Summary
We report a high seroprevalence of hepatitis E virus (HEV) in pigs in the Lao PDR. HEV seroprevalence was 51.2% (300/586) amongst abattoir pigs and 15.3% (46/301) amongst village pigs. The age distribution suggested previous in-village HEV pig infections. These findings suggest a zoonotic risk associated with village-based smallholder pig farming.
Keywords: Hepatitis E virus, HEV, Smallholder farming, Pigs, Laos
1. Introduction
Hepatitis E virus (HEV) is an enterically transmitted zoonotic viral agent that causes acute jaundice in humans with evidence that pigs and other mammals play a role in maintenance and transmission. In mainland Southeast Asia, human HEV infections have been described in Vietnam and Thailand (Corwin et al., 1996; Meng et al., 1999) and recently in Lao PDR (Laos), where of 400 patients admitted to hospital in Vientiane with hepatitis or jaundice, 15.4% had evidence of past infection with hepatitis E and 7 (1.7%) had serology and/or PCR evidence for acute hepatitis E infection (P.N. Newton, unpublished results). The people of Laos are largely rural and reliant on village-based agriculture. In many villages pigs are an important source of food and cash. In this study we examined the seroprevalence of HEV antibodies in pigs in villages and at abattoirs throughout Laos to better understand the potential role of pigs in the transmission of HEV to humans.
2. Materials and methods
No animal samples were obtained specifically for this study. Pig sera from two archival collections that were originally collected for classical swine fever virus sero-surveillance were tested: (a) sera were collected from pigs (n = 1912) being slaughtered at abattoirs in 16 provinces (89% of the total of 18 provinces) between January and August 2001, and a subset was selected using random number tables (n = 586; 30.6%) aiming for 40 sera per province when possible (Table 1); (b) randomly selected pigs were venesected in villages in March 1998 in all nine districts of Vientiane Municipality, resulting in the sampling of 301 pigs (total n = 1902; 15.9%) from 50 randomly selected villages (Table 1). Samples were stored at −20°C until analysis.
Table 1. HEV seropositivity for pig sera collected at slaughter and in villages.
| Province | Districta | Sampleb | Collection period | n | HEV positive villagesc n (%) | HEV Ig positive n (%) |
|---|---|---|---|---|---|---|
| Attapeu | N/A | A | Jan–Aug 2001 | 39 | NR | 14 (36.0) |
| Bhorikamxai | N/A | A | Jan–July 2001 | 40 | NR | 20 (50.0) |
| Bokeo | N/A | A | Jan–July 2001 | 40 | NR | 14 (35.0) |
| Champassack | N/A | A | Jan–Aug 2001 | 40 | NR | 10 (25.0) |
| Huaphan | N/A | A | May 2001 | 7 | NR | 6 (85.7) |
| Khammouane | N/A | A | Jan–Aug 2001 | 40 | NR | 22 (55.0) |
| Luang Namtha | N/A | A | Jan–Aug 2001 | 40 | NR | 25 (62.5) |
| Luang Prabang | N/A | A | Feb–June 2001 | 17 | NR | 8 (47.1) |
| Oudomxai | N/A | A | Jan–Aug 2001 | 40 | NR | 24 (60.0) |
| Phongsaly | N/A | A | Jan–Aug 2001 | 40 | NR | 16 (40.0) |
| Salavane | N/A | A | Jan–July 2001 | 40 | NR | 14 (35.0) |
| Savannakhet | N/A | A | Jan–July 2001 | 40 | NR | 30 (75.0) |
| Sekong | N/A | A | Jan–July 2001 | 40 | NR | 29 (72.5) |
| Vientiane Municipality | N/A | A | Jan–Aug 2001 | 40 | NR | 19 (47.5) |
| Xayabuly | N/A | A | Jan–July 2001 | 40 | NR | 18 (45.0) |
| Xieng Kouang | N/A | A | Jan–Aug 2001 | 43 | NR | 31 (72.1) |
| Total | A | 586 | 300 (51.2) | |||
| Vientiane Municipality | Hadxaifong | V | March 1998 | 31 | 5 (83.3) | 10 (31.3) |
| Vientiane Municipality | Pak Ngeum | V | March 1998 | 68 | 3 (33.3) | 6 (8.8) |
| Vientiane Municipality | Xaithany | V | March 1998 | 36 | 2 (33.3) | 5 (13.9) |
| Vientiane Municipality | Sikottabong | V | March 1998 | 55 | 2 (20.0) | 7 (12.7) |
| Vientiane Municipality | Sisattanak | V | March 1998 | 22 | 2 (66.6) | 3 (13.6) |
| Vientiane Municipality | Naxaithong | V | March 1998 | 22 | 2 (50.0) | 2 (9.1) |
| Vientiane Municipality | Chantabuly | V | March 1998 | 31 | 4 (57.1) | 7 (22.6) |
| Vientiane Municipality | Sangthong | V | March 1998 | 23 | 1 (33.3) | 3 (13.0) |
| Vientiane Municipality | Xaysettha | V | March 1998 | 13 | 1 (50.0) | 3 (23.1) |
| Total | V | 301 | 23 (46.0) | 46 (15.3) |
N/A: not available.
A: abattoir pigs; V: village pigs.
NR: not relevant.
Detection of HEV Ig was performed with an ELISA (Innis et al., 2002), using a recombinant capsid protein antigen of HEV (rHEV) Sargodha 1987 strain. The positivity cut-off (>40WR U/ml of total Ig) was determined by calculating the mean WR U/ml ± 3 SD of 30 Thai HEV-negative pigs. Samples that were ELISA positive were confirmed by Western blotting using SDS-PAGE 12% pre-cast gel (NuPAGE Novex, Invitrogen, Carlsbad, CA, USA) and nitrocellulose WB strips, and were considered positive for rHEV capsid protein antibody if positive and negative controls gave expected results and a band was clearly visible at 56 kDa MW.
Data analysis was performed using STATA version 8 (Stata Corp., College Station, TX, USA). Sample HEV IgG prevalence was calculated as the proportion of HEV serologically positive pigs in the sampled population (village, district, province or age group) and significant differences between sample groups (P ≤ 0.05) were determined using Student’s t-test for paired samples and ANOVA.
3. Results and discussion
The average HEV seroprevalence for abattoir pig samples was 51.2% (300/586), with provincial prevalence rates ranging from 25.0% (Champassak) to 85.7% (Huaphan) (Table 1). Among village pigs in Vientiane Municipality, the overall HEV seroprevalence was 15.3% (46/301); district seroprevalence rates ranged from 8.8% (Pak Ngeum) to 31.3% (Hadx-aifong) (Table 1). HEV seropositive pigs were recognized in 23 villages (46.0%) in Vientiane Municipality. There were no significant differences in the HEV seroprevalence between provinces and districts (P>0.05).
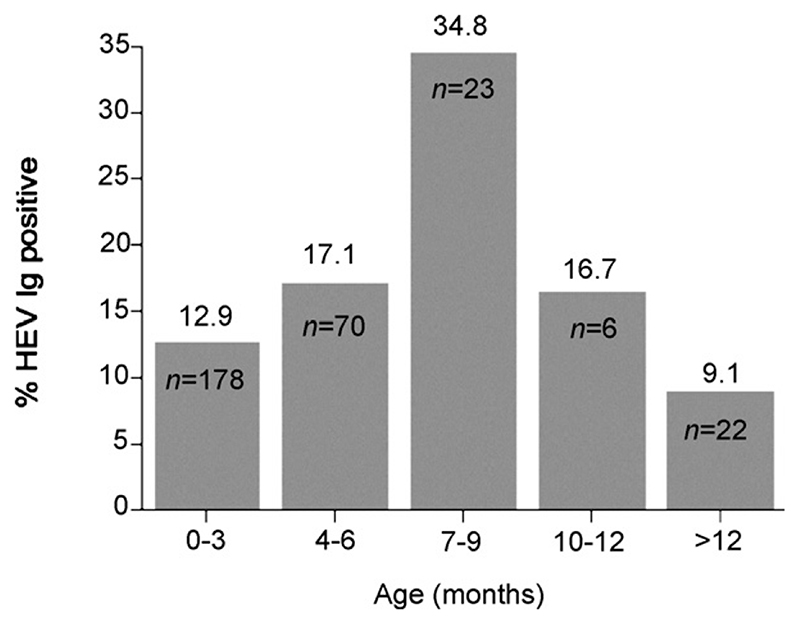
The age-stratified seroprevalence suggests previous HEV infections in village-raised pigs. While passively acquired maternal antibodies would be detected up to approximately 3 months of age, the results suggest new HEV infections beyond 3 months with seroprevalence peaking at 7–9 months (Figure 1). Similar patterns of HEV age-related seropositivity have been described in other studies with peaks between 4 and 6 months of age (Meng et al., 1999; Wibawa et al., 2004) with lower seroprevalence rates in adult pigs (Meng et al., 1999). Age dependent differences in HEV seroprevalence rates may explain the higher seroprevalence in abattoir samples as these pigs would generally be from the 6–9 month age range. However, differences between village and abattoir samples may also be caused by other factors such as geographical locality, and farming and husbandry practises.
Figure 1. Comparison of HEV seropositivity in pigs of different age ranges.
The high level of pig HEV seropositivity, the suggestion of acute HEV infections in pigs, frequent mixing of pigs and humans, and poor sanitation in Lao villages suggests the potential for pig to human transmission (Wibawa et al., 2004). The common practice in Lao villages of fertilizing vegetables with pig manure and raising pigs over fishponds may provide additional sources for human HEV infection. Furthermore, the consumption of uncooked pig meat, a common practice in Laos, is recognized as a potential route of human HEV infection (Wibawa et al., 2004) and a source for Trichinella spiralis infection (Sicard et al., 1976). Raising the awareness of the importance of the adequate cooking of meat, the boiling of drinking water and washing of vegetables are likely to be effective public health interventions to prevent HEV infections. However, to conclusively establish a link between pig and human HEV infections in Laos further genetic and epidemiological studies are required.
Acknowledgements
This study was funded by the Wellcome Trust of Great Britain, as part of the Wellcome Trust-Mahosot Hospital-Oxford University Tropical Medicine Research Collaboration. We wish to thank Duangrat Mongkolsirichaikul and Kittinun Hussem of the Armed Forces Research Institute for Medical Science, Thailand, for the hepatitis E virus diagnostic testing. The collection of the pig sera was funded by the Australian Center for International Agricultural Research, the Department of Livestock and Fisheries of Lao PDR and CSIRO Livestock Industries during project AS1/9438. We thank Dr Rattanaphone Phetsouvanh, Dr Mayfong Mayxay, the Directors of Mahosot Hospital and the Ministries of Health, and Agriculture and Forestry of the Lao PDR for their support.
Footnotes
Conflicts of interest statement
The authors have no conflicts concerning the work described in this paper.
References
- Corwin AL, Khiem HB, Clayson ET, Pham KS, Vo TT, Vu TY, Cao TT, Vaughn D, Merven J, Richie TL, Putri MP, et al. A water-borne outbreak of hepatitis E virus transmission in southwestern Vietnam. Am J Trop Med Hyg. 1996;54:559–562. doi: 10.4269/ajtmh.1996.54.559. [DOI] [PubMed] [Google Scholar]
- Innis BL, Seriwatana J, Robinson RA, Shrestha MP, Yarbough PO, Longer CF, Scott RM, Vaughn DW, Myint KS. Quantitation of immunoglobulin to hepatitis E virus by enzyme immunoassay. Clin Diagn Lab Immunol. 2002;9:639–648. doi: 10.1128/CDLI.9.3.639-648.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XJ, Dea S, Engle RE, Friendship R, Lyoo YS, Sirinarumitr T, Urairong K, Wang D, Wong D, Yoo D, Zhang Y, et al. Prevalence of antibodies to the hepatitis E virus in pigs from countries where hepatitis E is common or is rare in the human population. J Med Virol. 1999;59:297–302. [PubMed] [Google Scholar]
- Sicard D, Fontan R, Richard-Lenoble D, Gentilini M. Human trichinosis. A recent epidemic in Vientiane (Laos) (apropos of 32 cases) Bull Soc Pathol Exot Filiales. 1976;521:521–525. [PubMed] [Google Scholar]
- Wibawa ID, Muljono DH, Mulyanto, Suryadarma IG, Tsuda F, Takahashi M, Nishizawa T, Okamoto H. Prevalence of antibodies to hepatitis E virus among apparently healthy humans and pigs in Bali, Indonesia: Identification of a pig infected with a genotype 4 hepatitis E virus. J Med Virol. 2004;73:38–44. doi: 10.1002/jmv.20059. [DOI] [PubMed] [Google Scholar]