Abstract
For the past two decades, we have advanced in our understanding on the mechanisms implicated in the formation of brain circuits. The connection between the cortex and thalamus has deserved much attention, as thalamocortical connectivity is crucial for sensory processing and motor learning. Classical dye tracing studies in wild type and knock-out mice initially helped to characterize the developmental progression of this connectivity and revealed key transcription factors involved. With the recent advances of technical tools to specifically label subsets of projecting neurons, knock-down genes individually and/or modify their activity, the field has gained further understanding on the rules operating in thalamocortical circuit formation and plasticity. In this review, I will recapitulate the most relevant discoveries that have been made on this field, from development to early plasticity processes. I will emphasize how the implementation of new tools has helped the field to progress and what I consider to be the perspective and open questions for the future.
Keywords: Thalamus; axon guidance, spontaneous activity; somatosensory system; cortical maps; calcium waves
Introduction
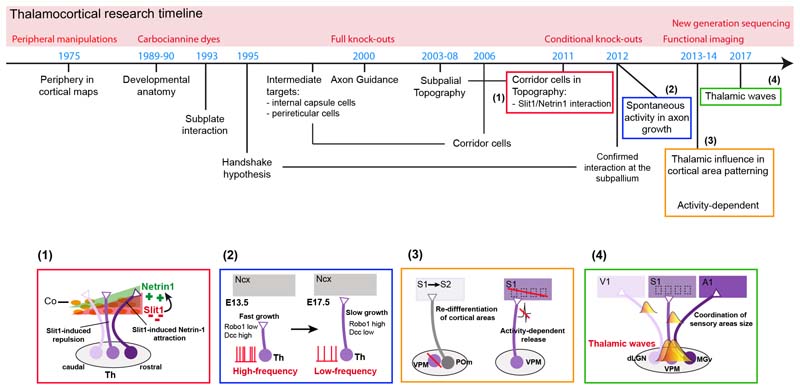
The thalamocortical system has been widely used as a model to study basic axon guidance mechanisms, to decipher the factors involved in the development of cortical maps, to understand how sensory systems develop, and to study the mechanisms involved in anatomical and functional circuit plasticity upon sensory loss. The reason for this great interest is because the thalamus has unique anatomical and functional specificities. Anatomically, TCAs conform a large ipsilateral projection, which develops embryonically crossing distinct anatomical boundaries. TCAs are highly topographically organized within a tract conveying sensory maps into the cortical territories. And finally, regarding their function, TCAs play a central role in sensory processing and perception as carry information received from the periphery to the specialized cortical areas. The application of novel techniques to this field, such as the use of conditional mouse lines or the in utero electroporation, has greatly contributed over the past years to boost our understanding on the role of TCAs in cortical development and sensory function (Figure 1).
Figure 1. Developmental timeline in the thalamocortical research.
Timeline representing the major discoveries in the field of thalamocortical development over the past 40 years. The major techniques used to achieve those findings are highlighted. The panels below emphasised four key findings that were made in the last few years. (1) The mechanism that allow rostral and intermediate TCAs to topographically arrange depend on interaction of guidance cues and receptors at the corridor cells. (2) The velocity of axonal growth in TCAs is controlled by activity-dependent gene expression. (3) The specification of a cortical area directly depends on the subtype of thalamic input that receives, being the higher-order mode the default signature. Moreover, this thalamic influence depends on neurotransmitter release from thalamic neurons. (4) Prenatal thalamic neurons are able to generate calcium waves of spontaneous activity among sensory nuclei that influence cortical areas size and plasticity.
Here, I will give a perspective of the major discoveries made for the thalamocortical system and then review how technical advances have rapidly helped the field to dissect the role of intrinsic versus extrinsic, mainly thalamic, mechanisms involved in the development of sensory cortical areas.
Axon guidance to the cortex
The ability to trace axons in fixed embryonic tissue offered by the carbocyanine dye technique, pioneered the field by the end of the last century. Using these anterograde and retrograde tracers to label axons and cells, several authors described for the first time the timing and trajectory of the thalamocortical connectivity and associated projections [1–8]. Briefly, thalamic axons exit the thalamus, traverse the pre-thalamus and avoid the hypothalamic region to cross the diencephalic-telencephalic boundary to the subpallium. Once in the subpallium, they cross distinct anatomical territories, interact with other developing axons, and cross the pallial-subpallial boundary to enter the neocortex where they arrive around E15.5 in mice (reviewed in [9–12]).
After the first anatomical description of the pathway followed by the TCAs, a new wave of discoveries was initiated in the late 90’s. By using full knock-out mice for genes expressed in the origin, along the pathway or in the target structure, several transcription factors were identified to be involved in thalamocortical pathfinding such as Emx2, Pax6, Tbr1, Gbx2, Nkx2.1 or Ebf1 [13–15]. Moreover, axon guidance molecules were also implicated. To cross the diencephalic-telencephalic boundary (DTB), TCAs need a Slit-mediated repulsion from the hypothalamus [16–18]. Once in the subpallium, TCAs are attracted toward the corridor, a dynamic region at the mantle of the medial ganglionic eminence (MGE) populated by GABAergic neurons originated at the lateral ganglionic eminence (LGE) [19]. This cellular bridge provides a permissive environment for TCAs to cross the MGE and to arrive to the cortex through the action of members of the Nrg1 family of guidance molecules. The position of these corridor cells at the mantle of the MGE is controlled by the midline repellent Slit2 [20]. It is believed that a fine control of the location of these corridor cells during evolution contributed to the switch from an external to an internal path of the TCAs that is characteristic in mammals [20].
In addition to the corridor cells, other cells provide guidance information to TCAs. Neurons located at the prethalamic, perireticular and internal capsule regions, with early projections to the thalamus, also influence TCA pathfinding at the DTB and at subpallial levels [21–23]. In fact, in mutant mice in which internal capsule projecting cells are displaced, TCAs do not cross the DTB [15]. In addition, the role of striatal cells is gaining importance in the guidance of TCAs at the subpallium, as many of their projections are located in the vicinity of TCAs. The modification of the position of these striatal cells or their elimination directly leads to consistent TCAs pathfinding errors [24]. Moreover, these cells might influence TCAs trajectory indirectly by modifying the position of corridor cells in a Frizzled-dependent manner [25].
The guidance of TCAs is also influenced by early pioneer cortical cells located in the subplate, a deep transient cortical cell population that projects to the thalamus [3]. It was proposed more than 15-years ago that the correct formation of the thalamo-cortical reciprocal connectivity relies on the early interaction between subplate axons and TCAs, the so called ‘handshake hypothesis’ formulated by Molnar and Blakemore [26]. By performing tracing studies the authors showed the intimate anatomical apposition of cortical axons and TCAs at the pallial subpallial boundary (PSPB) suggesting that these axons help each other’s guidance [22, 27]. However, this hypothesis remained for decades under debate as other studies showed that these axons only interdigitate in some portions of their trajectory or even repel each other in vitro [28–30].
After those years, the field entered in the era of analysing mutant mice in which the thalamocortical connectivity was affected by the lack of specific transcription factors. However, those studies did not clarify whether the handshake hypothesis was right, as results were overall contradictory. Some of the mutant mice did show deficits in TCAs pathfinding while in others, either the development of corticofugal axons was normal or both corticofugal axons and TCAs were severely affected [15, 31–34]. Conditional mutagenesis recently shed light into this issue and supported the subapallial interaction between both axonal tracts as a pre-requisite for their correct development. Specific genetic elimination of corticofugal axons without affecting the thalamus or any other subpallial structure leads to a scenario in which TCAs do not progress through the PSPB and thus, do not reach the cortex [35]. Actually, the opposite genetic experiment was done in parallel and showed that TCAs are also required in vivo to guide corticothalamic axons into the corridor and thalamus [36], altogether supporting the handshake hypothesis at least in a particular stage and region at the subpallium.
Acquiring Topography
One of the most important aspects for the correct function of the thalamocortical system is that its connectivity must be well arranged topographically. TCAs from the distinct principal sensory-modality nuclei target primarily modality-matched cortical areas. The dorsolateral geniculate nucleus (dLGN) projects to the primary visual cortex (V1), the ventromedial posterior (VPM) to the somatosensory area (S1) and the ventro-medial geniculate nucleus (vMG) to the primary auditory area (A1). This topography is acquired embryonically and at two consecutive subpallial structures, the corridor and the striatum [10, 11, 37]. Gradients of axon guidance molecules such as Netrin-1, Slit1, or Ephrin-A5 are expressed in register in both structures and play a role in the fine topographical positioning of TCAs [38–42]. However, a specific chemoattractive response is achieved at the corridor. By combining in vitro chemotaxis assays with the analysis of TCAs defects in specific mutant mice, it was determined that the rostral positioning of TCAs is achieved at the corridor by a modulation of Netrin-1 responsiveness by Slit1 [41] (Figure 1). Rostral TCAs specifically express a novel Robo1 co-receptor, FLRT3, which is a member of the Fibronectine leucine-rich repeat transmembrane proteins. By a series of in vitro biochemical assays, it was shown that in the presence of Slit1, both Robo1 and FLRT3 receptors are required to induce Netrin-1 attraction, via the upregulation of DCC receptor at the plasma membrane [42]. Thus, TCAs expressing FLRT3 can modulate Netrin-1 responsiveness in a context-dependent manner in developing TCAs.
Anyhow, it is more than likely that the specific expression of axon guidance receptors in the distinct TCAs subpopulations is genetically determined by unique transcription factors alone or in combination. For example, Lhx2, a transcription factor of the LIM homeodomain family is expressed by all early postmitotic thalamic neurons but downregulated in a region-specific manner in a late postmitotic stage [43]. While rostral and intermediate thalamic neurons switch off Lhx2, this transcription factor remains active only thalamic neurons from the auditory nucleus [43]. When Lhx2 was ectopically upregulated in rostral or intermediate thalamic nuclei, TCAs from those territories fail to reach their corresponding cortical areas and abnormally acquire an auditory fate targeting A1 [43]. These experiments suggest that there must be a transcriptional code that determines the fate of thalamocortical neurons from the distinct sensory nuclei. In fact, the development of the genome-wide analysis has helped to test this possibility through an unbiased approach. Using a genetic dual labelling method in mice, Gezelius and colleagues purified distinct sensory thalamic neurons and revealed their transcriptome profiling. Genes such as Sp9, Hs6st2, Crabp2 or Cck, were found to be expressed at specific sensory nuclei during embryonic development [44]. Complementary to this, other studies using new generation sequencing revealed the transcriptional profile of the distinct sensory thalamic nuclei at early postnatal stages [45].
The final topography of TCAs is also influenced by intracortical mechanisms. After several decades of intense debate, it is well accepted that the positional identity of cortical areas occurs independently of the thalamus, as generally precedes the thalamocortical innervation [46]. However, as we will recapitulate in the next sections, TCAs influence the final anatomical and functional organization of cortical territories at perinatal-early postnatal stages. Anyhow, intracortical mechanisms also guide TCAs to specific cortical territories influencing their final topographical disposition. The adaptation of the in utero electroporation technique as a successful gene delivery system in the mouse embryo allowed to produce gene manipulation in a time- and region-specific manner [47–49]. When the regional identity of the subplate cells was shifted out of register to the respective cortical plate by the in utero overexpression of Fgf8, TCAs tracked this shift by producing ectopic arbors in the “matching” subplate area [50]. These results strongly suggested the existence of positional information in the subplate that guides TCAs to the correct cortical territory, and matched previous data describing that TCAs must interact with subplate cells to recognize cortical areas [27, 51–54]. Specific elimination of subplate cells in S1 of the cat, showed that corresponding TCAs neither enter in S1 nor in any remaining sensory area with the intact subplate. Therefore, subplate cells are an essential cortical check-point for the spatial guidance of TCAs. It is likely that this positional information might depend on activity-based competition among TCAs, as thalamic axons interact with subplate cells through functional synapses [52, 53, 55–57]. Anyhow, TCAs have a remarkable capacity to reorganize themselves as was shown to occur in the Semaphorin6A mutant mice. In this mouse, visual TCAs are profoundly derailed embryonically provoking a topographical shift in the targeting of somatosensory axons to V1 [58]. Postnatally, the misrouted dLGN axons reach the V1 through alternative routes and re-establish a normal pattern of thalamocortical connectivity [58].
Controlling axon growth and branching
As aforementioned, TCAs are guided toward their correct targets by the interaction with axon guidance molecules and /or intermediate targets. Arriving on time to meet specific target cells is indispensable to achieve a correct functional circuit. Spontaneous activity, which is present in almost all the brain structures at embryonic stages, has been shown to influence early developmental processes such as neurotransmitter specification, axon pathfinding or axonal targeting [59–64]. Spontaneous activity in the form of calcium activity in developing neurons and axon growth cones has gained much attention in the last years. The development of novel techniques for imaging intracellular calcium ion concentration has helped to identify calcium as a second messenger inside the cell that mediates a wide spectrum of cellular functions. Calcium spikes are produced at distinct frequencies by embryonic thalamic neurons and in fact, is in this parameter where it is encoded the transcriptional regulation of genes that modulate TCAs growth rate (Figure 1). When TCAs traverse the subpallium their growth rate is increased and decelerates when they reach to the cortex [65]. This change in axonal growth rate is controlled by a developmental decrease in the frequency of calcium spikes at the cell soma [65]. The activity-dependent regulation of TCAs growth occurs via the transcriptional regulation Robo1 and Dcc genes. Robo1 acts as a brake while Dcc functions as an accelerator for growth [65, 66].
Spontaneous neural activity is also influential for the process of axonal branch formation in the thalamocortical system. Pioneer work by Herrmann and Shatz in the mid 90’s showed that when the sodium channel blocker tetrodotoxin (TTX) was infused into the kitten visual cortex fewer branches of visual TCAs were formed in V1[67]. Such activity-dependent branch formation was corroborated later using organotypic co-cultures assays first [68–70], and subsequently by specific genetic manipulation in vivo [71–74].
Influencing cortical territories, area identity and size
Although the patterning of cortical areas is grossly achieved by intrinsic cortical mechanisms, thalamocortical input has a critical role in shaping and delineating cortical areas later in development, both anatomically and functionally. As mentioned before, each cortical area is reciprocally connected with a distinct subset of thalamic neurons which are allocated in well-defined nuclei. Primary cortical areas have typical cytoarchitectonical features, especially S1 and V1, and thus, have been extensively used to understand the role of thalamocortical input in shaping cortical differentiation. Within the sensory areas, there is a topographical representation of the sensory periphery on the cortical surface. This point-to-point representation on the cortical field is due to the fact that neighbouring neurons respond to the activation of neighbouring peripheral receptors.
One of the first demonstrations of the impact of the peripheral input on the development of sensory cortical areas came from seminal studies in the early 70’s. When the infraorbital nerve, which conveys input from the whiskers to S1 was sectioned at birth, the representation of the injured whiskers in the barrel field was impaired or disappeared producing a concomitant increase in barrels representing the intact whiskers [75–77]. Importantly, manipulation in later stages did not lead to map defects [78] providing strong evidence that an intact periphery, before barrel pattern becomes visible, is a basic condition for a development of a normal map in S1. However, these studies were not dissecting out whether the relevant influencing factor for the development of cortical maps was the periphery itself or the neural activity that flows through the system. Moreover, the changes in cortical maps could be indirect and due to alterations in subcortical territories such as the thalamus.
The development of conditional loss-of-function mouse models in which a specific gene is deleted in cortical or thalamic neurons helped to dissect out the role of the thalamocortical input into the process of cortical map formation. Genetic manipulations of thalamic structures, such as the decrease in size of the dLGN or the ablation of the VPM nuclei, confirmed the importance of the thalamic input as an extrinsic factor essential for the differentiation and establishment of primary versus secondary cortical territories in both, visual and somatosensory systems [79–81]. In the absence of primary (first-order) TCAs, cortical areas will remain in the default differentiation stage, which is that of secondary areas (Figure 1). Thus, TCAs from the first-order nuclei provide specific information to cortical area patterning. This information could involve activity-dependent mechanisms. Indeed, perturbations in the neurotransmitter release of TCAs lead to the reduction or complete abolishment of the barrel-field in the somatosensory system [72–74, 82].
The above-mentioned results show that thalamic input is critical for cortical sensory areas development. However, the time at which TCAs are necessary is less understood. In fact, the thalamic influence over the formation of cortical sensory representations occurs much earlier than thought. By perturbing the embryonic topography of the thalamocortical axons specifically at the basal ganglia, Lokmane and colleagues showed that the ordering of TCAs is required for the transfer of the whisker representation to the barrel field [83]. Somatosensory TCAs target abnormally V1 at embryonic stages and, although they recover and rewire into S1 postnatally, fail to form an anatomical and functional map in the barrel field [83, 84]. Thus, these results strongly suggest that the correct postnatal development of cortical maps may depend on early prenatal events involving a “memory” on the first thalamocortical interactions. The discovery of the intrinsic capability of the thalamus to generate thalamocortical spontaneous waves of activity embryonically might support this view. Thalamic neurons from different sensory modalities engage in a prenatal communication by means of spontaneous calcium waves that propagate to the cortex [85]. Embryonic perturbation of these waves leads to changes in cortical areas size by the specific activity-dependent regulation of genes in thalamocortical neurons (Figure 1). The enlargement of S1 that it is triggered by the perturbation of the thalamic waves or the elimination of the eyes occurs independently of sensory experience [85] supporting again an early role of the thalamocortical communication in cortical areas development.
Although it is believed that the spontaneous activity from the periphery drives the correlated patterns seen in central stations [86–88], the embryonic emergence of the thalamic calcium waves is independent of the peripheral input as thalamic waves persist in an embryonic enucleated mouse model in which retinal input never reached the thalamus [85]. Nevertheless, it is possible that later in life when most of the in vivo recordings have been made, peripheral input imposes or coordinates the pattern of activity in the distinct developing sensory stations, including the thalamus.
Concluding remarks & Future perspective
In this review, I have focused on recapitulating the mechanisms involved in the guidance, targeting and plasticity of the thalamocortical connectivity, specially emphasizing on how the development of specific techniques has helped the field to understand these processes. The rapid progression of techniques such as the in utero electroporation for manipulation of gene function in vivo, the generation of specific Cre-lox mouse lines for controlling spatial and temporal targeting of genes and the incorporation of new generation sequencing for transcriptomic analysis of specific neurons have been crucial. Now, we know the basic rules involved in the establishment of the thalamocortical connectivity, so we are in a privileged position to engage further challenging questions. For example, direct reprogramming of cortical postmitotic neurons has been successfully achieved [89]. We could then test whether thalamocortical neurons could be reprogrammed from one sensory-modality subset into another. Furthermore, endogenous brain cells such the postnatal reactive astrocytes have been reprogrammed into functional neurons in vitro [90, 91] as well as in vivo [92], at least in models of brain injury. Unpublished data from our laboratory suggest that thalamic astrocytes can be reprogrammed into functional thalamic neurons in vitro (A. Herrero and G. López-Bendito, unpublished). If this is also the case in vivo, reprogrammed astrocytes could be used in the future as a strategy to restore sensory circuits in sensory-deprived animal models later in life. Finally, 3D brain organoids offer nowadays an exceptional opportunity to study aspects of human brain development and disease [93]. Sensory processing disorders have been widely associated to neurodevelopmental disorders such as Autism or Schizophrenia [94–96], but the mechanisms involved in their generation remain poorly understood. In this sense, future studies could implicate the generation of brain organoids from patients with Autism Spectrum Disorder to better understand the possible developmental errors in the formation of sensory circuits that may underlie the sensory processing disorders associated.
Acknowledgements
The writing of this review has been motivated by the recent receipt of the IBRO Kemali International Prize for Research in the Field of Basic and Clinical Neuroscience. We thank the members of the López-Bendito laboratory for discussions and useful comments on the manuscript. This work was supported by the Spanish MINECO BFU2015-64432-R, the Spanish State Research Agency through the “Severo Ochoa” Programme for Centres of Excellence in R&D (SEV-2013-0317), the PROMETEO/2017/149, and the European Commission Grant ERC-2014-CoG-647012. G. L-B is an EMBO YIP Investigator and a FENS-Kavli scholar. We apologize to the authors that could not be cited due to space limitations.
References
- [1].Ghosh A, Shatz CJ. Pathfinding and target selection by developing geniculocortical axons. J Neurosci. 1992;12:39–55. doi: 10.1523/JNEUROSCI.12-01-00039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Catalano SM, Robertson RT, Killackey HP. Early ingrowth of thalamocortical afferents to the neocortex of the prenatal rat. Proc Natl Acad Sci U S A. 1991;88:2999–3003. doi: 10.1073/pnas.88.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].McConnell SK, Ghosh A, Shatz CJ. Subplate neurons pioneer the first axon pathway from the cerebral cortex. Science. 1989;245:978–982. doi: 10.1126/science.2475909. [DOI] [PubMed] [Google Scholar]
- [4].De Carlos JA, O’Leary DD. Growth and targeting of subplate axons and establishment of major cortical pathways. J Neurosci. 1992;12:1194–1211. doi: 10.1523/JNEUROSCI.12-04-01194.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Blakemore C, Molnar Z. Factors involved in the establishment of specific interconnections between thalamus and cerebral cortex. Cold Spring Harb Symp Quant Biol. 1990;55:491–504. doi: 10.1101/sqb.1990.055.01.048. [DOI] [PubMed] [Google Scholar]
- [6].Molnár Z. Mechanisms Underlying the Early Establishment of Thalamocortical Connections in the Rat. 1998:1–23. doi: 10.1523/JNEUROSCI.18-15-05723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Molnar Z, Cordery P. Connections between cells of the internal capsule, thalamus, and cerebral cortex in embryonic rat. J Comp Neurol. 1999;413:1–25. doi: 10.1002/(sici)1096-9861(19991011)413:1<1::aid-cne1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- [8].Agmon A, Yang LT, Jones EG, O’Dowd DK. Topological precision in the thalamic projection to neonatal mouse barrel cortex. J Neurosci. 1995;15:549–561. doi: 10.1523/JNEUROSCI.15-01-00549.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lopez-Bendito G, Molnár Z. Thalamocortical development: how are we going to get there? Nature Reviews Neuroscience. 2003;4:276–289. doi: 10.1038/nrn1075. [DOI] [PubMed] [Google Scholar]
- [10].Leyva-Díaz E, Lopez Bendito G. In and out from the cortex: Development of major forebrain connections. Neuroscience. 2013;254:26–44. doi: 10.1016/j.neuroscience.2013.08.070. [DOI] [PubMed] [Google Scholar]
- [11].Garel S, Lopez-Bendito G. Inputs from the thalamocortical system on axon pathfinding mechanisms. Curr Opin Neurobiol. 2014;27:143–150. doi: 10.1016/j.conb.2014.03.013. [DOI] [PubMed] [Google Scholar]
- [12].Molnar Z, Garel S, Lopez-Bendito G, Maness P, Price DJ. Mechanisms controlling the guidance of thalamocortical axons through the embryonic forebrain. Eur J Neurosci. 2012;35:1573–1585. doi: 10.1111/j.1460-9568.2012.08119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bishop KM, Goudreau G, O’Leary DD. Regulation of area identity in the mammalian neocortex by Emx2 and Pax6. Science. 2000;288:344–349. doi: 10.1126/science.288.5464.344. [DOI] [PubMed] [Google Scholar]
- [14].Mallamaci A, Muzio L, Chan CH, Parnavelas J, Boncinelli E. Area identity shifts in the early cerebral cortex of Emx2-/- mutant mice. Nat Neurosci. 2000;3:679–686. doi: 10.1038/76630. [DOI] [PubMed] [Google Scholar]
- [15].López-Bendito G, Chan C-H, Mallamaci A, Parnavelas J, Molnár Z. Role of Emx2 in the development of the reciprocal connectivity between cortex and thalamus. J Comp Neurol. 2002;451:153–169. doi: 10.1002/cne.10345. [DOI] [PubMed] [Google Scholar]
- [16].Braisted J, Tuttle R, O’leary D. Thalamocortical axons are influenced by chemorepellent and chemoattractant activities localized to decision points along their path. Dev Biol. 1999;208:430–440. doi: 10.1006/dbio.1999.9216. [DOI] [PubMed] [Google Scholar]
- [17].Bagri A, Marín O, Plump A, Mak J, Pleasure S, Rubenstein J, Tessier-Lavigne M. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron. 2002;33:233–248. doi: 10.1016/s0896-6273(02)00561-5. [DOI] [PubMed] [Google Scholar]
- [18].López-Bendito G, Flames N, Ma L, Fouquet C, Di Meglio T, Chedotal A, Tessier-Lavigne M, Marín O. Robo1 and Robo2 cooperate to control the guidance of major axonal tracts in the mammalian forebrain. J Neurosci. 2007;27:3395–3407. doi: 10.1523/JNEUROSCI.4605-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lopez-Bendito G, Cautinat A, Sanchez JA, Bielle F, Flames N, Garratt AN, Talmage DA, Role LW, Charnay P, Marin O, Garel S. Tangential neuronal migration controls axon guidance: a role for neuregulin-1 in thalamocortical axon navigation. Cell. 2006;125:127–142. doi: 10.1016/j.cell.2006.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bielle F, Marcos-Mondejar P, Keita M, Mailhes C, Verney C, Nguyen Ba-Charvet K, Tessier-Lavigne M, Lopez-Bendito G, Garel S. Slit2 Activity in the Migration of Guidepost Neurons Shapes Thalamic Projections during Development and Evolution. Neuron. 2011;69:1085–1098. doi: 10.1016/j.neuron.2011.02.026. [DOI] [PubMed] [Google Scholar]
- [21].Metin C, Godement P. The ganglionic eminence may be an intermediate target for corticofugal and thalamocortical axons. J Neurosci. 1996;16:3219–3235. doi: 10.1523/JNEUROSCI.16-10-03219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Molnar Z, Adams R, Blakemore C. Mechanisms underlying the early establishment of thalamocortical connections in the rat. J Neurosci. 1998;18:5723–5745. doi: 10.1523/JNEUROSCI.18-15-05723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mitrofanis J, Guillery RW. New views of the thalamic reticular nucleus in the adult and the developing brain. Trends Neurosci. 1993;16:240–245. doi: 10.1016/0166-2236(93)90163-g. [DOI] [PubMed] [Google Scholar]
- [24].Uemura M, Nakao S, Suzuki ST, Takeichi M, Hirano S. OL-Protocadherin is essential for growth of striatal axons and thalamocortical projections. Nat Neurosci. 2007;10:1151–1159. doi: 10.1038/nn1960. [DOI] [PubMed] [Google Scholar]
- [25].Morello F, Prasad AA, Rehberg K, Vieira de Sa R, Anton-Bolanos N, Leyva-Diaz E, Adolfs Y, Tissir F, Lopez-Bendito G, Pasterkamp RJ. Frizzled3 Controls Axonal Polarity and Intermediate Target Entry during Striatal Pathway Development. J Neurosci. 2015;35:14205–14219. doi: 10.1523/JNEUROSCI.1840-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Molnar Z, Blakemore C. Guidance of thalamocortical innervation. Ciba Found Symp. 1995;193:127–149. doi: 10.1002/9780470514795.ch7. discussion 192-129. [DOI] [PubMed] [Google Scholar]
- [27].Molnar Z, Adams R, Goffinet AM, Blakemore C. The role of the first postmitotic cortical cells in the development of thalamocortical innervation in the reeler mouse. J Neurosci. 1998;18:5746–5765. doi: 10.1523/JNEUROSCI.18-15-05746.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bicknese AR, Sheppard AM, O’Leary DD, Pearlman AL. Thalamocortical axons extend along a chondroitin sulfate proteoglycan-enriched pathway coincident with the neocortical subplate and distinct from the efferent path. J Neurosci. 1994;14:3500–3510. doi: 10.1523/JNEUROSCI.14-06-03500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Miller B, Chou L, Finlay BL. The early development of thalamocortical and corticothalamic projections. J Comp Neurol. 1993;335:16–41. doi: 10.1002/cne.903350103. [DOI] [PubMed] [Google Scholar]
- [30].Bagnard D, Chounlamountri N, Püschel A, Bolz J. Axonal surface molecules act in combination with semaphorin 3a during the establishment of corticothalamic projections. Cereb Cortex. 2001;11:278–285. doi: 10.1093/cercor/11.3.278. [DOI] [PubMed] [Google Scholar]
- [31].Hevner R, Miyashita-Lin E, Rubenstein J. Cortical and thalamic axon pathfinding defects in Tbr1, Gbx2, and Pax6 mutant mice: evidence that cortical and thalamic axons interact and guide each other. J Comp Neurol. 2002;447:8–17. doi: 10.1002/cne.10219. [DOI] [PubMed] [Google Scholar]
- [32].Jones L, López-Bendito G, Gruss P, Stoykova A, Molnár Z. Pax6 is required for the normal development of the forebrain axonal connections. Development. 2002;129:5041–5052. doi: 10.1242/dev.129.21.5041. [DOI] [PubMed] [Google Scholar]
- [33].Stoykova A, Fritsch R, Walther C, Gruss P. Forebrain patterning defects in Small eye mutant mice. Development. 1996;122:3453–3465. doi: 10.1242/dev.122.11.3453. [DOI] [PubMed] [Google Scholar]
- [34].Tuttle R, Nakagawa Y, Johnson JE, O’leary DD. Defects in thalamocortical axon pathfinding correlate with altered cell domains in Mash-1-deficient mice. Development. 1999;126:1903–1916. doi: 10.1242/dev.126.9.1903. [DOI] [PubMed] [Google Scholar]
- [35].Chen Y, Magnani D, Theil T, Pratt T, Price DJ. Evidence that descending cortical axons are essential for thalamocortical axons to cross the pallial-subpallial boundary in the embryonic forebrain. PLoS One. 2012;7:e33105. doi: 10.1371/journal.pone.0033105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Deck M, Lokmane L, Chauvet S, Mailhes C, Keita M, Niquille M, Yoshida M, Yoshida Y, Lebrand C, Mann F, Grove EA, et al. Pathfinding of Corticothalamic Axons Relies on a Rendezvous with Thalamic Projections. Neuron. 2013;77:472–484. doi: 10.1016/j.neuron.2012.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Garel S, Yun K, Grosschedl R, Rubenstein JLR. The early topography of thalamocortical projections is shifted in Ebf1 and Dlx1/2 mutant mice. Development. 2002;129:5621–5634. doi: 10.1242/dev.00166. [DOI] [PubMed] [Google Scholar]
- [38].Dufour A, Seibt J, Passante L, Depaepe V, Ciossek T, Frisen J, Kullander K, Flanagan J, Polleux F, Vanderhaeghen P. Area specificity and topography of thalamocortical projections are controlled by ephrin/Eph genes. Neuron. 2003;39:453–465. doi: 10.1016/s0896-6273(03)00440-9. [DOI] [PubMed] [Google Scholar]
- [39].Bonnin A, Torii M, Wang L, Rakic P, Levitt P. Serotonin modulates the response of embryonic thalamocortical axons to netrin-1. Nat Neurosci. 2007;10:588–597. doi: 10.1038/nn1896. [DOI] [PubMed] [Google Scholar]
- [40].Powell AW, Sassa T, Wu Y, Tessier-Lavigne M, Polleux F. Topography of thalamic projections requires attractive and repulsive functions of Netrin-1 in the ventral telencephalon. PLoS Biol. 2008;6:e116. doi: 10.1371/journal.pbio.0060116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bielle F, Marcos-Mondejar P, Leyva-Diaz E, Lokmane L, Mire E, Mailhes C, Keita M, Garcia N, Tessier-Lavigne M, Garel S, Lopez-Bendito G. Emergent growth cone responses to combinations of Slit1 and Netrin 1 in thalamocortical axon topography. Curr Biol. 2011;21:1748–1755. doi: 10.1016/j.cub.2011.09.008. [DOI] [PubMed] [Google Scholar]
- [42].Leyva-Diaz E, Del Toro D, Menal MJ, Cambray S, Susin R, Tessier-Lavigne M, Klein R, Egea J, Lopez-Bendito G. FLRT3 Is a Robo1-Interacting Protein that Determines Netrin-1 Attraction in Developing Axons. Curr Biol. 2014;24:494–508. doi: 10.1016/j.cub.2014.01.042. [DOI] [PubMed] [Google Scholar]
- [43].Marcos-Mondejar P, Peregrin S, Li JY, Carlsson L, Tole S, Lopez-Bendito G. The Lhx2 Transcription Factor Controls Thalamocortical Axonal Guidance by Specific Regulation of Robo1 and Robo2 Receptors. Journal of Neuroscience. 2012;32:4372–4385. doi: 10.1523/JNEUROSCI.5851-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gezelius H, Moreno-Juan V, Mezzera C, Thakurela S, Rodriguez-Malmierca LM, Pistolic J, Benes V, Tiwari VK, Lopez-Bendito G. Genetic Labeling of Nuclei-Specific Thalamocortical Neurons Reveals Putative Sensory-Modality Specific Genes. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Frangeul L, Pouchelon G, Telley L, Lefort S, Luscher C, Jabaudon D. A cross-modal genetic framework for the development and plasticity of sensory pathways. Nature. 2016;538:96–98. doi: 10.1038/nature19770. [DOI] [PubMed] [Google Scholar]
- [46].Grove EA, Fukuchi-Shimogori T. Generating the cerebral cortical area map. Annu Rev Neurosci. 2003;26:355–380. doi: 10.1146/annurev.neuro.26.041002.131137. [DOI] [PubMed] [Google Scholar]
- [47].Saito T, Nakatsuji N. Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Dev Biol. 2001;240:237–246. doi: 10.1006/dbio.2001.0439. [DOI] [PubMed] [Google Scholar]
- [48].Shimogori T, Ogawa M. Gene application with in utero electroporation in mouse embryonic brain. Dev Growth Differ. 2008;50:499–506. doi: 10.1111/j.1440-169X.2008.01045.x. [DOI] [PubMed] [Google Scholar]
- [49].Tabata H, Nakajima K. Efficient in utero gene transfer system to the developing mouse brain using electroporation: visualization of neuronal migration in the developing cortex. Neuroscience. 2001;103:865–872. doi: 10.1016/s0306-4522(01)00016-1. [DOI] [PubMed] [Google Scholar]
- [50].Shimogori T, Grove EA. Fibroblast growth factor 8 regulates neocortical guidance of area-specific thalamic innervation. J Neurosci. 2005;25:6550–6560. doi: 10.1523/JNEUROSCI.0453-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ghosh A, Antonini A, McConnell SK, Shatz CJ. Requirement for subplate neurons in the formation of thalamocortical connections. Nature. 1990;347:179–181. doi: 10.1038/347179a0. [DOI] [PubMed] [Google Scholar]
- [52].Ghosh A, Shatz CJ. A role for subplate neurons in the patterning of connections from thalamus to neocortex. Development. 1993;117:1031–1047. doi: 10.1242/dev.117.3.1031. [DOI] [PubMed] [Google Scholar]
- [53].Allendoerfer KL, Shatz CJ. The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annu Rev Neurosci. 1994;17:185–218. doi: 10.1146/annurev.ne.17.030194.001153. [DOI] [PubMed] [Google Scholar]
- [54].O’Leary DD, Schlaggar BL, Tuttle R. Specification of neocortical areas and thalamocortical connections. Annu Rev Neurosci. 1994;17:419–439. doi: 10.1146/annurev.ne.17.030194.002223. [DOI] [PubMed] [Google Scholar]
- [55].Friauf E, McConnell SK, Shatz CJ. Functional synaptic circuits in the subplate during fetal and early postnatal development of cat visual cortex. J Neurosci. 1990;10:2601–2613. doi: 10.1523/JNEUROSCI.10-08-02601.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kanold PO, Luhmann HJ. The subplate and early cortical circuits. Annu Rev Neurosci. 2010;33:23–48. doi: 10.1146/annurev-neuro-060909-153244. [DOI] [PubMed] [Google Scholar]
- [57].Constantinople CM, Bruno RM. Deep Cortical Layers Are Activated Directly by Thalamus. Science. 2013;340:1591–1594. doi: 10.1126/science.1236425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Little GE, Lopez-Bendito G, Runker AE, Garcia N, Pinon MC, Chedotal A, Molnar Z, Mitchell KJ. Specificity and plasticity of thalamocortical connections in Sema6A mutant mice. PLoS Biol. 2009;7:e98. doi: 10.1371/journal.pbio.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Mizuno H, Hirano T, Tagawa Y. Evidence for activity-dependent cortical wiring: formation of interhemispheric connections in neonatal mouse visual cortex requires projection neuron activity. Journal of Neuroscience. 2007;27:6760–6770. doi: 10.1523/JNEUROSCI.1215-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Wang C-L, Zhang L, Zhou Y, Zhou J, Yang X-J, Duan S-m, Xiong Z-Q, Ding Y-Q. Activity-dependent development of callosal projections in the somatosensory cortex. J Neurosci. 2007;27:11334–11342. doi: 10.1523/JNEUROSCI.3380-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hanson MG, Landmesser LT. Normal patterns of spontaneous activity are required for correct motor axon guidance and the expression of specific guidance molecules. Neuron. 2004;43:687–701. doi: 10.1016/j.neuron.2004.08.018. [DOI] [PubMed] [Google Scholar]
- [62].Borodinsky L, Root C, Cronin J, Sann S, Gu X, Spitzer N. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature. 2004;429:523–530. doi: 10.1038/nature02518. [DOI] [PubMed] [Google Scholar]
- [63].Demarque M, Spitzer NC. Activity-dependent expression of Lmx1b regulates specification of serotonergic neurons modulating swimming behavior. Neuron. 2010;67:321–334. doi: 10.1016/j.neuron.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Yamamoto N, Bendito GL. Shaping brain connections through spontaneous neural activity. European Journal of. 2012 doi: 10.1111/j.1460-9568.2012.08101.x. [DOI] [PubMed] [Google Scholar]
- [65].Mire E, Mezzera C, az EL-Di, Paternain AV, Squarzoni P, Bluy L, Castillo-Paterna M, pez MiaJeLo, n SPi, Tessier-Lavigne M, Garel S, et al. Spontaneous activity regulates Robo1 transcription to mediate a switch in thalamocortical axon growth. Nature neuroscience. 2012:1–12. doi: 10.1038/nn.3160. [DOI] [PubMed] [Google Scholar]
- [66].Castillo-Paterna M, Moreno-Juan V, Filipchuk A, Rodriguez-Malmierca L, Susin R, Lopez Bendito G. DCC functions as an accelerator of thalamocortical axonal growth downstream of spontaneous thalamic activity. EMBO reports. 2015;16:851–862. doi: 10.15252/embr.201439882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Herrmann K, Shatz CJ. Blockade of action potential activity alters initial arborization of thalamic axons within cortical layer 4. Proc Natl Acad Sci U S A. 1995;92:11244–11248. doi: 10.1073/pnas.92.24.11244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Uesaka N, Hayano Y, Yamada A, Yamamoto N. Interplay between laminar specificity and activity-dependent mechanisms of thalamocortical axon branching. J Neurosci. 2007;27:5215–5223. doi: 10.1523/JNEUROSCI.4685-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Yamamoto N, Kurotani T, Toyama K. Neural connections between the lateral geniculate nucleus and visual cortex in vitro. Science. 1989;245:192–194. doi: 10.1126/science.2749258. [DOI] [PubMed] [Google Scholar]
- [70].Yamamoto N, Yamada K, Kurotani T, Toyama K. Laminar specificity of extrinsic cortical connections studied in coculture preparations. Neuron. 1992;9:217–228. doi: 10.1016/0896-6273(92)90161-6. [DOI] [PubMed] [Google Scholar]
- [71].Hayano Y, Sasaki K, Ohmura N, Takemoto M, Maeda Y, Yamashita T, Hata Y, Kitada K, Yamamoto N. Netrin-4 regulates thalamocortical axon branching in an activity-dependent fashion. Proc Natl Acad Sci U S A. 2014;111:15226–15231. doi: 10.1073/pnas.1402095111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Narboux-Neme N, Evrard A, Ferezou I, Erzurumlu RS, Kaeser PS, Laine J, Rossier J, Ropert N, Sudhof TC, Gaspar P. Neurotransmitter Release at the Thalamocortical Synapse Instructs Barrel Formation But Not Axon Patterning in the Somatosensory Cortex. Journal of Neuroscience. 2012;32:6183–6196. doi: 10.1523/JNEUROSCI.0343-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Suzuki A, Lee LJ, Hayashi Y, Muglia L, Itohara S, Erzurumlu RS, Iwasato T. Thalamic adenylyl cyclase 1 is required for barrel formation in the somatosensory cortex. Neuroscience. 2015;290:518–529. doi: 10.1016/j.neuroscience.2015.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Li H, Fertuzinhos S, Mohns E, Hnasko TS, Verhage M, Edwards R, Sestan N, Crair MC. Laminar and columnar development of barrel cortex relies on thalamocortical neurotransmission. Neuron. 2013;79:970–986. doi: 10.1016/j.neuron.2013.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Van der Loos H, Woolsey TA. Somatosensory cortex: structural alterations following early injury to sense organs. Science. 1973;179:395–398. doi: 10.1126/science.179.4071.395. [DOI] [PubMed] [Google Scholar]
- [76].Killackey HP, Belford G, Ryugo R, Ryugo DK. Anomalous organization of thalamocortical projections consequent to vibrissae removal in the newborn rat and mouse. Brain Res. 1976;104:309–315. doi: 10.1016/0006-8993(76)90623-5. [DOI] [PubMed] [Google Scholar]
- [77].Weller WL, Johnson JI. Barrels in cerebral cortex altered by receptor disruption in newborn, but not in five-day-old mice (Cricetidoe and Muridae) Brain Res. 1975;83:504–508. doi: 10.1016/0006-8993(75)90844-6. [DOI] [PubMed] [Google Scholar]
- [78].Woolsey TA, Wann JR. Areal changes in mouse cortical barrels following vibrissal damage at different postnatal ages. The Journal of Comparative Neurology. 1976;170:53–66. doi: 10.1002/cne.901700105. [DOI] [PubMed] [Google Scholar]
- [79].Vue TY, Lee M, Tan YE, Werkhoven Z, Wang L, Nakagawa Y. Thalamic Control of Neocortical Area Formation in Mice. Journal of Neuroscience. 2013;33:8442–8453. doi: 10.1523/JNEUROSCI.5786-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Chou SJ, Babot Z, Leingartner A, Studer M, Nakagawa Y, O’Leary DD. Geniculocortical input drives genetic distinctions between primary and higher-order visual areas. Science. 2013;340:1239–1242. doi: 10.1126/science.1232806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Pouchelon G, Gambino F, Bellone C, Telley L, Vitali I, Luscher C, Holtmaat A, Jabaudon D. Modality-specific thalamocortical inputs instruct the identity of postsynaptic L4 neurons. Nature. 2014;511:471–474. doi: 10.1038/nature13390. [DOI] [PubMed] [Google Scholar]
- [82].Martini FJ, Moreno-Juan V, Filipchuk A, Valdeolmillos M, Lopez-Bendito G. Impact of thalamocortical input on barrel cortex development. Neuroscience. 2018;368:246–255. doi: 10.1016/j.neuroscience.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Lokmane L, Proville R, Narboux-Neme N, Gyory I, Keita M, Mailhes C, Lena C, Gaspar P, Grosschedl R, Garel S. Sensory map transfer to the neocortex relies on pretarget ordering of thalamic axons. Curr Biol. 2013;23:810–816. doi: 10.1016/j.cub.2013.03.062. [DOI] [PubMed] [Google Scholar]
- [84].Lokmane L, Garel S. Map transfer from the thalamus to the neocortex: inputs from the barrel field. Semin Cell Dev Biol. 2014;35:147–155. doi: 10.1016/j.semcdb.2014.07.005. [DOI] [PubMed] [Google Scholar]
- [85].Moreno-Juan V, Filipchuk A, Anton-Bolanos N, Mezzera C, Gezelius H, Andres B, Rodriguez-Malmierca L, Susin R, Schaad O, Iwasato T, Schule R, et al. Prenatal thalamic waves regulate cortical area size prior to sensory processing. Nat Commun. 2017;8:14172. doi: 10.1038/ncomms14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ackman JB, Burbridge TJ, Crair MC. Retinal waves coordinate patterned activity throughout the developing visual system. Nature. 2012;490:219–225. doi: 10.1038/nature11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Siegel F, Heimel JA, Peters J, Lohmann C. Peripheral and Central Inputs Shape Network Dynamics in the Developing Visual Cortex In Vivo. Current biology : CB. 2012 doi: 10.1016/j.cub.2011.12.026. [DOI] [PubMed] [Google Scholar]
- [88].Colonnese MT, Khazipov R. “Slow activity transients” in infant rat visual cortex: a spreading synchronous oscillation patterned by retinal waves. J Neurosci. 2010;30:4325–4337. doi: 10.1523/JNEUROSCI.4995-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Rouaux C, Arlotta P. Direct lineage reprogramming of post-mitotic callosal neurons into corticofugal neurons in vivo. Nat Cell Biol. 2013 doi: 10.1038/ncb2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Heinrich C, Blum R, Gascon S, Masserdotti G, Tripathi P, Sanchez R, Tiedt S, Schroeder T, Gotz M, Berninger B. Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol. 2010;8:e1000373. doi: 10.1371/journal.pbio.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Heinrich C, Gascon S, Masserdotti G, Lepier A, Sanchez R, Simon-Ebert T, Schroeder T, Gotz M, Berninger B. Generation of subtype-specific neurons from postnatal astroglia of the mouse cerebral cortex. Nat Protoc. 2011;6:214–228. doi: 10.1038/nprot.2010.188. [DOI] [PubMed] [Google Scholar]
- [92].Guo Z, Zhang L, Wu Z, Chen Y, Wang F, Chen G. In Vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell. 2014;14:188–202. doi: 10.1016/j.stem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Arlotta P. Organoids required! A new path to understanding human brain development and disease. Nat Methods. 2018;15:27–29. doi: 10.1038/nmeth.4557. [DOI] [PubMed] [Google Scholar]
- [94].Linke AC, Jao Keehn RJ, Pueschel EB, Fishman I, Muller RA. Children with ASD show links between aberrant sound processing, social symptoms, and atypical auditory interhemispheric and thalamocortical functional connectivity. Dev Cogn Neurosci. 2018;29:117–126. doi: 10.1016/j.dcn.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Mikkelsen M, Wodka EL, Mostofsky SH, Puts NAJ. Autism spectrum disorder in the scope of tactile processing. Dev Cogn Neurosci. 2018;29:140–150. doi: 10.1016/j.dcn.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Anderson EJ, Tibber MS, Schwarzkopf DS, Shergill SS, Fernandez-Egea E, Rees G, Dakin SC. Visual Population Receptive Fields in People with Schizophrenia Have Reduced Inhibitory Surrounds. J Neurosci. 2017;37:1546–1556. doi: 10.1523/JNEUROSCI.3620-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]