Summary
Objective
Thiamin deficiency complicates severe Plasmodium falciparum malaria in Thailand and may contribute to acidosis. We therefore estimated the frequency of biochemical thiamin deficiency in patients presenting with uncomplicated falciparum malaria in southern Laos.
Methods
Red cell transketolase activation coefficients (a) were measured in 310 patients presenting with uncomplicated falciparum malaria and 42 days after starting treatment.
Results
Twelve per cent of patients had biochemical evidence of severe deficiency (a values >1.31) at presentation, declining to 3% 42 days later.
Conclusion
Thiamin deficiency was common in Lao patients admitted with uncomplicated P. falciparum infection and was reduced following treatment of malaria and multivitamin supplementation. The role of this preventable and treatable disorder in malaria and other acute infections, and the incidence of beriberi in rural Laos, needs further investigation.
Keywords: Plasmodium falciparum, malaria, beriberi, thiamin, Laos
Introduction
Plasmodium falciparum malaria remains a serious public health problem throughout most of the tropics. In the Western Pacific region there are an estimated 2.2 million cases and ~10 000 deaths per year (Hay et al. 2004). The Lao PDR (Laos) is currently changing uncomplicated falciparum malaria treatment policy from chloroquine, which has very poor efficacy, to artemisinin-based combination therapy (ACT) (Mayxay et al. 2004). Malnutrition is also a major public health problem in Laos (Phimmasone et al. 1996) and there is evidence that micronutrient deficiencies and malnutrition increase the burden of malaria morbidity and mortality (Caulfield et al. 2004).
An important but currently under-recognized nutritional deficiency in this region is beriberi, or clinical thiamin deficiency. Thiamin deficiency may present with a variety of clinical syndromes including peripheral neuropathy, myocardial dysfunction, encephalopathy and lactic acidosis. With the advent of mechanical rice milling in the late nineteenth century, beriberi became a dominant public health problem in south and southeast Asia, responsible for a considerable burden of mortality, especially amongst infants (Carpenter 2000; Thurnham 2005). With the identification of the etiology, changes in diet, supplementation and targeted health programmes to ensure adequate thiamin intake, the incidence of this disease in the more accessible, wealthier parts of Asia declined markedly and interest in the disease has waned. However, there have been recent reports suggesting that it remains an important public health problem in Asia, especially amongst relatively vulnerable people, such as refugees (World Health Organization 1999; McGready et al. 2001; Luxemburger et al. 2003), the elderly (Juguan et al. 1999) and infants (Luxemburger et al. 2003; Soukaloun et al. 2003). In Laos, beriberi was acknowl-edged as an important disease in the 1960s and 1970s (Prentice 1963; Pottier 1979) and was rediscovered in the early 1990s as a major cause of infant death in hospitals (Soukaloun et al. 2003).
Severe falciparum malaria is associated with metabolic acidosis, which arises as a consequence of tissue hypoxia, caused by obstruction of the microcirculation, anaemia and decreased hepatic clearance of lactate, and acute renal failure (Dondorp et al. 2004). In China 70 years ago, malaria and typhoid were thought to be associated with beriberi (Platt & Gin 1934; Ying 1934; Ching-Lang 1936; Neumann et al. 1979). More recently Krishna et al. (1999) hypothesized that, as thiamin deficiency can cause acidosis and that as the dietary conditions for beriberi are common in Asia, thiamin deficiency may contribute to the acidosis of malaria. Malaria increases glucose demand and requirement for lactate disposal, which are compromised by lack of thiamin, which is an essential co-factor for the pyruvate dehydrogenase complex.
Thiamin deficiency is conventionally assessed using functional assays for the thiamin-dependent red cell transketolase enzyme in ex vivo washed red cells. The activation coefficient (α) is the ratio of red cell transketolase activity after thiamin has been added to the basal in vitro activity before addition of thiamin. Higher coefficients represent greater thiamin deficiency. Krishna et al. (1999) demonstrated that adults presenting with malaria in western Thailand had higher α values than controls and that α values were higher in those with severe malaria than in those with uncomplicated malaria. α values were also significantly higher in those who died than in those who survived and in those with raised vs. normal plasma lactate. To determine whether thiamin deficiency occurs in uncomplicated falciparum in children and adults in Laos, we examined the frequency of thiamin deficiency among patients aged ≥1 year presenting with falciparum malaria in southern Laos.
Patients and methods
Study site, patients and clinical procedures
The study was conducted between June and October 2002 and 2003 at Phalanxay District Clinic, Savannakhet Province, southern Laos, in parallel with clinical trials of antimalarial treatment (see Mayxay et al. 2004) with 42-day follow-up. Patients presenting with symptoms and signs of acute uncomplicated malaria and a P.falciparum- positive blood smear were enrolled in the study provided that they, or their attending relatives, fulfilled the following criteria. (i) Gave fully informed written consent, (ii) had asexual P.falciparum density of 5000–200 000/μl, (iii) were not pregnant or lactating, (iv) were aged ≥1 year, (v) had axillary temperature ≥37.5 °C or history of fever in the previous 3 days and were likely to stay in hospital until parasite clearance and complete 42-days follow-up, (vi) had not taken a full course of any antimalarial in the previous 3 days, (vii) did not have signs of severe malaria (World Health Organization 2000) or a history of allergy or contraindication to the study drugs. At recruitment to the malaria trial and at follow-up, patients were not asked specifically about symptoms of beriberi, such as para-esthesia and oedema. Body mass index [BMI; weight in kg/(height in m)2] was calculated for adults >15 years and Z-scores of height for age for children ≤5 years (Anon 1995) were calculated using WHO Anthro 2005 (Anon 2005).
Venous blood was collected into sodium heparin tubes, centrifuged and washed in phosphate buffered saline three times immediately after collection, before treatment and after 42 days from the start of treatment. Washed red cell samples were stored at -30 °C for a maximum of 3 months and then at -70 °C until shipment to the UK on dry ice and analysis.
Patients were randomized to receive either chloroquine (CQ) 25 mg base/kg body weight over 3 days plus sulphadoxine-pyrimethamine 25 mg/1.25 mg/kg stat on the second day (CQ + SP) or artesunate 4 mg/kg/day for 3 days plus mefloquine 15 mg/kg on the second day and 10 mg/kg on the third day (MAS3) or coformulated artemether-lumefantrine (artemether 20 mg and lume-fantrine 120 mg per tablet) one dose every 12 h for 3 days (LAM3). Doses were one tablet for patients weighing less than 15 kg, two tablets for patients of 15–24 kg, three tablets for 25–34 kg and four tablets for ≥35 kg. Patients were also supplemented with one multivitamin tablet (containing thiamin 1 mg, B2 1 mg and B6 1 mg) and ferrous sulphate 200 mg/day for duration of follow-up, as is the standard practice at this hospital. Ethical clearance for the study was granted by the Ethical Committee of the Faculty of Medical Sciences, National University of Laos, and Oxford Tropical Research Ethics Committee, University of Oxford, UK.
Laboratory investigations
Parasite counts and haematocrit were performed daily until parasite clearance for two consecutive days and then weekly from day 7. Red cell transketolase activity assays were performed as described previously (see Krishna et al. 1999) by a modification of the nicotinamide-adenine dinucleotide-dependent method with ribose-5-phosphate as the substrate (Bayoumi & Rosalki 1976) except that samples were collected into acid citrate dextrose in the earlier study and into sodium heparin in this study. We have used categories of α of <1.15, 1.15–1.24, ≥1.25 and >1.31. Different upper reference ranges for α are described and we have used the more conservative α of >1.31 (Krishna et al. 1999) as defining definite severe biochemical deficiency. In support of this cutoff, all α measured from blood samples from 45 healthy UK volunteers (including 10 described by Krishna et al. 1999) were ≤1.31 [mean α (range) 1.16 (1.01-1.31)]. Plasmodium falciparum red cell parasitaemia in vitro and storage of ex vivo human samples for 18 months at -70 °C have been shown not to affect α values (Krishna et al. 1999). The samples were stored at -30 °C in the field for up to 3 months before being transferred to -80 °C. We then compared the α of aliquots, from the same blood samples, from patients recruited to a study of thiamin deficiency at Mahosot Hospital (unpublished) stored at these two temperatures for ≥5 months, assayed on the same day on the same run.
Statistical analysis
Data were analysed using Stata (v8, Stat Corp.). Comparisons between two groups were made by the Mann- Whitney U, Student’s paired and unpaired t, anova, chisquare, and Fisher’s exact tests, as appropriate.
Results
Patients
Of the 330 patients recruited to the clinical trial, 310 were eligible for and consented to participate in this study; 270 samples were available from patients on the day of admission and 270 from day 42 and 239 had paired samples. Clinical features, outcome measures and red cell transketolase data are presented in Table 1.
Table I. Admission demographic, clinical and laboratory details for patients with uncomplicated falciparum malaria in Laos stratified by red cell transketolase activity alpha (α).
| Variable | α on admission | α on day 42 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | α < 1.15 | α = 1.15-1.24 | α ≥ 1.25 | α > 1.31 | All | α < 1.15 | α = 1.15-1.24 | α ≥ 1.25 | α > 1.31 | |
| Number (%) | 270 | 103 (38) | 86 (32) | 81 (30) | 31 (12) | 270 | 130 (48) | 101 (37) | 39 (14) | 9 (3) |
| Age/years† | 11 (1-80) | 12 (1-80) | 8 (1-48) | 10 (1-78) | 12 (2-63) | 11 (1-80) | 12 (1-49) | 10 (1-80) | 11 (2-62) | 9 (2-62) |
| No. ≥I5 years (%) | 105 (39) | 48 (47) | 32 (37) | 25 (31) | 12 (39) | 108 (40) | 55 (42) | 39 (39) | 14 (36) | 4 (44) |
| No. <5 years (%) | 55 (20) | 15 (15) | 22 (26) | 19 (23) | <5 (19) | 51 (19) | 25 (19) | 20 (20) | «(15) | 3 (33) |
| Male (%) | 166 (62) | 63 (61) | 52 (60) | 51 (63) | 17 (55) | 168 (62) | 84 (65) | 63 (62) | 21 (54) | «(«7) |
| Days ill‡ | 9.9 (8.7-11.4) | 9.7 (7.7-12.3) | 9.4 (7.5-11.8) | 10.9 (8.6-13.9) | 10.2 (6.5-15.8) | 10.4 (9.1-12.0) | 11.4 (9.5-13.7) | 10.8 (8.5-13.7) | 7.0 (4.6-10.8) | 6.3 (2.6-15.2) |
| Prior malaria (%)¶ | 73 (27) | 28 (27) | 28 (33) | 17 (22) | 5 (18) | 81 (30) | 43 (34) | 28 (28) | 10 (26) | 3 (33) |
| BMI for adult ≥I5 years, kg/m2 * | 20.3 (19.8-20.8) | 20.5 (19.9-21.2) | 20.1 (19.2-21.1) | 20.0 (18.7-21.2) | 19.6 (17.5-21.8) | 20.3 (19.8-20.8) | 20.3 (19.5-21.2) | 20.6 (19.8-21.3) | 19.9 (18.7-21.1) | 19.4 (17.4-21.4) |
| Children ≤5 years HAZ† | -1.31 (-5.17-7.27) | -2.18 (-5.17-3.88) | -1.1 (5.17-3.49) | -1.19 (-5.18-7.27) 0.47 (-1.97-1.38) | -1.03 (-5.18-7.27) | -0.67 (-4.55-7.27) | -1.59 (-5.17-1.63) | -0.32 (-5.18-3.43) 1.94 (1.06-3.43) | ||
| Per cent with HAZ<-2 | 38 | 53 | 38 | 25 | 0 | 30 | 31 | 33 | 14 | 0 |
| Haematocrit (%)† | 36 (18-50) | 36 (21-47) | 35 (22-45) | 35 (18-50) | 36 (27-50) | 36 (18-50) | 37 (18-50) | 35 (21-50) | 36 (26-44) | 37 (27-44) |
| Parasitaemia (/μl)‡ | 26 126 (23 297-29 699) | 23 233 (19 368-27 868) | 32 986 (26 913-40 428) | 23 680 (19 134-29 307) | 18 387 (13 035-25 938) | 26 740 (23 900-29 917) | 23 918 (20 481-27 934) | 32 985 (26 913-40 428) | 23 954 (18 116-31 673) | 22 885 (14 601-35 871) |
| Temperature (°C)† | 38.0 (36.0-41.0) | 37.9 (36.0-41.0) | 37.9 (36.0-40.7) | 38.0 (36.0-40.8) | 38.1 (36.0-40.8) | 38.0 (36.0-40.8) | 37.9 (36.0-40.7) | 38.0 (36.0-40.7) | 38.1 (36.0-40.8) | 38.5 (37.0-40.8) |
| Pulse/min† | 96 (64-140) | 96 (66-130) | 100 (68-130) | 96 (64-140) | 94 (64-140) | 96 (64-140) | 100 (64-140) | 96 (66-140) | 96 (70-130) | 96 (70-130) |
| Blood pressure systolic (mmHg)* | 101 (99-103) | 103 (100-106) | 102 (98-105) | 99 (95-102) | 102 (95-108) | 102 (100-104) | 103 (100-106) | 101 (97-104) | 101 (96-106) | 103 (84-123) |
| Respiratory rate/min† | 26 (14-60) | 26 (14-60) | 27 (14-38) | 28 (15-52) | 26 (16-36) | 26 (14-60) | 26 (14-52) | 26 (14-52) | 26 (15-60) | 30 (20-40) |
| Liver palpable (%) | 21 (8) | 8 (8) | 5 («) | 8 (10) | 4(13) | 19 (7) | 7(5) | 9 (9) | 3 (8) | 2 (22) |
| Spleen palpable (%) | 58 (22) | 21 (20) | 15 (18) | 22 (28) | 9 (30) | 54 (20) | 21 (16) | 24 (24) | 9 (23) | 2 (22) |
| Cure/failure/reinfection | 238/9/17 | 97/2/4 | 72/4/8 | 69/3/5 | 30/1/0 | 249/7/14 | 118/2/10 | 94/3/4 | 37/2/0 | 9/0/0 |
| PCT/days† | 2 (2-5) | 2 (2-4) | 2 (2-5) | 2 (2-4) | 2 (2-3) | 2 (2-5) | 2 (2-4) | 2 (2-5) | 2 (2-4) | 2 (2-3) |
| FCT/h† | 26 (7-94) | 28 (7-78) | 26 (8-94) | 26 (7-86) | 30 (7-86) | 26 (7-94) | 26 (7-94) | 27 (7-77) | 24 (7-86) | 35 (12-86) |
| Day 42 haematocrit (%)† | 37 (25-47) | 38 (28-46) | 37 (25-46) | 37 (30-47) | 37 (30-44) | 37 (25-48) | 37 (28-48) | 37 (25-46) | 37 (30-47) | 37 (34-47) |
P > 0.06 for all comparisons except parasitaemia (see text).
*Mean (95% confidence interval, CI).
†Median (range).
‡Geometric mean (95% CI).
¶Unknown for three patients.
BMI, body mass index; HAZ, Z-score of height for age.
Treatment, clinical and parasitological outcomes
Patients were treated with CQ + SP (90), MAS3 (83) or LAM3 (97). All survived; 238/270 (88%) were cured, four (1.5%) had RI treatment failure, five (1.9%) had RIII treatment failure, six (2.2%) were lost to follow-up and 17 (6.3%) had re-infections. Full details of therapeutic responses are published elsewhere (Mayxay et al. 2004). For all 330 patients recruited to the trial, the 42-day cure rate, adjusted for re-infections, was 100% (106/106), 97% (93/96) and 92% (94/102) in the MAS3, LAM3 and CQ + SP groups, respectively. The cure rate was significantly higher in the MAS3, compared with CQ + SP, groups (P = 0.003). The mean (95% confidence interval, CI) parasite clearance time (days) was significantly shorter in the patients treated with MAS3 [2.07 (2.0-2.1)] and LAM3 [2.08 (2.0-2.1)] compared with those treated with CQ + SP [2.9 (2.8-3.0)], (P < 0.001 and <0.001, respectively) (Mayxay et al. 2004).
Red cell transketolase activity
The mean (95% CI; range) difference in α for the 26 sample duplicates stored at -30 and -80 °C was 0.018 (-0.0130.049; -0.11-0.27). An Altman-Bland plot (not shown) showed no evidence of a relationship between the difference and mean α.
The mean (95% CI; range) admission α was 1.20 (1.18-1.22; 0.67-2.24) and the percentage of patients with α values that were <1.15, 1.15-1.24, ≥1.25 and >1.31 were 38%, 32%, 30% and 12%, respectively. The different categories of α were compared in two ways, by anova across all categories and between those with α ≤ and >1.31. There were no significant relationships (P > 0.05) between either admission basal red cell transketolase activity or α categories and any of the admission clinical and demo-graphic variables, including age and the duration of illness. However, mean (95% CI) geometric parasitaemia was lower in those with α > 1.31 [18 387 (13 035-25 938)/μl] than those with α < 1.31 [27 345 (24 221-30 868/μl] (P = 0.03). The percentage of all children (<5 years) with a Z-score of height for age (HAZ) < -2, suggesting severe stunting, was 38%. There was a tendency for those with severe stunting to be more common in those with normal admission α (53%) than those with abnormal α (<1.151.24 (38%), 1.25-1.31(33%) and >1.31 (0%)) (P = 0.07) (Table 1).
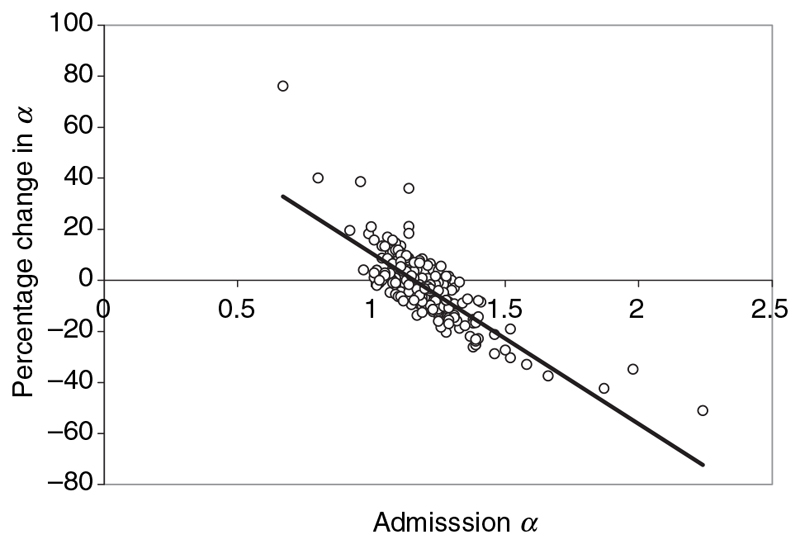
The mean (95% CI; range) day 42 α was 1.15 (1.14–1.16; 1.0–1.55) and the percentage of patients with α (values that were <1.15, 1.15–1.24, ≥1.25 and >1.31 were 48%, 37%, 14% and 3%, respectively). For the 239 patients with paired samples, the mean (95% CI) percentage change in α was –3 (–5 to –1)% and a paired t-test comparison of a on the day of admission and at day 42 suggested a significant reduction in a (P < 0.0001). The percentage change in a between admission and day 42 and the admission α were significantly correlated (R 2 = 0.639, P < 0.0001), suggesting that the higher the admission α, the greater percentage of reduction in convalescence (Figure 1).
Figure I. Percentage change in α between admission α and day 42 and admission α (equation y = -67 x + 78, R 2 = 0.639, P < 0.0001).
The mean α on admission and at day 42 did not differ significantly between the three different treatment groups (P > 0.2) or when the CQ-SP group was compared with the two ACT groups combined (P > 0.2). Mean day 0 and 42 α did not significantly differ between patients with treatment failure or success and they were not significantly related to parasite clearance time, fever clearance time, admission or day 42 haematocrit.
Discussion
This study in rural southern Laos suggests that 30% of the population presenting with malaria had evidence for biochemical thiamin deficiency, as defined by α and that 12% had unequivocal evidence for severe biochemical deficiency. None had overt clinical signs of beriberi and no patient had severe malaria or died. This study differs from that of Krishna et al. (1999) in that children (1–14 years) and patients who were treated as outpatients were included in this study but that patients with severe malaria were excluded.
At 42-day follow-up there was a significant reduction in mean α and in the proportion of patients with raised α, suggesting that the patients did have significant thiamin deficiency on admission. However, as patients received thiamin supplementation it cannot be determined whether this improvement in thiamin status was a consequence of recovery from malaria or thiamin supplementation or both. Patients were supplemented with thiamin 1 mg/day, which is the approximate recommended adult daily intake (World Health Organization 1999). As this is unlikely to have been sufficient to reverse severe biochemical deficiency (World Health Organization 1999), it implies that at least part of the reduction in α was a result of resolution of malaria. Further limitations of the study include the lack of dietary information and the exclusion of children <1 year, whilst in Vientiane the median (range) age of infants presenting with clinical beriberi was 3 (1–9) months (Soukaloun et al. 2003). The long sample storage period is unlikely to have affected α (Krishna et al. 1999) and if it did, it would have tended to underestimate the prevalence of thiamin deficiency (Puxtey et al. 1985). We are uncertain why admission parasitaemia was lower in those with severe deficiency – all patients had uncomplicated malaria and the finding may be a type I error.
The prevalence of biochemical thiamin deficiency may be underestimated by the α activation coefficient, as in chronic deficiency the in vitro activation of red cell transketolase may be subnormal (Neumann et al. 1979). As younger red cells have higher red cell transketolase activity (Spooner et al. 1979; Puxtey et al. 1985), the preferential invasion and destruction of relatively young red cells during falciparum malaria (Pasvol et al. 1980) may reduce the admission basal red cell transketolase activity and variably affect the α activation coefficient (Spooner et al. 1979). In addition, the suppression of erythropoiesis in association with malaria will delay the production of new red cells (Abdalla & Wickramasinghe 2004) and hence delay the recovery of the red cell transketolase activity of the pool of red cells in convalescence.
Lao people have multiple risk factors for thiamin deficiency. The consumption of polished rice, alcohol and thiaminase-containing foods such as ‘paa dek’ (fermented fish paste), thiamin antagonists such as betel nut and the hard physical labour of rural rice farming are likely to be important in adults (Vimokesant et al. 1975; Thurnham 2005). Traditional prolonged post-partum Lao maternal food avoidances may lead to fatal wet beriberi in infants and neurological symptoms in nursing mothers (Soukaloun et al. 2003). Malaria and other febrile episodes in pregnant and post-partum women may further predispose to beriberi in their infants by further depleting the short-term body stores.
That an increase in body temperature by 1 °C increases basal metabolic rate by 10% may explain the mechanism whereby malaria, and probably other febrile illness, increase the utilization of thiamin and precipitate deficiency (Thurnham 2005). Indeed, it is likely that biochemical thiamin deficiency is also associated with other febrile diseases (Ying 1934; Thurnham 2005). It has also been suggested that biochemical thiamin deficiency predisposes to infection (Anderson et al. 1986).
There is a considerable burden of thiamin deficiency in this community and using the criteria of World Health Organization (1999); Table 7), it would be described as a ‘moderate’(ly) severe public health problem. The clinical significance of this preventable and treatable disorder in malaria and other acute infections, and the incidence of beriberi in rural Laos, needs further investigation. Beriberi is conventionally regarded as a disease of the riceconsuming societies of south and southeast Asia. However, there have been reports from Africa, from both societies that consume rice, in the Gambia (Rolfe et al. 1993) and those that do not consume rice, in Ghana (Neumann et al. 1979), South Africa (Seftel et al. 1972) and Ethiopia (Mengistu & Maru 1979) (World Health Organization 1999). In view of the importance of acidosis in childhood malaria (Newton et al. 2005) it would be important to examine the thiamin status of children with severe malaria in Africa.
Déficience en thiamine et malaria falciparum non compliquée au Laos.
Objectif
La déficience en thiamine complique la malaria sévère à Plasmodium falciparum en Thaïlande et peut contribuer a de l’acidose. Nous avons estime la frequence de la déficience biochimique en thiamine chez des patients presentant une malaria falciparum non compliquee dans le sud du Laos.
Methode
Les coefficients ‘α’ de l’activation de la transcetolase dans les globules rouges ont ete mesures chez 310 patients avec une malaria falciparum non compliquee a leur presentation et a 42 jours apres instauration du traitement.
Resultats
2% des patients avaient une evidence biochimique de déficience sévère (valeurs de α > 1,31) a la presentation. Ce taux diminuait a 3% lorsque mesure 42 jours plus tard.
Conclusion
La déficience en thiamine était commune chez les patients Laotiens admis avec une infection non compliquee a P. falciparum. Cette déficience a ete reduite apres traitement de la malaria et supplementation en multivitamines. Le role de cette déficience evitable et traitable dans la malaria et dans d’autres infections aigues, ainsi que l’incidence du beriberi chez les Laotiens ruraux, necessite des recherches supplementaires.
mots clés
Plasmodium falciparum, malaria, béribéri, thiamine, Laos
Deficiencia de tiamina y malaria no complicada por falciparum en Laos.
Objetivo
La deficiencia de tiamina complica la malaria severa por Plasmodium falciparum en Tailandia y puede contribuir a la acidosis. El objetivo de este estudio fue estimar la frecuencia de deficiencia bioquímica de tiamina en pacientes que presentan malaria no complicada por falciparum en el sur de Laos.
Método
Los coeficientes (α) de activación de la transquetolasa de los eritrocitos fueron medidos en 310 pacientes al momento de presentarse con malaria no complicada por falciparum, así como 42 días después de comenzar tratamiento.
Resultados
Un doce por ciento de los pacientes presentaban evidencia bioquímica de deficiencia severa (valores α > 1.31) al momento de presentarse con malaria. Este valor disminuía a un 3% 42 días después.
Conclusión
La deficiencia de tiamina era común en pacientes de Laos admitidos con malaria no complicada por P.falciparum, y se redujo después del tratamiento de la malaria y de la suplementación con un complejo multivitamínico. Sería conveniente investigar más a fondo el papel de este desorden prevenible y tratable, en malaria y otras infecciones agudas, y la incidencia de beriberi en Laos rural,.
palabras clave
Plasmodium falciparum, malaria, beriberi, tiamina, Laos
Acknowledgements
We thank all the patients involved in this study, and Manisack Phommasansack, Pitta Sengkeomahavong, Bounmy Syphachanh, Ammala Phomsimone, Kaiam-phone Phonkeopaseuth, all medical assistants and nurses in Phalanxay District Hospital; Pranom Phongmany, Odai Xaysitthideth, Mr. Phomma, Rose McGready, Francois Nosten, David Thurnham and anonymous referees for their help. We are very grateful for the support of the Minister of Health, His Excellency Dr. Ponmek Dalaloy, and the Directors of Hygiene and Preventive Medicine, Drs Douangchanh Keo-Asa and Bounlay Phommasack, Lao PDR and His Excellency the British Ambassador to the Lao PDR. This study was funded by a grant from the British Embassy, Bangkok, with additional support from the Wellcome Trust of Great Britain.
References
- Abdalla SH, Wickramasinghe SN. The bone marrow in human malaria. In: Abdalla SH, Pasvol G, editors. Malaria - A Hematological Perspective. Imperial College Press; London: 2004. pp. 213–248. [Google Scholar]
- Anderson SH, Vickery CA, Nicol AD. Adult thiamine requirements and the continuing need to fortify processed cereals. The Lancet. 1986;2:85–89. doi: 10.1016/s0140-6736(86)91618-1. [DOI] [PubMed] [Google Scholar]
- Anon. WHO Technical Report Series No. 854. World Health Organization; Geneva: 1995. [Accessed 4 October 2006]. Physical status: the use and interpretation of anthropometry Expert Committee Report 1995. Available at: http://whqlibdoc.who.int/trs/WHO_TRS_854.pdf. [PubMed] [Google Scholar]
- Anon. [Accessed 4 October 2006];WHO Anthro 2005 software. 2005 Available at: http://www.who.int/childgrowth/software/en/
- Bayoumi RA, Rosalki SB. Evaluation of methods of coenzyme activation of erythrocyte enzymes for detection of deficiency of vitamins B1, B2 and B6. Clinical Chemistry. 1976;22:327–335. [PubMed] [Google Scholar]
- Carpenter KJ. Beriberi, White Rice and Vitamin B. University Presses of California; Columbia and Princeton: 2000. [Google Scholar]
- Caulfield LE, Richard SA, Black RE. Undernutrition as an underlying cause of malaria morbidity and mortality in children less than five years old. American Journal of Tropical Medicine and Hygiene. 2004;71(Suppl 2):55–63. [PubMed] [Google Scholar]
- Ching-Lang K. Infantile beriberi in Shanghai. Chinese Medical Journal. 1936;50:324–340. [Google Scholar]
- Dondorp AM, Chau TT, Phu NH, et al. Unidentified acids of strong prognostic significance in severe malaria. Critical Care Medicine. 2004;32:1683–1688. doi: 10.1097/01.ccm.0000132901.86681.ca. [DOI] [PubMed] [Google Scholar]
- Hay SI, Guerra CA, Tatem AJ, Noor AM, Snow RW. The global distribution and population at risk of malaria: past, present, and future. Lancet Infectious Diseases. 2004;4:327–336. doi: 10.1016/S1473-3099(04)01043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juguan JA, Lukito W, Schultink W. Thiamine deficiency is prevalent in a selected group of urban Indonesian elderly people. Journal of Nutrition. 1999;129:366–371. doi: 10.1093/jn/129.2.366. [DOI] [PubMed] [Google Scholar]
- Krishna S, Taylor AM, Supanaranond W, et al. Thiamine deficiency complicates malaria in southeast Asian adults. Lancet. 1999;353:546–549. doi: 10.1016/s0140-6736(98)06316-8. [DOI] [PubMed] [Google Scholar]
- Luxemburger C, White NJ, ter Kuile F, et al. Beri-beri: the major cause of infant mortality in Karen refugees. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2003;97:251–255. doi: 10.1016/s0035-9203(03)90134-9. [DOI] [PubMed] [Google Scholar]
- Mayxay M, Khanthavong M, Lindegårdh N, et al. Randomized comparison of chloroquine plus sulfadoxine-pyrimethamine versus artesunate plus mefloquine versus artemether-lumefantrine in the treatment of uncomplicated falciparum malaria in the Lao People’s Democratic Republic. Clinical Infectious Diseases. 2004;39:1139–1147. doi: 10.1086/424512. [DOI] [PubMed] [Google Scholar]
- McGready R, Simpson JA, Cho T, et al. Postpartum thiamine deficiency in a Karen displaced population. American Journal of Clinical Nutrition. 2001;74:808–813. doi: 10.1093/ajcn/74.6.808. [DOI] [PubMed] [Google Scholar]
- Mengistu M, Maru M. Dry beriberi: a clinical report from northwest Ethiopia. Ethiopian Medical Journal. 1979;17:29–32. [PubMed] [Google Scholar]
- Neumann CG, Swendseid ME, Jacob M, Stiehm R, Dirige O. Biochemical evidence of thiamine deficiency in young Ghanaian children. American Journal of Clinical Nutrition. 1979;32:99–104. doi: 10.1093/ajcn/32.1.99. [DOI] [PubMed] [Google Scholar]
- Newton CR, Valim C, Krishna S, et al. The prognostic value of measures of acid/base balance in pediatric falciparum malaria, compared with other clinical and laboratory parameters. Clinical Infectious Diseases. 2005;41:948–957. doi: 10.1086/432941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasvol G, Weatherall DJ, Wilson RJM. The increased susceptibility of young red cells to invasion by the malaria parasite Plasmodium falciparum . British Journal of Haematology. 1980;45:285–295. doi: 10.1111/j.1365-2141.1980.tb07148.x. [DOI] [PubMed] [Google Scholar]
- Platt BS, Gin SY. Some observations on a preliminary study of beri-beri in Shanghai. Transactions of Ninth Congress of Far Eastern Association of Tropical Medicine, Nanking. 1934;2:407–413. [Google Scholar]
- Phimmasone K, Douangpoutha I, Fauveau, Pholensa P. Nutritional status of children in the Lao PDR. Journal of Tropical Pediatrics. 1996;42:5–11. doi: 10.1093/tropej/42.1.5. [DOI] [PubMed] [Google Scholar]
- Pottier R. Le Sytème de Sante Lao et ses possibilities de développement. PhD thesis Universitè Rêne Descartes Published as Santê et Société au Laos (1973-1978) (no date) Comité de Coopération avec le Laos; Paris, France: 1979. [Google Scholar]
- Prentice CRM. The land of the million elephants - two years with a medical team in Laos. The Lancet. 1963;2:289–292. doi: 10.1016/s0140-6736(63)90188-0. [DOI] [PubMed] [Google Scholar]
- Puxtey JAH, Haskew AE, Ratcliffe JG. Changes in erythrocyte transketolase activity and the thiamine pyrophosphate effect during storage of blood. Annals Clinical Biochemistry. 1985;22:423–427. doi: 10.1177/000456328502200417. [DOI] [PubMed] [Google Scholar]
- Rolfe M, Walker RW, Samba KN, Cham K. Urban beriberi in The Gambia, west Africa. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1993;87:114–115. doi: 10.1016/0035-9203(93)90449-z. [DOI] [PubMed] [Google Scholar]
- Seftel HC, Metz J, Lakier JB. Cardiomyopathies in Johannesburg Bantu. South African Medical Journal. 1972;46:1707–1713. [PubMed] [Google Scholar]
- Soukaloun D, Kounnavong S, Pengdy B, et al. Dietary and socioeconomic factors associated with infantile beri beri in breastfed Lao infants. Annals of Tropical Paediatrics. 2003;23:181–186. doi: 10.1179/027249303322296493. [DOI] [PubMed] [Google Scholar]
- Spooner RJ, Percy RA, Rumley AG. The effect of erythrocyte ageing on some vitamin and mineral dependent enzymes. Clin Biochem. 1979;12:289–290. doi: 10.1016/s0009-9120(79)80132-0. [DOI] [PubMed] [Google Scholar]
- Thurnham DI. Beriberi. In: Caberllero B, Allen L, Prentice A, editors. Encyclopedia of Human Nutrition. 2nd. Academic Press; London: 2005. pp. 269–278. [Google Scholar]
- Vimokesant SL, Hilker DM, Nakornchai S, Rungruangsak K, Dhanamitta S. Effects of betel nut and fermented fish on the thiamine status of northeastern Thais. American Journal of Clinical Nutrition. 1975;28:1458–1463. doi: 10.1093/ajcn/28.12.1458. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Thiamine Deficiency and its Prevention and Control in Major Emergencies. Geneva: 1999. WHO/NHD/99.13. [Google Scholar]
- World Health Organization. Severe falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2000;94(Suppl 1):1–90. [PubMed] [Google Scholar]
- Ying YY. Beriberi in typhoid and its clinical significance. Transactions of the Ninth Congress of Far Eastern Association of Tropical Medicine. 1934;2:415. [Google Scholar]