Abstract
G protein-coupled angiotensin II receptors, AT1R and AT2R, are integral components of the renin–angiotensin system (RAS) that regulates blood pressure and fluid balance in humans. While AT1R is a well-established target of angiotensin receptor blockers (ARBs) for managing hypertension and a prime system for studying biased signaling, AT2R has been recognized as a promising target against neuropathic pain and lung fibrosis. In this review, we discuss how recent structural advances illuminate ligand-binding modes and subtype selectivity, shared and distinct features of the receptors, their transducer-coupling patterns, and downstream signaling responses. We also underscore the key ATR aspects that require further studies to fully appreciate the mechanistic framework that fine-tunes their cellular and physiological functions, providing untapped potential for drug discovery.
Background and introduction to angiotensin receptors
Angiotensin II (AngII) (see Glossary) is a peptide hormone that plays a major role in the RAS via acting on AT1R and AT2R subtypes, which belong to the superfamily of G protein-coupled receptors (GPCRs) (Figure 1) [1]. AT1R is expressed in many different cell types and signals via classical Gq-mediated pathways to elicit various responses related to regulation of blood pressure, electrolyte and water balance, and renal function [1]. Small-molecule angiotensin receptors blockers (ARBs), including losartan, candesartan, telmisartan, eprosartan, valsartan, irbesartan, olmesartan, and azilsartan, are clinically used as highly effective antihypertensive drugs [2]. AT2R has typically low expression in adults, which can be upregulated during pathology, and does not signal via canonical G protein pathways; thus, its signaling mechanisms and function remain controversial [3]. In general, AT2R counterbalances the action of AT1R, while also offering tissue and cell protective function, for which it has been considered as a promising target against lung fibrosis [4]. An experimental AT2R-selective agonist C21 has recently entered phase II clinical trials for the treatment against lung damage in patients infected with COVID-19 (Clinical Trial Numberi NCT04452435). Additionally, antagonizing AT2R receptor using selective inhibitor EMA-401 has been shown effective for reducing neuropathic pain [5].
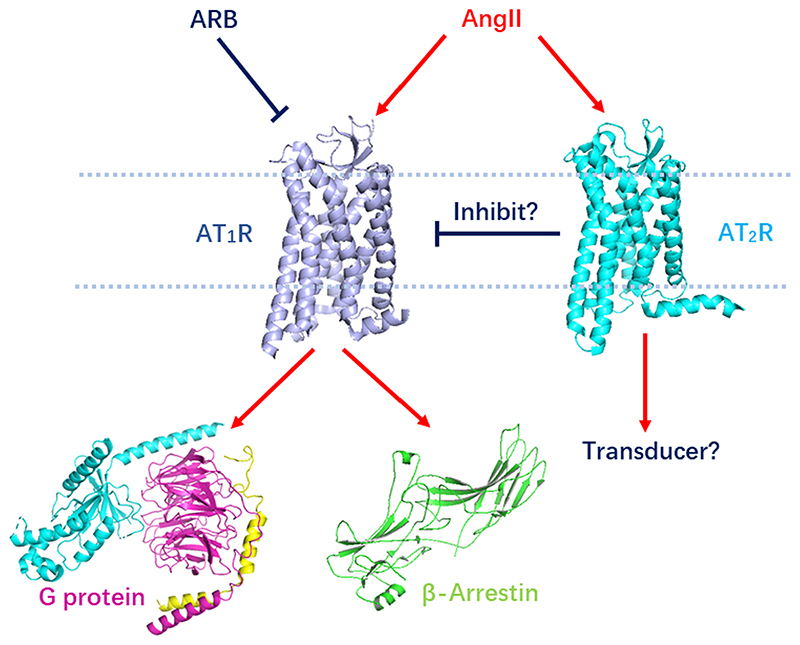
Figure 1. AT1R/AT2R signaling pathways.
AngII isthenatural ligand of AT1R and AT2R. AngII activates AT1R that signals through Gq protein and β arrestin, while the transducer of AT2R is still elusive. ARBs inhibit AT1R by blocking the AngII binding, while AT2R putatively inhibit AT1R by forming inactive AT1R–AT2R heterodimer. Abbreviations: AngII, angiotensin II; ARB, angiotensin receptor blocker; AT1R, angiotensin 1 receptor; AT2R, angiotensin 2 receptor.
Among the two subtypes of angiotensin receptors, AT1R is a prototypical GPCR that signals by engaging with two intracellular transducers, heterotrimeric G proteins and versatile scaffolding proteins called β-arrestins (βarr). In recent years, it has been shown that for some GPCRs either specific mutations [6,7] or ligands can selectively tilt the balance of engagement either towards G protein or βarr axis [8]; a phenomenon widely referred to as biased signaling [9,10]. Therapeutic efficacy of biased ligands is increasingly appreciated for their ability to reduce side effects usually associated with balanced ligands [11,12]. Therefore, understanding the underlying mechanisms and structural rearrangements in GPCRs, induced by biased ligands, is essential for designing the next generation of safer drugs. AT1R has been widely studied to understand the structural mechanism of bias. In fact, βarr-biased AT1R ligands result in improved cardiac performance even after exerting antihypertensive effects, making them preferred alternatives to clinically used ARBs [13].
During the past several years, many breakthrough studies shed light on molecular mechanisms related to function of both angiotensin receptors. In this review, we primarily focus on structural aspects of ligand-receptor interaction with an emphasis on subtype selectivity, receptor activation, and biased agonism. We underscore the insights obtained by high-resolution crystal structures of AT1R and AT2R in complex with antagonists, agonists, and biased ligands (Table 1), and discuss how this information improved our current understanding of receptor activation and signaling mechanisms.
Table 1. Available crystal structures of AT1R and AT2R.
| Receptor subtype | PDB ID | Resolution, Å | Ligand | Ligand type | Receptor State | Fusion partner | Complex partner | Refs | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | AT1R | 4YAY | 2.9 | ZD7155 | Small-molecule antagonist | inactive | N-term BRIL | [15] | |
| 2 | AT1R | 4ZUD | 2.8 | Olmesartan | Small-molecule antagonist | inactive | N-term BRIL | [18] | |
| 3 | AT1R | 6DO1 | 2.9 | s-AngII | Peptide partial agonist | active | ICL3 BRIL | Nanobody AT110i1 | [20] |
| 4 | AT1R | 6OS2 | 2.7 | TRV026 | Peptide-biased agonist | active | ICL3 BRIL | Nanobody AT110i1le | [21] |
| 5 | AT1R | 6OS1 | 2.8 | TRV023 | Peptide-biased agonist | active | ICL3 BRIL | Nanobody AT110i1le | [21] |
| 6 | AT1R | 6OS0 | 2.9 | AngII | Peptide endogenous agonist | active | ICL3 BRIL | Nanobody AT110i1 | [21] |
| 7 | AT2R | 5UNG | 2.8 | Comp 1 | Small-molecule antagonist, AT2R selective | active-like | N-term BRIL | [30] | |
| 8 | AT2R | 5UNF | 2.8 | Comp 1 | Small-molecule antagonist, AT2R selective | active-like | N-term BRIL | [30] | |
| 9 | AT2R | 5UNH | 2.9 | Comp 2 | Small-molecule antagonist, dual AT1R/AT2R | active-like | N-term BRIL | [30] | |
| 10 | AT2R | 5XJM | 3.2 | s-AngII | Peptide partial agonist | active | ICL3 BRIL | Fab 4A03 | [32] |
| 11 | AT2R | 6JOD | 3.2 | AngII | Peptide endogenous agonist | active | N-term BRIL | Fab 4A03 | [33] |
| 12 | AT2R | 7C6A | 3.4 | s-AngII | Peptide partial agonist | active | ICL3 BRIL | Fab SRP2070 | [42] |
Structural snapshot of ARB binding
Initial insights into the overall AT1R structure and AngII binding emerged from homology modeling based on methionine proximity assay data [14]. The first high-resolution room-temperature crystal structure of AT1R in complex with a small molecule antagonist ZD7155 was obtained by serial femtosecond crystallography with an X-ray free electron laser (XFEL) (Table 1) [15]. The structure revealed canonical GPCR heptahelical transmembrane (7TM) architecture with an extracellular N terminus, three intracellular loops (ICLs), and three extracellular loops (ECLs) connecting seven transmembrane helices (TM1–7), followed by a short amphiphilic helix 8 (H8), and an intracellular C terminus. Two disulfide bonds stabilize conformations of the N terminus as well as the ECL2 that adopts a β-hairpin secondary structure as in most peptide receptors.
Bound to an antagonist, the receptor was captured in a distinct inactive state, based on the orientation of microswitches critical for activation and the conformations of TM5, TM6, and TM7. All residues within the conserved allosteric sodium-binding site in class A GPCRs [16] remain intact in AT1R except for the substitution of Ser7.46 [17] to Asn, which likely disrupts sodium binding but instead stabilizes the inactive state by forming two hydrogen bonds with Asn1113.35. In the absence of sodium ions, Asn1113.35Ala mutation showed 300-fold higher affinity for AngII [18]. Another potential inactive state lock is formed by the DRY motif residue Arg1263.50 and Asn2356.30.
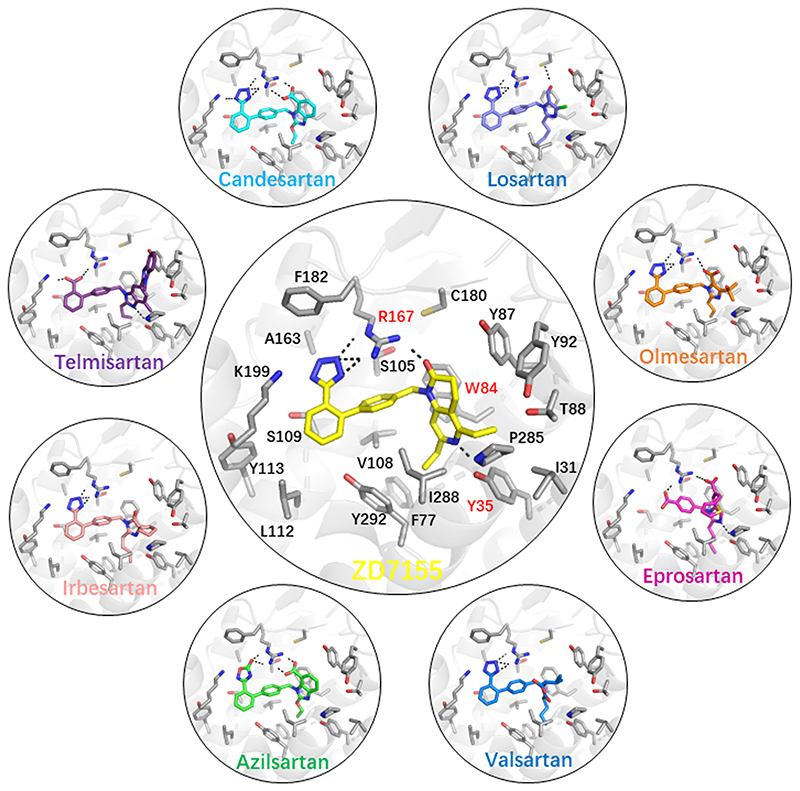
The high-affinity antagonist ZD7155, a precursor of the antihypertensive drug candesartan, binds deep in a large orthosteric ligand-binding pocket, interacting with residues from TM1-3, TM7, and ECL2 (Figure 2). The ligand is anchored by three key residues, Arg167ECL2, Tyr351.39, and Trp842.60, which play essential roles but have not been previously implicated in binding of different ARBs. The positively charged Arg167ECL2 forms ionic and hydrogen bonds with the acidic tetrazole and the naphthyridin-2-one groups of ZD7155, while Tyr351.39 and Trp842.60 form an additional hydrogen bond and a π-stacking interaction with the naphthyridin-2-one, respectively. Docking other common ARBs into the crystal structure along with site-directed mutagenesis study suggested that all these compounds bind in a similar pose as ZD7155 and interact with the same three key receptor residues. Their relative contributions to the total binding energy, however, vary for different compounds, which, due to their diverse chemical structures, are also engaged in additional interactions with other binding site residues. For example, the short alkyl tail present in several ARBs extends into the narrow hydrophobic pocket surrounded by Tyr351.39, Phe772.53, Val1083.32, Ile2887.39, and Tyr2927.43.
Figure 2. Binding of angiotensin receptor blockers (ARBs) to angiotensin 1 receptor (AT1R).
The crystal structure of AT1R in complex with ZD7135 (central large circle) revealed key anchoring interactions of the ligand with three critical residues Arg167ECL2, Tyr351.39, and Trp842.60, as labelled in red. Docking of various ARBs in the AT1R crystal structure (small circles) demonstrated that they bind in the orthosteric ligand-binding pocket in similar poses by forming extensive interactions with the same three anchoring residues as well as with other common and specific residues. All ligand-receptor hydrogen bonds and ionic interactions are shown as broken lines.
It has been shown that small modifications of ligands can lead to changes in the ligand’s mode of action despite retaining the same ligand binding mode. A follow-up study revealed the molecular basis for diverse pharmacological properties of olmesartan derivatives [18]. Substitution of the carboxyl group attached to the imidazole moiety of the inverse agonist olmesartan by the carbamoyl group turned it into a neutral antagonist, likely because of a switch of interaction from Arg167ECL2 to Tyr872.63. Further addition of a 4-hydroxybenzyl group to the biphenyltetrazole moiety of olmesartan converted the ligand into a partial agonist. The 4-hydroxybenzyl group forms extensive interactions with the ‘toggle switch’ residue Trp2536.48, implicated in the activation of many GPCRs [16], and with a cluster consisting of Lys1995.42, His2566.51, Gln2576.52, and Thr2606.55, which were previously identified to be important for the ligand-dependent activation of AT1R [15].
Structural basis of AT1R activation
Further understanding of endogenous ligand binding and receptor activation mechanisms arrived with crystal structures of AT1R in complex with the endogenous peptide agonist AngII and its derivative [Sar1,Ile8]-angiotensin II (s-AngII) that acts as a partial agonist, which have been obtained in the presence of a conformation stabilizing nanobody AT110i1 mimicking receptor interaction with G protein (Table 1) [19–21].
The crystal structures revealed that AngII binds to AT1R in an extended conformation, with its C terminus reaching deep inside the ligand-binding pocket and its N terminus pointing to the extra-cellular side, and forms both polar and nonpolar contacts with AT1R residues (Figure 3), in agreement with previous homology-modeling studies [14]. The N termini of AT1R and AngII, together with the β-hairpin of ECL2, form a twisted four-strand β sheet. As revealed by the crystal structures and confirmed by site-directed mutagenesis, Tyr351.39, Trp842.60, Arg167ECL2, and Lys1995.42 are involved in both ARB and peptide binding, however, Phe182ECL2 and Ile2887.39 interact only with peptides [19,20]. The last residue of AngII, Phe8, is involved in triggering activation-related conformational changes in AT1R by pulling TM5 inward via Lys1995.42 that forms a salt bridge with the C terminus of AngII and by pushing on Leu1123.34 with its bulky side chain. As a result, Leu1123.34 changes its rotameric state to occupy the former position of Tyr2927.43, which leads to rotation of TM3 around its axis. This rotation flips Asn1113.35, breaking its hydrogen bonds with Asn2957.46 and eliminating one of the major inactive state locks. Mutations of either of these two residues to Ala induce the constitutive activation of AT1R [22], reinforcing the notion that both Asn1113.35 and Asn2957.46 stabilize the inactive conformation of AT1R and play essential roles for the AT1R activation.
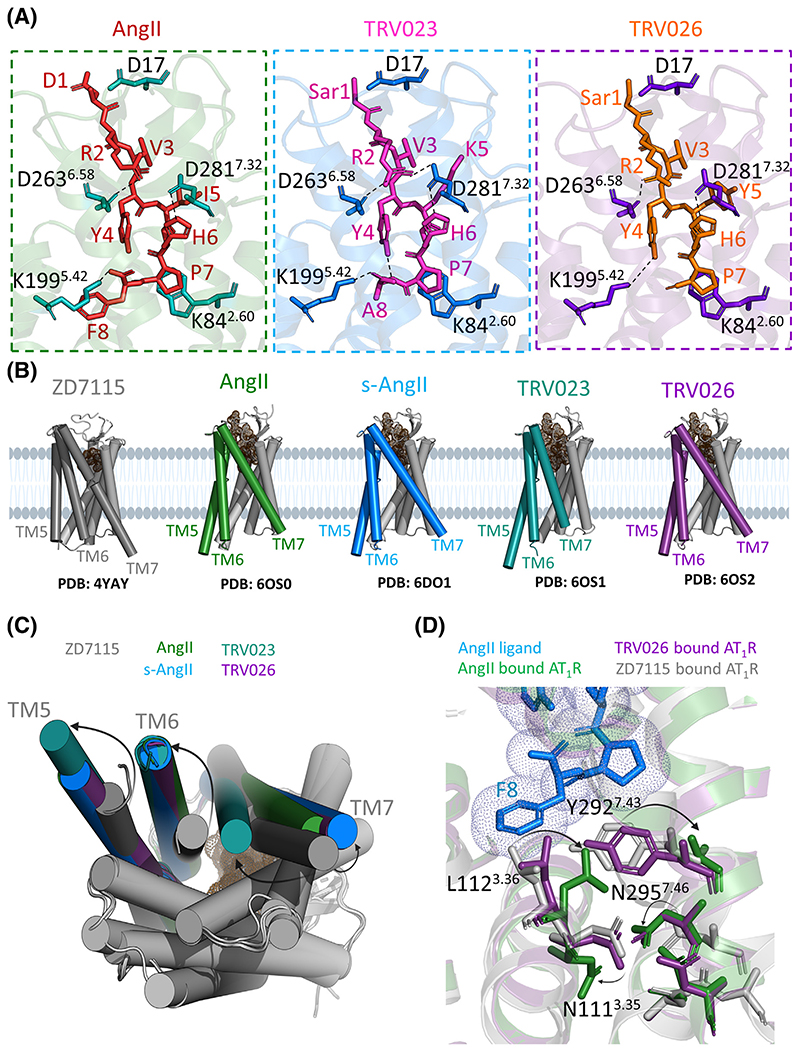
Figure 3. Molecular mechanisms of AT1R activation and biased signaling.
(A) Structural details of peptide ligand binding to AT1R: endogenous agonist AngII (PDB ID 6OS0) and βarr-biased ligands TRV023 (PDB ID 6OS1) and TRV026 (PDB ID 60S2). The structures show key residues interacting with respective peptides. Overall, all peptide ligands show similar placement of their peptide backbone in the receptor orthosteric pocket and similar interactions with key residues including D17, K842.60, D2636.58, and D2817.32. (B) Agonist binding induces large conformational changes in TM5, TM6, and TM7 as highlighted in specific colors for different ligands. (C) An overlay of AT1R structures bound to different ligands viewed from the intracellular side. Importantly, TM6 and TM5 move out of the receptor core whereas TM7 moves inward compared to AT1R bound to antagonist ZD7115 (PDB ID 4YAY). For agonists, AngII and s-AngII, TM6 shows maximal movement, while TM5 moves the least. In case of biased agonists, TRV023 and TRV026, it is TM7 that shows the highest diversity in its movement pattern, which is distinct for each biased agonist. For TRV023, TM7 shows maximal inward movement, whereas for other ligands it drifts away in the opposite direction at different magnitudes. (D) Conformational switches induced by ligand binding. The full endogenous agonist AngII (blue sticks) induces an inward movement of L1123.36 and N2957.46 and an outward movement of N1113.35 and Y2927.43 (green sticks) with respect to their conformations in AT1R-ZD7155 (grey sticks). Surprisingly, in case of TRV026 (purple sticks), L1123.36, N1113.35, and Y2927.43 show very little if any conformational changes compared to AT1R-ZD7155 residues (gray sticks). It is the reorientation of N2957.46 that is critical and seems to be sufficient to put AT1R in a conformation that can accommodate βarr as seen in the AT1R crystal structures bound to various βarr-biased agonists. Abbreviations: AngII, angiotensin II; AT1R, angiotensin 1 receptor; βarr, β arrestin; s-AngII, [Sar1,Ile8]-angiotensin II; TM, transmembrane helix.
The release of the inactive state lock results in a sequence of conformational changes that propagate towards the intracellular side of the receptor leading to an outward shift of TM6 by 11 Å along with an outward shift of TM5 and an inward shift of TM7. These TM shifts are stabilized by rearrangements of conserved microswitches, such as Tyr3027.53 of the NPxxY motif that forms potential water-mediated hydrogen bond with Tyr2155.58. Additionally, ICL2 that is unstructured in the inactive state of AT1R adopts a short α helix, connecting the DRY motif residues Arg1263.50 and Asp1253.49 with Arg137ICL2 and Arg140ICL2, as well as with Asp112 from the conformation-stabilizing nanobody via an extensive ionic network. It has been observed that upon activation of class A GPCRs, Arg3.50 switches from an ionic lock with Asp3.49 to an extended conformation that can engage in interactions with the prolonged α5 helix of G protein [23]. In the active-like structures of AT1R in complex with s-AngII and AngII, Arg1263.50 remains in the inactive conformation, highlighting singularity of AT1R activation pattern or potential artifact from using the nanobody. The main difference between the partial agonist s-AngII and the full agonist AngII is that the less bulky C-terminal residue Ile8 in s-AngII does not induce a TM3 rotation [19,20].
Structural insights into biased agonism
While biased signaling by AT1R has been well established over a decade ago [13], its structural basis was poorly understood until recently. Initial insights were obtained in a study using double electron-electron resonance (DEER) spectroscopy, a pulsed electron paramagnetic resonance (EPR) technique, which can effectively measure the distribution of distances between two selected residues that are appropriately labeled [19]. The recent studies using the DEER approach on AT1R system provided a map of global changes that occur in the receptor when it transitions from its basal state to a fully activated state (receptor bound to endogenous ligand AngII) as well as to other distinct conformations that AT1R can sample upon binding to biased ligands of different efficacies. Expectedly, these studies revealed an outward movement of TM6 away from the AT1R core and an inward movement of TM7 upon receptor activation. Importantly, this approach also underscored the spectrum of conformational populations that the receptor samples both in ligand-bound and in ligand-free states. For example, about 60% of receptor population was found to exist in the open TM6 conformation, even in the presence of full agonist, AngII, suggesting the requirement for a transducer binding to fully enrich the active receptor population. Even in the ligand-free apo state, about 10% of the receptor population appears to sample active-like open conformation, which may explain the constitutive activity of AT1R [21,24].
Analysis of receptor conformations in the presence of G protein- and βarr-biased ligands has started to illuminate some critical aspects of receptor structural changes that may be linked to preferred transducer coupling and distinct functional outcomes. For example, Gq-biased ligands, namely TRV055 and TRV056, appear to induce a less-pronounced outward movement of TM6, a subtle inward shift of TM7, and a TM5 movement towards TM6. βarr-biased ligands, such as TRV023, TRV026, TRV027, and TRV034, show significant diversity in conformational features that they impart to the receptor, although their overall functional responses are similar. These four ligands appear to induce different degrees of the outward TM6 movement in the receptor accompanied by additional differences in the TM5, TM7, and H8 regions (Figure 3) [19]. These intriguing observations based on the DEER spectroscopy measurements were further corroborated and expanded by crystal structures of the receptor in complex with different biased ligands (Table 1), and molecular dynamics simulation studies using these structural templates [19–21]. It was observed that βarr-biased agonists disrupt the two critical intermolecular interactions, that is, Arg1263.50-Asn2356.30 and Asn1113.35-Asn2957.46, differently than the natural agonist AngII does. For example, AngII induces inward movements of Leu1123.36 and Asn2957.46 and outward movements of Asn1113.35, Tyr2927.43, and Asn2987.41, but in case of βarr-biased ligands, the reorientation of Asn2957.46 appears to be sufficient to stabilize a receptor conformation able to accommodate βarrs [19–21]. Molecular dynamics simulation studies suggest that AT1R can primarily sample two different active-like conformations: a canonical conformation and an alternative conformation. While the canonical conformation can accommodate both, the Gq α5 helix and the finger loop of βarr, the alternative conformation can only allow the docking of the βarr finger loop but not the Gq α5 helix [24]. It is important to note here that analogous conclusions were drawn from extensive earlier biochemical and simulation studies, even before the structural templates were available, which is certainly reassuring [25–29]. While the complete picture will emerge only when high-resolution structural snapshots of ternary complexes become available, these important studies certainly start to provide a structural mechanism for understanding biased agonism and pave the way for structure-based design of biased ligands [21,24].
Structural framework of subtype selectivity
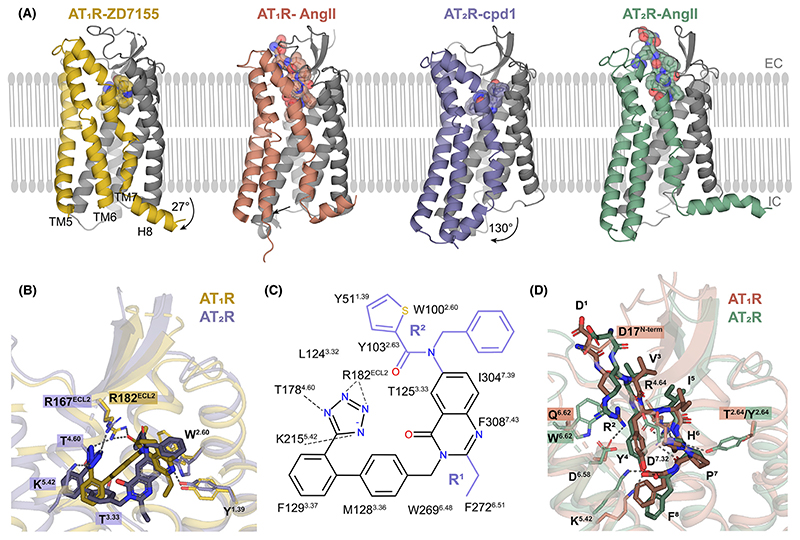
Structural basis for selectivity and diversity between AT1R and AT2R has been illuminated by the structures of AT2R in complex with small molecule ligands Comp1 (AT2R selective) and Comp2 (dual AT1R/AT2R), derived from AT1R antagonists (Table 1) [30]. Unexpectedly, the receptor bound to putative antagonists was crystallized in a distinct active-like state, based on the conformation of activation-related microswitches and positions of the intracellular ends of TM5–7 (Figure 4A). However, in contrast to other active state GPCR structures, H8 flips over from its canonical orientation parallel to the membrane by ~130° to interact with the intracellular tips of TM5 and TM6, effectively blocking interactions with G proteins and βarrs, in agreement with reports that AT2R does not signal through these canonical GPCR transducers [31]. Subsequent AT2R structures determined in complex with the partial agonist s-AngII [27] and the endogenous agonist AngII [32,33] revealed no density for H8 in the s-AngII-bound structure and a more canonical orientation of H8 parallel to the lipid membrane in the AT2R-AngII structure, suggesting that H8 potentially regulates AT2R signaling. One caveat, however, is that the s-AngII structure has been obtained using the AT2R construct with a BRIL partner fused in ICL3, which could interfere with the conformation of H8 observed in the structures with Comp1 and Comp2.
Figure 4. Structural details of AT1R/AT2R selectivity.
(A) Overall conformations of AT1R bound to antagonist ZD7155 (yellow, PDB ID 4YAY) and to endogenous agonist AngII (red, PDB ID 6OS0) and of AT2R bound to small molecule antagonists Compound 1 (purple, PDB ID 5UNG) and to endogenous agonist AngII (green, PDB ID 6JOD). Largest conformational changes are observed for TM5–7 and H8, highlighted in different colors. (B) Distinct binding modes for small-molecule antagonist binding to AT1R (yellow, PDB ID 4YAY) versus AT2R (purple, PDB ID 5UNG). (C) Schematic diagram of AT2R interactions with a small-molecule antagonist. Chemical structure of Compound 1 contains a common quinazolinone–biphenyl–tetrazole structure–activity relationship scaffold with two substituent groups, R1 and R2, colored in purple. (D) Comparison of AngII binding to AT1R (red, PDB ID 6OS0) versus AT2R (green, PDB ID 6JOD). Abbreviations: AngII, angiotensin II; AT1R, angiotensin 1 receptor; AT2R, angiotensin 2 receptor; TM, transmembrane helix.
Because of the active-like AT2R conformation and low conservation between the receptor sub-types (7 identical out of 13 residues in the ligand-binding site), the pocket shape for antagonist binding differs dramatically between AT2R and AT1R (Figure 4B). As a result, the dual antagonist Comp2 binds to AT2R in a different conformation of its common scaffold compared with binding of ZD7155 and other ARBs to AT1R. Despite the different binding pose, the residues equivalent to the main anchors of ARBs in AT1R, ArgECL2, Tyr1.39, and Trp2.60, also provide critical interactions in AT2R. Additionally, the tetrazole group of AT2R compounds is engaged in new polar interactions with Thr1253.33, Thr1784.60, and Lys2155.42. Further insights in selectivity were obtained by using molecular docking, site-directed mutagenesis, and structure–activity relationship (SAR) analysis [30]. Docking of these selective and dual ligands in crystal structures of AT1R and AT2R, as well as in models of the AT1R active state and AT2R inactive state, has demonstrated that, although the conformational state of AT2R has little effect on binding, all tested compounds are incompatible with docking in the model of the AT1R active state, corroborating the mode of action of these ligands as antagonists of AT1R. Additionally, the obtained docking scores qualitatively reflect the relative affinities of the tested ligands at both receptors. The SAR analysis of compounds with a common quinazolinone–biphenyl–tetrazole scaffold suggests that R1 derivatives are critical for AT2R selectivity, while R2 substituents define selectivity toward AT1R (Figure 4C). The n-propyl group in the R1 position fills hydrophobic subpockets in both receptors, which are composed of different residues. Smaller R1 substituents (ethyl and methyl) induce a shift of the whole scaffold in AT1R, resulting is less-optimal interactions and reduced affinity, while in AT2R the size of the n-propyl moiety does not affect the ligand-binding pose. The aromatic substituents in R2 position bind tightly to a hydrophobic subpocket in AT2R, explaining a reduced binding affinity in case of smaller R2 groups. Such a subpocket is absent in AT1R, making it less sensitive to variations in the R2 group size and allowing to accommodate larger R2 substitutents.
In contrast to distinct binding modes of small-molecule antagonists, the endogenous peptide agonist AngII binds to both receptors (Table 1) in a similar conformation with its C-terminal residue Phe8 reaching deep inside the pocket, the five C-terminal residues Tyr4-Phe8 adopting a C-shaped conformation, and the N terminus stretching toward the extracellular opening of the pocket (Figure 4D). While the five C-terminal residues of AngII closely follow each other in both receptors with rmsd = 1.2 Å, the N-terminal residues deviate by as much as 3–4 Å. The most divergent Asp1 of AngII forms several polar interactions with Asp17N-term in AT1R, but no interactions in AT2R in agreement with a higher selectivity of Ang(2–8) towards AT2R [34]. Arg2 makes salt bridges with conserved Asp6.58 and Asp7.32 in both receptors; however, in AT2R, its conformation is constrained by a stacking interaction with Trp2836.62 (Gln2676.62 in AT1R), making the salt bridges less optimal. Accordingly, removal of two N-terminal residues, Ang(3–8), makes the peptide even more selective towards AT2R [34]. Other marked differences include His6 that forms a hydrogen bond with Tyr1042.64 in AT2R. In AT1R, the equivalent residue Thr882.64 is too short for a hydrogen bond, and His6 interacts with Asp2817.32 instead. Mutation of His6 to Tyr has been shown to dramatically increase peptide selectivity towards AT2R, likely because of potential clashes between Tyr6 and TM7 backbone in AT1R [35].
Concluding remarks and future perspectives
In this review, we have discussed recent structural studies on angiotensin receptors to underscore the basis of receptor activation, subtype selectivity, and biased agonism. The recent surge of structural data on these receptors dramatically improved our understanding of ligand–receptor interaction and receptor activation; however, the next frontier is to visualize the receptors in complex with signal transducers, namely the Gq protein, βarrs, and others. Such structural snapshots combined with biochemical and biophysical characterizations and cellular studies, should further decipher the mechanistic framework of signaling in AT2R and biased agonism in AT1R.
Going forward, there are several key areas that require focused investigation to fully decipher the activation and signaling paradigms of the angiotensin receptor system (see Outstanding questions). For example, it would be interesting to see whether the results of AT1R studies, one of the most well characterized receptors in terms of biased signaling, will be applicable to other GPCRs and provide a better conceptual framework for understanding biased agonism? Similarly, it remains to be seen if the growing structural information will guide the development of biased ligands and the design of follow up studies to better understand the mechanistic insights into biased signaling. AT1R also presents a peculiar example, where the two isoforms of βarrs have distinct contributions in ERK1/2 MAP kinase activation. While βarr2 is supportive and its knockdown inhibits agonist-induced phosphorylation of ERK1/2, depletion of βarr1 has the opposite effect [36]. Thus, it remains to be explored if these isoform-specific functional outcomes are governed by distinct structural features of AT1R-βarr1/2 complexes? In fact, recent studies have indeed started to provide some important structural clues into this functional diversity of βarr isoforms [37,38].
Although recent AT2R crystal structures provided important insights into receptor subtype selectivity and offered initial clues on receptor activation, the mechanism of signal transduction by AT2R remains poorly understood. We still lack information on the precise conformational changes involved in AT2R activation, particularly with respect to receptor dynamics and potential regulation of signaling by H8. Addressing these questions will require dissecting AT2R signaling mechanisms biochemically and using complementary methods, such as NMR, EPR, and single molecule fluorescence [39]. It also remains to be seen if additional crystal structures of AT2R, for example, in complex with small molecule drug candidates, the agonist C21 and the antagonist EMA401, may help to clarify molecular determinants of receptor activation and can be further leveraged for rational design of more efficient drugs. Finally, robust identification of AT2R signal transducers and deciphering their structures in complex with AT2R by X-ray crystallography or cryo-EM [40] should break the long-thought ‘Enigma’ code of AT2R signaling [41].
Highlight.
Angiotensin receptors are some of the most-studied receptor systems to understand subtype selectivity of ligands and biased agonism from structural perspective.
Several crystal structures of AT1R and AT2R have allowed direct visualization of ligand binding at high resolution including those of ARBs and biased angiotensin II analogs.
Biophysical studies using EPR spectroscopy and molecular dynamics simulation have provided further insights into structural changes in the AT1R that govern distinct transducer coupling and biased signaling.
Structural coverage of AT1R and AT2R now provide a previously lacking frame-work to design and characterize subtype-selective ligands and tailored biased ligands.
Outstanding questions.
How can the structural templates available now be leveraged to design subtype selective ligands and biased ligands?
What is the structural framework for differential transducer-coupling of AT1R in response to agonist stimulation? For example, how does the structure of AT1R differ between Gq- versus βarr-bound conformations?
How does ligand-bias at the receptor level manifests at the receptor–transducer coupling? For example, how does the structure of receptor–transducer complexes differ from each other in response to biased vs. unbiased ligands?
What is the role of H8 in signal transduction of AT2R?
Which transducers couple to AT2R and how do they interact with the receptor?
Glossary.
- Angiotensin II
a peptide hormone consisting of eight amino acids involved in regulation of blood pressure, water content, and sodium levels.
- Angiotensin receptor blockers
antagonists and inverse agonists of AT1R that stabilize an inactive conformation of the receptor, and are used clinically as antihypertensive drugs.
- Biased agonism
the ability of ligands to preferentially trigger selective transducer-coupling and downstream signaling responses, that is, either heterotrimeric G proteins or βarrs.
- Orthosteric and allosteric sites
the binding pocket occupied by the natural agonist is typically referred to as orthosteric, while other binding sites on the receptor are referred to as allosteric sites.
- Subtype selectivity
the ability of ligands to selectively recognize and activate or inhibit a specific subtype of a given receptor.
Acknowledgements
Research in the laboratory of H.Z. is funded by the National Key R&D Program of China (2018YFA0508100), the National Natural Science Foundation of China (81722044, 91753115, 21778049, 81861148018), and the National Science and Technology Major Project of China (2018ZX09711002). V.C., A.M., and A.L. were supported by the Russian Foundation for Basic Research (RFBR) according to the research project No. 18-54-80016. V.C. acknowledges that the University of Southern California is his primary affiliation. Research on AT1R in the laboratory of A.K.S. is funded by a DST-BRICS grant [DST/IMRCD/BRICS/PilotCall2/GPCRArrSig/2018 (G)] and IIT Kanpur.
Footnotes
Declaration of interests
None to be declared.
Resources
References
- 1.de Gasparo M, et al. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- 2.Taylor AA, et al. Angiotensin receptor blockers: pharmacology, efficacy, and safety. J Clin Hypertens (Greenwich) 2011;13:677–686. doi: 10.1111/j.1751-7176.2011.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porrello ER, et al. The angiotensin II type 2 (AT2) receptor: an enigmatic seven transmembrane receptor. Front Biosci (Landmark Ed) 2009;14:958–972. doi: 10.2741/3289. [DOI] [PubMed] [Google Scholar]
- 4.Paz Ocaranza M, et al. Counter-regulatory reninangiotensin system in cardiovasculardisease. Nat Rev Cardiol. 2020;17:116–129. doi: 10.1038/s41569-019-0244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rice ASC, et al. EMA401, an orally administered highly selective angiotensin II type 2 receptor antagonist, as a novel treatment for postherpetic neuralgia: a randomised, double-blind, placebo-controlled phase 2 clinical trial. Lancet. 2014;383:1637–1647. doi: 10.1016/S0140-6736(13)62337-5. [DOI] [PubMed] [Google Scholar]
- 6.Dwivedi-Agnihotri H, et al. Distinct phosphorylation sites in a prototypical GPCR differently orchestrate β-arrestin interaction, trafficking, and signaling. Sci Adv. 2020;6:eabb8368. doi: 10.1126/sciadv.abb8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lotta LA, et al. Human gain-of-function MC4R variants show signaling bias and protect against obesity. Cell. 2019;177:597–607.:e9. doi: 10.1016/j.cell.2019.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whalen EJ, et al. Therapeutic potential of beta-arrestin-and G protein-biased agonists. Trends Mol Med. 2011;17:126–139. doi: 10.1016/j.molmed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shukla AK, et al. Emerging structural insights into biased GPCR signaling. Trends Biochem Sci. 2014;39:594–602. doi: 10.1016/j.tibs.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Wootten D, et al. Mechanisms of signalling and biased agonism in G protein-coupled receptors. Nat Rev Mol Cell Biol. 2018;19:638–653. doi: 10.1038/s41580-018-0049-3. [DOI] [PubMed] [Google Scholar]
- 11.Ranjan R, et al. Biased opioid receptor ligands: gain without pain. Trends Endocrinol Metab. 2017;28:247–249. doi: 10.1016/j.tem.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Violin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28:416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Violin JD, et al. Selectively engaging beta-arrestins at the an-g otens n II type 1 receptor reduces blood pressure and ncreases cardiac performance. J Pharmacol Exp Ther. 2010;335:572–579. doi: 10.1124/jpet.110.173005. [DOI] [PubMed] [Google Scholar]
- 14.Fillion D, et al. Structure of the human angiotensin II type 1 (AT1) receptor bound to angiotensin II from multiple chemoselective photoprobe contacts reveals a unique peptide binding mode. J Biol Chem. 2013;288:8187–8197. doi: 10.1074/jbc.M112.442053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, et al. Structure of the angiotensin receptor revealed by serial femtosecond crystallography. Cell. 2015;161:833–844. doi: 10.1016/j.cell.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katritch V, et al. Allosteric sodium in class A GPCR signaling. Trends Biochem Sci. 2014;39:233–244. doi: 10.1016/j.tibs.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ballesteros JA, Weinstein H. Integrated methods for the construction of three dimensional models and computational probing of structure-function relations in G-protein coupled receptors. Methods Neurosci. 1995;25:366–428. [Google Scholar]
- 18.Zhang H, et al. Structural basis for ligand recognition and functional selectivity at angiotensin receptor. J Biol Chem. 2015;290:29127–29139. doi: 10.1074/jbc.M115.689000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wingler LM, et al. Angiotensin analogs with divergent bias stabilize distinct receptor conformations. Cell. 2019;176:468–478.:e11. doi: 10.1016/j.cell.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wingler LM, et al. Distinctive activation mechanism for angiotensin receptor revealed by a synthetic nanobody. Cell. 2019;176:479–490.:e12. doi: 10.1016/j.cell.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wingler LM, et al. Angiotensin and biased analogs induce structurally distinct active conformations within a GPCR. Science. 2020;367:888–892. doi: 10.1126/science.aay9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unal H, Karnik SS. Constitutive activity in the angiotensin II type 1 receptor: discovery and applications. Adv Pharmacol. 2014;70:155–174. doi: 10.1016/B978-0-12-417197-8.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weis WI, Kobilka BK. The molecular basis of G protein-coupled receptor activation. Annu Rev Biochem. 2018;87:897–919. doi: 10.1146/annurev-biochem-060614-033910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suomivuori CM, et al. Molecular mechanism of biased signaling in a prototypical G protein-coupled receptor. Science. 2020;367:881–887. doi: 10.1126/science.aaz0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.St-Pierre D, et al. Angiotensin II cyclic analogs as tools to investigate AT1R biased signaling mechanisms. Biochem Pharmacol. 2018;154:104–117. doi: 10.1016/j.bcp.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 26.Cabana J, et al. Identification of distinct conformations of the angiotensin-II type 1 receptor associated with the Gq/11 protein pathway and the beta-arrestin pathway using molecular dynamics simulations. J Biol Chem. 2015;290:15835–15854. doi: 10.1074/jbc.M114.627356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Domazet I, et al. Characterization of angiotensin II molecular determinants involved in AT1 receptor functional selectivity. Mol Pharmacol. 2015;87:982–995. doi: 10.1124/mol.114.097337. [DOI] [PubMed] [Google Scholar]
- 28.Zimmerman B, et al. Differential beta-arrestin-dependent conformational signaling and cellular responses revealed by angiotensin analogs. Sci Signal. 2012;5:ra33. doi: 10.1126/scisignal.2002522. [DOI] [PubMed] [Google Scholar]
- 29.Clement M, et al. Activation induces structural changes in the liganded angiotensin II type 1 receptor. J Biol Chem. 2009;284:26603–26612. doi: 10.1074/jbc.M109.012922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang H, et al. Structural basis for selectivity and diversity in angiotensin II receptors. Nature. 2017;544:327–332. doi: 10.1038/nature22035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akazawa H, et al. Angiotensin II type 1 and type 2 receptor-induced cell signaling. Curr Pharm Des. 2013;19:2988–2995. doi: 10.2174/1381612811319170003. [DOI] [PubMed] [Google Scholar]
- 32.Asada H, et al. Crystal structure of the human angiotensin II type 2 receptor bound to an angiotensin II analog. Nat Struct Mol Biol. 2018;25:570–576. doi: 10.1038/s41594-018-0079-8. [DOI] [PubMed] [Google Scholar]
- 33.Asada H, et al. The crystal structure of angiotensin II type 2 receptor with endogenous peptide hormone. Structure. 2020;28:418–425.:e4. doi: 10.1016/j.str.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Bosnyak S, et al. Relative affinity of angiotensin peptides and novel ligands at AT1 and AT2 receptors. Clin Sci (Lond) 2011;121:297–303. doi: 10.1042/CS20110036. [DOI] [PubMed] [Google Scholar]
- 35.Magnani F, et al. Electronic sculpting of ligand-GPCR subtype selectivity: the case of angiotensin II. ACS Chem Biol. 2014;9:1420–1425. doi: 10.1021/cb500063y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SH, et al. beta-arrestin 2-dependent activation of ERK1/2 is required for ADP-induced paxillin phosphorylation at Ser(83) and microglia chemotaxis. Glia. 2012;60:1366–1377. doi: 10.1002/glia.22355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baidya M, et al. Key phosphorylation sites in GPCRs orchestrate the contribution of beta-Arrestin 1 in ERK1/2 activation. EMBO Rep. 2020;21:e49886. doi: 10.15252/embr.201949886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Namkung Y, et al. Functional selectivity profiling of the angiotensin II type 1 receptor using pathway-wide BRET signaling sensors. Sci Signal. 2018;11:eaat1631. doi: 10.1126/scisignal.aat1631. [DOI] [PubMed] [Google Scholar]
- 39.Gusach A, et al. Beyond structure: emerging approaches to study GPCR dynamics. Curr Opin Struct Biol. 2020;63:18–25. doi: 10.1016/j.sbi.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Ishchenko A, et al. Structural biology of G protein-coupled receptors: new opportunities from XFELs and cryoEM. Curr Opin Struct Biol. 2018;51:44–52. doi: 10.1016/j.sbi.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sadybekov A, Katritch V. Breaking the Enigma Code of angiotensin II type 2 receptor signaling. Structure. 2020;28:390–392. doi: 10.1016/j.str.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyagi H, et al. The discovery of anew antibody for BRIL-fused GPCR structure determination. Sci Rep. 2020;10:11669. doi: 10.1038/s41598-020-68355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]