Abstract
Background
The World Health Organization recommends not transfusing African children hospitalized with uncomplicated severe anemia (hemoglobin 4-6g/dl). However, high mortality and readmission rates suggest less restrictive transfusion strategies might improve outcomes.
Methods
The TRACT factorial open-label trial randomized Ugandan and Malawian children 2 months to 12 years with hemoglobin 4-6g/dl and no severity signs to immediate transfusion with 20 or 30ml/kg whole-blood equivalent (volume depending on second randomization) or no immediate transfusion (control, transfusion with 20ml/kg triggered subsequently by new severity signs or hemoglobin<4g/dl). Three other randomizations investigated transfusion blood volume, post-discharge micronutrients and-or cotrimoxazole. The primary endpoint was 28 day mortality.
Results
Fifteen-hundred-sixty-five children (median 26 months; 977(62%) with malaria) were randomized to immediate transfusion (n=778) or control (n=787) and followed for 180 days (71(5%) lost-to-follow-up). Seven-hundred-seventy-eight (100%) transfusion vs. 386(49%) control children were transfused during admission (median 1.3 vs. 24.9 hours from randomization). Mean (standard deviation) whole-blood equivalent received was 314(228) vs. 142(224) milliliters, respectively. Seven(1%) transfused vs. 13(2%) control children died before 28 days (hazard ratio[HR]=0.54 (95% CI 0.22-1.36) p=0.19), and 35(4%) vs. 47(6%), respectively, died before 180 days (HR=0.75 (0.48-1.15)), without evidence of interaction with other randomizations (p>0.2) nor evidence of between-group differences in readmissions or serious adverse events, nor hemoglobin recovery at 180 days. Length-of-stay was mean 0.9 days longer in the controls.
Conclusions
There was no evidence of between-group differences in clinical outcomes over 6 months. Triggered transfusion (control group) reduced blood transfusion requirements but increased length-of-stay and required clinical and repeated hemoglobin monitoring.
(Funded by the Medical Research Council and Department for International Development (through a concordat with MRC) Grant Number MR/JO 12483, United Kingdom; TRACT Current Controlled Trials number, ISRCTN84086586)
Severe anemia (hemoglobin <6g/dl) is a leading cause of hospital admission in children in subSaharan Africa.1,2 Outcomes remain unsatisfactory, with high rates of reported in-hospital (9-10%)2,3 and 6-month (12%)4 mortality, and readmission.4 Given the major burden of pediatric anemia on health services, coupled with scarce resources of donated blood (<5 units donated/1000 population/year, despite substantial external funding),5 World Health Organization (WHO) guidelines encourage restrictive transfusion approaches, specifically not transfusing stable children with hemoglobin 4-6g/dl.6 The underpinning evidence base is weak;7 further, adherence is poor,8,9 additionally hampered by inconsistent hemoglobin threshold recommendations for transfusion for malaria. No guidance is provided on clinical and hemoglobin monitoring strategies post-admission to identify those children subsequently developing new severity criteria warranting transfusion.6
The TRansfusion and TReatment of severe Anemia in African Children Trial (TRACT; ISRCTN84086586) compared four interventions. Here, we report the comparison of immediate transfusion versus no immediate transfusion (control, usual-care, with transfusion subsequently triggered by new severity signs or fall in hemoglobin to <4g/dl) in children with uncomplicated severe anemia (4-6g/dl).10
Methods
We performed an open-label, multicenter, factorial randomized trial in three hospitals in Uganda and one in Malawi. One stratum enrolled children aged 2 months to 12 years hospitalized with uncomplicated severe anemia (hemoglobin 4-6g/dl without evidence of reduced conscious level, respiratory distress, acute hemoglobinuria,11 or disclosed sickle cell disease). Children with known chronic disease (kidney or liver failure, malignancies, congenital heart disease) or admitted for burns, trauma or surgery were excluded, as were children with previous transfusions during the same admission and exclusively breast-fed children (see Supplementary Appendix for complete Methods).
For the portion of the study reported here, children were randomly assigned 1:1 to immediate transfusion or control (no immediate transfusion). However, as we also evaluated volume of transfused blood (see accompanying manuscript(reference)), children in the immediate transfusion group were further randomized 1:1 to transfusion with 30ml/kg whole-blood (15ml/kg packed/settled cells12) or 20ml/kg whole-blood (10ml/kg packed/settled cells12).
Children were simultaneously factorially randomized to 3 months post-discharge adjunctive micronutrient supplementation or iron-folate alone (usual care), and to 3 months post-discharge cotrimoxazole prophylaxis or not (not reported herein).
The present report uses the following pre-specified primary comparison for this randomization ---immediate transfusion (pooling 20ml/kg and 30ml/kg groups) vs. control. Note children with severity signs and/or hemoglobin <4g/dl (complicated severe anemia) at screening were also randomized to 30ml/kg or 20ml/kg (transfusions volume randomization reported separately).13
Ethics committees of Imperial College London, UK, Makerere University, Uganda, and the College of Medicine, Blantyre, Malawi approved the protocol, which is posted at NEJM.org
Screening and Randomization
In children with severe anemia signs or symptoms (e.g., severe pallor14), hemoglobin was measured (Hemocue®),15 and clinical assessment, performed. When prior written consent from parents or legal guardians could not be obtained, ethics committees approved verbal assent with delayed written informed consent as soon as practical.16 Otherwise, informed written consent was obtained from parents or guardians before randomization, stratified by trial center alone (since no children in this randomization had severity features). The statistician in London generated and kept the sequential randomization list, computer-generated using variably-sized permuted blocks. Randomization used consecutively numbered packets containing randomized links to opaque sealed envelopes, ensuring allocation concealment (see Supplementary Appendix, Methods).
Study Procedures
Children were managed on general pediatric wards; ventilatory facilities were unavailable. Basic infrastructural support for emergency care, patient monitors, bedside hemoglobin, glucose and lactate point-of care tests were provided. Local blood transfusion services provided blood free of charge, pre-screened for transfusion-transmissible infections and prepared using standard procedures, but without leucocyte reduction. Second transfusions, if indicated in immediate-transfusion participants, followed the originally-assigned randomized volume. Additional transfusions in children continuing to fulfil or develop de novo (in the control group) severity criteria (hemoglobin <4g/dl or severity signs above) were 20ml/kg whole-blood equivalent, irrespective of randomization. Furosemide or other diuretics were not prescribed. Other treatments, including anti-malarials and antibiotics, followed national guidelines.
Bedside observations were performed at admission and every 30 minutes for the first 2 hours, then 4, 8, 16, 24, and 48h after the start of the first transfusion (immediate group) or randomization (control group). Hemoglobin was assessed 8-hourly in the first 24 hours, then at 48 hours, or if triggered by clinical deterioration, using Hemocue®. Patients were actively monitored for serious adverse events (SAEs), particularly suspected cardiac or pulmonary overload or transfusion-related events following modified guidelines recommended by the United Kingdom’s Serious Hazards of Transfusion initiative.18 Post-discharge, children were clinically assessed and hemoglobin measured at 28, 90 and 180 days post-randomization. Children exited the trial at 180 days. Clinicians were unblinded; laboratory tests were assayed blinded.
End Points
The primary outcome was mortality through 28 days from randomization. Secondary outcomes were mortality at 48 hours, 90 days and 180 days; development of new profound anemia (<4g/dl) during acute admission or development of severe anemia (<6g/dl) post discharge; hospital readmission; proportion achieving correction of anemia (>9g/dl19 following WHO guidelines6); suspected transfusion reactions (febrile reactions, TRALI (Transfusion-Related-Acute-Lung-Injury)); SAEs; costs and cost-effectiveness. Adverse events were graded using Common Toxicity Criteria for Adverse Events v4.0.20 An independent Endpoint Review Committee reviewed cause of deaths, suspected transfusion reactions, suspected respiratory and neurological events, and allergic reactions, blinded to randomized group.
Statistical Analysis
We determined that enrollment of 1553 children would provide 80% power to detect a 50% relative reduction in 28-day mortality from 9%2,3 control to 4.5% immediate transfusion assuming 6% lost-to-follow-up by 6 months (allowing for different primary endpoint timing in other randomizations) and two-sided alpha=0.013 (four comparisons across randomizations, as detailed in the Supplementary Appendix, Methods). An independent Data Monitoring Committee reviewed interim data (three annual meetings) using Haybittle-Peto criterion (p<0.001). Randomized groups were compared following intention-to-treat using log-rank tests or competing-risks methods for time-to-event outcomes, exact tests for binary outcomes and generalized estimating equations with independent working correlation for global tests of repeated measures. Primary analyses were stratified by randomization stratification factors. Economic analysis estimated costs (in 2018 US dollars) and health outcomes (in life-years gained) over 180 days using health resource use and unit costs for each country. Analyses used Stata v15.1 and R v3.5.1. Confidence intervals are not adjusted for multiple testing. See Supplementary Methods for details. All authors vouch for the data and analysis; all authors participated in writing the manuscript.
Results
Between September 2014 and May 2017, 1565 children were randomized-- 778 to immediate transfusion (388 to 30ml/kg, 390 to 20ml/kg) an 787 to control-- and are included in all analyses (Fig. 1). Baseline characteristics were balanced between randomized groups (Table 1, Supplementary Appendix, Table S1). Nine-hundred-eighty-four (63%) had Plasmodium falciparum malaria, but HIV infection, culture-proven bacteremia and severe malnutrition were uncommon (<4%). Although known sickle cell disease patients were ineligible, 7 were identified post-randomization. Batch genotyping at trial completion increased confirmed sickle cell disease participants to 340/1549 (22%).
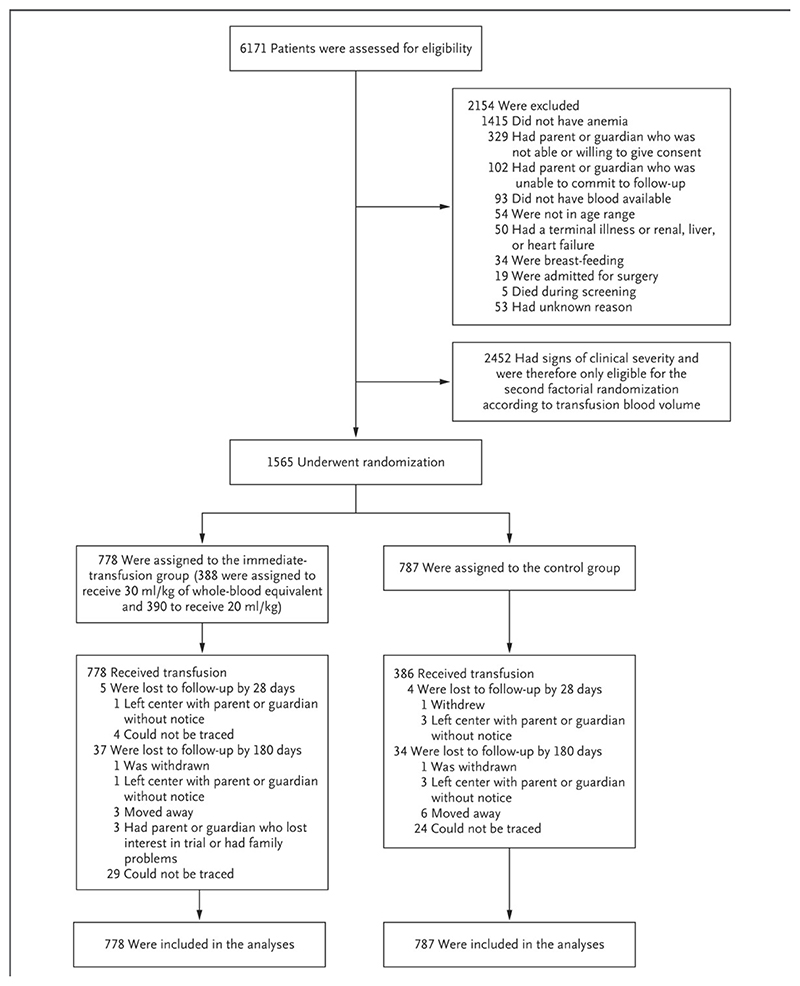
Figure 1. CONSORT diagram.
Screening, Randomization, and Follow-up. In the control group (no immediate transfusion), transfusion with 20 ml of whole-blood equivalent per kilogram of body weight was triggered by the development of new signs of clinical severity, which included impaired consciousness (prostration or unconsciousness), increased difficulty in breathing (respiratory distress), hemoglobinuria (grade 6 or higher) in the current illness, or a hemoglobin level of less than 4 g per deciliter. The data regarding the children who were lost to follow-up are presented for days 0 through 28 and days 0 through 180 days (i.e., the data regarding children lost to follow-up by 28 days are a subset of the data of those lost to follow-up by 180 days). Screening did not take place on the days when there was no available blood for transfusion.
Table 1. Baseline Characteristics of Children Randomized to Immediate vs. Control Transfusion Strategy.
| Immediate (N=778) | Control (N=787) | Total (N=1565) | |
|---|---|---|---|
| Age (months) | 27 (13, 50) | 26 (12, 50) | 26 (12,50) |
| Male | 440 (57%) | 442 (56%) | 882 (56%) |
| Hemoglobin (g/dl) | 5.2 (4.5, 5.7) | 5.1 (4.6, 5.7) | 5.1 (4.6,5.7) |
| Weight (kg) | 10.3 (8.4, 14.0) | 10.5 (8.1, 14.5) | 10.4 (8.2,14.2) |
| Mid upper arm circumference (cm) | 14.2 (13.3, 15.1) | 14.2 (13.4, 15.5) | 14.2 (13.4,15.2) |
| Heart rate (beats per minute) | 144 (130, 157) | 145 (131, 156) | 144 (130,157) |
| History of fever in this illness | 749 (96%) | 757 (96%) | 1506 (96%) |
| Axillary temperature* at screening | 37.1 (36.7, 37.8) | 37.1 (36.6, 37.8) | 37.1 (36.7,37.8) |
| Fever (>37.5°C) | 252 (32%) | 269 (34%) | 521 (33%) |
| Hypothermia (<36.0°C) | 27 (3%) | 25 (3%) | 52 (3%) |
| Systolic blood pressure (mmHg) | 91.5 (85, 98) | 92 (85, 99) | 92 (85,99) |
| Diastolic blood pressure (mmHg) | 56 (49, 63) | 55 (49, 63) | 55.5 (49,63) |
| Oxygen saturation (%) | 98 (97, 99) | 98 (96, 99) | 98 (97,99) |
| Respiratory rate (breaths per minute) | 38 (32, 44) | 38 (32, 46) | 38 (32,46) |
| Shock† | 100 (13%) | 112 (14%) | 212 (14%) |
| Severe dehydration (skin turgor or sunken eyes) | 38 (5%) | 29 (4%) | 67 (4%) |
| HIV positive | 15/736 (2%) | 17/736 (2%) | 32/1472 (2%) |
| Malaria slide or RDT positive | 497 (64%) | 487 (62%) | 984 (63%) |
| Positive blood culture | 29/702 (4%) | 33/696 (5%) | 62/1398 (4%) |
| C-reactive protein (mg/dl) | 54.2 (19.1, 100.2) | 55.8 (18.9, 107.4) | 54.9 (19, 101.3) |
| Lactate (mmol/L) | 2.4 (1.7, 3.2) | 2.3 (1.7, 3.2) | 2.3 (1.7, 3.2) |
| Previous blood transfusion in this illness | 11 (1%) | 7 (1%) | 18 (1%) |
| Blood transfusion ever (prior to this illness) | 194 (25%) | 150 (19%) | 344 (22%) |
| Sickle cell disease ascertained post-randomization | 0 | 7 | 7 (<1%) ** |
| Sickle cell disease ascertained by genotyping‡ | 172/772 (22%) | 168/777 (22%) | 340/1549 (22%) |
measured using a digital thermometer
Any one of capillary refill time >2 seconds, temperature gradient or weak pulse
Seven children were identified as having sickle cell disease during their primary admission. Four children were found through further questions to the carer to have sickle cell after consent and randomization within this uncomplicated anemia strata, and three were diagnosed following clinical symptoms during admission.
From batch genotyping after the end of the trial (n=16 with missing results).
Note: showing numbers (%) or median and interquartile ranges (IQR). There was no evidence of change imbalance in baseline characteristics (p>0.05) except for oxygen saturation (median (IQR) 98 (97, 99) in immediate vs. 98 (96, 99) in control p=0.005) and blood transfusion ever (prior to this illness) (p=0.006)
Adherence
778(100%) immediate transfusion vs. 386(49%) control children received a transfusion during admission, a median (IQR) 1.3 (0.9,1.7) vs. 24.9 (9.2,49.8) hours from randomization, respectively (Supplementary Appendix, Fig. S1, Table S2). The major trigger for transfusion in controls was hemoglobin <4g/dl (295/386,76%); 57(15%) had new clinical severity signs and 7(2%), new sickle cell diagnoses. Three(1%) and 24(6%) of these transfusions occurred before or after 48 hours, respectively, with last hemoglobin 4-6g/dl and no new severity signs recorded. Only 5(1%) control children meeting transfusion criteria did not receive one.
Seven-hundred-sixty-six(99%) immediate transfusion vs. 379(98%) controls received first transfusions within ±3ml/kg of their randomized whole-blood equivalent volume. First transfusion was whole blood in 395(51%) immediate transfusion vs. 218(56%) control; median(IQR) storage age of blood was 12(6-19) vs. 11(6-18) days, respectively); 748(96%) immediate vs. 346(90%) control children received a single transfusion; maximum 4 vs. 2 transfusions in first 48 hours, respectively (Supplementary Appendix, Table S2). During the primary admission, the mean (standard deviation) total milliliters whole-blood equivalent transfused was 314(228) immediate transfusion vs. 142(224) control.
Mortality
At day 28 (primary endpoint) and day 180 (end of follow-up), 1556(99%) and 1494(95%) children, respectively, had known vital status. By day 28, 7(1%) immediate transfusion (1(0.3%) 30ml/kg, 6(1%) 20ml/kg) and 13(2%) control children had died (hazard ratio(HR) (immediate cf. control)=0.54 (95% CI 0.22-1.36); p=0.19; Table 2; Figure 2a, and Supplementary Appendix, Fig.S2). Ten of 13 deaths in the control group had received a transfusion (Supplementary Appendix, Table S3; a median 9.0h (IQR 6.8-30.6h) post-randomization). By day 180, 35(4%) in the immediate vs. 47(6%) in the control group had died (HR=0.75 (0.48-1.15)). (See Supplementary Appendix, Results for cause of death and subgroup analyses.)
Table 2. Secondary and Other Outcomes.
| Immediate N participants (% of 778) | Control N participants (% of 787) | Total N participants (% of 1565) | Hazard Ratio (95% CI) | p | |
|---|---|---|---|---|---|
| Death by 48 hours* | 0 (0%) | 2 (0%) | 2 (0%) | - | * |
| Death by 28 days (primary outcome) | 7 (1%) | 13 (2%) | 20 (1%) | 0.54 (0.22, 1.36) | 0.19 |
| Death by 90 days* | 24 (3%) | 31 (4%) | 55 (4%) | 0.78 (0.46, 1.32) | * |
| Death by 180 days* | 35 (4%) | 47 (6%) | 82 (5%) | 0.75 (0.48, 1.15) | * |
| Correction of anemia (Hb>9g/dl during admission)* | 399 (51%) | 43 (5%) | 442 (28%) | 11.73 (8.69,15.84)† | * |
| Development of new profound anemia (Hb<4g/dl) during admission* | 11 (1%) | 309 (39%) | 320 (20%) | 0.03 (0.02, 0.05) † | * |
| Development of severe anemia (Hb<6g/dl) post discharge* | 106 (14%) | 142 (18%) | 248 (16%) | 0.73 (0.56, 0.94)† | * |
| Readmission to hospital* | 123 (16%) | 113 (14%) | 236 (15%) | 1.09 (0.84, 1.40)† | * |
| Any serious adverse event* [number of events] | 152 (20%) [198] | 151 (19%) [201] | 303 (2519%) [399] | 1.02 (0.82, 1.28) | 0.85 |
| Any anemia SAE [number of events] | 78 (9%) [97] | 83 (9%) [107] | 161 (9%) [204] | - | 0.74& |
| Any malaria SAE [number of events] | 54 (7%) [61] | 51 (7%) [56] | 105 (7%) [117] | - | 0.76& |
| Any sepsis SAE [number of events] | 22 (3%) [27] | 28 (4%) [33] | 50 (3%) [60] | - | 0.47& |
| Any hemoglobinuria SAE [number of events] | 24 (3%) [27] | 15 (2%) [16] | 39 (%2) [43] | - | 0.15& |
| Suspected allergic reactions* ** | 6 (<1%) | 2 (<1%) | 8 (<1%) | - | 0.17& |
| Suspected transfusion-related lung injury or circulatory overload* ‡ | 0 (0%) | 0 (0%) | 0 (0%) | - | - |
secondary outcome prespecified in the protocol; p-values not reported except for adverse events following journal policy.
estimated from competing risks sub-hazard regression.
suspected pulmonary overload or transfusion-related acute lung injury (TRALI) or transfusion-related cardiac overload (TACO)
grades in Supplementary Appendix, Table S5.
Chi-squared test
Fisher’s Exact test
Note: confidence intervals have not been adjusted for multiple testing and inferences drawn from the intervals may not be reproducible.
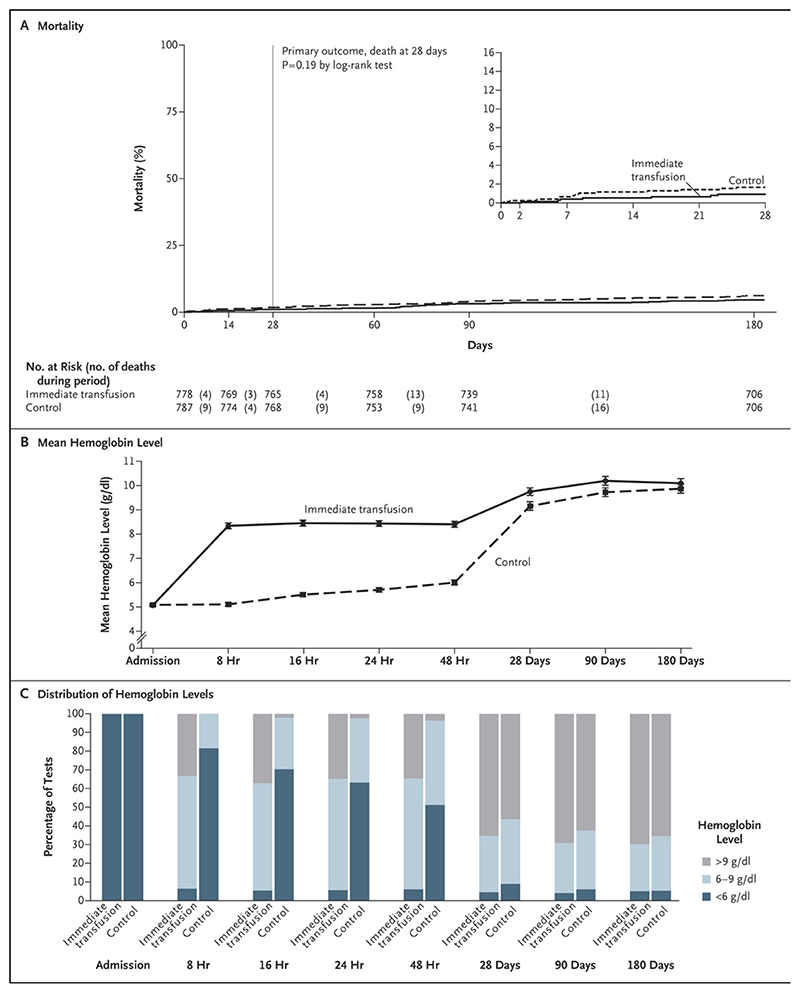
Figure 2. Key Outcomes, Admission through 180 Days.
The time window for the 180-day visit was 120 to 240 days after randomization (99.3% of the children were seen after 170 days). The inset in Panel A shows the same data on an enlarged y axis. Panel B shows the mean hemoglobin level during the first 48 hours and through 180 days. Panel C shows distribution of hemoglobin levels of less than 6 g per deciliter, 6 to 9 g per deciliter, and higher than 9 g per deciliter according to the indicated time point. Additional details are provided in Table S4A and S4B in the Supplementary Appendix. Key outcomes (a) Mortality through 180 days (b) Mean hemoglobin over 180 days (c) Proportions with hemoglobin <6g/dl, 6-9g/dl and >9g/dl over 180 days
Hemoglobin Recovery
As expected, hemoglobin increases over the first 48h were substantially greater with immediate transfusion (Figs. 2b and c). At 48 hours, mean hemoglobin was +2.42g/dl (95% CI 2.17,2.67) higher with immediate transfusion; absolute mean(SD) was 8.4(1.7) in the immediate group vs. 7.0(1.7) in controls transfused before 48 hours (n=249) and 5.5(1.1) in controls not transfused before 48 hours (n=535). Controls transfused after 12 hours had similar post-transfusion hemoglobin increases to controls transfused within 12 hours (Supplementary Appendix, Fig. S3). During admission, hemoglobin recovery to >9g/dl occurred faster, and new profound anemia (<4g/dl) less frequently, with immediate transfusion (Table 2, Supplementary Appendix, Figs. S4aS4c). Differences in hemoglobin had attenuated substantially at 28 days (+0.60g/dl (95% CI +0.35,+0.86), higher with immediate transfusion) and 90 days (+0.48g/dl (+0.22,+0.73)). At day 180 there was no evidence of differences in mean hemoglobin (+0.23g/dl (-0.03,+0.49)) or proportions ≤9g/dl or <6g/dl (Figs. 2b and c).
Other Clinical Outcomes
Median hospital-stay (IQR) was 3 (3,4) vs 4 (3,6) days in immediate transfusion vs. control (HR=1.62 (95% CI 1.46-1.80), Supplementary Appendix, Fig. S5) with mean 4.0 vs. 4.9 hospitalization-days in the primary admission respectively. By day-180, 123(16%) immediate transfusion vs. 113(14%) control children had been readmitted to hospital (HR=1.09 (95% CI 0.84-1.40), Table 2, Supplementary Appendix, Fig. S6), mostly for anemia, malaria or sepsis. 152(20%) immediate transfusion vs. 151(19%) control children experienced SAEs (p=0.85, Table 2, Supplementary Appendix, Table S4). Transfusion-specific SAEs included non-life-threatening allergic events in 6 immediate transfusion vs. 2 control children (Supplementary Appendix, Table S5).
Costs and Cost-effectiveness
The main cost drivers were hospital length-of-stay (mean $29.70 immediate transfusion vs. $34.30 control), blood transfusions (mean $22.20 vs. $11.78 (half of the control group received a transfusion), respectively), and hemoglobin tests (mean $8.46 vs. $8.41, respectively), resulting in total unadjusted costs per child of $72.09 in the immediate transfusion vs. $66.46 in the control group, $5.63 less in controls (Supplementary Appendix, Tables S6-8). Sensitivity analyses suggested total unadjusted savings might increase to $22.8 using standard blood units (450mls) and/or increased prices. Total unadjusted costs were similar if zero and 2 post-randomization hemoglobin tests were done within 48 hours for immediate transfusion and control groups, respectively (Supplementary Appendix, Table S9). Life-years over 180 days were similar in immediate transfusion (0.487) and control groups (0.482). Overall, the control strategy (including triggered transfusion) was less costly than immediate transfusion, albeit estimated to be less effective (Supplementary Appendix, Table S10).
Predictors of Control Group Transfusion
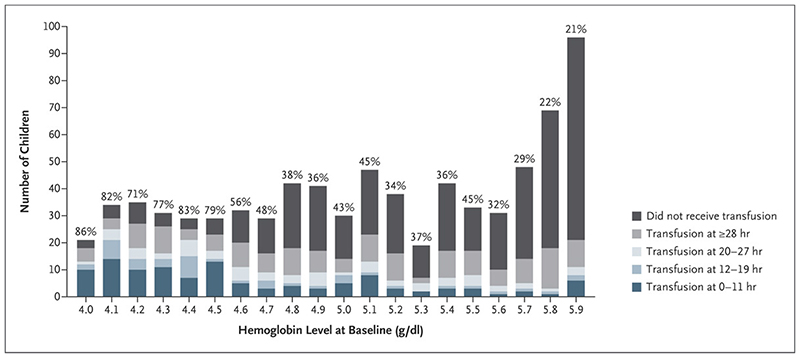
Time to transfusion was considered in 776 control children, excluding 11 identified post-randomization as having severity criteria at admission (7 sickle cell disease (all transfused), 4 preexisting hemoglobinuria (three transfused)). Three-hundred-forty-eight (45%) received a transfusion by 96 hours. Admission hemoglobin was the strongest predictor (Supplementary Appendix, Table S11); 79% of children with hemoglobin ≤4.5g/dl were transfused vs. 35% with hemoglobin >4.5g/dl (Fig. 3). Immediately transfusing all children with hemoglobin ≤4.5g/dl (23% of controls) would have captured 142(41%) triggered transfusions. There was no evidence that undiagnosed sickle cell disease affected transfusion. Predictors of transfusion for <4g/dl were similar (data not shown).
Figure 3. Proportion transfused in control group by baseline hemoglobin.
Transfusion Status among the Children in the Control Group, Stratified According to Baseline Hemoglobin Level. The percentages above the bars represent the percentage of children who received a transfusion among those who had the indicated hemoglobin level at baseline.
Discussion
While mortality rates were too low either to demonstrate or refute any benefits, our large multicenter trial demonstrated that in children with uncomplicated severe anemia, immediate transfusion resulted in fewer children who developed profound anemia (hemoglobin<4g/dl), an absolute indication for transfusion, and superior early hemoglobin recovery (to >9g/dl), compared to controls. However, those findings did not translate into fewer readmissions (15% of children) or fewer anemia-related SAEs (9%).
A key limitation of the present study was the lower overall mortality (2%) than the 9% predicted from other studies of uncomplicated severe anemia in African children, which had consistently higher mortality.3,21,22 One reason may be that screening was halted when no donor blood units were available; thus, no children in the study died awaiting transfusion, in contrast to previous reports.8,21,22 Trial hemovigilance may have also improved outcomes, as very few adverse transfusion reactions were documented. Clinical and hemoglobin monitoring (8-hourly through 24 hours) identified new severity criteria warranting transfusion in the control group, leading to 49% receiving a potentially life-saving transfusion (94% per-protocol), albeit substantially later than had they been in the immediate transfusion group. WHO guidelines were largely based on observational studies7,21,22 and recommend not transfusing patients with uncomplicated severe anemia (4-6g/dl). Guidelines, however, do not address monitoring during admission or anticipate post-admission development of complicated severe anemia. The trial did not withhold transfusion in controls in that situation, as this would be unethical and inconsistent with good clinical practice; however, this approach may also have led to lower mortality than originally hypothesized. The TRACT control group therefore reflected a pragmatic strategy of conserving blood for high-risk children identified post-admission. The low control mortality suggests that monitoring and surveillance may be important in reducing poor outcomes compared to not transfusing at all. However, the trial retained good power for readmissions. Strategies to prevent readmissions should therefore be a key focus of future interventional trials to reduce morbidity in this high-risk group.
Worldwide consensus transfusion guidelines for stable children within intensive care units recommend transfusion at hemoglobin <7g/dl23 but explicitly highlight the need for further trials, particularly with 5-7g/dl.7 Children in high-income countries likely have steady-state hemoglobin 11-14g/dl, whereas African children in areas where malaria and α+thalassemia are common,24 typically have hemoglobin levels of 9-11g/dl.4,25 The impact of lower immediate transfusion thresholds in Africa may therefore reflect differences in steady-state. We cannot exclude a small absolute mortality benefit in children receiving immediate transfusion in our trial; however, 7/13 deaths in the control group were transfused within 10 hours of randomization, and we found no differences in morbidity. Given the burden of pediatric severe anemia in sub-Saharan Africa, immediate transfusion in uncomplicated severe anemia risks overburdening the blood transfusion services compared with close monitoring and targeted transfusion.
Nevertheless, hospital length-of-stay was longer in control children, with potential implications for out-of-pocket costs for parents. Hemoglobin monitoring also uses resources (financial and staff), although at a lower rate (estimated costs ~$20 per blood unit vs. ~$1 per hemoglobin test). Thus, overall, a control (triggered) transfusion strategy costs less than immediate transfusion but also is slightly less effective, making triggered transfusion with monitoring the cost-effective option. Limitations include the substantial uncertainty in blood unit costs, which vary by country, blood unit size and type (Supplementary Appendix, Table S6),26 and in hemoglobin monitoring costs. Our within-trial economic analysis does not capture longer-term risks and benefits, which could change the value-for-money estimates. Ideally, greater efforts should be put into achieving an adequate and reliable blood supply. However, health services should consider the option of providing hemoglobin measurements for monitoring and continuing with the WHO’s restrictive transfusion strategy, to avert the more substantial costs to the blood transfusion services of providing immediate blood to all with hemoglobin 4-6g/dl.
Trial strengths include broad eligibility criteria, which enhanced generalizability, with a large subgroup with malaria, and high adherence to randomized strategy (94%) and follow-up (>95%). The hidden sickle cell disease burden in 22% with uncomplicated severe anemia should prompt universal screening of admitted children with severe anemia27. Reassuringly, undiagnosed sickle cell disease did not predict transfusion in the control group, indicating that sickle cell disease without life-threatening complications can be managed without immediate transfusion. An important limitation is lack of hemoglobin measurements >48 hours post-admission, specifically at discharge.
Overall, there was no evidence of differences in clinical outcomes between immediate transfusion and a control (triggered) transfusion strategy. The control strategy reduced blood requirements by 60% but increased length-of-stay by 20% and required clinical and hemoglobin monitoring. Although we cannot exclude the possibility of a small mortality benefit from immediate transfusion, mortality was very low with both strategies, and immediate transfusion failed to improve re-admissions over the following 6 months, which remained high in both groups.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Supplementary Material
Acknowledgements
We thank all participants and staff from all the centers participating in the TRACT trial. This paper is published with permission from the Director of KEMRI.
Support
TRACT was funded by a grant from the UK Medical Research Council (MRC) [MR/J012483/1]. Cipla Limited donated the cotrimoxazole for the trial. The MRC Clinical Trials Unit at UCL receives core support from the MRC [MC_UU_12023/26], through a concordat with the Department for International Development. ASW is a National Institutes of Health Research Senior Investigator. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Contributor Information
Kathryn Maitland, Department of Paediatrics, Kenya Medical Research Institute (KEMRI)/Wellcome Trust Research Programme, Kilifi, Kenya; Department of Paediatrics, Imperial College London UK
Sarah Kiguli, Department of Paediatrics, Makerere University and Mulago Hospital, Kampala
Peter Olupot-Olupot, Department of Paediatrics, Makerere University and Mulago Hospital, Kampala; Busitema University Faculty of Health Sciences, Mbale Campus and Mbale Regional Referral Hospital Mbale
Charles Engoru, Busitema University Faculty of Health Sciences, Mbale Campus and Mbale Regional Referral Hospital Mbale
Macpherson Mellewa, Soroti Regional Referral Hospital, Soroti (CE, FO, MN): all in Uganda. College of Medicine and Malawi-Liverpool-Wellcome Trust Clinical Research Programme, Blantyre, Malawi
Pedro Saramago Goncalves, Centre for Health Economics, University of York, UK
Ayub Mpoya, Kenya Medical Research Institute (KEMRI)/Wellcome Trust Research Programme, Kilifi, Kenya
George Chagaluka, Soroti Regional Referral Hospital, Soroti (CE, FO, MN): all in Uganda. College of Medicine and Malawi-Liverpool-Wellcome Trust Clinical Research Programme, Blantyre, Malawi
Neil Kennedy, School of Medicine, Dentistry and Biomedical Science, Queen’s University, Belfast; Uganda Blood Transfusion Services (BTS) National BTS, Kampala, Imperial College London UK(DKB) Mbale BTS, Imperial College London UK (BW) Malawi BTS, Blantyre, Malawi,Imperial College London UK (BM)
Eva Nabawanuka, Department of Paediatrics, Makerere University and Mulago Hospital, Kampala
Sophie Uyoga, Kenya Medical Research Institute (KEMRI)/Wellcome Trust Research Programme, Kilifi, Kenya
Gary Frost, Department of Nutrition Research Section, Imperial College London UK
Imelda Bates, Liverpool School of Tropical Medicine and Hygiene, Liverpool UK.
Jennifer A. Evans, Department of Paediatrics, University Hospital of Wales, Cardiff, UK
Thomas N. Williams, Department of Paediatrics, Kenya Medical Research Institute (KEMRI)/Wellcome Trust Research Programme, Kilifi, Kenya; Department of Paediatrics, Imperial College London UK
Elizabeth C George, Medical Research Council Clinical Trials Unit at University College London
Diana M. Gibb, Medical Research Council Clinical Trials Unit at University College London
A. Sarah Walker, Medical Research Council Clinical Trials Unit at University College London
References
- 1.Stevens GA, Finucane MM, De-Regil LM, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995-2011: a systematic analysis of population-representative data. Lancet Glob Health. 2013;1:e16-25. doi: 10.1016/S2214-109X(13)70001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pedro R, Akech S, Fegan G, Maitland K. Changing trends in blood transfusion in children and neonates admitted in Kilifi District Hospital, Kenya. Malar J. 2010;9:307. doi: 10.1186/1475-2875-9-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calis JC, Phiri KS, Faragher EB, et al. Severe anemia in Malawian children. The New England journal of medicine. 2008;358:888–99. doi: 10.1056/NEJMoa072727. [DOI] [PubMed] [Google Scholar]
- 4.Phiri KS, Calis JC, Faragher B, et al. Long term outcome of severe anaemia in Malawian children. PloS one. 2008;3:e2903. doi: 10.1371/journal.pone.0002903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Global status report on blood safety and availability 2016. World Health Organization; Geneva: 2017. World Health Organization. [Google Scholar]
- 6.Pocket book of hospital care for children: Second edition Guidelines for the management of common childhood illnesses. World Health Organization; Geneva: 2013. [PubMed] [Google Scholar]
- 7.Maitland K, Ohuma EO, Mpoya A, Uyoga S, Hassall O, Williams TN. Informing thresholds for paediatric transfusion in Africa: the need for a trial. Wellcome Open Res. 2019 doi: 10.12688/wellcomeopenres.15003.1. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiguli S, Maitland K, George EC, et al. Anaemia and blood transfusion in African children presenting to hospital with severe febrile illness. BMC Med. 2015;13:21. doi: 10.1186/s12916-014-0246-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Opoka RO, Ssemata AS, Oyang W, et al. High rate of inappropriate blood transfusions in the management of children with severe anemia in Ugandan hospitals. BMC Health Serv Res. 2018;18:566. doi: 10.1186/s12913-018-3382-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mpoya A, Kiguli S, Olupot-Olupot P, et al. Transfusion and Treatment of severe anaemia in African children (TRACT): a study protocol for a randomised controlled trial. Trials. 2015;16:593. doi: 10.1186/s13063-015-1112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olupot-Olupot P, Engoru C, Uyoga S, et al. High Frequency of Blackwater Fever Among Children Presenting to Hospital With Severe Febrile Illnesses in Eastern Uganda. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017;64:939–46. doi: 10.1093/cid/cix003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uyoga S, Mpoya A, Olupot-Olupot P, et al. Haematological quality and age of donor blood issued for paediatric transfusion to four hospitals in sub-Saharan Africa. Vox Sang. 2019 doi: 10.1111/vox.12764. Under consideration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maitland K, Olupot-Olupot P, Kiguli S, et al. Transfusion volume for children in African with severe anemia. The New England journal of medicine. 2019 doi: 10.1056/NEJMoa1900100. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olupot-Olupot P, Prevatt N, Engoru C, et al. Evaluation of the diagnostic accuracy and cost of different methods for the assessment of severe anaemia in hospitalised children in Eastern Uganda [version 1; referees: 1 approved] Wellcome Open Res. 2018;3:130. doi: 10.12688/wellcomeopenres.14801.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medina Lara A, Mundy C, Kandulu J, Chisuwo L, Bates I. Evaluation and costs of different haemoglobin methods for use in district hospitals in Malawi. Journal of clinical pathology. 2005;58:56–60. doi: 10.1136/jcp.2004.018366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maitland K, Molyneux S, Boga M, Kiguli S, Lang T. Use of deferred consent for severely ill children in a multi-centre phase III trial. Trials. 2011;12:90. doi: 10.1186/1745-6215-12-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ala F, Allain JP, Bates I, et al. External financial aid to blood transfusion services in sub-Saharan Africa: a need for reflection. PLoS medicine. 2012;9:e1001309. doi: 10.1371/journal.pmed.1001309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williamson L, Cohen H, Love E, Jones H, Todd A, Soldan K. The Serious Hazards of Transfusion (SHOT) initiative: the UK approach to haemovigilance. Vox Sang. 2000;78(Suppl 2):291–5. [PubMed] [Google Scholar]
- 19.Lacroix J, Hebert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. The New England journal of medicine. 2007;356:1609–19. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- 20.Common Terminology Criteria for Adverse Events (CTCAE) National Institutes of Health National Cancer Institute; 2009. May 28th, [Google Scholar]
- 21.English M, Ahmed M, Ngando C, Berkley J, Ross A. Blood transfusion for severe anaemia in children in a Kenyan hospital. Lancet. 2002;359:494–5. doi: 10.1016/S0140-6736(02)07666-3. [DOI] [PubMed] [Google Scholar]
- 22.Lackritz EM, Campbell CC, Ruebush TK, 2nd, et al. Effect of blood transfusion on survival among children in a Kenyan hospital. Lancet. 1992;340:524–8. doi: 10.1016/0140-6736(92)91719-o. [DOI] [PubMed] [Google Scholar]
- 23.Doctor A, Cholette JM, Remy KE, et al. Recommendations on RBC Transfusion in General Critically Ill Children Based on Hemoglobin and/or Physiologic Thresholds From the Pediatric Critical Care Transfusion and Anemia Expertise Initiative. Pediatr Crit Care Med. 2018;19:S98–S113. doi: 10.1097/PCC.0000000000001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wambua S, Mwacharo J, Uyoga S, Macharia A, Williams TN. Co-inheritance of alpha+-thalassaemia and sickle trait results in specific effects on haematological parameters. Br J Haematol. 2006;133:206–9. doi: 10.1111/j.1365-2141.2006.06006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staedke SG, Maiteki-Sebuguzi C, DiLiberto DD, et al. The Impact of an Intervention to Improve Malaria Care in Public Health Centers on Health Indicators of Children in Tororo, Uganda (PRIME): A Cluster-Randomized Trial. Am J Trop Med Hyg. 2016;95:358–67. doi: 10.4269/ajtmh.16-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loua A, Nikiema JB, Kasilo OM, Tayou TC. Blood safety and availability in the WHO African region. Global Surgery. 2018;4:1–7. [Google Scholar]
- 27.Macharia AW, Mochamah G, Uyoga S, et al. The clinical epidemiology of sickle cell anemia In Africa. Am J Hematol. 2018;93:363–70. doi: 10.1002/ajh.24986. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.