Abstract
Introduction
In addition to tau pathology and neuronal loss, neuroinflammation occurs in progressive supranuclear palsy (PSP). However, the prognostic value of the in vivo imaging markers for these processes in PSP remains unclear. We test the primary hypothesis that baseline in vivo imaging assessment of neuroinflammation in subcortical regions predicts clinical progression in patients with PSP.
Methods
Seventeen patients with PSP–Richardson’s syndrome underwent a baseline multimodal imaging assessment, including [11C]PK11195 positron emission tomography (PET) to index microglial activation, [18F]AV-1451 PET for tau pathology and structural MRI. Disease severity was measured at baseline and serially up to 4 years with the Progressive Supranuclear Palsy Rating Scale (PSPRS) (average interval of 5 months). Regional grey-matter volumes and PET ligand binding potentials were summarised by three principal component analyses (PCAs). A linear mixed-effects model was applied to the longitudinal PSPRS scores. Single-modality imaging predictors were regressed against the individuals’ estimated rate of progression to identify the prognostic value of baseline imaging markers.
Results
PCA components reflecting neuroinflammation and tau burden in the brainstem and cerebellum correlated with the subsequent annual rate of change in the PSPRS. PCA-derived PET markers of neuroinflammation and tau pathology correlated with regional brain volume in the same regions. However, MRI volumes alone did not predict the rate of clinical progression.
Conclusions
Molecular imaging with PET for microglial activation and tau pathology can predict clinical progression in PSP. These data encourage the evaluation of immunomodulatory approaches to disease-modifying therapies in PSP and the potential for PET to stratify patients in early phase clinical trials.
Introduction
Neuroinflammation has been recognised as a common pathogenic process in progressive supranuclear palsy (PSP)1 2 and other tauopathies such as Alzheimer’s disease3 (AD) and frontotemporal dementia,4–6 together with genetic, epidemiological and imaging associations. For example, activated microglia are found in the neighbourhood of neurofibrillary tangles, even during early stages of disease,1 and are directly synaptotoxic.3 Neuroinflammation, including microglial activation, interacts with tau pathology to promote cell dysfunction and death in preclinical models of tauopathy.
Positron emission tomography (PET) radioligands have been developed to assess neuroinflammation and tau pathology accumulation in vivo in clinical cohorts. [11C]PK11195 is a widely used PET tracer that binds primarily to activated microglia in PSP7–9 and other neurodegenerative disorders.10 11 The ligand [18F]AV-1451 is widely used to assess tau pathology in AD and can be informative in PSP (see12 for review) despite lower sensitivity to PSP tau isoforms and off-target binding in some regions.13 14 However, it has not been shown whether either of these PET biomarkers of neuroinflammation and tau pathology predict longitudinal clinical progression in patients with PSP.
Our main hypothesis was that inflammation in the subcortical regions associated with PSP pathology promotes disease progression. We therefore test whether baseline in vivo measures of neuroinflammation ([11C]PK11195 PET) predict the annual rate of clinical progression in patients with PSP–Richardson’s syndrome. We test secondary hypotheses regarding the predictive value of baseline cortical inflammation, tau pathology ([18F]AV-1451 PET) and regional brain volume (structural MRI).
Methods
Participants
As part of the Neuroimaging of Inflammation in Memory and Other Disorders study,15 we recruited 17 people with a clinical diagnosis of probable PSP according to Movement Disorder Society (MDS) 1996 criteria.16 These participants also met the later MDS-PSP 2017 criteria for PSP–Richardson’s syndrome.17 Participants underwent a baseline neuropsychological assessment followed by an MRI scan and two PET scans with [11C]PK11195 (11C-labelled R-enantiomer of PK11195) and [18F]AV-1451 (also known as 18F-flortaucipir). The cross-sectional baseline data of [11C]PK11195 and [18F]AV-1451 have been published.7 Disease severity was measured at baseline visit and serially up to 4 years using the Progressive Supranuclear Palsy Rating Scale (PSPRS).18 Assessments were at an average of 5-month intervals (SD±2.3 months). Postmortem confirmation of PSP pathology was available in each of the eight patients who subsequently donated their brain to the Cambridge Brain Bank. For all 17 participants, the clinical diagnosis was reviewed and confirmed at follow-up.
MRI and PET data acquisition and preprocessing
Full details of the imaging protocols have been published elsewhere.9 19 In brief, patients underwent 3 T MRI, together with dynamic PET imaging of [11C]PK11195 and [18F]AV-1451 for 75 and 90 min, respectively. MP-RAGE (Magnetization Prepared Rapid Acquisition Gradient Echo) T1-weighted MRI was acquired on Siemens Magnetom Tim Trio and Verio scanners (Siemens Healthineers, Erlangen, Germany), while PET scans were performed on a GE Advance and GE Discovery 690 PET/CT (GE Healthcare, Waukesha, USA). The two PET scanners used identical emission data acquisition protocols and image reconstruction algorithms (see Malpetti et al for discussion and more details7). Each patient underwent both [11C]PK11195 and [18F]AV-1451 PET scans using the same scanner (n=11 GE Discovery scanner, n=6 GE Advance scanner).7 Median (mean and SD) of the time interval between the baseline clinical assessment and the imaging scans were 0.0 (1.1±1.5) months for MRI, 2.0 (2.7±2.0) months for [11C]PK11195 PET and 1.0 (1.9±1.8) months for [18F]AV-1451 PET.
For each subject, the aligned dynamic PET image series for each scan was rigidly coregistered to the T1-weighted MRI image. Grey-matter volumes and non-displaceable binding potential (BPND) values for each tracer were calculated in 83 cortical and subcortical regions of interest (ROIs) using a modified version of the Hammersmith atlas (www.brain-development.org), which includes parcellation of the brainstem and cerebellar dentate nucleus. Each T1 image was spatially normalised using Advanced Normalization Tools (http://www.picsl.upenn.edu/ANTS/), and the inverse transform was applied to a version of the Hammersmith atlas to bring ROIs to native T1 space. The T1-weighted images were segmented into grey matter, white matter and cerebrospinal fluid (CSF) with SPM12 (www.fil.ion.ucl.ac.uk) and were used to determine regional grey matter, white matter and CSF volumes, and to calculate the total intracranial volume (TIV=grey matter+white matter+CSF) in each participant. Regional grey-matter volumes included in further analyses were corrected for TIV. Prior to kinetic modelling, regional PET data were corrected for partial volume effects from the CSF by dividing by the mean regional grey-matter plus white-matter fraction determined from Statistical Parametric Mapping (SPM) segments smoothed to PET spatial resolution. For [11C]PK11195, supervised cluster analysis was used to determine the reference tissue time–activity curve, and BPND values were calculated in each ROI using a simplified reference tissue model with vascular binding correction.20 For [18F]AV-1451, BPND values were quantified in each ROI using a basis function implementation of the simplified reference tissue model,21 with superior cerebellar cortex grey matter as the reference region. This cerebellar region was selected as reference region given postmortem evidence showing minimal tau pathology in PSP (see pathology data in Passamonti et al 19).
Statistical analyses
Grey-matter volumes and BPND values for each ligand were combined across the two hemispheres to derive 43 bilateral ROIs,7 9 19 which were next included in separate principal component analyses (PCAs) for each imaging modality. Varimax rotation was applied in each PCA to maximise interpretability and specificity of the resulting components. Components with eigenvalues of >1 were retained, explaining >80% of cumulative variance.
A linear mixed model was applied to longitudinal PSPRS scores collected from the first research visit to estimate the clinical annual rate of change at group level and then extract a patient-specific estimate of disease progression. The model included the estimation of a random intercept and slope, with time (in years) as independent variable and PSPRS scores as dependent variable. The effect of time on clinical changes has been also tested via likelihood ratio tests of the model described previously against the null model without the time effect. Linear mixed-effects analyses were performed using R software and lme4 package (R Core Team, 2012).
To test whether specific neuroanatomical patterns of grey-matter volume, microglial activation and tau pathology predict clinical progression, linear regression models were applied with the estimated rate of change (slope) as dependent variable, and each method-specific PCA component as predictor. First, we tested for significant regressions on slope with each modality-specific subcortical component as predictor, in accordance with our main hypothesis. Then, we explored the predictive value of cortical components running separate linear regression analyses for each imaging method and component. Age, education and sex were included as nuisance covariates. We tested associations between rate of change of clinical scores, duration from symptom onset to the baseline research visit, and the first PSPRS score.
Analogous linear regression models were then estimated with the intercept of the clinical severity as dependent variable. This identifies a cross-sectional association between imaging markers and clinical severity at baseline, which was estimated at individual level from the linear mixed-effects model on longitudinal PSPRS scores. For cross-sectional analyses, we expected to find significant associations with subcortical imaging components.7 9 22 23
Lastly, the modality-specific subcortical components were included in cross-modality Pearson correlations to test for associations between the strength of regional volumes, neuroinflammation and tau pathology.
Data availability
Anonymised data may be shared by request to the senior author from a qualified investigator for non-commercial use (data sharing may be subject to restrictions according to consent and data protection legislation).
Results
The demographics and clinical and cognitive variables of our sample are summarised in table 1. Fifteen out of 17 patients died within 5 years from the baseline research assessment (median=2.5 years, mean±SD=2.2 ± 1.0 (range 0.4–4.5) years from baseline visit). Eight of these 15 donated to the Cambridge Brain Bank, where post mortem examination confirmed PSP pathology in all eight. In table 1, we report demographic, clinical characteristics and group comparisons for two subgroups of patients, categorised by dividing the group along the median of time interval between study baseline and death. Age, years of education, baseline PSPRS and annual PSPRS rate of change were compared with independent-samples t-tests; sex was compared using χ2 test.
Table 1.
Demographic and clinical characteristics
| Total patient group | Patient survival≤2.5 years | Patient survival>2.5 years | Difference | |
| N | 17 | 9 | 8 | |
| Sex (F/M) | 7/10 | 4/5 | 3/5 | χ2=0.08, p=0.772 |
| Age (mean±SD) | 68.3±5.7 | 68.6±7.1 | 68.0±4.1 | t(15)=0.20, p=0.848 |
| Education (mean±SD) | 12.1±1.9 | 11.8±1.8 | 12.5±2.1 | t(15)=−0.77, p=0.452 |
| Symptom duration (mean±SD) | 4.7±1.5 | 4.6±1.7 | 4.8±1.5 | t(15)=−0.28, p=0.786 |
| PSPRS baseline (mean±SD) | 41.2±14.5 | 45.1±14.8 | 37.0±13.7 | t(15)=1.19, p=0.255 |
| Clinical progression following baseline–PSPRS points/year (mean±SD) | 6.2±1.5 | 6.4±0.7 | 5.9±2.1 | t(15)=0.75, p=0.468 |
Statistics are reported for the total patient group and for subgroups based on median split of survival from baseline research assessment to death (median=2.5 years, mean±SD=2.2±1.0).
PSPRS, Progressive Supranuclear Palsy Rating Scale; t(), t-test.
PCA of imaging data
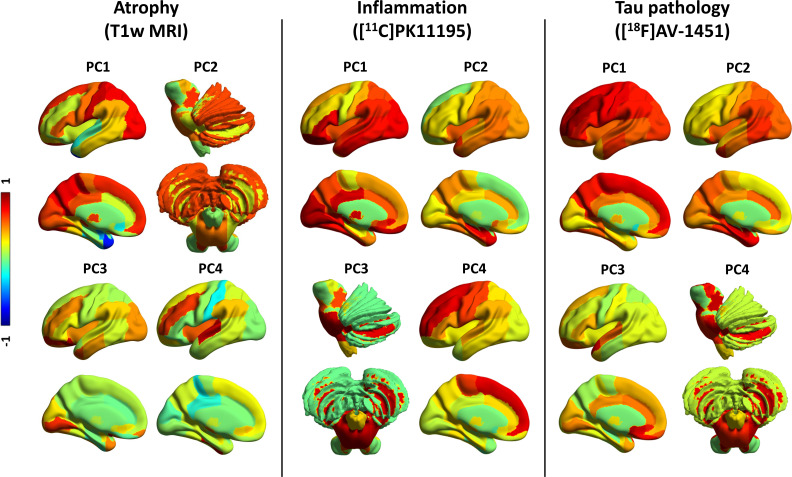
For grey-matter volumes, seven components were identified, which explained 80.3% of the total variance. Figure 1 (left panel) provides a pictorial representation of the first four components, and online supplemental table 1 details regional weights in all seven components. Component 1 was widely distributed, including medial frontal cortex, thalamus, occipitoparietal regions, posterior cingulate cortex and postcentral cortex (32.0% of the total variance). Component 2 (11.8% variance) was weighted to midbrain, substantia nigra and pons, nucleus accumbens and putamen, as well as amygdala, hippocampus and precentral cortex, cerebellar grey-matter and dentate nucleus. Component 3 (10.0% variance) loaded onto the orbitofrontal cortex, anterior temporal lobe and lingual gyrus. Component 4 (7.9% variance) included the superior temporal gyrus, fusiform gyrus, middle inferior temporal lobe and insula.
Figure 1.
PCs for grey-matter volume, inflammation and tau pathology. First four PCs for grey-matter volumes (left panel), [11C]PK11195 BPND (middle panel) and [18F]AV-1451 BPND (right panel). The colours represent the rotated weights (range: from −1 to 1) of the brain regions for each component. BPND, non-displaceable binding potential; PC, principal component.
jnnp-2020-325549supp001.pdf (131.7KB, pdf)
For [11C]PK11195 BPND and [18F]AV-1451 BPND, each PCA identified four components, which collectively and respectively explained 81.4% and 81.8% of the data variance, as reported in Malpetti et al.7 For [11C]PK11195 (figure 1, middle panel; online supplemental table 2), component 1 loaded onto posterior cortical regions, the orbitofrontal cortex and cerebellar grey matter; component 2 grouped together medial and superior temporal lobes, insula and temporoparietal junction; component 3 was weighted to brainstem regions (ie, midbrain and pons), dentate nucleus and cerebellar white matter; while component 4 included superior and medial frontal regions. For [18F]AV-1451 (figure 1, right panel; online supplemental table 3), component 1 reflected global cortical binding; component 2 grouped insula and medial temporal lobe regions; component 3 loaded onto anterior superior temporal gyrus and frontal subgenual cortex; component 4 was weighted towards subcortical areas, including midbrain, pons, substantia nigra, thalamus, cerebellar dentate nucleus and white matter.
Clinical progression as measured by longitudinal PSPRS
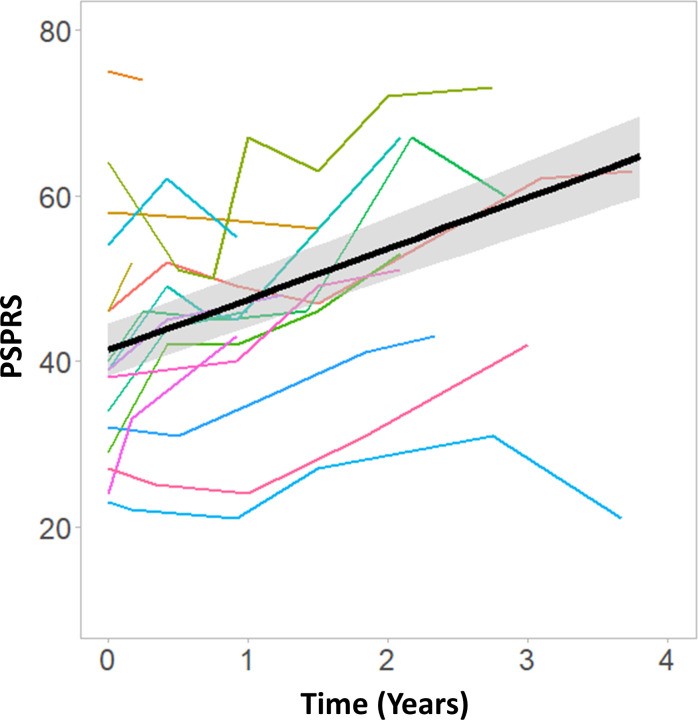
The linear mixed model on longitudinal PSPRS progression after baseline, considering the first research visit as baseline, indicated a significant effect of time (, ean=6.15 points/year, SD=1.06; figure 2 and table 1). The model comparison against the null model confirmed the significant effect of time (∆χ2=42.61, ∆df=3, p<0.0001). We also applied an analogous model over the whole longitudinal clinical assessment period, including all scores available from patients’ initial clinical diagnosis visit to their latest clinical visit. This confirmed a similar annual rate of change in PSPRS (mean=7.20 points/year, SD=1.18).
Figure 2.
Clinical severity as measured by the PSPRS over time. Coloured lines chart the time course of the PSPRS score in individual patients. The black line represents the linear fit at group level. PSPRS, Progressive Supranuclear Palsy Rating Scale.
Single-modality prediction models
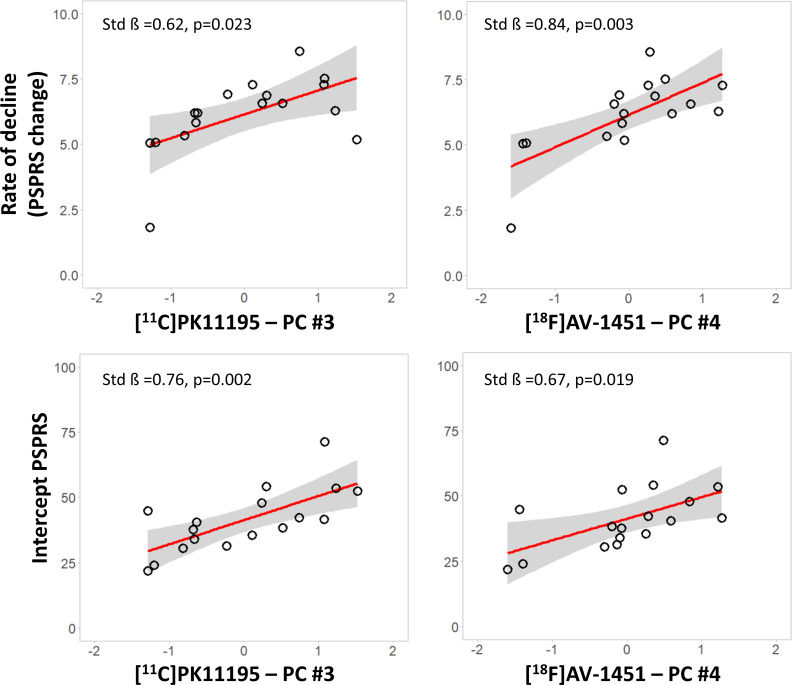
We tested whether imaging markers in subcortical components predicted longitudinal PSPRS progression, applying linear regression models for each modality-specific subcortical components (MRI component 2, [11C]PK11195 component 3 and [18F]AV-1451 component 4). Correcting for age, education and sex, the annual rate of clinical progression was related positively with (1) the [11C]PK11195 subcortical component 3 (standardised beta=0.624, p=0.023) and (2) the [18F]AV-1451 subcortical component 4 (standardised beta=0.840, p=0.003) (figure 3, top row). Applying regression models on the slope with single cortical components, age, education and sex as predictors, we found that no other components had significant correlations with the annual rate of clinical progression (p>0.05 after false discovery rate (FDR) correction for multiple comparisons). For MRI, no component showed an association with clinical rate of change (p>0.05 after FDR correction for multiple comparisons). The regression model on clinical progression with symptom duration as single regressor was not significant (standardised beta=−0.06, p=0.822 uncorrected). Similarly, clinical severity at baseline (PSPRS) was not associated with clinical progression (standardised beta=0.33, p=0.196).
Figure 3.
Inflammation and tau PET markers are associated with clinical severity and progression. Significant regression analyses of annual change in PSPRS scores (top row) and intercept PSPRS scores (bottom row) against baseline scores for each modality-specific principal component (X axis—residual component values corrected for covariates): [11C]PK11195 PET (left panel) and [18F]AV-1451 PET (right panel). Estimated parameters are reported for each model with age, education and sex as covariates. PET, positron emission tomography; PSPRS, Progressive Supranuclear Palsy Rating Scale.
We tested whether imaging markers in subcortical components were related to baseline variation in disease severity. Across the three modality-specific subcortical components, linear regression models with individual PSPRS intercept scores as the dependent variable, correcting for age, education and sex, indicated significant associations with the [11C]PK11195 subcortical component #3 (standardised beta=0.755, p=0.002), and [18F]AV-1451 subcortical component #4 (standardised beta=0.673, p=0.019) (figure 3, bottom row). However, there was no significant association between intercept and any of the grey-matter components or cortical PET components (all p>0.05 after FDR correction for multiple comparisons). The regression model on clinical intercept scores with symptom duration (standardised beta=0.43, p=0.086) was not significant.
Intermodality correlations between subcortical imaging components
Simple correlations across subjects between MRI and PET subcortical components of clinical slope were significant for MRI component #2 with [11C]PK11195 component 3 (r=−0.584, p=0.014 uncorrected, p=0.014 FDR correction) and [18F]AV-1451 component 4 (r=−0.626, p=0.007 uncorrected, p=0.011 FDR correction). The correlation between [11C]PK11195 component 3 and [18F]AV-1451 component 4 was also significant (r=0.769, p<0.0001 uncorrected, p<0.0001 FDR correction).7
Discussion
Our main finding is that subcortical neuroinflammation is associated with clinical severity of PSP at baseline, and with faster subsequent clinical progression. A similar effect is found for estimated subcortical tau pathology, with the caveats related to interpreting [18F]AV1451 binding in PSP. The PET markers were associated with each other and with structural MRI measures for atrophy in the same regions. However, subcortical grey-matter volumes were not correlated with subsequent clinical progression and were not significantly related to clinical severity at baseline. Similarly, clinical severity at baseline was not predictive of clinical progression in the following years, suggesting that the annual rate of clinical changes is approximately constant throughout different stages of disease.
Several studies in PSP have explored the association between changes in clinical severity and in vivo neuroimaging markers for microglial activation (eg, [11C]PK11195 PET8 9), tau pathology (eg, [18F]AV-1451 PET19 22–25) and atrophy (eg, structural MRI26–28). This study, however, focusses on the prognostic (or predictive) potential of baseline multimodal imaging markers. The baseline uptakes of both [11C]PK11195 and [18F]AV-1451 in PSP-related subcortical regions correlated with the subsequent annual rate of change in severity, as measured by PSPRS. Note that we are not testing whether the progression of PET markers compares with progression of disease severity. The progression of [18F]AV-1451 uptake has been compared with MRI-derived atrophy progression,29 revealing greater atrophy changes than PET signal changes over time. Instead, for prognostic value, we found that grey-matter volumetric measures were weaker predictors than PET markers, despite the correlation of subcortical grey-matter volumes with [11C]PK11195 and [18F]AV-1451 binding. The latter correlation suggests a close relationship between not only microglial activation and tau pathology,1 7 but also with neurodegeneration in those subcortical regions commonly associated with pathological hallmarks of PSP.30 Our findings align with studies of AD, another tauopathy, in which baseline in vivo PET markers of tau pathology and microglial activation predicted clinical progression, outperforming structural MRI.31
The correlation of in vivo PET measures with baseline disease severity has been reported in previous studies.7 9 22 23 Using [11C]PK11195 PET to assess neuroinflammation, a study reported a positive association was observed between clinical severity and ligand binding in pallidum, midbrain and pons.9 There was a strong association between [11C]PK11195 binding and [18F]AV-1451 in these subcortical regions, although inconsistent findings are reported in studies using [18F]AV-1451in PSP (see Leuzy et al 12). The sporadic lack of significant correlates of [18F]AV-1451 in PSP is often attributed to the low affinity of the ligand for 4R-tau pathology. However, in relatively small studies, sessional variance of clinical rating scales may also reduce power. Therefore, our estimate of baseline clinical severity used the intercept extracted from the linear mixed-effects model of longitudinal PSPRS scores rather than single baseline assessment.
The null result for structural MRI predictors might be surprising, given previous reports on the utility of visual and volumetric atrophy assessments in the midbrain and other subcortical regions, including caudate, putamen, globus pallidus, subthalamus and thalamus, as an in vivo biomarker in patients with PSP.32 Indeed, structural MRI has provided the most studied and validated diagnostic biomarkers in PSP. However, a biomarker’s properties for diagnostics (ie, presence of PSP32 33) or correlates of severity (ie, at baseline) do not imply the property of prognostication.
Overall, our findings on the in vivo association between imaging markers of different pathological processes and their prognostic relevance accord with postmortem data1 2 and suggest a key role for microglial activation and tau burden on neurodegeneration, and consequent clinical progression. A growing literature supports a role for neuroinflammation in driving tau spreading and neurodegeneration in tauopathies (see Vogels et al 3 for review). Furthermore, genome-wide association studies implicate inflammatory pathways in the aetiology of tauopathies.34 35 For example, Jabbari et al reported an association between a common variation at the leucine-rich repeat kinase 2 (LRRK2) locus and survival from symptom onset to death in patients with PSP.35 This relationship may be mediated by the effect of increased LRRK2 expression in microglia proinflammatory responses,36 promoting spread and accumulation of misfolded tau protein, analogous to AD.3 This hypothesis is supported by the association of dysregulated expression of the microglial-related gene CXCR4, regional accumulation of neurofibrillary tangles and increased risk of PSP.37 The role of early neuroinflammation in tauopathies is supported by PET evidence of microglial activation preceding PET evidence of aggregated tau and symptoms in carriers of mutations of the microtubule associated protein tau gene (MAPT).5 A preliminary study of longitudinal changes in microglial activation in two patients with PSP showed stable microglial activation across 6–10 months8 but may have lacked power. However, preclinical evidence with PET in tau transgenic mice suggests that inflammation increases longitudinally and predicts greater tau accumulation and lesser performance over time.38
There are several limitations to our study. We recruited according to clinical diagnostic criteria, and although clinicopathological correlations of PSP-Richardson’s syndrome are very high, including 8 of 8 cases in our study with post mortem pathology, they are not perfect. Moreover, the average rate of change in severity was 6–7 points per year on the PSPRS, which is lower than several previous observational studies18 39 and clinical trials.40 41 This may partially be due to selection criteria that favoured patients robust enough to undergo three brain scans at baseline. However, our cases were otherwise typical, and 15 out of 17 died within 5 years from baseline (mean 2.2 years±1.0). The modest size of our cohort prevented the application of complex models for the direct comparison between MRI and PET predictors, such as multiple linear regression or linear mixed models with several independent variables. The replication of these findings with larger and multicenter clinical cohorts will be important to establish generalisability of our results. Other limitations relate to the PET tracers used. [11C]PK11195 binds to the 18 kDa translocator protein which is overexpressed in activated microglia, but also in other cell types, like astrocytes and vascular smooth muscle cells, although it has been found selective for activated microglia over reactive astrocytes.42 There are also caveats for [18F]AV-1451, namely, its off-target binding (monoamine oxidase, choroid plexus and neuromelanin) and lower affinity for PSP tau compared with AD-related tau (see Malpetti et al and Leuzy et al 7 12 for discussion regarding limitations of this ligand). Nonetheless, the topological distribution of [18F]AV-1451 binding and correlations with severity maintain utility for this ligand even in PSP.
In conclusion, our results support the relevance of neuroinflammation for progression of PSP–Richardson’s syndrome. We suggest that [11C]PK11195 may be a valuable biomarker for clinical trials in PSP, complementary to structural MRI. The PET markers may be useful for stratification of patients based on prognosis and for evaluation of therapeutic response, supporting the development of immunomodulatory strategies for disease-modifying treatments in PSP, alone or in conjunction with treatments directed against tau and other pathogenic pathways.
Acknowledgments
We thank our participant volunteers for their participation in this study, and the radiographers/technologists at the Wolfson Brain Imaging Centre and Addenbrooke’s PET/CT Unit, and the research nurses of the Cambridge Centre for Parkinson-plus for their invaluable support in data acquisition. We thank the East Anglia Dementias and Neurodegenerative Diseases Research Network (DeNDRoN) for help with subject recruitment, and Drs Istvan Boros, Joong-Hyun Chun and other WBIC RPU staff for the manufacture of the radioligands. We thank Avid (Lilly) for supplying the precursor for the production of [18F]AV-1451 used in this study. We also thank Frank Hezemans for consultation on statistical methods.
Footnotes
Twitter: @M_Malpetti, @timrittman
Contributors: Conceptualisation and design: MM, JBR, JTOB and FIA; data analysis: MM, PSJ, TDF and YTH; data curation and methodology: MM, PSJ, DS, TR, TDF, YTH and JBR; interpretation: MM, LP, JBR, JTOB; data collection: LP, DS, TR, TDF, YTH, PVR, WRB-J, FIA and JBR; first draft: MM; supervision: JBR and JTOB. All authors revised and approved the final version of the manuscript.
Funding: This study was cofunded by the National Institute for Health Research (NIHR) Biomedical Research Centre and Biomedical Research Unit in Dementia based at Cambridge University Hospitals NHS Foundation Trust and the University of Cambridge (146281; BRC-1215-20014), including their financial support for the Cambridge Brain Bank; the PSP Association ('MAPT-PSP' award); the Wellcome Trust (103838); the Cambridge Trust & Sidney Sussex College Scholarship; the Medical Research Council (MR/P01271X/1); Evelyn Trust and the Cambridge Centre for Parkinson-Plus (RG95450). The views expressed are those of the author and not necessarily those of the NIHR or the Department of Health and Social Care.
Competing interests: Unrelated to this work, JBR serves as an associate editor to Brain and is a non-remunerated trustee of the Guarantors of Brain, Darwin College and the PSP Association (UK). He provides consultancy to Asceneuron, Biogen, UCB and has research grants from AZ-Medimmune, Janssen, Lilly as industry partners in the Dementias Platform UK. Unrelated to this work, JTOB has received honoraria for work as DSMB chair or member for TauRx, Axon, Eisai, has acted as a consultant for Roche, and has received research support from Alliance Medical and Merck.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Anonymised data may be shared by request to the senior author from a qualified investigator for non-commercial use (data sharing may be subject to restrictions according to consent and data protection legislation).
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Participants had mental capacity to take part in the study and provided written informed consent. The protocol was approved by the National Research Ethics Service East of England Cambridge Central Committee (reference: 13/EE/0104) and the UK Administration of Radioactive Substances Advisory Committee.
References
- 1. Ishizawa K, Dickson DW. Microglial activation parallels system degeneration in progressive supranuclear palsy and corticobasal degeneration. J Neuropathol Exp Neurol 2001;60:647–57. 10.1093/jnen/60.6.647 [DOI] [PubMed] [Google Scholar]
- 2. Fernández-Botrán R, Ahmed Z, Crespo FA, et al. Cytokine expression and microglial activation in progressive supranuclear palsy. Parkinsonism Relat Disord 2011;17:683–8. 10.1016/j.parkreldis.2011.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vogels T, Murgoci A-N, Hromádka T. Intersection of pathological tau and microglia at the synapse. Acta Neuropathol Commun 2019;7:109. 10.1186/s40478-019-0754-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bright F, Werry EL, Dobson-Stone C, et al. Neuroinflammation in frontotemporal dementia. Nat Rev Neurol 2019;15:540–55. 10.1038/s41582-019-0231-z [DOI] [PubMed] [Google Scholar]
- 5. Bevan-Jones WR, Cope TE, Jones PS, et al. In vivo evidence for pre-symptomatic neuroinflammation in a MAPT mutation carrier. Ann Clin Transl Neurol 2019;6:373–8. 10.1002/acn3.683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Malpetti M, Rittman T, Jones PS, et al. In vivo PET imaging of neuroinflammation in familial frontotemporal dementia. J Neurol Neurosurg Psychiatry 2021;92:319–22. 10.1136/jnnp-2020-323698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Malpetti M, Passamonti L, Rittman T. Neuroinflammation and tau co‐localize in vivo in progressive supranuclear palsy. Ann Neurol 2020:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gerhard A, Trender-Gerhard I, Turkheimer F, et al. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in progressive supranuclear palsy. Mov Disord 2006;21:89–93. 10.1002/mds.20668 [DOI] [PubMed] [Google Scholar]
- 9. Passamonti L, Rodríguez PV, Hong YT, et al. [11C]PK11195 binding in Alzheimer disease and progressive supranuclear palsy. Neurology 2018;90:e1989–96. 10.1212/WNL.0000000000005610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stefaniak J, O'Brien J, O’Brien J. Imaging of neuroinflammation in dementia: a review. J Neurol Neurosurg Psychiatry 2016;87:21–8. 10.1136/jnnp-2015-311336 [DOI] [PubMed] [Google Scholar]
- 11. Boche D, Gerhard A, Rodriguez-Vieitez E, et al. Prospects and challenges of imaging neuroinflammation beyond TSPO in Alzheimer's disease. Eur J Nucl Med Mol Imaging 2019;46:2831–47. 10.1007/s00259-019-04462-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leuzy A, Chiotis K, Lemoine L, et al. Tau PET imaging in neurodegenerative tauopathies-still a challenge. Mol Psychiatry 2019;24:1112–34. 10.1038/s41380-018-0342-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lowe VJ, Curran G, Fang P, et al. An autoradiographic evaluation of AV-1451 tau PET in dementia. Acta Neuropathol Commun 2016;4:1–19. 10.1186/s40478-016-0315-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marquié M, Normandin MD, Meltzer AC, et al. Pathological correlations of [F-18]-AV-1451 imaging in non-alzheimer tauopathies. Ann Neurol 2017;81:117–28. 10.1002/ana.24844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bevan-Jones WR, Surendranathan A, Passamonti L, et al. Neuroimaging of inflammation in memory and related other disorders (NIMROD) study protocol: a deep phenotyping cohort study of the role of brain inflammation in dementia, depression and other neurological illnesses. BMJ Open 2017;7:e013187. 10.1136/bmjopen-2016-013187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP International workshop. Neurology 1996;47:1–9. 10.1212/WNL.47.1.1 [DOI] [PubMed] [Google Scholar]
- 17. Höglinger GU, Respondek G, Stamelou M, et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder Society criteria. Mov Disord 2017;32:853–64. 10.1002/mds.26987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Golbe LI, Ohman-Strickland PA. A clinical rating scale for progressive supranuclear palsy. Brain 2007;130:1552–65. 10.1093/brain/awm032 [DOI] [PubMed] [Google Scholar]
- 19. Passamonti L, Vázquez Rodríguez P, Hong YT. F-AV-1451 positron emission tomography in Alzheimer’s disease and progressive supranuclear palsy. Brain 2017;3:aww340. 10.1093/brain/aww340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yaqub M, van Berckel BNM, Schuitemaker A, et al. Optimization of supervised cluster analysis for extracting reference tissue input curves in (R)-[(11)C]PK11195 brain PET studies. J Cereb Blood Flow Metab 2012;32:1600–8. 10.1038/jcbfm.2012.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gunn RN, Lammertsma AA, Hume SP, et al. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage 1997;6:279–87. 10.1006/nimg.1997.0303 [DOI] [PubMed] [Google Scholar]
- 22. Smith R, Schain M, Nilsson C, et al. Increased basal ganglia binding of 18 F-AV-1451 in patients with progressive supranuclear palsy. Mov Disord 2017;32:108–14. 10.1002/mds.26813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Whitwell JL, Lowe VJ, Tosakulwong N, et al. [18 F]AV-1451 tau positron emission tomography in progressive supranuclear palsy. Mov Disord 2017;32:124–33. 10.1002/mds.26834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cho H, Choi JY, Hwang MS, et al. Subcortical 18 F-AV-1451 binding patterns in progressive supranuclear palsy. Mov Disord 2017;32:134–40. 10.1002/mds.26844 [DOI] [PubMed] [Google Scholar]
- 25. Schonhaut DR, McMillan CT, Spina S, et al. 18 F-flortaucipir tau positron emission tomography distinguishes established progressive supranuclear palsy from controls and Parkinson disease: A multicenter study. Ann Neurol 2017;82:622–34. 10.1002/ana.25060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Whitwell JL, Xu J, Mandrekar JN, et al. Rates of brain atrophy and clinical decline over 6 and 12-month intervals in PSP: determining sample size for treatment trials. Parkinsonism Relat Disord 2012;18:252–6. 10.1016/j.parkreldis.2011.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsai RM, Lobach I, Bang J, et al. Clinical correlates of longitudinal brain atrophy in progressive supranuclear palsy. Parkinsonism Relat Disord 2016;28:29–35. 10.1016/j.parkreldis.2016.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dutt S, Binney RJ, Heuer HW, et al. Progression of brain atrophy in PSP and CBS over 6 months and 1 year. Neurology 2016;87:2016–25. 10.1212/WNL.0000000000003305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Whitwell JL, Tosakulwong N, Schwarz CG, et al. MRI Outperforms [18F]AV-1451 PET as a Longitudinal Biomarker in Progressive Supranuclear Palsy. Mov Disord 2019;34:105–13. 10.1002/mds.27546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sintini I, Schwarz CG, Senjem ML, et al. Multimodal neuroimaging relationships in progressive supranuclear palsy. Park Relat Disord 2019;66:56–61. 10.1016/j.parkreldis.2019.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Malpetti M, Kievit RA, Passamonti L, et al. Microglial activation and tau burden predict cognitive decline in Alzheimer's disease. Brain 2020;143:1588–602. 10.1093/brain/awaa088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Whitwell JL, Höglinger GU, Antonini A, et al. Radiological biomarkers for diagnosis in PSP: where are we and where do we need to be? Mov Disord 2017;32:955–71. 10.1002/mds.27038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Correia MM, Rittman T, Barnes CL, et al. Towards accurate and unbiased imaging-based differentiation of Parkinson's disease, progressive supranuclear palsy and corticobasal syndrome. Brain Commun 2020;2:fcaa051. 10.1093/braincomms/fcaa051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Broce I, Karch CM, Wen N. Immune-Related genetic enrichment in frontotemporal dementia: an analysis of genome-wide association studies. PLoS Med 2018;15:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jabbari E, Koga S, Valentino RR, et al. Genetic determinants of survival in progressive supranuclear palsy: a genome-wide association study. Lancet Neurol 2021;20:107-116. 10.1016/S1474-4422(20)30394-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moehle MS, Webber PJ, Tse T, et al. Lrrk2 inhibition attenuates microglial inflammatory responses. J Neurosci 2012;32:1602–11. 10.1523/JNEUROSCI.5601-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bonham LW, Karch CM, Fan CC, et al. Cxcr4 involvement in neurodegenerative diseases. Transl Psychiatry 2018;8:73. 10.1038/s41398-017-0049-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Eckenweber F, Medina-Luque J, Blume T, et al. Longitudinal TSPO expression in tau transgenic P301S mice predicts increased tau accumulation and deteriorated spatial learning. J Neuroinflammation 2020;17:1–12. 10.1186/s12974-020-01883-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Litvan I, Kong M. Rate of decline in progressive supranuclear palsy. Mov Disord 2014;29:463–8. 10.1002/mds.25843 [DOI] [PubMed] [Google Scholar]
- 40. Bang J, Lobach IV, Lang AE, et al. Predicting disease progression in progressive supranuclear palsy in multicenter clinical trials. Parkinsonism Relat Disord 2016;28:41–8. 10.1016/j.parkreldis.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boxer AL, Lang AE, Grossman M, et al. Davunetide in patients with progressive supranuclear palsy: a randomised, double-blind, placebo-controlled phase 2/3 trial. Lancet Neurol 2014;13:676–85. 10.1016/S1474-4422(14)70088-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Banati RB. Visualising microglial activation in vivo. Glia 2002;40:206–17. 10.1002/glia.10144 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2020-325549supp001.pdf (131.7KB, pdf)
Data Availability Statement
Anonymised data may be shared by request to the senior author from a qualified investigator for non-commercial use (data sharing may be subject to restrictions according to consent and data protection legislation).
Data are available upon reasonable request. Anonymised data may be shared by request to the senior author from a qualified investigator for non-commercial use (data sharing may be subject to restrictions according to consent and data protection legislation).