Abstract
Background and Purpose
Fatigue is a common symptom among stroke survivors and in general practice. However, the clinical significance of fatigue and its relationship to incident stroke is unclear. The aim of this study was examine the relationship between self-reported fatigue and the incidence of stroke in a general population.
Methods
This was a prospective population-based study. The study population was 15,654 men and women aged 39-79 years recruited in 1993-1997 and followed till March 2016. Fatigue was assessed at 18 months after baseline using the vitality domain of the Short Form 36 questionnaire (SF36-VT). Cox proportional hazard models were constructed to describe the prospective relationship between baseline fatigue and incident stroke adjusting for age, sex, systolic blood pressure, cholesterol, physical activity, smoking status, alcohol consumption, fruit and vegetable consumption, diabetes mellitus, body mass index (BMI), vitamin supplement use, education level, Townsend deprivation index and occupational social class. Incident stroke was ascertained using death certificates and hospital record linkage data.
Results
Through 249,248 person years of follow up, 1,509 incident strokes occurred. Participants who reported the highest level of fatigue (Quartile 4) were more likely to be women, more likely to be multi-morbid and to perceive their health as fair or poor. We observed approximately 50% relative risk increase in stroke risk (HR 1.49 (95% CI 1.29-1.71)) in those who reported highest level of fatigue compared to those who reported the lowest level of fatigue (Q4 vs. Q1). This relationship remained unaltered regardless of anaemia status, presence or absence of chronic bronchitis, thyroid dysfunction or depression.
Conclusions
Self-report fatigue assessed by vitality domain of SF-36 predicts risk of future stroke at the general population level. Identifying and addressing stroke risk factors in those who report fatigue in general practice may have substantial benefit at the population level.
Index terms: Stroke, fatigue, psychosocial, stroke risk factors, non-traditional risk factors
Introduction
Fatigue is defined as a subjective experience involving malaise and an aversion to mental or physical activity [1]. It is well established that stroke survivors experience significant fatigue [2]. Furthermore, among the general population fatigue is a common complaint, featuring in 25% of general practice consultations, and is associated with increased all-cause and cardiovascular mortality [3,4]. However, there are no studies which have focused the relationship between fatigue and incident stroke.
Nonetheless, there is indirect evidence for a relationship between fatigue and incident stroke. Increased risk of stroke is associated with related factors such as; poor sleep quality [5–7], chronic stress [8], depression [9] and vital exhaustion (a concept that incorporates symptoms of both fatigue and anxiety) [10,11]. Furthermore, there are several plausible mechanisms through which fatigue may influence stroke risk. Fatigue may be ‘cause specific’, that is, a symptom of a specific disease process with shared risk factors for stroke such as; cardiac failure, anaemia and thyroid disease [12–14] Fatigue may act as a marker for a range of subclinical pathological processes relevant to stroke pathogenesis including; chronic inflammation, metabolic derangement [15,16], damage to the cerebral microvasculature and neuro-hormonal disturbance [17,18]. Fatigue may also exert a negative influence on psychosocial function and motivation, thereby reducing physical activity participation and impacting upon dietary choices [19–21].
Therefore, the aim of this study was to examine the prospective relationship between self-reported general fatigue assessed using well validated SF-36 vitality domain and incident stroke in a large population based study of apparently healthy men and women of the European Prospective Investigation into Cancer (EPIC)-Norfolk cohort.
Materials and Methods
Because of the sensitive nature of the data collected for this study, requests to access the dataset should be made only by qualified researchers trained in human subject confidentiality protocols and should be directed to the corresponding author. The study population was drawn from the EPIC-Norfolk study. This is a prospective cohort study of a sample of 39-79 year olds in the general population in Norfolk, UK. All GP practices were contacted in the Norfolk area and participants were recruited from the registers of the 35 participating practices between 1993 and 1997. Baseline data were collected at recruitment using postal questionnaires and participants were followed until death or the latest data extraction, 2nd of March 2016. A study flow diagram is available in supplementary material, Figure I. Ethical approval was obtained from the Norwich Ethics Committee. To be eligible in the current study, participants were required to give signed informed consent, have no history of stroke or transient ischaemic attack (TIA) at baseline health check or before the completion of the SF36-VT questionnaire, which was collected 18 months after enrolment. Participants were also required to have complete data for all key confounders (cholesterol levels, smoking status, systolic blood pressure and body mass index). All data were analysed anonymously. Due to hospital record and stroke register linkage outcome data were available for all patients. Further details concerning the recruitment methods of the EPIC-Norfolk study have been described in detail elsewhere [22].
Participants were asked to complete the Short Form 36 (SF36) questionnaire 18 months after enrolment. Of the eight domains within the SF36 the vitality domain (SF36-VT) was used as the method for the evaluation of self-reported fatigue. This method has been validated against comparable generic health surveys (such as the Nottingham health questionnaire) in studies of both diseased and general populations [23, 24]. Participants were asked to rate “how much of the time over the past 4 weeks... 1) Did you feel full of life? 2) Did you have a lot of energy? 3) Did you feel worn out? 4) Did you feel tired?” Participant responses were collated and transformed into a scale ranging from 1-100 and divided into vitality quartiles designated 1-4, with quartile 1 representing the group with the most vitality (henceforth referred to as the least fatigued quartile) and quartile 4 representing the group with the least vitality (henceforth referred to as the most fatigued quartile).
Incident stroke was ascertained by identifying the ICD 9 codes, 430–438 or ICD 10, 60-69 on death certificates and hospital record linkage data through East Norfolk Commission Record (ENCORE). This method has been shown to be highly sensitive and specific for stroke case ascertainment [25]. Covariates commonly associated with fatigue and stroke in general practice were assessed at baseline. Height, weight and systolic blood pressure were measured at the first health check (1993-1997). Body mass index was calculated using the formula (weight[kg]/(height[m2]) and categorised according to World Health Organisation definitions. Non-fasting blood samples were also collected, including non-fasting cholesterol, TSH (values >4.0 mU/l were considered indicative of low thyroid dysfunction) and haemoglobin (anaemia was defined as Hb <14.0 g/dL in men and <12.0 g/dL in women). All covariates were collected by trained staff according to standardised protocols within the EPIC Norfolk study [22].
The Health and Lifestyle questionnaire was administered at baseline and included detailed questions on demographic information, health behaviours and past medical history. Social class was categorised according to the Registrar General’s occupation classification scheme and sub-divided into manual or non-manual categories. Education status of participants was defined according to completion of secondary education examinations (O’level or A’level) or degree status. Participants were asked to describe their smoking habits and classified as current, non-current and never smokers. Baseline comorbidities were assessed through the question ‘Has your doctor ever told you have the following?’ followed by a list of conditions which included cancer, diabetes, heart attack and stroke. Medication use was also captured using this survey. Use of antidepressant medications was used to identify those individuals with comorbid depression. In addition, the EPIC Physical Activity Questionnaire (EPAQ2) was used to categorise individuals as inactive, moderately inactive, moderately active or active and the EPIC Food Frequency Questionnaire was used to quantify participant’s intake of fruit, vegetables and alcohol.
Data were analysed using SPSS version 25.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics are presented for the sample across fatigue quartiles, quartile one (Q1) represents SF36 scores 100-76, quartile two (Q2) represents SF36 scores 75-66, quartile 3 (Q3) represents SF36 scores 65-51 and quartile 4 (Q4) represents SF36 scores 50-0. Sample characteristics was compared between fatigue quartiles using the ANOVA test for normally distributed data, Kruskal-Wallis test for non-normally distributed data and Chi-squared test for categorical data. The association between covariates and incident stroke was tested using the same methods. Potentially significant confounders (using 80% significance level) and clinically relevant stroke risk factors were chosen and brought forward into the final models. Despite evidence of an association between physical and social functioning domains of the SF36 and stroke, these were not included in the final models due to a high level of collinearity with fatigue scores. Cox proportional hazards models were constructed to examine the association between fatigue (defined as a reduced vitality reported using the SF36-VT) and incident stroke using the first quartile (least fatigued group) as the reference category. The first of these was unadjusted (model A). The second included the traditional risk factors for stroke; age, sex, systolic blood pressure, cholesterol, self-reported diabetes mellitus, body mass index (BMI) (model B). Behavioural risk factors were added in the subsequent model, including; self-reported smoking status, alcohol intake, fruit and vegetable consumption and physical activity (model C). The penultimate model additionally included measures of socioeconomic status; education, social class, Townsend Score (model D). The final model included all elements of model D plus vitamin supplement usage (model E). Effect sizes are expressed as hazard ratios (HR) with 95 % confidence intervals (CI).
Sensitivity analysis were carried out by excluding participants who experienced stroke in the first two years of the study to rule out reverse causality. In addition, two additional models were created further adjusting for conditions that may cause fatigue, model F which is model E + haemoglobin values and model H which is model E + comorbidities (cancer, chronic bronchitis, past-history of myocardial infarction). Stratified analyses were carried out to examine the impact of presence or absence of co-morbidities that cause fatigue (anaemia, cancer and COPD) and by factors previously found to affect the association between vital exhaustion and stroke (sex and smoking status). Finally, the analysis was carried out for specific stroke subtype (ischaemic and haemorrhagic) and outcome (fatal and non-fatal stroke). A Kaplan Meier curve was constructed to examine the relationship between fatigue and incident stroke over time. The relationship between the vitality and all other domains within the SF36 was examined using Spearman rank correlation.
Results
In total 25,636 participants attended the baseline health assessment, of these 7536 were excluded due to missing SF36 vitality data. This was because not all participants who returned SF36 at 18 months after enrolment attended health assessment and vice versa. A further 375 were excluded due to prevalent stroke and 2071 were excluded due to missing data for key confounders. Most variables were missing data for less than 1% of participants, however cholesterol values and fruit and vegetable intake were missing for a larger proportion. Variables with a high proportion (>10%) of missing data not included in the principle analysis including; haemoglobin levels and thyroid function (quantified by thyroid stimulating hormone). In total 15,654 participants were included in the analysis (see Figure 1). There was no material difference between the baseline characteristics of the participants included and those who were excluded (Supplementary Table I). In this sample, there were a total of 1,509 cases of incident stroke captured through 249,248 person years of follow-up (mean 17.77 years). Outcome data were available for all participants. SF36 vitality scores were correlated with all other SF36 domains. However, the correlation was strongest for the relationship between fatigue and mental health domains (mental health component summary score Spearman’s rho= 0.64, p=0.000) and less for physical domains (physical component summary score rho=0.458, p=0.000) (see Supplementary table II).
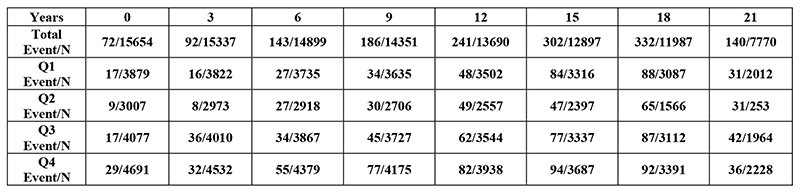
Figure 1. Kaplan Meier graph and lifetable demonstrating stroke events vs number at risk in each fatigue quartile.
Table 1 shows the baseline characteristics across the fatigue quartiles. Participants who reported the greatest level of fatigue (quartile 4) were more likely to be female and more likely to be obese or overweight. Participants in this group were more likely to have comorbidities including COPD, diabetes mellitus, history of cancer and cardiovascular disease. There was no significant difference between the mean ages across four quartiles. Higher fatigue score was also associated with lower Townsend score and educational attainment. Poor scores within physical and social functioning domains of the SF36 were highly correlated with fatigue and incident stroke. Self-reported general health was also worst in those who reported the highest level of fatigue.
Table 1. Baseline sample characteristics by SF36-VT quartiles of 15,654 EPIC-Norfolk participants (1993-1997).
| Characteristic | SF-36 vitality score quartiles | |||
|---|---|---|---|---|
| Q1 (100-76) |
Q2 (75-66) |
Q3 (65-51) |
Q4 (50-0) |
|
| Total N (%) | 3879 | 3007 | 4077 | 4691 |
| Age (years) mean (SD) | 59.25 (8.67) | 58.97 (0.09) | 59.29 (9.25) | 58.80 (9.42) |
| Sex | ||||
| Male N (%) | 1935 (49.88) | 1380 (45.89) | 1770 (43.41) | 1821 (38.82) |
| Female N (%) | 1944 (50.12) | 1627 (54.11) | 2307 (56.59) | 2870 (61.18) |
| Body Mass Index (kg/m2) | ||||
| < 18.5 | 12 (0.31) | 12 (0.40) | 19 (0.47) | 31 (0.66) |
| 18.5 – 24.9 | 1701 (43.85) | 1275 (42.40) | 1599 (39.22) | 1815 (38.69) |
| 25.0 – 30.0 | 1783 (45.96) | 1376 (45.76) | 1861 (45.65) | 2021 (43.08) |
| Over 30.0 | 383 (9.87) | 344 (11.44) | 598 (14.67) | 824 (17.57) |
| Systolic BP (mmHg) mean (SD) | 135.38 (17.74) | 134.57 (18.04) | 134.99 (18.54) | 134.47 (18.11) |
| Serum cholesterol (mmol/l) mean (SD) | 6.15 (1.12) | 6.16 (1.16) | 6.18 (1.17) | 6.18 (1.16) |
| Diabetes Mellitus (self-reported) | 64 (1.65) | 49 (1.63) | 69 (1.69) | 130 (2.77) |
| Use of antidepressant drugs | 75 (1.93) | 81 (2.69) | 184 (4.51) | 400 (8.53) |
| Myocardial Infarction (self-reported) | 60 (1.55) | 67 (2.23) | 112 (2.75) | 202 (4.31) |
| Cancer (self-reported) | 167(4.31) | 149 (4.96) | 207 (5.08) | 332 (7.08) |
| COPD (self-reported) | 231 (5.96) | 209 (6.95) | 410 (10.06) | 539 (11.49) |
| Fruit intake (g/day) median (IQR) | 222.55 (196.00) | 224.40 (191.55) | 217.10 (190.93) | 203.90 (191.75) |
| Vegetable intake (g/day) median (IQR) | 230.55 (157.80) | 228.63 (143.68) | 229.04 (138.69) | 217.12 (141.66) |
| Alcohol consumption (g/day) median (IQR) | 4.50 (8.50) | 4.00 (9.00) | 4.00 (8.50) | 3.00 (8.00) |
| Smoking Status (self-reported) | ||||
| Current | 372 (9.59) | 261 (8.68) | 415 (10.18) | 599 (12.77) |
| Former | 1586 (40.89) | 1259 (41.87) | 1756 (43.07) | 1936 (41.27) |
| Never | 1921 (49.52) | 1487 (49.45) | 1906 (46.75) | 2156 (45.96) |
| Physical Activity (self-reported) | ||||
| Inactive | 858 (22.12) | 754 (25.07) | 1124 (27.57) | 1569 (33.45) |
| Moderately Inactive | 1104 (28.46) | 931 (30.96) | 1201 (29.46) | 1372 (29.25) |
| Moderately Active | 976 (25.16) | 720 (23.94) | 1035 (25.39) | 1009 (21.51) |
| Active | 941 (24.26) | 602 (20.02) | 717 (17.59) | 741 (15.79) |
| Townsend score median (IQR) | -2.69 (2.34) | -2.81 (2.00) | -2.67 (2.63) | -2.54 (2.55) |
| Social Class | ||||
| Professional | 280 (7.22) | 279 (9.28) | 306 (7.51) | 310 (6.61) |
| Managerial | 1563 (40.29) | 1176 (39.11) | 1558 (38.21) | 1678 (35.77) |
| Skilled non-manual | 636 (16.40) | 485 (16.13) | 711 (17.44) | 828 (17.65) |
| Skilled manual | 848 (21.86) | 665 (22.12) | 850 (20.85) | 1043 (22.23) |
| Semi-skilled manual | 444 (11.45) | 321 (10.68) | 541 (13.27) | 647 (13.79) |
| Non-skilled | 108 (2.78) | 81 (2.69) | 111 (2.72) | 185 (3.94) |
| Educational Attainment | ||||
| Degree or Higher | 545 (14.05) | 436 (14.50) | 573 (14.05) | 606 (12.92) |
| A-Level | 1673 (43.13) | 1282 (42.63) | 1665 (40.84) | 1863 (39.71) |
| O-Level | 403 (10.39) | 318 (10.58) | 439 (10.77) | 531 (11.32) |
| No Qualification | 1258 (32.43) | 971 (32.29) | 1400 (34.34) | 1691 (36.05) |
| Vitamin use | 1652 (42.59) | 1318 (43.83) | 1811 (44.42) | 2138 (45.58) |
| Anaemia N (%) | 767 (29.23) | 583 (27.64) | 768 (26.6) | 844 (25.39) |
The data presented as mean and (SD) for normally distributed continuous variables, median and interquartile range (IQR) for non-normally distributed variables, and number (N) and percentage (%) for categorical variables. P values indicate levels of significance across all quartiles, these were calculated using student’s T test and T test for unequal variance for normally distributed data and the Kruskal-Wallis test for non-normally distributed data. P values for categorical variables were calculated using the Chi squared test. f The following data had missing values: anaemia (30% missing).
Figure 1 illustrates the unadjusted relationship between stroke incidence and fatigue quartile, fatigue was associated with an excess risk of stroke throughout the follow up period. Table 2 shows results of the Cox proportional hazards models. The hazard ratio for incident stroke was 1.49 (95% CI 1.29-1.71) in the fully adjusted model (model E). This increased to 1.59 (95% CI 1.35-1.86) when analyses were confined to the ischaemic stroke. Exclusion of those who had stroke within 2 years of reporting fatigue did not change the effect size. The effect of fatigue in haemorrhagic stroke was limited (a non-significant 12% increase in post estimate hazard ratio). The effect of fatigue on stroke fatality also did not reach the level of statistical significance. Additional adjustment for comorbidities in Model G (cancer, COPD, prior myocardial infarction, thyroid dysfunction and anaemia) marginally increased the effect size to 1.55 (1.26-1.91) (see Table 1).
Table 2.
Cox proportional hazards model and hazard ratios (with corresponding 95% confidence intervals) for incident stroke of any type, and by stroke sub-type and fatal strokes in the EPIC Norfolk study.
| Hazard Ratios | SF-36 vitality score quartiles | P value* | |||
|---|---|---|---|---|---|
| Model | Q1 (100-75) |
Q2 (75-66) |
Q3 (65-51) |
Q4 (50-0) |
|
| All strokes | |||||
| Events/population | 345/3879 | 267/3007 | 400/4077 | 497/4691 | 1509/15654 |
| Model A HR (95 % CI) | 1.00 | 1.01 (0.86-1.18) | 1.15 (1.00-1.33) | 1.29 (1.13-1.48) | <0.001 |
| Model B HR (95 % CI) | 1.00 | 1.02 (0.87-1.20) | 1.19 (1.03-1.38) | 1.49 (1.30-1.72) | <0.001 |
| Model C HR (95 % CI) | 1.00 | 1.04 (0.88-1.22) | 1.20 (1.03-1.38) | 1.49 (1.29-1.71) | <0.001 |
| Model D HR (95 % CI) | 1.00 | 1.04 (0.88-1.22) | 1.19 (1.03-1.38) | 1.48 (1.28-1.70) | <0.001 |
| Model E HR (95 % CI) | 1.00 | 1.04 (0.89-1.22) | 1.20 (1.04-1.39) | 1.49 (1.29-1.71) | <0.001 |
| Model F HR (95 % CI)* | 1.00 | 1.02 (0.84-1.25) | 1.16 (0.96-1.39) | 1.52 (1.27-1.80) | <0.001 |
| Model G HR (95 % CI)* | 1.00 | 1.12 (0.88-1.41) | 1.20 (0.96-1.49) | 1.55 (1.26-1.91) | <0.001 |
| Ischaemic stroke | |||||
| Events | 272/3879 | 218/3007 | 327/4077 | 416/4691 | 1233/15654 |
| Model A HR (95 % CI) | 1.00 | 1.04 (0.87-1.24) | 1.197 (1.02-1.41) | 1.37 (1.18-1.60) | <0.001 |
| Model B HR (95 % CI) | 1.00 | 1.06 (0.89-1.27) | 1.233 (1.05-1.45) | 1.59 (1.36-1.85) | <0.001 |
| Model C HR (95 % CI) | 1.00 | 1.08 (0.90-1.29) | 1.244 (1.06-1.46) | 1.59 (1.36-1.86) | <0.001 |
| Model D HR (95 % CI) | 1.00 | 1.08 (0.90-1.29) | 1.241 (1.06-1.46) | 1.58 (1.35-1.84) | <0.001 |
| Model E HR (95 % CI) | 1.00 | 1.08 (0.91-1.29) | 1.247 (1.06-1.47) | 1.59 (1.36-1.86) | <0.001 |
| Haemorhagic stroke | |||||
| Events/ Population | 73/3879 | 49/3007 | 73/4077 | 81/4691 | 276/15654 |
| Model A HR (95 % CI) | 1.00 | 0.87 (0.61-1.25) | 0.99 (0.72-1.37) | 0.99 (0.72-1.36) | 0.87 |
| Model B HR (95 % CI) | 1.00 | 0.89 (0.62-1.28) | 1.03 (0.75-1.43) | 1.14 (0.83-1.57) | 0.50 |
| Model C HR (95 % CI) | 1.00 | 0.88 (0.62-1.27) | 1.02 (0.73-1.41) | 1.11 (0.80-1.53) | 0.67 |
| Model D HR (95 % CI) | 1.00 | 0.88 (0.61-1.27) | 1.02 (0.74-1.41) | 1.11 (0.81-1.54) | 0.65 |
| Model E HR (95 % CI) | 1.00 | 0.88 (0.62-1.27) | 1.02 (0.74-1.41) | 1.12 (0.81-1.55) | 0.64 |
| Fatal stroke | |||||
| Events/ Population | 145/3879 | 95/3007 | 162/4077 | 216/4691 | 618/15654 |
| Model A HR (95 % CI) | 1.00 | 0.95 (0.73-1.23) | 1.01 (0.80-1.26) | 1.22 (0.99-1.50) | 0.106 |
| Model B HR (95 % CI) | 1.00 | 0.91 (0.70-1.18) | 0.96 (0.76-1.20) | 1.23 (1.00-1.53) | 0.030 |
| Model C HR (95 % CI) | 1.00 | 0.89 (0.69-1.16) | 0.88 (0.70-1.11) | 1.14 (0.92-1.42) | 0.065 |
| Model D HR (95 % CI) | 1.00 | 0.93 (0.71-1.21) | 0.89 (0.71-1.13) | 1.16 (0.93-1.45) | 0.078 |
| Model E HR (95 % CI) | 1.00 | 0.93 (0.71-1.21) | 0.89 (0.71-1.12) | 1.16 (0.93-1.44) | 0.083 |
Model A: unadjusted; Model B: age, sex (first man) systolic, BP, cholesterol, DM (first), BMI; Model C: B + smoking, alcohol intake, fruit and vegetable intake, physical activity; Model D: C+ education, social class (first), Townsend Score; Model E: D +vitamin use; Model F: E + haemaglobin levels; Model G: F+prior MI, cancer, chronic bronchitis and high TSH. *Model F and Model G include data with missing values. * P test for trend across vitality quartiles.
The effect size was similar between never smokers compared to smokers, and between men and women. The analysis was also stratified by comorbidities which are known to be associated with fatigue. The presence of anaemia, depression, thyroid dysfunction, COPD and cancer did not attenuate the effect of fatigue, although the precision of all estimates was affected by the low event rates in these subgroups. However, the effect size was lower amongst participants who had previously experienced myocardial infarction HR1.27 [95% CI 0.60-2.70]) and sub-analysis of participants reporting poor or moderate health (HR 1.16 [95% CI 0.93-1.44]) (see Supplementary Table III).
Discussion
In this population-based prospective cohort study we demonstrate the independent association between self-reported fatigue and the risk of incident stroke in a general population. This effect was large and remained persistant over more than 20 years of follow up. It represented a 59% increase in the relative risk of ischaemic stroke in those who reported greatest fatigue (Q4) compared to those in the lowest level of fatigue with a clear linear dose response relationship. Those in the most fatigued group were more likely to; be female, suffer from comorbidities and report lower scores in all domains of the SF36, particularly the mental health domain. To the best of our knowledge, this is the first report demonstrating the link between self-reported fatigue and stroke in a general population which may have clinical implication in preventing stroke.
Notwithstanding the paucity in research into general fatigue, there is some evidence that supports the plausibility of our findings. Several have evaluated the relationship between exhaustion or vital exhaustion (a triad of depression, demoralisation and irritability) and stroke [10]. Three of these studies were evaluated within a meta-analysis of 17 papers concerning vital exhaustion and cardiovascular events. This concluded that vital exhaustion increased the risk of all cardiovascular disease by 53% and of stroke by 46% although the latter relationship was not statistically significant [26]. The studies were limited by low event rates and this association was found only to be significant amongst women and smokers [10,11, 27]. A separate study concerning vital exhaustion in Russia found that exhausted men aged 25–64-years were at a substantially higher risk of stroke than non-exhausted men over 14 years of follow-up (HR 2.6) [28]. Furthermore, we have previously shown that fatigue increased the hazards of cardiovascular mortality by 45% [4].
There is also evidence available to support the association between the factors that underlie fatigue and incident stroke. Fatigue therefore, may be used as an umbrella concept that can capture the experience of multiple interrelated adverse health states relevant to the pathogenesis of stroke. Fatigue in this study was associated with worse mental and physical health. It has previously been established that fatigue may be a result of sleep disorder [29] or psychosocial stress [30], which have been associated with incident stroke under various labels including: non-restorative sleep [5–7] work pressure [31], major life events [11], chronic stress [8] and depression [9]. A meta-analysis of 14 studies involving a total of 10,130 strokes found a 33% increased risk of incident stroke in those reporting perceptions of psychosocial stress [32]. The mechanisms behind these associations are unclear, however it has been proposed that both poor sleep and chronic stress may lead to measurable disturbances in normal homeostatic mechanisms, such as endocrine dysfunction, metabolic syndrome [33] and hypertension [34] which may contribute to the pathogenesis of stroke. Furthermore, psychosocial stress and poor sleep may mediate stroke risk by adversely affecting dietary choices and participation in physical activity [20, 21].
In addition to the role of psychosocial factors it is likely that poor physical health accounts for a portion of the observed relationship between fatigue and stroke. In this context fatigue may be a useful composite marker for the presence and severity of comorbidity. Furthermore, fatigue may be a symptom of disturbances of physical function that are often undiagnosed and unaccounted for in risk quantification such as metabolic syndrome [15] and chronic inflammatory states [16]. It is also possible that fatigue may be a premonitory symptom of stroke. For example, there is some evidence that cardiovascular events are preceded by fatigue [17] and it is possible that stroke sufferers experience subclinical infarcts prior to the major event, resulting in fatigue [35, 36]. However, exclusion of incident strokes occurring within the first two years of follow up reduces the likelihood that our results are due to reverse causality.
Our study has several strengths. Unlike previous studies on this topic we chose a broad definition of fatigue which could be assessed using an intuitive tool (SF36-VT). The SF36 has been extensively evaluated in the British population and has excellent internal consistency [24, 37, 38, 39, 40], test re-test reliability (correlation coefficient 0.84) [38] and construct validity when compared with alternative fatigue scales [24, 40] While comprehensive fatigue scales may allow fatigue to be quantified with greater precision, the brevity and simplicity of this scale may make it a more suitable tool in clinical practice [40]. This study drew from a large population and included a higher number of incident strokes than observed in previous studies. Furthermore, the detail available in this prospective cohort allow us to control for various relevant confounders and examine the mediating effect of known causes of fatigue including anaemia and depression. We also had complete follow up in through data linkage and use of disease registries. Most importantly, participants in our cohort were recruited from general practice registries similar baseline characteristics to the national population (with the exception of a slightly lower prevalence of smokers) [25]. Therefore, it is likely that these results are applicable across other Caucasian populations.
There are a number of noteworthy limitations to this study. As a prospective cohort study, we cannot exclude the impact of residual confounding by known or unknown confounders. Secondly, it should be noted that this study measured fatigue only at baseline and cannot account for changes in fatigue status or severity over time. However, such random variation is likely to result in an underestimation of the effect size. We excluded a proportion due to missing data on SF-36VT, however, there was no material difference between sample characteristics of those included and excluded. We didn’t distinguish individuals with chronic fatigue syndrome, which is a distinct disease entity and these individuals may have different risk profile than the general population. While causality cannot be directly implied, we have demonstrated the prospective relationship through several sensitivity and mediating analyses as well as provided plausible biomedical causal mechanisms.
The increase in ischaemic stroke risk associated with fatigue is substantial. However, at present, fatigue is a neglected symptom in clinical practice [1,4]. Therefore, these findings may provide impetus for consideration of fatigue as an important risk marker. Unlike traditional biomarkers, fatigue may facilitate a more holistic evaluation of an individual’s wellbeing [29] and could be considered alongside other ‘non-disease specific’ facets of health such as cognitive impairment, isolation, frailty and polypharmacy [37, 38]. This may identify individuals with a high cardiovascular risk who would be overlooked by existing scoring systems and facilitate early detection of modifiable risk factors [41].
Furthermore, fatigue itself could be approached as a modifiable risk factor [4]. There are a number of effective management strategies for fatigue designed for specific patient populations that may be applicable for the general population. Non-pharmacological management, such as graded exercise programs, fatigue management education and psychotherapy have been found to be safe and effective in multiple chronic conditions [42–45]. Insomnia may also be treated effectively using similar measures [46]. Pharmacological intervention, be it through de-prescribing culprit drugs or prescribing drugs such as antidepressants are likely to be less widely applicable [42, 44]. At a societal level, addressing issues such as income inequality and long working hours may be important to address root causes of fatigue [47, 48]. Further research is required to examine the potential effect of fatigue treatment on preventing stroke.
Conclusions
The recognition that fatigue is an important adverse health state, may be used to improve risk quantification in stroke and incentivise physicians to identify and treat relevant risk factors in fatigued individuals. Therefore, assessment of fatigue via SF-36 vitality domain as part of may provide an opportunity to reduce future burden of stroke. Future studies should focus on further elucidating the mechanisms underlying fatigue in the general population. We recommend that fatigue be considered as part of a holistic assessment of cardiovascular disease risk in general practice.
Supplementary Material
Acknowledgements
The authors would like to thank the participants and staff of the EPIC-Norfolk cohort and collaborating GP practices in Norfolk, UK.
Sources of Funding
The EPIC-Norfolk study (DOI 10.22025/2019.10.105.00004) has received funding from the Medical Research Council (MR/N003284/1 and MC-UU_12015/1) and Cancer Research UK (C864/A14136). We are grateful to all the participants who have been part of the project and to the many members of the study teams at the University of Cambridge who have enabled this research. The first author is also grateful for the support of the Flora-Gow Murray Scholarship which allowed this project to be undertaken.
Footnotes
Contributorship
PKM conceived the study. GB performed literature review, data analysis under supervision by SRN and PKM. RNL is responsible for data linkage. NJW and KTK are PIs of EPIC-Norfolk Study. GB, SRN and PKM drafted the manuscript and all authors contributed to the writing of the paper. PKM is the guarantor.
Conflict of Interest
The authors have no conflicts of interest to declare.
Contributor Information
Genevieve Barlas, Institute of Applied Health Sciences, University of Aberdeen, Aberdeen AB25 2ZD, Scotland, UK. 01224 437841.
Robert L Luben, Email: robert.luben@phpc.cam.ac.uk, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK.
Samuel R Neal, Email: s.neal.15@aberdeen.ac.uk., Institute of Applied Health Sciences, University of Aberdeen, Aberdeen AB25 2ZD, Scotland, UK
Nicholas J Wareham, Email: nick.wareham@mrc-epid.cam.ac.uk, MRC Epidemiology Unit, Cambridge, UK.
Kay-Tee Khaw, Email: kk101@medschl.cam.ac.uk, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK.
Phyo K Myint, Email: phyo.myint@abdn.ac.uk, Institute of Applied Health Sciences, University of Aberdeen, Aberdeen AB25 2ZD, Scotland, UK.
References
- 1.Sharpe M, Wilks D. Fatigue. BMJ. 2002;325:480–3. doi: 10.1136/bmj.325.7362.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Staub F, Bogousslavsky J. Fatigue after Stroke: A Major but Neglected Issue. Cerebrovasc Dis. 2001;15:75–81. doi: 10.1159/000047685. [DOI] [PubMed] [Google Scholar]
- 3.Cullen W, Kearney Y, Bury G. Prevalence of fatigue in general practice. Irish Ir J Med Sci. 2002;171:10–12. doi: 10.1007/BF03168931. [DOI] [PubMed] [Google Scholar]
- 4.Basu N, Yang X, Luben RN, Whibley D, et al. Fatigue is associated with excess mortality in the general population: results from the EPIC-Norfolk study. BMC Medicine. 2016;20:122. doi: 10.1186/s12916-016-0662-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sofi F, Cesari F, Casini A, Macchi C, Abbate R, Gensini GF. Insomnia and risk of cardiovascular disease: a meta-analysis. Eur J Prev Cardiolog. 2014;21:57–64. doi: 10.1177/2047487312460020. Insomnia and risk of cardiovascular disease: a metaanalysis. [DOI] [PubMed] [Google Scholar]
- 6.Wu M-P, Lin H-J, Weng S-F, Ho C-H, Wang J-J, Hsu Y-W. Insomnia Subtypes and the Subsequent Risks of Stroke: Report From a Nationally Representative Cohort. Stroke. 2014;45:1349–54. doi: 10.1161/STROKEAHA.113.003675. [DOI] [PubMed] [Google Scholar]
- 7.Hsu C-Y, Chen Y-T, Chen M-H, Huang C-C, Chiang C-H, Huang P-H, et al. The association between insomnia and increased future cardiovascular events: a nationwide population-based study. Psychosomatic Medicine. 2015;77:743–51. doi: 10.1097/PSY.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 8.May M, McCarron P, Stansfeld S, Ben-Shlomo Y, Gallacher J, Yarnell J, Smith G, Elwood P, Ebrahim S. Does psychological distress predict the risk of ischemic stroke and transient ischemic attack? The Caerphilly study. Stroke. 2002;33:7–12. doi: 10.1161/hs0102.100529. [DOI] [PubMed] [Google Scholar]
- 9.Pan A, Sun Q, Okereke OI, Rexrode KM, Hu FB. Depression and risk of stroke morbidity and mortality: a meta-analysis and systematic review. JAMA. 2011;306:1241–9. doi: 10.1001/jama.2011.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz SW, Carlucci C, Chambless LE, Rosamond WD. Synergism between smoking and vital exhaustion in the risk of Ischemic stroke: evidence from the ARIC study. Ann Epidemiology. 2004;14:416–24. doi: 10.1016/j.annepidem.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Kornerup H, Marott JL, Schnohr P, Boysen G, Barefoot J, Prescott B. Vital exhaustion increases the risk of ischemic stroke in women but not in men: results from the Copenhagen City Heart Study. J Psychosom Res. 2010;68:131–7. doi: 10.1016/j.jpsychores.2009.08.009. Vital exhaustion increases the risk of ischemic stroke in women but not in men: Results from the Copenhagen City Heart Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirk J, Douglass R, Nelson E, Jaffe J, Lopez A, Ohler J, et al. Chief complaint of fatigue: a prospective study. J Fam Pract. 1990;30:33–39. [PubMed] [Google Scholar]
- 13.Squizzato A, Gerdes VEA, Brandjes DPM, Buller HR, Stam J. Thyroid Diseases and Cerebrovascular Disease. Stroke. 2005;36:2302–10. doi: 10.1161/01.STR.0000181772.78492.07. [DOI] [PubMed] [Google Scholar]
- 14.Haeusler K, Laufs U, Endres M. Chronic Heart Failure and Ischemic Stroke. Stroke. 2011;42:2977–82. doi: 10.1161/STROKEAHA.111.628479. [DOI] [PubMed] [Google Scholar]
- 15.Maloney EM, Boneva RS, Lin J-MS, Reeves WC. Chronic fatigue syndrome is associated with metabolic syndrome: results from a case-control study in Georgia. Metabolism. 2010;59:1351–7. doi: 10.1016/j.metabol.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 16.Solenski NJ. Emerging risk factors for cerebrovascular disease. Curr Drug Targets. 2007;8:802–16. doi: 10.2174/138945007781077364. [DOI] [PubMed] [Google Scholar]
- 17.Appels A. Exhaustion and coronary heart disease: the history of a scientific quest. Patient Educ Couns. 2004;55:223–9. doi: 10.1016/j.pec.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Cheung N, Rogers S, Mosley TH, Klein R, Couper D, Wong TY. Vital exhaustion and retinal microvascular changes in cardiovascular disease: atherosclerosis risk in communities study. Psychosom Med. 2009;71:308–12. doi: 10.1097/PSY.0b013e318190f009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaput J-P. Sleep patterns, diet quality and energy balance. Physiol Behav. 2014;134:86–91. doi: 10.1016/j.physbeh.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Ament W, Verkerke GJ. Exercise and fatigue. Sports Med. 2009;39:389–422. doi: 10.2165/00007256-200939050-00005. [DOI] [PubMed] [Google Scholar]
- 21.Myint PK, Luben RN, Wareham NJ, Bingham SA, Khaw K-T. Combined effect of health behaviours and risk of first ever stroke in 20 040 men and women over 11 years’ follow-up in Norfolk cohort of European Prospective Investigation of Cancer (EPIC Norfolk): prospective population study. BMJ. 2009;338:b349. doi: 10.1136/bmj.b349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Day N, Oakes S, Luben R, Khaw K-T, Welch A, Wareham N. EPIC-Norfolk: study design and characteristics of the cohort. European Prospective Investigation of Cancer. Br J Cancer. 1999;80(Suppl 1):95–103. [PubMed] [Google Scholar]
- 23.Jenkinson C, Wright L, Coulter A. Criterion validity and reliability of the SF-36 in a population sample. Qual Life Res. 1994;3:7–12. doi: 10.1007/BF00647843. [DOI] [PubMed] [Google Scholar]
- 24.O’Connor PJ. Evaluation of four highly cited energy and fatigue mood measures. J Psychosom Res. 2004;57:435–41. doi: 10.1016/j.jpsychores.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Sinha S, Myint PK, Luben RN, Khaw KT. Accuracy of death certification and hospital record linkage for identification of incident stroke. BMC Med Res Methodol. 2008;8:74. doi: 10.1186/1471-2288-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen R, Bavishi C, Haider S, Thankachen J, Rozanski A. Meta-Analysis of Relation of Vital Exhaustion to Cardiovascular Disease Events. Am J of Cardiol. 2017;119:1211–6. doi: 10.1016/j.amjcard.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Schuitemaker G, Dinant G, Van DerPol G, Verhelst A, Appels A. Vital Exhaustion as a Risk Indicator for First Stroke. Psychosomatics. 2004;45:114–118. doi: 10.1176/appi.psy.45.2.114. [DOI] [PubMed] [Google Scholar]
- 28.Gafarov V, Voevoda M, Gromova E, et al. Cardiovascular Diseases and Vital Exhaustion: Longitudinal study in Russia/Siberia (WHO Monica-Psychosocial Program) Russian Journal of Cardiology. 2016;4:115–23. [Google Scholar]
- 29.Elwood P, Hack M, Pickering J, et al. Sleep disturbance, stroke, and heart disease events: evidence from the Caerphilly cohort. J Epidemiol Community Health. 2006;60:69–73. doi: 10.1136/jech.2005.039057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jason LA, Evans M, Brown M, et al. What is Fatigue? Pathological and Nonpathological Fatigue. PM R. 2010;2:327–31. doi: 10.1016/j.pmrj.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 31.Suadicani P, Andersen LL, Holtermann A, et al. Perceived psychological pressure at work, social class, and risk of stroke: A 30-year follow-up in Copenhagen male study. J Occup Environ Med. 2011;53:1388–95. doi: 10.1097/JOM.0b013e31823c149d. [DOI] [PubMed] [Google Scholar]
- 32.Booth J, Connelly L, Lawrence M, Chalmers C, Joice S, Becker C, et al. Evidence of perceived psychosocial stress as a risk factor for stroke in adults: a meta-analysis. BMC Neurology. 2015;15:233. doi: 10.1186/s12883-015-0456-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jennings JR, Muldoon MF, Hall M, Buysse DJ, Manuck SB. Self-reported Sleep Quality is Associated With the Metabolic Syndrome. Sleep. 2007;30:219–23. doi: 10.1093/sleep/30.2.219. [DOI] [PubMed] [Google Scholar]
- 34.Sparrenberger F, Cichelero FT, Ascoli AM, Foneseca F, Weiss G, Berwanger O, et al. Does psychosocial stress cause hypertension? A systematic review of observational studies. J Hum Hypertens. 2009;23:12–9. doi: 10.1038/jhh.2008.74. [DOI] [PubMed] [Google Scholar]; Appels A, Höppener P, Mulder P. A questionnaire to assess premonitory symptoms of myocardial infarction. Int J Cardiol. 1987;17:15–24. doi: 10.1016/0167-5273(87)90029-5. [DOI] [PubMed] [Google Scholar]
- 35.Fanning JP, Wong AA, Fraser JF. The epidemiology of silent brain infarction: a systematic review of population-based cohorts. BMC Med. 2014;12:119. doi: 10.1186/s12916-014-0119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bang OY, Ovbiagele B, Kim JS. Nontraditional Risk Factors for Ischemic Stroke: An Update. Stroke. 2015;46:3571–8. doi: 10.1161/STROKEAHA.115.010954. [DOI] [PubMed] [Google Scholar]
- 37.Jenkinson C, Wright L, Coulter A. Criterion validity and reliability of the SF-36 in a population sample. Quality of Life Research. 1994;3:7–12. doi: 10.1007/BF00647843. [DOI] [PubMed] [Google Scholar]
- 38.Ruta D, Abdalla M, Garratt A, Coutts A, Russell I. SF 36 health survey questionnaire: I. Reliability in two patient based studies. Quality and Safety in Health Care. 1994;3:180–185. doi: 10.1136/qshc.3.4.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burholt V, Nash P. Short Form 36 (SF-36) Health Survey Questionnaire: normative data for Wales. Journal of Public Health. 2011;33:587–603. doi: 10.1093/pubmed/fdr006. [DOI] [PubMed] [Google Scholar]
- 40.Whitehead L. The Measurement of Fatigue in Chronic Illness: A Systematic Review of Unidimensional and Multidimensional Fatigue Measures. J Pain Symptom Manage. 2009;37:107–128. doi: 10.1016/j.jpainsymman.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 41.Bowling CB, Booth I, Safford MM, Whitson HE, Ritchie CS, Wadley VG, et al. Nondisease-specific problems and all-cause mortality in the REasons for Geographic and Racial Differences in Stroke study. J Am Geriatr Soc. 2013;61:73. doi: 10.1111/jgs.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith MEB, Haney E, McDonagh M, Pappas M, Daeges M, Wasson N, et al. Treatment of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Systematic Review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162(12):841. doi: 10.7326/M15-0114. [DOI] [PubMed] [Google Scholar]
- 43.Wu S, Kutlubaev MA, Chun H-YY, Cowey E, Pollock A, et al. Interventions for poststroke fatigue. Cochrane Database of Syst Rev? 2015;7:CD007030. doi: 10.1002/14651858.CD007030.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan F, Amatya B, Galea M. Management of Fatigue in Persons with Multiple Sclerosis. Front Neurol. 2014;5:177. doi: 10.3389/fneur.2014.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cramp F, Hewlett S, Almeida C, Kirwan JR, Choy EH, Chalder T, et al. Non-pharmacological interventions for fatigue in rheumatoid arthritis. Cochrane Database of Syst Rev? 2013;8:CD008322. doi: 10.1002/14651858.CD008322.pub2. [DOI] [PubMed] [Google Scholar]
- 46.Unbehaun T, Spiegelhalder K, Hirscher V, Riemann D. Management of insomnia: update and new approaches. Nat Sci Sleep. 2010;2:127–38. doi: 10.2147/nss.s6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuo FE, Sullivan WC. Aggression and Violence in the Inner City: Effects of Environment via Mental Fatigue. Environment and Behavior. 2001;33:543–71. [Google Scholar]
- 48.Hickie IB, Hooker AW, Bennett BK, Hadzi-Pavlovic D, Wilson AJ, Lloyd AR. Fatigue in selected primary care settings: sociodemographic and psychiatric correlates. Medical Journal of Australia. 1996;164:585–8. doi: 10.5694/j.1326-5377.1996.tb122199.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.