Abstract
Rationale
Hypoxia followed by reoxygenation promotes inflammation by activating NF-κB transcription factors in endothelial cells (EC). This process involves modification of the signalling intermediary TRAF6 with polyubiquitin chains. Thus cellular mechanisms that suppress TRAF6 ubiquitination are potential therapeutic targets to reduce inflammation in hypoxic tissues.
Objective
In this study, we tested the hypothesis that endothelial activation in response to hypoxia-reoxygenation can be influenced by Cezanne, a deubiquitinating enzyme that cleaves ubiquitin from specific modified proteins.
Methods and Results
Studies of cultured endothelial cells (EC) demonstrated that hypoxia (1% oxygen) induced Cezanne via p38 MAP kinase-dependent transcriptional and post- transcriptional mechanisms. Hypoxia-reoxygenation had minimal effects on pro-inflammatory signalling in unmanipulated EC but significantly enhanced Lys-63 polyubiquitination of TRAF6, activation of NF-κB and expression of inflammatory genes following silencing of Cezanne. Thus although hypoxia primed cells for inflammatory activation it simultaneously induced Cezanne which impeded signalling to NF-κB by suppressing TRAF6 ubiquitination. Similarly, ischemia induced Cezanne in the murine kidney in vascular EC, glomerular EC, podocytes and epithelial cells, and genetic deletion of Cezanne enhanced renal inflammation and injury in murine kidneys exposed to ischemia followed by reperfusion.
Conclusions
We conclude that inflammatory responses to ischemia are controlled by a balance between ubiquitination and deubiquitination and that Cezanne is a key regulator of this process. Our observations have important implications for therapeutic targeting of inflammation and injury during ischemia-reperfusion.
Keywords: Endothelium, hypoxia, inflammatory activation
Introduction
Tissues are exposed to hypoxia followed by reoxygenation during ischemia-reperfusion which occurs in several clinical settings including organ transplantation, percutaneous coronary intervention and bypass grafting1,2. Hypoxia-reoxygenation promotes inflammation by activating NF-κB transcription factors which induce adhesion proteins (e.g. VCAM-1, E-selectin) and other inflammatory molecules3–9. NF-κB transcription factors are regulated by an intricate signalling network that governs their intracellular localisation and transcriptional activity10. In the basal state, NF-κB dimers are inactivated by binding to inhibitor of κB (IκB) proteins which sequester NF-κB in the cytoplasm by masking its nuclear localization sequence. IκB kinase (IKK) promotes NF-κB activation by phosphorylating IκBα, a modification that targets it for ubiquitin- mediated degradation, and by phosphorylating RelA NF-κB subunits to enhance DNA binding and transcriptional activation11.
Recent studies have shed light on the signalling events that control NF-κB activation by hypoxia. In normoxic conditions, IKK proteins are targeted for hydroxylation by prolyl hydroxylase domain 1 (PHD1), a modification that leads to their ubiquitination and degradation4. Hypoxia can promote nuclear localization of NF-κB by preventing PHD1-mediated repression of IKK4, and by inducing the expression of several NF-κB sub-units6. The mechanism of NF-κB activation by hypoxia also involves modification of TRAF6 proteins with a non-canonical form of polyubiquitin (linked through Lys63) that is known to activate IKK12,13. Despite these insights, the mechanisms for NF-κB activation by hypoxia are understood incompletely and may vary according to particular cell types.
We previously discovered a regulator of NF-κB called Cezanne (Cellular Zinc-finger Anti-NF-κB; also known as OTUD7B) and cloned full length cDNA from EC14. Our subsequent studies revealed that Cezanne can be induced by TNFα, IL-1 and functions as an inhibitor of NF-κB, thus forming a negative feedback loop in inflammatory cytokine signalling15,16, and a recent report indicated that Cezanne can also influence non-canonical NF-κB activation17. The molecular mechanism for the anti-inflammatory effects of Cezanne was recently illuminated by our group who demonstrated that this molecule belongs to a novel family of deubiquitinating enzymes18. Thus Cezanne suppresses activation of NF-κB in response to TNFα by cleaving polyubiquitin chains from RIP1 which is a component of the TNFR complex15,16. Cezanne is related to a protein called A20 (TNFAIP3) which can also suppress NF-κB activation via deubiquitination of signalling proteins19,20. However, although A20 and Cezanne possess partially overlapping biochemical properties, they exert unique functions by targeting distinct forms of polyubiquitin20,21. Here, we examined whether Cezanne can influence inflammatory responses to hypoxia-reoxygenation in EC using both in vitro and in vivo models.
Methods
Reagents and antibodies
Anti-Cezanne (Proteintech Europe Ltd), Anti-RelA (p65), anti-κBα, anti-TRAF6, anti-Lamin B (Santa Cruz Biotechnology), phosphorylated anti-ATF2 (Thr71), phosphorylated anti-RelA (Ser536) (Cell Signalling Technology), anti-ubiquitin (Invitrogen), anti- Lys63 polyubiquitin, anti-GAPDH (Merck-Millipore), anti-α-tubulin (Sigma-Aldrich) anti-kidney injury marker-1 (R&D Systems) and polyclonal goat anti-rabbit and anti-mouse conjugated horse radish peroxidise (HRP) (Dako) antibodies were obtained commercially. Rabbit anti-Cezanne polyclonal antibodies were raised and subsequently affinity purified as described15. Pharmacological inhibitors of p38 (CT8730, UCB Celltech & Sb202190, Merck Chemical) and actinomycin D (Sigma-Aldrich) were obtained commercially and dissolved in DMSO.
Endothelial cells and exposure to hypoxia
Human coronary microvascular EC (HCMEC) and human dermal microvascular EC (HDMEC) were obtained commercially (Promocell). Human umbilical vein endothelial cells (HUVEC) were collected using collagenase. EC were cultured as described previously15. Confluent HUVEC cultures were exposed to 1% - 5% O2 using the Ruskinn Hypoxia Workstation prior to re-oxygenation (20% O2; normoxia) as described9. Hypoxia is defined as exposure of cells to 1% O2 unless stated otherwise.
RNA interference
Cell cultures were transfected with siRNA sequences that are known to silence Cezanne (5’-GAAUCUAUCUGCCUUUGGA-3’; Dharmacon14 or SMARTpool ON- TARGETplus Human Otud7b siRNA), ATF2 (Dharmacon or SMARTpool ON-TARGETplus Human ATF2 siRNA) or p38α (Dharmacon) using the Neon transfection system (Life Technologies) and were then incubated in antibiotic-free growth medium for 48 h before analysis. Alternatively, they were transfected with non-targeting scrambled controls (Silencer Negative control # 1 siRNA; Ambion). To control for the possible effects of mRNA processing, cells were transfected with siRNA targeting SHP2 (Dharmacon) which is unlikely to be involved in TRAF signalling.
Comparative real time PCR
RNA was extracted using the EZNA Total RNA Kit I (Omega Bio- tek) and reverse transcribed into cDNA using qScript cDNA Supermix (Quanta Biosciences). Transcript levels were quantified by comparative real-time PCR using gene-specific primers for human Cezanne (sense, 5’-ACAATGTCCGATTGGCCAGT-3’; antisense, 5’- ACAGTGGGATCCACTtCaCATTC-3’), human E-selectin (sense, 5’- GCTCTGCAGCTCGGACAT-3’; antisense, 5’-GAAAGTCCAGCTACCAAGGGAAT-3’), human VCAM-1 (sense, 5’- GGTGGGACACAAATAAGGGTTTTGG -3’; antisense, 5’- CTTGCAATTCTTTTACAGCCTGCC-3’), human ICAM-1 (sense, 5’- GTCCCCTCAAAAGTCATCC-3’; antisense, 5’-AACCCCATTCAGCGTCACCT-3’), human β- actin (sense, 5’-CtGGAACGGTGAAGGTGACA-3’; antisense, 5’- AAGGGACTTCCTGTAACAATGCA-3’), murine VCAM-1 (sense, 5’- GTCAAAGAGGGAGACACCGT-3’; antisense, 5’-CGAGCCATCCACAGACTTTA-3’), murine E-selectin (sense, 5’-TTCGTGTaCcAATGCATCCT-3’; antisense, 5’- GGCTTCCATAGTCAGGGTGT-3’), murine ß-actin (sense, 5’-AGCGCAAGTACTCTGTGTGG- 3’; antisense, 5’-CTTGCTGAtCcaCAtCtGCT-3’), rat/murine Cezanne (sense, 5’- GGTTGGCAGCAGTTCTATCA-3’; antisense, 5’-CAAAGCTGCCCAGTTTGTTA-3’) and rat ß- actin (sense, 5’-aGcGCAAGTACTCTGTGTGG-3’; antisense, 5’- CTTGCTGATCCACATCTGCT-3’) using PerfeCTa SYBR Green Supermix (Quanta Biosciences) and the CFX96 Real-Time PCR Detection System (BioRad). Reactions were performed in triplicate. Relative gene expression was calculated by comparing the number of thermal cycles that were necessary to generate threshold amounts of product. Data were pooled from three independent experiments and mean values were calculated with standard deviations.
Western blotting
Levels of particular proteins were measured in cytosolic or nuclear lysates prepared using the Nuclear Extraction Kit (Active Motif) by Western blotting using specific primary antibodies, HRP-conjugated secondary antibodies and chemiluminescent detection.
Immunoprecipitation of TRAF6
Cells were lysed using 30 mM Tris-HCl (pH 7.6), 120 mM NaCl, 1% Triton X-100, 2 mM KCl, 2 mM EDTA, 10% glycerol and Complete Protease Inhibitor Cocktails (Roche). Lysates were clarified by low-speed centrifugation and pre-cleared using protein-G-sepharose before immunoprecipitation using anti-TRAF6 antibodies. Beads were then washed extensively using lysis buffer. Precipitated material or lysates were analysed by Western blotting.
Animals
Male Fisher rats (F344, RT1lvl) (170 to 210 g) were obtained commercially (Charles River, Sulzfeld, Germany). Transgenic ‘gene-trapped’ (GT) mice22 in which the Cezanne gene is disrupted by removal of exons 4-7 and insertion of a splice acceptor site, LacZ open reading frame, neomycin resistance gene (Neo) and polyA tail in the third intron (B6-Otud7btml(NCOM)Cmhd; CezanneGT/GT; C57BL/6 background) were obtained from the Canadian Mouse Mutant Repository. Splicing of the LacZ-Neo-polyA sequences to the third exon of Cezanne was confirmed by RT-PCR (Online Fig. I) and is predicted to generate a fusion protein comprising the peptide MTLDMDAVLSDFVRSTGAEPGLARDLLE (encoded by exon 3 of Cezanne) linked to LacZ. Baseline phenotyping analysis carried out at the Toronto Centre for Phenogenomics as part of the North American Conditional Mouse Mutagenesis Project revealed that CezanneGT/GT mice are normal in terms of gross appearance, histopathology of multiple tissues, weight gain, glucose tolerance and clinical chemistry (http://www.europhenome.org).
Renal ischemia/reperfusion
For the induction of renal ischemia/reperfusion in rats, the abdomen was opened under inhalation anesthesia using isofluorane. The left renal artery was clamped with an atraumatic vascular clamp for 45 min. During this time the right kidney was removed. The left kidney was reperfused for 2-72 h. Renal ischemia/reperfusion in mice was carried out under inhalation anesthesia using isofluorane. In wild-type and Cezanne GT/GT mice, the left renal artery was clamped with an atraumatic vascular clamp for 45 min and subsequently reperfused for 6 h. Ischemia and subsequent reperfusion were confirmed at a macroscopic level by monitoring changes in tissue appearance. Principles of NIH Guide for the Care and Use of Laboratory Animals as well as the German Law on the Protection of Animals and UK Home Office regulations were followed.
Immunohistochemistry and morphological studies
Sections made from formalin-fixed, paraffin-embedded tissues were incubated in xylene for 5 min, hydrated by sequential exposure to decreasing concentrations of ethanol (100% to 50%) and water. Heat-mediated antigen retrieval was carried out in tris-sodium citrate in a standard microwave. The sections were then blocked for endogenous peroxidase activity and incubated in 20% goat serum prior to overnight incubation with primary antibodies followed by HRP-conjugated secondary antibodies and substrate (EnVision™+ Kits; Dako). Sections were then counterstained with hematoxylin and visualized by bright field microscopy. For morphological studies, sections were stained with hematoxylin and eosin prior to histological assessment. Tissue injury was assessed independently by two experienced renal researchers and classified as healthy or mild/moderate/severe injury as we described9. Granulocytes were identified by staining using the napthol AS-D chloroacetate esterase kit (Sigma).
Statistics
Differences between samples were analysed using an unpaired Student’s t-test or ANOVA (*p<0.05, **p<0.01, ***p<0.001).
Results
Hypoxia induces Cezanne via transcriptional and post-transcriptional mechanisms
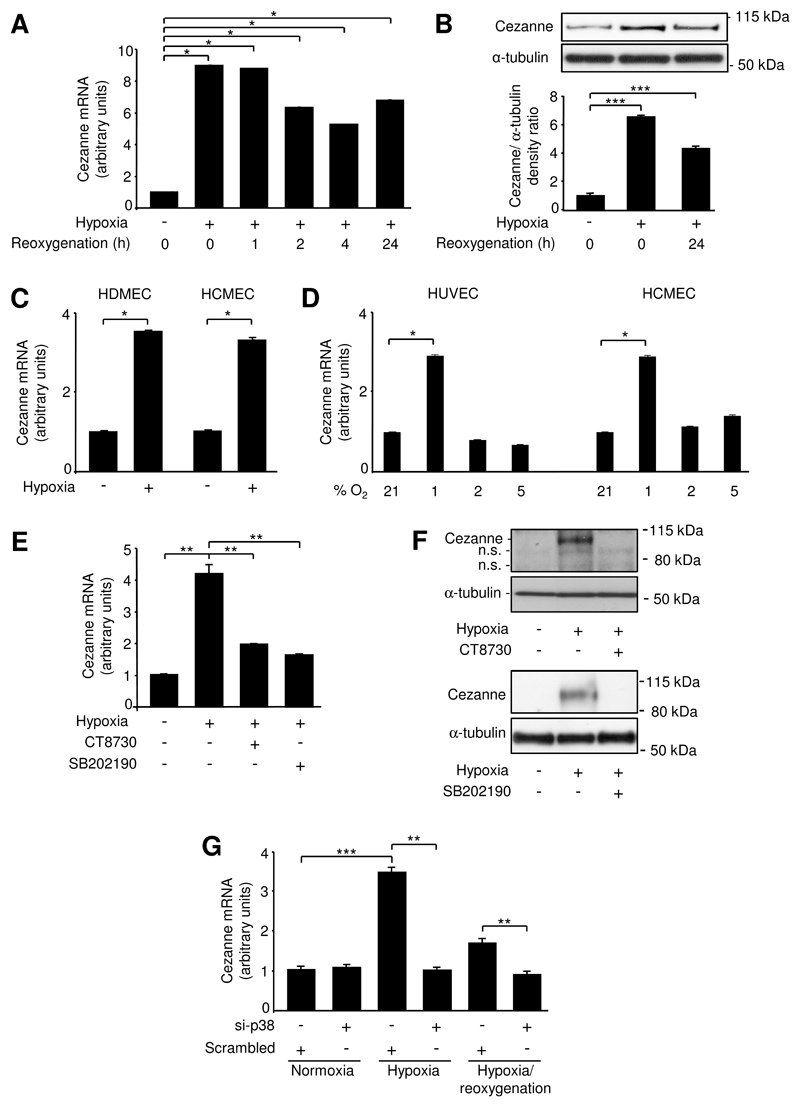
Studies of cultured HUVEC revealed that Cezanne mRNA and protein levels were enhanced by exposure to 1% O2 and remained elevated for at least 24 h following reoxygenation (Fig. 1A,B). This level of hypoxia (1% O2) also induced Cezanne in cultured microvascular EC at both the mRNA (Fig. 1C) and protein levels (Online Fig. II). By contrast, exposure of EC to 2% or 5% O2 had little or no effect on Cezanne expression in HUVEC or HCMEC (Fig. 1D). Thus cellular responses to hypoxia were studied using 1% O2 conditions in all subsequent experiments.
Figure 1. Hypoxia induced Cezanne via p38.
(A,B) HUVEC were exposed to hypoxia (4 h) or hypoxia followed by reoxygenation (1-24 h) or remained untreated. (A) Cezanne transcript levels were quantified by real-time PCR. Data were pooled from 3 independent experiments. (B) Cytosolic lysates were tested by Western blotting using anti-Cezanne antibodies and by using anti-α-tubulin antibodies to assess total protein levels. Representative blots (upper panels) and results from densitometry analysis of five experiments (lower panel) are shown. (C) HDMEC or HCMEC were exposed to hypoxia (4 h) or remained untreated. Cezanne transcript levels were quantified by real-time PCR. Data were pooled from 3 independent experiments. (D) HUVEC or HCMEC were exposed to 1%, 2%, 5% or normoxia (21%) for 4 h. Cezanne transcript levels were quantified by real-time PCR. Data were pooled from 3 independent experiments. (E,F) HUVEC were exposed to CT8730 (1 μM) or SB202190 (50 μM) for 1 h or were treated with vehicle alone and then exposed to hypoxia (4 h) or remained untreated. (E) Cezanne transcript levels were quantified by real-time PCR. (F) Cytosolic lysates were tested by Western blotting using anti-Cezanne antibodies and by using anti-α-tubulin antibodies to assess total protein levels. n.s., non-specific band. (G) HUVEC were treated with p38α-specific siRNA (si-p38) or with a scrambled, non-targeting sequence and were then exposed to hypoxia (4 h) or hypoxia (4 h) followed by reoxygenation (4 h) or remained untreated (normoxia). Cezanne transcript levels were quantified by real-time PCR.
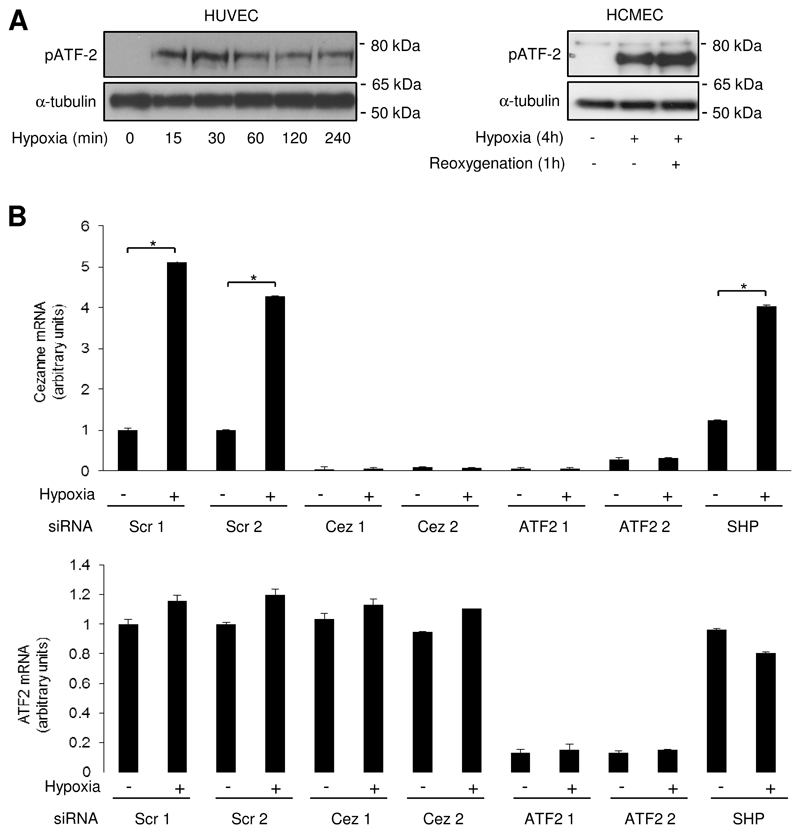
We studied the molecular mechanism for Cezanne induction and focussed on the role of p38 MAP kinase and the transcription factor HIF1α, which are known to be activated by hypoxia8,23,24. Activation of HIF1α in response to dimethyloxallyl glycine, desferrioxamine or CoCl2 did not influence Cezanne expression (Online Fig. III) and silencing of HIF1α or HIF2α did not suppress Cezanne expression in hypoxic EC (Online Fig. IV), indicating that induction of Cezanne by hypoxia is HIF-independent. By contrast, pharmacological inhibition of p38 using either CT8730 or SB202190 (Fig. 1E,F) or gene silencing of p38α (Fig. 1G) suppressed the induction of Cezanne by hypoxia, indicating that p38 positively regulates Cezanne expression. We examined the mechanism of Cezanne induction by p38, which is known to influence gene expression via members of the activating transcription factor (ATF) family and also by enhancing mRNA stability25. We observed that ATF2 was phosphorylated in response to hypoxia in both HUVEC and microvascular EC (Fig. 2A). Thus, gene silencing studies were carried out to assess the function of ATF2 in hypoxic EC. We observed that silencing of ATF2 using two different siRNA sequences suppressed the induction of Cezanne by hypoxia (Fig. 2B). By contrast, transfection using non-targeting scrambled sequences or using sequences that target an irrelevant mRNA (SHP2) demonstrated that transfection and mRNA processing per se did not alter Cezanne induction by hypoxia (Fig. 2B). Finally, Cezanne expression was reduced by two different siRNAs designed to target Cezanne, thus validating the quantitative real-time PCR readout (Fig. 2B). Thus we conclude that ATF2 positively regulates Cezanne expression in hypoxic EC. p38 also enhanced the stability of Cezanne transcripts in hypoxic EC as pharmacological inhibition of p38 using SB202190 destabilized Cezanne mRNA in actinomycin D chase experiments (Online Fig. V). Thus we conclude that p38 induces Cezanne through both transcriptional and post-transcriptional mechanisms.
Figure 2. Hypoxia induced Cezanne via ATF2.
(A) HUVEC or HCMEC were exposed to hypoxia for 15 min-4 h or remained untreated (normoxia). Some cultures were then reoxygenated for 1 h. Cytosolic lysates were tested by Western blotting using anti-phosphorylated ATF2 antibodies or by using anti-α-tubulin antibodies to assess total protein levels. Data are representative of three independent experiments. (B) HUVEC were treated with siRNA sequences that target Cezanne (Cez 1, Dharmacon; Cez 2, SMARTpool), ATF2 (ATF2 1, Dharmacon; ATF2 2, SMARTpool) or SHP2, or with scrambled, non-targeting sequences as a control (Scr). They were then exposed to hypoxia (4 h) or remained untreated (normoxia). Levels of Cezanne or ATF2 were quantified by real-time PCR. Data were pooled from 3 independent experiments.
Cezanne suppresses inflammatory activation in response to hypoxia-reoxygenation by inhibiting RelA phosphorylation
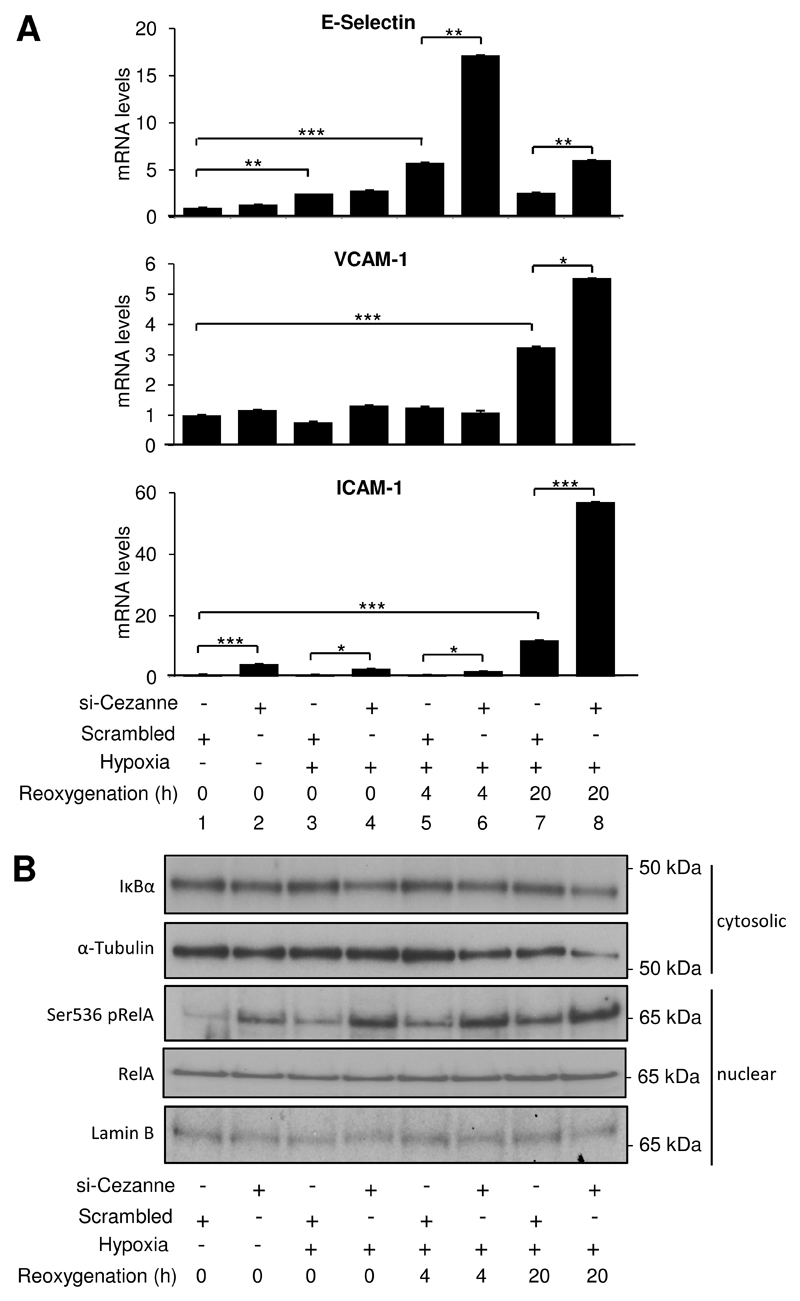
We used a previously validated RNA interference method (15 and Fig. 2B) to examine whether Cezanne regulates pro-inflammatory responses to hypoxia-reoxygenation. Silencing of Cezanne in HUVEC had no effect on basal levels of E-selectin or VCAM-1 in unstimulated cells (Fig. 3A, compare 1 & 2) but enhanced their subsequent induction by hypoxia-reoxygenation (compare 5 & 6 and 7 & 8). By contrast, silencing of Cezanne enhanced the expression of ICAM-1 in EC exposed to normoxia or hypoxia-reoxygenation (Fig. 3A, lower panel). Silencing of Cezanne using a pool of siRNA sequences from an alternative source also significantly enhanced the induction of E-selectin, VCAM-1 and ICAM-1 in EC exposed to hypoxia-reoxygenation (Online Fig. VI).
Figure 3. Cezanne suppressed inflammatory activation in response to hypoxia-reoxygenation.
HUVEC were treated with Cezanne-specific siRNA (si-Cezanne from Dharmacon) or with a scrambled, non-targeting sequence and were then exposed to hypoxia (4 h) or hypoxia (4 h) followed by reoxygenation (4-20 h) or remained untreated. (A) Levels of E-selectin, VCAM- 1 or ICAM-1 transcripts were quantified by real-time PCR. Data were pooled from three independent experiments. (B) Cytosolic or nuclear lysates were tested by Western blotting using anti-IκBα, anti-RelA or anti-Ser536 phosphorylated RelA antibodies and by using anti-α-tubulin or anti-Lamin B antibodies to assess total protein levels. Data are representative of three independent experiments.
As E-selectin, VCAM-1 and ICAM-1 are induced by NF-κB, we examined whether Cezanne influences the activation of this transcription factor in response to hypoxia. Exposure of EC to hypoxia-reoxygenation enhanced Ser536 phosphorylation of RelA NF-κB sub-units but did not influence IκBα stability or nuclear localization of NF-κB (Fig. 3B). Thus hypoxia-reoxygenation activates NF-κB in EC through a non-canonical pathway that involves RelA phosphorylation. Although silencing of Cezanne led to a modest reduction in IκBα expression (Fig. 3B, upper panel), the biological significance is questionable since there was no noticeable effect of Cezanne siRNA on NF-κB nuclear localisation (Fig. 3B, lower panels). By contrast, silencing of Cezanne led to a pronounced elevation in Ser536 phosphorylation of RelA in cells exposed to hypoxia-reoxygenation (Fig. 3B, centre panel), indicating that endogenous Cezanne negatively regulates NF-κB activation and pro-inflammatory transcriptional responses under these conditions.
Cezanne prevents Lys-63 polyubiquitination of TRAF6 in response to hypoxia-reoxygenation
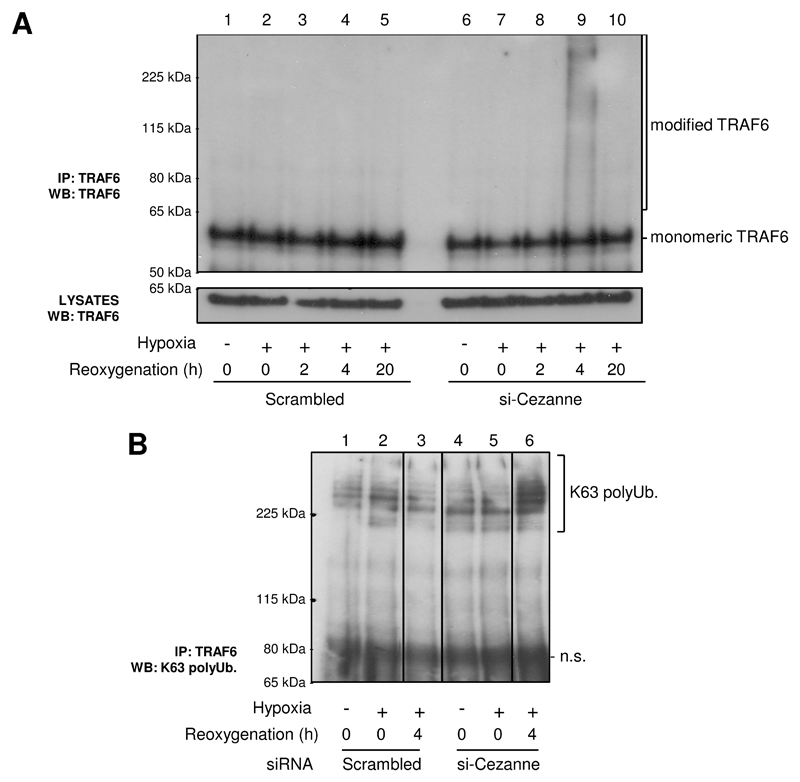
We examined whether Cezanne influences Lys63-polyubiquitination of TRAF6, a modification that promotes NF-κB activation in response to hypoxia12. Analysis of TRAF6 immunoprecipitates revealed that hypoxia-reoxygenation induced high molecular weight forms of TRAF6 that co-precipitated with Lys63 polyubiquitin in EC that were pre-treated with Cezanne-specific siRNA but not in control cultures (Fig. 4A, compare 4 and 9; Fig. 4B, compare 3 and 6). Kinetic studies demonstrated that hypoxia-reoxygenation for 4-8 h led to modification of TRAF6 in the absence of Cezanne, whereas earlier (2 h) and later time points (16-24 h) had little or no effect (Online Fig. VII). It is plausible that modification of TRAF6 requires at least 4 h exposure to hypoxia due to the requirement of de novo synthesis of a component of the ubiquitination machinery. These data indicate that endogenous Cezanne suppresses TRAF6 polyubiquitination in response to hypoxia-reoxygenation.
Figure 4. Cezanne suppressed TRAF6 polyubiquitination in response to hypoxia-reoxygenation.
HUVEC were treated with Cezanne-specific siRNA (si-Cezanne from Dharmacon) or with a scrambled, non-targeting sequence and were then exposed to hypoxia (4 h) or hypoxia (4 h) followed by reoxygenation (2-20 h) or remained untreated. (A) TRAF6 immunoprecipitates or lysates were tested by Western blotting using anti-TRAF6 antibodies. (B) TRAF6 immunoprecipitates were tested by Western blotting using antibodies that recognise Lys63-polyubiquitin. n.s., non-specific. Data are representative of three independent experiments that gave closely similar results.
Cezanne reduces inflammation and injury in response to ischemia-reperfusion
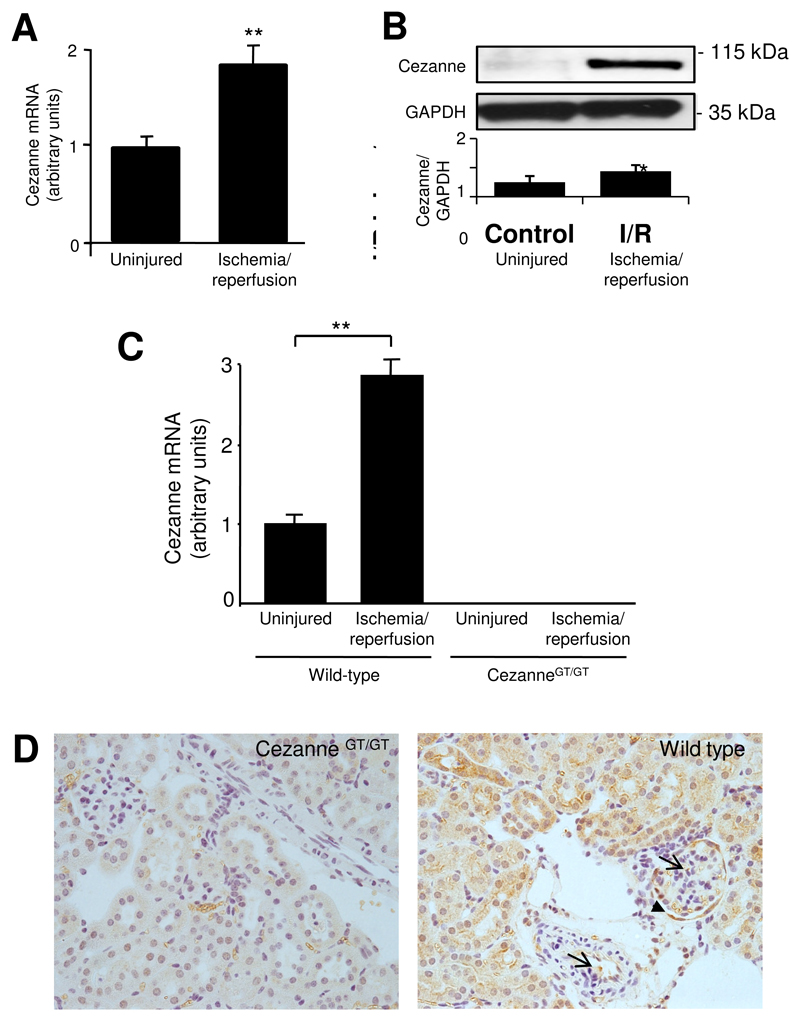
Our finding that Cezanne can be induced by hypoxia in vitro led us to examine the expression of Cezanne in tissues exposed to ischemia in vivo. We observed by quantitative RT-PCR (Figs. 5A and 5C) and Western blotting (Fig. 5B) that Cezanne was induced in rat or murine kidneys exposed to ischemia-reperfusion. Immunohistochemistry was carried out to assess the cellular localization of Cezanne expression in ischemic tissues. This was performed exclusively using the murine model so that tissues from CezanneGT/GT mice could be used to control for staining specificity. Immunohistochemistry using anti-Cezanne antibodies revealed staining in vascular EC, glomerular EC, podocytes and tubular epithelial cells in wild-type mice exposed to ischemia-reperfusion, whereas tissues from CezanneGT/GT mice were negative (Fig. 5D). Thus we conclude that ischemia-reperfusion induces Cezanne in multiple cell types in the kidney including EC.
Figure 5. Cezanne was induced by renal ischemia in vivo.
(A,B) Expression levels of Cezanne were assessed in left kidneys of male Fisher rats (n=4) that were exposed to ischemia followed by reperfusion. Basal levels of Cezanne were measured in the uninjured right kidney of each animal. (A) Cezanne transcript levels were quantified in kidneys exposed to ischemia-reperfusion (6 h) and uninjured kidneys by real-time PCR and normalised by quantifying β-actin transcripts. (B) Lysates from kidneys exposed to ischemia-reperfusion (6 h) or uninjured kidneys were tested by Western blotting using anti-Cezanne antibodies or by using anti-α-tubulin antibodies to assess total protein levels. A representative blot and results from densitometry analysis are shown. (C,D) Left kidneys of wild-type or CezanneGT/GT mice (n=6 per group) were exposed to ischemia followed by reperfusion for 6 h whereas contralateral kidneys were uninjured. (C) Cezanne transcript levels were quantified in kidneys exposed to ischemia-reperfusion and uninjured kidneys by real-time PCR and normalised by quantifying β-actin transcripts. (D) Cezanne expression was assessed by immunohistochemistry in kidneys exposed to ischemia-reperfusion. Positive staining was observed in wild-type tissues in vascular and glomerular EC (arrows), in podocytes (arrowhead) and epithelial cells.
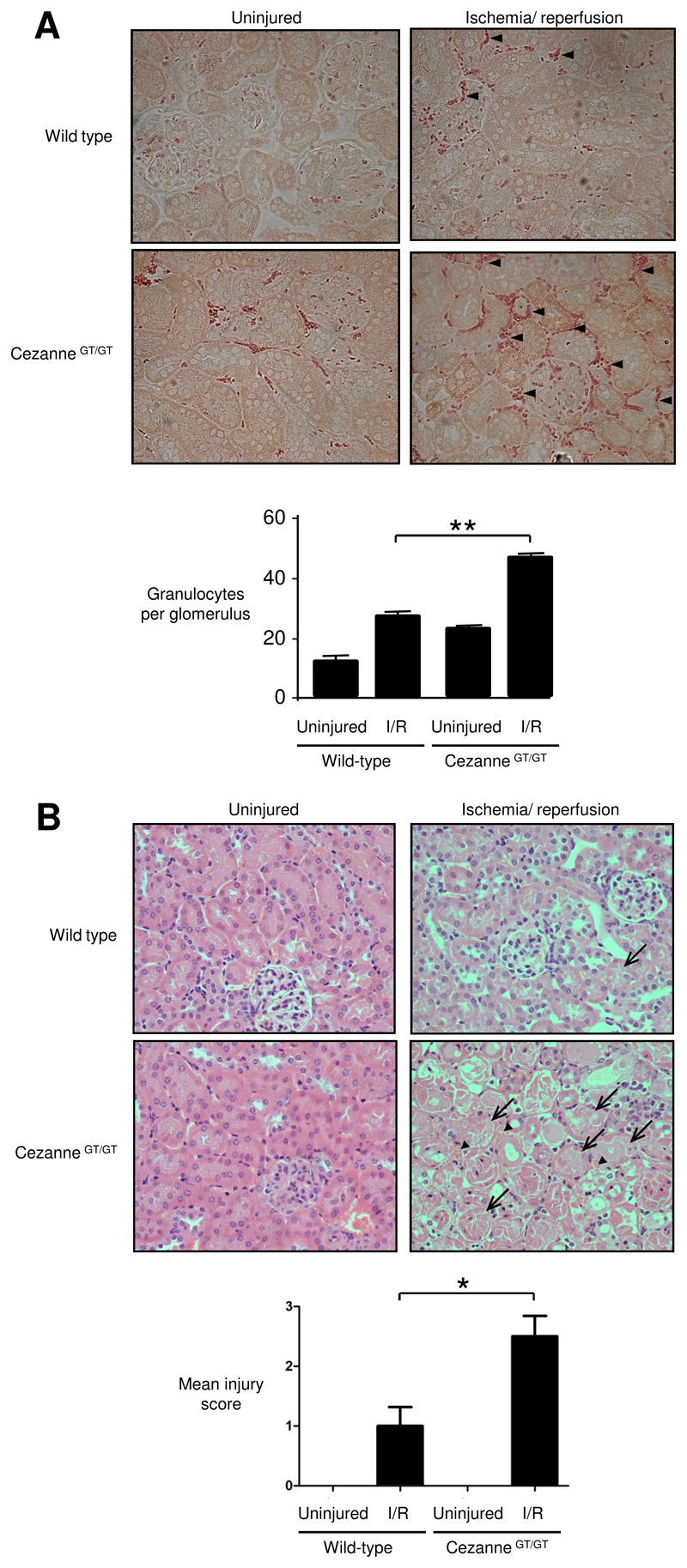
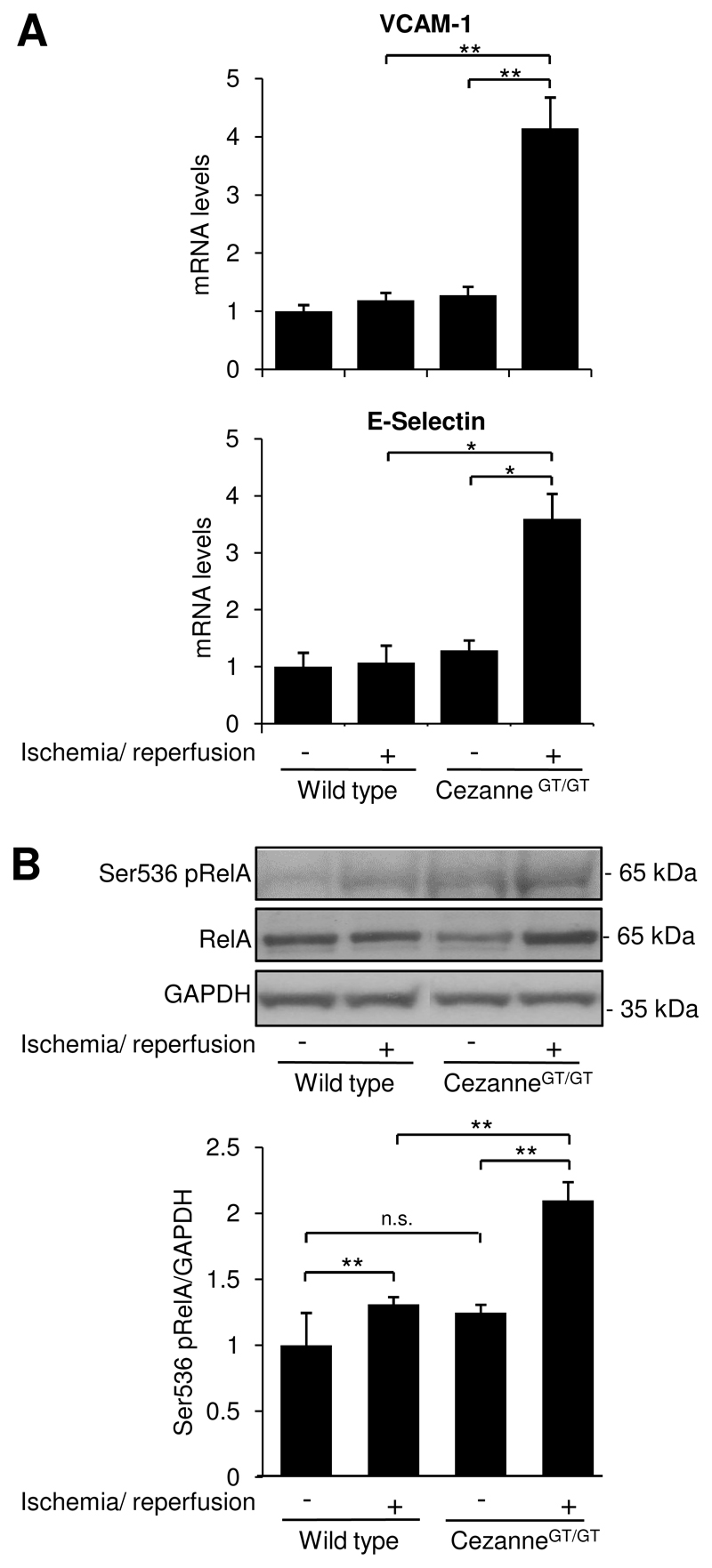
The influence of Cezanne on NF-κB activation, inflammation and injury was assessed by comparing responses to ischemia-reperfusion in wild-type and CezanneGT/GT mice. In the first instance, we demonstrated by quantitative RT-PCR that Cezanne was expressed in several tissues in wild-type mice (highest in brain) but was absent from peripheral blood cells (Online Fig. VIII). Renal ischemia-reperfusion promoted the accumulation of granulocytes (Fig. 6A) and enhanced tissue injury (Fig. 6B and Online Fig. IX) in kidneys of wild-type mice. Of note, granulocyte accumulation and tissue injury in response to ischemia-reperfusion were significantly greater in CezanneGT/GT mice compared to wild-type animals (Fig. 6 and Online Fig. IX). Similarly, expression of VCAM-1 and E-selectin (Fig. 7A) and Ser536 phosphorylation of RelA in response to ischemia-reperfusion were enhanced in CezanneGT/GT mice compared to wild-type animals (Fig. 7B). Thus we conclude that ischemia induces local expression of Cezanne which protects the kidney from NF-κB-dependent inflammation and injury in response to reperfusion.
Figure 6. Cezanne reduced inflammation and injury in response to ischemia-reperfusion.
Left kidneys of wild-type or CezanneGT/GT mice (n=6 per group) were exposed to ischemia followed by reperfusion for 6 h whereas contralateral kidneys were uninjured. (A) Granulocytes were identified by napthol AS-D chloroacetate esterase staining. Representative images are shown and positive cells are indicated (arrow heads) (upper panels). Granulocytes were counted manually and data were pooled from multiple animals per group (lower panels). (B) Tissue sections were stained with hematoxylin and eosin prior to histological assessment. Representative images are shown with areas of acute tubular necrosis (arrows) and haemorrhage (arrow heads) indicated (upper panels). Quantitative assessment of tissue injury was carried out by two experienced renal researchers and average scores are shown (lower panels). 0, healthy; 1, mild injury; 2, moderate injury; 3, severe injury.
Figure 7. Cezanne suppressed NF-κB activation and adhesion molecule expression in response to ischemia-reperfusion.
Left kidneys of wild-type or CezanneGT/GT mice (n=6 per group) were exposed to ischemia followed by reperfusion for 6 h whereas contralateral kidneys were uninjured. (A) Levels of VCAM-1 or E-selectin transcripts were quantified by real-time PCR. (B) Lysates were tested by Western blotting using anti-RelA or anti-Ser536 phosphorylated antibodies or by using anti-GAPDH antibodies to assess total protein levels. Representative blots and results from densitometry analysis are shown.
Discussion
Recent studies have shown that the assembly of polyubiquitin chains on specific signalling intermediaries promotes signalling to NF-κB in response to several stimuli including cytokines, microbial products and hypoxia12,26. Our previous studies revealed that Cezanne is a deubiquitinating enzyme that suppresses NF-κB activation in response to TNFα or IL-1 by removing polyubiquitin chains from signalling intermediaries15,18. Here we demonstrate that Cezanne can also inhibit NF-κB-dependent inflammatory activation in response to hypoxia- reoxygenation by reducing Lys63 polyubiquitination of TRAF6. To our knowledge, Cezanne is the first example of a deubiquitinating enzyme that controls inflammatory responses to hypoxia.
Inflammatory signalling activates multiple delayed negative feedback loops that precisely control the kinetics of NF-κB and MAP kinase activation. That is, NF-κB induces molecules that feedback to inhibit signalling to NF-κB (e.g. IκBα, A20, Cezanne15,27,29) and/or suppress the activity of inflammatory MAP kinases (e.g. MKP-1, GADD45ß30,31). Here we describe a novel form of cross-talk between the NF-κB and MAP kinase pathways where p38-dependent induction of Cezanne during hypoxia suppresses NF-κB activity. Importantly, although hypoxia primed EC for NF-κB activation, it simultaneously induced Cezanne which blunted signalling to NF-κB during reoxygenation. Thus hypoxia-reoxygenation had minimal effects on NF-κB activation and adhesion molecule expression in unmanipulated EC but significantly enhanced inflammatory activation following silencing of Cezanne. This feed-forward inhibition pathway differs from typical delayed feedback systems because here the ‘accelerator’ (signalling to NF- ĸB) and ‘brake’ (Cezanne) are applied simultaneously. TNFR signalling provides a classic example of simultaneous activation of positive and negative regulators as it activates a pro- apoptotic pathway and simultaneously signals to NF-κB which induces multiple anti-apoptotic molecules32,33. Thus, although TNFα can prime cells for apoptosis, the execution of TNFα- mediated apoptosis usually relies on additional factors that suppress NF-κB (e.g. viral infection34). By analogy, although hypoxia can prime EC for inflammatory activation, the expression and/or activity of Cezanne determines whether inflammatory signalling proceeds in response to reoxygenation. Given that Cezanne can be modulated by shear stress, TNFα, IL-1 and reactive oxygen species15,16, future studies should address whether these factors influence inflammatory responses to hypoxia-reoxygenation by altering the expression and/or activity of Cezanne.
We translated our in vitro findings to a murine model by demonstrating that renal inflammation and injury in response to ischemia/reperfusion is enhanced by genetic deletion of Cezanne. Although several papers have studied the function of Cezanne in cultured cells, this is the first demonstration that Cezanne protects against inflammation and injury in vivo. Since Cezanne was shown to be expressed in renal tissues but not in peripheral blood, we suggest that local expression of this molecule is responsible for protection. However, we cannot rule out the possibility of systemic effects entirely since a recent study revealed that Cezanne influences B cell function17. Immunohistochemistry revealed that Cezanne was expressed in vascular and glomerular EC in ischemic kidneys as well as epithelial cells. Thus the function of Cezanne identified in ischemic kidneys may be due, in part, to its induction in EC in response to hypoxia. The expression of Cezanne in ischemic tissues may also be related to inflammatory cytokines which are known to induce Cezanne in cultured cells15. Future studies using tissue-specific conditional knockout mice should now be carried out to define the specific cell populations that are regulated by Cezanne during ischemia. Further studies are also required to examine whether Cezanne is a pleiotropic molecule that possesses divergent functions since baseline phenotyping studies revealed that Cezanne knockout mice have a reduced startle response and lower serum cholesterol levels (http://www.europhenome.org).
Our observation that endogenous Cezanne can reduce inflammation and injury in response to ischemia-reperfusion has important implications for therapeutic targeting of inflammation during transplantation of vascularised organs, bypass grafting, percutaneous coronary intervention of arteries and other procedures that involve reperfusion. Future studies should examine the potential role of Cezanne induction in ischemic preconditioning, a procedure that is known to protect tissues from subsequent inflammation and injury in response to ischemia-reperfusion35. In addition, strategies to enhance Cezanne expression (e.g. pharmacologically) in ischemic tissues may inform the development of novel therapies to reduce ischemia-reperfusion injury.
Acknowledgements
The study was funded by Kidney Research UK and the British Heart Foundation. We thank the Canadian Mouse Mutant Repository, Toronto Centre for Phenogenomics and the North American Conditional Mouse Mutagenesis Project for providing CezanneGT/GT mice and baseline phenotyping data. We also acknowledge Dr Lauryl Nutter (Toronto Centre for Phenogenomics) for assistance in accessing phenotyping data and Amy Lewis and Monica Neilan (University of Sheffield, UK) for technical support. The authors do not have conflicting financial interests.
Non-Standard Abbreviations
- EC
endothelial cells
- MAP kinase
mitogen-activated protein kinase
- NF-κB
nuclear factor κB
- IκB
inhibitor of κB
- TRAF6
tumour necrosis factor receptor associated factor 6
- IKK
IκB kinase
References
- 1.Perico N, Cattaneo D, Sayegh MH, Remuzzi G. Delayed graft function in kidney transplantation. Lancet. 2004;364:1814–1827. doi: 10.1016/S0140-6736(04)17406-0. [DOI] [PubMed] [Google Scholar]
- 2.Prasad A, Stone GW, Holmes DR, Gersh B. Reperfusion Injury, Microvascular Dysfunction, and Cardioprotection The “Dark Side” of Reperfusion. Circulation. 2009;120:2105–2112. doi: 10.1161/CIRCULATIONAHA.108.814640. [DOI] [PubMed] [Google Scholar]
- 3.Oliver KM, Garvey JF, Ng CT, Veale DJ, Fearon U, Cummins EP, Taylor CT. Hypoxia Activates NF-kappa B-Dependent Gene Expression Through the Canonical Signaling Pathway. Antioxid Redox Signal. 2009;11:2057–2064. doi: 10.1089/ars.2008.2400. [DOI] [PubMed] [Google Scholar]
- 4.Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, Seeballuck F, Godson C, Nielsen JE, Moynagh P, Pouyssegur J, Taylor CT. Prolyl hydroxylase-1 negatively regulates I kappa B kinase-beta, giving insight into hypoxia-induced NF kappa B activity. Proc Natl Acad Sci USA. 2006;103:18154–18159. doi: 10.1073/pnas.0602235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Natarajan R, Fisher BJ, Jones DG, Ghosh S, Fowler AA. Reoxygenating microvascular endothelium exhibits temporal dissociation of NF-kappa B and AP-1 activation. Free Radic Biol Med. 2002;32:1033–1045. doi: 10.1016/s0891-5849(02)00813-4. [DOI] [PubMed] [Google Scholar]
- 6.Walmsley SR, Print C, Farahi N, Peyssonnanx C, Johnson RS, Cramer T, Sobolewski A, Condliffe AM, Cowburn AS, Johnson N, Chilvers ER. Hypoxia-induced neutrophil survival is mediated by HIF-1 alpha-dependent NF-kappa B activity. J Exp Med. 2005;201:105–115. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. NF-kappa B links innate immunity to the hypoxic response through transcriptional regulation of HIF-1 alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan S, McNicholas WT, Taylor CT. A critical role for p38 map kinase in NF-kappa B signaling during intermittent hypoxia/reoxygenation. Biochem Biophys Res Comm. 2007;355:728–733. doi: 10.1016/j.bbrc.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Lutz J, Luong LA, Strobl M, Deng M, Huang H, Anton M, Zakkar M, Enesa K, Chaudhury H, Haskard DO, Baumann M, et al. The A20 gene protects kidneys from ischaemia/reperfusion injury by suppressing pro-inflammatory activation. J Mol Med. 2008;86:1329–1339. doi: 10.1007/s00109-008-0405-4. [DOI] [PubMed] [Google Scholar]
- 10.Van der Heiden K, Cuhlmann S, Luong LA, Zakkar M, Evans PC. Role of nuclear factor kappa B in cardiovascular health and disease. Clin Sci. 2010;118:593–605. doi: 10.1042/CS20090557. [DOI] [PubMed] [Google Scholar]
- 11.Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. I kappa B kinases phosphorylate NF-kappa B p65 subunit on serine 536 in the transactivation domain. J Biol Chem. 1999;274:30353–30356. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- 12.An J, Mo D, Liu H, Veena MS, Srivatsan ES, Massoumi R, Rettig MB. Inactivation of the CYLD Deubiquitinase by HPV E6 Mediates Hypoxia-Induced NF-kappa B Activation. Cancer Cell. 2008;14:394–407. doi: 10.1016/j.ccr.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verhelst K, Carpentier I, Beyaert R. Regulation of TNF-induced NF-κB activation by different cytoplasmic ubiquitination events. Cytokine Growth Factor Rev. 2011;22:277–286. doi: 10.1016/j.cytogfr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Evans PC, Taylor ER, Coadwell J, Heyninck K, Beyaert R, Kilshaw PJ. Identification and characterization of two novel A20-like proteins. Biochem J. 2001;357:617–623. doi: 10.1042/0264-6021:3570617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enesa K, Zakkar M, Chaudhury H, Luong L, Rawlinson L, Mason JC, Haskard DO, Dean JLE, Evans PC. NF-kappaB suppression by the deubiquitinating enzyme Cezanne: a novel negative feedback loop in pro-inflammatory signaling. J Biol Chem. 2008;83:7036–7045. doi: 10.1074/jbc.M708690200. [DOI] [PubMed] [Google Scholar]
- 16.Enesa K, Ito K, Luong LA, Thorbjornsen I, Phua C, To Y, Dean J, Haskard DO, Boyle J, Adcock I, Evans PC. Hydrogen peroxide prolongs nuclear localization of NF-kappa B in activated cells by suppressing negative regulatory mechanisms. J Biol Chem. 2008;283:18582–18590. doi: 10.1074/jbc.M801312200. [DOI] [PubMed] [Google Scholar]
- 17.Hu H, Brittain GC, Chang J-H, Peubla-Osorio N, Jin J, Zal A, Xiao Y, Cheng X, Chang M, Fu Y-X, Zal T, et al. OTUD7B controls non-canonical NF-kB activation through deubiquitination of TRAF3. Nature. 2013;494:371–374. doi: 10.1038/nature11831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans PC, Smith TS, Lai MJ, Williams MG, Burke DF, Heyninck K, Kreike MM, Beyaert R, Blundell TL, Kilshaw PJ. A novel type of deubiquitinating enzyme. J Biol Chem. 2003;278:23180–23186. doi: 10.1074/jbc.M301863200. [DOI] [PubMed] [Google Scholar]
- 19.Evans PC, Ovaa H, Hamon M, Kilshaw PJ, Hamm S, Bauer S, Ploegh HL, Smith TS. Zinc-finger protein A20, a regulator of inflammation and cell survival, has de-ubiquitinating activity. Biochem J. 2004;378:727–734. doi: 10.1042/BJ20031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wertz IE, O’Rourke KM, Zhou HL, Eby M, Aravind L, Seshagiri S, Wu P, Wiesmann C, Baker R, Boone DL, Ma A, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappa B signalling. Nature. 2004;430:694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 21.Bremm A, Freund SMV, Komander D. Lys11-linked ubiquitin chains adopt compact conformations and are preferentially hydrolyzed by the deubiquitinase Cezanne. Nature Struc Mol Biol. 2010;17(8):939–947. doi: 10.1038/nsmb.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skarnes WC, von Melchner H, Wurst W, Hicks G, Nord AS, Cox T, Young SG, Ruiz P, Soriano P, Tessier-Lavigne M, Conklin BR, et al. A public gene trap resource for mouse functional genomics. Nat Genet. 2004;6:543–544. doi: 10.1038/ng0604-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seko Y, Takahashi M, Tobe K, Kadowaki T, Yazaki Y. Hypoxia and hypoxia/reoxygenation activate p65(PAK), p38mitogen-activated protein kinase (MAPK), and stress-activated protein kinase (SAPK) in cultured rat cardiac myocytes. Biochem Biophys Res Comm. 1997;239:840–844. doi: 10.1006/bbrc.1997.7570. [DOI] [PubMed] [Google Scholar]
- 24.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 25.Dean JLE, Brook M, Clark AR, Saklatvala J. p38 mitogen-activated protein kinase regulates cyclooxygenase-2 mRNA stability and transcription in lipopolysaccharide-treated human monocytes. J Biol Chem. 1999;274:264–269. doi: 10.1074/jbc.274.1.264. [DOI] [PubMed] [Google Scholar]
- 26.Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 27.Arenzanaseisdedos F, Thompson J, Rodriguez MS, Bachelerie F, Thomas D, Hay RT. Inducible Nuclear Expression of Newly Synthesized I-Kappa-B-Alpha Negatively Regulates Dna-Binding and Transcriptional Activities of Nf-Kappa-B. Mol Cell Biol. 1995;15:2689–2696. doi: 10.1128/mcb.15.5.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson DE, Ihekwaba ACE, Elliott M, Johnson JR, Gibney CA, Foreman BE, Nelson G, See V, Horton CA, Spiller DG, Edwards SW, et al. Oscillations in NF-kappa B signaling control the dynamics of gene expression. Science. 2004;306:704–708. doi: 10.1126/science.1099962. [DOI] [PubMed] [Google Scholar]
- 29.Opipari AW, Boguski MS, Dixit VM. The A20 cDNA Induced by Tumor Necrosis FactorAlpha Encodes A Novel Type of Zinc Finger Protein. J Biol Chem. 1990;265:14705–14708. [PubMed] [Google Scholar]
- 30.Chi HB, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM, Flavell RA. Dynamic regulation of pro-and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci USA. 2006;103:2274–2279. doi: 10.1073/pnas.0510965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Smaele E, Zazzeroni F Papa S, Nguyen DU, Jin RG, Jones J, Cong R, Franzoso G. Induction of gadd45 beta by NF-kappa B downregulates pro-apoptotic JNK signalling. Nature. 2001;414:308–313. doi: 10.1038/35104560. [DOI] [PubMed] [Google Scholar]
- 32.Micheau O Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 33.Karin M, Lin A. NF-kappa B at the crossroads of life and death. Nature Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 34.Viswanathan K, Frueh K, DeFilippis V. Viral hijacking of the host ubiquitin system to evade interferon responses. Curr Opin Microbiol. 2010;13:517–523. doi: 10.1016/j.mib.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joo JD, Kim M, D’Agati VD, Lee HT. Ischemic preconditioning provides both acute and delayed protection against renal ischemia and reperfusion injury in mice. J Am Soc Nephrol. 2006;17:3115–3123. doi: 10.1681/ASN.2006050424. [DOI] [PubMed] [Google Scholar]