Abstract
Introduction
Large epidemiological studies have demonstrated the link between metabolic syndrome and cancer development, including colorectal cancer. However, the influence of metabolic syndrome on disease progression is less well studied, particularly in the post-surgical setting. This study investigates the effect of metabolic syndrome on colorectal cancer recurrence (all-site and liver-specific) after curative surgery for Stage I-III disease.
Materials and Methods
Consecutive patients who underwent curative resection for Stage I-III colorectal cancer in a single UK centre were prospectively recruited. Disease-free and overall survival with metabolic syndrome as a factor, were determined using the Kaplan-Meier technique. Hazard ratios for all-site and liver-specific recurrence were determined using univariable and multivariable Cox-regression models.
Results
1006 patients were recruited and followed up for a median of 50 months (IQR 30-67). 177 patients (17.6%) met the criteria for metabolic syndrome. 245 patients (25.4%) developed recurrence, 161 (16.0%) of these had liver recurrence. The presence of metabolic syndrome was associated with a reduction in disease-free survival from 69 to 58 months (p<0.001) and overall survival from 74 to 61 months (p<0.001). Metabolic syndrome was an independent predictor of all-site (HR 1.76; p<0.001) and liver-specific (HR 1.74; p=0.01) recurrence.
Conclusion
Metabolic syndrome is a predictor of all-site and liver-specific recurrence after primary resection for stage I-III colorectal cancer.
Keywords: Metabolic syndrome, colorectal cancer, surgery, recurrence, liver
1. Introduction
Colorectal cancer (CRC) has a huge global footprint, with 1.8 million new cases annually, accounting for over 10% of all cancer incidence and making it the third most frequently diagnosed cancer overall [1, 2]. Despite stabilisation in incidence and mortality in high-income countries, rapid rises are seen in low-income and transitioning countries, meaning that global incidence is set to eclipse 2.2 million by 2030 [3]. In terms of mortality, CRC ranks second among all cancers, with over 800 000 deaths per year across the world, largely attributable to metastatic disease [2]. For patients with stage I-III disease, surgical treatment offers relatively high cure rates, with recurrence at 5 years ranging from 5% (stage I) to 33% (stage III) [4]. Distant recurrence is twice as common as local recurrence, with the liver being the most common secondary site [5]. Therefore, it is important to identify novel modifiable risk factors, in order to better stratify patients for adjuvant therapy and/ or intensification of follow up after primary resection, as well as introducing primary intervention that may reduce the risk of recurrence.
Metabolic syndrome (MetS) describes a pro-inflammatory state associated with insulin resistance and obesity [6, 7]. The harmonised definition is of a constellation of interrelated risk factors for cardiovascular disease and type-2 diabetes, including hypertension, dyslipidaemia (raised triglyceride (TG) and lowered high-density lipoprotein cholesterol (HDL-C)), raised fasting glucose, and central obesity [8]. From a liver perspective, it has been shown that non-alcoholic fatty liver disease, encompassing hepatic steatosis and non-alcoholic steatohepatitis (NASH), is a hepatic manifestation of MetS [9–11].
MetS has been linked with the development of several cancers, including CRC [12–14]. Two large epidemiological studies with over 35 000 patients, focused our attention on the link between MetS and CRC mortality, particularly in men [15, 16]. The mechanisms for this were proposed to be insulin resistance (increased bioavailability of insulin-like growth factor-1 (IGF-1)), aromatase activity, adipokine production, angiogenesis, glucose utilisation, and oxidative stress [12, 17]. In terms of prognosis, obesity, as a single metabolic parameter, has been repeatedly shown to predict poorer outcomes, in terms of overall survival (OS) in men and women, and disease-free survival (DFS) in men, in large scale studies of Stage II and III CRC [18, 19]. Furthermore, our previous work highlights the increased risk of recurrent colorectal liver metastases following curative liver resection in patients with liver steatosis [20]. However, the direct implication of MetS, as a combined phenotype of dysregulated metabolic parameters, on CRC disease progression, has not been studied in detail, particularly in patients having curative bowel resection. To this end, we investigated the relationship between MetS and CRC recurrence in the liver and at other sites, after primary resection for stage I-III disease. We hypothesised that the presence of MetS was associated with an increased recurrence rate after curative surgery. The specific aims of this study were to: (i) assess the influence of MetS on post-operative disease-free (DFS) and overall (OS) survival and; (ii) ascertain whether MetS is a predictor of all-site and liver-specific disease recurrence in the post-operative setting.
2. Methods
Between 2010 and 2017, consecutive patients with stage I–III CRC who were treated surgically with curative intent were enrolled. Ethical approval was obtained through the UK National Research Ethics Service and all patients were prospectively recruited as part of an ongoing UK National Institute of Health Research Clinical Research Network study (UKCRN ID 6067; NCT03309722). Exclusion criteria included evidence of a hereditary tumour, or the presence of multiple tumours. No patients were excluded based upon age, BMI or American Society of Anaesthesia (ASA) grade. Other results and further details from this ongoing study have been previously reported [21–26]. Study oversight activities and monitoring were performed at an independent clinical research organisation. Pathological verification of diagnosis and staging was in accordance with the Association of Coloproctology of Great Britain and Ireland guidelines [27]. Participants were followed up until 31st December 2019.
A database of electronically stored, prospectively collected anonymised data was compiled for statistical analysis. Surgical procedures were carried out at University Hospital Southampton NHS Trust, UK. All patients were discussed in the CRC multidisciplinary meeting prior to surgery to confirm resectability and operative approach (laparoscopic, open, trans-anal), based on disease characteristics, local expertise, and available resources. Patients were not offered or withheld (neo)adjuvant therapy based on metabolic parameters. Data collected included: patient demographics, metabolic parameters (see below), clinical parameters (e.g. pathological stage and tumour markers) and follow up information (recurrence and/ or death).
At baseline (immediately before the primary resection), all participants donated a fasting early morning blood sample for analysis. Plasma glucose, TGs and HDL-C were measured utilising the routine laboratory techniques of the host institution. Height and body weight were measured without shoes and outer clothing at time of first appointment. Blood pressure (BP) was measured using a digital monitor. Detailed medical history relating to diabetes and dyslipidaemias was obtained at diagnosis.
Criteria used to determine MetS in this study were any three of the following: BMI >30kg/m2 (as an index of central obesity); fasting TG ≥1.7mmol/L (or drug treatment for elevated TG), HDL-C <1.0 mmol/L (men) or <1.3 mmol/L (women; or drug treatment for reduced HDL-C); systolic BP ≥130 mmHg, diastolic BP ≥85 mmHg (or antihypertensive drug treatment in a patient with a history of hypertension); fasting glucose ≥5.6 mmol/L (or drug treatment of elevated glucose).
Participants were followed up post-treatment with 6-monthly serum carcinoembryonic antigen (CEA) for three years, two CT scans (chest, abdomen and pelvis) in the first three years, and colonoscopy at 1-year and 5-years, as per national guidelines [28]. DFS was defined as the period of time from index procedure to first radiological evidence of disease relapse. OS was the duration from index procedure to death.
Statistical analysis was performed using IBM SPSS Statistics (version 26) and Stata (version 16). Tests for normality were performed using Kolmogorov–Smirnov and Shapiro–Wilk tests. The association with recurrence was assessed using Cox-regression in univariable and multivariable analysis. Hazard ratios (HRs) for disease recurrence were obtained with 95% confidence intervals. Survival analysis was conducted using the Kaplan-Meier method with p-values obtained by log rank tests. Seeing as the proportion of patients with disease recurrence or death did not reach 50% in the study period, median values for DFS and OS could not be calculated and mean values are shown instead. All values are listed to 3 decimal places unless otherwise stated.
3. Results
3.1. Demographics
One thousand and six patients who underwent curative resection for stage I-III CRC were included. The median age was 72.4 years (range 25 to 93), 56.7% were ≥ 70 years and 59% were male. The distribution of primary tumours between right colon (proximal to splenic flexure), left colon (splenic flexure, descending and sigmoid colon) and rectum was 36.6%, 28.6% and 34.8%, respectively (Supplementary Table 1a). The types of operative procedure and their frequencies are shown in Supplementary Table 1b, with 62% of procedures performed laparoscopically. One quarter of the entire cohort (25.4%) developed distant recurrence during the follow-up period, with 67.5% in the liver (n=161), 20.3% in the lung (n=53) and 12.2% at other sites (n=31). Of the patients with liver metastases, 77 were operable (45% of all liver metastases). Median follow up was 50 months (IQR 30-67 months).
With respect to metabolic parameters: 25.5% of the entire cohort had BMI >30kg/m2, 42.8% had fasting TG ≥1.7mmol/L (or drug treatment for elevated TG), 49.6% had fasting HDL-C <1.0 mmol/L (men) or <1.3 mmol/L (women) (or drug treatment for reduced HDL-C), 60.3% had systolic BP ≥130 mmHg and/or diastolic BP ≥85 mmHg (or antihypertensive drug treatment), 36.7% had fasting glucose ≥5.6 mmol/L (or drug treatment for diabetes mellitus (DM) and overall, 17.6% met criteria for MetS (Table 1). Pathological staging (TNM 7th edition) gave overall frequencies of T- and N-stages as follows: T1 (8%), T2 (18 %), T3 (53%), T4 (20%), N0 (61%), N1 (27%) and N2 (12%).
Table 1. Description of the cohort with respect to demographic, metabolic and clinical variables.
| Variable | Recurrence (n=245) | No Recurrence (n=761) | Entire cohort (n=1006) | |
|---|---|---|---|---|
| Age | ||||
| ≥70 years | 130 (53%) | 440 (58%) | 570 (57%) | |
| BMI | ||||
| ≥30 | 80 (33%) | 177 (23%) | 257 (26%) | |
| TG score | ||||
| Yes | 116 (47%) | 315 (41%) | 431 (43%) | |
| HDL-C score | ||||
| Yes | 124 (51%) | 375 (49%) | 499 (50%) | |
| BP score | ||||
| Yes | 148(60%) | 459 (60%) | 607 (60%) | |
| DM score | ||||
| Yes | 106 (43%) | 263 (35%) | 369 (37%) | |
| Metabolic syndrome | ||||
| Yes | 59 (24%) | 118 (16%) | 177 (18%) | |
| T-Stage | ||||
| 4 | 83 (34%) | 119 (16%) | 202 (20%) | |
| N-Stage | ||||
| 2 | 59 (24%) | 66 (9%) | 125 (12%) | |
| LN Ratio | ||||
| ≥0.05 | 136 (56%) | 210 (27%) | 344 (34%) | |
| EMVI | ||||
| Yes | 133 (45%) | 192 (25%) | 325 (32%) | |
| Tumour perforation | ||||
| Yes | 16 (7%) | 27 (4%) | 43 (4%) | |
| Neoadjuvant Chemotherapy | ||||
| Yes | 44 (18%) | 94 (12%) | 138 (14%) | |
| Resection Margin | ||||
| R1 | 21 (9%) | 22 (3%) | 43 (4%) | |
| Serum CEA | ||||
| ≥50 | 26 (10%) | 26 (3%) | 52 (5%) | |
| NLR | ||||
| ≥4 | 100 (41%) | 227 (30%) | 327 (33%) | |
| Differentiation | ||||
| Wellmoderate | 188 (76%) | 604 (79%) | 792 (79%) | |
| Tumour Site | ||||
| Rectum | 91 (37%) | 259 (34%) | 350 (35%) | |
LN ratio – proportion of resected lymph nodes which contained tumour; EMVI – extramural vascular invasion; NLR – neutrophil to lymphocyte ratio from pre-operative blood sample.
3.2. Disease-free and overall survival with metabolic syndrome as a factor
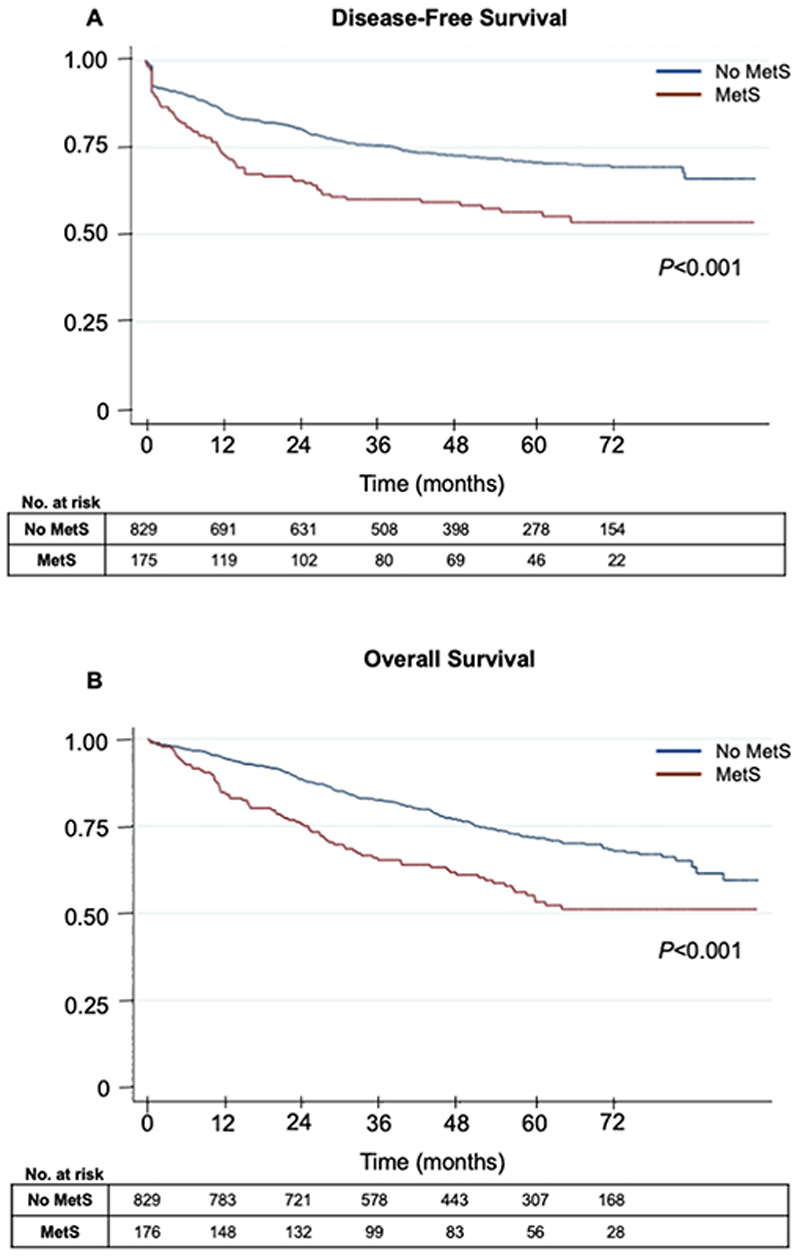
The first question was whether MetS influenced DFS and OS following curative surgery for stage I-III CRC. DFS for the entire cohort was 68.6 [95% CI 66.2-71.0] months. For the subgroup with MetS, DFS was 57.7 [51.4-64.1] months, which was significantly lower than the subgroup without MetS, where DFS was 70.8 [68.3-73.4] months (p<0.001, log rank test). OS was 71.5 [69.3-73.6] months for the entire cohort, and 61.1 [55.5-66.6] months and 73.7 [71.4-76.0] months in the MetS and non-MetS subgroups, respectively (p<0.001, log rank test). Kaplan-Meier curves are shown in Figure 1 with descriptive statistics shown in Supplementary Table 2.
Figure 1. The effect of MetS on DFS and OS following curative resection.
Kaplan-Meir curves for the effect of MetS on (A) DFS and (B) OS following curative primary colorectal resection. Number at risk provided in tables. Log rank test used to derive p values.
3.3. Univariable analysis of metabolic and clinical variables associated with disease recurrence (entire cohort)
To demonstrate the individual associations between metabolic/ clinical variables and all-site recurrence, univariable analysis was conducted. Of the metabolic parameters, BMI >30kg/m2 (HR 1.70) TG (HR 1.30), HDL-C (HR 1.31), DM (HR 1.50) and MetS (HR 1.34) were significantly associated with all-site recurrence (Table 2), whereas BP (HR 1.09) was not. Of the clinical parameters, T-stage (T3 and T4), N-stage, LN ratio, EMVI, tumour perforation, R1 resection, serum CEA, NLR ≥4 and left-sided tumours were all significantly associated with all-site recurrence, with HRs ranging from 1.42 (left colonic tumours) to 10.2 (T4 tumours). Neoadjuvant chemotherapy and histological grade were not.
Table 2. Results of univariable and multivariable analysis of metabolic and clinical risk factors for all-site recurrence after primary colorectal resection.
| Variable | Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age | |||||
| ≥70 | 0.959 (0.739-1.244) | 0.751 | 0.985(0.743-1.306) | 0.919 | |
| BMI | |||||
| ≥30 | 1.698 (1.289-2.236) | <0.001 | 1.382(1.017-1.879) | 0.039 | |
| TG score | |||||
| Yes | 1.319 (1.018-1.711) | 0.037 | 0.994(0.685-1.443) | 0.975 | |
| HDL-C score | |||||
| Yes | 1.273 (0.981-1.651) | 0.069 | 1.117(0.772-1.616) | 0.556 | |
| BP score | |||||
| Yes | 1.092(0.835-1.429) | 0.519 | 1.012(0.749-1.369) | 0.936 | |
| DM score | |||||
| Yes | 1.496(1.152-1.943) | 0.003 | 1.408(1.049-1.891) | 0.023 | |
| Metabolic syndrome | |||||
| Yes | 1.935(1.436-2.607) | <0.001 | 1.767(1.295-2.411) | <0.001* | |
| T-Stage | |||||
| 4 | 10.214 (4.459-23.40) | <0.001 | 4.765(1.976-11.49) | 0.001 | |
| N-Stage | |||||
| 2 | 4.410 (3.126-6.226) | <0.001 | 2.352(1.242-4.455) | 0.009 | |
| LN Ratio | |||||
| ≥0.05 | 2.984(2.3-3.872) | <0.001 | 1.919(1.436-2.565) | <0.001* | |
| EMVI | |||||
| Yes | 3.282 (2.527-4.261) | <0.001 | 1.683(1.245-2.274) | 0.001 | |
| Tumour perforation | |||||
| Yes | 2.456(1.453-4.149) | 0.001 | 0.850(0.454-1.592) | 0.612 | |
| Neoadjuvant Chemotherapy | |||||
| Yes | 1.113(0.762-1.627) | 0.578 | 0.875(.546-1.404) | 0.581 | |
| Resection Margin | |||||
| R1 | 4.134(2.659 -6.427) | <0.001 | 2.203(1.292-3.757) | 0.004 | |
| Serum CEA | |||||
| ≥50 | 3.795(2.281-6.316) | <0.001 | 2.521(1.441-4.410) | 0.001 | |
| NLR | |||||
| ≥4 | 1.962(1.510-2.549) | <0.001 | 1.811(1.352-2.424) | <0.001 | |
| Differentiation | |||||
| Well | 1.343(0.921-1.957) | 0.125 | 1.272(0.853-1.896) | 0.239 | |
| Tumour Site | |||||
| Rectum | 1.242 (0.904-1.705) | 0.181 | 1.753(1.200-2.561) | 0.004 | |
In this model the repeated factors were removed (for LN ratio, N stage was removed; for MetS, the metabolic factors which constitute MetS were removed).
LN ratio – proportion of resected lymph nodes which contained tumour; EMVI – extramural vascular invasion; NLR – neutrophil to lymphocyte ratio from pre-operative blood sample.
3.4. Multivariable analysis of metabolic and clinical variables associated with disease recurrence (entire cohort)
Multivariable analysis was then undertaken to assess the relative contributions of metabolic and clinical variables on all-site recurrence. Cox regression modelling showed that BMI >30kg/m2 (HR 1.38), DM (HR 1.41) and MetS (HR 1.77) were independently associated with all-site recurrence, with MetS demonstrating a greater hazard ratio than its constituent variables. TG, HDL-C and BP were not significantly associated with all-site recurrence in this model (Table 2). Similarly, T-stage (T3 and T4), N-stage, LN ratio, EMVI, R1 resection, serum CEA, NLR ≥4 and left-sided tumours were all independently associated with all-site recurrence, with HRs ranging from 1.46 (left colonic tumours) to 4.77 (T4 tumours). Tumour perforation, neoadjuvant chemotherapy and histological grade were not.
3.5. Multivariable analysis of metabolic and clinical variables associated with liver recurrence
Having identified MetS as a predictor of all-site recurrence, its association with liver-specific recurrence was examined by multivariable analysis. MetS was independently associated with liver recurrence (HR 1.66), as was serum CEA ≥50 (HR 3.28), NLR ≥4 (HR 1.67) and LN ratio ≥0.05 (HR 2.19). The association between R1 resection and liver recurrence failed to reach significance (HR 1.93; p=0.056). Age >70 years (HR 1.04) and neoadjuvant chemotherapy (HR 1.15) were not significantly linked with liver recurrence in this model (Table 3).
Table 3. Results of multivariable analysis of MetS (adjusted for clinical variables) for liver recurrence after primary colorectal resection. T-stage was treated as stratum effect modifier with p <0.001 across strata.
| Variable | P value | Adjusted HR | 95% CI | |
|---|---|---|---|---|
| Lower | Upper | |||
| MetS | 0.019 | 1.657 | 1.085 | 2.532 |
| Serum CEA ≥50 | 0.001 | 3.284 | 1.660 | 6.499 |
| NLR ≥4 | 0.008 | 1.670 | 1.143 | 2.438 |
| LN ratio ≥0.05 | <0.001 | 2.190 | 1.498 | 3.203 |
| Resection margin (R1) | 0.056 | 1.927 | 0.983 | 3.780 |
| Age >70 | 0.838 | 1.040 | 0.717 | 1.508 |
| Neoadjuvant chemotherapy | 0.583 | 1.152 | 0.695 | 1.908 |
4. Discussion
This study was designed to evaluate the effect of MetS on all-site and liver-specific recurrence following primary resection in 1006 stage I-III CRC patients. The key findings were that: (i) DFS and OS were significantly reduced in patients with MetS and; (ii) MetS was an independent predictor of all-site and liver-specific recurrence.
Our study is in keeping with the general consensus that MetS has a negative influence on CRC progression. Shen et al. studied a Chinese cohort of 503 patients with all stages of CRC, including 13% with Stage IV disease, and demonstrated a significant reduction in OS (63.0 to 48.0 months) and DFS (20.5 to 12.0 months) in patients with MetS [29]. Interestingly, subgroup analysis showed that MetS was an independent risk factor for recurrence in colonic but not rectal cancer, and for liver-specific recurrence in both colon and rectal cancers. This discrepancy may be attributed to a modest sample size and inclusion of Stage IV patients. In addition, it is unclear whether their use of the term “recurrence” refers to local recurrence or metastatic recurrence. We purposefully chose not to distinguish between colonic and rectal cancers because our focus was on distant recurrence, which is far less skewed towards either colonic or rectal primaries than local recurrence [30, 31].
Another similar study by You et al. described a Chinese cohort of 1069 patients with non-metastatic CRC, followed up for a median of 60 months [32]. Despite geographical and ethnic differences, the prevalence of MetS was similar to our cohort, at 20.7%. Rather surprisingly, patients with MetS were shown to have reduced DFS but no difference in OS. This is inconsistent with our data and data from the FIESTA study of CRC patients who had radical surgery, which demonstrated a marked reduction in OS (51 vs 170 months) in patients with MetS compared to those without [33]. We suspect that although MetS influences cancer-specific mortality, OS in this subgroup is also likely to be determined by diabetes and cardiovascular-related diseases, which are innately linked with MetS. Other factors that may explain decreased OS in patients with metabolic dysfunction include delayed diagnosis, aggressiveness of the disease and a lessened response to treatment [34]. Moreover, a 2013 meta-analysis clearly supports the link between MetS and decreased survival in CRC patients [35].
Vargas and colleagues made some inroads into the molecular basis for the link between MetS and CRC prognosis [36]. Using tissue from a training cohort of 80 post-surgical Stage II patients (later validated in a similar cohort), they identified six MetS-related genes, the overexpression of which, stratified patients for poorer DFS: apolipoprotein A-II (APOA2), apolipoprotein C1 (APOC1), apolipoprotein C2 (APOC2), apolipoprotein D (APOD), ATP-Binding Cassette Sub-Family A Member 1 (ABCA1), and leptin receptor(LEPR). Interestingly, both APOC1 and APOD are associated with hepatic steatosis [37, 38]. This may explain our previously reported findings regarding increased risk of metastatic CRC recurrence in patients with fatty liver [20].
Before drawing conclusions from these data, certain limitations must be pointed out. First of all, despite being a relatively large study conducted on an ethnically diverse population, it will not adequately represent all ethnic groups. Furthermore, the management of CRC (including national screening programmes, surgical technique, (neo)adjuvant treatment and follow up) varies across the globe and outcomes will be influenced by this. Similarly, the threshold for and intensity of treatment of MetS and its constituent disorders will also vary between regions. Lastly, this study focussed on the impact of pre-operative MetS on CRC recurrence. An assessment of how surgery alters metabolic variables, and the relationship between post-treatment MetS and disease recurrence was not directly examined, but could form the basis of future studies.
Conclusion
MetS is an independent predictor of all-site and liver-specific recurrence after primary resection of Stage I-III CRC, with associated reduction in DFS and OS. Our findings support the consideration of metabolic parameters in multidisciplinary decision making, as part of ongoing efforts to personalise treatment in patients with CRC.
Supplementary Material
Acknowledgements
The authors would like to thank the patients who enrolled in the study and the Clinical Trials Assistants (University of Southampton) who recruited them.
Funding source
This work is funded by Southampton NIHR-BRC and a Cancer Research UK grant to ZZH (A29908) and AHM (A10013). The funders had no direct involvement in study design, the collection, analysis and interpretation of data, writing of the report, or decision to submit the article for publication.
Footnotes
Competing Interests
The authors report no competing interests.
References
- 1.Ferlay J, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. International journal of cancer. 2019;144:1941–53. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, et al. Global cancer statistics 2018 GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Arnold M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–91. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 4.Osterman E, Glimelius B. Recurrence Risk After Up-to-Date Colon Cancer Staging, Surgery, and Pathology: Analysis of the Entire Swedish Population. Diseases of the colon and rectum. 2018;61:1016–25. doi: 10.1097/DCR.0000000000001158. [DOI] [PubMed] [Google Scholar]
- 5.Augestad KM, et al. Metastatic spread pattern after curative colorectal cancer surgery. A retrospective, longitudinal analysis Cancer epidemiology. 2015;39:734–44. doi: 10.1016/j.canep.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Sakkinen PA, et al. Clustering of procoagulation, inflammation, and fibrinolysis variables with metabolic factors in insulin resistance syndrome. American journal of epidemiology. 2000;152:897–907. doi: 10.1093/aje/152.10.897. [DOI] [PubMed] [Google Scholar]
- 7.Eckel RH, et al. The metabolic syndrome. Lancet. 2005;365:1415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 8.Alberti KG, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 9.Nassir F, et al. Pathogenesis and Prevention of Hepatic Steatosis. Gastroenterology & hepatology. 2015;11:167–75. [PMC free article] [PubMed] [Google Scholar]
- 10.Marchesini G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–50. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 11.Yang KC, et al. Association of Non-alcoholic Fatty Liver Disease with Metabolic Syndrome Independently of Central Obesity and Insulin Resistance. Scientific reports. 2016;6 doi: 10.1038/srep27034. 27034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cowey S, Hardy RW. The metabolic syndrome: A high-risk state for cancer? The American journal of pathology. 2006;169:1505–22. doi: 10.2353/ajpath.2006.051090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russo A, et al. Metabolic syndrome and cancer risk. European journal of cancer. 2008;44:293–7. doi: 10.1016/j.ejca.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Stocks T, et al. Blood glucose and risk of incident and fatal cancer in the metabolic syndrome and cancer project (me-can): analysis of six prospective cohorts. PLoS medicine. 2009;6:e1000201. doi: 10.1371/journal.pmed.1000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trevisan M, et al. Markers of insulin resistance and colorectal cancer mortality. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2001;10:937–41. [PubMed] [Google Scholar]
- 16.Colangelo LA, et al. Colorectal cancer mortality and factors related to the insulin resistance syndrome. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2002;11:385–91. [PubMed] [Google Scholar]
- 17.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. The American journal of clinical nutrition. 2007;86:s836–42. doi: 10.1093/ajcn/86.3.836S. [DOI] [PubMed] [Google Scholar]
- 18.Sinicrope FA, et al. Body mass index at diagnosis and survival among colon cancer patients enrolled in clinical trials of adjuvant chemotherapy. Cancer. 2013;119:1528–36. doi: 10.1002/cncr.27938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinicrope FA, et al. Obesity is an independent prognostic variable in colon cancer survivors. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16:1884–93. doi: 10.1158/1078-0432.CCR-09-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamady ZZ, et al. Fatty liver disease as a predictor of local recurrence following resection of colorectal liver metastases. The British journal of surgery. 2013;100:820–6. doi: 10.1002/bjs.9057. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, et al. miR-153 supports colorectal cancer progression via pleiotropic effects that enhance invasion and chemotherapeutic resistance. Cancer research. 2013;73:6435–47. doi: 10.1158/0008-5472.CAN-12-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bullock MD, et al. Stratifying risk of recurrence in stage II colorectal cancer using deregulated stromal and epithelial microRNAs. Oncotarget. 2015;6:7262–79. doi: 10.18632/oncotarget.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cellura D, et al. miR-19-Mediated Inhibition of Transglutaminase-2 Leads to Enhanced Invasion and Metastasis in Colorectal Cancer. Molecular cancer research: MCR. 2015;13:1095–105. doi: 10.1158/1541-7786.MCR-14-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ling H, et al. The clinical and biological significance of MIR-224 expression in colorectal cancer metastasis. Gut. 2016;65:977–89. doi: 10.1136/gutjnl-2015-309372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhome R, et al. Exosomal microRNAs derived from colorectal cancer-associated fibroblasts: role in driving cancer progression. Aging. 2017;9:2666–94. doi: 10.18632/aging.101355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanley CJ, et al. Targeting the Myofibroblastic Cancer-Associated Fibroblast Phenotype Through Inhibition of NOX4. Journal of the National Cancer Institute. 2018:110. doi: 10.1093/jnci/djx121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langman G, et al. Association of Coloproctology of Great Britain & Ireland (ACPGBI): Guidelines for the Management of Cancer of the Colon, Rectum and Anus 2017 - Pathology Standards and Datasets. Colorectal disease: the official journal of the Association of Coloproctology of Great Britain and Ireland. 2017;19(Suppl 1):74–81. doi: 10.1111/codi.13708. [DOI] [PubMed] [Google Scholar]
- 28.Leong K, et al. Association of Coloproctology of Great Britain & Ireland (ACPGBI): Guidelines for the Management of Cancer of the Colon, Rectum and Anus 2017 - Follow Up, Lifestyle and Survivorship. Colorectal disease: the official journal of the Association of Coloproctology of Great Britain and Ireland. 2017;19(Suppl 1):67–70. doi: 10.1111/codi.13706. [DOI] [PubMed] [Google Scholar]
- 29.Shen Z, et al. Metabolic syndrome is an important factor for the evolution of prognosis of colorectal cancer: survival, recurrence, and liver metastasis. American journal of surgery. 2010;200:59–63. doi: 10.1016/j.amjsurg.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Adam IJ, et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994;344:707–11. doi: 10.1016/s0140-6736(94)92206-3. [DOI] [PubMed] [Google Scholar]
- 31.Manfredi S, et al. Incidence and patterns of recurrence after resection for cure of colonic cancer in a well defined population. The British journal of surgery. 2006;93:1115–22. doi: 10.1002/bjs.5349. [DOI] [PubMed] [Google Scholar]
- 32.You J, et al. Metabolic syndrome contributes to an increased recurrence risk of nonmetastatic colorectal cancer. Oncotarget. 2015;6:19880–90. doi: 10.18632/oncotarget.4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng F, et al. Preoperative metabolic syndrome and prognosis after radical resection for colorectal cancer: The Fujian prospective investigation of cancer (FIESTA) study. International journal of cancer. 2016;139:2705–13. doi: 10.1002/ijc.30404. [DOI] [PubMed] [Google Scholar]
- 34.Campbell PT, et al. Impact of body mass index on survival after colorectal cancer diagnosis: the Cancer Prevention Study-II Nutrition Cohort. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30:42–52. doi: 10.1200/JCO.2011.38.0287. [DOI] [PubMed] [Google Scholar]
- 35.Esposito K, et al. Colorectal cancer association with metabolic syndrome and its components: a systematic review with meta-analysis. Endocrine 2013;44:634-47. and colorectal cancer. Molecular oncology. 2014;8:1469–81. doi: 10.1007/s12020-013-9939-5. [DOI] [PubMed] [Google Scholar]
- 36.Vargas T, et al. Genes associated with metabolic syndrome predict disease-free survival in stage II colorectal cancer patients. A novel link between metabolic dysregulation and colorectal cancer Molecular oncology. 2014;8:1469–81. doi: 10.1016/j.molonc.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muurling M, et al. Overexpression of APOC1 in obob mice leads to hepatic steatosis and severe hepatic insulin resistance. Journal of lipid research. 2004;45:9–16. doi: 10.1194/jlr.M300240-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Labrie M, et al. Apolipoprotein D Transgenic Mice Develop Hepatic Steatosis through Activation of PPARgamma and Fatty Acid Uptake. PloS one. 2015;10:e0130230. doi: 10.1371/journal.pone.0130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.