Abstract
Neorickettsia sennetsu has been described from Japan and Malaysia, causing a largely forgotten infectious mononucleosis-like disease. Because it is believed to be contracted from eating raw fish, frequently consumed in the Lao PDR, we looked for evidence of N. sennetsu among Lao patients and fish. A buffy coat from 1 of 91 patients with undifferentiated fever was positive by 16S rRNA amplification and sequencing and real-time polymerase chain reactions (PCR) targeting two N. sennetsu genes. Lao blood donors and patients with fever, hepatitis, or jaundice (N = 1,132) had a high prevalence (17%) of immunofluorescence assay IgG anti-N. sennetsu antibodies compared with 4% and 0% from febrile patients (N = 848) in Thailand and Malaysia, respectively. We found N. sennetsu DNA by PCR, for the first time, in a fish (Anabas testudineus). These data suggest that sennetsu may be an under-recognized cause of fever and are consistent with the hypothesis that it may be contracted from eating raw fish.
Introduction
In 1954, Rickettsia sennetsu was reported on the Japanese island of Kyushu as the first documented cause of a disease resembling infectious mononucleosis.1–6 The disease was characterized by fever, weakness, anorexia, generalized lymphadenopathy, hepatosplenomegaly, and peripheral blood mononucleosis with atypical lymphocytes. The incubation period was ~14 days, and no fatalities were reported. The causative agent was isolated from patients’ peripheral blood, lymph nodes, and bone marrow and subsequently renamed Ehrlichia sennetsu and most recently Neorickettsia sennetsu.1–9 Neorickettsia are obligate intracellular bacteria closely related to Ehrlichia and Anaplasma and, unlike Rickettsia, grow in host membrane-lined cytoplasmic vacuoles.5 The disease on Kyushu came to be known as sennetsu fever from the Japanese for infectious mononucleosis and was the first agent shown to cause this syndrome; Epstein-Barr virus as a cause was described 14 years later. The other described Neorickettsia species parasitize trematode flukes and mammals. N. helminthoeca infects flukes and canids, causing salmon poisoning disease in dogs in the Unites States, whereas N. risticii causes Potomac horse fever in North America and Europe and is probably transmitted by trematodes of snails, insects, bats, and swallows.5,9
Sennetsu was shown to be transmissible to mice and then to humans in Japan. The feeding of raw gray mullet (Mugil cephalus, a diadromous fish) to 96 people caused a sennetsu- like illness in 5% of subjects, and N. sennetsu was cultured from the blood of 4 of those who became ill, suggesting that the infection was linked to eating raw fish. Investigation of the epidemiology of sennetsu in Japan identified a closely related organism, the “SF agent,” in Stellantchasmus falcatus fluke metacercaria in M. cephalus, but this agent is not known to cause human disease, and in non-human primates, oral ingestion of the SF agent was not infectious.3–6,10,11 Analysis of the 16S rRNA sequence of the SF agent suggests that it is most closely related to N. risticii and N. sennetsu.10 The Miyayama strain of N. sennetsu has recently been sequenced.9 Thus, the source of N. sennetsu was not identified, and the disease apparently vanished in Japan, but in 1985, antibodies against N. sennetsu in sera from febrile patients in peninsular Malaysia and culture from at least one patient were mentioned but have not been formally reported.12,13 In Lao, raw fish and fermented fish paste are commonly eaten, and we speculated that N. sennetsu could be a local cause of undifferentiated fever.
Materials and Methods
Patients
Sera from Lao patients with unexplained fever and hepatitis from Vientiane, unexplained fever at Phalanxay, Savannakhet Province (southern Laos), and blood bank controls from Vientiane were used (Table 1).14 Sera from Malaysia (N = 40) and Thailand were also tested (Table 1).15 These studies were approved by the Ethical Review Committee of the Faculty of Medical Sciences, National University of Laos. The index patient with N. sennetsu infection gave written informed consent for the publication of his clinical details. The study of the non-Lao sera was approved by the University of Marseille Ethics Committee.
Table 1. Seroprevalence of IgG antibody titres against N. sennetsu among Lao and Thai patients.
| IgG 1/titer | Blood bank [no. (%)]* |
Hepatitis [no. (%)]† |
Unexplained fever– Vientiane [no. (%)]‡ |
Combined Vientiane [no. (%)]§ |
Unexplained fever— Phalanxay [no. (%)]¶ |
Thai [no. (%)]** |
|---|---|---|---|---|---|---|
| Age range (years) | 17-50 | 0.4-83 | 16-78 | 0.4-83 | 1-80 | 15-87 |
| Geometric mean (95% CI) | 408 (290-575) | 369 (300-454) | 247 (194-314) | 336 (290-388) | 264(192-362) | 189 (167-211) |
| 0 | 159 (82) | 313 (80) | 273 (86) | 745 (83) | 189 (83) | 776 (96) |
| 50 | 2 (1.0) | 1 (0.3) | 0 | 3 (0.3) | 5 (2.2) | 6 (0.7) |
| 100 | 3 (1.6) | 11 (2.8) | 14 (4.4) | 28 (3.1) | 6 (2.6) | 7 (0.9) |
| 200 | 7 (3.6) | 22 (5.6) | 14 (4.4) | 43 (4.8) | 9 (3.9) | 11 (1.4) |
| 400 | 10 (5.2) | 15 (3.9) | 10 (3.1) | 35 (3.9) | 10 (4.4) | 8 (1.0) |
| 800 | 6 (3.1) | 18 (4.6) | 6 (1.9) | 30 (3.3) | 8 (3.5) | 4 (0.5) |
| 1,600 | 7 (3.6) | 10 (2.6) | 2 (0.7) | 19 (2.1) | 2 (0.9) | 0 |
| Total | 194 | 390 | 319 | 903 | 229 | 812 |
2003, adult (> 15 years) blood donors presenting at Lao Red Cross Blood Bank, Vientiane, in March 2003.
2001-2004, patients, all consenting acutely jaundiced patients and/or those with elevation, by at least three times the upper limit of normal, of either AST or ALT or both, presenting at Mahosot Hospital, Vientiane.
2001-2002, all consenting adult (> 15 years) febrile patients presenting at Mahosot Hospital, Vientiane, with negative blood cultures after a 7-day incubation and, if appropriate, negative malaria smears.
Sum of the patients listed in the first three columns.
2003-2004, all consenting febrile patients presenting at Phalanxay District Hospital, Savannakhet Province, who were malaria smear negative and in whom follow-up was possible.
2001-2003, patients with undifferentiated febrile illness.15 Median (range) age, 38 (15-87) years; 78.2% male, from five hospitals in Thailand—four in the northeast (Udon Thani Hospital, Udon Thani Province, Maharat Nakhon Rachasima Hospital, Nakhon Rachasima province, Loei Hospital, Loei province, and Banmai Chaiyapod Hospital, Burirum province) and one in the south (Chumphon Hospital, Chumphon province).
Fish
We tested 238 samples from 88 fish species in three sets: 1) 98 intestines of fish purchased from fresh fish markets in Vientiane and preserved in 90% ethanol, 2) DNA from intestines and stomachs from 20 fish each of Channa gachua and Trichopsis vittata, and 3) 10 samples of the 10 most commonly consumed fish species in Vientiane obtained fresh from local markets, frozen at -80°C, and sent on dry ice to Marseille (see Supplementary Material A and B, which can be found online at www.ajtmh.org).16
Molecular methods
DNA samples, extracted using the QIAamp Tissue kit (QIAGEN, Hilden, Germany) from human blood or fish tissue were screened by polymerase chain reaction (PCR) using primers EHR16SD and EHR16SR, targeting a 345-bp fragment of the 16S rRNA gene of Anaplasmataceae.17 Positive PCR results were confirmed by two specific realtime PCRs on a Lightcycler (Roche Biochemicals, Mannheim, Germany) with primers and Taqman probes targeting the gltA and the Omp85 genes of N. sennetsu (see Supplementary Material C, which can be found online at www.ajtmh.org).18 PCR products were purified with the QIAquick PCR Purification Kit (QIAGEN) and sequencing performed on an ABI PRISM 310 DNA Sequencer (Applied Biosystems Inc., Foster City, CA). The sequences obtained were identified by comparison with sequences available in GenBank using the BLAST software.
Serology
Specific microimmunofluorescence (IFA) and Western blot assays were performed in Marseille, France, using whole cell antigens of N. sennetsu strain Miyayama (ATTC VR 367) and O. tsutsugamushi serotypes Karp, Kato, Gilliam, and Kawasaki for IgG and IgM with methods similar to those previously reported.14,19 For O. tsutsugamushi, an IFA result was considered positive if any of the following were detected: 1) positive antibody titers > 1:128 for immunoglobulin G (IgG) and > 1:64 for IgM, 2) seroconversion, or 3) > 4-fold increase in titers between acute- and the convalescent-phase serum.14 For N. sennetsu, an IFA result was considered positive for reciprocal IgG antibody titers ≥ 100 or seroconversion. For Western blot analysis, the procedure used was similar to the method previously described for rickettsiae.19 Briefly, the N. sennetsu whole cell antigens were resuspended in sterile distilled water and adjusted to 2 mg protein/mL. Twenty microliters of the preparation was electrophoresed at 100 V for 2 hours through a separating gel containing 10% polyacrylamide using a Mini-Protean II cell apparatus (BioRad, Hercules, CA). A mixture of prestained molecular mass standards (Kaleidoscope; Bio-Rad) was used to estimate the molecular masses of the separated antigens. Antigens were transferred onto a 0.45-μm-pore nitrocellulose membrane that was electrophoresed for 1 hour at 4°C and 100 V. The blots were blocked overnight at 4°C with 5% non-fat milk powder in Tris-buffered saline (TBS) and washed with distilled water. Serum specimens (diluted at ratio of 1:200) were applied to the blots for 1 hour at room temperature. After three separate washes in TBS, the blots were incubated for 1 hour with peroxidase-conjugated goat anti-human IgG. After three washes in TBS, blots were revealed in a solution of 4-chloro- 1-naphtol (Sigma, St. Louis, MO).
Statistical analysis. Data were analyzed with Stata v10 (College Station, TX), Wilcoxon matched pairs signed-rank tests were used to compare titers, and the χ2 and McNemar tests were used to compare proportions.
Results
Case report
A 17-year-old unemployed Lao man from a rice farming family in Vientiane Province presented at Mahosot Hospital in April 2003 with fever, headache, and weakness for 2 weeks. On admission, he was pale, jaundiced, and febrile, with hepato-splenomegaly and palpable inguinal and cervical lymph nodes. He was anemic (hematocrit 19%) with a normal peripheral white cell count (6.7 x 109/L), except relative and absolute lymphocytosis (neutrophils 38%, lymphocytes 60%, monocytes 2%; local upper reference range for lymphocytes < 45% and < 3.6 x 109/L, respectively), a normal platelet count (180,000/mm3), and a negative malaria blood smear. Atypical lymphocytes were not searched for. He had an elevated erythrocyte sedimentation rate (ESR) (75 mm in the first hour) but negative hemocultures after 7 days of incubation. Serum direct bilirubin 20.5 μmol/L (reference range < 4.3 μmol/L) and total bilirubin 32.0 μmol/L (< 14.5 μmol/L), aspartate aminotransferase (AST) 86 IU/L (< 37 IU/L) and alanine aminotransferase (ALT) 43 IU/L (< 40 IU/L) were raised. Abdominal ultrasound showed homogenous splenomegaly. In 2000, he had been admitted to the hospital with splenomegaly but did not receive blood transfusions. He had not visited any other provinces in Laos or been abroad. On the second day, he was empirically treated with ofloxacin (200 mg twice a day) for 7 days, was afebrile on the third day, and was discharged home on the fifth day. When reviewed in December 2006, he was well but had 8 cm of spleen palpable below the left costal margin without palpable hepatomegaly or lymphadenopathy. His red cells were not G6PD deficient by the methemoglobin reduction test, but hemoglobin cellulose acetate electrophoresis showed the presence of hemoglobin H. He remembered eating boiled snakehead fish (Channa species), including their intestines, in the weeks before becoming ill. He had eaten raw homemade “padek” or raw fermented fish paste two to three times a week for many years. The fish used to make the padek, from the Nam Ngum lake, were pa khao, pa kadert, pa kheng, pa khor, pa xieum, and pa khagneng, which are probably Puntius sp., Trichogaster trichopterus, Anabas testudineus, Channa striata, Ompok bimaculatus, and Mystus multiradiatus, respectively.16,20 The patient’s sera from admission and December 2006 were negative for antiN. sennetsu antibodies. PCR targeting the 16S rRNA gene of Anaplasmataceae from the admission buffy coat was positive and gave a sequence with 100% homology with N. sennetsu strain Miyayama (Genbank accession number FJ905928). A dendrogram representing the phylogenetic relationships between this sequence (Patient 1) and that of other Anaplasmataceae (16S rRNA gene sequence comparison) is given in Figure 1. N. sennetsu was confirmed by two specific real-time PCRs with Taqman probes with cycle thresholds at 33.4 and 36.0 for the gltA and Omp85 genes, respectively. Sequences from these PCR products were 100% identical to that of N. sennetsu (Genbank accession numbers FJ905932 and FJ905933 for gltA and omp85, respectively). None of the remaining available 90 buffy coats from Lao patients were PCR positive for N. sennetsu.
Figure 1. Dendrogram representing phylogenetic relationships between sequences derived from Fish 1 (Channa gachua), Fish 2 (Trichopsis vittata), Fish 3 (Anabas testudineus), and Patient 1 and other Anaplasmataceae (16S rRNA gene sequence comparison). The MEGA 4.1 software (http://www.megasoftware.net) was used to infer the tree by using the neighbor-joining method Kimura-2 parameter. The support of each branch, as determined from 1,000 bootstrap samples, is indicated by the value at the node.
Sennetsu in fish
Of the initial 98 fish collected (Supplementary Material A), intestines from one individual each of Channa gachua and Trichopsis vittata were positive by standard and real-time PCR targeting the 16S rRNA gene. The percentage homologies with N. sennetsu of the two sequences obtained from these fish were 92.2 (C. gachua, Fish 1; Figure 1) and 95.8% (T. vittata, Fish 2; Figure 1), respectively (GenBank accession numbers FJ905929 and FJ905930, respectively). Of the 20 flesh samples of C. gachua and T. vittata, two specimens of C. gachua were PCR positive for the same organism closely related to N. sennetsu, with standard and real-time PCR targeting the 16S rRNA gene. One fresh gill tissue sample from Anabas testudineus (Fish 3; Figure 1) had a 16S rRNA sequence with 99.1% homology with N. sennetsu strain Miyayama (Genbank accession number FJ905931) that was confirmed by the two specific real-time PCRs with Taqman probes with cycle thresholds of 30 and 32 for the gltA and Omp85 genes, respectively. Sequences from these PCR products were 100% identical to that of N. sennetsu.
Sennetsu seroprevalence in humans
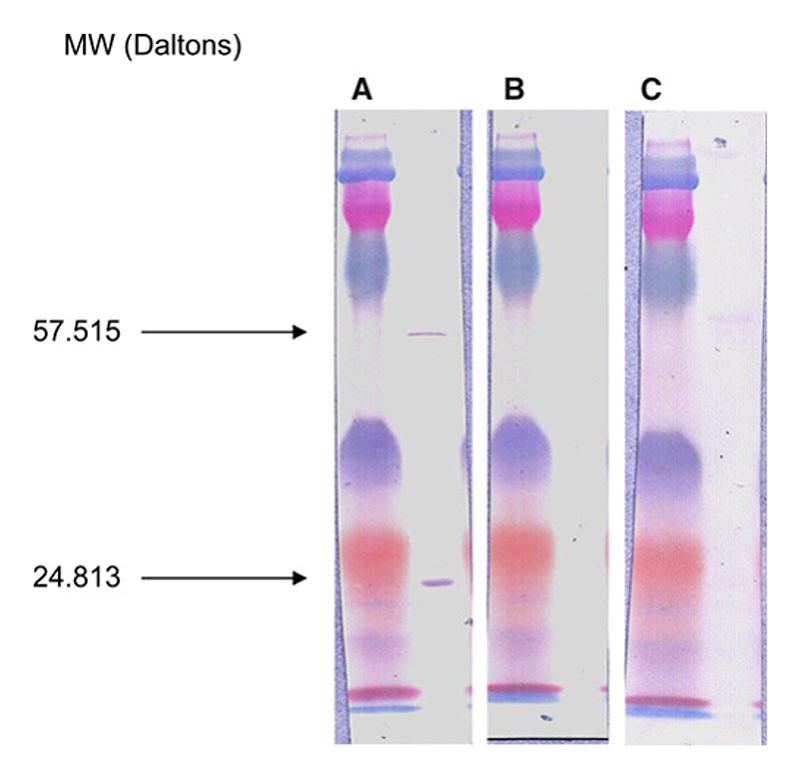
Prevalence of IgG antibodies in Lao (blood donors and patients with unexplained fever and jaundice/hepatitis in Vientiane and unexplained fever at Phalanxay) ranged from 14.4% to 17%, whereas seroprevalences in patients from Malaysia and northeast Thailand were 0% and 4%, respectively (Table 1). Seroprevalence of IgG antibodies against O. tsutsugamushi (> 1/128) in sera from Malaysia, Thailand, and Lao were 15%, 16%, and 15%, respectively. Western blot analysis was performed on 12 representative sera of patients with N. sennetsu antibodies (Figure 2A; N = 6), no antibodies (Figure 2B; N = 3), or O. tsutsugamushi antibodies (Figure 2C; N = 3). A specific band for N. sennetsu was found at a molecular mass of 24.813 Da for the six sera with N. sennetsu antibodies. A second band at 57.515 Da was also detected in all patients with N. sennetsu antibodies that was also weakly positive in the three patients with O. tsutsugamushi antibodies (Figure 2C). However, we did not find evidence for cross-reactivity between antibodies against O. tsutsugamushi and N. sennetsu; we compared the presence of admission anti- O. tsutsugamushi and N. sennetsu IgG reciprocal titers > 128 and 100, respectively, from the Vientiane unexplained fever and hepatitis/jaundice studies (N = 702; McNemar test, P < 0.0001). The admission IgG titers against these two organisms were also significantly different (Wilcoxon matched pairs signed-rank test, P = 0.003). Therefore, the high prevalence of anti- N. sennetsu antibodies in Laos is unlikely to be caused by prior O. tsutsugamushi infection.
Figure 2. Western immunoblotting of (A) a N. sennetsu-positive serum sample (a febrile Lao patient with an IFA IgG anti-sennetsu titer of 1/400), (B) a negative serum sample, and (C) an O. tsutsugamushi-positive serum sample. This figure appears in color at www.ajtmh.org.
Discussion
A search for sennetsu in central Laos showed one patient with a clinical illness compatible with Japanese reports of 50 years ago1–6 and N. sennetsu DNA in his buffy coat. Although sennetsu causes splenomegaly and may cause jaundice, the patient also had hemoglobin H disease, and these clinical findings may have been a consequence of the hemoglobinopathy rather than neorickettsiosis. The positive PCR result cannot have arisen from contamination because N. sennetsu has not been amplified in the Marseille laboratory before. The primers and probes used are specific for N. sennetsu compared with the genomes and GenBank database. We tested and amplified N. sennetsu with three different sets of primers and probes with 100% sequence homology. Thus, it is unlikely that we amplified sequences from related Neorickettsia species; however, because there are very few data on this genus, further work is required to study the diversity of Neorickettsia in humans, other vertebrates, and invertebrates. The high prevalence of antibodies against this organism in Laos suggest that sennetsu neorickettsiosis is a common infection. The microimmunofluorescence assay is apparently specific, because it is negative in sera of patients from Europe (Raoult and others, unpublished data), there was no significant cross-reactivity between antibodies against O. tsutsugamushi and N. sennetsu (above), and it was confirmed for the first time by Western blot. However, it remains possible that the antibodies found here are caused, in some cases, by exposure to organisms closely related to N. sennetsu and not N. sennetsu itself.
In cell culture, N. sennetsu are resistant to penicillin, erythromycin, co-trimoxazole, and, surprisingly, chloramphenicol, but sensitive to doxycycline, ciprofloxacin, and higher concentrations of rifampicin.21 Our patient was serendipitously treated with ofloxacin, which is likely to have been efficacious, but whether the patient would have recovered without antibiotic treatment is not known.
This is the first detection of N. sennetsu in a patient using molecular methods and the first case formally reported since 1966, extending the known range of sennetsu 1,500 km into the central mainland of southeast Asia.4,5 Since the 1980s, sen- netsu had apparently disappeared as a public health problem in Kyushu—perhaps because the consumption of gray mullet, the putative source, has declined with increased prosperity and change in food habits (S. Yamamoto, Y. Minanishima, and Y. Rikihisa, personal communication). In Japan, it was speculated that sennetsu was contracted from raw fish, but no proof was obtained.4,5,13 We found evidence for the first time of N. sennetsu in a fish, the climbing perch (A. testudineus), suggesting that this is at least one of the sources of human infection in Laos. Indeed the Lao patient with sennetsu had consumed climbing perch, which occurs throughout the Mekong River Basin, Sri Lanka to China, Indonesia, and the Philippines and is an important food fish.20 Climbing perch is the first identified potential source of sennetsu, and, because it is widely distributed and eaten, sennetsu may be prevalent in countries where it is consumed. However, we only studied the possible role of fish, which, although consistent with the hypothesis that the consumption of raw fish is important, leaves many uncertainties in the epidemiology of this disease. In Japan, N. sennetsu was also isolated from rodents.22 Raw fish are widely eaten in eastern Asia, as demonstrated by the widespread abundant (~40 million people infected) human Opisthorchis and Clonorchis liver fluke infections, also contracted from eating raw fish, in Thailand, Cambodia, Vietnam, Laos, China, Siberia, Korea, and Japan.23 Raw and undercooked fish are commonly eaten in Laos; within one southern province, frequent consumption of raw or insufficiently cooked fish was reported by 75.1% of villagers.24 If the hypothesis that N. sennetsu is contracted from raw/undercooked fish or from trematodes within fish is correct, the geographical distribution of sennetsu may be similar to that of liver flukes in East Asia. S. falcatus is also widespread in east Asia, including Laos,25 but whether it is involved in the epidemiology of sennetsu remains to be determined.
Because sennetsu is sensitive to tetracyclines21 and may be prevented by appropriate cooking, it would be an important diagnosis to make. Further research on the geographic distribution, incidence, and transmission of this disease and other Neorickettsia are needed, especially because a raw fish diet is widely expanding worldwide, including in the United States and East Asia.23,26 We speculate that the growing consumption of raw fish may be associated with new causes of infectious mononucleosis-like syndrome and suggest investigating for Neorickettsia infections in patients when a viral cause is not identified.
Supplementary Material
Acknowledgments
The authors thank all the patients who participated in this study, the doctors and nursing staff, the staff of the LARReC, the Mekong River Commission, and the Microbiology Laboratory of Mahosot Hospital, especially Vilada Chansamouth, Kaviphone Phouthavongs, Suriyasak Thongpaseuth, Anisone Changthongthip, Viengmone Davong, Olay Lattana, Manivanh Vongsouvath, Sengkham Symanivong, and Sengmani Symanivong. We are especially indebted to Christopher Barlow of the Mekong River Commission for his help in the collection of fish specimens, Kenichiro Yamada for translating Japanese papers, Drs. Seigo Yamamoto and Yoichi Minanishima for their advice in Myazaki, Dr. G. Dasch for the sennetsu isolate, and Professors Nicholas White and Nicholas Day and Dr. Stuart Blacksell, anonymous referees, and all the staff of the Mahidol Oxford Research Unit for support. We are very grateful to the Minister of Health, His Excellency Dr. Ponmek Dalaloy, and the Director of the Curative Department, Ministry of Health, Professor Sommone Phounsavath for support for this study.
Financial support
The work in Laos was a part of the Wellcome Trust- Mahosot Hospital-Oxford Tropical Medicine Research Collaboration funded by the Wellcome Trust of Great Britain. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Reprint requests: Paul Newton, Wellcome Trust-Mahosot Hospital-Oxford Tropical Medicine Research Collaboration, Microbiology Laboratory, Mahosot Hospital, Vientiane, Lao PDR, E-mail: paul@tropmedres.ac
Disclaimer: The authors declare that they have no conflict of interest.
Contributor Information
Paul N. Newton, Wellcome Trust-Mahosot Hospital-Oxford Tropical Medicine Research Collaboration, Microbiology Laboratory, Mahosot Hospital, Vientiane, Lao PDR, and Centre for Tropical Medicine, Nuffield Department of Clinical Medicine, University of Oxford, Churchill Hospital, Oxford OX3 7LJ, UK, Tel/Fax: 856-21-242168.
Jean-Marc Rolain, Email: jean-marc.rolain@univmed.fr, Jean-Marc Rolain, Unité des Rickettsies, Université de la Méditerranée, Marseille, France, Tel: 33-4-91-32-43-75, Fax: 33-4-91-38-77-72.
Bouachanh Rasachak, Email: rbouachanh@yahoo.com, Wellcome Trust- Mahosot Hospital-Oxford Tropical Medicine Research Collaboration, Microbiology Laboratory, Mahosot Hospital, Vientiane, Lao PDR, Tel/ Fax: 856-21-242168.
Mayfong Mayxay, Email: mayfong@tropmedres.ac, Wellcome Trust-Mahosot Hospital-Oxford Tropical Medicine Research Collaboration, Microbiology Laboratory, Mahosot Hospital, Vientiane, Faculty of Medicine, University of Health Sciences, Vientiane, Lao PDR, Centre for Tropical Medicine, Nuffield Department of Clinical Medicine, University of Oxford, Churchill Hospital, Oxford OX3 7LJ, UK, Tel/Fax: 856-21-242168.
Khamtanh Vathanatham, Email: khamtanhv@yahoo.com, Living Aquatic Resources Research Centre (LARReC), Vientiane, Lao PDR, Tel/Fax: 856-21-242168.
Pise th Seng, Email: sengpiseth@yahoo.fr, Unité des Rickettsies, Université de la Méditerranée, Marseille, France, Tel: 33-4-91-32-43-75, Fax: 33-491-38-77-72.
Rattanaphone Phetsouvanh, Email: rphetsouvanh@yahoo.co.uk, Wellcome Trust-Mahosot Hospital-Oxford Tropical Medicine Research Collaboration, Microbiology Laboratory, Mahosot Hospital, Vientiane, Lao PDR, and Centre for Tropical Medicine, Nuffield Department of Clinical Medicine, University of Oxford, Churchill Hospital, Oxford OX3 7LJ, UK, Tel/Fax: 856-21-242168.
Te Thammavong, Email: redblood@laotel.com, Lao Red Cross, Blood Transfusion Service, Vientiane, Lao PDR, Tel/Fax: 856-21-242168.
Jamaayah Zahidi, Email: jamazahidi@yahoo.com, Jamaayah Zahidi, Institute of Medical Research, Kuala Lumpur, Malaysia.
Yupin Suputtamongkol, Email: ysuputtamongkol@gmail.com, Department of Medicine, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand.
Bounkong Syhavong, Email: bounkongs@yahoo.com, Wellcome Trust-Mahosot HospitalOxford Tropical Medicine Research Collaboration, Microbiology Laboratory, Mahosot Hospital, Vientiane, Lao PDR, Tel/Fax: 856-21242168.
Didier Raoult, Unité des Rickettsies, Université de la Méditerranée, Marseille, France, Tel: 33-491-32-43-75, Fax: 33-4-91-38-77-72.
References
- 1.Misao T, Kobayashi Y. Studies on mononucleosis. 1 report. Isolation of the infectious agent [in Japanese] Fukuoka Med J. 1954;45:519. [Google Scholar]
- 2.Fukuda T, Kitao T, Keida Y. Studies on the causative agent of “Hyuganetsu” disease. 1. Isolation of the agent and its inoculation trial in human beings [in Japanese] Med Biol. 1954;32:200–209. [Google Scholar]
- 3.Misao TY, Kobayashi Y, Shirakawa M. La mononucleose infecteuse (fibvre ganglionaire) [in French] Sang. 1978;28:785–805. [PubMed] [Google Scholar]
- 4.Tachibana N. Sennetsu fever: the disease, diagnosis, and treatment. In: Leive L, editor. Microbiology. American Society for Microbiology; Washington, DC: 1986. pp. 205–208. [Google Scholar]
- 5.Rikihisa Y. The tribe Ehrlichieae and ehrlichial diseases. Clin Microbiol Rev. 1991;4:286–308. doi: 10.1128/cmr.4.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirai K. Study of infectious mononucleosis in Kumamoto Prefecture. 1. Isolation of a Rickettsia sennetsu-like organism from patients with epidemic glandular fever [in Japanese] Kumamoto Igakkai Zasshi. 1966;40:1159–1173. [PubMed] [Google Scholar]
- 7.Dumler JS, Barbet AF, Bekker CP, Dasch GA, Palmer GH, Ray SC, Rikihisa Y, Rurangirwa FR. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila . Int J Syst Evol Microbiol. 2001;51:2145–2165. doi: 10.1099/00207713-51-6-2145. [DOI] [PubMed] [Google Scholar]
- 8.Rikihisa Y, Zhang C, Kanter M, Cheng Z, Ohashi N, Fukuda T. Analysis of p51, groESL and the major antigen P51 in various species of Neorickettsia an obligatory intracellular bacterium that infects trematodes and mammals. J Clin Microbiol. 2004;42:3823–3826. doi: 10.1128/JCM.42.8.3823-3826.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hotopp JC, Lin M, Madupu R, Crabtree J, Angiuoli SV, Eisen JA, Seshadri R, Ren Q, Wu M, Utterback TR, Smith S, et al. Comparative genomics of emerging human ehrlichiosis agents. PLoS Genet. 2006;2:e21. doi: 10.1371/journal.pgen.0020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen B, Rikihisa Y, Yamamoto S, Kawabata N, Fuerst PA. Characterization of the SF agent, an Ehrlichia sp. isolated from the fluke Stellantchasmus falcatus by 16S rRNA base sequence, serological, and morphological analyses. Int J Syst Bacteriol. 1996;46:149–154. doi: 10.1099/00207713-46-1-149. [DOI] [PubMed] [Google Scholar]
- 11.Shishido A, Honjo S, Suganuma M, Ohtaki S, Hikita M, Fujiwara T, Takasaka M. Studies on infectious mononucleosis induced in the monkey by experimental infection with Rickettsia sennetsu . Jpn J Med Sci Biol. 1965;18:73–83. doi: 10.7883/yoken1952.18.73. [DOI] [PubMed] [Google Scholar]
- 12.Holland CJ, Ristic M, Huxsoll DL, Cole AI, Rapmund G. Adaptation of Ehrlichia sennetsu to canine blood monocytes: preliminary structural and serological studies with cell culture-derived Ehrlichia sennetsu . Infect Immun. 1985;48:366–371. doi: 10.1128/iai.48.2.366-371.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ristic M. Current strategies in research on ehrlichioisis. In: Williams JC, Kakoma I, editors. Ehrlichiosis: A Vector Borne Disease of Animals and Humans. Kluwer Academic Publishers; Dordecht: 1990. pp. 136–153. [Google Scholar]
- 14.Phongmany S, Rolain JM, Phetsouvanh R, Blacksell SD, Soukkhaseum V, Rasachack B, Phiasakha K, Soukkhaseum S, Frichithavong K, Chu V, Keolouangkhot V, et al. Rickettsial infections and fever, Vientiane, Laos. Emerg Infect Dis. 2006;12:256–262. doi: 10.3201/eid1202.050900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suttinont C, Losuwanaluk K, Niwatayakul K, Hoontrakul S, Intaranongpai W, Silpasakorn S, Suwancharoen D, Panlar P, Saisongkorh W, Rolain JM, Raoult D, et al. Causes of acute, undifferentiated, febrile illness in rural Thailand: results of a prospective observational study. Ann Trop Med Parasitol. 2006;100:363–370. doi: 10.1179/136485906X112158. [DOI] [PubMed] [Google Scholar]
- 16.Kottelat M. Fishes of Laos. WHT Publications; Colombo, Sri Lanka: 2001. [Google Scholar]
- 17.Parola P, Roux V, Camicas JL, Baradji I, Brouqui P, Raoult D. Detection of ehrlichiae in African ticks by polymerase chain reaction. Trans R Soc Trop Med Hyg. 2000;94:707–708. doi: 10.1016/s0035-9203(00)90243-8. [DOI] [PubMed] [Google Scholar]
- 18.Inokuma H, Brouqui P, Drancourt M, Raoult D. Citrate synthase gene sequence: a new tool for phylogenetic analysis and identification of Ehrlichia . J Clin Microbiol. 2001;39:3031–3039. doi: 10.1128/JCM.39.9.3031-3039.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rolain JM, Gouriet F, Brouqui P, Larrey D, Janbon F, Vene S, Jarnestrom V, Raoult D. Concomitant or consecutive infection with Coxiella burnetii and tickborne diseases. Clin Infect Dis. 2005;40:82–88. doi: 10.1086/426440. [DOI] [PubMed] [Google Scholar]
- 20.Mekong River Commission. The MRC Mekong Fish Database CD. Mekong River Commission; Vientiane, Lao PDR: 2003. [Google Scholar]
- 21.Brouqui P, Raoult D. In vitro susceptibility of Ehrlichia sennetsu to antibiotics. Antimicrob Agents Chemother. 1990;34:1593–1596. doi: 10.1128/aac.34.8.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukuda T, Sasahara T, Kitao T. Studies on the causative agent of Hyuga fever. 10. The vector. Jpn J Infect Dis. 1962;36:235–241. [PubMed] [Google Scholar]
- 23.Sripa B. Concerted action is needed to tackle liver fluke infections in Asia. PLoS Negl Trop Dis. 2008;2:e232. doi: 10.1371/journal.pntd.0000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sayasone S, Odermatt P, Phoumindr N, Vongsaravane X, Sensombath V, Phetsouvanh R, Choulamany X, Strobel M. Epidemiology of Opisthorchis viverrini in a rural district of southern Lao PDR. Trans R Soc Trop Med Hyg. 2007;101:40–47. doi: 10.1016/j.trstmh.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 25.Sithithaworn P, Sukavat K, Vannachone B, Sophonphong K, Ben-Embarek P. Epidemiology of food-borne trematodes and other parasite infections in a fishing community on the Nam Ngum reservoir, Lao PDR. SE Asian J Trop Med Publ Hlth. 2006;37:1083–1090. [PubMed] [Google Scholar]
- 26.Unnevehr L, Roberts T. Food safety incentives in a changing world food system. Food Contr. 2002;13:73–76. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.