Abstract
Keywords: Kupffer cell, lipid metabolism, iron metabolism, anemia, non-alcoholic fatty liver disease (NAFLD), anemia of inflammation (AI)
Background
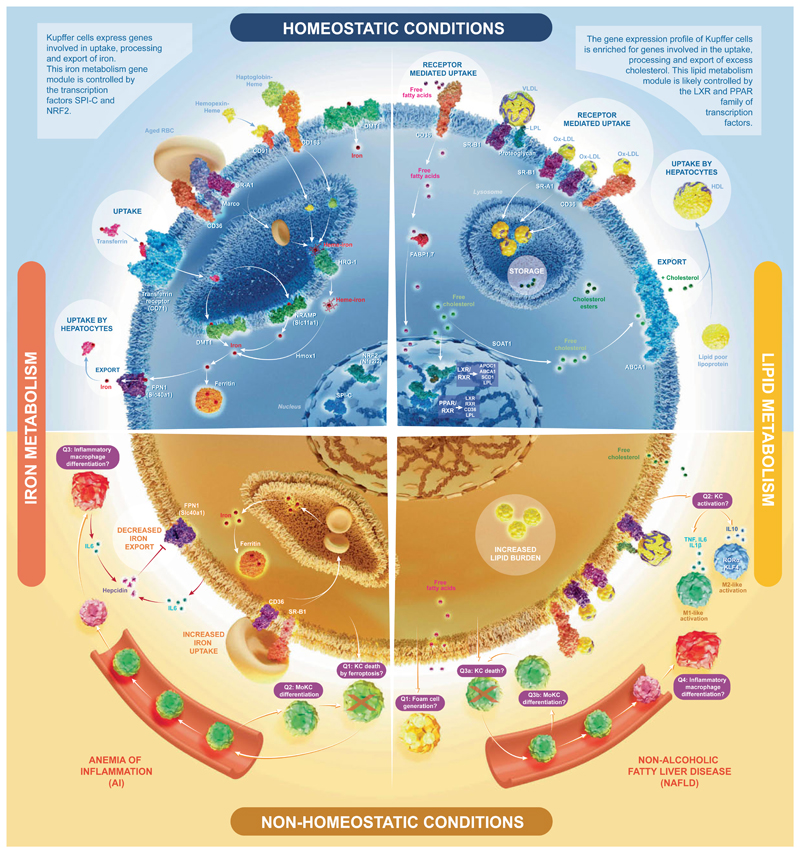
Macrophages perform distinct ‘accessory’ functions in their tissue of residence.1 Comparison of the transcriptome of Kupffer cells (KCs) with other macrophages found that KCs express genes associated with iron and lipid metabolism.2 All proteins proposed to be involved in iron and lipid uptake, processing and export shown here have been found to be highly expressed by KCs (bulk transcriptomics,2) and not to be expressed by contaminating cells by single-cell transcriptomics (unpublished data).
Iron metabolism as an accessory function of KCs
Iron present in aged red blood cells (RBCs) needs to be recycled efficiently. Although hepatocytes represent the main iron storing cells in the body they are not well equipped to phagocytose aged RBCs. KCs are ideally localized in the sinusoid and express genes involved in uptake, processing and export of iron.3 This iron metabolism gene module is controlled by the transcription factors SPI-C and NRF2. The liver plays a major role in the regulation of iron metabolism. Hepatocytes do not only store excess iron, but they are also the primary source of hepcidin, which by binding to ferroportin causes its degradation and the suppression of iron export from cells, including macrophages. Inflammatory mediators such as IL6 induce hepcidin production, which results in iron sequestration by macrophages. Uncontrolled or prolonged KC activation during severe infections leads to pathological cytokine production by KCs, resulting in increased hepcidin production and decreased transferrin production by hepatocytes. This leads to iron deprivation by decreased iron transport in circulation and increased iron sequestration by KCs often causing the so-called anemia of inflammation (AI).4 The excessive uptake of stressed RBCs during inflammation results in KC death (possibly through ferrop-tosis – question 1 [Q1]), and a massive recruitment of monocytes to the liver.4 Some of these monocytes differentiate into monocyte-derived KCs, but whether these cells can selfmaintain in the liver for prolonged periods is unclear and may depend of the inflammatory context (Q2). A fraction of the monocytes recruited may also differentiate into short-lived inflammatory macrophages that further fuel inflammation (Q3).
Lipid metabolism as a plausible accessory function of KCs
Mammalian cells cannot degrade the sterol ring of cholesterol. Cholesterol is eliminated by hepatic biliary excretion. While lipid metabolism is a general function of macrophages, who need to process lipids from dying cells they phagocytose, it is potentially an additional accessory function for KCs.5 The gene expression profile of KCs is enriched, compared with other tissue macrophages, for genes involved in the uptake, processing and export of excess cholesterol to extracellular high-density lipoprotein acceptors for transport to hepatocytes. Expression of many of these genes are driven by the transcription factors LXRa, RXRa, PPARδ and PPARγ, which are also expressed by KCs.2
Despite this enriched lipid metabolism signature, the precise roles of KCs in homeostatic lipid metabolism, as well as in conditions of excess lipid such as non-alcoholic fatty liver disease (NAFLD) are unknown (reviewed in5). In terms of NAFLD, there are a number of questions that need to be answered. Q1) Lipid laden foam cells have been reported in the liver during NAFLD, however it remains unclear whether these arise from KCs or infiltrating macrophages or a combination of both.6 Q2) KC activation is thought to be one of the main driving forces of the inflammation seen during NAFLD,7 However, although it has been proposed that skewing KCs towards an M2-like phenotype (driven by RORa and KLF4 induced IL10) is protective in NAFLD,8,9 type 2 immunity has been shown to exacerbate NAFLD while mice lacking IL4/IL13 are protected.10 Thus, the exact role of M1/M2 like macrophages in NAFLD pathogenesis remains to be elucidated, this is likely due to the shortcomings of the M1/M2 nomenclature in vivo.11 Q3) In addition to the above, it is also unclear if KCs can persist during NAFLD or are killed by the excess fat and replaced by recruited monocyte-derived KCs (MoKCs). In a mouse model of NASH, we have recently identified MoKCs suggesting KC death and replacement is ongoing during NAFLD.12 However, these do not seem to persist following return to normal chow, raising the question of whether these cells are capable of self-renewal or if the return to steady state results in MoKC loss. A long-term model of NAFLD, will be required to assess this. Q4) In addition to potentially becoming MoKCs, monocytes recruited to the liver during NAFLD may also become short-lived pro-inflammatory macrophages distinct from KCs. This remains to be examined with more specific markers to distinguish between KCs and other hepatic macrophages, such as Clec4F. This will be important to understand, as blocking monocyte recruitment using CCR2 inhibitors has been shown to be protective in NAFLD.13 Answering these questions represents an important goal for future research.
Importantly, NAFLD has been associated with iron overload in both KCs and hepatocytes in one-third of patients.14 Inflammatory mediators induced by lipid overload lead to increased hepcidin levels, which in turn lead to impaired iron export in KCs and hepatocytes. Iron overload in turn aggravates NAFLD and iron deprivation ameliorates disease symptoms. The central role of KCs in iron and lipid metabolic pathways highlights KCs as prime therapeutic targets for metabolic diseases.
Footnotes
Conflict of interest
MG reports grants from the FWO and the ERC. CS reports grants from the Wellcome Trust and from the FWO.
Please refer to the accompanying ICMJE disclosure forms for further details.
Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jhep.2018.02.013.
References
- [1].Okabe Y, Medzhitov R. Tissue biology perspective on macrophages. Nat Immunol. 2016;17:9–17. doi: 10.1038/ni.3320. [DOI] [PubMed] [Google Scholar]
- [2].Scott CL, Zheng F, De Baetselier P, Martens L, Saeys Y, De Prijck S, et al. Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat Commun. 2016;7:10321. doi: 10.1038/ncomms10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nairz M, Theurl I, Swirski FK, Weiss G. “Pumping iron-“how macrophages handle iron at the systemic, microenvironmental, and cellular levels. Pflugers Arch. 2017;469:397–418. doi: 10.1007/s00424-017-1944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Theurl I, Hilgendorf I, Nairz M, Tymoszuk P, Haschka D, Asshoff M, et al. On-demand erythrocyte disposal and iron recycling requires transient macrophages in the liver. Nat Med. 2016;22:945–951. doi: 10.1038/nm.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Remmerie A, Scott CL. Macrophages and lipid metabolism. Cell Immunol. 2018 doi: 10.1016/j.cellimm.2018.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Leroux A, Ferrere G, Godie V, Cailleux F, Renoud M-L, Gaudin F, et al. Toxic lipids stored by Kupffer cells correlates with their pro-inflammatory phenotype at an early stage of steatohepatitis. J Hepatol. 2012;57:141–149. doi: 10.1016/j.jhep.2012.02.028. [DOI] [PubMed] [Google Scholar]
- [7].Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol. 2017;17:306–321. doi: 10.1038/nri.2017.11. [DOI] [PubMed] [Google Scholar]
- [8].Luo W, Xu Q, Wang Q, Wu H, Hua J. Effect of modulation of PPAR-γ activity on Kupffer cells M1/M2 polarization in the development of nonalcoholic fatty liver disease. Sci Rep. 2017;7:44612. doi: 10.1038/srep44612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Han Y-H, Kim H-J, Na H, Nam M-W, Kim J-Y, Kim J-S, et al. RORα induces KLF4-mediated M2 polarization in the liver macrophages that protect against nonalcoholic steatohepatitis. Cell Rep. 2017;20:124–135. doi: 10.1016/j.celrep.2017.06.017. [DOI] [PubMed] [Google Scholar]
- [10].Hart KM, Fabre T, Sciurba JC, Gieseck RL, Borthwick LA, Vannella KM, et al. Type 2 immunity is protective in metabolic disease but exacerbates NAFLD collaboratively with TGF-β. Sci Transl Med. 2017;9:eaal3694. doi: 10.1126/scitranslmed.aal3694. [DOI] [PubMed] [Google Scholar]
- [11].Nahrendorf M, Swirski FK. Abandoning M1/M2 for a network model of macrophage function. Circ Res. 2016;119:414–417. doi: 10.1161/CIRCRESAHA.116.309194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Devisscher L, Scott CL, Lefere S, Raevens S, Bogaerts E, Paridaens A, et al. Non-alcoholic steatohepatitis induces transient changes within the liver macrophage pool. Cell Immunol. 2017:74–83. doi: 10.1016/j.cellimm.2017.10.006. [DOI] [PubMed] [Google Scholar]
- [13].Krenkel O, Puengel T, Govaere O, Abdallah AT, Mossanen JC, Kohlhepp M, et al. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. Hepatology. 2017 doi: 10.1002/hep.29544. [DOI] [PubMed] [Google Scholar]
- [14].Aigner E, Weiss G, Datz C. Dysregulation of iron and copper homeostasis in nonalcoholic fatty liver. World J Hepatol. 2015;7:177–188. doi: 10.4254/wjh.v7.i2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]