood and brain samples taken at the appropriate pre-treatment time for analysis of compound.
Results
MDI-222 potentiates Ca2+ flux in recombinant homomeric AMPARs
In cells stably-expressing hGluA2 AMPAR, the application of glutamate (100 μM) alone did not elicit a signal due to rapid AMPAR desensitisation. However, in the presence of MDI-222, the application of glutamate consistently increased intracellular Ca2+ levels to a maximum extent (123 ± 7%) comparable to the positive control (LY404187; Table 1). The effect of MDI-222 in hGluA2 AMPAR was concentration-dependent and had a pEC50 of 5.3 (EC50 = 5.0 μM) that was comparable to UoS12258 (pEC50 = 5.19, EC50 = 6.5 μM; Ward et al., 2017).
Table 1. Potentiation by glutamate-induced Ca2+ flux in homomeric assemblies of different AMPAR subtypes.
| AMPAR subtype | pEC50 (n) | Max. % response (n) a |
|---|---|---|
| hGluA1i b | 4.6, 4.7 (2) | 30 ± 2 (4) |
| hGluA2i | 5.3 ± 0.1 (9) | 123 ± 7 (9) |
| hGluA3i | 4.5 ± 0.1 (3) | 53 ± 3 (3) |
| hGluA4i | 5.1 ± 0.1 (4) | 76 ± 1 (4) |
| rGluA2i | 5.4 ± 0.1 (4) | 76 ± 8 (4) |
the maximum response is defined in terms of the positive control LY404187
MDI-222 was tested 4 times and gave a consistent max response (30 ± 2%) but in only 2/4 assays was it possible to reliably calculate pEC50 values.
Values shown are mean ± standard error of the mean
MDI-222 had comparable effects at all four homomeric human AMPAR subtypes (i.e., GluA1, GluA2, GluA3 and GluA4) and showed no marked differences between the rat and human GluA2 AMPARs (Table 1). There were no effects of MDI-222 on glutamate-induced currents in cell lines expressing either GluN1A/GluN2B-containing NMDARs or GluK1 or GluK2 kainate receptors measured either using the Ca2+ flux assay or whole-cell patch clamp electrophysiology (data not shown). The selectivity of MDI-222 for AMPAR was further emphasized by the fact that when tested at a concentration of 10 μM, it demonstrated no significant binding to a panel of 79 different receptors, ion channels and transporters (CEREP).
MDI-222 potentiates whole-cell glutamate-induced currents in hGluA2 AMPARs
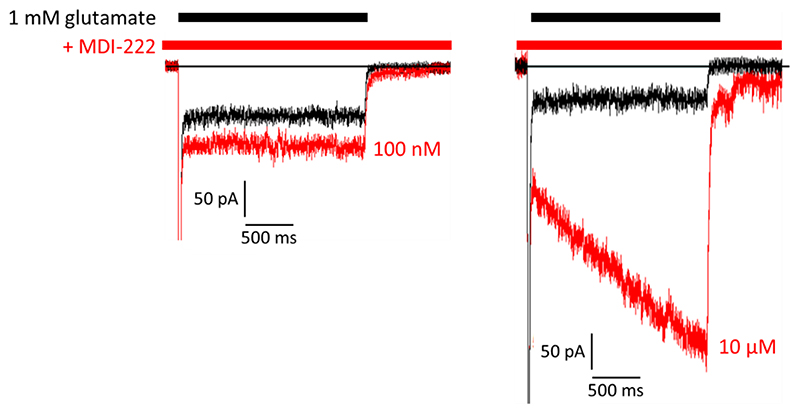
The functional effects of MDI-222 on hGluA2 AMPARs were further assessed using whole-cell voltage-clamp electrophysiology to measure potentiation of inward currents evoked by 1 mM glutamate (Figure 2). MDI-222 significantly potentiated glutamate-evoked charge transfer (area under the curve) by 121 ± 7% (P<0.05 paired t-test, n = 9) at 100 nM and 702 ± 40% at 10 μM (P<0.05, paired t-test, n = 3).
Figure 2. Representative traces showing glutamate (1 mM)-induced currents recorded in the absence and presence of 100 nM and 10 pM MDI-222 (100 nM and 10 pM) in HEK cells stably expressing human GluA2 receptors.
MDI-222 potentiates AMPAR-mediated responses in native rat AMPARs
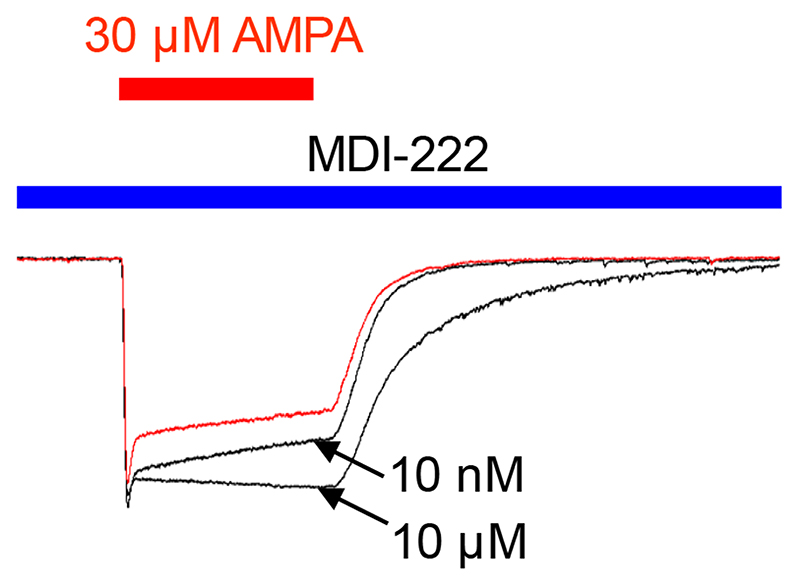
The effect on native neuronal AMPARs was assessed by measuring the effects of 1 nM to 10 μM MDI-222 on AMPA-induced whole cell currents (30 μM, 2 s pulses every 30 s) recorded from rat cultured hippocampal neurons. Representative current traces from a neuron incubated with AMPA alone or AMPA plus 10 nM or 10 μM MDI-222 are overlaid in Figure 3.. The potentiation of AMPA-induced currents was quantified in terms of the effects of MDI-222 on total charge transfer (essentially the area under the curve), and the rates of desensitisation and deactivation. These parameters are summarised in Table 2 which clearly shows a significant but modest effect on charge transfer and desensitisation at minimum effective concentrations of 1 and 10 nM, respectively. However, there was no effect on deactivation except at the highest concentration tested (10 μM). As regards total charge transfer, 10 μM MDI-222 gave only a modest (62%) increase in the charge transfer.
Figure 3. Representative traces showing the potentiation of a 30 μM AMPA-induced current by 10 μM and 10 pM MDI-222 in cultured isolated rat hippocampal neurons. Importantly, when applied in the absence of AMPA, MDI-222 did not invoke an inward current, indicating that it needed the presence of agonist to be effective.
Table 2. Effects of MDI-222 on various aspects of the AMPA-mediated inward current measured in isolated rat hippocampal neurons.
| Conc. MDI-222 (no. of cells) | Responses, normalised to 30 pM AMPA alone | ||
|---|---|---|---|
| Charge transfer | Desensitisation | Deactivation | |
| 1 nM (5) | 1.14 ± 0.05 * | 0.77 ± 0.10 | 1.06 ± 0.04 |
| 10 nM (4) | 1.37 ± 0.09 * | 0.81 ± 0.05 * | 1.19 ± 0.15 |
| 100 nM (4) | 1.33 ± 0.12 * | 0.76 ± 0.03 * | 1.29 ± 0.17 |
| 1 μM (4) | 1.28 ± 0.14 * | 0.72 ± 0.04 * | 1.33 ± 0.17 |
| 10 μM (9) | 1.62 ± 0.15 * | 0.25 ± 0.08 * | 2.79 ± 0.43 * |
Statistically significant changes compared to AMPA alone (* P<0.05 paired t-test). Values shown are mean ± standard error of the mean.
MDI-222 potentiates the dentate gyrus population spike amplitude in anaesthetised rats
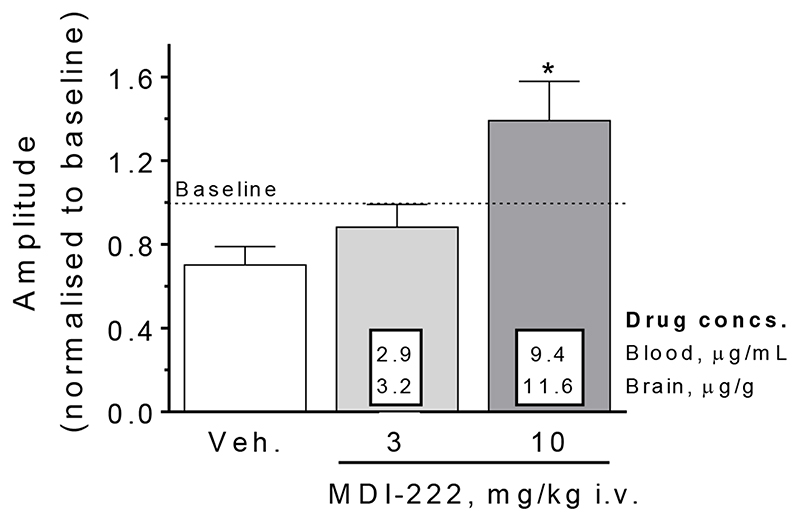
In order to demonstrate that MDI-222 could modulate electrophysiological activity in vivo, anaesthetised rats were given either 3 or 10 mg/kg compound i.v. and the amplitude of the electrically-evoked dentate gyrus population spike was measured. Figure 4 shows that a 10 (but not 3) mg/kg i.v. dose of MDI-222 was able to significantly increase population spike amplitude from 70% of baseline in the vehicle-treated animals to 139% of baseline (P<0.05, n=3). Analysis of brain samples taken immediately after the experiment showed mean brain drug concentrations of 11.6 μg/g.
Figure 4. MDI-222 (10 mg/kg i.v.) was able to increase the amplitude of the electrically-evoked population spike recorded in the dentate gyrus of anaesthetised rats (the 30% reduction in amplitude within the vehicle group relative to baseline is presumed to be related to the i.v. injection). Veh. = vehicle (99% PEG200/1% DMSO). Values shown are mean ± standard error of the mean (n=3/group).
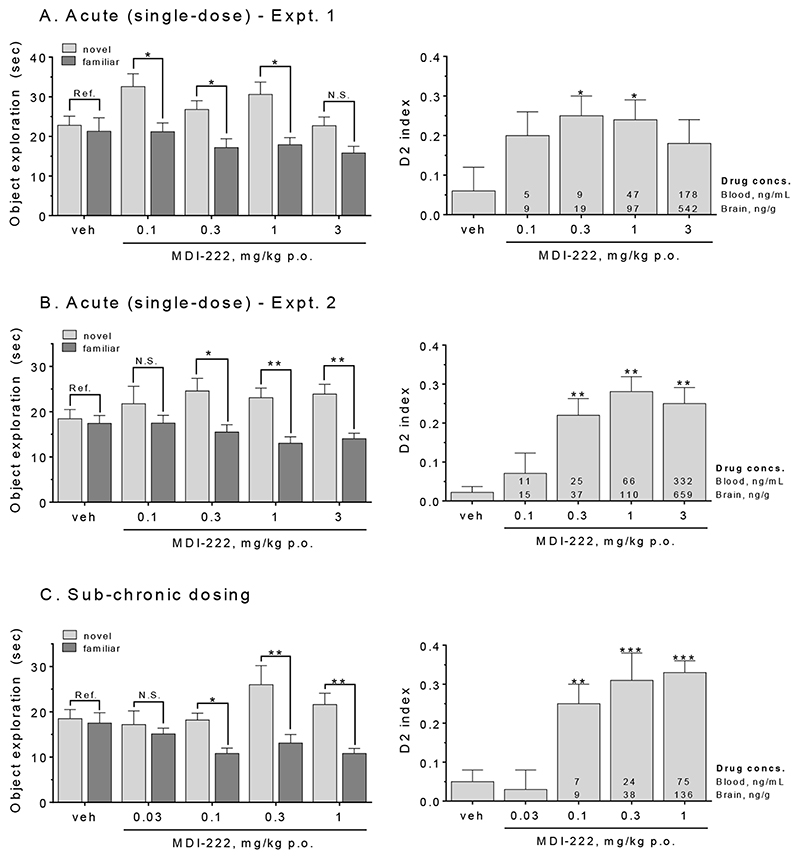
Novel object recognition
Two separate acute, single-dose administration studies of the effects of MDI-222 in the novel object recognition assay were conducted. In Experiment 1, doses of 0.1, 0.3 and 1 (but not 3) mg/kg p.o. MDI-222 resulted in a significant increase in the time spent exploring the novel relative to familiar objects (Figure 5A, left-hand panel). This translated into a significant increase the D2 index at 0.3 and 1 mg/kg (but not 0.1 or 3) mg/kg (Figure 5A, right-hand panel). In Experiment 2, p.o. doses of 0.3, 1 and 3 mg/kg all produced a significant increase both in the exploration of the novel relative to familiar object as well as the D2 index (Figure 5B). At the minimal effective dose for the D2 index of 0.3 mg/kg, the respective mean blood and brain drug concentrations were 9 ng/mL and 16 ng/g for Experiment 1 and 25 ng/mL and 37 ng/g for Experiment 2, with the differences between experiments presumably reflecting a variability in the formulation of the compound that was a consequence of its poor solubility.
Figure 5. MDI-222 enhanced performance in the novel object test of recognition memory.
A. (Experiment 1) and B (Experiment 2). After acute (single) doses, 0.3 and 1 mg/kg p.o. MDI-222 significantly increased the time spent exploring the novel compared to familiar object (left-hand panel) as well as the D2 index (right hand panel). C. MDI-222 also improved performance following sub-chronic (7-day) dosing, with the minimal effective dose being 0.1 mg/kg p.o. Values shown are mean ± SEM (n=11/18/group). *, ** = P<0.05 and 0.01 for within group exploration of familiar versus novel object (left-hand panels) or D2 index score relative to vehicle-treated animals (right-hand panels).
When administered sub-chronically for 7 days, MDI-222 significantly increased the exploration of the novel versus familiar object at doses of 0.1, 0.3 and 1 mg/kg p.o. with these same three doses also significantly increasing the D2 index (Figure 5C). At the minimally effective dose of 0.1 mg/kg, mean blood and brain levels were 7 ng/mL and 9 ng/g, respectively.
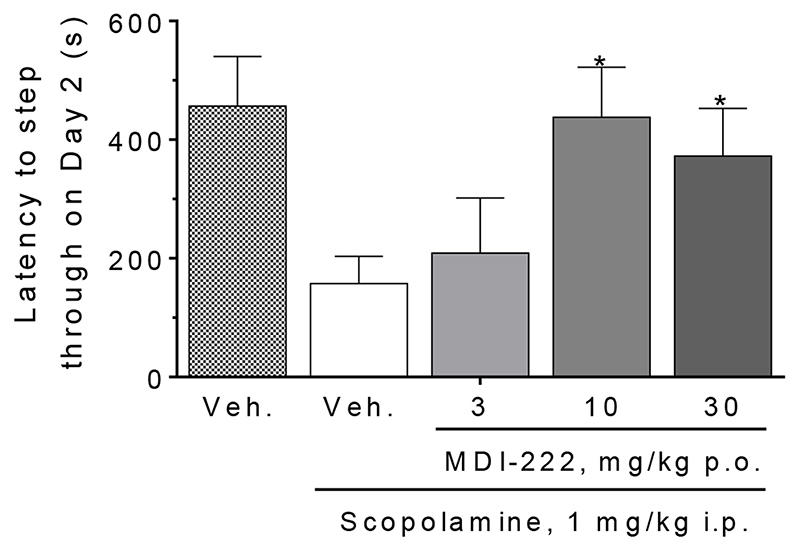
MDI-222 reverses a scopolamine-induced deficit in passive avoidance
Compared to vehicle-treated animals, scopolamine (1 mg/kg i.p.) produced a much shorter latency for rats to step through from the safe to the aversive chamber (Figure 6; respective latencies for vehicle- and scopolamine-treated rats = 457 ± 83 and 157 ± 46 s), indicating that scopolamine impairs the “remembering” of the conditioning trial on Day 1. Doses of 10 and 30 mg/kg p.o. MDI-222 were able to significantly reverse the scopolamine-induced deficit. These effects could not be attributed to changes in exploratory behaviour since on either the training or recall day, activity in the open field was not altered by drug treatment (data not shown). Accordingly, in this assay, the MED of MDI-222 was 10 mg/kg p.o.
Figure 6. MDI-222 reversed a scopolamine-induced deficit in performance in the rat passive avoidance assay.
Vehicles used were saline (scopolamine, given i.p.) and 1% methyl cellulose (MDI-222). Values shown are mean ± standard error of the mean (n=6/group). *, P<0.05 versus the veh. + scopolamine group.
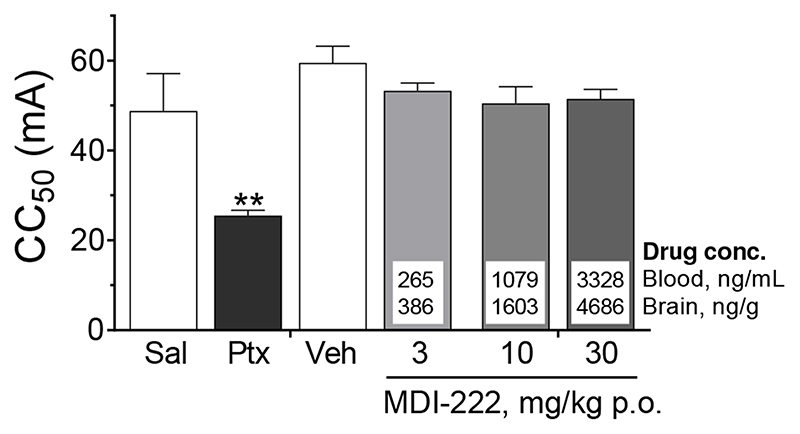
MDI-222 is not proconvulsant in the maximal electroshock seizure threshold (MEST) test
In the MEST test, corneal application of electrical current (CC50 approx. 60-70 mA, 0.1 ms duration) in the rat induces tonic and full tonic-clonic seizures. As expected, the GABAA receptor blocker picrotoxin produced a robust proconvulsant effect, reducing the CC50 by almost half relative to saline-treated controls (CC50 values of 49 ± 8 and 26 ± 1 for saline and picrotoxin-treated animals respectively; Figure 7). In contrast, MDI-222 did not significantly affect the seizure threshold, with CC50 values (53 ± 2, 51 ± 4 and 52 ± 2 at 3, 10 and 30 mg/kg p.o., respectively) being between 10-15% lower than vehicle (1% methylcellulose)-treated rats (CC50 = 60 ± 4). This lack of effect of MDI-222 was despite excellent brain exposures (measured in a satellite group of animals), with the highest dose tested, 30 mg/kg p.o., resulting in brain drug concentrations of around 4,700 ng/g.
Figure 7. MDI-222 does not reduce seizure threshold in the maximal electroshock seizure threshold test.
MDI-222 was dosed orally in a 1% methylcellulose vehicle with a pre-treatment time of 0.5 h prior to testing. In these studies, the effects of picrotoxin (Ptx, 2 mg/kg i.p.) versus animals treated with saline (Sal) were used as a positive control. Values shown are mean ± standard error of the mean (n=12/group) with a satellite group of animals (n=2-3/dose) being dosed in order to generate blood and drug concentrations. **, P <0.01 compared to vehicle-treated animals.
Discussion
MDI-222 is a relatively weak and non-selective potentiator of the human AMPAR as measured using the Ca2+ flux assay (pEC50 values ranging from 4.5 to 5.4 across the hGluA1, hGluA2, hGluA2 and hGluA2 subtypes). Moreover, there were no human-to-rat species differences since the pEC50 values of MDI-222 at hGluA2 and rGluA2 were comparable (pEC50 values of 5.3 and 5.4, respectively). The compound did not have activity at other members of the ionotropic glutamate receptor (iGluR) family, demonstrating no effects on GluN1a/GluN2B heteromeric NMDARs or homomeric GluK1 or GluK2 receptors in either the Ca2+ flux or electrophysiological assays. In addition, MDI-222 did not bind a panel of 70 different receptors, ion channels and transporters, further emphasising its selectivity for AMPARs. MDI-222 was the back-up compound to the lead molecule UoS12258 (Ward et al., 2017). This latter compound was also a relatively weak AMPAR PAM, having a pEC50 of 5.6 in both the Ca2+ flux and whole-cell patch clamp assays but was much more potent that MDI-222, with, for example, the maximal potentiation in the charge transfer (i.e., area under the curve) in rat primary hippocampal neurons being 62% for MDI-222 (Table 2) and 310% for UoS12258 (Ward et al., 2017).
The apparently modest in vitro AMPA PAM efficacy of MDI-222 nevertheless translated into a significant functional effect in vivo in that MDI-222 was able to potentiate the dentate gyrus population spike amplitude in anaesthetised rats (Figure 3) and enhanced cognitive performance in the NOR assay, with minimum effective doses of 0.3 and 0.1 mg/kg p.o. when dosed acutely and sub-chronically, respectively (Figure 4). The MED for MDI-222 in the acute-dosing NOR assay is the same as that obtained for the “more potent” UoS12258 (0.3 mg/kg p.o.). However, the MED of UoS12258 in the NOR following sub-chronic dosing (0.01 mg/kg p.o.; Ward et al., 2017) was appreciably lower than that seen with MDI-222 (0.1 mg/kg p.o.). On the other hand, in the rat passive avoidance assay, both MDI-222 and UoS12258 were able to reverse a scopolamine-induced deficit with similar MED values (10 and 3 mg/kg p.o., respectively). Hence, despite their differences in intrinsic efficacy, both MDI-222 (a low-impact AMPAR PAM) and UoS12258 (a high-impact AMPAR PAM) have a broadly similar potency with respect to cognitive enhancement. In contrast, the compounds had markedly different effects in the MEST assay of proconvulsant activity. Hence, MDI-222 had no effect on the seizure threshold, even at a dose (30 mg/kg p.o.) that gave excellent brain exposure with drug concentrations of approximately 4.7 μg/g (Figure 7). In contrast, UoS12258 produced a marked reduction in the threshold such that at a dose (100 mg/kg p.o.) giving a brain exposure of around 5.0 μg/g, the proconvulsant effect was comparable to that of the positive control picrotoxin (Ward et al., 2017).
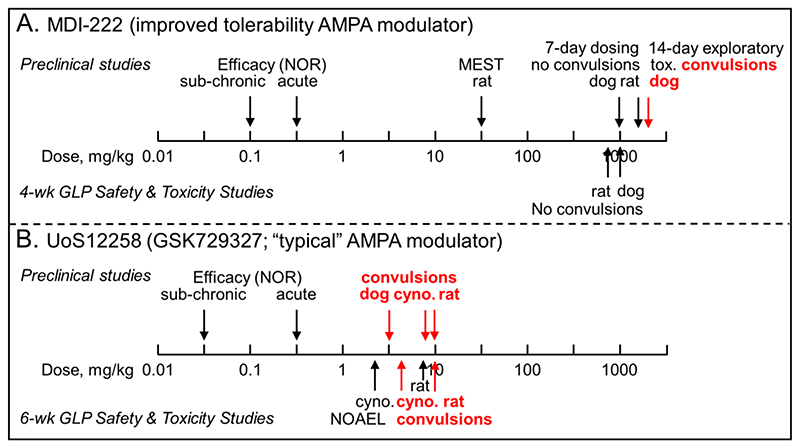
Despite possessing a proconvulsant activity in the MEST assay UoS12258 progressed into preclinical and clinical development, since at the time this was considered to be an inherent, mechanism-related side effect that could not be separated out from the cognition-enhancing effects. Furthermore, in 6-week GLP preclinical Safety and Toxicity studies, UoS12258 demonstrated convulsant activity in all three species studied (rat, dog and cynomolgus monkey) with the no adverse effect levels being defined at 2.5 and 3 mg/kg p.o. for cynomolgus monkey and rat, respectively (Figure 8). Thereafter, UoS12258 was evaluated in single-dose (0.25-1.75 mg) Phase 1 studies in man where further development was halted due to a prolonged half-life (~130 h), which raised the possibility of drug accumulation to levels beyond those considered acceptable from a safety point of view.
Figure 8. Comparison of the preclinical safety profiles showing the much safer profile of MDI-222 compared to UoS12258.
A. MDI-222 was not proconvulsant in the maximal electroshock threshold test (MEST) assay at a dose of 30 mg/kg p.o. and neither did it cause convulsions during 7-day dose ranging studies (maximum doses in rat and dog = 1,600 and 1,000 mg/kg p.o., respectively) or 4-week GLP Safety and Toxicity studies (maximum doses in rat and dog = 800 and 1,000 mg/kg p.o., respectively). To overcome solubility-limiting sub-proportional concentrations at higher doses, 14-day exploratory toxicity studies were conducted using a nanomilled formulation. In plasma drug concentrations equivalent to a dose of ~2,000 mg/kg of the standard formulation, convulsions were observed. B. UoS12258 was proconvulsant in the MEST assay at a dose of 10 mg/kg p.o. (data not shown for clarity; Ward et al., 2017) and during dose-range finding studies caused convulsions in dogs, cynomolgus (cyno.) monkeys and rats at doses of 3, 9 and 10 mg/kg p.o., respectively. Because of the sensitivity of dogs, the cynomolgus monkey was chosen as the second species, along with rat, for 6-week GLP Safety and Toxicity studies in which convulsions were observed at doses of 4 and 10 mg/kg in cynomolgus monkeys and rats, respectively with the no adverse effect levels (NOAEL) being 2.5 and 7.5 mg/kg, respectively. For both compounds, the efficacy assay used to define the therapeutic margin was the novel object recognition (NOR) assay. Doses shown are mg/kg p.o.
In contrast to UoS12258, MDI-222 had a much more benign preclinical safety profile. Specifically, and consistent with its lack of a proconvulsant liability in the MEST assay, in 7-day dose-range finding study, doses of up to 1,600 mg/kg in rat (corresponding to plasma Cmax concentrations of around 15,000 ng/mL) and 1,000 mg/kg in dog (4-8,000 ng/mL, depending on gender) were well tolerated and did not produce convulsions (Figure 8). There were also no convulsions observed in the subsequent 4-week, GLP safety assessment studies in both rat or dog even at the highest doses tested (800 mg/kg in rat and 1,000 mg/kg in dog, corresponding to respective plasma Cmax concentrations of 13-17,000 and 8-13,000 ng/mL).
During the 4-week safety assessment studies, systemic exposures on Day 28 relative to Day 1 were up to 6-fold lower in rat and 9-fold lower in dog, and non-dose-proportional systemic concentrations were observed at elevated doses. To improve systemic concentrations and investigate the potential metabolic induction, an additional 14-day exploratory toxicity investigation was conducted. In these studies, dogs were dosed with a nanomilled formulation of MDI-222 that produced a plasma exposure (Cmax = 23,000 ng/mL) equivalent to around 2,000 mg/kg of the “standard” formulation and convulsions were observed.
Given that the minimal effective dose of MDI-222 in the NOR assay is either 0.3 mg/kg p.o. when dosed acutely (corresponding to plasma drug concentrations of 9 and 25 ng/mL in Expts. 1 and 2, respectively) or 0.1 mg/kg p.o. when given sub-chronically (plasma concentration = 7 ng/mL), then there is a very large, ≥1,000-fold therapeutic index between plasma concentrations required for efficacy (7-25 ng/mL) and those shown to cause convulsions in dogs (23,000 ng/mL). Despite this very attractive safety profile, further development of MDI-222 was halted due to nonmechanism-related developability issues. These included poor solubility, high protein binding and an activation of the pregnane X receptor linked to a potential for drug-drug interactions.
In summary, MDI-222 is an AMPAR PAM which produced cognition enhancing effects in rats with a significantly improved tolerability profile over its predecessor UoS12258 and other molecules of this class. Although further development of MDI-222 was not possible, these studies nevertheless demonstrate the feasibility of dissociating the desirable cognition enhancing effects from the unwanted proconvulsant or convulsant liabilities that are often considered to be inherent in compounds that enhance AMPAR-mediated neurotransmission. The dissociation between the cognition enhancing and proconvulsant/convulsant effects as a consequence of increasing AMPAR function has recently been also demonstrated with additional compounds (Kunugi et al., 2019) and suggests that the therapeutic potential of AMPAR PAMs may finally achieve clinical utility, not only in diseases associated with cognitive dysfunction, but also other disorders associated with a dysfunction of glutamatergic neurotransmission and more specifically AMPARs, such as depression (Zanos et al., 2016).
Acknowledgements
We thank and acknowledge the wider team of scientists that were involved with this work at GlaxoSmithKline.
Funding
The work described in this article was funded by GlaxoSmithKline.
Footnotes
Declaration of conflicting interests
All authors are or have been employees and shareholders of GlaxoSmithKline. SW is grant holder of Wellcome Trust funded project Transforming the treatment of schizophrenia: Design and development of AMPA receptor modulators with a much improved safety profile as novel drugs for treating the cognitive dysfunction associated with schizophrenia and other CNS disorders grant WT-103096/Z/13/Z. The authors declare no other potential conflicts of interest associated with the research, authorship and/or publication of this article.
References
- Black MD. Therapeutic potential of positive AMPA modulators and their relationship to AMPA receptor subunits. A review of preclinical data. Psychopharmacology (Berl) 2005;179:154–63. doi: 10.1007/s00213-004-2065-6. [DOI] [PubMed] [Google Scholar]
- Buccafusco J. Emerging cognitive enhancing drugs. Expert Opin Emerg Drugs. 2009;14:577–89. doi: 10.1517/14728210903257796. [DOI] [PubMed] [Google Scholar]
- Chang PK, Verbich D, McKinney RA. AMPA receptors as drug targets in neurological disease - advantages, caveats, and future outlook. Eur J Neurosci. 2012;35:1908–16. doi: 10.1111/j.1460-9568.2012.08165.x. [DOI] [PubMed] [Google Scholar]
- Dauvermann MR, Lee G, Dawson N. Glutamatergic regulation of cognition and functional brain connectivity: insights from pharmacological, genetic and translational schizophrenia research. Br J Pharmacol. 2017;174:3136–3160. doi: 10.1111/bph.13919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley JM, Wilkinson KA. Synaptic AMPA receptor composition in development, plasticity and disease. Nat Rev Neurosci. 2016;17:337–50. doi: 10.1038/nrn.2016.37. [DOI] [PubMed] [Google Scholar]
- Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: the last 25 years. Neuron. 2013;80:704–17. doi: 10.1016/j.neuron.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito I, Tanabe S, Kohda A, Sugiyama H. Allosteric potentiation of quisqualate receptors by a nootropic drug aniracetam. J Physiol. 1990;424:533–43. doi: 10.1113/jphysiol.1990.sp018081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunugi A, Tanaka M, Suzuki A, et al. TAK-137, an AMPA-R potentiator with little agonistic effect, has a wide therapeutic window. Neuropsychopharmacology. 2019;44:961–970. doi: 10.1038/s41386-018-0213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Goodman L, Fourie C, et al. AMPA receptors as therapeutic targets for neurological disorders. Adv Protein Chem Struct Biol. 2016;103:203–61. doi: 10.1016/bs.apcsb.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Partin KM. AMPA receptor potentiators: from drug design to cognitive enhancement. Curr Opin Pharmacol. 2015;20:46–53. doi: 10.1016/j.coph.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell R, Lynch G, Gall C, et al. Brain vacuolation resulting from administration of the Type II Ampakine CX717 is an artifact related to molecular structure and chemical reaction with tissue fixative agents. Toxicol Sci. 2018;162:383–395. doi: 10.1093/toxsci/kfx277. [DOI] [PubMed] [Google Scholar]
- Reuillon T, Ward SE, Beswick P. AMPA receptor positive allosteric modulators: Potential for the treatment of neuropsychiatric and neurological disorders. Curr Top Med Chem. 2016;16:3536–3565. doi: 10.2174/1568026616666160627114507. [DOI] [PubMed] [Google Scholar]
- Tang CM, Shi QY, Katchman A, Lynch G. Modulation of the time course of fast EPSCs and glutamate channel kinetics by aniracetam. Science. 1991;254:288–90. doi: 10.1126/science.254.5029.288. [DOI] [PubMed] [Google Scholar]
- Tsai JJ, Wu T, Leung H, et al. Perampanel, an AMPA receptor antagonist: From clinical research to practice in clinical settings. Acta Neurol Scand. 2018;137:378–391. doi: 10.1111/ane.12879. [DOI] [PubMed] [Google Scholar]
- Ward SE, Bax BD, Harries M. Challenges for and current status of research into positive modulators of AMPA receptors. Br J Pharmacol. 2010;160:181–90. doi: 10.1111/j.1476-5381.2010.00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SE, Harries M, Aldegheri L, et al. Discovery of N-[(2S)-5-(6-Fluoro-3-pyridinyl)-2,3-dihydro-1H-inden-2-yl]-2-propanesulfonamide, a novel clinical AMPA receptor positive modulator. J Med Chem. 2010;53:5801–5812. doi: 10.1021/jm1005429. [DOI] [PubMed] [Google Scholar]
- Ward SE, Harries M, Aldegheri L, et al. Integration of lead optimization with crystallography for a membrane-bound ion channel target: discovery of a new class of AMPA receptor positive allosteric modulators. J Med Chem. 2011;54:78–94. doi: 10.1021/jm100679e. [DOI] [PubMed] [Google Scholar]
- Ward SE, Pennicott LE, Beswick P. AMPA receptor-positive allosteric modulators for the treatment of schizophrenia: an overview of recent patent applications. Future Med Chem. 2015;7:473–91. doi: 10.4155/fmc.15.4. [DOI] [PubMed] [Google Scholar]
- Ward SE, Beswick P, Calcinaghi N, et al. Pharmacological characterization of N-[(2S)-5-(6-fluoro-3-pyridinyl)-2,3-dihydro-1H-inden-2-yl]-2-propanesulfonamide: a novel, clinical AMPA receptor positive allosteric modulator. Br J Pharmacol. 2017;174:370–385. doi: 10.1111/bph.13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley ML, Waters KA, Gartlon JE, et al. Evaluation of the pro-cognitive effects of the AMPA receptor positive modulator, 5-(1-piperidinylcarbonyl)-2,1,3-benzoxadiazole (CX691), in the rat. Psychopharmacology. 2009;202:343–354. doi: 10.1007/s00213-008-1325-2. [DOI] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–6. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]