Abstract
Background and Purpose
Blood pressure variability (BPV) from beat-to-beat is associated with an increased risk of cardiovascular events and enables rapid assessment of BPV, but the underlying causes of elevated BPV are unclear.
Methods
In consecutive patients within 4-6 weeks of TIA or non-disabling stroke (Oxford Vascular Study), continuous non-invasive BP was measured beat-to-beat over 5 minutes (Finometer). Arterial stiffness was measured by carotid-femoral pulse wave velocity (PWV, Sphygmocor). After automated and manual data cleaning, associations between BP variability (rCV, residual coefficient of variation), demographic factors and arterial stiffness were determined for both systolic (SBP) and diastolic (DBP) BP, by ANOVA and linear models. Relationships between demographic factors and arterial stiffness were determined by interaction terms and mediation.
Results
Among 1013 patients, 54 (5.3%) were in AF and 51 (5%) had low quality recordings. In a general linear model including the remaining 908 participants, SBPV was most strongly associated with age (p=0.00003), BMI (p=0.003), and arterial stiffness (p=0.008), with weaker independent associations with current smoking (p=0.01) and a low diastolic BP (p=0.046). However, whilst there was a linear increase in SBPV with BMI in men, in women SBPV was lowest for a BMI in the normal range, but was greater below 20 or above 30 (ANOVA p=0.012, BMI-sex interaction p=0.03). Although BMI and PWV were partially independent, increased PWV mediated approximately 32% of the relationship between increased BMI and SBPV (p<0.001).
Conclusions
Vascular aging, manifest as arterial stiffness, was a strong determinant of increased SBPV and partially mediated the effect of increased BMI. However, although high BMI was independently associated with SBPV in both sexes, a low BMI was associated with increased SBPV only in women. SBPV may partially mediate the relationship between BMI and cardiovascular events, whilst obesity may provide a modifiable target to reduce SBPV and cardiovascular events.
Introduction
Patients with episodic hypertension after a cerebrovascular event have a high risk of recurrent stroke,1, 2 residual visit-to-visit variability in blood pressure (BPV) on treatment has a poor prognosis despite good control of mean BP,3 and benefits of some antihypertensive drugs in the prevention of stroke may partly result from reduced variability in SBP.4–6 Strong associations between visit-to-visit BP variability, cardiovascular events, renal impairment and cognitive decline have now been demonstrated in population-based cohorts,7, 8 patients with diabetes,9 renal impairment,10 cognitive decline11 and other groups,12 with similar predictive value of BP variability from day-to-day on home readings,13–15 with a significant reduction in BPV with specific antihypertensives.4, 6 However, both visit-to-visit and home BP variability require a prolonged period of assessment, good patient compliance and follow-up visits, limiting their use in acute assessment or for assessing treatment response in clinical practice and future trials.
Variability in blood pressure from one beat to the next (beat-to-beat BPV) is also associated with an increased risk of recurrent stroke or cardiovascular events in patients with a TIA or minor stroke, with a similar physiological profile to home day-to-day BPV16 and has at least similar predictive value,15 whilst enabling BPV assessment at a single visit. However, the determinants of beat-to-beat BPV in at-risk individuals, and its covariance with other major cardiovascular risk factors is unclear. Furthermore, beat-to-beat BPV is itself composed of multiple components contributing to total BPV, from physiological rhythms reflecting breathing and intact autonomic function to increased BPV associated with stiff arteries and impaired baroreceptor function in older patients with impaired compensatory mechanisms.16 To assess the potential clinical utility of beat-to-beat BPV, and to identify populations at an increased risk of elevated BPV, it is necessary to understand the determinants of beat-to-beat BPV in at risk populations, and how this varies by demographic characteristics.
Therefore, in patients attending a TIA and minor stroke clinic, we determined association between beat-to-beat BPV variability with arterial stiffness and major cardiovascular risk factors and which demographic features determine a likely pathological excess of beat-to-beat BPV.
Methods
Study population
Consecutive, consenting patients with TIA or minor stroke (NIHSS <5) were recruited between September 2010 and October 2019, as part of the Phenotyped Cohort of the Oxford Vascular Study (OXVASC).15, 16 Participants were recruited at the OXVASC daily emergency assessment clinic, following a referral after attendance at the Emergency Department or from primary care, usually within 24 hours. Patients were referred after transient neurological symptoms or symptoms consistent with a minor stroke, not requiring direct admission to hospital. The OXVASC population consists of >92,000 individuals registered with about 100 primary-care physicians in Oxfordshire, UK.17 All consenting patients had a standardised medical history and examination, ECG, blood tests and a stroke protocol MRI brain and contrast-enhanced MRA (or CT-brain and carotid Doppler ultrasound or CT-angiogram), an echocardiogram and 5 day ambulatory cardiac monitor. All patients were assessed by a study physician, reviewed by the senior study neurologist (PMR) and are followed-up face-to-face at 1, 3, 6 and 12 months, and 2, 5 and 10 years. Medication is standardly prescribed according to guidelines, most commonly with dual antiplatelets (aspirin and clopidogrel), high dose statins (atorvastatin 40-80mg) and a combination of perindopril and indapamide, with the possible addition of amlodipine, to reach a target of <130/80 on home telemetric blood pressure monitoring.16 Access to the data will be openly considered on application to the chief investigator (PMR).
As part of the OXVASC Phenotyped Cohort, a routine prospective cardiovascular physiological assessment is performed at the 1 month follow-up visit between September 2010 and September 2019. Participants were excluded if they were under 18 years, had severe cognitive impairment, were pregnant or had autonomic failure, a recent myocardial infarction, unstable angina, heart failure (NYHA 3-4 or ejection fraction <40%) or untreated bilateral carotid stenosis (>70%). OXVASC is approved by the Oxfordshire Research Ethics Committee.
After 15-20 minutes supine rest, beat-to-beat BPV was measured over 5 minutes in a quiet, dimly-lit, temperature-controlled room (21-230C). Continuous ECG and non-invasive blood pressure were acquired at 200Hz (Finometer, Finapres Medical Systems, The Netherlands), via a Powerlab 8/30 with LabChart Pro software (ADInstruments, USA). Waveforms were preferentially recorded from the middle phalanx of the middle finger. Automated calibration (‘Physiocal’) was performed until the recording was stable, but turned off during each test. Estimated brachial waveforms (Finometer) were calibrated offline by linear regression to 2-3 supine, oscillometric brachial readings, performed immediately prior to the monitoring period on the contralateral arm, with manual exclusion of artefacts. In patients with a significant deterioration in recording quality during the first five minutes, the test was stopped, and the calibration procedure repeated. If necessary the finger cuff was moved to an adjacent finger or the proximal phalanx of the same finger, or the hand was warmed with a hand warmer. Prior to physiological assessment, two sitting clinic BPs, 5 minutes apart, were measured at ascertainment and one month in the non-dominant arm, by trained personnel.
Analysis
BPV on beat-to-beat monitoring was calculated over 5 minutes. Ectopic beats and artefacts were automatically detected from the R-R interval of the ECG, visually reviewed and removed by linear interpolation of R-R interval. Blood pressure artefacts were automatically detected, manually reviewed, and removed by linear interpolation to adjacent normal beats with in-house software. Patients in atrial fibrillation during the recording were excluded. Systolic and diastolic BPV were calculated as the standard deviation (SD) and the coefficient of variation (CV=SD/mean), before and after de-trending of the recording about a linear regression across 5 minutes. All recordings were reviewed blinded to clinical data, after automated and manual data cleaning, to assess for the quality of recording (3- excellent quality; 2- adequate quality for analysis; 3-unuseable, poor quality recording), based upon the presence of artefacts or drift in the baseline measurement.
Associations with demographic indices were determined by general linear models and by logistic regression for the top decile of BPV on each index. Models were performed for univariate associations; adjusted for age and sex and for age, sex and cardiovascular risk factors at study entry (current smoking, history of hypertension, diabetes).
Analyses were performed in R, Matlab r2015 or Windows Excel.
Results
1031 assessments were performed in 1013 eligible, consecutive, consenting patients between September 2010 and October 2019, with 18 patients assessed twice after separate clinical presentations. 54 / 1013 (5.3%) patients were in atrial fibrillation during the recording, whilst 51 (5%) patients had inadequate recordings. Patients with atrial fibrillation or poor recording quality were older, had higher blood pressure and were more likely to have a history of hypertension (table 1).
Table 1. Characteristics of patients included and excluded from the analysis.
Participants were excluded if they were in AF during the assessment, or if their recording was of poor quality with a high probability of artefactual recordings. p-values are given for ANOVA for continuous variables, and chi-squared tests for categorical variables. AF = atrial fibrillation; FHx = family history; BP = blood pressure.
| All | Included | In AF | Poor data | p-val |
|---|---|---|---|---|
| 1013 | 908 (90) | 54 (5) | 51 (5) | |
| 67 (13.3) | 66.1 (13.3) | 77.8 (8.9) | 72.2 (11.1) | <0.001 |
| 427 (42) | 387 (43) | 18 (33) | 22 (44.9) | 0.35 |
| 453 (44) | 390 (43) | 36 (67) | 27 (55.1) | <0.001 |
| 116 (11) | 104 (11.5) | 7 (13) | 5 (10.2) | 0.88 |
| 160 (15) | 148 (16) | 6 (11) | 6 (12.2) | 0.39 |
| 535 (53) | 478 (53) | 32 (59) | 24 (49) | 0.62 |
| 303 (30) | 274 (30) | 12 (22) | 17 (34.7) | 0.12 |
| 54 (5.3) | - | 54 (100) | - | - |
| 74 (7) | 37 (4.1) | 35 (65) | 2 (4.1) | <0.001 |
| 314 (31) | 277 (31) | 20 (37) | 16 (32.7) | 0.67 |
| 16 (1.6) | 10 (1.1) | 6 (11) | - | <0.001 |
| 133 (19) | 132 (18) | 137 (24) | 145 (27) | <0.001 |
| 78 (12) | 78 (11) | 83 (15) | 77 (8.9) | 0.01 |
Mean SBP was strongly correlated with SD of SBP (supplementary figure 1), but with no correlation with CV-SBP or CV-DBP, before or after detrending of the recording (rCV). However, there was an inverse correlation between CV-DBP and mean DBP before and after detrending (supplementary figure 1).
Beat-to-beat SBP variability increased with age (table 2) with a slightly greater SBPV in women, that was not significant after adjustment for age. The other key univariate and adjusted associations of increased beat-to-beat SBPV were increased BMI and increased arterial stiffness (table 2). After adjustment for age, sex and other cardiovascular risk factors, current smoking was also associated with increased SBPV. A history of hypertension, diabetes or dyslipidaemia were not associated. Only age was associated with DBPV in univariate analysis, although BMI was associated with DBPV after adjustment for age, sex and cardiovascular risk factors (supplementary table 2).
Table 2. Linear models for baseline demographic measures and systolic blood pressure (SBP) level and variability.
SBP variability is analysed as the residual coefficient of variation (rCV), unadjusted or adjusted (adj) for age and sex, or for age, sex and cardiovascular risk factors (CV-RFs). Independent variables include hypertension (HTN) reported by patients at baseline, or defined by patient report and current treatment at baseline. Current treatment and pulse wave velocity measures are given at the time of the physiological assessment. p-val – p value; LDL – low density lipoprotein; HDL – high density lipoprotein; TSH – thyroid stimulating hormone; BMI – body mass index.
| Unadjusted | Adj. Age+Sex | Adj CV-RFs | Unadjusted | Adj. Age+Sex | Adj CV-RFs | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | p-val | β | p-val | β | p-val | β | p-val | β | p-val | β | ||
| 0.34 | <0.001* | 0.34 | <0.001* | 0.35 | <0.001* | 0.02 | 0.001* | 0.02 | 0.001* | 0.02 | ||
| 0.47 | 0.692 | -0.13 | 0.909 | -0.15 | 0.898 | 0.27 | 0.039* | 0.24 | 0.064 | 0.26 | 0.057 | |
| 7.83 | <0.001* | 5.51 | <0.001* | 6.33 | <0.001* | 0.03 | 0.804 | -0.11 | 0.415 | -0.06 | 0.728 | |
| 5.04 | <0.001* | 1.93 | 0.171 | 2.26 | 0.124 | -0.07 | 0.636 | -0.26 | 0.111 | -0.25 | 0.139 | |
| 1.62 | 0.377 | 1.21 | 0.499 | 1.09 | 0.547 | 0.31 | 0.123 | 0.33 | 0.109 | 0.39 | 0.056 | |
| 1.4 | 0.274 | -0.53 | 0.675 | -0.87 | 0.526 | -0.15 | 0.278 | -0.23 | 0.119 | -0.24 | 0.115 | |
| 1.52 | 0.313 | -0.69 | 0.645 | -1.85 | 0.239 | -0.15 | 0.383 | -0.24 | 0.162 | -0.27 | 0.123 | |
| -0.13 | 0.91 | 0.39 | 0.738 | -0.75 | 0.561 | 0.04 | 0.793 | 0.11 | 0.418 | 0.01 | 0.958 | |
| -0.6 | 0.708 | 3.09 | 0.055 | 3.61 | 0.041* | 0.18 | 0.311 | 0.41 | 0.026* | 0.42 | 0.036* | |
| -3.89 | 0.877 | 2.83 | 0.908 | 3.52 | 0.885 | -1.12 | 0.689 | -0.24 | 0.932 | -0.06 | 0.982 | |
| -0.01 | 0.732 | 0.02 | 0.443 | 0.01 | 0.571 | 0 | 0.21 | 0.01 | 0.024* | 0.01 | 0.032* | |
| -0.1 | 0.411 | 0.02 | 0.842 | 0.01 | 0.938 | 0.03 | 0.046* | 0.03 | 0.01* | 0.04 | 0.005* | |
| 0 | 0.958 | -0.06 | 0.085 | -0.06 | 0.077 | 0 | 0.503 | 0 | 0.294 | 0 | 0.243 | |
| -0.16 | 0.68 | 0.1 | 0.789 | 0.17 | 0.654 | 0.01 | 0.759 | 0.01 | 0.805 | 0.03 | 0.506 | |
| -0.01 | 0.555 | -0.01 | 0.564 | -0.01 | 0.593 | 0 | 0.282 | 0 | 0.229 | 0 | 0.285 | |
| 1.16 | 0.323 | 0.32 | 0.793 | 0.71 | 0.563 | -0.05 | 0.704 | -0.2 | 0.127 | -0.2 | 0.15 | |
| 0.23 | 0.487 | 0.13 | 0.684 | 0.19 | 0.558 | -0.05 | 0.139 | -0.06 | 0.105 | -0.05 | 0.13 | |
| 4.18 | 0.002* | 1.11 | 0.42 | -5.96 | 0.168 | -0.04 | 0.805 | -0.21 | 0.175 | 0.16 | 0.736 | |
| 1.11 | 0.439 | -0.79 | 0.578 | -1.19 | 0.736 | -0.2 | 0.215 | -0.28 | 0.078 | -0.58 | 0.143 | |
| Pulse Wave Velocity | 2.43 | <0.001 | 2.29 | <0.001 | 2.35 | <0.001 | 0.14 | <0.001 | 0.11 | 0.002 | 0.11 | 0.002 |
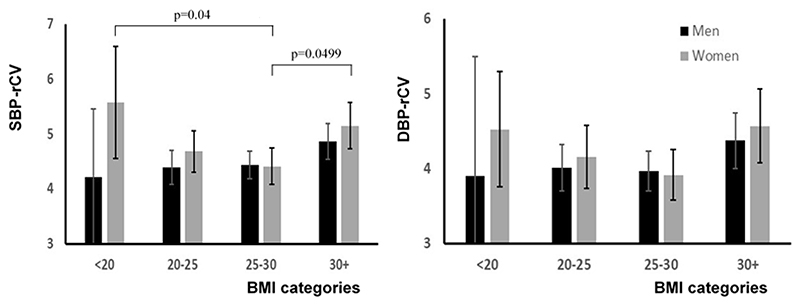
BMI was the strongest clinical risk factor associated with increased BPV. It was not associated with mean or maximum SBP but only with SBPV (supplementary figure 2), and was negatively correlated with age (p<0.0001). There was a significant difference in BPV between standardly defined BMI groups in women (ANOVA p=0.012), with a U-shaped relationship (figure 1), such that SBPV in women with a BMI between 25-30 was significantly lower than women with a BMI below 20 (post-hoc Tukey HSD p=0.04) or greater than 30 (p=0.0499). The elevated SBPV in women with a low BMI did not reflect a specific excess of high BPV in this group, with a similarly shaped distribution of SBPV across different levels of BMI. In contrast, there was no difference between absolute BMI categories in men (figure 2), with a linear increase in BPV when BMI was defined by quintiles (supplementary figure 1). This pattern predominantly reflected differences in weight, with a more linear increase in BPV across quintiles of weight in men, with no change across quintiles of height (supplementary figure 2).
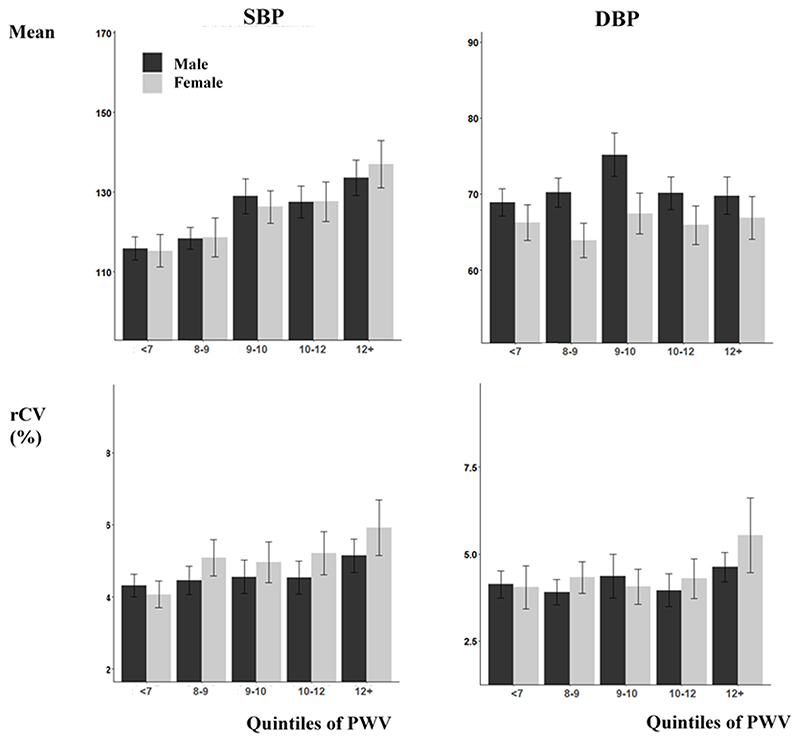
Figure 1. Relationship between increased carotid-femoral pulse wave velocity beat-to-beat BP variability, stratified by sex.
Results are presented as the mean and confidence interval for each group. rCV is calculated as the coefficient of variation of de-trended recordings. Y-axes are set to the 95% range of each measure across the whole population.
Figure 2. Mean values of rCV for systolic (SBP) and diastolic (DBP), stratified by sex and standard thresholds for BMI.
Results are presented as the mean and confidence interval for each group. rCV is calculated as the coefficient of variation of de-trended recordings. There is a significant difference between groups for SBP-rCV for women only (ANOVA p=0.014), with significant post-hoc tests adjusted for multiple comparisons shown (Tukey test).
The difference in the pattern of change with BMI between sexes was evident in a multivariate model, with a significant association between BMI and SBPV or DBPV when including all indices significantly associated with BPV after adjustment for age, sex and CV risk factors (table 2). Furthermore, there was a significant interaction between BMI with sex for SBPV, but not for DBPV, reflecting the linear increase in SBPV in men but not women. This was confirmed on stratifying the population by sex, with a significant association between BMI and BPV in men, exceeding even the association between age and BPV, whilst in women there was no overall linear association with the only strong determinant being the relationships between age and low DBP with SBPV (table 3), reflecting the non-linear pattern in women.
Table 3. Multivariate associations and interactions between BMI and sex for variability in systolic (SBP) and diastolic (DBP) blood pressure.
Beta-coefficients and p-values (p-val) are given from general linear models, adjusted for significant covariates and for the interaction between BMI and sex, or stratified by sex. BP variability is measured as the residual coefficient of variation after de-trending of data (rCV).
| SBP | DBP | SBP | DBP | ||||
|---|---|---|---|---|---|---|---|
| β | p-val | β | p-val | β | p-val | β | p-val |
| Men | |||||||
| 0.02 | 0.0003** | 0.05 | 0.33 | 0.02 | 0.01* | 0.05 | 0.49 |
| 0.22 | 0.11 | 0.69 | 0.65 | - | - | - | - |
| -0.01 | 0.046* | -0.07 | 0.21 | -0.01 | 0.29 | -0.05 | 0.48 |
| 0.47 | 0.01* | 3.6 | 0.09 | 0.39 | 0.07 | 3.0 | 0.23 |
| 0.04 | 0.003** | 0.35 | 0.02* | 0.07 | 0.0002** | 0.49 | 0.02* |
| Women | |||||||
| 0.02 | 0.0002** | 0.06 | 0.31 | 0.03 | 0.004** | 0.07 | 0.43 |
| 1.78 | 0.015* | 9.1 | 0.26 | - | - | - | - |
| -0.01 | 0.03* | -0.07 | 0.18 | -0.02 | 0.04* | -0.12 | 0.22 |
| 0.50 | 0.01* | 3.7 | 0.08 | 0.66 | 0.06 | 4.9 | 0.20 |
| 0.07 | 0.0003** | 0.51 | 0.02* | 0.02 | 0.45 | 0.2 | 0.35 |
| -0.06 | 0.03* | -0.31 | 0.29 | - | - | - | - |
| 0.015 | 0.06 | -0.01 | 0.88 | ||||
| 2.31 | 0.01* | 1.29 | 0.21 | ||||
| -0.01 | 0.07 | -0.07 | 0.29 | ||||
| 0.60 | 0.008* | 0.47 | 0.07 | ||||
| 0.10 | 0.008* | 0.11 | 0.01* | ||||
| 0.06 | 0.01* | 0.029 | 0.28 | ||||
| -0.07 | 0.03* | -0.042 | 0.27 | ||||
In addition to BMI, arterial stiffness was the factor most strongly associated with BPV, even compared to age. A linear increase in SBPV with arterial stiffness was seen in both men and women (figure 2), with a steeper relationship in women than in men but with no significant interaction with sex (p=0.38). This association was sufficiently strong that there was no association between age and SBPV after adjustment for PWV (adjusted for age and sex: age p=0.19; sex p=0.007; PWV p=0.002).
There was no univariate association between BMI and PWV (β=0.03, p=0.17), but after adjustment for age and sex there was a strong association (β=0.06, p<0.001), which persisted after adjustment for other cardiovascular risk factors (β=0.05, p=0.008). In a combined model allowing for the sex*BMI interaction, BMI and PWV independently predicted SBPV, with no residual significant association between either age or DBP with SBPV, although associations remained for sex and smoking (table 3). There was no interaction between smoking and BMI for BPV, and no association between thyroid stimulating hormone level and either BMI or BPV. In a causal mediation analysis allowing for adjustment for age and sex, there was also a significant indirect mediation effect of the relationship between BMI and SBPV by PWV (ACME 0.008 p<0.001; ADE 0.016 p=0.3; total effect 0.024), explaining 32% of the relationship between BMI and SBPV. There was no significant indirect mediation by BMI of the relationship between PWV and SBPV (ACME p=0.12, 10% variance explained).
Discussion
In an at-risk population with recent TIA or minor stroke, beat-to-beat BP variability over 5 minutes was associated with increased BMI and arterial stiffness. There was a linear increase in SBPV with BMI in men, but both low and high BMI were associated with increased SBPV in women. PWV and BMI were independently associated with SBPV, but PWV also mediated a proportion of the effect of BMI on SBPV. Associations between current smoking and increased SBPV persisted despite adjustment, but associations between both age and falling DBP were not significant after adjustment for PWV, indicating a likely primary role for arterial stiffness in mediating the relationship between age, DBP or BMI with beat-to-beat BPV.
Despite the large number of studies demonstrating that visit-to-visit and day-to-day BP variability are associated with an increased risk of cardiovascular events1–4, 7–9, 11, 12, 18, few studies have determined the prognostic significance of beat-to-beat blood variability,15, 19 despite its widespread use in the assessment of autonomic function in both research and clinical practice.20, 21 We previously demonstrated in an earlier report from this population that beat-to-beat BP variability was associated with a 47% increased risk of stroke and 37% increased risk of cardiovascular events per standard deviation of beat-to-beat BPV,15 compared with 24% and 33% for day-to-day BP variability. One other study demonstrated that beat-to-beat BPV was increased in acute stroke and associated with poor outcome,19, 22 albeit with SD as the principle index of BPV, whilst beat-to-beat BPV was associated with markers of end-organ injury and vascular aging, both in this population, 16 and in limited studies in other populations.23 This was also consistent with limited studies using intra-arterial continuous blood pressure measurements.24, 25
However, there is as yet little evidence identifying the determinants and clinical characteristics of patients with increased beat-to-beat BPV. We previously demonstrated in a much smaller sample that beat-to-beat BPV was associated with aortic stiffness and pulsatility, as well as with markers of cardiovascular reactivity,16 and that SBPV increases with age with an increased skew of the distribution of SBPV in a subset of the population. In this report, increased BMI was the strongest clinical factor associated with increased SBPV. However, the relationship between BMI and SBPV was complex, with a linear association in men. However, in women there was a U-shaped relationship, with increased SBPV in women with both a reduced and increased BMI compared to normal BMI, consistent with an increased risk of mortality for patients when both over and underweight. Although this could reflect ‘physiological’ variability in women with a reduced BMI that could be beneficial, the marked positive skew of the distribution of SBPV in women with a BMI below 20 suggests that this is not the case, and that the elevation in SBPV is driven by patients with an excess of pathological SBPV, even in women with a reduced BMI. This may reflect increased autonomic instability and sympathetic overactivity in obese patients, but could reflect reverse causation, with frailer patients being predisposed both to being underweight and to having increased BPV. Alternatively, elevated SBPV may mediate some of the relationship between obesity or being underweight and cardiovascular risk, and represent a new treatment target.
This study confirmed the previously demonstrated association between arterial stiffness and beat-to-beat BPV, with no significant association between age and SBPV after adjustment for PWV. Furthermore, the mediation analysis suggests that it is partly increased arterial stiffness in patients with increased BMI that results in increased SBPV, although this does not explain the increased SBPV in patients with reduced BMI. One possible link between arterial stiffness, increased BMI and SBPV is an enhanced inflammatory cascade in obesity leading to endothelial dysfunction, increased arterial stiffness and atherosclerosis, and potentially increased SBPV,26, 27 either directly or indirectly, with recent trials of anti-inflammatory interventions reducing recurrent cardiovascular events,28 independently of mean blood pressure effects.29 This also implies that weight loss in the obese has the potential to reduce cardiovascular morbidity, and stroke in particular, through reductions in arterial stiffness and therefore reductions in blood pressure variability. Furthermore, given the negative correlation between age and BMI, this supports the hypothesis that the rising incidence of stroke in younger patients, and women in particular, may partly be driven by increasing weight that may partly be mediated by increased SBPV.
There are limitations to our study. Firstly, all patients were assessed after a cerebrovascular event, limiting generalizability to other disease groups. However, this population are at an increased risk of recurrent stroke,30 and this risk has been shown to be associated with beat-to-beat BPV in this group.15 Secondly, 5% of patients did not have adequate recordings, despite repeated measures to improve quality, particularly in elderly patients who may be at a particularly increased risk of stroke. As such, the prevalence of elevated BPV may be underestimated, with consequent underestimation of the risk associated with elevated BPV. Thirdly, we measured beat-to-beat BPV in a highly controlled environment, using expensive equipment. Development of more cost-effective methods would be essential to apply beat-to-beat BPV to routine clinical practice. Fourthly, we extensively cleaned and de-trended the data, improving precision of measurement but also limiting its direct applicability to clinical practice. As such, further development is required to standardize methods of acquisition, data cleaning and analysis of beat-to-beat BPV in a practical method for use in clinical practice. Finally, obesity may cause systematic bias in the assessment of PWV through artificially increasing the measured distance between the carotid and femoral applanation sites, whilst also affecting accuracy of blood pressure measurement.
Overall, beat-to-beat SBPV reflected both age-associated arterial stiffness and changes in BMI. This suggests a potential role for weight loss to reduce SBPV in the obese, with a resulting reduction in cardiovascular risk, but further research is required in under-weight women to determine why SBPV may be increased, and whether this is a marker of frailty or an independent treatable factor. Furthermore, it implies that measures that reduce SBPV, such as amlodipine,4 may be particularly effective at reducing cardiovascular risk in patients who are overweight, or women who are underweight.
Conclusions
Beat-to-beat BP variability reflects increased arterial stiffness and an increased BMI in both sexes, and a low BMI in women alone. This may be a potentially modifiable mechanism resulting in the increased risk of cardiovascular events due to increased arterial stiffness and alterations in BMI.
Supplementary Material
Acknowledgments
This work uses data provided by patients and collected by the NHS as part of their care and support and would not have been possible without access to this data. The NIHR recognises and values the role of patient data, securely accessed and stored, both in underpinning and leading to improvements in research and care.
Sources of Funding
The Oxford Vascular Study is funded by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC), Wellcome Trust, Wolfson Foundation, British Heart Foundation and the European Union’s Horizon 2020 programme (grant 666881, SVDs@target). PMR is in receipt of a NIHR Senior Investigator award. AJSW and this work is funded by a Wellcome Trust Clinical Research Development Fellowship (206589/Z/17/Z) and British Heart Foundation Project Grant (PG/16/38/32080). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Non-standard Abbreviations and Acronyms
- ACME
Average causal mediation effects
- ADE
Average direct effect
- BPV
Blood pressure variability
- CV
Coefficient of Variation
- OXVASC
Oxford Vascular Study
- PWV
Pulse Wave Velocity
- rCV
Residual coefficient of variation
- SD
Standard deviation
Footnotes
Author contribution statement
AJSW devised, acquired, supervised and analysed the physiological assessments, devised, analysed and supervised the statistical analysis and drafted, analysed, edited and submitted the manuscript. AL, SM and LL acquired and analysed the physiological assessments. PM established and supervised the OXVASC study, devised, initiated and supervised the physiological studies and statistical analyses and edited and supervised the manuscript.
Disclosure/conflict of interest
There are no conflicts of interest.
References
- 1.Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010;375:938–948. doi: 10.1016/S0140-6736(10)60309-1. [DOI] [PubMed] [Google Scholar]
- 2.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlof B, Sever PS, Poulter NR. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 3.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlof B, Poulter NR, Sever PS, Ascot B, Investigators MRCT. Effects of beta blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010;9:469–480. doi: 10.1016/S1474-4422(10)70066-1. [DOI] [PubMed] [Google Scholar]
- 4.Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: A systematic review and meta-analysis. Lancet. 2010;375:906–915. doi: 10.1016/S0140-6736(10)60235-8. [DOI] [PubMed] [Google Scholar]
- 5.Webb AJ, Fischer U, Rothwell PM. Effects of beta-blocker selectivity on blood pressure variability and stroke: A systematic review. Neurology. 2011;77:731–737. doi: 10.1212/WNL.0b013e31822b007a. [DOI] [PubMed] [Google Scholar]
- 6.Webb AJ, Wilson M, Lovett N, Paul N, Fischer U, Rothwell PM. Response of day-to-day home blood pressure variability by antihypertensive drug class after transient ischemic attack or nondisabling stroke. Stroke. 2014;45:2967–2973. doi: 10.1161/STROKEAHA.114.005982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muntner P, Whittle J, Lynch AI, Colantonio LD, Simpson LM, Einhorn PT, Levitan EB, Whelton PK, Cushman WC, Louis GT, et al. Visit-to-visit variability of blood pressure and coronary heart disease, stroke, heart failure, and mortality: A cohort study. Ann Intern Med. 2015;163:329–338. doi: 10.7326/M14-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muntner P, Shimbo D, Tonelli M, Reynolds K, Arnett DK, Oparil S. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: Findings from nhanes iii, 1988 to 1994. Hypertension. 2011;57:160–166. doi: 10.1161/HYPERTENSIONAHA.110.162255. [DOI] [PubMed] [Google Scholar]
- 9.Chiriaco M, Pateras K, Virdis A, Charakida M, Kyriakopoulou D, Nannipieri M, Emdin M, Tsioufis K, Taddei S, Masi S, et al. Association between blood pressure variability, cardiovascular disease and mortality in type 2 diabetes: A systematic review and meta-analysis. Diabetes Obes Metab. 2019;21:2587–2598. doi: 10.1111/dom.13828. [DOI] [PubMed] [Google Scholar]
- 10.Gosmanova EO, Mikkelsen MK, Molnar MZ, Lu JL, Yessayan LT, Kalantar-Zadeh K, Kovesdy CP. Association of systolic blood pressure variability with mortality, coronary heart disease, stroke, and renal disease. J Am Coll Cardiol. 2016;68:1375–1386. doi: 10.1016/j.jacc.2016.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oishi E, Ohara T, Sakata S, Fukuhara M, Hata J, Yoshida D, Shibata M, Ohtsubo T, Kitazono T, Kiyohara Y, et al. Day-to-day blood pressure variability and risk of dementia in a general japanese elderly population: The hisayama study. Circulation. 2017;136:516–525. doi: 10.1161/CIRCULATIONAHA.116.025667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ, McManus RJ. Blood pressure variability and cardiovascular disease: Systematic review and meta-analysis. BMJ. 2016;354:i4098. doi: 10.1136/bmj.i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johansson JK, Puukka PJ, Virtanen R, Jula AM. Beat-to-beat, ambulatory hour-to-hour, and home day-to-day variabilities in blood pressure, pulse pressure, and heart rate in comparison with each other and with target-organ damage. Blood Press Monit. 2015;20:113–120. doi: 10.1097/MBP.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 14.Kikuya M, Ohkubo T, Metoki H, Asayama K, Hara A, Obara T, Inoue R, Hoshi H, Hashimoto J, Totsune K, et al. Day-by-day variability of blood pressure and heart rate at home as a novel predictor of prognosis: The ohasama study. Hypertension. 2008;52:1045–1050. doi: 10.1161/HYPERTENSIONAHA.107.104620. [DOI] [PubMed] [Google Scholar]
- 15.Webb AJS, Mazzucco S, Li L, Rothwell PM. Prognostic significance of blood pressure variability on beat-to-beat monitoring after transient ischemic attack and stroke. Stroke. 2018;49:62–67. doi: 10.1161/STROKEAHA.117.019107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webb AJ, Rothwell PM. Physiological correlates of beat-to-beat, ambulatory, and day-to-day home blood pressure variability after transient ischemic attack or minor stroke. Stroke. 2014;45:533–538. doi: 10.1161/STROKEAHA.113.003321. [DOI] [PubMed] [Google Scholar]
- 17.Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, Redgrave JN, Bull LM, Welch SJ, Cuthbertson FC, et al. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (oxford vascular study) Lancet. 2005;366:1773–1783. doi: 10.1016/S0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]
- 18.Shimbo D, Abdalla M, Falzon L, Townsend RR, Muntner P. Studies comparing ambulatory blood pressure and home blood pressure on cardiovascular disease and mortality outcomes: A systematic review. J Am Soc Hypertens. 2016;10:224–234.:e217. doi: 10.1016/j.jash.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dawson SL, Manktelow BN, Robinson TG, Panerai RB, Potter JF. Which parameters of beat-to-beat blood pressure and variability best predict early outcome after acute ischemic stroke? Stroke. 2000;31:463–468. doi: 10.1161/01.str.31.2.463. [DOI] [PubMed] [Google Scholar]
- 20.van Wijnen VK, Finucane C, Harms MPM, Nolan H, Freeman RL, Westerhof BE, Kenny RA, Ter Maaten JC, Wieling W. Noninvasive beat-to-beat finger arterial pressure monitoring during orthostasis: A comprehensive review of normal and abnormal responses at different ages. J Intern Med. 2017;282:468–483. doi: 10.1111/joim.12636. [DOI] [PubMed] [Google Scholar]
- 21.Romano SM, Lazzeri C, Chiostri M, Gensini GF, Franchi F. Beat-to-beat analysis of pressure wave morphology for pre-symptomatic detection of orthostatic intolerance during head-up tilt. J Am Coll Cardiol. 2004;44:1891–1897. doi: 10.1016/j.jacc.2004.07.046. [DOI] [PubMed] [Google Scholar]
- 22.Eames PJ, Blake MJ, Dawson SL, Panerai RB, Potter JF. Dynamic cerebral autoregulation and beat to beat blood pressure control are impaired in acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2002;72:467–472. doi: 10.1136/jnnp.72.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei FF, Li Y, Zhang L, Xu TY, Ding FH, Wang JG, Staessen JA. Beat-to-beat, reading-to-reading, and day-to-day blood pressure variability in relation to organ damage in untreated chinese. Hypertension. 2014;63:790–796. doi: 10.1161/HYPERTENSIONAHA.113.02681. [DOI] [PubMed] [Google Scholar]
- 24.Parati G, Pomidossi G, Albini F, Malaspina D, Mancia G. Relationship of 24-hour blood pressure mean and variability to severity of target-organ damage in hypertension. J Hypertens. 1987;5:93–98. doi: 10.1097/00004872-198702000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Veerman DP, de Blok K, van Montfrans A. Relationship of steady state and ambulatory blood pressure variability to left ventricular mass and urinary albumin excretion in essential hypertension. Am J Hypertens. 1996;9:455–460. doi: 10.1016/0895-7061(95)00437-8. [DOI] [PubMed] [Google Scholar]
- 26.Della Corte V, Tuttolomondo A, Pecoraro R, Di Raimondo D, Vassallo V, Pinto A. Inflammation, endothelial dysfunction and arterial stiffness as therapeutic targets in cardiovascular medicine. Curr Pharm Des. 2016;22:4658–4668. doi: 10.2174/1381612822666160510124801. [DOI] [PubMed] [Google Scholar]
- 27.Tuttolomondo A, Casuccio A, Della Corte V, Maida C, Pecoraro R, Di Raimondo D, Vassallo V, Simonetta I, Arnao V, Pinto A. Endothelial function and arterial stiffness indexes in subjects with acute ischemic stroke: Relationship with toast subtype. Atherosclerosis. 2017;256:94–99. doi: 10.1016/j.atherosclerosis.2016.10.044. [DOI] [PubMed] [Google Scholar]
- 28.Ridker PM, Libby P, MacFadyen JG, Thuren T, Ballantyne C, Fonseca F, Koenig W, Shimokawa H, Everett BM, Glynn RJ. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: Analyses from the canakinumab anti-inflammatory thrombosis outcomes study (cantos) Eur Heart J. 2018;39:3499–3507. doi: 10.1093/eurheartj/ehy310. [DOI] [PubMed] [Google Scholar]
- 29.Rothman AM, MacFadyen J, Thuren T, Webb A, Harrison DG, Guzik TJ, Libby P, Glynn RJ, Ridker PM. Effects of interleukin-1beta inhibition on blood pressure, incident hypertension, and residual inflammatory risk: A secondary analysis of cantos. Hypertension. 2020;75:477–482. doi: 10.1161/HYPERTENSIONAHA.119.13642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothwell PM, Giles MF, Chandratheva A, Marquardt L, Geraghty O, Redgrave JN, Lovelock CE, Binney LE, Bull LM, Cuthbertson FC, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (express study): A prospective population-based sequential comparison. Lancet. 2007;370:1432–1442. doi: 10.1016/S0140-6736(07)61448-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.