Abstract
Purpose
To assess the relationships of microstructural damage in the cerebral white matter (WM), as measured by diffusion tensor imaging (DTI), with clinical parameters and magnetic resonance imaging (MRI) measures of focal tissue damage in patients with multiple sclerosis (MS).
Materials and Methods
Forty-five relapsing-remitting (RR) MS patients (12 male, 33 female; median age = 29 years, Expanded Disability Status Scale (EDSS) = 1.5, disease duration = 3 years) were studied. T2-lesion masks were created and voxelwise DTI analyses performed with Tract-Based Spatial Statistics (TBSS).
Results
T2-lesion volume (T2-LV) was significantly (P < 0.05, corrected) correlated with fractional anisotropy (FA) in both lesions and normal-appearing WM (NAWM). Relationships (P = 0.08, corrected) between increasing EDSS score and decreasing FA were found in the splenium of the corpus callosum (sCC) and along the pyramidal tract (PY). All FA associations were driven by changes in the perpendicular (to primary tract direction) diffusivity. No significant global and voxelwise FA changes were found over a 2-year follow-up.
Conclusion
FA changes related to clinical disability in RR-MS patients with minor clinical disability are localized to specific WM tracts such as the sCC and PY and are driven by changes in perpendicular diffusivity both within lesions and NAWM. Longitudinal DTI measurements do not seem able to chart the early disease course in the WM of MS patients.
Keywords: multiple sclerosis, clinical disability, diffusion tensor imaging, tract-based spatial statistics, lesions
MEASURING RELEVANT PATHOLOGY in multiple sclerosis (MS) by magnetic resonance imaging (MRI) remains a challenge. For instance, the frequency of acute inflammatory lesions correlates only weakly with changes in clinical disability. While this may in part be explained by the heterogeneity in lesion pathology and the impact of lesion location, it is important to note that normal-appearing white matter (NAWM) is also abnormal in MS (1). Furthermore, brain atrophy occurs as an early feature of the disease (2,3). Assessment of specific cerebral tissue characteristics by nonconventional MRI techniques may thus be a valuable option for a more comprehensive understanding of MS pathology in vivo. In this context, diffusion tensor imaging (DTI) allows for a detailed and regional quantification of microstructural integrity in WM tracts. By fitting a diffusion tensor model to all voxels within the brain, estimation of fractional anisotropy (FA: a measure of diffusion directionality), mean diffusivity (MD), and parallel and perpendicular diffusivities of the diffusion tensor (4) are possible.
However, attempts to correlate DTI metrics with measures of clinical disability have produced conflicting results so far (5–12). Also, changes in DTI metrics over time in MS patients were not detected by all studies (13–16). Methodological differences might serve to explain at least some of this controversy. In this respect, the recent application (9) of a novel voxelwise analysis approach (17) suggested that this may be more sensitive than a global analysis of DTI data.
Several pieces of evidence from animal models (18–21) also indicate that more specific pathophysiological information may be derived from DTI via measurement of parallel and perpendicular diffusivities, which have been proposed in these studies as markers of axon and myelin damage, respectively. In human MS studies, increased perpendicular diffusivity has been observed in the corpus callosum (22,23), internal capsule (22), and pyramidal tract (24). Furthermore, perpendicular diffusivity in the whole WM was found to correlate with callosal size and clinical disability in another study (9). Also, callosal perpendicular diffusivity has been associated with functional connectivity in the primary sensorimotor cortex (25) and with a behavioral task relying on the efficiency of interhemispheric communication (26).
Against this background, we studied a cohort of relapsing-remitting (RR)-MS patients with minor clinical disability in order to: 1) assess correlations of WM microstructural damage, reflected by FA, with clinical parameters and MR measures of focal tissue damage, using a DTI technique for voxelwise analysis called Tract-Based Spatial Statistics (TBSS) (17); 2) test the differential contribution of axon and myelin damage (assessed by tensor diffusivities) in those WM regions where FA showed significant associations; and 3) assess changes in WM FA over an observation period of 2 years.
Materials And Methods
Patients
The study comprised 45 outpatients with RR MS who underwent an extensive cross-sectional MRI examination including DTI. Eight patients were on diseasemodifying treatment (DMT) and seven were still in the stage of a clinically isolated syndrome (27), but converted to clinically definite MS during follow-up. Thus, the diagnosis of RR MS was ultimately based on the observation of at least two relapses in all individuals (28). Apart from these cross-sectional data we also collected longitudinal information on a subgroup of individuals who were followed at regular intervals after the institution of treatment with glatiramer acetate (GA). These repeat MRI scans were performed at 6 (n = 14), 12 (n = 9), and 24 months (n = 8). Clinical disability was assessed according to the Expanded Disability Status Scale (EDSS) and information regarding previous relapses and treatment were recorded. In the longitudinal cohort, 15 patients started treatment with GA after study entry. Table 1 summarizes the demographic, clinical, and MRI features of the whole patient cohort.
Table 1. Demographic, Clinical, and MR Features in Our Cohort of Relapsing-Remitting Multiple Sclerosis (RR MS) Patients at Study Entry.
| Features | |
|---|---|
| Number of patients | 45 |
| Males/females | 12/33 |
| Median age, years (range) | 29 (19-45) |
| Median disease duration, | 3 (0.8-27) |
| years (range) | |
| Median EDSS score (range) | 1.5 (0-4) |
| Median number of previous | 2 (0*-20) |
| relapses (range) | |
| Median T2-lesion volume, mL (range) | 2.19 (0.32-27.53) |
Seven patients were in the stage of a clinically isolated syndrome (CIS) suggestive of MS at cross-sectional assessment and then converted into clinically definite RR MS over the follow-up.
The study was approved by the local Ethical Committee and written informed consent was obtained from all patients.
MR Data Acquisition
Scans were obtained on a 1.5 T Gyroscan Intera (Philips Medical Systems, Best, The Netherlands) MR scanner with a maximum gradient strength of 20 mT m Diffusion-weighted (DW) data were acquired using echo-planar imaging (EPI) (TR = 4000 msec; TE = 89 msec; 16 × 6-mm-thick axial slices; voxel size = 0.9 × 0.9 × 6.0 mm). The diffusion weighting was distributed in six directions using a b-value of 1000 sec mm−2.
A dual-echo turbo spin-echo sequence (TR/TE1/TE2 = 2300/20/70 msec, 256 × 256 matrix, 250 mm field of view, 46 × 3-mm-thick axial slices), yielding proton density-weighted (PD-W) and T2-weighted (T2-W) images, was acquired in a transverse plane parallel to the line connecting the anterior commissure (AC) to the posterior commissure (PC). A T1-weighted (T1-W) image (TR = 680 msec, TE = 14 msec) was also acquired after administration of gadolinium (Gad)-DTPA (0.1 mol/kg body weight).
Data Analysis
Diffusion Data Analysis
DW images were first corrected for MRI eddy currents and head motion using affine registration to a reference volume, ie, the volume without diffusion weighting (b = 0). FMRIB’s Diffusion Toolbox (FDT), part of the FMRIB Software Library (FSL v. 4.0, www.fmrib. ox.ac.uk/fsl (29)) was then used to fit a diffusion tensor model to the data at each brain voxel. From this model, voxelwise images of FA, MD, and diffusivity parallel (λ1) and perpendicular ((λ2+λ3)/2) to the principal diffusion direction were computed for the whole brain.
To test for local linear correlations of WM FA with clinical and MR measures we used TBSS v. 1.1 (17). First, individual FA images were nonlinearly aligned to a high-resolution standard-space average of 58 well-aligned, good-quality FA images from healthy subjects using spline-based free-form deformation (implemented in the Image Registration Toolkit, www.doc.ic.ac.uk/~dr). The cross-subject mean FA image was computed and used to generate a WM tract “skeleton,” which was thresholded at FA >0.3. The resulting skeleton included 62,618 1 × 1 × 1 mm WM voxels (corresponding to one-third of WM voxels of the unthresholded skeleton), assumed to contain the major WM tracts. Individual FA images were warped onto this group skeleton for statistical comparisons by searching perpendicular from the skeleton for maximum FA values. Maximum FA values were chosen in order to restrict the analysis to the centers of WM tracts (where maximum FA values will be found), rather than considering the voxels on the edges of tracts, which will suffer from partial volume effects.
The randomise program within FSL was used to carry out permutation-based testing (30). Cluster-size thresholding was used for statistical inference. Clusters were defined by first thresholding the raw t-statistic map at t > 2, and finding contiguous clusters of suprathreshold voxels, using 26-neighbour connectivity. The null distribution of the cluster-size statistic was built up over 5000 random permutations of group membership, with the maximum size (across space) recorded at each permutation. The 95th percentile of this distribution (a cluster size of ≈600 voxels on the WM skeleton) was then used as the clustersize threshold, ie, clusters were thresholded at a level of P < 0.05, fully corrected for multiple comparisons across space (ie, controlling the family-wise error—the chance of one or more false positives anywhere on the WM skeleton).
We also carried out multiple F-tests with TBSS, using voxel-based thresholding corrected for multiple comparisons across space, in order to investigate which single regressor was most significantly associated with FA in the WM skeleton.
TBSS was also used to test for longitudinal FA changes after 6, 12, and 24 months using a paired t-test design within randomise, and to investigate relationships between changes in FA and changes in disability scores (EDSS, pyramidal and cerebellar functional scores) over time in the subgroup of patients with longitudinal datasets, considering the maximum time interval available for each patient (24 months in six patients, 18 months in three patients, 12 months in four patients, and 6 months in four patients).
Finally, we defined two sets of regions of interest (ROIs) based on: 1) the WM regions whose FA values significantly correlated with EDSS score cross-sectionally, and 2) the WM regions showing significant FA decreases over time. We applied the ROIs onto the FA images and tested for correlations between FA changes in the ROIs and changes in disability scores (EDSS, pyramidal and cerebellar functional scores) within the maximum time interval available for each patient.
MS Lesion Analysis
A single observer, blinded to clinical data, identified and segmented T2 lesions, employing a segmentation technique based on user-supervised local thresholding (Jim 4.0, Xinapse System, Leicester, UK). Lesion borders were determined primarily on PD-W images, but information from T2-W images was also considered. T2-lesion volume (T2-LV) was estimated by multiplying lesion area by slice thickness. Lesion masks were binarized, transformed into MNI152 standard space, and averaged to create a group mean lesion mask.
Statistical Analysis
Values of FA, parallel, and perpendicular diffusivities averaged across the WM skeleton were correlated with T2-LV, disease duration, and number of previous relapses using Pearson’s correlation coefficient, whereas correlations with EDSS, which is an ordinal rating scale, were performed with Spearman’s rank order correlation coefficient (SROC).
Voxelwise relationships of cerebral WM FA with clinical and MR measures and longitudinal FA changes were tested using randomise within TBSS (see details in Diffusion Data Analysis). Within clusters showing significant correlations between FA and EDSS score, we computed mean FA and parallel and perpendicular diffusivities for each patient and looked for correlations with EDSS, pyramidal and cerebellar functional scores using SROC.
For all statistical analysis, values of P < 0.05 were considered significant (corrected for multiple comparisons where necessary). SPSS v. 11.0 (Chicago, IL) was used to perform statistical calculations.
Results
Cross-Sectional Analysis
Global Correlations of White Matter FA With MR and Clinical Measures
We first computed a global FA measure by averaging FA across the whole WM skeleton. Significant correlations were found between this global FA measure and T2-LV (r = −0.65, P < 0.01), disease duration (r = −0.35, P < 0.05), and number of previous relapses (r = −0.50, P < 0.01). No significant correlation was found between the global FA measure and EDSS score (r = −0.12, NS). The significant correlations were driven by perpendicular diffusivity changes (correlations with perpendicular diffusivity: T2-LV: r = 0.70, P < 0.01; disease duration: r = 0.39, P < 0.01; number of previous relapses: r = 0.50, P < 0.01). Much lower and nonsignificant levels of correlation were found with parallel diffusivity averaged across the WM skeleton.
Voxelwise Local Correlations of White Matter FA With MR and Clinical Measures
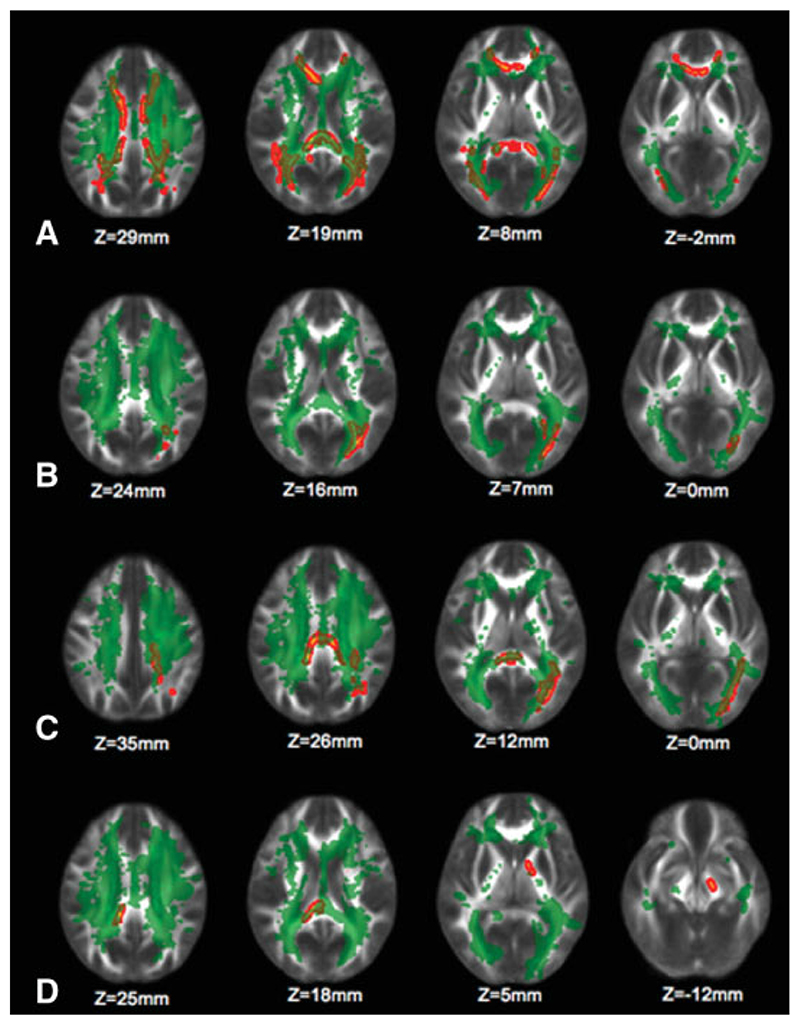
We next tested for correlations of local, voxelwise FA values with MR and clinical measures. WM regions with a significant inverse correlation between (local) FA and (global) T2-LV were located both within lesional areas and in regions of NAWM (Fig. 1A), including the splenium of the corpus callosum (sCC), a region where FA changes also correlated with the EDSS score (see details below).
Figure 1.
Clusters in red-yellow indicate those voxels whose FA values have a significant negative correlation (across patients) with total T2-lesion volume (T2-LV) (A), disease duration (B), number of previous relapses (C), and EDSS score (D). The significant regions have been thickened for better visibility. The green overlay shows the group mean lesion mask. The background image is the group mean FA (in MNI152 standard space). Images are shown in radiological convention. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com].
A trend (P = 0.08, corrected) for decreased FA with increasing disease duration was found in the left forceps major and the left inferior fronto-occipital/inferior longitudinal fascicle (IFO/ILF) (Fig. 1B). Most of the disease duration clusters overlapped with the mean lesion mask (Fig. 1B).
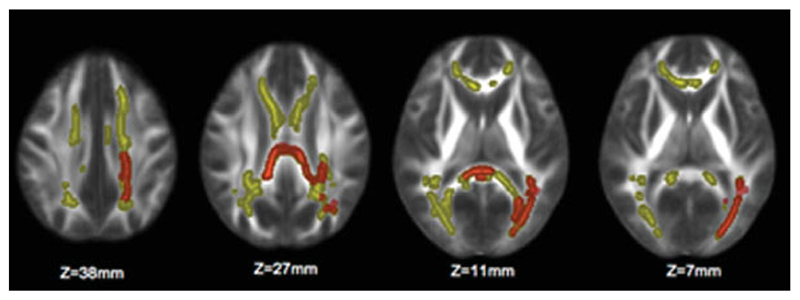
The number of relapses before study entry showed a significant inverse correlation with FA in the left superior corona radiata (SCR), left superior longitudinal fascicle (SLF), bilateral body of the CC, sCC, and left IFO/ILF. Most of these clusters overlapped with the mean lesion mask (Fig. 1C). These clusters also overlapped with those clusters whose FA values showed a significant inverse correlation with T2-LV (Fig. 2).
Figure 2.
Yellow clusters represent brain areas with a significant inverse correlation between T2-lesion volume (T2-LV) and FA (ie, where higher T2-LV is correlated with a significant decrease in FA). Regions overlapping with the significant inverse correlation between FA and the number of previous relapses are marked in orange. The significant regions have been thickened for better visibility. The background image is the group mean FA. Images are shown in radiological convention. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com].
We found a trend (P = 0.08, corrected) for correlation between increasing EDSS score and decreasing FA in the sCC and in a region of the pyramidal tract (PY) spanning from the left posterior limb of the internal capsule (PLIC) to the left cerebral peduncle (CP) (Fig. 1D). The sCC cluster, but not the PY cluster, overlapped with the mean lesion mask (Fig. 1D) and overlapped with the sCC cluster that showed a significant correlation between FA and T2-LV (cf. Fig. 1A,D). ROI analysis of the sCC and PY clusters showed that the correlation between mean FA and EDSS score was moderate (sCC cluster: r = −0.38; PY cluster: r = −0.48; P = 0.001 for both). Correction for age (r = −0.43, P = 0.003 in the sCC cluster and r = −0.49, P = 0.001 in the PY cluster) and disease duration (r = −0.41, P = 0.007 in the sCC cluster and r = −0.54, P < 0.001 in the PY cluster) did not significantly change the strength of the correlation within the two ROIs. T2-LV by itself also did not significantly correlate with the EDSS score (r = 0.20: P = 0.17).
To test for the differential contribution of parallel and perpendicular diffusivities to the EDSS score, we also separately correlated EDSS score with parallel and perpendicular diffusivities within those clusters that showed a trend for correlation between FA and EDSS score. EDSS showed a significant positive correlation with perpendicular diffusivity only (r = 0.42, P = 0.004 in the sCC cluster and r = 0.38, P = 0.01 in the PY cluster) but not with parallel diffusivity (r = 0.08, P = 0.56 in the sCC cluster and r = −0.09, P = 0.53 in the PY cluster).
Because of the topography of observed DTI findings we also tested for a specific association with the pyramidal and cerebellar functional scores of the EDSS. Only the FA values in the PY cluster correlated significantly with both pyramidal and cerebellar scores (r = −0.30, P = 0.04 and r = −0.38, P = 0.01, respectively). This correlation was driven by perpendicular diffusivity, which was significantly correlated with both pyramidal and cerebellar scores (r = 0.33, P = 0.03 and r = 0.38, P = 0.01, respectively), but not by the parallel diffusivity.
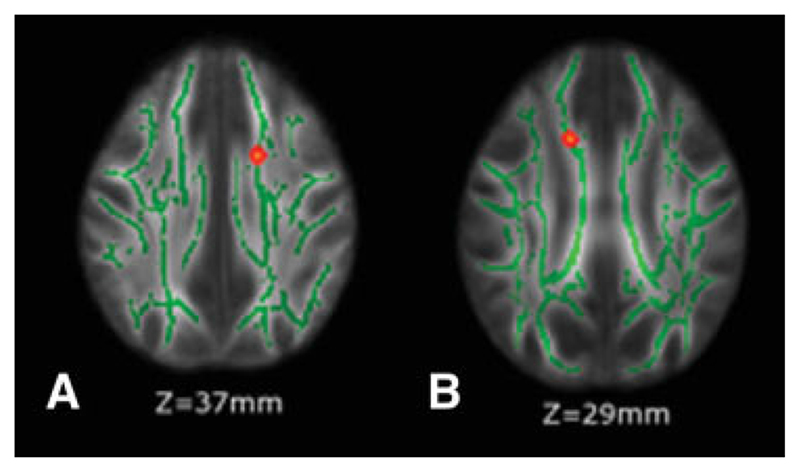
Multiple regression analysis within TBSS showed that the only single F-contrast surviving voxel-based thresholding (corrected for multiple comparisons across space) was the correlation between FA and T2-LV, where significant voxels were found in the left SCR and in the right body of the CC (Fig. 3).
Figure 3.
Results from multiple regression analysis (see Results for details) show voxels (in red-yellow, thickened for better visibility) where an increase in T2-lesion volume (T2-LV) was significantly correlated with an FA decrease. These voxels colocalize with the left superior corona radiata (SCR) (A) and the right body of the corpus callosum (CC) (B). The significant regions have been thickened for better visibility. Green is the WM skeleton, thresholded at FA >0.3. The background image is the group mean FA. Images are shown in radiological convention. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com].
Longitudinal Analysis
Global FA Changes Over Time
No significant changes over time (study entry, 6, 12, and 24 months) were found for FA values averaged across the WM skeleton.
Voxelwise Local FA Changes Over Time
No significant differences were found after 6 and 12 months. After 24 months, significant FA decreases in the genu of the CC and left forceps minor and significant FA increases in the left SLF and bilateral IFO/ILF were found. These differences disappeared after controlling for T2-LV at both baseline and 24 months and also after controlling for age.
Correlations of FA Changes With Clinical Score Changes in the Total White Matter and Within Selected ROIs
These correlations were not assessed because of the absence of significant FA changes over time.
Discussion
This study attempted to investigate the contribution of cerebral WM microstructure, measured by DTI, to the pathophysiological understanding of RR MS, in particular regarding its clinical-morphological relationships. For this purpose, we also assessed the different tensor diffusivities and used a novel DTI technique for voxelwise analysis.
In the cross-sectional analysis of our patient cohort, we found a significant inverse correlation between T2-lesion volume and FA values across the whole WM. This relationship was seen not only in those WM tracts overlapping with MS lesions but also in regions of NAWM. The fact that parts of WM tracts whose FA values showed a significant negative correlation with T2-lesion volume (genu and splenium of the corpus callosum and cingulum) were located in the NAWM suggests an association between the accrual of lesion damage and NAWM changes. Wallerian degeneration of axons and changes in myelin distant from lesioned white matter tracts are a likely explanation (31,32). However, this does not exclude a further component of NAWM damage, such as a diffuse axonopathy, which may develop in parallel and independent of MS lesion accrual (1,33).
Previous studies have found significant relationships between DTI metrics in regions of NAWM and local or total T2-lesion volume and used this as evidence for the presence of Wallerian degeneration. In particular, this mechanism of damage has been demonstrated for the normal-appearing periventricular and frontal lobe areas (6), internal capsule (22), whole corpus callosum (22,23,34,35), midbody of the corpus callosum (7), and pyramidal tract (24,36).
Our findings also confirm and extend the results of a previous study (9), where a significant correlation between local FA and total T2-lesion volume was shown in 15 MS patients (mainly RR) in regions with the highest lesion probability and in callosal regions of NAWM. Although our mean T2-lesion volume was lower compared to the previous study, in our MS patients regions of significant correlations were not only confined to the corpus callosum and periventricular regions, but also extended to the superior corona radiata, and to the superior and inferior longitudinal fascicle. These findings confirm that FA is a sensitive measure for detecting direct damage caused by MS lesions but also degeneration in regions distant from focal damage (ie, NAWM).
The association of T2-lesion load with WM damage measured by FA is also supported in the current study by the voxelwise multiple regression analysis, in which T2-lesion volume turned out to be the only single variable associated with FA reductions in the WM, specifically in tracts such as the superior corona radiata and body of the corpus callosum.
Increased disease duration showed here a significant but only low-to-moderate correlation with decreasing FA values in the whole WM. In a previous study (10), MD values computed from several ROIs of the NAWM did not significantly correlate with disease duration. An explanation for this discrepancy may be the fact that we considered for analysis the whole WM, including MS lesions that have lower FA values than NAWM (6,37). The voxelwise analysis of our patient cohort showed that the WM regions most significantly related to disease duration were located in the forceps major and inferior longitudinal fascicle. The fact that these regions lie in lesion areas and that they overlap with the regions showing a significant correlation with T2-lesion volume indirectly suggests lesion accrual over time as the driving force for these correlations.
A similar explanation is probably shared by the observed significant association of decreasing FA values across the whole WM with the number of relapses before study entry. This is supported by the similarity (with respect to T2-LV correlations) of WM regions identified by our voxelwise approach and showing strong associations, such as the body and splenium of the corpus callosum, superior corona radiata, superior longitudinal fascicle, and inferior longitudinal fascicle. Most of these WM tracts again overlapped with the mean T2-lesion mask and with regions whose FA values showed a significant inverse correlation with T2-lesion volume.
In our population of RR MS patients, clinical disability, measured by the EDSS score, did not significantly correlate with either the total T2-LV or the FA in the whole WM. This finding is in line with previous studies where no significant relationships were found between EDSS score and FA values in different regions of interest of the NAWM in RR (6,7), early (5), secondary, and primary progressive (6) MS patients. By contrast, Ciccarelli et al (10) reported on a significant correlation between EDSS score and FA in the whole (supra- and infratentorial) NAWM in a cohort of RR MS patients.
However, using a voxel-based analysis technique we found in this study a statistical trend toward a significant correlation between increasing EDSS scores and decreasing FA values in the splenium of the corpus callosum and in a substantial part of the pyramidal tract spanning from the posterior limb of the internal capsule to the cerebral peduncle. In this context it appears important to note that the FA values in the pyramidal cluster also correlated significantly with both pyramidal and cerebellar subscores of the EDSS. The presence of a trend but not of a clear-cut statistical significance may be explained by the fact that our cohort of RR MS patients had a low range of disability (median EDSS of 1.5). These findings suggest that FA changes related to clinical disability in RR MS patients with very low disability and relatively short disease duration are not widespread but localized to specific tracts of the WM. Moreover, these correlations were unchanged after correcting for age and disease duration, suggesting that our findings are not dependent on variables that may per se reduce FA values in the WM (38).
When assessing the contribution of parallel and perpendicular diffusivity in those WM regions showing significant associations of FA with MR and clinical measures, we found that these were driven predominantly by increases in perpendicular diffusivity. Changes in parallel and perpendicular diffusivity have been suggested to relate to axon or myelin damage, respectively, in mouse models of MS (18–21). Moreover, perpendicular diffusivity, together with FA and MD, was shown to be a robust predictor of myelin content in postmortem human brain, prior to and after fixation (39). However, this correspondence between axon and myelin damage and tensor diffusivities is still controversial. Thus, in the study by Cader et al (9), the significant relationship between local FA and normalized callosal cross-sectional area, assumed to be an indirect in vivo index of axon density in the corpus callosum (40), was determined primarily by increases in the perpendicular diffusivity. Furthermore, evidence from another study suggested that increases in perpendicular, but not parallel, diffusivity in NAWM may be deemed an in vivo “signature” of Wallerian degeneration (22). Finally, in a rat model of spinal MS, both parallel and perpendicular diffusivities were significantly associated with axon histopathological features distal to focal lesions (41). Thus, while we cannot resolve the histopathological implications of parallel versus perpendicular diffusivity, at least our study further attests to the predominant role of perpendicular diffusivity in clinical-morphological correlations in MS. Consistent with the global analysis on the WM skeleton, we also found that in the splenium of the corpus callosum and in a substantial part of the pyramidal tract, FA decreases related to increasing clinical disability were driven by significant increases in perpendicular diffusivity, without significant changes in the parallel one. Previous studies have found significant associations between EDSS score and increases in perpendicular diffusivity in the corpus callosum (9) and in the lateral columns of the spinal cord (corticospinal tracts) (12).
In conclusion, while the employed analysis techniques yielded some interesting insights and associations on a cross-sectional level, their use did not allow us to detect significant WM microstructural changes over time. We found no global FA changes over time across the whole WM and some voxelwise local FA changes disappeared after controlling for T2-lesion volume and for age. Other investigators, using histogram analysis, also failed to find DTI changes over 2 years in the whole and normal-appearing brain tissue in early RR MS patients (15). Moreover, in the same study, no significant differences in longitudinal rates of change between patients and normal controls were found (15). These negative results may suggest a limited role for global DTI assessment in following the early disease course in MS. Alternatively, other reasons may be the relatively short length of follow-up in both studies (2 years) and, at least here, the relatively small number of patients assessed over time, which might have underpowered the statistical analysis. Finally, also a beneficial effect of disease-modifying treatment in delaying the evolution of WM microstructural damage in MS patients of this study cannot be ruled out.
Acknowledgment
We thank Saad Jbabdi for help with image analysis.
References
- 1.Miller DH, Thompson AJ, Filippi M. Magnetic resonance studies of abnormalities in the normal appearing white matter and grey matter in multiple sclerosis. J Neurol. 2003;250:1407–1419. doi: 10.1007/s00415-003-0243-9. [DOI] [PubMed] [Google Scholar]
- 2.De Stefano N, Battaglini M, Smith SM. Measuring brain atrophy in multiple sclerosis. J Neuroimaging. 2007;17(Suppl 1):10S–15S. doi: 10.1111/j.1552-6569.2007.00130.x. [DOI] [PubMed] [Google Scholar]
- 3.Giorgio A, Battaglini M, Smith SM, De Stefano N. Brain atrophy assessment in multiple sclerosis: importance and limitations. Neuroimaging Clin N Am. 2008;18:675–686. doi: 10.1016/j.nic.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- 5.Griffin CM, Chard DT, Ciccarelli O, et al. Diffusion tensor imaging in early relapsing-remitting multiple sclerosis. Mult Scler (Houndmills, Basingstoke, England) 2001;7:290–297. doi: 10.1177/135245850100700504. [DOI] [PubMed] [Google Scholar]
- 6.Filippi M, Cercignani M, Inglese M, Horsfield MA, Comi G. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56:304–311. doi: 10.1212/wnl.56.3.304. [DOI] [PubMed] [Google Scholar]
- 7.Hasan KM, Gupta RK, Santos RM, Wolinsky JS, Narayana PA. Diffusion tensor fractional anisotropy of the normal-appearing seven segments of the corpus callosum in healthy adults and relapsing-remitting multiple sclerosis patients. J Magn Reson Imaging. 2005;21:735–743. doi: 10.1002/jmri.20296. [DOI] [PubMed] [Google Scholar]
- 8.Wilson M, Tench CR, Morgan PS, Blumhardt LD. Pyramidal tract mapping by diffusion tensor magnetic resonance imaging in multiple sclerosis: improving correlations with disability. J Neurol Neurosurg Psychiatry. 2003;74:203–207. doi: 10.1136/jnnp.74.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cader S, Johansen-Berg H, Wylezinska M, et al. Discordant white matter N-acetylasparate and diffusion MRI measures suggest that chronic metabolic dysfunction contributes to axonal pathology in multiple sclerosis. NeuroImage. 2007;36:19–27. doi: 10.1016/j.neuroimage.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 10.Ciccarelli O, Werring DJ, Wheeler-Kingshott CA, et al. Investigation of MS normal-appearing brain using diffusion tensor MRI with clinical correlations. Neurology. 2001;56:926–933. doi: 10.1212/wnl.56.7.926. [DOI] [PubMed] [Google Scholar]
- 11.Lin X, Tench CR, Morgan PS, Niepel G, Constantinescu CS. ‘Importance sampling’ in MS: use of diffusion tensor tractography to quantify pathology related to specific impairment. J Neurol Sci. 2005;237:13–19. doi: 10.1016/j.jns.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 12.Ciccarelli O, Wheeler-Kingshott CA, McLean MA, et al. Spinal cord spectroscopy and diffusion-based tractography to assess acute disability in multiple sclerosis. Brain. 2007;130:2220–2231. doi: 10.1093/brain/awm152. [DOI] [PubMed] [Google Scholar]
- 13.Caramia F, Pantano P, Di Legge S, et al. A longitudinal study of MR diffusion changes in normal appearing white matter of patients with early multiple sclerosis. Magn Reson Imaging. 2002;20:383–388. doi: 10.1016/s0730-725x(02)00519-2. [DOI] [PubMed] [Google Scholar]
- 14.Schmierer K, Altmann DR, Kassim N, et al. Progressive change in primary progressive multiple sclerosis normal-appearing white matter: a serial diffusion magnetic resonance imaging study. Mult Scler (Houndmills, Basingstoke, England) 2004;10:182–187. doi: 10.1191/1352458504ms996oa. [DOI] [PubMed] [Google Scholar]
- 15.Rashid W, Hadjiprocopis A, Davies G, et al. Longitudinal evaluation of clinically early relapsing-remitting multiple sclerosis with diffusion tensor imaging. J Neurol. 2008;255:390–397. doi: 10.1007/s00415-008-0678-0. [DOI] [PubMed] [Google Scholar]
- 16.Cassol E, Ranjeva JP, Ibarrola D, et al. Diffusion tensor imaging in multiple sclerosis: a tool for monitoring changes in normalappearing white matter. Mult Scler (Houndmills, Basingstoke, England) 2004;10:188–196. doi: 10.1191/1352458504ms997oa. [DOI] [PubMed] [Google Scholar]
- 17.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 18.Budde MD, Kim JH, Liang HF, Russell JH, Cross AH, Song SK. Axonal injury detected by in vivo diffusion tensor imaging correlates with neurological disability in a mouse model of multiple sclerosis. NMR Biomed. 2008;21:589–597. doi: 10.1002/nbm.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 20.Sun SW, Liang HF, Schmidt RE, Cross AH, Song SK. Selective vulnerability of cerebral white matter in a murine model of multiple sclerosis detected using diffusion tensor imaging. Neurobiol Dis. 2007;28:30–38. doi: 10.1016/j.nbd.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Q, Butzkueven H, Gresle M, et al. MR diffusion changes correlate with ultra-structurally defined axonal degeneration in murine optic nerve. NeuroImage. 2007;37:1138–1147. doi: 10.1016/j.neuroimage.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 22.Henry RG, Oh J, Nelson SJ, Pelletier D. Directional diffusion in relapsing-remitting multiple sclerosis: a possible in vivo signature of Wallerian degeneration. J Magn Reson Imaging. 2003;18:420–426. doi: 10.1002/jmri.10379. [DOI] [PubMed] [Google Scholar]
- 23.Oh J, Henry RG, Genain C, Nelson SJ, Pelletier D. Mechanisms of normal appearing corpus callosum injury related to pericallosal T1 lesions in multiple sclerosis using directional diffusion tensor and 1H MRS imaging. J Neurol Neurosurg Psychiatry. 2004;75:1281–1286. doi: 10.1136/jnnp.2004.039032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin F, Yu C, Jiang T, Li K, Chan P. Diffusion tensor tractography-based group mapping of the pyramidal tract in relapsing-remitting multiple sclerosis patients. AJNR Am J Neuroradiol. 2007;28:278–282. [PMC free article] [PubMed] [Google Scholar]
- 25.Lowe MJ, Beall EB, Sakaie KE, et al. Resting state sensorimotor functional connectivity in multiple sclerosis inversely correlates with transcallosal motor pathway transverse diffusivity. Hum Brain Mapp. 2008;29:818–827. doi: 10.1002/hbm.20576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warlop NP, Achten E, Debruyne J, Vingerhoets G. Diffusion weighted callosal integrity reflects interhemispheric communication efficiency in multiple sclerosis. Neuropsychologia. 2008;46:2258–2264. doi: 10.1016/j.neuropsychologia.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 27.Miller D, Weinshenker BFM, et al. Differential diagnosis of suspected multiple sclerosis: a consensus approach. Mult Scler. 2008;14:1157–1174. doi: 10.1177/1352458508096878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polman C, Reingold S, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald criteria.”. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 29.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 30.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen IV, McQuaid S, Mirakhur M, Nevin G. Pathological abnormalities in the normal-appearing white matter in multiple sclerosis. Neurol Sci. 2001;22:141–144. doi: 10.1007/s100720170012. [DOI] [PubMed] [Google Scholar]
- 32.Trapp BD, Bo L, Mork S, Chang A. Pathogenesis of tissue injury in MS lesions. J Neuroimmunol. 1999;98:49–56. doi: 10.1016/s0165-5728(99)00081-8. [DOI] [PubMed] [Google Scholar]
- 33.Rovaris M, Gass A, Bammer R, et al. Diffusion MRI in multiple sclerosis. Neurology. 2005;65:1526–1532. doi: 10.1212/01.wnl.0000184471.83948.e0. [DOI] [PubMed] [Google Scholar]
- 34.Ciccarelli O, Werring DJ, Barker GJ, et al. A study of the mechanisms of normal-appearing white matter damage in multiple sclerosis using diffusion tensor imaging—evidence of Wallerian degeneration. J Neurol. 2003;250:287–292. doi: 10.1007/s00415-003-0992-5. [DOI] [PubMed] [Google Scholar]
- 35.Ge Y, Law M, Johnson G, et al. Preferential occult injury of corpus callosum in multiple sclerosis measured by diffusion tensor imaging. J Magn Reson Imaging. 2004;20:1–7. doi: 10.1002/jmri.20083. [DOI] [PubMed] [Google Scholar]
- 36.Pagani E, Filippi M, Rocca MA, Horsfield MA. A method for obtaining tract-specific diffusion tensor MRI measurements in the presence of disease: application to patients with clinically isolated syndromes suggestive of multiple sclerosis. NeuroImage. 2005;26:258–265. doi: 10.1016/j.neuroimage.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Bammer R, Augustin M, Strasser-Fuchs S, et al. Magnetic resonance diffusion tensor imaging for characterizing diffuse and focal white matter abnormalities in multiple sclerosis. Magn Reson Med. 2000;44:583–591. doi: 10.1002/1522-2594(200010)44:4<583::aid-mrm12>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 38.Salat DH, Tuch DS, Greve DN, et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005;26:1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 39.Schmierer K, Wheeler-Kingshott CA, Tozer DJ, et al. Quantitative magnetic resonance of postmortem multiple sclerosis brain before and after fixation. Magn Reson Med. 2008;59:268–277. doi: 10.1002/mrm.21487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evangelou N, Esiri MM, Smith S, Palace J, Matthews PM. Quantitative pathological evidence for axonal loss in normal appearing white matter in multiple sclerosis. Ann Neurol. 2000;47:391–395. [PubMed] [Google Scholar]
- 41.DeBoy CA, Zhang J, Dike S, et al. High resolution diffusion tensor imaging of axonal damage in focal inflammatory and demyelinating lesions in rat spinal cord. Brain. 2007;130:2199–2210. doi: 10.1093/brain/awm122. [DOI] [PubMed] [Google Scholar]