Summary
Background
It is unclear whether adjuvant or early salvage radiotherapy following radical prostatectomy is more appropriate for men who present with localised or locally advanced prostate cancer. We aimed to prospectively plan a systematic review of randomised controlled trials (RCTs) comparing these radiotherapy approaches.
Methods
We used a prospective framework for adaptive meta-analysis (FAME), starting the review process while eligible trials were ongoing. RCTs were eligible if they aimed to compare (immediate) adjuvant radiotherapy (ART) versus early salvage radiotherapy (SRT), following radical prostatectomy in men with intermediate or high-risk, localised or locally advanced prostate cancer. We searched trial registers and conference proceedings until April 2019 to identify eligible RCTs. By establishing the ARTISTIC collaboration with relevant trialists, we were able to anticipate when eligible trial results would emerge, and we developed and registered a protocol prior to knowledge of the trial results (CRD42019132669). We included a harmonised definition of PSA-driven, event-free survival (EFS), and predicted when we would have sufficient power to assess whether ART was superior to SRT. Investigators supplied results for EFS, both overall and within pre-defined patient subgroups. Hazard ratios (HRs) for the effects of radiotherapy timing on EFS and subgroup interactions were combined using fixed-effect meta-analysis.
Findings
We identified 3 eligible trials and were able to obtain updated EFS results for 2153 men (100% of those randomised). Median follow-up ranged from 60 to 78 months. 1075 men were randomised to receive ART and 1078 to a policy of SRT, of whom, 421 (39%) had commenced treatment at the time of analysis. Patient characteristics were balanced within trials and overall. Men had median age of around 65 years and most (78%) had a Gleason sum score of 7. All trials were assessed as having low risk of bias.
Based on 270 EFS events, the meta-analysis showed no evidence that EFS was improved with ART compared to a policy of SRT (HR=0.95, 95% CI=0.75-1.21, p=0.70), with only a 1% change in 5-year EFS (89% vs. 88%). Results were consistent across trials (heterogeneity p=0.18; I2=42%). Although power is limited, we did not see any strong evidence of a difference in the treatment effect according to any of the patient or disease characteristics assessed.
Interpretation
This collaborative, and prospectively-designed systematic review and meta-analysis suggests that ART does not improve EFS in men with localised or locally advanced prostate cancer. Until data on long-term outcomes are available, early salvage treatment would seem the preferable treatment policy as it offers the opportunity to spare many men from RT and its associated side-effects.
Funding
This work is funded by the UK Medical Research Council study (MC_UU_12023/25)
Keywords: Prostate Cancer, radiotherapy, systematic review, prospective meta-analysis
Introduction
It is unclear whether adjuvant or early salvage radiotherapy following radical prostatectomy is more appropriate for men who present with localised or locally advanced prostate cancer. Three published randomised controlled trials (RCTs)(1–3) showed that adjuvant radiotherapy to the prostate bed gave better biochemical control than no adjuvant radiotherapy. However, results were inconsistent regarding the longer-term outcomes of progression-free survival, metastases-free survival and overall survival. Adjuvant radiotherapy was not universally recommended in these patients therefore, and uptake of adjuvant radiotherapy has been variable(4). Easier access to more sensitive PSA tests has enabled earlier detection of biochemical relapse, and the possibility of earlier salvage treatment. As a result, trials comparing adjuvant radiotherapy (ART) and early salvage radiotherapy strategies (SRT) were initiated independently (5–7).
The three trials focused on different primary outcomes (time free of metastases (RADICALS(5)); event-free survival (GETUG-AFU 17(6)); and biochemical failure (RAVES(7)), and each was powered accordingly. Investigators acknowledged the difficulty in adequately powering these trials for longer-term, definitive outcomes due to the relatively good prognosis of these men. Therefore, there was a clear need to synthesise the results of these trials in a systematic review, to give a more reliable answer as to whether ART or SRT is most appropriate.
In 2014, whilst recruitment to all three trials was ongoing, representatives from the RADICALS, GETUG-AFU 17 and RAVES trial teams and the Meta-analysis Group of the MRC Clinical trials Unit at UCL met to discuss the feasibility and value of a prospectively designed individual participant data (IPD) meta-analysis of the three trials (8). However, recognising that IPD would not be available from the trials until long-term follow-up is completed, we planned an aggregate data systematic review in the first instance. Such systematic reviews are usually planned retrospectively, with prior knowledge of some or all trial results, which can introduce potential bias in to the review and meta-analysis methods. Instead, under the auspices of the ARTISTIC collaboration, we began to prospectively plan a systematic review and series of meta-analyses before trial results were known(9), to assess the effects of ART versus SRT in these men(9).
Methods
All methods were pre-specified in a protocol, submitted (April 2019) for registration in PROSPERO, prior to data collection or analysis (CRD42019132669). We used a prospective framework for adaptive meta-analysis (FAME (9)), which reduces the likelihood of bias in the selection of studies, assessment of risk of bias, outcome definition, and in the timing and conduct of planned analyses. The approach has been used in six prior systematic review in prostate cancer(10–12). In summary, we applied FAME key principles: 1) starting the systematic review process whilst all trials were ongoing or yet to report; 2) searching comprehensively for all published, unpublished and eligible trials; 3) liaising with trial teams to develop and maintain a detailed picture of how information and results are likely to accumulate; 4) predicting the feasibility and timing of reliable meta-analysis; 5) interpreting results taking account of available and unavailable data, and assessing the value of updating the systematic review and meta-analysis.
Eligibility criteria
All eligible trials should have randomised men with intermediate or high-risk, localised or locally advanced prostate cancer, with no evidence of distant metastases, and who had a radical prostatectomy prior to enrolment into the trial. They should have aimed to compare ART versus a policy of deferred, early SRT following radical prostatectomy. Randomisation should have precluded prior knowledge of treatment assigned, and should have occurred more than 4 weeks but no longer than 22 weeks after radical prostatectomy. Men should have had post-operative PSA not greater than 0.2ng/ml, and had one or more high risk features including pT stage 3 or 4; Gleason 7-10; pre-operative PSA≥10ng/ml and / or positive surgical margins. They should not have received either prior radiotherapy, or androgen deprivation therapy (pre- or post- prostatectomy).
For this prospective systematic review and meta-analysis of aggregate data(8, 9), we aimed to include trials that were still recruiting patients.
Search strategy
Eligible trials were identified through searches of ClinicalTrials.gov and the WHO trials registry platform. We used prostate cancer and radiotherapy as keywords, to be as inclusive as possible, and limited the search results to randomised controlled trials. We also searched the online archive of conference abstracts from the American Society of Clinical Oncology (ASCO) and ASCO Genitourinary Cancer Symposium using the terms prostate, radiotherapy and random; and reviewed all submitted abstracts in the genitourinary and prostate cancer sessions of the European Society of Medical Oncology (ESMO) annual meeting (2016-2019) from to identify reports of any additional eligible trials, limiting the search using the term radiotherapy. Searches were carried out initially in May 2014 and updated periodically until final submission of the manuscript in July 2020.
Outcomes
The primary outcome measure for this first stage of the meta-analysis is event-free survival (EFS). We agreed a harmonised definition of EFS as the time from randomisation until the first evidence of either: biochemical recurrence (PSA ≥0.4ng/ml and rising after completion of any post-operative radiotherapy); clinical progression/radiological progression; initiation of a non-trial treatment; death from prostate cancer; or a PSA level of ≥ 2.0 ng/ml at any time after randomisation. Patients last reported as alive with no recorded clinical or biochemical event or non-trial treatment initiated were censored on the date of most recent follow-up. Patients without an EFS event who died from causes other than prostate cancer were censored on the date of death.
We also planned to assess the effects of radiotherapy timing on time free of metastases, prostate-cancer specific survival and overall survival in subsequent staged meta-analyses, to be conducted when we have sufficient statistical power.
Data collection
Data relating to the trial designs, in particular in relation to the methods of randomisation, were extracted from trial protocols and supplemented by trialists. We also sought summaries of patient baseline characteristics (age, PSA, performance status, tumour stage, Gleason sum score, surgical margins, seminal vesicle involvement, extracapsular extension, lymph node involvement) and interventions, and results for the outcome of EFS overall and within predefined patient subgroups directly from the trial teams.
Risk of Bias
Risk of bias assessments were carried out for each of the trials for the outcome of EFS, using the Cochrane risk of bias 2 tool(13, 14). This amendment of our protocol was to reflect the recent release of the revised tool. A low risk of bias was desirable for all domains.
Analysis
Prospectively planning the meta-analysis
We anticipated that approximately 120 events would have occurred in the SRT arm across the three trials(5–7) by autumn 2019. Assuming a 5-year baseline survival of 88%, we anticipated this would give >90% power to detect a 5% difference in EFS between immediate and early salvage radiotherapy and >99% power for a 10% difference. This provided a firm basis and for planning a reliable meta-analysis of trial results.
As events for the longer-term outcomes are accumulating slowly, there is insufficient power to assess the effects on these. Therefore, we will review control arm event rates for these outcomes regularly, and will carry out further planned meta-analyses following a similar process.
Measures of treatment effect and data synthesis
For the primary analysis, we combined the hazard ratios across trials using the fixed-effect model(15) to give a pooled hazard ratio representing the overall risk of an event on ART compared with SRT. Chi-square heterogeneity tests and the I2 statistic (16)were used to assess statistical heterogeneity and a DerSimonian and Laird random effects model(17) was also used to assess the robustness of the results to the choice of model.
Provided there were sufficient data available, we aimed to assess whether the treatment effect varied according to whether or not the trials included planned use of hormone therapy. We also planned to investigate whether the treatment effect was consistent across subgroups of men. Subgroups were defined by: pre-surgical PSA (≤10 ng/ml, >10ng/ml); Gleason score (≤6, 7, ≥8); involvement of seminal vesicles (involved / not involved); surgical margins (positive / negative) and CAPRA-S(18) risk group (Low / Intermediate and High), which takes into account a number of patient and disease characteristics at baseline in order to predict risk(18). Individual interaction HRs for each trial were calculated from the ratio of the estimated HRs for each subgroup (e.g., the HR for Low risk divided by the HR for Intermediate and High risk >=8) and combined these across trials using a fixed-effect meta-analysis(19, 20). All p-values were two-sided.
Role of the funding source
The funding body for ARTISTIC (UK Medical Research Council, MC_UU_12023/25) had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the results included in the study, although not to the underlying trial data, and had final responsibility for the decision to submit for publication.
Results
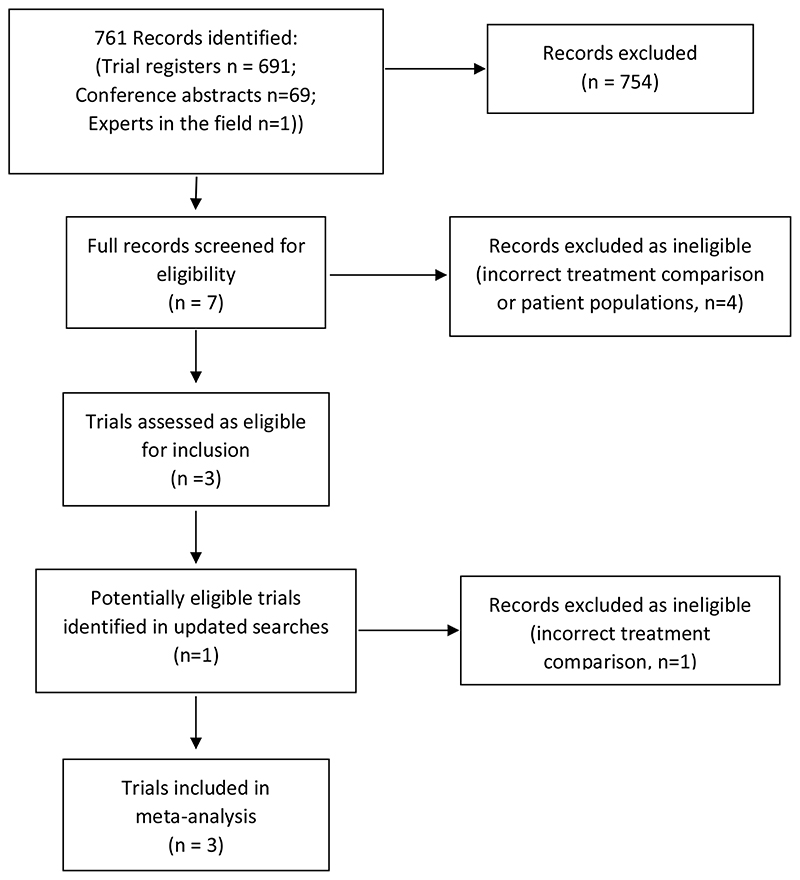
Our initial searches of clinical trial registers and conference abstract searches retrieved 760 records. One additional trial was identified through discussion with the RADICALS-RT trial investigators. After removing duplicates and clearly ineligible records, we screened seven potentially eligible trials (Figure 1). Four trials were excluded either because they made a different treatment comparison and/or because they were conducted in patients with more advanced disease. Three trials, RADICALS-RT, GETUG-AFU 17 and RAVES were retained as being eligible for inclusion. Updated searches conducted in 2016 identified a further potentially eligible trial, however, this was subsequently excluded because it compared adjuvant RT to no RT, rather than with an early salvage policy(21).
Figure 1. PRISMA diagram showing study identification.
RADICALS-RT(5) recruited 1396 patients in UK, Denmark, Canada and Ireland from November 2007 until December 2016; GETUG-AFU 17(6) recruited 424 patients in France between April 2008 and June 2016; and RAVES(7) recruited 333 patients in Australia and New Zealand between March 2009 and December 2015. Median follow-up ranged from 60 months to 73 months. Whilst the GETUG-AFU 17(6) and RADICALS-RT(5) trials were designed to assess whether ART was superior to SRT, the RAVES trial(7) was designed to assess whether SRT was non-inferior to ART in terms of biochemical failure. The RT schedule was similar in all trials, 64Gy in 32 fractions or 66Gy in 33 fractions. RADICALS-RT also permitted 52.5Gy in 20 fractions. For all trials, patients randomised to receive ART should have commenced it within 6 months after surgery. SRT was triggered at a level of 0.2ng/ml PSA for RAVES; at 0.2ng/ml and rising in GETUG-AFU 17; and 0.1ng/ml or 3 consecutive rises still below 0.1ng/ml for RADICALS-RT. Initiation of SRT following these triggers varied across the trials, as did intended use of hormone therapy RT (Table 1). All of the included trials were judged to have low risk of bias (Table 2).
Table 1. Trial Characteristics.
| Trial | Accrual period | Key eligibility criteria | Use of hormone therapy | RT field | Radiotherapy (RT) schedule | Adjuvant RT timing | Early salvage RT timing | Trigger for early salvage RT | Primary outcome measure | Trial design |
|---|---|---|---|---|---|---|---|---|---|---|
| RADICALS- RT(5) | 11/2007 – 12/2016 | 1 or more of: Positive margins pT3a / pT3b / pT4 Gleason 7-10 | Men could chose to enter a second randomisation to no hormones / 6m / 24m. Men not randomised could receive hormone therapy off protocol | RT to prostate bed | 66/33# OR 52.5/20# | ≤ 6m of radical prostatectomy | ≤ 2m of trigger PSA | PSA > 0.1 ng/ml and rising OR 3 consecutive rising PSA levels | Freedom from distant metastases | Superiority |
| GETUG- AFU 17(6) | 04/2008 – 06/2016 | pT3a / pT3b / pT4 and Positive margins and extracapsular extension | All men received hormone therapy alongside RT either in the adjuvant or early salvage setting | RT to prostate bed | 66/33# | ≤ 6m of radical prostatectomy | As soon as possible after PSA relapse and before PSA=1ng/ml | PSA ≥ 0.20 ng/ml and rising | Event free survival | Superiority |
| RAVES(7) | 03/2009 – 12/2015 | pT2 / pT3a / pT3b and either Positive margins or extracapsular extension | No use of hormone therapy | RT to prostate bed | 64/32# | ≤ 6m of radical prostatectomy | ≤ 4m of trigger PSA | PSA ≥ 0.20 ng/ml | Freedom from biochemical failure | Non-inferiority |
PSA= prostate specific antigen; RT= radiotherapy
Table 2. Risk of bias assessment.
| Domain | RADICALS-RT (5) | GETUG-AFU-17(6) | RAVES(7) |
|---|---|---|---|
| 1. Risk of bias arising from the randomisation process |
Low risk
Was allocation sequence random? YES, minimisation with stratification by Gleason sum score, margin status, RT schedule and study centre. Was allocation sequence concealed? YES, central randomisation at the MRC Clinical Trials Unit at UCL using a computer-implemented algorithm Did baseline differences suggest a problem? NO, arms are well balanced |
Low risk
Was allocation sequence random? YES, minimisation with stratification by study centre, pT stage and Gleason grade to avoid significant imbalances between the arms Was allocation sequence concealed? YES, central randomisation using an internet based service, or via central randomisation at the Institut Bergonié Did baseline differences suggest a problem? NO, arms are well balanced |
Low risk
Was allocation sequence random? YES, minimisation algorithm. Patients are stratified by pre-operative PSA; Gleason score; margin positivity; seminal vesicle involvement; and radiotherapy institution Was allocation sequence concealed? YES, internet based randomisation system Did baseline differences suggest a problem? NO, arms are well balanced |
| 2. Risk of bias due to deviations from the intended interventions |
Low risk
Were participants aware of their assigned intervention during the trial? Yes – blinding is not possible in a radiotherapy trial Were carers and people delivering the interventions aware of participants’ assigned intervention during the trial? Yes – blinding is not possible in a radiotherapy trial Were there deviations from the intended intervention that arose because of the trial context? No – the trial context did not cause changes to intervention Was an appropriate analysis used to estimate the effect of assignment to intervention? Yes – full ITT analysis provided for the metaanalysis outcome of EFS |
Low risk
Were participants aware of their assigned intervention during the trial? Yes – blinding is not possible in a radiotherapy trial Were carers and people delivering the interventions aware of participants’ assigned intervention during the trial? Yes – blinding is not possible in a radiotherapy trial Were there deviations from the intended intervention that arose because of the trial context? No – the trial context did not cause changes to intervention Was an appropriate analysis used to estimate the effect of assignment to intervention? Yes – full ITT analysis provided for the metaanalysis outcome of EFS |
Low risk
Were participants aware of their assigned intervention during the trial? Yes – blinding is not possible in a radiotherapy trial Were carers and people delivering the interventions aware of participants’ assigned intervention during the trial? Yes – blinding is not possible in a radiotherapy trial Were there deviations from the intended intervention that arose because of the trial context? No – the trial context did not cause changes to intervention Was an appropriate analysis used to estimate the effect of assignment to intervention? Yes – full ITT analysis provided for the metaanalysis outcome of EFS |
| 3. Risk of bias due to missing outcome data |
Low risk
Were data available for all, or nearly all, participants randomized? YES, results for event-free survival provided for all patients randomised |
Low risk
Were data for this outcome available for all, or nearly all, participants randomized? YES, results for event-free survival provided for all patients randomised |
Low risk
Were data for this outcome available for all, or nearly all, participants randomized? YES, results for event-free survival provided for all patients randomised |
| 4. Risk of bias in measurement of the outcome |
Low risk
Was method of measuring the outcome inappropriate? NO, used an agreed meta-analysis definition of event-free survival that was suitable across each different trial designs Could measurement of the outcome have differed between intervention groups? NO, used an agreed definition of event-free survival that was suitable for the different intervention groups, and before results were known Outcome assessor aware of intervention received? YES, but unlikely to influence PSA based biochemical failure (the dominant event in the composite outcome). Relatively few clinical and radiological progressions or deaths were reported and unlikely to be affected by outcome assessor |
Low risk
Was method of measuring the outcome inappropriate? NO, used an agreed meta-analysis definition of event-free survival that was suitable across each different trial designs Could measurement of the outcome have differed between intervention groups? NO, used an agreed definition of event-free survival that was suitable for the different intervention groups, and before results were known Outcome assessor aware of intervention received? YES, but unlikely to influence PSA based biochemical failure (the dominant event in the composite outcome). Relatively few clinical and radiological progressions or deaths were reported and unlikely to be affected by outcome assessor |
Low risk
Was method of measuring the outcome inappropriate? NO, used an agreed meta-analysis definition of event-free survival that was suitable across each different trial designs Could measurement of the outcome have differed between intervention groups? NO, used an agreed definition of event-free survival that was suitable for the different intervention groups, and before results were known Outcome assessor aware of intervention received? YES, but unlikely to influence PSA based biochemical failure (the dominant event in the composite outcome). Relatively few clinical and radiological progressions or deaths were reported and unlikely to be affected by outcome assessor |
| 5. Risk of bias in selection of the reported result |
Low risk
Were the data that produced this result analysed in accordance with a pre-specified analysis plan finalized before unblinded outcome data were available for analysis? YES, the data were analysed and supplied in accordance with the meta-analysis protocol that was registered before trial results were known. This is distinct from the trial analysis. |
Low risk
Were the data that produced this result analysed in accordance with a pre-specified analysis plan finalized before unblinded outcome data were available for analysis? YES, the data were analysed and supplied in accordance with the meta-analysis protocol that was registered before trial results were known. This is distinct from the trial analysis. |
Low risk
Were the data that produced this result analysed in accordance with a pre-specified analysis plan finalized before unblinded outcome data were available for analysis? YES, the data were analysed and supplied in accordance with the meta-analysis protocol that was registered before trial results were known. This is distinct from the trial analysis. |
| Overall judgement | Low risk | Low risk | Low risk |
PSA= prostate specific antigen; RT= radiotherapy
All three trials aimed to recruit men with localised or locally advanced prostate cancer, with similar, but non-identical, definitions: RADICALS-RT allowed men with pT3 and pT4 disease; GETUG-AFU 17 was restricted to men with pT3 or pT4a (with bladder neck invasion) disease and positive surgical margins (R1) only; and the RAVES trial included men with at least one of positive margins (pT2 or pT3) or extracapsular extension (pT3). Furthermore, men without extracapsular extension were excluded from the GETUG AFU-17 trial, but not from the RAVES or RADICALS trials.
The baseline characteristics of the included men largely represent the eligibility criteria of the three trials (Table 3). Median age was 64 (GETUG-AFU 17 and RAVES) or 65 years (RADICALS-RT) with men ranging in age from 37 years to 79 years. The majority of men had either stage pT3a or b disease (1719/2153, 80%), positive surgical margins (1526/2153, 71%) and extracapsular extension (1656/2153, 77%).
Table 3. Patient Characteristics.
| RADICALS-RT(5) | GETUG-AFU 17(6) | RAVES(7) | ||||
|---|---|---|---|---|---|---|
| ART | SRT | ART | SRT | ART | SRT | |
| Patients randomised | 697 | 699 | 212 | 212 | 166 | 167 |
| Median follow up (range) | 60 months (2-132 months) | 75 months (0 – 130 months) | 78 months (1- 122 months) | |||
| Median Age (interquartile range) | 65 (60-68) | 65 (60-68) | 64 (60-68) | 64(59-68) | 64 (60-68) | 64 (59 – 68) |
| Median pre-operative PSA (interquartile range) | 7.8 (5.8-11.4) | 8.0 (5.6-11.6) | Not available | Not available | 7.4 (5.5-10.2) | 7.4 (5.3 – 10.4) |
| Stage | ||||||
| pT2 | 163 (23%) | 176 (25%) | 0 | 0 | 37 (22%) | 39 (23%) |
| pT stage 3a/b | 529 (76%) | 519 (74%) | 208 (99%) | 206 (98%) | 129 (78%) | 128 (77%) |
| pT4 | 5 (1%) | 4 (1%) | 3 (1%) | 5 (2%) | 0 | 0 |
| Gleason score | ||||||
| ≤6 | 48 (7%) | 48 (7%) | 21 (10%) | 22 (10%) | 8 (5%) | 8 (5%) |
| 7 | 537 (77%) | 528 (76%) | 173 (82%) | 167 (78%) | 132 (80%) | 134 (80%) |
| ≥8 | 112 (16%) | 123 (17%) | 17 (8%) | 23 (11%) | 26 (16%) | 25 (15%) |
| Positive margins | 439 (63%) | 443 (63%) | 211 (100%) | 210 (100%) | 110 (66%) | 113 (68%) |
| Seminal vesicle involvement | ||||||
| Yes | 129 (19%) | 132 (19%) | 44 (21%) | 46 (22%) | 31 (19%) | 33 (20%) |
| No | 568 (81%) | 567 (81%) | 167 (79%) | 165 (78%) | 135 (81%) | 134 (80%) |
| Unknown | 0 | 0 | 1 | 1 | 0 | 0 |
| Extracapsular extension | ||||||
| Yes | 492 (71%) | 483 (69%) | 212 (100%) | 212 (100%) | 129 (78%) | 128 (77%) |
| No | 205 (29%) | 215 (31%) | 0 | 0 | 37 (22%) | 39 (23%) |
| Unknown | 0 | 1 | 0 | 0 | 0 | 0 |
| Lymph node involvement | ||||||
| Involved | 38 (10%) | 28 (7%) | 0 | 0 | 1 (<1%) | 0 |
| Not involved | 335 (90%) | 374 (93%) | 212 (100%) | 212 (100%) | 165 (99%) | 167 (100%) |
| Nx | 324 | 297 | 0 | 0 | 0 | 0 |
| CAPRA-S Risk group* | Not estimable | Not estimable | ||||
| Low (0-2) | 58 (8%) | 55 (8%) | 22 (13%) | 21 (13%) | ||
| Intermediate (3-5) | 382 (55%) | 384 (55%) | 100 (60%) | 98 (59%) | ||
| High (6+) | 257 (37%) | 260 (37%) | 44 (27%) | 48 (29%) | ||
The GETUG AFU-17 trial did not record pre-operative PSA levels and therefore, CAPRA-S scores(18) which comprise scores based on a number of patient and disease characteristics at baseline, including pre-operative PSA levels, cannot be calculated for the trial.
PSA= prostate specific antigen; ART= Adjuvant radiotherapy; SRT = early salvage radiotherapy
Effects of RT timing on EFS
We were able to include updated EFS results for 2053 men, representing 100% of men randomised in the three trials, and 270 events had been recorded. 1075 men were randomised to receive ART and 1078 to a policy of early salvage RT. At the time of this analyses, only 421 men (39%) randomised to early salvage RT had received post-operative RT. Although EFS events were dominated by biochemical failures, as expected, the proportion of patients free of biochemical failure at 5 years was high (RAVES: 87%; RADICALS-RT: 88% and GETUG-AFU 17: 94%).
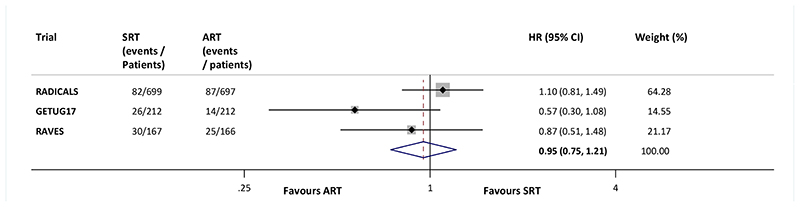
Pooling the EFS results of the three trials in a meta-analysis gives an overall fixed effect HR of 0.95 (95%CI 0.75 to 1.21, p=0.70). With a baseline EFS rate of 88% at 5 years, this translated to no difference between SRT and ART, at 5 years (1%, 95% CI: -2% to 3%). Although RADICALS is the largest trial, the other two trials are contributing almost 40% of the total weight to the meta-analysis. Results were broadly consistent across trials (Heterogeneity p=0.18, Inconsistency I2=42%); Fig. 2), and the results from a random effects model were very similar (HR=0.89, 95% CI 0.62 to 1.27, p=0.52).
Figure 2. Effect of radiotherapy timing on event free survival.
Each filled square denotes the HR for that trial comparison, with the horizontal lines showing the 95% confidence interval (CI). The size of the square is directly proportional to the amount of information contributed by a trial. The diamond represents a (fixed-effect) meta-analysis of the trial HRs, with the centre of this diamond indicating the HR and the extremities the 95% CI. ART= Adjuvant radiotherapy; SRT= early salvage radiotherapy; HR = hazard ratio; CI = confidence interval
Effects of RT timing on EFS by patient characteristics
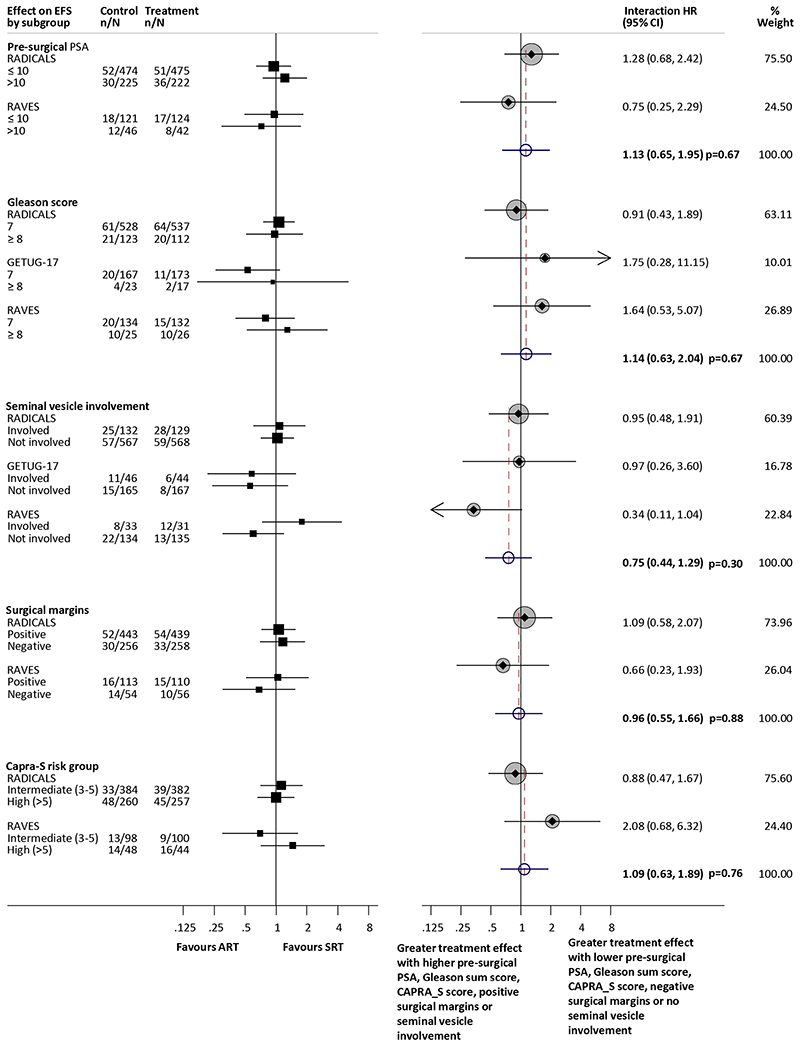
Results were supplied for the effect of radiotherapy timing on EFS by all pre-specified subgroups for the RADICALS-RT and RAVES trials. However, the GETUG-AFU 17 trial did not record pre-operative PSA, and all men had positive surgical margins and extracapsular extension. Therefore GETUG-AFU 17 has not been included in the analysis of EFS by pre-operative PSA, surgical margins or CAPRA_S risk group. Furthermore, due to the very low numbers of events reported in men with a Gleason Sum score of ≤6 and for Low CAPRA_S risk group for both the RAVES and RADICALS trials, it was not possible to estimate a HR within these groups. Therefore, the interaction analysis of Gleason sum score compares EFS in men with sum scores of 7 with those who have a score of ≥8 and the analysis of CAPRA_S risk group compares EFS in men with intermediate (3–5) and high (>5) risk Based on the available data, there was no good evidence that the effect on EFS of adjuvant radiotherapy varied according to any of our predefined subgroups: pre-surgical PSA (interaction HR=1.13, 95%CI 0.65–1.95, p=0.67, Gleason sum score (interaction HR=1.14, 95% CI 0.63–2.04, p=0.67), seminal vesicle involvement (interaction HR=0.75, 95%CI 0.44–1.29, p=0.30), surgical margins (interaction HR=0.96, 95% CI 0.55–1.66, p=0.88) or CAPRA_S risk group (interaction HR=1.09, 95% CI 0.63-1.89, p=0.76; Fig. 3).
Figure 3. Effect of radiotherapy timing on EFS by pre-surgical PSA (ng/ml), Gleason sum score, seminal vesicle involvement, surgical margins and CAPRA-S risk group.
Each filled square denotes the HR for each subgroup of men defined by, age at randomisation, performance status, clinical T stage, and Gleason sum score within each trial, with the horizontal lines showing the 95% confidence interval (CI). The size of the square is directly proportional to the amount of information contributed by a subgroup. Each filled circle denotes the HR for the interaction between the effect of radiotherapy and these subgroups for each trial, with the horizontal lines showing the 95% CI. The size of each circle is directly proportional to the amount of information contributed by a trial. The open circle represents a (fixed-effect) meta-analysis of the interaction HRs, with the horizontal line showing the 95% CI. ART= Adjuvant radiotherapy; SRT= early salvage radiotherapy; HR = hazard ratio; CI = confidence interval
Discussion
Summary of results
Based on our findings, the systematic use of ART following prostatectomy does not improve PSA-driven EFS in men with localised or locally advanced prostate cancer. EFS rates are high, at around 88% after 5 years in both groups, despite around 60% of men randomised to receive SRT not having initiated treatment by the time of this analysis. There was no evidence to suggest that the effect of adjuvant RT on EFS varied according to pre-surgical PSA, Gleason sum score, seminal vesicle involvement, surgical margins or CAPRA_S risk group.
Strengths
By using the prospective FAME approach, and working collaboratively with trialists, we have been able to overcome some of the limitations associated with a standard aggregate data meta-analysis. Firstly, we reduced the potential for bias in the selection of studies by specifying eligibility criteria and conducting searches for eligible trials whilst they were ongoing or unreported. We also limited the potential for bias in the analysis, by harmonising the EFS outcome definition and planning all analyses (including subgroup analyses) in advance of the trial results being known. This is further reflected in a low risk of bias assessment for each domain for each trial. Furthermore, working with the trialists we were able to included up-to-date EFS results from 100% of men randomised in all eligible trials, and the timing of this analysis was determined based on having sufficient power. Therefore the meta-analysis represents the totality of randomised evidence about the effects of radiotherapy timing in men with localised or locally advanced prostate cancer, and our prospective and collaborative approach has allowed a more consistent, thorough and timely investigation of effects than is typically possible with aggregate data meta-analysis. The results provide context for the individual trials and maximise their usefulness and impact on clinicians, patients and policy makers.
For the trial teams, involved in the ARTISTIC collaboration, prospectively planning the systematic review and meta-analysis has helped the trialists to re-assure participants and funders that there was value in continuing, and an IDMC for one of the trials that the primary outcome should be amended. It has also provided an opportunity to discuss and resolve issues and ultimately to address the clinical questions the trials set out to answer. In this way, the ARTISTIC collaboration has operated in much like that seen in IPD meta-analysis, and prospective IPD meta-analysis (9, 22).
Limitations
Prospective meta-analysis typically utilises individual participant data (IPD), and the advantages of collecting IPD for meta-analysis are well documented(22, 23) but IPD for these trials will not be available for many years. Therefore, to obtain an early signal regarding the impact of radiotherapy timing on the intermediate outcome of EFS, we adopted a prospective and collaborative aggregate data approach. Despite exceeding the anticipated number of events needed to detect an absolute improvement of 5% with ART with 90% power, we did not have sufficient power to detect a very small (<5%) benefit. That said, we found no evidence of an absolute effect of ART on 5-year EFS (0% (95% CI -1% to 3%). Given that the large benefits of radiotherapy on early biochemical outcomes in men with prostate cancer both in the localised or locally advanced(1–3) and metastatic settings(12) have failed to translate into clear long-term benefits, a clinically meaningful benefit of ART would seem unlikely. However, as there is no evidence currently that PSA-failure is a reliable surrogate of survival or other clinically-driven outcomes in the localised prostate cancer setting, the ARTISTIC collaboration will continue to work together to monitor accumulating events across the trials and plan meta-analyses of the long term outcomes.
Although the three trials have results that are broadly consistent, we were unable to explore the effect of giving hormone therapy alongside RT on EFS as we had planned. GETUG-AFU 17 gave concomitant radiotherapy and hormone therapy; RAVES used radiotherapy alone; and RADICALS included an optional second randomisation to either long (24m) or short (6m) duration hormones or to no hormones. Men who did not opt for this randomisation could receive hormones off-protocol. Whilst it may be tempting to speculate that use of concomitant hormone treatment may modify the effect of radiotherapy timing on EFS, power in the GETUG-AFU 17 trial is limited. Therefore, until the results of the RADICALS hormone duration randomisation are available, the overall HR of 0.98 for EFS remains the most reliable.
Due to the low event rate overall the power of the patient subgroup analyses is limited. Nevertheless, we do not see any indication of a benefit of ART in any of the subgroups assessed and therefore based on the evidence available our main conclusion holds true across for all patients included in the meta-analysis. As very few patients across all three trials had nodal involvement (N+ disease), we were unable to assess the effect radiotherapy timing in this population.
Context of what is known
Prior RCTs assessing the effects of ART in localised and locally advanced prostate cancer did not compare the approach with a policy of early salvage treatment. Indeed one criticism of the earlier trials (1, 2) was that relatively few men randomised to observation received SRT at all, and those who did had relatively high PSA levels before SRT was initiated. In the more recent Finnish trial(21), although 86% of men randomised to the observation arm were reported to have received SRT, median PSA levels were 0.7ng/ml at the time SRT was initiated. Thus, the SRT policy cannot be considered to be ‘early’ as in the three trials included in this meta-analysis. Like the earlier trials, the Finnish trial concluded that there was a large improvement in biochemical recurrence with ART compared to observation, but evidence of a clear benefit on longer-term clinical outcomes is lacking. When making treatment choices, the lack of evidence of a benefit of ART must be considered alongside adverse effects of this treatment. All three trials have reported increases in specific side-effects with ART, including increased urinary morbidity (RADICALS-RT); grade 2 or greater genito-urinary toxicity (RAVES) and grade 2 or greater late genito-urinary and erectile dysfunction toxicities (GETUG-AFU 17).
What this means for research and practice
Based on this prospectively designed meta-analysis, ART following prostatectomy does not improve PSA-driven event free survival compared to policies of early SRT in men with localised or locally advanced prostate cancer. Early salvage RT policies therefore seem to offer the opportunity to spare, or at least postpone, radiotherapy and thus associated adverse effects, for many men with no obvious disadvantage to EFS. Most men included in these trials do well – with around 88% remaining event-free 5 years after prostatectomy. Based on these findings, the likelihood that delaying RT would have a deleterious effect on longer-term outcomes is low, but we will complete further meta-analyses on these clinically important outcomes as data from the included trials mature.
Take home message / conclusions
We have found no clear evidence that ART offers an advantage over early SRT following prostatectomy for men diagnosed with locally advanced or localised prostate cancer. Furthermore, a high proportion of men remain event free following surgery with the salvage approach, which we believe should be considered as the standard of care. Guidelines and policy should be reviewed to reflect this.
Acknowledgements
The ARTISTIC project has been funded by the UK Medical Research Council through their funding of the MRC Clinical Trials Unit at UCL (Grant: MC_UU_12023/25). We are grateful to each of the RADICALS-RT, GETUG-AFU 17 and RAVES trials teams in particular to Holly Pickering, Howard Kynaston, Noel Clarke, Charles Catton, Peter Meidahl Petersen, Mark Frydenberg, Jarad Martin, Gillian Duschesne and Scott Willians (full details of all trial teams can be found in the individual trial publications) and all of the men who participated in these trials.
Footnotes
Author Contributions
All authors were involved in devising and agreeing the final protocol for this work. CV and DF carried out the analyses. CV drafted the manuscript with substantive input from JFT. All authors reviewed and commented on the draft manuscript and agreed the final version for submission. Pre-publication results from the trials were supplied with the permission of the trial teams and sponsors and were prepared and supplied for the analyses by CB, AC, CFB, CBr and SC.
Declaration of Interest
CV, DF, AK, MP, PR, AC, CB, MB, SC, SF, CBr and JT report no financial conflicts of interest in relation to this work. CP reports grants received from Bayer; personal fees received from Bayer and Janssen and other (including speaker fees, advisory board membership and honoraria) form Bayer, AAA and Janssen, outside the submitted work.
PS reports honoraria, speaker fees and advisory board fees from Ipsen, Astellas, Bouchara, Takeda and Ferring, during the conduct of the study; as well as other relationships and activities from Janssen, Bayer and Sanofi, all outside the submitted work.
CFB reports grants from New Zealand Health Research Council, Australian National Health and Medical Research Council, Auckland Hospital Charitable Trust, TROG Seed Funding, and Genesis Oncology Trust during the conduct of the study.
MS reports grants and non-financial support from Astellas, Clovis Oncology, Janssen, Novartis, Pfizer and Sanofi; and personal fees from Eli Lilly and Janssen, outside the submitted work. IL reports other financial relationships from Sanofi, Ipsen and Astellas, outside the submitted work.
MKP reports grants and non-financial support from Astellas, Clovis Oncology, Novartis, Pfizer, and Sanofi, outside the submitted work.
Contributor Information
Claire L Vale, MRC Clinical Trials Unit at UCL, London, UK.
David Fisher, MRC Clinical Trials Unit at UCL, London, UK.
Andrew Kneebone, Northern Sydney Cancer Centre, Sydney, Australia.
Christopher Parker, Royal Marsden NHS Foundation Trust and Institute of Cancer Research, Sutton, UK.
Maria Pearse, Auckland City Hospital, Auckland, New Zealand.
Pierre Richaud, Insitut Bergonie, Bordeaux, France.
Paul Sargos, Insitut Bergonie, Bordeaux, France.
Matthew R Sydes, MRC Clinical Trials Unit at UCL, London, UK.
Christopher Brawley, MRC Clinical Trials Unit at UCL, London, UK.
Meryem Brihoum, Unicancer, Paris, France.
Chris Brown, NHMRC Clinical Trials Centre, University of Sydney, Australia.
Sylvie Chabaud, Centre Léon Bérard, Lyon, France.
Adrian Cook, MRC Clinical Trials Unit at UCL, London, UK.
Silvia Forcat, MRC Clinical Trials Unit at UCL, London, UK.
Carol Fraser-Browne, Auckland City Hospital, Auckland, New Zealand.
Igor Latorzeff, Clinique Pasteur, Toulouse, France.
Mahesh KB Parmar, MRC Clinical Trials Unit at UCL, London, UK.
Jayne F Tierney, MRC Clinical Trials Unit at UCL, London, UK.
References
- 1.Bolla M, Poppel H, Collette L, van Cangh P, Vekemans K, da Pozzo L, et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911) The Lancet. 2005;366:572–8. doi: 10.1016/S0140-6736(05)67101-2. [DOI] [PubMed] [Google Scholar]
- 2.Thompson IM, Tangen CM, Paradelo J, Scott Lucia M, Troyer D, Medssing E, et al. Adjuvant Radiotherapy for Pathological T3N0M0 Prostate Cancer Significantly Reduces Risk of Metastases and Improves Survival: Long-Term Followup of a Randomized Clinical Trial. J Urol. 2009;181:956–62. doi: 10.1016/j.juro.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiegel T, Bottke D, Steiner U, Siegmann A, Golz R, Storkel S, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009;27:2924–30. doi: 10.1200/JCO.2008.18.9563. [DOI] [PubMed] [Google Scholar]
- 4.Sineshaw HM, Gray PJ, Efstathiou JA, Jemal A. Declining Use of Radiotherapy for Adverse Features After RadicalProstatectomy: Results From the National Cancer Data Base. Eur Urol. 2015;68:768–74. doi: 10.1016/j.eururo.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Parker C, Sydes MR, Catton C, Kynaston H, Logue J, Murphy C, et al. Radiotherapy and androgen deprivation in combination after local surgery (RADICALS): a new Medical Research Council/National Cancer Institute of Canada phase III trial of adjuvant treatment after radical prostatectomy. BJU Int. 2007;99(6):1376–9. doi: 10.1111/j.1464-410X.2007.06844.x. [DOI] [PubMed] [Google Scholar]
- 6.UNICANCER. Triptorelin and Radiation Therapy in Treating Patients Who Have Undergone Surgery for Intermediate-Risk Stage III or Stage IV Prostate Cancer 2008 [ClinicalTrials.gov trial record for NCT00667069] Available from: https://clinicaltrials.gov/ct2/show/NCT00667069?term=NCT00667069&rank=1.
- 7.Pearse M, Fraser-Browne C, Davis ID, Duchesne GM, Fisher R, Frydenberg M, et al. A phase III trial to investigate the timing of radiotherapy for prostate cancer with high-risk features: background and rationale of the RAVES trial (Radiotherapy Adjuvant Versus Early Salvage) BJU Int. 2014;113:7–12. doi: 10.1111/bju.12623. [DOI] [PubMed] [Google Scholar]
- 8.Seidler AL, Hunter KE, Cheyne S, Ghersi D, Berlin JA, Askie L. A guide to prospective meta-analysis. BMJ. 2019;367:l5342. doi: 10.1136/bmj.l5342. [DOI] [PubMed] [Google Scholar]
- 9.Thomas J, Askie LM, Berlin JA, Elliott J, Ghersi D, Simmonds M, et al. Prospective approaches to accumulating evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane; London: 2019. [Google Scholar]
- 10.Vale CL, Burdett S, Rydzewska LH, Albiges L, Clarke NW, Fisher D, et al. Addition of docetaxel or bisphosphonates to standard of care in men with localised or metastatic, hormone-sensitive prostate cancer: a systematic review and meta-analyses of aggregate data. Lancet Oncol. 2016;17(2):243–56. doi: 10.1016/S1470-2045(15)00489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rydzewska LHM, Burdett S, Vale CL, Clarke NW, Fizazi K, Kheoh T, et al. Adding abiraterone to androgen deprivation therapy in men with metastatic hormone-sensitive prostate cancer: A systematic review and meta-analysis. Eur J Cancer. 2017;84:88–101. doi: 10.1016/j.ejca.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burdett S, Boeve LM, Ingleby FC, Fisher DJ, Rydzewska LH, Vale CL, et al. Prostate radiotherapy for metastatic hormone-sensitive prostate cancer: a STOPCAP systematic review and meta-analysis. Eur Urol. 2019;76(1):115–24. doi: 10.1016/j.eururo.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:I4989. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT, Sterne JAC, Savovic J, Page MJ, Hrobjartsson A, Boutron I, et al. A revised tool for assessing risk of bias in randomized trials Cochrane Methods Cochrane Database of Systematic Reviews. 2016;10 doi: 10.1002/14651858.CD201601. [DOI] [Google Scholar]
- 15.Yusuf S, Collins R, Peto R, Furberg CD, Stampfer MJ, Goldhaber SZ, et al. Intravenous and intracoronary fibrinolytic therapy in acute myocardial infarction: Overview of results on mortality, reinfarction and side-effects from 33 randomized controlled trials. Eur Heart J. 1985;6:556–85. doi: 10.1093/oxfordjournals.eurheartj.a061905. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemporary clinical trials. 2007;28(2):105–14. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Cooperberg MR, Hilton JF, Carroll PR. The CAPRA-S score: A straightforward tool for improved prediction of outcomes after radical prostatectomy. Cancer. 2011;117(22):5039–46. doi: 10.1002/cncr.26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher DJ, Copas AJ, Tierney JF, Parmar MKB. A critical review of methods for the assessment of patient-level interactions in individual patient data (IPD) meta-analysis of randomised trials, and guidance for practitioners. J Clin Epidemiol. 2011;64:949–67. doi: 10.1016/j.jclinepi.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 20.Fisher DJ, Carpenter JR, Morris TP, Freeman SC, Tierney JF. Meta-analytical methods to identify who benefits most from treatments: daft, deluded, or deft approach? BMJ. 2017;356:j573. doi: 10.1136/bmj.j573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hackman G, Taari K, Tammela TL, Matikainen M, Kouri M, J T, et al. Randomised Trial of Adjuvant Radiotherapy Following Radical Prostatectomy Versus Radical Prostatectomy Alone in ProstateCancer Patients with Positive Margins or Extracapsular Extension. Eur Urol. 2019;76:586–95. doi: 10.1016/j.eururo.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Tierney JF, Vale CL, Riley R, Tudur Smith C, Stewart LA, Clarke M, et al. Individual participant data (IPD) meta-analyses of randomised controlled trials: Guidance on their use. PLoS Med. 2015;12(7):e1001855. doi: 10.1371/journal.pmed.1001855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart LA, Tierney JF. To IPD or Not to IPD? Advantages and disadvantages of systematic reviews using individual patient data. Eval Health Prof. 2002;25(1):76–97. doi: 10.1177/0163278702025001006. [DOI] [PubMed] [Google Scholar]