Abstract
Recently, an increased interest toward enzymatic carboligation was observed, as biocatalytic carbon–carbon bond formation is a common obstacle in retrosynthetic planning. The construction of extended 2-oxoacid frameworks by 2-oxoacid aldolases and enzymes acting as aldolases is a potent tool for synthetic chemists since a broad spectrum of downstream reactions through functional group interconversions gives access to a plethora of compound classes. In the search for selective biocatalysts, successful protein engineering efforts and high throughput screenings from biodiversity expand the structural diversity of nucleophile and electrophile substrates. Several successful examples with an emphasis on reactions catalyzed by class II aldolases and enzymes acting as class II aldolases are highlighted, including reactions in which both enantiomeric products and in selected cases even diastereomeric products are accessed.
Introduction
As biocatalysis is progressively implemented in pharmaceutical processes it is notable that many functional group interconversions are feasible; however, the demand in selective carbon—carbon forming reactions remains underexploited [1–5]. The biocatalytic alternatives to conventional synthetic routes contribute to the sustainability of the overall chemical process since the parameters of enzymatic reactions align predominantly with the principles of green chemistry especially regarding chemoselectivity, regioselectivity, and stereoselectivity [6,7]. Nonetheless, the construction of a complex organic framework starting from simple achiral building blocks is a challenge with available biocatalytic tools. The current limitations are found in the stereoselectivity in the carboligation reaction and the lack of complementary catalysts in case selective enzymes were identified. Furthermore, the substrate scope is considered rather narrow, which reduces drastically the potential applications. Fortunately, recent efforts brought significant improvements in selectivity and performance of biocatalysis in selective carbon—carbon bond formation [8–10]. A class of lyases labeled as aldolases is recently getting rediscovered since it reliably performs the highly desired biocatalytic carbon—carbon bond formation [11]. Particularly, pyruvic acid aldolases deserve more attention in view of their potential for the biocatalytic community. The modularity of the aldol reaction catalyzed by pyruvic acid aldolases elongating a broad pool of aldehydes with 2-oxoacids into 4-hydroxy-2-oxoacid products is impressive. The constructed carbon frameworks with this approach are highly valuable due to the manifoldness of the established downstream routes such as 2,4-dihydroxy acids, 2-amino-4-dihydroxy acids, or 3-hydroxy acids [12]. In the last few years, the attractiveness of aldolase-mediated carbon—carbon bond formation has risen due to the progress in enzyme discoverynd protein engineering efforts. Several obstacles such as the narrow substrate scope and a lack of stereoselectivity of this reaction have been targeted and the progress will be highlighted in this review.
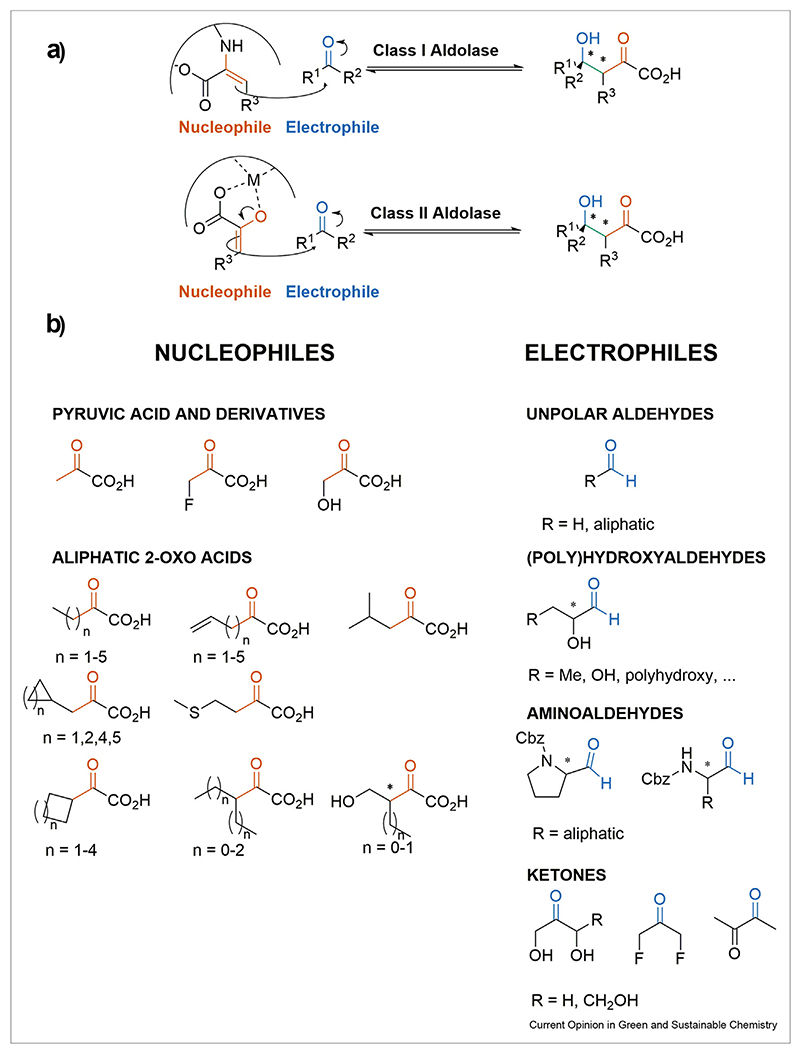
A pyruvic acid, or more generally, 2-oxoacid aldolase-mediated reaction forges a carbon—carbon bond between a 2-oxoacid, acting as a nucleophile in its enolized form and a second carbonyl acting as an electrophile (Scheme 1a). This furnishes an aldol adduct with up to two stereocenters with optimal atom efficiency. To be in a reactive state, the nucleophile referred to as ‘aldol donor’ undergoes enolization promoted by an active site base [13]. Hence, the active site of aldolases is designed based on the ability to shield this highly reactive intermediate from the bulk solution. Consequently, the nucleophile binding site of the enzyme has a rather stringent substrate selectivity tolerating only a few structural alterations because of its high steric and electronic constraints. These constraints are required to maintain a highly ordered bi-substrate transition state that occurs upon the admission of the electrophile i.e., ‘aldol acceptor’ to assure a perfect stereochemical outcome. The most established aldolases are the so-called ‘class I aldolases’ with an active site lysine to form a Schiff base with the nucleophile (Scheme 1a) [14]. Remarkably, the emerging protocols of aldolase-mediated reactions rely almost exclusively on the hitherto underappreciated metal-dependent class II aldolase family. In these enzymes, the activation of the nucleophile proceeds via enolization driven by a divalent Lewis acidic metal ion located in the active site of the enzyme (Scheme 1a). The range of metals in class II aldolases are found among a variety of transition metals such as nickel, cobalt, manganese, or zinc but also alkaline earth metal magnesium [15]. In their sequence, usually, one characteristic protein domain is found that allows the assignment of the aldolases beyond the catalytic species. The most promising class II aldolases belong to the same Pfam family [PF03328] named 4-hydroxy-2-oxo-heptane-1,7-dioic acid (HpcH)/HpaI aldolase/citrate lyase family (Scheme 2a). The manifoldness in the genomic context of this family suggests a diverse range of physiological activities. HpcH aldolases, however, have been identified to either participate in the last steps of the degradation of biphenyl or polychlorinated biphenyl in a retro-aldol manner or as the related 5-oxo-4-deoxy-d-glucarate aldolases (GarL) in the catabolism of d-glucarate/galactarate [16–18].
Scheme 1. Aldolase-mediated carboligation with the current scope of nucleophiles and electrophiles.
(a) Aldolase-mediated aldol reaction furnishing elongated 2-oxo acid frameworks. A carbonyl compound as an electrophile (Blue) and a 2-oxo acid as a nucleophile (Orange) form a carbon-carbon bond (green). The mechanism of class I and class II aldolases are depicted: The Mechanism of class I aldolases bearing an active site lysine residue and below class II aldolases in which an active site divalent metal ion binds to an enolized pyruvic acid that attacks the carbonyl of an aldehyde. (b) The expanded nucleophile scope and electrophiles for the aldol reaction of 4-hydroxy-2-oxo acids mediated by pyruvic acid aldolases.
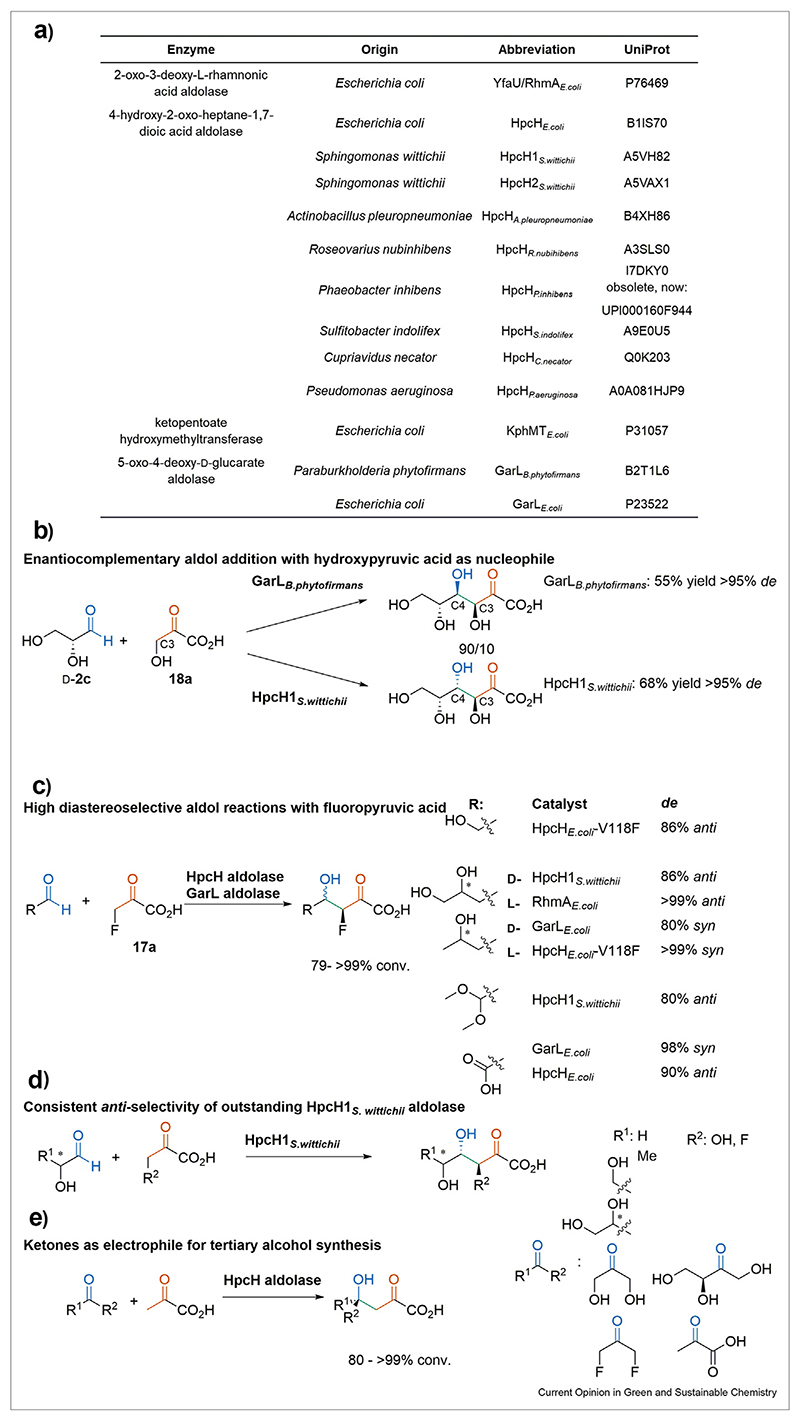
Scheme 2. An overview of potent class II aldolases and enzymes acting as class II aldolases and examples of stereoselective aldol additions for the synthesis of (poly)substituted 2-oxo acids.
(a) A list of class II aldolases and enzymes acting as aldolases discussed in this review, including their source, UniProt code, an abbreviation used in this review. (b) Hydroxypyruvic acid as a nucleophile in an aldol reaction with d-glyceraldehyde by 4-hydroxy-2-oxo-heptane-1,7-dioic acid aldolase (HpcH aldolase) or 5-oxo-4-deoxy-d-glucarate aldolase (GarL aldolase) from various organisms [22]. (c) Fluoropyruvic acid as a nucleophile in class II aldolase-mediated reactions [29]. (d) 2-hydroxyaldehydes as substrates for HpcH1S.wittichii aldolase showing antiselectivity with fluoropyruvic acid and hydroxypyruvic acid. (e) Ketones as an electrophile for the creation of tertiary alcohols [23].
Enzymes of the [PF03328] mechanistic type adopt the common TIM barrel fold and are hexamers (i.e., trimer of dimers) binding one metal cofactor per subunit. The metal itself binds by an aspartate and a glutamate over a water bridge. An arginine residue in the active site is responsible for the binding of the nucleophile carboxylate and together with an adjacent histidine are identified to be crucial for enzymatic activity, participating in the activation of metal-bound water for proton transfer during catalysis [19,20]. Hence, mostly small nucleophiles as pyruvic acid and its analogs were explored due to the restrictive architecture of the active site.
Recent findings with pyruvic acid and 3-substituted analogs as nucleophiles
Pyruvic acid is not only eponymous for pyruvic acid aldolases, but also the best-investigated nucleophile [21]. Fortunately, class II aldolases accept its analogs, hydroxypyruvic acid and fluoropyruvic acid, which enables the scope of accessible products. The most successful electrophiles within these studies are small chiral (poly)hydroxylated aliphatic aldehydes such as glycolaldehyde, glyceraldehyde, lactaldehyde or erythrose. A notable observation is that enzymes of the HpcH/HpaI aldolase/citrate lyase family accept both the S-configuration and the R-configuration at the 2-hydroxy group in the electrophilic aldehyde. Furthermore, although both syn- and anti-diastereoselectivities are described, the configuration of the chiral center at C3 is exclusively S-oriented [22,23].
Hydroxypyruvic acid as nucleophile
In case hydroxypyruvic acid is the selected nucleophile, the hydroxy substituent on the C3-position enables the construction of a 3,4-dihydroxy-2-oxoacid motif with the control over both stereocenters (Scheme 2b), which is a long-lasting goal within aldolase research [22]. Due to its self-activating capacity, the spontaneous homo-aldol addition of hydroxypyruvic acid is an obstacle, which must be overcome [23]. In a screening of 571 class II aldolases, 19 wild type enzymes of the HpcH/HpaI/citrate lyase family, tolerating hydroxypyruvic acid as a nucleophile, were found [22]. The remarkably selective enzyme HpcH1S.wittichii (Scheme 2b and 2d), which catalyzes the aldol addition of hydroxypyruvic acid to enantiopure 2—hydroxyaldehydes yielding aldol, adducts with a remarkably high diasteroselectivity and isolated yields ranging from 47% to 68% with the products showing solely S,S-configuration. Moreover, d- or l-glyceraldehyde have given 68% and 61% isolated yields, respectively, l-lactaldehyde (47% isol. yield) and d-erythrose (57% isol. yield, >95% de) the excellent de of the anti-3,4-hydroxy-2—oxoacid product highlights the great potential of the catalyst. HpcH1S.wittichii lacks only d-lactaldehyde in this distinct selectivity with 55% de [22]. The conversion rate of hydroxypyruvic acid is relying on an active site phenylalanine side chain in HpcH1S.wittichii in which a CH-π interactions to the C3 of the enolized nucleophile occurred. Replacing the phenylalanine residue with other aromatic amino acids such as tyrosine offers a 2-fold improvement [24]. High selectivity is also found with GarLB.phytofirmans with noteworthy enantiocomplementary 3S,4R-configuration obtained with similar yield (Scheme 2b, 55%) [22].
Fluoropyruvic acid as nucleophile
Fluorine substituents provide unique options to new organofluorine core structures, offering great pharmacological potential [25,26]. The groundbreaking work of Berry and co-workers with fluoropyruvic acid as nucleophile utilizes class I aldolases, thus, not the scope of this review and is reviewed here in detail [26–28]. With class II aldolase HpcHE.coli a change of the nucleophile to fluoropyruvic acid over pyruvic acid resulted in a 500-fold decrease in the turnover number and was accompanied by substrate inhibition. This may be overcome in a continuous substrate addition process [29].
A broad scope of aldehydes is suitable as electrophiles for fluoropyruvic acid in an aldol addition mediated by members of the HpcH/HpaI/citrate lyase family (Scheme 2c) [29]. A screening found high acceptance toward 2-hydroxyaldehydes, aliphatic aldehydes, 2,2-dimethoxyacetaldehyde, and even glyoxylic acid. Exclusively the 3S-configuration is present in the products; however, the configuration and enantiopurity of the second chiral center is largely dependent on the electrophile, e.g., the S-configuration in l-lactaldehyde leads to a strong preference toward the anti-isomer regardless of the employed HpcH aldolase (HpcHE.coli: 99% conv. 92% de, HpcH1S.wittichii 100% conv. 90% de, HpcH2s.wittichii 87% conv. >99% de). In case d-lactaldehyde is employed GarLE.coli furnishes the syn-product in a complementary fashion (97% conv., 80% de). The only exception remains d-lactaldehyde with 1:1 ratio of syn/anti product. An observation that is also made in case d-lactaldehyde reacts with hydroxypyruvic acid as nucleophile [22]. Furthermore, the anti-product (up to >99% de) is obtained in case l-glyceraldehyde is employed with the HpcH aldolases and GarLE.coli with up to 97% conversion [29].
The de may be reduced over time toward the thermodynamic product and therefore requires kinetic control [27,30]. Lowering the catalyst loading boosts the anti-selectivity with the electrophiles glycoaldehyde and 2,2-dimethoxyacetaldehyde from 39–59% to 71–93% de-values anti, which is accompanied by a drop in conversion [29]. Surprisingly, in the case of glyoxylic acid as electrophile GarLE.coli turned out as a potent catalyst with extraordinary diastereomeric selectivity toward syn (99% de) and full conversion whereas HpcHE.coli produced the anti-product (92% de) [29].
Ketones as alternative electrophiles for pyruvic acid analogs
The pyruvic acid self-addition has revealed the feasibility of ketones acting as electrophiles for an aldol reaction. The produced tertiary alcohols are a widespread motif in bioactive compounds [31]. Since conventional organic synthesis uses hazardous metals and chemicals biocatalytic alternatives were developed such as the use of thiamine-dependent lyases and hydroxynitrile lyases to furnish 2-alkyl-2-hydroxy ketones, 2-alkyl-2-hydroxy-3-diketones, 3-hydroxy ketones, and ketone cyanohydrins [32]. Recent successful efforts used dihydroxyacetone phosphate-dependent class II aldolases furnished tertiary alcohol moieties in branched-chain sugars in an enantioselective fashion [33]. Hence, the feasibility of HpcH aldolase for the synthesis of tertiary alcohols bearing a carboxy group has been probed with hydroxyacetone and analogs thereof. It turned out that a single hydroxyl group adjacent to the ketone is not sufficient that hydroxyacetone is accepted as an electrophile; however, dihydroxyketone is readily accepted by a broad set of HpcH aldolases (Scheme 2e) [23]. The most promising enzyme candidates are HpcH2S.wittichii, HpcHA.pleuropneumoniae, HpcHS.indolifex, and HpcHR.nubihibens with pyruvic acid and 2-oxobutyric acid as nucleophiles. Besides dihydroxyketone, its fluoro-analog, 2,3-butadione, and keto sugar l-erythrulose are also accepted. The best catalyst performs aldol addition onto the ketones at similar rates as with aldehydes; however, it was required to provide the ketones in a fourfold excess to shift the equilibrium toward product formation. Yields are up to 75% with dihydroxyacetone and 90% with l-erythrulose and 2-oxobutyric acid by using HpcH2S.wittichii as a catalyst. Pyruvic acid as nucleophile has a slight edge since with dihydroxyacetone as an electrophile and 92% of the aldol product were isolated. The products are found to be in S-configuration at the chiral center at C4 [23].
The extension of the aliphatic chain length of the nucleophilic 2-oxoacids
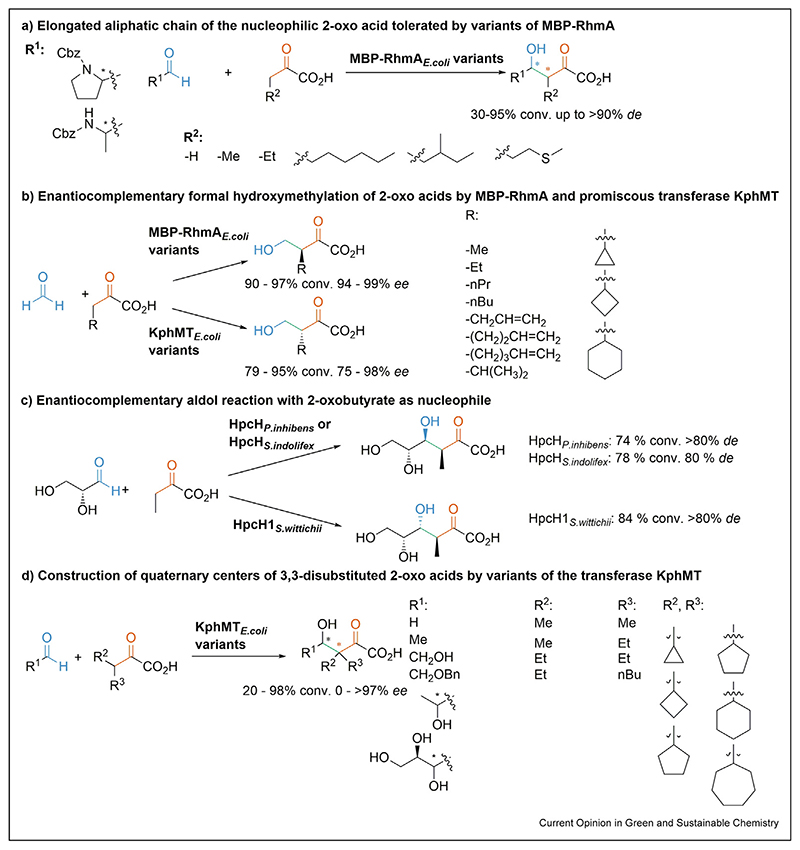
The limitation of the size of the nucleophile is one of the key obstacles working with 2-oxo acid aldolases. Hence, one focus point of recent investigation was the enlargement of the aliphatic chain of the employed 2-oxo acids. Structure-guided protein engineering with the purpose of active site enlargement of class II aldolase 2-oxo-3-deoxy-l-rhamnonate aldolase (RhmAE.coli) also annotated as YfaU, which was fused to the maltose-binding protein (MBP) to improve soluble expression [34]. The engineering efforts ultimately enabled a broad scope of aliphatic 2-oxo acids as nucleophiles such as 2-oxobutyric acid, 2-oxopentanoic acid, 2-oxooctanoic acid, oxoleucine, and oxomethionine (Scheme 3a). Bulky residues within the active site were replaced and the resulting variants MBP-RhmAE.coli-W23V, MBP-RhmAE.coli-L216A, and MBP-RhmAE.coli-W23V/L216A were probed with both enantiomers of Cbz-protected prolinal and alaninal that are attractive electrophiles as the obtained aldol products are precursors for proline, pipecolic acid, and even more widespread 4-amino-3-hydroxy acid motif [35,36]. Conversion ranged from 30 to 95%, while both enantiomers of the electrophiles were accepted. The chiral center in the aldol product is preferentially S-configured at the 3-position with des up to >90% [35]. Best diastereoselectivity is founh oxomethionine (91% conv.), or 2-oxooctanoic acid (40% conv.) and (R)-Cbz-alaninal (syn, selectivity of >90% de). An apparent change in the active site orientation in case oxoleucine acted as a nucleophile (50% conv.) lead to a switch in the diastereomeric outcome (anti 90% de) with (S)-Cbz-alaninal.
Scheme 3. The expanded nucleophilic scope of class II aldolases.
(a) Protected aminoaldehydes as electrophiles for class II aldolase MBP-2-oxo-3-deoxy-l-rhamnonate aldolase (RhmAE.coli) and its variants [35]. (b) Enantiocomplementary aldol addition of formaldehyde with MBP-RhmAE.coli and oxopentoate methyltransferase (KphMTE.coli) variants [38]. (c) Stereocomplementary aldol addition with 2-oxobutyric acid as a nucleophile and exemplarily with d-glyceraldehyde as electrophile mediated by HpcH aldolases [39]. (d) Recently identified KphMTE.coli variants are probed with 3,3-disubstituted 2-oxo acids and tolerate large (poly)hydroxylated aldehydes to form quaternary centers with low to high enantioselectivity [40].
A similar engineering attempt expanded the active site of promiscuous tetrahydrofolate dependent oxopantoate hydroxymethyltransferase (KphMTE.coli) of the Pfam family [PF02548]. In a co-factor independent mechanism KphMTE.coli utilizes a Lewis-acidic divalent transition metal such as cobalt to act as a class II aldolase [37]. With the variants KphMTE.coli-I202A and KphMTE.coli-I212A a similar nucleophile scope as that of MBP-RhmAE.coli is observed. The synthetic potential of these variants lies in the stereocomplementary behavior of MBP-RhmAE.coli, which was revealed using formaldehyde as an electrophile (Scheme 3b). Furthermore, similarly to MBP-RhmAE.coli also KphMTE.coli variants tolerate 3-cyclopropyl-2-oxoacid and 3-cyclobutyl-2-oxoacid whereas 3-cyclohexyl-2-oxoacid is exclusively tolerated by MBP-RhmAE.coli [38].
An NMR-based screening assay revealed that 2-oxobutyric acid is well tolerated by several wild-type HpcH aldolases [39]. 2-oxobutyric acid was tested on R-configured 2-hydroxyaldehyde electrophiles. In case d-glyceraldehyde was employed, both syn-diastereomers and anti-diastereomers are accessible with up to >84% product formation and >80% de using HpcH1S.wittichii and HpcHP.inhibens (Scheme 3c). The anti-selectivity correlates with the other 3-substituted nucleophiles when HpcH1S.wittichii is employed (d-glyceraldehyde 70% de, d-lactaldehyde 50% de, d-erythrose 80% de). The bulky d-ribose leads to a significant drop in selectivity accompanied by a switch in diastereoselectivity (syn 20% de). HpcHS.indolifex with a remarkably high syn-preference [39] has the potential to become a working horse for biocatalytic aldol reactions since its consistent selectivity found with the tested nucleophiles is highly desired.
KphMTE.coli wild type and variants also are suitable for the unprecedented construction of quaternary carbon centers [32,40]. It turns out that ramifications at the 3-positions within the nucleophile present already in the natural oxopantoate pathway were also tolerated in case KphMTE.coli acts in an aldolase fashion. This activity allows the construction of 3,3,3-trisubstituted 2-oxoacids with gem-alkyl and spirocyclic centers (Scheme 3d). Besides small aldehydes such as formaldehyde, acetaldehyde, and R- or S-lactaldehyde, C4-aldosugars and C5-aldosugars are tolerated as electrophiles. Bulky cyclopentyl- (90% conv.), cyclohexyl- (20% conv.), and cycloheptyl-2-oxoacetic acid (40% conv.) are tolerated as nucleophile onto formaldehyde in an analytical scale screening and the aldol products are directly decarboxylated and esterified on a preparative scale. Small 3,3-disubstituted 2-oxo acids provide reasonable stereoselectivity with up to >80% ee with formaldehyde as an electrophile. The most active variants KphMTE.coli-I202A and KphMTE.coli-I212A and the corresponding double mutant provide more than 90% conversion. The variant KphMTE.coli-V214G is particularly potent for the ligation of 2-oxoisovaleric acid and d-erythrose. Furthermore, it turns out that KphMTE.coli variants enantioselectively convert only the S-enantiomer of Ketoisoleucine. Molecular models suggest that the steric repulsion of active site residues limits the accessibility of the enolate intermediate [40].
Follow-up chemistry for 4-hydroxy-2-oxo acids produced by an aldol reaction
The versatility of 2-oxoacid intermediates in biocatalytic multistep routes or as a platform for broad product portfolios is well described [41–43]. The scarcity of one-pot one-step reactions for downstream modifications of aldol products can be explained by the reversibility of the aldol reaction [23,34,44]. Therefore, inactivation of the aldolase by precipitation or removal of the active site metal ion is often required for further follow-up chemistry [29]. This methodology enables the reduction of the 2-oxo group of the product by dehydrogenase and the amination by a u-transaminase (Scheme 4a).
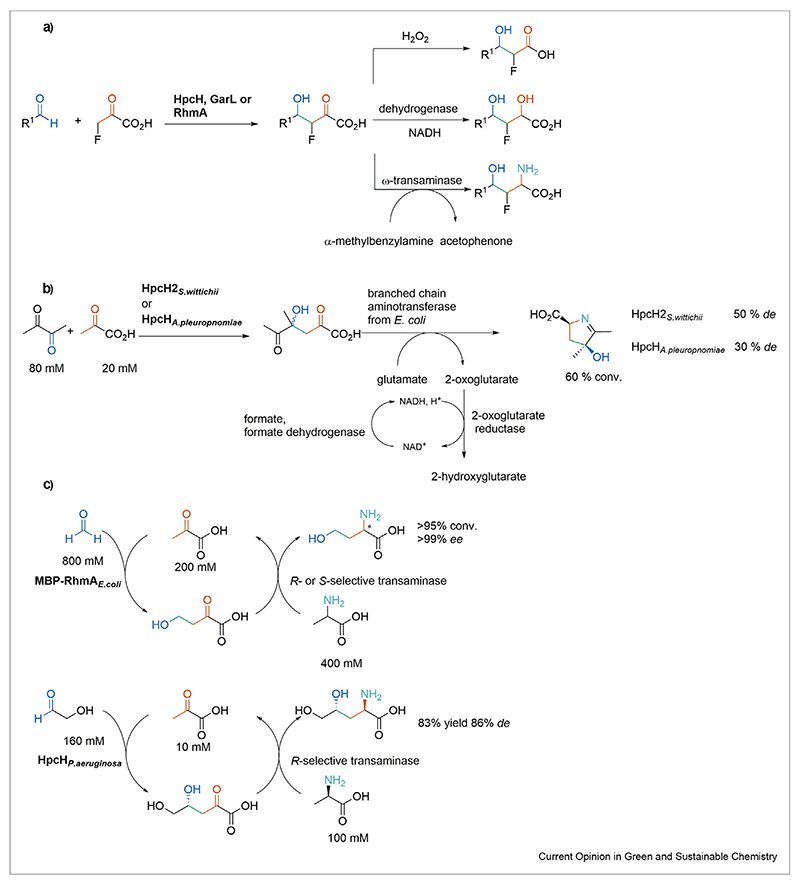
Scheme 4. Biocatalytic follow-up chemistry for 2-oxo acids furnished by aldol addition.
(a) One-pot two-step follow-up sequence for fluoro-aldol products [29]. (b) One-pot simultaneous cascade of an aldol reaction and a subsequent trans-amination [23]. (c) One-pot cyclic cascade of an MBP-RhmAE.coli mediated aldol addition and transamination [34] and HpcHP.aeruginosa and trans-amination [45].
A remarkable example is a one-pot one-step cascade couples an aldol reaction of pyruvic acid and butanedione mediated by HpcH2S.wittichii and HpcHA.pleuropneumoniae with the amination of a branched-chain aminotransferase from Escherichia coli (Scheme 4b). The equilibrium shift is driven by the removal of the deaminated amino donor by 2-oxoglutarate reductase coupled with a cofactor recycling system. A one-pot cyclic reactions setup starting from pyruvic acid, formaldehyde, and alanine as amino source provided an efficient route for the synthesis of both enantiomers of homoserine. MBP-RhmAE.coli and enantiocomplementary set of ω-transaminase tolerated 400 mM substrate loading and produced homoserine between 86% and >95%, respectively, with >99% ee for both enantiomers (Scheme 4c). A related approach synthesized l-syn or anti-4-hydroxyglutamic acid and d-anti-4,5-dihydroxynorvaline with 83–95% yield. In the case of d-anti-4,5-dihydroxynorvaline extraordinary 86% de was achieved in a preparative scale experiment (Scheme 4c) [45]. Cornerstones for the experimental setup proved to be the slow addition of the electrophile in the aldol addition and the monitoring of the reaction time.
The oxidative decarboxylation with stoichiometric concentrations of H2O2 allows the synthesis of a carboxylic acid with a loss of a carbon atom and enables subsequent esterification, which reduces the polarity of products [28,35,38]. Since esterification often demands strong acidic or basic condition that potentially leads to a loss in the enantiopurity of the products, suitable conditions are required to maintain the ee in the product [38].
Conclusion and outlook
The implementation of inherently sustainable enzyme-driven catalysts into chemical processes is advancing with the recent success in the development of novel biocatalytic carbon—carbon bond-forming reactions [10,11]. Recent efforts have expanded the notoriously small pool of nucleophiles of the so-called ‘pyruvic acid aldolase-mediated reactions. On the one hand, the finding of naturally occurring enzymes by high throughput methodologies allowed the tolerance of substituents at C3 of pyruvic acids. Hence, unprecedented stereoselectivity was identified by several novel enzymes, although, the examples with excellent diastereoselectivity are still limited to suitable nucleophile-electrophile pairing. On the other hand, protein engineering of the active site enabled aldolases and enzymes acting as aldolase to accept aliphatic and cyclic 2-oxo acid nucleophiles. This allows the synthesis of various precursors for bioactive compounds in a highly stereoselective fashion. Furthermore, the presented studies show class II aldolase-mediated reactions on a preparative scale with high substrate loading, which is advantageous for industrial applicability. The recent progress in this field offers an insight into the potential of these catalysts. It turns out that the obtained multifunctionalized building blocks are perfectly suited as starting point to provide a whole platform of different compounds as a diverse set of downstream routes are accessible and several one-pot protocols show their feasibility.
Biocatalytic aldol reaction still has limitations but is clearly on the upswing. One challenge is the supply of highly oxidized starting material for aldol reactions. Therefore, upstream reactions which provide the tedious 2-oxo acid and aldehyde substrates in situ would boost the applicability of pyruvic acid aldolase reactions [46]. It also would allow biomass-derived compounds as alternative starting material to construct the nucleophilic and electrophilic compounds that bear similar functionalization patterns.
Acknowledgement
This research was funded in whole by the Austrian Science Fund (FWF) [J4242-B21]. For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. The author is thankful to Pere Clapés and Mélanie Hall for valuable suggestions and proofreading the manuscript.
Abbreviations
- HpcH
4-hydroxy-2-oxo-heptane-1,7-dioic acid aldolase
- GarL
5-oxo-4-deoxy-d-glucarate aldolases
- RhmA
2-oxo-3-deoxy-l-rhamnonate aldolase
- KphMT
oxopantoate hydroxymethyltransferase
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
* * of outstanding interest
- 1.Wu S, Snajdrova R, Moore JC, Baldenius K, Bornscheuer U. Biocatalysis: enzymatic synthesis for industrial applications. Angew Chem Int Ed. 2020;60:88–119. doi: 10.1002/anie.202006648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winkler CK, Schrittwieser JH, Kroutil W. Power of biocatalysis for organic synthesis. ACS Cent Sci. 2021;7:55–71. doi: 10.1021/acscentsci.0c01496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galanie S, Entwistle D, Lalonde J. Engineering biosynthetic enzymes for industrial natural product synthesis. Nat Prod Rep. 2020;37:1122–1143. doi: 10.1039/c9np00071b. [DOI] [PubMed] [Google Scholar]
- 4.Patel RN. Biocatalysis for synthesis of pharmaceuticals. Bioorg Med Chem. 2018;26:1252–1274. doi: 10.1016/j.bmc.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 5.Truppo MD. Biocatalysis in the pharmaceutical industry: the need for speed. ACS Med Chem Lett. 2017;8:476–480. doi: 10.1021/acsmedchemlett.7b00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheldon RA, Brady D. Broadening the scope of biocatalysis in sustainable organic synthesis. ChemSusChem. 2019;12:2859–2881. doi: 10.1002/cssc.201900351. [DOI] [PubMed] [Google Scholar]
- 7.Sheldon RA. Fundame ntals of green chemistry: efficiency in reaction design. Chem Soc Rev. 2012;41:1437–1451. doi: 10.1039/c1cs15219j. [DOI] [PubMed] [Google Scholar]
- 8.Liao C, Seebeck FP. S-adenosylhomocysteine as a methyl transfer catalyst in biocatalytic methylation reactions. Nat Catal. 2019;2:696–701. [Google Scholar]
- 9.Black MJ, Biegasiewicz KF, Meichan AJ, Oblinsky DG, Kudisch B, Scholes GD, Hyster TK. Asymmetric redox-neutral radical cyclization catalysed by flavin-dependent ‘ene’-reductases. Nat Chem. 2020;12:71–75. doi: 10.1038/s41557-019-0370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zetzsche LE. Narayan ARH: Broadening the scope of bio-catalytic C-C bond formation. Nat Rev Chem. 2020;4:334–346. doi: 10.1038/s41570-020-0191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huffman MA, Fryszkowska A, Alvizo O, Borra-Garske M, Campos KR, Canada KA, Devine PN, Duan D, Forstater JH, Grosser ST, Halsey HM, et al. Design of an in vitro biocatalytic cascade for the manufacture of islatravir. Science. 2019;366:1255–1259. doi: 10.1126/science.aay8484. [DOI] [PubMed] [Google Scholar]
- 12.Li T, Cui X, Cui Y, Sun J, Chen Y, Zhu T, Li C, Li R, Wu B. Exploration of transaminase diversity for the oxidative conversion of natural amino acids into 2-ketoacids and high-value chemicals. ACS Catal. 2020;10:7950–7957. [Google Scholar]
- 13.Daniels AD, Campeotto I, Van Der Kamp MW, Bolt AH, Trinh CH, Phillips SEV, Pearson AR, Nelson A, Mulholland AJ, Berry A. Reaction mechanism of N-acetylneuraminic acid lyase revealed by a combination of crystallography, QM/MM simulation and mutagenesis. ACS Chem Biol. 2014;9:1025–1032. doi: 10.1021/cb500067z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Windle CL, Müller M, Nelson A, Berry A. Engineering aldolases as biocatalysts. Curr Opin Chem Biol. 2014;19:25–33. doi: 10.1016/j.cbpa.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobes RD, Simpson RT, Vallee BL, Rutter WJ. A functional role of metal ions in a class II aldolase. Biochemistry. 1969;8:585–588. doi: 10.1021/bi00830a018. [DOI] [PubMed] [Google Scholar]
- 16.Masai E, Sugiyama K, Iwashita N, Shimizu S, Hauschild JE, Hatta T, Kimbara K, Yano K, Fukuda M. The bphDEF metacleavage pathway genes involved in biphenyl/polychlorinated biphenyl degradation are located on a linear plasmid and separated from the initial bphACB genes in Rhodococcus sp. strain RHA1. Gene. 1997;187:141–149. doi: 10.1016/s0378-1119(96)00748-2. [DOI] [PubMed] [Google Scholar]
- 17.Stringfellow JM, Turpin B, Cooper RA. Sequence of the Escherichia coli C homoprotocatechuic acid degradative operon completed with that of the 2,4-dihydroxyhept-2-ene-1,7-dioic acid aldolase-encoding gene (HpcH) Gene. 1995;166:73–76. doi: 10.1016/0378-1119(95)00596-8. [DOI] [PubMed] [Google Scholar]
- 18.Hubbard BK, Koch M, Palmer DRJ, Babbitt PC, Gerlt JA. Evolution of enzymatic activities in the enolase superfamily: Characterization of the (d)-glucarate/galactarate catabolic pathway in Escherichia coli. Biochemistry. 1998;37:14369–14375. doi: 10.1021/bi981124f. [DOI] [PubMed] [Google Scholar]
- 19.Rea D, Fülöp V, Bugg TDH, Roper DI. Structure and mechanism of HpcH: a metal ion dependent class II aldolase from the homoprotocatechuate degradation pathway of Escherichia coli. J Mol Biol. 2007;373:866–876. doi: 10.1016/j.jmb.2007.06.048. [DOI] [PubMed] [Google Scholar]
- 20.Rea D, Hovington R, Rakus JF, Gerlt JA, Fülöp V, Bugg TDH, Roper DI. Crystal structure and functional assignment of YfaU, a metal ion dependent class II aldolase from Escherichia coli K12. Biochemistry. 2008;47:9955–9965. doi: 10.1021/bi800943g. [DOI] [PubMed] [Google Scholar]
- 21.Fesko K, Gruber-Khadjawi M. Biocatalytic methods for C - C bond formation. ChemCatChem. 2013;5:1248–1272. [Google Scholar]
- 22.De Berardinis V, Guérard-Hélaine C, Darii E, Bastard K, Hélaine V, Mariage A, Petit JL, Poupard N, Sánchez-Moreno I, Stam M, Gefflaut T, et al. Expanding the reaction space of aldolases using hydroxypyruvate as a nucleophilic substrate. Green Chem. 2017;19:519–526. [* The first description of several high selective HpcH aldolases] [Google Scholar]
- 23.Laurent V, Gourbeyre L, Uzel A, Hélaine V, Nauton L, Traïkia M, de Berardinis V, Salanoubat M, Gefflaut T, Lemaire M, Guérard-Hélaine C. Pyruvate aldolases catalyze cross-aldol reactions between ketones: highly selective access to multifunctionalized tertiary alcohols. ACS Catal. 2020;10:2538–2543. [* * The study shows the availability of ketones as substrates for Type II aldolases to construct challenging tertiary alcohol products] [Google Scholar]
- 24.Marsden SR, Mestrom L, Bento I, Hagedoorn P, Mcmillan DGG, Hanefeld U. CH-p Interactions promote the conversion of hydroxypyruvate in a class II pyruvate aldolase. Adv Synth Catal. 2019;361:2649–2658. [* A mechanistic study that reveals the beneficial influence of aromatic residues in the active site on substrate conversion] [Google Scholar]
- 25.Wu L, Maglangit F, Deng H. Fluorine biocatalysis. Curr Opin Chem Biol. 2020;55:119–126. doi: 10.1016/j.cbpa.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Windle CL, Berry A, Nelson A. Aldolase-catalysed stereoselective synthesis of fluorinated small molecules. Curr Opin Chem Biol. 2017;37:33–38. doi: 10.1016/j.cbpa.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 27.Stockwell J, Daniels AD, Windle CL, Harman TA, Woodhall T, Lebl T, Trinh CH, Mulholland K, Pearson AR, Berry A, Nelson A. Evaluation of fluoropyruvate as nucleophile in reactions catalysed by N-acetyl neuraminic acid lyase variants: scope, limitations and stereoselectivity. Org Biomol Chem. 2016;14:105–112. doi: 10.1039/c5ob02037a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howard JK, Müller M, Berry A, Nelson A. An enantio- and diastereoselective chemoenzymatic synthesis of α-fluoro β-hydroxy carboxylic esters. Angew Chem Int Ed. 2016;55:6767–6770. doi: 10.1002/anie.201602852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang J, Hait D, Head-Gordon M, Chang MCY. Chemoenzymatic platform for synthesis of chiral organofluorines based on type II aldolases. Angew Chem Int Ed. 2019;58:11841–11845. doi: 10.1002/anie.201906805. [* * This study provides a comprehensive insight into the possibilities to use type II aldolases for a fluoroorganics platform] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marsden SR, Mestrom L, McMillan DGG, Hanefeld U. Thermodynamically and kinetically controlled reactions in biocatalysis – from concepts to perspectives. ChemCatChem. 2020;12:426–437. [Google Scholar]
- 31.Liu Y-L, Lin X-T. Recent advances in catalytic asymmetric synthesis of tertiary alcohols via nucleophilic addition to ketones. Adv Synth Catal. 2019;361:876–918. [Google Scholar]
- 32.Gaggero N. Building up quaternary stereocenters through biocatalyzed direct insertion of carbon nucleophiles on ketones. Eur J Org Chem. 2019;2019:7615–7628. [Google Scholar]
- 33.Laurent V, Ekaterina D, Aujon A, Debacker M, Petit J-L, Hélaine V, Liptaj T, Breza M, Mariage A, Nauton L, Traïkia M, et al. Synthesis of branched-chain sugars with a DHAP-dependent aldolase: ketones are electrophile substrates of rhamnulose-1-phosphate aldolases. Angew Chem Int Ed. 2018;57:5467–5471. doi: 10.1002/anie.201712851. [DOI] [PubMed] [Google Scholar]
- 34.Hernandez K, Bujons J, Joglar J, Charnock SJ, Domínguez De María P, Fessner WD, Clapés P. Combining aldolases and transaminases for the synthesis of 2-amino-4-hydroxybutanoic Acid. ACS Catal. 2017;7:1707–1711. [Google Scholar]
- 35.Hernández K, Joglar J, Bujons J, Parella T, Clapés P. Nucleophile promiscuity of engineered class II pyruvate aldolase from E. Coli, YfaU. Angew Chem Int Ed. 2018;57:3583–3587. doi: 10.1002/anie.201711289. [* * The first study that broadly expanded the scope of aliphatic 2-oxo acids utilized by a class II aldolase] [DOI] [PubMed] [Google Scholar]
- 36.Hernández K, Gómez A, Joglar J, Bujons J, Parella T, Clapés P. 2-Keto-3-deoxy-l-rhamnonate aldolase (YfaU) as catalyst in aldol additions of pyruvate to amino aldehyde derivatives. Adv Synth Catal. 2017;359:2090–2100. [* The synthesis of an amino acid platform showed the manifoldness of follow-up chemistry for class II aldolase products] [Google Scholar]
- 37.Sugantino M, Zheng R, Yu M, Blanchard JS. Mycobacterium tuberculosis ketopantoate hydroxymethyltransferase: Tetrahydrofolate-independent hydroxymethyltransferase and enolization reactions with a-keto acids. Biochemistry. 2003;42:191–199. doi: 10.1021/bi020516q. [DOI] [PubMed] [Google Scholar]
- 38.Marín-Valls R, Hernández K, Bolte M, Joglar J, Bujons J, Clapés P. Chemoenzymatic hydroxymethylation of carboxylic acids by tandem stereodivergent biocatalytic aldol reaction and chemical decarboxylation. ACS Catal. 2019;9:7568–7577. [* * Enantiocomplementarity in biocatalytic aldol reactions is described or enantiocomplementary biocatalytic aldol reactions are described] [Google Scholar]
- 39.Laurent V, Uzel A, Hélaine V, Nauton L, Traïkia M, Gefflaut T, Salanoubat M, De Berardinis V, Lemaire M, Guérard-Hélaine C. Exploration of aldol reactions catalyzed by stereoselective pyruvate aldolases with 2-oxobutyric acid as nucleophile. Adv Synth Catal. 2019;361:2713–2717. [* * An NMR-based screening reveals that a panel of Pfam family PF03328 members offers aldol activity with strict stereoselectivity with 2-oxobutyric acid as a nucleophile] [Google Scholar]
- 40.Marín-Valls R, Hernández K, Bolte M, Parella T, Joglar J, Bujons J, Clapés P. Biocatalytic construction of quaternary centers by aldol addition of 3,3-disubstituted 2-oxoacid derivatives to aldehydes. J Am Chem Soc. 2020;142:19754–19762. doi: 10.1021/jacs.0c09994. [* * An unprecedented construction of quaternary carbon centers enables the preparation of previously unaccessible building blocks] [DOI] [PubMed] [Google Scholar]
- 41.Jambunathan P, Zhang K. Novel pathways and products from 2-keto acids. Curr Opin Biotechnol. 2014;29:1–7. doi: 10.1016/j.copbio.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 42.Wu S, Zhou Y, Wang T, Too H, Wang DIC, Li Z. Highly regio- and enantioselective multiple oxy- and aminofunctionalizations of alkenes by modular cascade biocatalysis. Nat Commun. 2016;7:11917. doi: 10.1038/ncomms11917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song Y, Li J, Shin H-D, Liu L, Du G, Chen J. Biotechnological production of alpha-keto acids: Current status and perspectives. Bioresour Technol. 2016;219:716–724. doi: 10.1016/j.biortech.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 44.Ogawa J, Yamanaka H, Mano J, Doi Y, Kodera T, Nio N, Smirnov SV, Natalya N, Kozlov Yl, Shimizu S. Synthesis of 4-hydroxyisoleucine by the aldolase – transaminase coupling reaction and basic characterization of the aldolase from Arthrobacter simplex AKU 626. Biosci Biotechnol Biochem. 2007;71:1607–1615. doi: 10.1271/bbb.60655. [DOI] [PubMed] [Google Scholar]
- 45.Guérard-Helaine C, Heuson E, Ndiaye M, Gourbeyre L, Lemaire M, a Hélaine V, Charmantray F, Petit J-L, Salanoubat M, de Berardinis V, Gefflaut T. Stereoselective synthesis of g-hydroxy-a-amino acids through aldolase-transaminase recycling cascades. Chem Commun. 2017;53:5465–6468. doi: 10.1039/c7cc00742f. [DOI] [PubMed] [Google Scholar]
- 46.Fryszkowska A, Devine PN. Biocatalysis in drug discovery and development. Curr Opin Chem Biol. 2020;55:151–160. doi: 10.1016/j.cbpa.2020.01.012. [DOI] [PubMed] [Google Scholar]