Abstract
Localized aerosol delivery of gene therapies is a promising treatment of severe pulmonary diseases including lung cancer, cystic fibrosis, COPD and asthma. The administration of drugs by inhalation features multiple benefits including an enhanced patient acceptability and compliance. The application of a spray dried powder formulation has advantages over solutions due to their increased stability and shelf life. Furthermore, optimal sizes of the powder can be obtained by spray drying to allow a deep lung deposition. The present study optimized the parameters involved with spray drying polyplexes formed by polyethylenimine (PEI) and nucleic acids in inert excipients to generate a nano-embedded microparticle (NEM) powder with appropriate aerodynamic diameter. Furthermore, the effects of the excipient matrix used to generate the NEM powder on the biological activity of the nucleic acid and the ability to recover the embedded nanoparticles was investigated. The study showed that bioactivity and nucleic acid integrity was preserved after spray drying, and that polyplexes could be reconstituted from the dry powders made with trehalose but not mannitol as a stabilizer. Scanning electron microscopy (SEM) showed trehalose formulations that formed fused, lightly corrugated spherical particles in the range between 1 and 5 μm, while mannitol formulations had smooth surfaces and consisted of more defined particles. After redispersion of the microparticles in water, polyplex dispersions are obtained that are comparable to the initial formulations before spray drying. Cellular uptake and transfection studies conducted in lung adenocarcinoma cells show that redispersed trehalose particles performed similar to or better than polyplexes that were not spray dried. A method for quantifying polymer and nucleic acid loss following spray drying was developed in order to ensure that equal nucleic acid amounts were used in all in vitro experiments. The results confirm that spray dried NEM formulations containing nucleic acids can be prepared with characteristics known to be optimal for inhalation therapy.
Keywords: Gene therapy, Spray drying, Nanoparticle formulation, Pulmonary delivery, Polyethylenimine
1. Introduction
Pulmonary delivery remains one of the most logical routes for region-selective treatment of lung diseases [1]. Advantages such as a large surface area, low proteolytic activity, avoidance of the first pass effect and thin epithelium barriers all contribute to provide strong opportunities to enhance therapeutic efficacy through increasing drug bioavailability [2]. In addition, the use of dry powder inhalers to deliver a drug formulation presents its own set of advantages due to their ease of use and their relatively high patient compliance rate [3]. While numerous conventional treatments have been developed in order to target local lung diseases, one of the most promising strategies for treating severe lung diseases involves the use of gene therapy. Small interfering RNA (siRNA), e.g., can silence pathologic gene expression that underlays the disorder being treated. Lung diseases such as cystic fibrosis, pulmonary tuberculosis, viral infections, asthma, chronic obstructive pulmonary disease (COPD) and lung cancer have all been investigated as potential targets in which siRNA might prove to be a promising therapeutic [4–7]. Despite their potential, siRNA therapeutics face numerous challenges that limit their safe and efficient application in lung disease treatment [8]. To overcome these hurdles associated with the delivery of therapeutic siRNA, nanoparticles have long been the preferred approach for encapsulating and protectiong nucleic acids. In the literature, a large variety of materials taking advantage of nanoformulation methods is reported to successfully deliver siRNA and achieve gene knockdown [9].
Cationic polymers are a class of non-viral vectors that represent a large population of nanoparticles being developed as efficient delivery vehicles of siRNA [10]. Polyethylenimine (PEI) is one of the most widely studied gene delivery vectors due to its highly modifiable amine rich structure. The high incidence of positively charged amines renders PEI the ability to electrostatically interact with nucleic acids to form nanoparticles termed “polyplexes” and facilitate cellular internalization of siRNA [11]. In order to achieve local delivery of the gene therapy to the lungs, however, it is necessary to formulate the nanoscale polyplexes into a microparticle powder comprised of a suitable matrix with aerodynamic diameters within the range of 1–5 μm [12]. After their inhalation, the matrix of nano-embedded microparticles (NEMs) must then dissolve in the lung lining fluid and release the embedded polyplexes for cellular uptake.
Spray drying is a widely used processing technique employed by chemical, materials, cosmetic, food and pharmaceutical industries [13]. This rapid, continuous, reproducible and scalable technique is very appealing in both the industrial and laboratory setting as a method to produce dry powder formulations intended for inhalation. However, the use of the spray drying to process nucleic acid polyplexes into microparticle powder requires optimization of processing parameters such as the excipient selection and drying conditions in order to retain polyplex bioactivity, redispersability, as well as stability [14,15].
The goal of this study was to optimize various spray drying parameters used to process PEI/bulk DNA (bDNA) polyplexes into NEMs, which will serve as a method development study for the formulation and analytics of siRNA polyplexes. The two well-known matrix stabilizing saccharides, mannitol and trehalose, were used in the spray drying process and the redispersibility of the corresponding NEM formulations was investigated. Additionally, various physical characteristics of the microparticles formed with the different excipients were examined. As polyplexes are a dynamic system made by electrostatic assembly which can be affected by shear forces, spraying could have a tremendous effect on the nanoparticle composition and concludingly their biological activity. This needs to be evaluated in order to achieve precise dosing and prevent overdosing as well as underdosing [16,17]. Hence, an important goal of the work was to develop a method to quantify the amount of polymer and nucleic acid that remained following spray drying. This method was established in a well-studied system of PEI and bDNA in order to prove its applicability, and to transfer it afterwards towards more complex systems containing nucleic acids e.g. siRNA and other amine based polymers. Ultimately, the cellular internalization and transfection efficiency of the redispersed PEI/ DNA polyplexes were assessed in lung adenocarcinoma A549 cells to pave the way for spray drying more sophisticated polyplex or micelleplex [18] formulations containing other polymers and/or siRNA with well established analytics.
2. Materials and methods
2.1. Materials
Hyperbranched polyethylenimine (PEI, 25 kDa) was obtained from BASF (Ludwigshafen, Germany). RPMI-1640 medium with L-glutamine and sodium bicarbonate, Dulbecco’s phosphate buffered saline (PBS), heat-inactivated fetal bovine serum (FBS), D-(+)-glucose, sodium bicarbonate, picrylsulfonic acid (TNBS) solution 5%, sodium pyruvate, 2-mercaptoethanol, dimethyl sulfoxide (DMSO, ≥99.7%), ethylenediaminetetraacetic acid (EDTA, 99.4-–100.06%), trypan blue (0.4%, sterile filtered) and bulk DNA (bDNA) from salmon sperm (6000 kDa, Fisher Scientific) were purchased from Sigma-Aldrich (Munich, Germany). SYBR Gold dye was obtained from Life Technologies (Carlsbad, CA, U.S.A.).
2.2. Preparation of polyplexes
To form polyplexes, 25 k PEI was dissolved in water to yield a 1.0 mg/mL solution and was then filtered through a 0.22 μm filter for sterilization. This stock solution was then diluted to pre-calculated concentrations with sterile 5 or 10% solutions of mannitol or trehalose and an equal volume was added to a defined amount of bDNA to prepare polyplexes at various N/P ratios (the molar ratio of nitrogen in PEI to phosphate in bDNA). This solution was incubated for 20 min at room temperature to allow polyplex formation. The amount of 25 k PEI required for a specific N/P ratio was calculated as follows (Eq. (1)):
| (1) |
where mPEI is the mass of PEI in μg and mDNA is the mass of bDNA in μg in each sample
2.3. Spray drying
Spray drying was used to incorporate the polyplexes into nanoembedded microparticles (NEMs). First, polyplexes were formed at various N/P ratios as described above containing 20 μg of bDNA, and 2 mL of the solution was mixed with 8 mL of an aqueous solution of different matrix excipients before spray drying. The matrix excipients tested were 5 or 10% (w/v) solutions of mannitol or trehalose in RNase free DI-water. All powder formulations were prepared using a Büchi B-290 spray dryer (Büchi Labortechnik, Essen, Germany) equipped with a 2-fluid nozzle (Büchi Labortechnik). In brief, the spray dryer was preconditioned using distilled water, and all powder formulations were prepared at identical drying conditions using the following parameters: atomizing air flow = 473 L/h, aspiration = 70%, pump ratio = 5%, nozzle cleaner = 0, inlet temperature = 65 °C, outlet temperature = 38 ± 1 °C. Nitrogen was the atomizing gas, air was dehumidified with a DeltaTherm-dehumidifier (DeltaTherm, Munich, Germany) and dry microparticles loaded with polyplexes (white powder) were contained in the collection vessel at the end of the glass cyclone, from where they were collected using a spatula and stored in a desiccator at room temperature for further experiments.
For measurement of polyplex redispersibility, spray-dried samples were dissolved in 70 μL of water, and the resulting suspension of polyplexes in water/matrix solution was vortexed for 15 s before measuring z-average, PDI, and zeta (ζ) potential as described below.
2.4. Hydrodynamic diameter and zeta (ζ) potential measurements
Hydrodynamic diameter measurements of freshly prepared or redispersed polyplexes were performed by dynamic light scattering (DLS) using a Zetasizer Nano ZS (Malvern Instruments Inc., Malvern, U.K.). To perform these measurements using attenuator levels between 6 and 8, the amount of spray-dried sample was adjusted adequately. Polyplexes prepared for spray drying were measured in their specific medium e.g. mannitol 10% where refractive index and viscosity were adjusted accordingly. For redispersed polyplexes, NEM were dissolved in a fashion to achieve equal excipient concentration as prior to spray drying. A total volume of 70 μL of each sample was added into a disposal cuvette (Brand GMBH, Wertheim, Germany) and the 173° backscatter angle measurement was read in triplicates with each run consisting of 15 scans. Results are represented as average size (nm) ± standard deviation. The particle size distribution was reflected in the PDI, which ranges from 0 for a monodisperse to 1.0 for an entirely heterogeneous dispersion. The samples were then diluted to 570 μL with filtered Nanopure water and transferred to a folded capillary cell (Malvern Instruments Inc., Malvern, U.K.), and ζ-potential measurements were taken. ζ-potential measurements were read in triplicates by laser Doppler anemometry (LDA), with each run consisting of 30-50 scans. Results are shown in mV ± standard deviation.
2.5. Scanning electron microscopy
The surface morphology and geometric size of the NEMs was examined by scanning electron microscopy (SEM) using an JSM-6510LVLGS, 25 kV (JEOL, Peabody, MA, U.S.A). For imaging, the NEM powder was sprinkled on a stub covered with double-sided carbon tape and sputter-coated with gold (Ernest Fullan) under vacuum for 40 s. The sizes of the microparticles were estimated from the SEM images processed with the Fiji distribution of ImageJ [19]. The histogram of the measured diameters was fit to a Gaussian distribution, from which average and standard deviation were calculated and outliers were shown.
2.6. Residual water content
The residual water content of the NEM powder after spray drying was determined by coulometric measurement using an Aqua 40.00 Karl Fischer Titrator with corresponding software from Analytik Jena AG (Jena, Germany). The samples (approx. 15 mg) were loaded into 2R vials, placed into the heating chamber and measured at 150 °C until the measurement drift reached the start drift ≤ +2 μg/min or until a final measurement time of 10 min. The start drift was established after approximately 1 h of equilibration showing a rate of less than 10 μg/min. Hydranal Coulomat AG (Riedel-deHaen, Seelze, Germany) was used as reagent. Before each session, the titrator was calibrated with a 1% water standard. The moisture content was calculated as the % weight of water relative to the overall sample weight.
2.7. Cascade impactor analysis
An 8-stage Andersen-Cascade-Impactor (ACI) (Thermo Andersen, Smyrna, GA, USA) fitted with a USP induction port and pre-seperator operated with a flow rate of 28.3 L × min-1 at 25 °C and 75% relative humidity, was used to evaluate the aerosol properties of the NEM powder. Prior to each ACI test, 55 mg of the formulation was loaded into 4 hydroxypropylmethylcellulose capsules each. A HandiHaler (Boehringer Ingelheim Pharma GmbH & Co. KG, Ingelheim, Germany) was used to discharge each capsule twice according to the manufacturer’s manual with an interval of 5 s in between. To enable a total volume of 4L passing through the instrument, the application time was set to 8.5 s. After each test, the ACI was disassembled and the powder that had deposited on each stage was weighed independently. The amount of nucleic acid deposited on each stage was analyzed as described under 2.8., and the mass median aerodynamic diameter (MMAD) and geometric standard deviation (GSD) were calculated as described in the literature [20]. The fine particle fraction (FPF) was calculated as the fraction of all particles deposited with an aerodynamic diameter below 5 μm [20].
2.8. Polymer and nucleic acid quantification following spray drying
In order to quantify the PEI and bDNA content that was encapsulated into the microparticles following spray drying, heparin competition assays were performed in order to disassociate the PEI-bDNA polyplexes within the NEM powder. Subsequently, the bDNA that was released from the polyplexes was determined by a modified SYBR® gold assay [18] while the PEI concentration was determined by TNBS assay [21]. First, the batch of NEM powder was dissolved in 2 mL borax buffer 0.1 M (Sodium Borax Decahydrate, Sigma Aldrich, Germany) in a volumetric flask. Following procedure was executed in triplicates: According to the theoretical content of bDNA, a volume was chosen to achieve approximately 0.04 μg of bDNA per N/P ratio. This solution was diluted to a final volume of 150 μL per sample with HPW in a tube. Afterwards, 75 μL of a 58 k IU heparin solution was added to each tube to achieve complete disassociation of polyplexes after 2hr. This solution was further diluted to 450 μL and distributed into a white 96 well plate (Thermo Scientific™ BioLite microwell plate, Thermo Scientific GmbH, Schwerte, Germany) in triplicates of 100 μL each.
For quantification of bDNA, 30 μL of a 4 × SYBR® gold solution was added to each well and incubated for 5 min in the dark at room temperature. The fluorescence of each sample was quantified using a Synergy 2 multi-mode microplate reader (BioTek Instrument, Winooski, VT, U.S.A.) at excitation wavelength of 485/20 nm and emission wavelength of 520/20 nm and compared to a freshly prepared standard curve of free bDNA incubated with SYBR® gold dye.
For quantification of 25 k PEI, 100 μL of batch dissolved solution in 2 mL borax was incubated with 30 μL of 3 mM TNBS solution in triplicates and absorbance at 405 nm was determined after a 1 h incubation with a quartz cuvette in a UV-1600PC spectrophotometer (VWR International GmbH, Darmstadt, Germany). The measured absorbance was compared to a freshly prepared standard curve of free 25 k PEI incubated with TNBS reagent. To verify each quantification assay, internal standards were prepared freshly and analyzed in parallel. These standards consisted of bDNA and PEI of known amounts which were chosen according to the theoretical amount of bDNA or PEI being analyzed in the sample, respectively. These were 0.04 μg bDNA per well for nucleic acid quantification and 0.48 to 1.2 μg PEI for polymer quantification. Measurements with deviations of less than 10% of the internal standard were considered as precise and taken into account for further analysis.
2.9. Cell culture
Human adenocarcinoma alveolar based lung cancer cells (A549) were obtained from ATTC (LG Promochem, Wesel, Germany) and cultured in RPMI-1640 medium with L-glutamine and sodium bicarbonate and supplemented with 10% heat inactivated fetal bovine serum and 1% penicillin/streptomycin (Corning Incorporated, Corning, NY, U.S.A.). Cells were grown in 75 cm2 cell culture flasks (Thermo Fisher Scientific, Waltham, MA, U.S.A.) at 37 °C and 5% CO2 and passaged every 2-3 days when they reached confluency.
2.10. In vitro cellular uptake
In 24-well plates, (Corning Incorporated, Corning, NY, U.S.A) 50,000 A549 cells were seeded and incubated overnight at 37 °C and 5% CO2. For each experiment, were prepared freshly with YOYO-labeled bDNA or redispersed after spray drying. Negative controls consisted of blank/untreated cells. Unless otherwise stated, cells were transfected for 24 h in 37 °C and 5% CO2 with a volume of fresh or redispersed polyplexes that contained 0.5 μg of YOYO-labeled bDNA within a total volume of 500 μL of serum containing cell culture media. Cells were then washed with PBS, trypsinized and spun down at 400 rfc for 5 min. After centrifugation, the supernatant was decanted, and the cells were washed three times and resuspended in 250 μL PBS/2 mM EDTA. Samples were analyzed via flow cytometry (Applied Biosystems Attune® Acoustic Focusing Cytometer, Life Technologies), and the median fluorescence intensity (MFI) was measured using 488 nm excitation and a 530/30 nm bandpass emission filter set. Samples were run in triplicates, with each sample gated by morphology based on forward/sideward scattering for a minimum of 10,000 viable cells. Analysis and presentation of the data was performed by GraphPad Prism 5.0 software calculating mean values and standard deviation.
2.11. In vitro transfection efficiency
In 24-well plates, 50,000 A549 cells were seeded and incubated overnight at 37 °C and 5% CO2. For each experiment, freshly prepared or redispersed polyplexes loaded with GFP plasmid (The Plasmid Factory, Bielefeld, Germany) were tested. Negative controls consisted of blank/untreated cells. Unless otherwise stated, cells were transfected for 48 h in 37 °C and 5% CO2 with a volume of fresh or redispersed polyplexes that contained 0.75 μg of GFP plasmid within a total volume of 500 μL of serum containing cell culture media. Cells were then washed with PBS, trypsinized and spun down at 400 rcf for 5 min. After centrifugation, the supernatant was decanted, and the cells were washed three times and resuspended in 250 μL PBS/2 mM EDTA. Samples were analyzed via flow cytometry (Applied Biosystems Attune® Acoustic Focusing Cytometer, Life Technologies), and the median fluorescence intensity (MFI) was measured using 488 nm excitation and a 530/30 nm bandpass emission filter set. Samples were run in triplicates, with each sample gated by morphology based on forward/side-ward scattering for a minimum of 10,000 viable cells. Analysis and presentation of the data was performed by GraphPad Prism 5.0 software calculating mean values and standard deviation.
2.12. Statistics
All results are given as mean value ± standard deviation (SD). Oneway ANOVA and two-way ANOVA with Bonferroni post-test were performed in GraphPad Prism software (Graph Pad Software, La Jolla, CA).
3. Results and discussion
In this proof-of-concept study, we investigated the preparation of nano-embedded microparticles (NEMs) from bulk DNA-loaded polyplexes that have aerosol diameters suitable for deep lung deposition through the use of spray drying. We subsequently studied the impact of spray drying parameters on their suitability for subsequent in vitro or in vivo transfection studies. The prototype polyplexes used here comprised of the well-studied polymer polyethylenimine (PEI) and doublestranded template DNA isolated from salmon sperm (bDNA). The use of these popular DNA transfection components allowed for the optimization of excipient matrix and various spray drying parameters. The resulting DNA-loaded NEMs were then evaluated with respect to physiochemical and aerosol characteristics, and polyplex redispersibility, cellular uptake and transfection efficiency in a lung epithelial cell model.
3.1. Dynamic light scattering measurements of spray dried PEI polyplexes
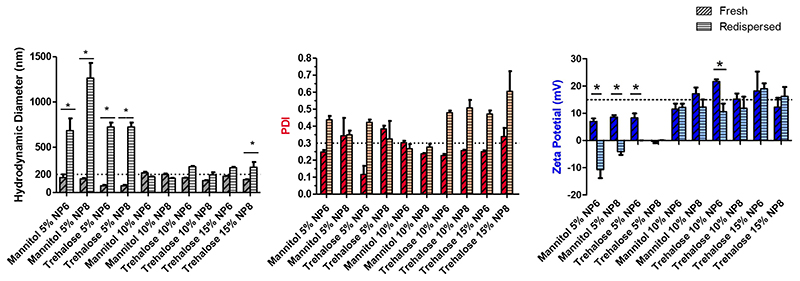
The bDNA-loaded polyplexes were processed into NEMs to provide a vehicle for the protection and efficient delivery of nucleic acids to the lungs. To this extent, it is crucial that the microparticle scaffold aides in stabilizing the polyplexes during their storage and facilitates reconstitution into individual nanoparticles upon contact with the aqueousbased environment lining the lung epithelia. Here, PEI polyplexes were encapsulated into a microparticle powder with various excipient matrices via spray drying to generate a stable and inhalable powder. Mannitol and trehalose were selected as the stabilizing excipients based on their well-known ability to stabilize macromolecules [22,23]. Previous studies conducted in the da Rocha lab reported optimal parameters for the generation of spray dried powder from PAMAM den-driplexes which we based our initial trials on [24]. These initial trials involved the use of aqueous 5% mannitol as the excipient matrix for the NEMs. Upon measuring redispersed PEI polyplexes via DLS, we found a 2-fold increase in particle diameter along with an increased overall negative surface charge at all N/P ratios that were tested (Fig. S1). With the apparent increase in particle aggregation and negative surface charge, we hypothesized that the spray drying parameters being used were causing an unacceptable amount of polymer loss leading to a change of N/P ratio. It needs to be taken into consideration that polyplex suspensions at N/P higher than 2.5 contain a considerable amount of free polymer [25]. However, if free polymer is lost, a new equilibrium between nucleic acid-bound and free polymer emerges [26]. Therefore, we modified the spray drying parameters and also investigated the use of the excipient trehalose at various concentrations on NEM redispersibility. Similar to our initial trials, the redispersed PEI polyplexes from NEM powder generated with 5% mannitol or 5% trehalose show a significant increase in particle diameter with reconstituted particles approximately > 700 nm (Fig. 1). Moreover, the 5% excipient formulations also show decreased surface charges upon redispersion. However, redispersed PEI polyplexes from the formulations with 10% mannitol and trehalose demonstrated no significant change in particle diameter and were all approximately 200 nm in size, and hence below our limit of 260 nm (dashed line Fig. 1). Literature suggests that smaller nanoparticles are likely phagocytosed by macrophages through their “secondary size” [27] which was defined as the size of their agglomerates on cell surfaces. Hence we hypothesize that the smaller the particles, the more likely the endocytosis by lung epithelia rather than macrophage phagocytosis. We therefore assume that particles below this limit allow macrophage escape to a greater extent than bigger particles and facilitate cell transfection [10,27]. Additionally, these formulations maintained their positive surface charge, indicating that the particles retained their polymer/nucleic acid composition along with their colloidal stability. The 10% excipient requirement to generate reconstitutable NEMs is higher than amounts used to redisperse PLGA nanospheres [28] or PAMAM dedriplexes [29] in other studies. One possible hypothesis for the higher amount of excipient needed for the reconstitution of polyamine nanoparticles lies in the large amount of water in the polyplexes that is lost during the spray drying process which in turn may necessitate larger amounts of matrix excipients acting in a wick-like manner during redispersion [30]. The redispersed mannitol NEM formulations had the more monodisperse particle distributions of all the formulations tested. Specifically, the redispersed 10% mannitol formulation was the most monodisperse with PDI values of approximately 0.272 ± 0.02, and hence below our limit of 0.3 (dashed line Fig. 1). The redispersed trehalose NEM formulations had more polydisperse particle distributions with PDI values all approximately 0.46 ± 0.10. The PDI values reported here are well corroborated in various other studies investigating spray dried polymeric nanoparticles with mannitol or trehalose [31]. Due to their favorable characteristics upon redispersion, NEM formulations that were generated with 10% mannitol or trehalose were selected for further characterization studies.
Fig. 1.
Hydrodynamic Diameter and zeta potential of redispersed NEM formulations. Dynamic light scattering characterization of fresh and redispersed PEI-bDNA polyplexes with various concentrations of mannitol or trehalose stabilizing excipient. Two-way ANOVA, repeated measurements, Bonferroni post-test, *P < 0.05.
3.2. Aerodynamic diameter measurements of spray dried PEI polyplexes
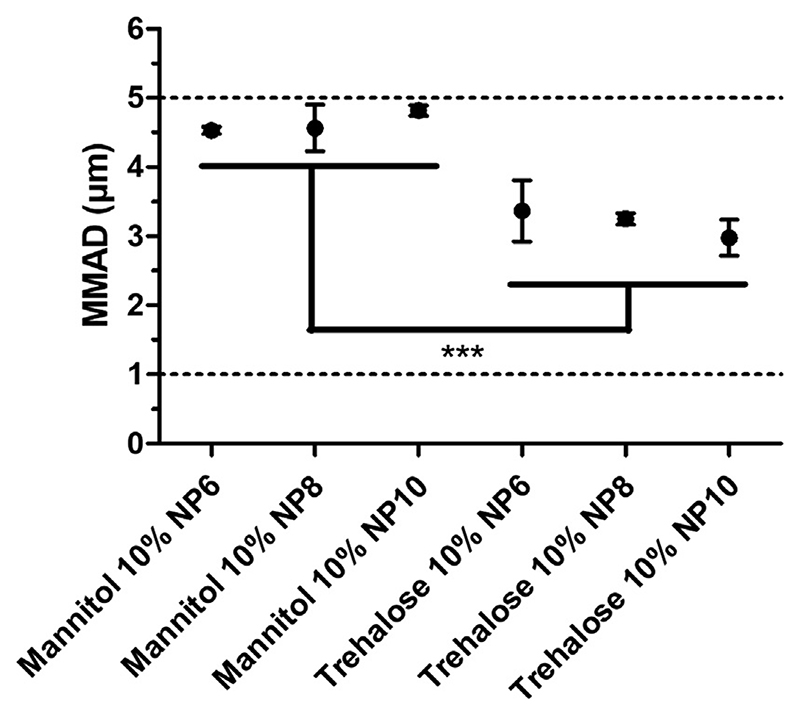
A crucial characteristic for the deposition of particles in the lung is their aerodynamic behavior. In order to evaluate the aerodynamic behavior of the NEM formulations obtained, an 8-stage Andersen-Cascade-Impactor (ACI) was utilized to determine the mass median aerodynamic diameter (MMAD), geometric standard deviation (GSD), and fine particle fraction (FPF). The NEM formulations analyzed were comprised of 10% mannitol or trehalose and contained PEI polyplexes at N/P 6, 8 or 10. All the NEM formulations tested showed an MMAD in the ideal range for bronchial/alveolar targeting (1-5 μm) as shown in Fig. 2 [32]. However, NEM formulations containing 10% trehalose as an excipient showed a significantly lower MMAD of approximately 3.17 ± 0.21 μm versus the 10% mannitol NEM formulations with approximately 4.67 ± 0.13 μm. Although not significant, GSD were slightly greater in trehalose formulations compared to mannitol formulations with 2.61 ± 0.26 μm vs. 2.01 ± 0.05 μm. Also not significantly different between both formulations is the FPF which was calculated as 72.6 ± 3.4% for trehalose formulations and as 67.5 ± 1.3% for mannitol formulations (Fig. S2). Aerosol performance strongly depends on MMAD, GSD and especially FPF representing the fraction reaching lower lung areas. By taking these findings into account we cannot state a significant difference between both formulations but accknolwedge a trend for better performance with trehalose formulations as MMAD were smaller and FPF greater. Additionally, there was no significant difference between the various N/P ratios within each excipient group tested, indicating that the amount of PEI that was used in the original formulation had little effect on the resulting aerodynamic behavior of the NEM particle. The larger microparticle diameters measured in the NEM formulations containing mannitol as an excipient may be explained by its crystalline nature upon drying. This is underlined by our XRD analysis where in the contrast to the amorphous halo of trehalose, mannitol showed distinct crystalline characteristics (Fig. S4). Peaks at 14.6° and 16.8° strongly suggest the β polymorph as shown by Nunes et al. [33] Additionally, published results of the β polymorph of mannitol show comparable patterns with Fig. S4, confirming the crystalline structure of spray dried mannitol NEM [34,35]. Amorphous sugars, such as trehalose (Fig. S3), are well known to be excellent at stabilizing biomacromolecules while crystalline sugars, such as mannitol, may lead to phase separation and destabilization [36]. This phenomenon might explain slightly larger microparticle sizes in the formulations containing mannitol as an excipient.
Fig. 2.
Aerodynamic diameter measurements. Aerodynamic diameter of NEM formulations made with 10% mannitol or trehalose stabilizing excipient. Dotted lines represent optimal range of microparticle size (1–5 μm) to achieve deep lung deposition. One-way ANOVA, Bonferroni post-test, ***P < 0.001.
3.3. Scanning electron microscopy
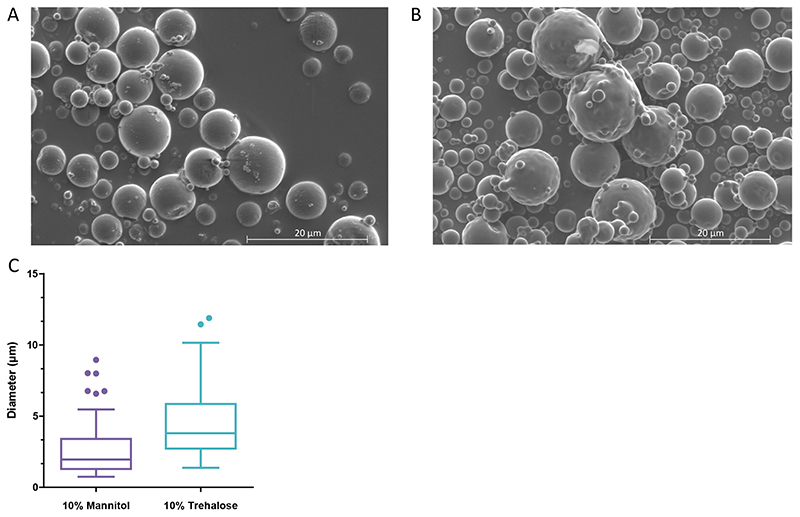
In order to further characterize the NEM formulations, scanning electron microscopy was employed to visualize the microparticles that were formed. Imaging of spray-dried 10% mannitol NEM samples showed that the microparticles were predominantly spherical, with a smooth surface and diameters of approximately 2.8 ± 2.1 μm (Fig. 3A). Conversely, 10% trehalose microparticles are predominantly fused spheres with a corrugated surface and diameters of approximately 4.60 ± 2.6 μm (Fig. 4B). The fused nature of the trehalose particles visualized here may be attributed to “moisture bridges” forming between molecules of the trehalose [37]. The difference in the apparent microparticle diameter for these formulations when comparing the SEM images and their MMAD stems from the surface characteristics of the particles measured. Smoother particle surface increases the particle’s overall density and ultimately its aerosol performance [38]. Therefore, the smoother surface of the mannitol formulations may account for the slight increase in aerodynamic diameter shown in the ACI studies for this formulation.
Fig. 3. Scanning electron microscope images. Representative SEM images of PEI-bDNA NEM formulations with (A) 10% mannitol excipient or (B) 10% trehalose excipient. (C) Diameter of microparticles measured via ImageJ imaging software. Bars depicting mean values and standard deviation, dots depicting outliers.
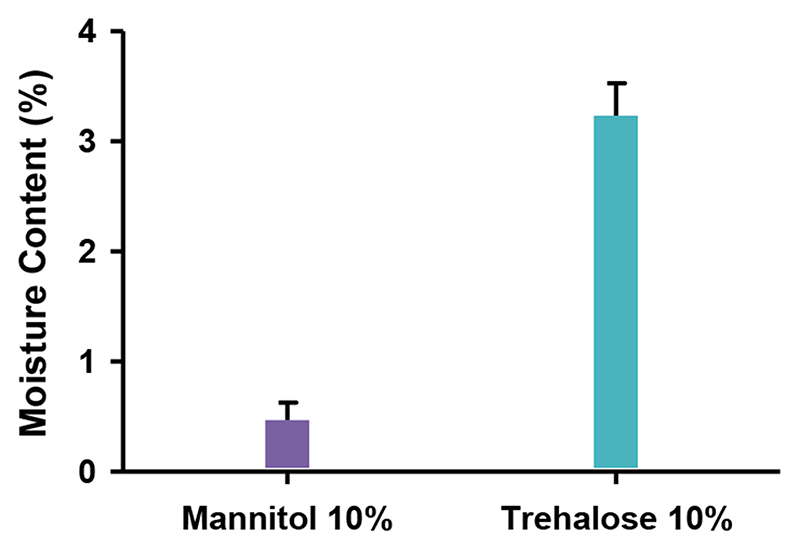
Fig. 4. Moisture content analysis. Moisture content of NEM formulations made with 10% mannitol or trehalose stabilizing excipient directly after the spray drying process.
3.4. Residual moisture content
It is well known that the residual moisture content following the generation of a inhalable powder plays one of the most crucial roles in determining its long-term stability, both physically and chemically [39]. Therefore, the residual moisture content of the NEM formulations was measured directly after the spray drying process. Immediately following the collection of the NEM powder, the 10% trehalose formulations showed a residual moisture content of 3.2% compared to 0.4% for the 10% mannitol formulations (Fig. 4). One cause for the slightly higher moisture content in the trehalose NEM powder may be due to the hygroscopic nature of the material [40]. Empirically, we experienced an extremely low solubility of the 10% mannitol NEM powder, which made it difficult to continue characterization studies of these formulations. The lower residual moisture content of the 10% mannitol formulations reflects their low solubility and may be explained by the hydrophobic nature of crystalline mannitol [41]. Conversely, the higher moisture content and amorphous state of trehalose allows for the sufficient rehydration and buffering of the polyplexes in the NEM formulation.
3.5. Quantification of polymer and nucleic acid content
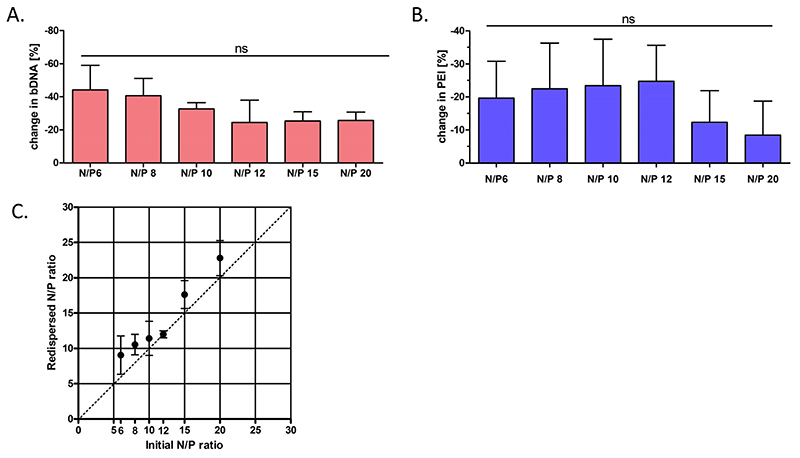
One of the main disadvantages of spray drying at the laboratory scale is that the overall yield can vary from 20 – 70% due to loss of product in the walls of the drying chamber [42]. Prior to any in vitro investigation of the NEM formulations’ efficiency upon reconstitution, it was crucial to quantify the amount of polymer and nucleic acid that may have been lost during the spray drying process. Therefore, the 10% trehalose NEM powder was redispersed and the reconstituted polyplexes underwent a competition assay with the polyanion heparin in order to dissociate PEI from the bulk DNA, similar to our previous studies [15]. With the polyplex disassociated, we then utilized SYBR® gold fluorescent dye to assess the relative amount of free, uncondensed DNA (Fig. 5A). As expected, there was about a 20 – 40% loss of bulk DNA in all of the formulations tested. While not statistically significant, there was an increase in the overall retained amount of DNA as the N/P ratio increased, ultimately leveling off at an N/P ratio of 12. To quantify the amount of PEI that was lost following the spray drying process, a modified TNBS assay was conducted following the reconstitution of the 10% trehalose NEM formulation (Fig. 5B). Here again, approximately 10 – 35% loss of PEI polymer was observed following the spray drying process. Interestingly, there was no observable trend in the overall amount of PEI lost following spray drying; however, there is a sharp increase in the amount of PEI retained in the NEM formulation at N/P ratio of 15 and higher, albeit not statistically significant. Following the quantification of the remaining PEI and bulk DNA in the spray dried microparticles, we were able to calculate the “redispersed” N/P ratio of the polyplexes within the 10% trehalose NEM formulation (Fig. 5C). When these are compared to the “initial” N/P ratio, it becomes clear that the spray drying process effectively increases the N/P ratio of the polyplexes contained within each 10% trehalose formulation by approximately 20% through the combined loss of both the PEI and DNA in the final NEM formulation. To the best of our knowledge, we are the first to investigate the impact of spray drying on the composition of electrostatically assembled polyplexes, which has an important impact on their subsequent performance in vitro and in vivo as well as on toxicity, especially in vivo [43]. In comparison to recent literature, this could lead to new insights in the performance of spray dried polyplexes. Schulze et. al. showed increased transfection efficiency of PEI polyplexes containing an EGFP reporter plasmid after spray drying compared to their freshly prepared counterpart [17]. While the glucose-PVA ratio in spray dried polyplexes and the amount of PVA in freshly prepared polyplexes remains unclear, the group did not evaluate if the increase in EGFP expression is caused by a changed composition of polymer and/or nucleic acid content. Concentration of the nucleic acid content through intensive excipient loss for example might explain this phenomenon. Also decreased cytotoxicity of redispersed polyplexes in a dose escalation assay could be explained by a loss of polymer which, due to its cationic charge, is the root cause for cytotoxicity [17,44]. In fact, a possible loss of polymer is supported by our findings. Hence, by applying our new developed method to this and other studies, we gain a better understanding of preparation and process effects on polyplex performance after spray drying.
Fig. 5.
Polymer and nucleic acid quantification. Quantification of (A) bulk DNA and (B) PEI in the 10% trehalose NEM formulations following spray drying (C) Comparing the redispersed N/P ratio with the initial N/P ratio of the PEI polyplexes loaded with bulk DNA. One Way ANOVA, Bonferroni post-test, P < 0.05, ns=non-significant, n = 4
3.6. In vitro cellular uptake of redispersed polyplexes
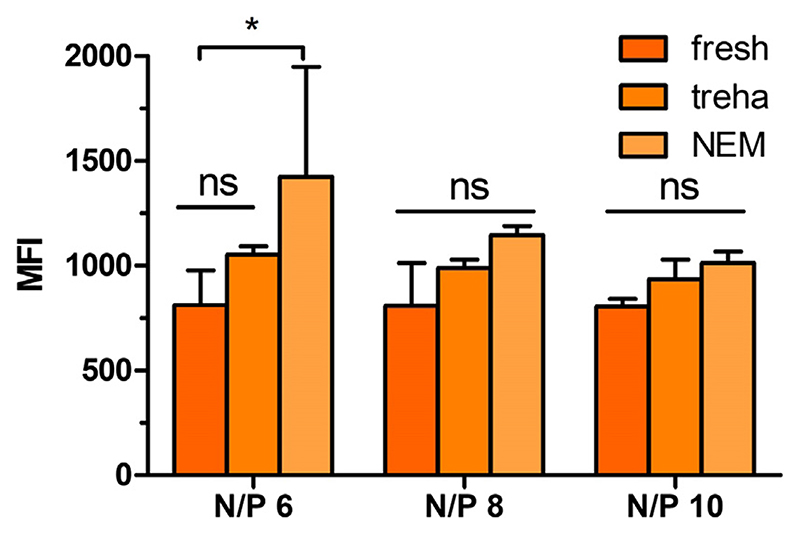
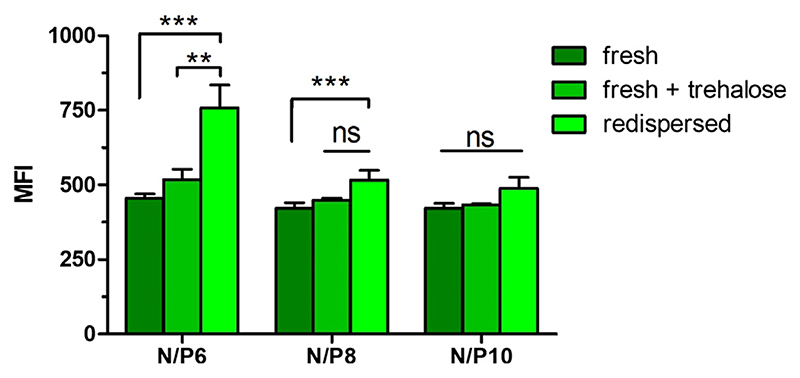
After quantifying the amount of bulk DNA in the 10% trehalose NEM formulation and ensuring that the polyplexes were able to be redispersed, the ability of the redispersed PEI/bDNA polyplexes to be delivered to cells was investigated. Therefore, YOYO-1 labeled DNA was used to form polyplexes with PEI at various N/P ratios and spray dried into NEM powder. An amount of each NEM powder correlating to 0.5 μg of YOYO-1 labeled DNA was then weighed out and lung adenocarcinoma A549 cells were treated for 24 h. Additionally, fresh polyplexes containing 0.5 μg of YOYO-1 DNA were transfected simultaneously. Following transfection, cells were submitted to flow cytometry analysis and the level of per-cell fluorescence was quantified. Cells treated with vehicle only (5% glucose) were included as negative controls. As seen in Fig. 6, a trend can be observed that freshly prepared polyplexes with trehalose showed greater uptake efficiencies in comparison to freshly prepared polyplexes. However, this trend is statistically not significant, indicating a rather small effect of trehalose on uptake efficiencies. Also, redispersed polyplexes from the 10% trehalose NEM formulations show higher uptake efficiencies than their freshly prepared counterparts with trehalose. Again, this observation is statistically not significant but underlines and confirms our findings described above discussing the increase of N/P ratios (Fig. 5) and the hypothesized changed in vitro performance through spray drying. However, NEM with N/P ratio of 6 show a significant increase of MFI values compared to their freshly prepared counterparts which can be explained by a comparably strong increase from N/P ratio 6 to 8 after spray drying. However, this increased cell uptake could also be partly a result of the slightly agglomerated particles as reflected in the increased PDIs (Fig. 1).
Fig. 6.
Cellular uptake of redispersed NEM formulations in A549 cells. Median fluorescence intensity (MFI) was determined by flow cytometry to evaluate the cellular uptake in a human non-small cell lung carcinoma cell line (A549) of fresh or redispersed PEI polyplexes from 10% trehalose NEM formulations at N/ P ratios of 6, 8 and 10 with 0.5 μg of YOYO-1 labeled bulk DNA in comparison to freshly prepared formulations in presence of trehalose. Blank samples consisted of A549 cells treated with 5% glucose only. Data points indicate mean ± SD (n = 3). Two-way ANOVA, Bonferroni post-test, *P < 0.05, ns = non-significant.
3.7. In vitro transfection efficiency redispersed polyplexes
To further investigate the bioactivity of redispersed polyplexes and their transfection efficiency following spray drying, we utilized a GFP expressing plasmid. The GFP plasmid was complexed with PEI at various N/P ratios and formed polyplexes similar to those loaded with bulk DNA (Fig. S5). Following spray drying using the same parameters outlined above, 10% trehalose NEM formulations were generated. Lung adenocarcinoma A549 cells were transfected with redispersed, fresh PEI/pEGFP polyplexes, or freshly prepared formulations in presence of trehalose for 48 h, and the level of GFP fluorescence per cell was quantified via flow cytometry (Fig. 7). Akin to the uptake studies, cells transfected with the redispersed polyplexes at the various N/P ratios exhibited similar, if not better gene transfection when compared to the fresh polyplex counterparts. Interestingly, the presence of trehalose in freshly prepared particles did not show the same positive effect on transfection efficacy, especially at N/P 6. Therefore, trehalose potentially influencing the permeability of the cell membrane and thereby increasing polyplex uptake and gene transfection as observed previously [45] does not explain our observations. Importantly, due to the ability to quantify the amount of PEI and nucleic acid within each NEM formulation, we are able to ensure that the same quantity of plasmid DNA was used in the transfection for both fresh and redispersed polyplexes, however, according to Fig. 5C the increased N/P ratio following spray drying could explain better transfection results. As hypothesized for the increased cellular uptake, faster sedimentation of larger particles may as well play a role, especially in the case of plasmid containing polyplexes at N/P 6 which showed stronger aggregation than higher N/ P formulations (Fig. S5) or bulk DNA containing polyplexes (Fig. 1). The results suggest, therefore, that the formulation of PEI/DNA polyplexes in the form of NEM does not negatively affect biological activity of the nucleic acid.
Fig. 7.
Transfection efficiency of redispersed NEM formulations in A549 cells. Median fluorescence intensity (MFI) was determined by flow cytometry to evaluate the transfection efficiency of pEGFP in a human non-small cell lung carcinoma cell line (A549) of fresh or redispersed PEI polyplexes from 10% trehalose NEM formulations at N/P ratios of 6, 8 and 10 with 0.75 μg of GFP plasmid in comparison to freshly prepared formulations in presence of trehalose. Blank samples consisted of A549 cells treated with 5% glucose only. Data points indicate mean ± SD (n = 3). Two-way ANOVA, Bonferroni post-test, **P < 0.01, ***P < 0.001, ns = non-significant.
4. Conclusion
Herein we demonstrate successful spray drying of PEI/DNA polyplexes into NEM powders intended for pulmonary gene delivery. Various spray drying processing parameters were optimized while the saccharides trehalose and mannitol were investigated as matrix excipients to stabilize the polyplexes in the resulting NEM formulations. The mannitol and trehalose NEMs were then characterized through various physical techniques, and the behavior of redispersed PEI-DNA polyplexes were compared to fresh unprocessed particles. Following these characterization studies, 10% trehalose was found to be an efficient stabilizer that preserved the size of the polyplexes following their resuspension. Microparticle powder analysis revealed fused, slightly corrugated particles with an aerodynamic diameter suitable for deep lung deposition. In vitro studies showed that the redispersed polyplexes maintained their uptake and transfection profiles in lung adenocarcinoma A549 cells when compared to fresh polyplexes. While previous studies describe spray drying of polymeric nanoparticles into NEMs, this study is, to the best of our knowledge, the first to quantify the extent of polymer and nucleic acid loss during the process and to recalculate the N/P ratio of the dried polyplexes accordingly. By quantifying the amount of DNA remaining in the resulting NEM powder, we were able to ensure that our in vitro transfections were executed without bias due to equal DNA amounts transfected. However, as discussed, the increase of the N/P ratio may have a positive impact on uptake and transfection and would have to be accounted for when preparing new batches of NEMs. Taken together, this study serves as a proof-of-concept for the processing of polymeric nanoparticles loaded with nucleic acids into a dry powder that is suitable for inhalation therapy. After optimizing the analytics for precise analysis of the composition of a well studied polyplex system, this study plants the basis for further studies with more complex polymers and siRNA. Along these lines, further optimization of the spray drying parameters to produce NEM powder from polymeric nanoparticles loaded with siRNA as well as formulation with biodegradable polymers is underway along with long term storage and stability studies of these NEM formulations.
Supplementary Material
Acknowledgements
This work was supported by the European Research Council [grant number ERC-2014-StG – 63783] and Wayne State School of Medicine, United States, for GRA support of Daniel Feldmann. The authors thank Ahmed El Mahmoudi (Technical University of Munich, Germany) for expert spray lab support. Sandro da Rocha accknowledges the National Science Foundation, United States under Grant No. 1643770.
References
- [1].Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Brit J Clin Pharmacol. 2003;56:588–599. doi: 10.1046/j.1365-2125.2003.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Patton JS, Byron PR. Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov. 2007;6:67–74. doi: 10.1038/nrd2153. [DOI] [PubMed] [Google Scholar]
- [3].Crompton GK. Dry powder inhalers: advantages and limitations. J Aerosol Med. 1991;4:151–156. doi: 10.1089/jam.1991.4.151. [DOI] [PubMed] [Google Scholar]
- [4].Xie Y, Kim NH, Nadithe V, Schalk D, Thakur A, Kilic A, Lum LG, Bassett DJP, Merkel OM. Targeted delivery of siRNA to activated T cells via transferrin-polyethylenimine (Tf-PEI) as a potential therapy of asthma. J Control Rel. 2016;229:120–129. doi: 10.1016/j.jconrel.2016.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Soutschek J, Akinc A, BramLage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J, John M, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- [6].Seguin RM, Ferrari N. Emerging oligonucleotide therapies for asthma and chronic obstructive pulmonary disease. Exp Opin Investig Drugs. 2009;18:1505–1517. doi: 10.1517/13543780903179294. [DOI] [PubMed] [Google Scholar]
- [7].Klinger-Strobel M, Lautenschlager C, Fischer D, Mainz JG, Bruns T, Tuchscherr L, Pletz MW, Makarewicz O. Aspects of pulmonary drug delivery strategies for infections in cystic fibrosis-where dowe stand? Exp Opin Drug Deliv. 2015;12:1351–1374. doi: 10.1517/17425247.2015.1007949. [DOI] [PubMed] [Google Scholar]
- [8].Chow MY, Lam JK. Dry powder formulation of plasmid DNA and siRNA for inhalation. Curr Pharm Des. 2015;21:3854–3866. doi: 10.2174/1381612821666150820105916. [DOI] [PubMed] [Google Scholar]
- [9].Draz MS, Fang BA, Zhang P, Hu Z, Gu S, Weng KC, Gray JW, Chen FF. Nanoparticle-mediated systemic delivery of siRNA for treatment of cancers and viral infections. Theranostics. 2014;4:872–892. doi: 10.7150/thno.9404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Merkel OM, Zheng M, Debus H, Kissel T. Pulmonary gene delivery using polymeric nonviral vectors. Bioconjug Chem. 2012;23:3–20. doi: 10.1021/bc200296q. [DOI] [PubMed] [Google Scholar]
- [11].Alexis F, Zeng J, Shu W. PEI nanoparticles for targeted gene delivery. CSH Protoc. 2006;2006 doi: 10.1101/pdb.prot4451. [DOI] [PubMed] [Google Scholar]
- [12].Heyder J. Deposition of inhaled particles in the human respiratory tract and consequences for regional targeting in respiratory drug delivery. Proc Am Thorac Soc. 2004;1:315–320. doi: 10.1513/pats.200409-046TA. [DOI] [PubMed] [Google Scholar]
- [13].Cal K, Sollohub K. Spray drying technique. I: hardware and process parameters. J Pharm Sci. 2010;99:575–586. doi: 10.1002/jps.21886. [DOI] [PubMed] [Google Scholar]
- [14].Heyder RS, Zhong Q, Bazito RC, da Rocha SRP. Cellular internalization and transport of biodegradable polyester dendrimers on a model of the pulmonary epithelium and their formulation in pressurized metered-dose inhalers. Int J Pharm. 2017;520:181–194. doi: 10.1016/j.ijpharm.2017.01.057. [DOI] [PubMed] [Google Scholar]
- [15].Elsayed M, Corrand V, Kolhatkar V, Xie Y, Kim NH, Kolhatkar R, Merkel OM. Influence of oligospermines architecture on their suitability for siRNA delivery. Biomacromol. 2014;15:1299–1310. doi: 10.1021/bm401849d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Okuda T, Morishita M, Mizutani K, Shibayama A, Okazaki M, Okamoto H. Development of spray-freeze-dried siRNA/PEI powder for inhalation with high aerosol performance and strong pulmonary gene silencing activity. J Control Rel. 2018;279:99–113. doi: 10.1016/j.jconrel.2018.04.003. [DOI] [PubMed] [Google Scholar]
- [17].Schulze J, Kuhn S, Hendrikx S, Schulz-Siegmund M, Polte T, Aigner A. Spray-dried nanoparticle-in-microparticle delivery systems (NiMDS) for gene delivery, comprising polyethylenimine (PEI)-based nanoparticles in a poly(vinyl alcohol) matrix. Small. 2018;14:e1701810. doi: 10.1002/smll.201701810. [DOI] [PubMed] [Google Scholar]
- [18].Jones SK, Lizzio V, Merkel OM. Folate receptor targeted delivery of siRNA and paclitaxel to ovarian cancer cells via folate conjugated triblock copolymer to overcome TLR4 driven chemotherapy resistance. Biomacromol. 2016;17:76–87. doi: 10.1021/acs.biomac.5b01189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].European-Pharmacopoeia-9.0. Aerodynamic Assessment of Fine Particles in: 2.9.18 Preparations for Inhalation. 2017 [Google Scholar]
- [21].Snyder SL, Sobocinski PZ. An improved 2,4,6-trinitrobenzenesulfonic acid method for the determination of amines. Anal Biochem. 1975;64:284–288. doi: 10.1016/0003-2697(75)90431-5. [DOI] [PubMed] [Google Scholar]
- [22].Naini V, Byron PR, Phillips EM. Physicochemical stability of crystalline sugars and their spray-dried forms: dependence upon relative humidity and suitability for use in powder inhalers. Drug Dev Ind Pharm. 1998;24:895–909. doi: 10.3109/03639049809097269. [DOI] [PubMed] [Google Scholar]
- [23].Seville PC, Li HY, Learoyd TP. Spray-dried powders for pulmonary drug delivery. Crit Rev Ther Drug Carrier Syst. 2007;24:307–360. doi: 10.1615/critrevtherdrugcarriersyst.v24.i4.10. [DOI] [PubMed] [Google Scholar]
- [24].Conti DS, Brewer D, Grashik J, Avasarala S, da Rocha SR. Poly(amidoamine) dendrimer nanocarriers and their aerosol formulations for siRNA delivery to the lung epithelium. Mol Pharm. 2014;11:1808–1822. doi: 10.1021/mp4006358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Boeckle S, von Gersdorff K, van der Piepen S, Culmsee C, Wagner E, Ogris M. Purification of polyethylenimine polyplexes highlights the role of free polycations in gene transfer. J Gene Med. 2004;6:1102–1111. doi: 10.1002/jgm.598. [DOI] [PubMed] [Google Scholar]
- [26].Zheng M, Pavan GM, Neeb M, Schaper AK, Danani A, Klebe G, Merkel OM, Kissel T. Targeting the blind spot of polycationic nanocarrier-based siRNA delivery. ACS Nano. 2012;6:9447–9454. doi: 10.1021/nn301966r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kuhn DA, Vanhecke D, Michen B, Blank F, Gehr P, Petri-Fink A, Rothen-Rutishauser B. Different endocytotic uptake mechanisms for nanoparticles in epithelial cells and macrophages. Beilstein J Nanotech. 2014;5:1625–1636. doi: 10.3762/bjnano.5.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Takashima Y, Saito R, Nakajima A, Oda M, Kimura A, Kanazawa T, Okada H. Spray-drying preparation of microparticles containing cationic PLGA nanospheres as gene carriers for avoiding aggregation of nanospheres. Int J Pharm. 2007;343:262–269. doi: 10.1016/j.ijpharm.2007.05.042. [DOI] [PubMed] [Google Scholar]
- [29].Bielski E, Zhong Q, Mirza H, Brown M, Molla A, Carvajal T, da Rocha SRP. TPP-dendrimer nanocarriers for siRNA delivery to the pulmonary epithelium and their dry powder and metered-dose inhaler formulations. Int J Pharm. 2017;527:171–183. doi: 10.1016/j.ijpharm.2017.05.046. [DOI] [PubMed] [Google Scholar]
- [30].Abdelwahed W, Degobert G, Stainmesse S, Fessi H. Freeze-drying of nanoparticles: formulation, process and storage considerations. Adv Drug Deliv Rev. 2006;58:1688–1713. doi: 10.1016/j.addr.2006.09.017. [DOI] [PubMed] [Google Scholar]
- [31].Agnoletti M, Bohr A, Thanki K, Wan F, Zeng X, Boetker JP, Yang M, Foged C. Inhalable siRNA-loaded nano-embedded microparticles engineered using microfluidics and spray drying. Eur J Pharm Biopharm. 2017;120:9–21. doi: 10.1016/j.ejpb.2017.08.001. [DOI] [PubMed] [Google Scholar]
- [32].Yang W, Peters JI, Williams RO., 3rd Inhaled nanoparticles-a current review. Int J Pharm. 2008;356:239–247. doi: 10.1016/j.ijpharm.2008.02.011. [DOI] [PubMed] [Google Scholar]
- [33].Nunes C, Suryanarayanan R, Botez CE, Stephens PW. Characterization and crystal structure of D-mannitol hemihydrate. J Pharm Sci. 2004;93:2800–2809. doi: 10.1002/jps.20185. [DOI] [PubMed] [Google Scholar]
- [34].Cares-Pacheco M, Vaca-Medina G, Calvet R, Espitalier F, Letourneau JJ, Rouilly A, Rodier E. Physicochemical characterization of D-mannitol polymorphs: the challenging surface energy determination by inverse gas chromatography in the infinite dilution region. Int J Pharm. 2014;475:69–81. doi: 10.1016/j.ijpharm.2014.08.029. [DOI] [PubMed] [Google Scholar]
- [35].Burger A, Henck JO, Hetz S, Rollinger JM, Weissnicht AA, Stottner H. Energy/ temperature diagram and compression behavior of the polymorphs of D-mannitol. J Pharm Sci. 2000;89:457–468. doi: 10.1002/(SICI)1520-6017(200004)89:4<457::AID-JPS3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- [36].Yu L. Amorphous pharmaceutical solids: preparation, characterization and stabilization. Adv Drug Deliv Rev. 2001;48:27–42. doi: 10.1016/s0169-409x(01)00098-9. [DOI] [PubMed] [Google Scholar]
- [37].Weng L, Ziaei S, Elliott GD. Effects of water on structure and dynamics of trehalose glasses at low water contents and its relationship to preservation outcomes. Sci Rep-UK. 2016;6:28795. doi: 10.1038/srep28795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chew NY, Tang P, Chan HK, Raper JA. How much particle surface corrugation is sufficient to improve aerosol performance of powders? Pharm Res. 2005;22:148–152. doi: 10.1007/s11095-004-9020-4. [DOI] [PubMed] [Google Scholar]
- [39].Wang W. Lyophilization and development of solid protein pharmaceuticals. Int J Pharm. 2000;203:1–60. doi: 10.1016/s0378-5173(00)00423-3. [DOI] [PubMed] [Google Scholar]
- [40].Nagase H, Endo T, Ueda H, Nakagaki M. An anhydrous polymorphic form of trehalose. Carbohydr Res. 2002;337:167–173. doi: 10.1016/s0008-6215(01)00294-4. [DOI] [PubMed] [Google Scholar]
- [41].Yu L, Mishra DS, Rigsbee DR. Determination of the glass properties of D-man-nitol using sorbitol as an impurity. J Pharm Sci. 1998;87:774–777. doi: 10.1021/js970224o. [DOI] [PubMed] [Google Scholar]
- [42].Rabbani NR, Seville PC. The influence of formulation components on the aero-solisation properties of spray-dried powders. J Control Rel. 2005;110:130–140. doi: 10.1016/j.jconrel.2005.09.004. [DOI] [PubMed] [Google Scholar]
- [43].Fahrmeir J, Gunther M, Tietze N, Wagner E, Ogris M. Electrophoretic purification of tumor-targeted polyethylenimine-based polyplexes reduces toxic side effects in vivo. J Control Rel. 2007;122:236–245. doi: 10.1016/j.jconrel.2007.05.013. [DOI] [PubMed] [Google Scholar]
- [44].Chen L, Simpson JD, Fuchs AV, Rolfe BE, Thurecht KJ. Effects of surface charge of hyperbranched polymers on cytotoxicity, dynamic cellular uptake and localization, hemotoxicity, and pharmacokinetics in mice. Mol Pharmaceutics. 2017 doi: 10.1021/acs.molpharmaceut.7b00611. [DOI] [PubMed] [Google Scholar]
- [45].Srinivasachari S, Liu Y, Zhang G, Prevette L, Reineke TM. Trehalose click polymers inhibit nanoparticle aggregation and promote pDNA delivery in serum. J Am Chem Soc. 2006;128:8176–8184. doi: 10.1021/ja0585580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.