Abstract
Recent studies have demonstrated that a detailed knowledge of the extent of angiographic coronary artery disease (CAD) is not a prerequisite for clinical decision making, and the clinical management of patients with CAD is more and more focused towards the identification of myocardial ischemia and the quantification of ischemic burden. In this view, non-invasive assessment of ischemia and in particular stress imaging techniques are emerging as preferred and non-invasive options. A quantitative assessment of regional myocardial perfusion can provide an objective estimate of the severity of myocardial injury and may help clinicians to discriminate regions of the heart that are at increased risk for myocardial infarction. Positron emission tomography (PET) has established itself as the reference standard for myocardial blood flow (MBF) and myocardial perfusion reserve (MPR) quantification. Cardiac magnetic resonance (CMR) is increasingly used to measure MBF and MPR by means of first-pass signals, with a well-defined diagnostic performance and prognostic value. The aim of this article is to review the currently available evidence on the use of both PET and CMR for quantification of MPR, with particular attention to the studies that directly compared these two diagnostic methods.
Keywords: Cardiac magnetic resonance (CMR), positron emission tomography (PET), quantitative perfusion imaging
Introduction
Until recently decision on revascularization of a coronary artery stenosis has been frequently based on the angiographic appearance of the coronary lesions, without a concomitant assessment of the functional severity of the stenosis and of the resulting ischemic burden, despite the well-established knowledge that the percentage of stenosis is a poor predictor of the functional severity of the lesion, in particular intermediate cases.1-3
Supportive evidence on the role of non-invasive imaging for the stratification of patients with stable coronary artery disease (CAD) came from the nuclear sub study of the COURAGE trial,4 which examined a subgroup of patients tested with single photon emission computed tomography (SPECT), exploring the effects of optimal medical treatment (OMT) vs percutaneous coronary intervention (PCI) on the ischemic burden and patients’ outcome. This study demonstrated that the combination of PCI and OMT was more effective in reducing the total ischemic burden than OMT alone and that the antiischemic effect of PCI was greater for patients with moderate to severe pre-treatment ischemia. The reduction of the ischemic burden was associated in a non risk-adjusted model with freedom from events. These trends constituted an important indication confirming the value of ischemia assessment to guide therapeutic decision making.
These findings confirmed a previous landmark study by Hachamovitch et al,5 who sought to show the survival benefit associated with revascularization vs medical therapy, stratifying the patients based on the extent of the ischemic burden detected by SPECT. In a large retrospective population of 10,627 patients, it was demonstrated that those with no or very low amounts of inducible ischemia (<10%-12%) were at very low risk and did not require invasive treatment, while for increasing amounts of ischemic myocardium there was a significant prognostic benefit by PCI.
The functional significance of coronary stenoses has also been assessed invasively. The usefulness of fractional flow reserve (FFR) in guiding revascularization procedures has been determined by a few cornerstone studies that compared FFR-guided revascularization with decision making guided by angiographic data. The DEFER Study demonstrated that PCI of coronary artery lesions with non-significant FFR is not of benefit.5 The FAME6 and the FAME 27 studies demonstrated that FFR-guided PCI in patients with multi-vessel CAD allows a functionally complete revascularization in these patients using a lower number of stents and with a significantly reduced event-rate in comparison to patients managed on the basis of their angiographic findings.
These studies delineated that a detailed knowledge of the extent of angiographic CAD is not a prerequisite for clinical decision-making and that functional measurements of the ischemic burden, a marker available using non-invasive techniques, is sufficient for decision making and provides prognostic information. On the basis of these scientific evidences, the clinical management of patients with CAD is progressively moving away from merely assessing the presence or absence of coronary artery lesions and is more and more focused towards the identification of myocardial ischemia and the quantification of ischemic burden.
In this view, non-invasive assessment of ischemia and in particular stress imaging techniques are emerging as preferred and non-invasive options. Most clinical perfusion imaging techniques only assess relative differences in myocardial perfusion. Non-quantitative methods might substantially underestimate perfusion compared to quantitative methods,8,9 especially when the appreciation of three-vessel disease10,11 and microcirculation12,13 are challenged. A quantitative estimate of regional myocardial perfusion can provide an objective measure of the severity of myocardial injury and may help clinicians to discriminate regions of the heart that are at increased risk for myocardial infarction. Myocardial perfusion reserve (MPR),14 defined as the ratio of the maximum myocardial blood flow (MBF)15 to the baseline, is an indicator of epicardial CAD as well as myocardial microvascular function abnormalities.
Experimental and clinical studies have shown that the functional severity of a coronary stenosis can be determined by measuring MBF and MPR. Positron emission tomography (PET) has established itself as the reference standard for MBF and MPR quantification.16-18 Cardiac magnetic resonance (CMR) is increasingly used to measure MPR by means of first-pass signals.19-21 The aim of this article is to review the evidence currently available on the use of both PET and CMR for quantification of MPR, with particular attention to the studies that directly compared these two diagnostic methods.
CMR Quantitative Perfusion Imaging
In the past two decades, first-pass perfusion cardiovascular magnetic resonance (CMR) has rendered an indispensable tool for the non-invasive detection of myocardial ischemia. By taking advantage of its high spatial resolution, non-invasive and non-toxic nature CMR perfusion imaging has achieved an improvement in sensitivity and specificity for the detection of CAD and has given further insights into the understanding of ischemic heart disease.22
In CMR perfusion studies, a paramagnetic gadolinium (Gd) containing contrast agent is injected during vasodilator-induced (adenosine or dipyridamole) stress and repeated app 15 minutes later at rest. The spatiotemporal distribution of Gd within the heart can be measured dynamically and the resultant blood and tissue enhancement data can be analyzed to estimate the rate of perfusion to each region of the myocardium.23,24
A potential advantage of perfusion CMR is its ability to quantify perfusion reserve within a myocardial segment. Although time-demanding, compared to visual interpretation, quantitative evaluation of myocardial perfusion properties with CMR, as expressed semi-quantitatively by MPR index and fully-quantitatively by absolute MBF, may provide additional clinically relevant information and an objective, stepwise correlation of myocardial perfusion impairment to the severity of coronary artery status.
A semi-quantitative analysis of myocardial perfusion is based on the assessment of the signal intensity changes over the course of the first pass of the contrast through the myocardium. The upslope integral technique has been the most effective semi-quantitative method that was studied and yields a high diagnostic accuracy in patients with suspected CAD.25,26 The accuracy of the upslope analysis may, however, be affected by differences in the contrast agent’s pharmacodynamics and pharmacokinetic properties. The use of fully quantitative perfusion analysis helps to avoid these problems.
In order to perform absolute quantification of MBF, a quantifiable relationship must exist between the signal intensity changes in the image and underlying blood flow. Most quantitative analysis methods require that the measured blood (arterial input function, AIF) and tissue (tissue function, TF) enhancement data are calculated and mathematically deconvolved in order to estimate the system impulse response function, from which myocardial perfusion can be computed.
There are two main deconvolution techniques: compartment kinetic modeling and Fermi-function deconvolution. With compartmental kinetic models, the forward flux of Gd from the blood to the myocardium is taken to represent absolute MBF.27,28 Fermi-function method is based on the calculation of the amount of Gd present within a region of myocardium.29 This technique is relatively robust to the effects of extracellular accumulation of the contrast agent during first pass of the contrast. Both of the techniques described above have been shown to correlate with myocardial perfusion over a wide range of flow. Multiple other techniques have been used for deconvolution, and are currently under validation.30
Quantitative CMR perfusion imaging has been validated against microspheres in animals,15 more established non-invasive imaging modalities (echocar-diography,31 SPECT,32,33 PET34-38) and invasive, catheter-based techniques39,40 for functional appreciation of coronary flow. The above research and clinical evidence demonstrated a strong correlation between quantitative CMR values and coronary artery status, and highlighted the prognostic value of the method in patients with CAD.41-44
PET Quantitative Perfusion Imaging
PET is considered the current gold standard technique for quantitative perfusion imaging, and has contributed substantially to the understanding of cardiac physiology and pathophysiology. Myocardial PET myocardial perfusion imaging improves diagnostic accuracy45 and provides a useful adjunct to assessment of regional perfusion abnormalities.46
With the use of suitable tracers and appropriate mathematic models, PET has been successfully applied in regional quantification of absolute MPR,3,47 and has been used for the sensitive detection of early abnormalities in coronary vascular function associated with CAD.48 Currently, 13NH3, H2, 15O, and 82Rb are the most widely used PET perfusion tracers. 82Rb is most widely used in clinical practice MBF tracer because it does not require a cyclotron on site and has a very short half-life. Several tracer kinetic models for quantification of PET-MBF have been successfully validated against the radiolabeled microsphere gold standard in animals. These models have to compensate for underestimation of radiotracer concentration due to the partial-volume effect, limited spatial resolution and motion of the heart.49
Quantitative perfusion indices measured by PET correlates inversely with the degree of coronary artery stenosis at angiography.3 Furthermore, recent studies have demonstrated that PET-derived MPR is an independent predictor of outcome, predicts major adverse cardiac events and cardiac death in patients with myocardial ischemia,50 reduced survival in patients with left ventricular systolic dysfunction51 and provides incremental risk stratification among diabetic patients without CAD.52
Because of its ability to provide non-invasive regional absolute quantification of MBF, PET has been widely used to assess myocardial perfusion pattern in a wide range of the cardiac pathology.
In healthy humans, PET has demonstrated significant variation in regional CFR related to parameters like gender and age, a finding that has important clinical implications.53,54 In asymptomatic subjects with cardiovascular risk factors, PET allows the early detection of functional coronary flow impairment, irrespective of any vessel structural alterations.55,56
However, PET has proven more clinically useful in the appreciation of functional significance of epicardial coronary lesions. In chronic stable angina, perfusion PET successfully distinguished between segments perfused to the normal and diseased vessels.57 Although affected by a certain degree of variation, PET-MPR is linearly related to the severity of CAD. The extent and severity of ischemia on PET provides incremental risk estimates of cardiac death and all-cause death compared with traditional coronary risk factors.58 Furthermore, the accurate study MBF by PET permitted an insight into the understanding and estimation of myocardial perfusion in human hibernating myocardium and its response to the available therapeutic options.59,60
In parallel, the feasibility of PET to assess MBF and MPR has offered an effective assessment of microvas-cular function, a parameter otherwise unable to exam by direct methods. This feature of PET permitted the demonstration of abnormal in a wide range of primary and secondary cardiomyopathies. In patients with hypertrophic cardiomyopathy (HCM), with the use of PET, it has been demonstrated impaired MPR corresponding to microvascular dysfunction and affecting both the hypertrophied and the non-hypertrophied segments.61 MBF and MPR impairment in context of HCM, detected with the use of PET, has allowed the assessment of response to the different therapeutic interventions,62-64 and has proven to be an independent predictor of pejorative outcome.65 Similarly, in patients with dilated cardiomyopathy (DCM), abnormal perfusion pattern demonstrated in multiple PET studies66-68 has been shown to be an independent predictor of subsequent cardiac events and clinical deterioration.69
CMR vs PET Quantification Perfusion Imaging
PET is well accepted as a validated technique for non-invasive quantitative measurements of myocardial perfusion using either 15O2-labeled water or 13NH3. CMR has demonstrated potential for clinical quantitative perfusion imaging and might be a good alternative for non-invasive quantitative evaluation of the myocardial perfusion and detection of CAD.70
Semi-quantitative perfusion CMR methods estimating MPR have proven effective in the appreciation of CAD. Myocardial perfusion ratio index has been shown to be closely related to the degree of stenosis.23,25 Initial studies comparing CMR semi-quantitative analysis methods with PET have shown good correlation in patients with CAD.34,35
In a pivotal prospective study by Schwitter et al,34 the quality of a multislice CMR approach was determined and compared with PET and quantitative coronary angiography (QA). A total of 48 patients and healthy subjects were studied by dipyridamole first-pass CMR using a multislice hybrid echo-planar pulse sequence and segmental signal intensity upslope quantitative measurements were compared to 13NH3 PET. Receiver-operator characteristic analysis of subendocardial upslope data revealed a sensitivity and specificity of 91% and 94%, respectively, for the detection of CAD as defined by PET (mean coronary flow reserve minus 2 SD of controls) and a sensitivity and specificity of 87% and 85%, respectively, in comparison with QA (diameter stenosis ≥50%). The number of pathological sectors per patient on PET and MR studies correlated linearly (slope, 0.94; r = 0.76). The presented MR approach identified patients with coronary artery stenoses and provided information on the amount of compromised myocardium, with best results being obtained when perfusion indices were assessed in the subendocardial layer, which is most sensitive to an ischemic challenge.
In another study comparing semi-quantitative adenosine perfusion CMR with quantitative measures of QA and PET in healthy volunteers and patients with CAD, Ibrahim et al35 compared upslope and peak-intensity indices, measured with the use of a multislice ultra-fast hybrid sequence to 13NH3 PET flow reserve measurements. Localization of coronary artery stenosis, based on the upslope index, yielded sensitivity, specificity and diagnostic accuracy of 69%, 89%, and 79%, respectively. Upslope index correlated with PET flow reserve (r: 0.70). A reduced coronary flow reserve (PET: 2.0, MRI: 1.3) was detected by the upslope index with sensitivity, specificity, and diagnostic accuracy of 86%, 84%, and 85%, respectively. Although MPR was underestimated by CMR as compared to PET, a close relationship was observed between MRI upslope index and PET estimates of flow reserve, yielding acceptable diagnostic performance for localization of CAD.
Improvement in imaging acquisition and post-processing methods permitted the development of quantitative algorithms in perfusion CMR. Few studies have examined its accuracy and diagnostic value against PET perfusion. Parkka et al36 studied the accuracy of first-pass CMR and kinetic modeling for quantitative analysis of MBF and MPR during dipyridamole infusion, compared to PET, in 18 healthy males. Using a perfusion-related parameter, the unidirectional influx constant (Ki), MBF was computed in three coronary artery territories. There was a significant correlation for both dipyridamole-induced flow and MPR between CMR and PET. However, in accordance with previous studies, MPR values provided with MRI were lower compared to PET (2.5 ± 1.0 vs 4.3 ± 1.8).
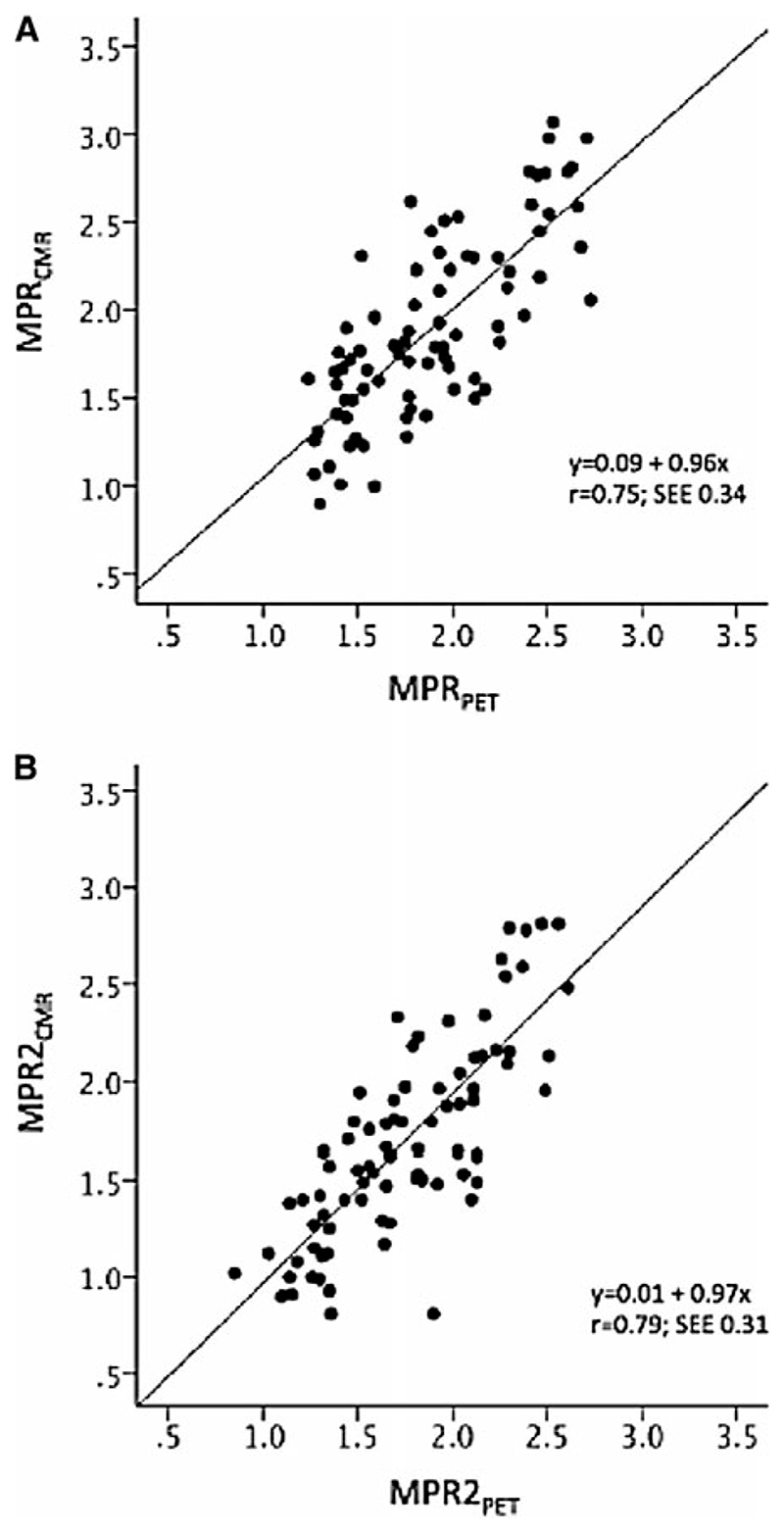
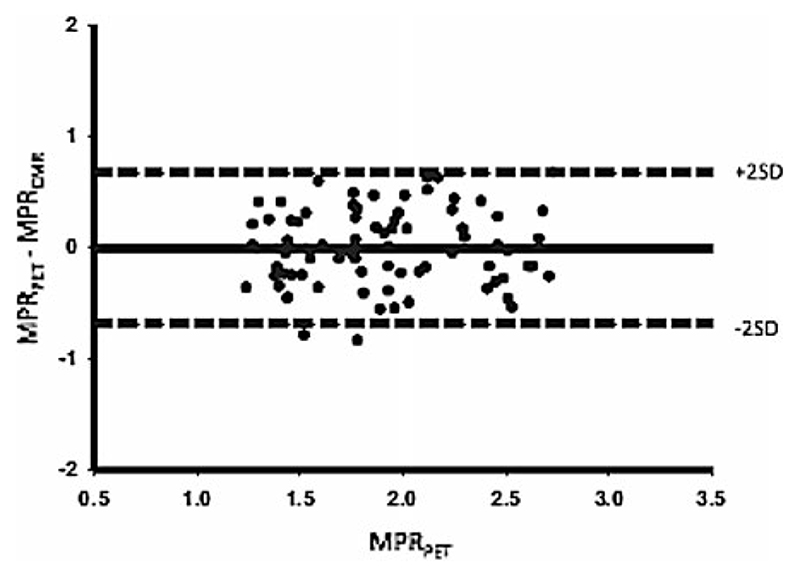
In the same concept, Fritz-Hansen et al38 assessed quantitative CMR with the Ki perfusion method in healthy volunteers, using 13NH3 PET as a reference method. Ten healthy males were examined with combined PET and CMR dipyridamole perfusion imaging in order to determine absolute MPR. CMR-derived myocardial and blood time concentration curves were fitted by a two-compartment perfusion model. A linear relationship was observed between CMR- and PET-derived MPR for regional and global data (Figure 1). A good agreement between the two methods to determine low or high perfusion reserves was found (Figure 2; Table 1).
Figure 1.
Scatter plots with fit lines comparing myocardial perfusion reserve (MPR) values from cardiac magnetic resonance (MPRCMR) and positron emission tomography (MPRPET) for the entire myocardial territory (A) and the mean of the lowest 2 segments in each territory (MPR2) (B) (Courtesy of Morton et al37).
Figure 2.
Bland-Altman plot showing the agreement between CMR- and PET-derived absolute MPR measurements (Courtesy of Morton et al37). Abbreviations as in Table 1.
Table 1. Published evidence comparing CMR and PET quantitative perfusion imaging: principal methodological characteristics and results.
| Year | Author | Reference | n | Type of participants | CMR perfusion protocol | Stressor | Quantitative analysis CRM method | PET radiotracer | Quantitative angiography (% significant stenosis) | Sensitivity* | Specificity* | Agreement between CMR/ PET-derived indices** |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2001 | Schwitter J | 34 | 66 | Healthy volunteers 18 and CAD 48 | 1.5 T mulislice hybrid echo-planar pulse sequence | Dipyridamole | Semi-quantitative | 13NH3 | Performed (>50%) | 91% | 94% | MBF: r. not stated MPR: r. 0.76x |
| 2002 | Ibrahim T | 35 | 44 | Healthy volunteers 19 and CAD 25 | 1.5 T, Multislice ultra-fast hybrid sequence | Adenosine | Semi-quantitative | 13NH3 | Performed (>50%) | 86% | 84% | MBF: r. not stated MPR: r. 0.70 |
| 2006 | Pãrkkã JP | 36 | 18 | Healthy volunteers | 1.5 T, saturation recovery turboFLASH sequence | Dipyridamole | Quantitative | (15O)H2O | Not performed | - | - | MBF: r. 0.70 MPR: r. 0.46 |
| 2008 | Fritz-Hansen | 38 | 10 | Healthy volunteers | 1.5 T, ECG-triggered saturation recovery turboFLASH sequence | Dipyridamole | Quantitative | 13NH3 | Not performed | - | - | MBF: r. 0.79 MPR.: r. Not stated |
| 2008 | Pack N | 72 | 5 | Healthy volunteers | 3 T, saturation recovery turboFLASH sequence | Adenosine | Quantitative | 13NH3 | Not performed | - | - | MBF: r. 0.85 MPR.: r: Not stated |
| 2012 | Morton G | 37 | 38 | CAD | 1.5 T, k-t balanced turbogradient echo sequence | Adenosine | Quantitative | 13NH3 | Performed (>70%) | - | - | MBF: r. 0.32 MPR: r. 0.79 |
n, Number of patients; CAD, coronary artery disease; CMR., cardiac magnetic resonance; PET, positron emission tomography; MBF, myocardial blood flow; MPR, myocardial perfusion reserve
Detection of CAD by CMR-derived MPR. against PET-derived MPR.
Correlation coefficient
These studies, with the use of quantitative CMR methods in healthy humans, enforced previous evidence that Ki constant can identify myocardial regions of occluded infarct-related arteries.71 CMR perfusion data were similar between the two studies, indicating that both saturation- and inversion-recovery imaging sequences are useable and that the method seems robust for use. However, the method seems to underestimate perfusion at stress compared to PET.
In parallel, model-independent analysis methods tested at 3 T CMR have provided similar results. Pack et al72 applied in five normal subjects adenosine perfusion CMR imaging with the use of a saturation recovery turboFLASH sequence and subsequent PET perfusion imaging. Regional and pixelwise quantitative perfusion estimates correlated with dynamic 13NH3 PET (r = 0.85) and were similar to results from other validated CMR studies. The authors succeeded to demonstrate that a model-independent analysis method can be used to quantify myocardial perfusion with dynamic contrast-enhanced perfusion CMR.
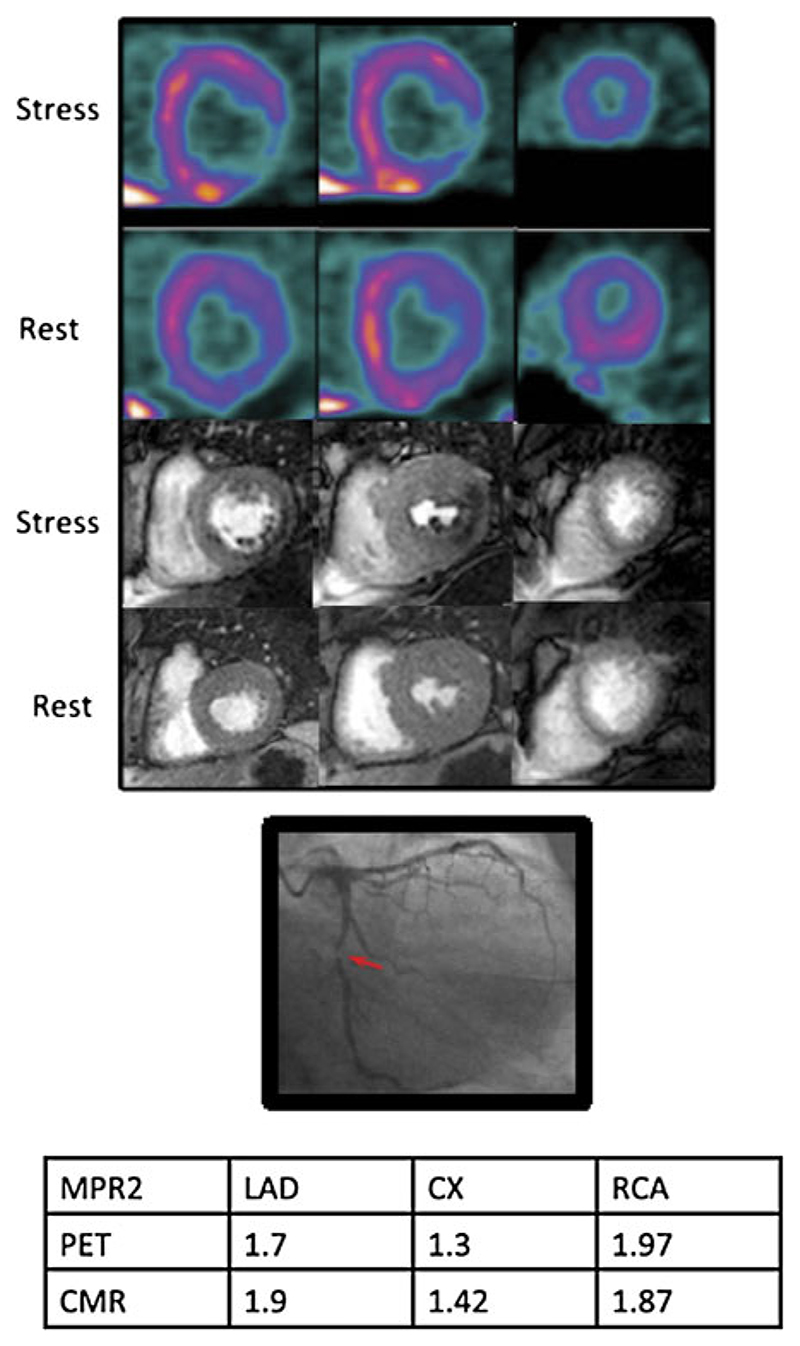
More recently, Morton et al compared MBF and MPR with CMR and PET in a cohort of 41 patients with known or suspected CAD. Patients underwent quantitative 13NH3 PET and adenosine CMR perfusion imaging before coronary angiography.37 CMR-derived indices correlated well with PET-derived measurements (r: 0.75). MBF and MPR for the 2 lowest scoring segments in each coronary territory also correlated strongly between the two techniques (r: 0.79). Absolute CMR perfusion values correlated significantly, but weakly, with PET values both at rest (r: 0.32) and during stress (r: 0.37). An MPR by PET < 1.44 predicted significant CAD with 82% sensitivity and 87% specificity, and MPR by CMR < 1.45 predicted significant CAD with 82% sensitivity and 81% specificity (Figure 3). This has been the single study so far to compare fully quantitative CMR against PET perfusion imaging in patients with CAD. Quantitative indices derived by the two techniques correlated strongly, and both techniques proved comparable and accurate. However, the correlation between the absolute perfusion values from PET and CMR was relatively weak, suggesting that a single absolute stress perfusion cutoff value is not superior for the detection of CAD for the moment. Interestingly, there was not any incremental value in combining MPR data from both PET and CMR for the diagnosis of CAD.
Figure 3.
PET (top), CMR (middle), and the x-ray angiogram of the left coronary artery of a 54-year-old patient with diabetes and exertional angina. Basal, mid, and apical slices have been taken from the PET study, which approximately correspond to the CMR slices. There is a stress-induced perfusion defect in the infero-lateral region from base to apex visible on both PET and CMR images. There is a corresponding severe stenosis of the proximal Cx. There was no other significant angiographic disease. Myocardial perfusion reserve of the lowest 2 segments (MPR2) for each territory are shown in the table. The MPR2 for the circumflex artery is below the cutoff of 1.44 and 1.45 for both PET and CMR, respectively (Courtesy of Morton et al37). PET, Positron emission tomography; CMR, cardiac magnetic resonance; Cx, circumflex coronary artery; LAD, left anterior descending coronary artery; RCA, right coronary artery.
Overall, although bibliographic evidence is poor, a good correlation has been steadily confirmed for relative and absolute quantitative perfusion CMR methods, when PET quantification techniques are considered as the reference standard. Both techniques have proven useful for the detection and estimation of coronary artery stenosis. The tendency to measure lower stress perfusion with CMR than with PET, observed in all studies, could in part be explained by the different methods used in both the acquisition and the post-processing of the exams. Different contrast agents with particular pharmacodynamic and pharmacokinetic properties, differences among stressors as well as difference in the mathematical models used for exam analysis, could partially explain this difference. Further research in this area will be needed.
CMR vs PET Spatial Resolution
In order to appreciate and compare the efficacy of CMR and PET perfusion imaging, special consideration should be given to the spatial resolution of each technique.
Improvements in hardware, pulse sequence development, and image reconstruction algorithms have enabled high-resolution imaging of first-pass myocardial perfusion with CMR, with spatial resolution of approximately 1 mm which is around 10× better than with nuclear perfusion imaging and 2-3 times better than with conventional perfusion CMR.73 In addition, perfusion studies have been performed at 3 T scanners and have demonstrated improved signal-to-noise ratio (SNR).74 Spatial coverage has also been improved by adopting 3D encoding methods combined with parallel imaging using either 3D SSFP or 3D FLASH.75
Although the spatial resolution of PET is generally better than SPECT (typically 4-7 mm with PET and 1015 mm with SPECT),76 it is ultimately dependent on the distance traveled by the positron between its point of emission and annihilation in tissue (positron range). Resolution is also determined by the residual kinetic energy at the time of annihilation event, random counts, and the detectors’ thickness.77 Compared to the conventional PET scanners crystals, novel PET crystals possess higher light outputs leading to improved energy and spatial resolution.78
Myocardial ischemia affects the subendocardial layers of the left ventricular myocardium earlier and more severely than the outer layers.79 The detection of subendocardial ischemia is considered a sensitive endpoint for the diagnosis of pathological alterations of myocardial blood supply.80-83 Due to the relatively low spatial resolution of nuclear myocardial perfusion imaging, SPECT and PET techniques have been limited to patients with LV hypertrophy.84-86 Only a few studies using 15O-labeled water PET reported a transmural perfusion ratio in normal hearts in animal experiments and in healthy volunteers,87 confirming the existence of transmural perfusion inhomogeneities also in normal hearts, with higher rest MBF in the subendocardium and a homogeneous MBF during stress.
The high spatial resolution of myocardial perfusion CMR, in comparison to PET, allows the visualization of subendocardial ischemia as a delayed wash-in of the contrast agent.40,80,87 Post-processing of perfusion CMR data can be used to quantify the imbalances between subendocardial and subepicardial myocardial perfusion and thus improving the diagnostic accuracy of ischemia.
Current Limitations and Future Perspectives
While PET and CMR offer an accurate, reproducible, and efficient appreciation of myocardial perfusion, which could potentially guide therapeutic stratification and management, they suffer from certain drawbacks that preclude their establishment as exams of choice for ischemia assessment at the moment.
PET is well accepted as a validated technique for non-invasive quantitative measurements of myocardial perfusion using either 15O2-labeled water or 13NH3. However, it has a relatively low spatial resolution, is associated with radiation exposure, and it is not widely available for clinical use.
CMR has higher spatial and temporal resolution, uses non-ionized tracers, and is more widely available. On the other hand, CMR cannot be used in patients with iatrogenic devices incompatible with the MRI environment. CMR might be a good alternative for non-invasive quantitative evaluation of the myocardial perfusion and detection of CAD.
Both perfusion techniques are limited by significant variability in post-processing quantification analysis. It is also possible that systematic errors could relate to the fact that PET88,89 and particularly CMR90 have only moderate inter-study reproducibility. The findings that MPR, a ratio of stress and rest perfusion values, correlates well but that the absolute perfusion values correlate relatively poorly suggests that the errors in quantification have a similar influence on both rest and stress perfusion values and were subsequently canceled by the calculation of MPR. These errors might be a result of either methodological or physiological factors.
This variation is potentially attributed to a combination of factors including variation in stress test response, image acquisition/quality, and variation in measurements at the time of post-processing. Reproducibility may also differ due to inherent pitfalls, such as differences in the expertise between centers. Therefore, any study must reasonably account for factors that systematically alter its accuracy and reproducibility. Defining these factors is a necessary prerequisite to their broad clinical application.
The final barrier is to demonstrate that perfusion quantification has additional benefit over visual analysis or semi-quantitative techniques. One of the justifications for absolute quantification has been improved detection of three-vessel disease.91 The incremental benefit of absolute quantification still needs to be established in larger clinical studies.
Nonetheless, the above limitations reflect the practical challenges encountered in both clinical practice and research. No quantitative perfusion analysis technique has been adopted in clinical practice at this time, and visual inspection performed by an experienced reporter remains the mainstay of clinical reporting.
Ongoing technical innovation with the development of improved hardware, software and novel technical approaches, such as novel spatial-temporal acceleration techniques,92,93 introduction of novel contrast media,94 and radiotracers95 promise improved diagnostic performance for the assessment of coronary artery status and myocardial ischemic burden and offer the potential for the exams to being employed as a clinical endpoint. Further development of each exam will substantially define each one’s distinct role in improving clinical care.
Acknowledgments
The authors acknowledge financial support from the Department of Health through the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy’s & St. Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust. The Division of Imaging Sciences receives also support as the Centre of Excellence in Medical Engineering (fundedby the Welcome Trust and EPSRC; Grant Number WT088641/Z/09/Z) as well as the BHF Centre of Excellence (British Heart Foundation Award RE/08/03). Dr Bratis acknowledges receiving training grant by the Hellenic Society of Cardiology. Dr Mahmoud acknowledges receiving training grant by the Egyptian Ministry of Higher Education. Dr Chiribiri receives grant support from Philips Healthcare. Dr Nagel received significant grant support from Bayer Schering Pharma and Philips Healthcare.
References
- 1.Piek JJ, Boersma E, di Mario C, Schroeder E, Vrints C, Probst P, et al. Angiographical and Doppler flow-derived parameters for assessment of coronary lesion severity and its relation to the result of exercise electrocardiography. DEBATE study group. Doppler Endpoints Balloon Angioplasty Trial Europe. Eur Heart J. 2000;21:466–74. doi: 10.1053/euhj.1999.1871. [DOI] [PubMed] [Google Scholar]
- 2.Gould KL, Kirkeeide RL, Buchi M. Coronary flow reserve as a physiologic measure of stenosis severity. J Am Coll Cardiol. 1990;15:459–74. doi: 10.1016/s0735-1097(10)80078-6. [DOI] [PubMed] [Google Scholar]
- 3.Uren NG, Melin JA, De Bruyne B, Wijns W, Baudhuin T, Camici PG. Relation between myocardial blood flow and the severity of coronary-artery stenosis. N Engl J Med. 1994;330:1782–8. doi: 10.1056/NEJM199406233302503. [DOI] [PubMed] [Google Scholar]
- 4.Shaw LJ, Berman DS, Maron DJ, Mancini GB, Hayes SW, Hartigan PM, et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the clinical outcomes utilizing revascularization and aggressive drug evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117:1283–91. doi: 10.1161/CIRCULATIONAHA.107.743963. [DOI] [PubMed] [Google Scholar]
- 5.Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107:2900–7. doi: 10.1161/01.CIR.0000072790.23090.41. [DOI] [PubMed] [Google Scholar]
- 6.Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’t Veer M, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–24. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 7.De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991–1001. doi: 10.1056/NEJMoa1205361. [DOI] [PubMed] [Google Scholar]
- 8.Christian TF, Rettmann DW, Aletras AH, Liao SL, Taylor JL, Balaban RS, et al. Absolute myocardial perfusion in canines measured by using dual-bolus first-pass MR imaging. Radiology. 2004;232:677–84. doi: 10.1148/radiol.2323030573. [DOI] [PubMed] [Google Scholar]
- 9.Berman DS, Kang X, Gransar H, Gerlach J, Friedman JD, Hayes SW, et al. Quantitative assessment of myocardial perfusion abnormality on SPECT myocardial perfusion imaging is more reproducible than expert visual analysis. J Nucl Cardiol. 2009;16:45–53. doi: 10.1007/s12350-008-9018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaacks SM, Ali A, Parrillo JE, Barron JT. How well does radionuclide dipyridamole stress testing detect three-vessel coronary artery disease and ischemia in the region supplied by the most stenotic vessel? Clin Nucl Med. 1999;24:35–41. doi: 10.1097/00003072-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Berman DS, Kang X, Slomka PJ, Gerlach J, de Yang L, Hayes SW, et al. Underestimation of extent of ischemia by gated SPECT myocardial perfusion imaging in patients with left main coronary artery disease. J Nucl Cardiol. 2007;14:521–8. doi: 10.1016/j.nuclcard.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–40. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 13.Patel AR, Epstein FH, Kramer CM. Evaluation of the microcirculation: Advances in cardiac magnetic resonance perfusion imaging. J Nucl Cardiol. 2008;15:698–708. doi: 10.1016/j.nuclcard.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Saadi N, Nagel E, Gross M, Bornstedt A, Schnackenburg B, Klein C, et al. Noninvasive detection of myocardial ischemia from perfusion reserve based on cardiovascular magnetic resonance. Circulation. 2000;101:1379–83. doi: 10.1161/01.cir.101.12.1379. [DOI] [PubMed] [Google Scholar]
- 15.Hsu LY, Groves DW, Aletras AH, Kellman P, Arai AE. A quantitative pixel-wise measurement of myocardial blood flow by contrast-enhanced first-pass CMR perfusion imaging: microsphere validation in dogs and feasibility study in humans. JACC Cardiovasc Imaging. 2012;5:154–66. doi: 10.1016/j.jcmg.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muzik O, Duvernoy C, Beanlands RS, Sawada S, Dayanikli F, Wolfe ER, Jr, et al. Assessment of diagnostic performance of quantitative flow measurements in normal subjects and patients with angiographically documented coronary artery disease by means of nitrogen-13 ammonia and positron emission tomography. J Am Coll Cardiol. 1998;31:534–40. doi: 10.1016/s0735-1097(97)00526-3. [DOI] [PubMed] [Google Scholar]
- 17.Camici PG, Rimoldi OE. The clinical value of myocardial blood flow measurement. J Nucl Med. 2009;50:1076–87. doi: 10.2967/jnumed.108.054478. [DOI] [PubMed] [Google Scholar]
- 18.Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124:2215–24. doi: 10.1161/CIRCULATIONAHA.111.050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manning WJ, Atkinson DJ, Grossman W, Paulin S, Edelman RR. First-pass nuclear magnetic resonance imaging studies using gadolinium-DTPA in patients with coronary artery disease. J Am Coll Cardiol. 1991;18:959–65. doi: 10.1016/0735-1097(91)90754-w. [DOI] [PubMed] [Google Scholar]
- 20.Wilke N, Jerosch-Herold M, Wang Y, Huang Y, Christensen BV, Stillman AE, et al. Myocardial perfusion reserve: Assessment with multisection, quantitative, first-pass MR imaging. Radiology. 1997;204:373–84. doi: 10.1148/radiology.204.2.9240523. [DOI] [PubMed] [Google Scholar]
- 21.Jerosch-Herold M, Kwong RY. Optimal imaging strategies to assess coronary blood flow and risk for patients with coronary artery disease. Curr Opin Cardiol. 2008;23:599–606. doi: 10.1097/HCO.0b013e328312c2f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagel E, al-Saadi N, Fleck E. Cardiovascular magnetic resonance: Myocardial perfusion. Vol. 25. Herz; 2000. pp. 409–16. [DOI] [PubMed] [Google Scholar]
- 23.Cullen JH, Horsfield MA, Reek CR, Cherryman GR, Barnett DB, Samani NJ. A myocardial perfusion reserve index in humans using first-pass contrast-enhanced magnetic resonance imaging. J Am Coll Cardiol. 1999;33:1386–94. doi: 10.1016/s0735-1097(99)00004-2. [DOI] [PubMed] [Google Scholar]
- 24.Gebker R, Schwitter J, Fleck E, Nagel E. How we perform myocardial perfusion with cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2007;9:539–47. doi: 10.1080/10976640600897286. [DOI] [PubMed] [Google Scholar]
- 25.Nagel E, Klein C, Paetsch I, Hettwer S, Schnackenburg B, Wegscheider K, et al. Magnetic resonance perfusion measurements for the noninvasive detection of coronary artery disease. Circulation. 2003;108:432–7. doi: 10.1161/01.CIR.0000080915.35024.A9. [DOI] [PubMed] [Google Scholar]
- 26.al-Saadi N, Gross M, Bornstedt A, Schnackenburg B, Klein C, Fleck E, et al. Comparison of various parameters for determining an index of myocardial perfusion reserve in detecting coronary stenosis with cardiovascular magnetic resonance tomography. Z Kardiol. 2001;90:824–34. doi: 10.1007/s003920170081. [DOI] [PubMed] [Google Scholar]
- 27.Ishida M, Ichihara T, Nagata M, Ishida N, Takase S, Kurita T, et al. Quantification of myocardial blood flow using model based analysis of first-pass perfusion MRI: Extraction fraction of Gd-DTPA varies with myocardial blood flow in human myocardium. Magn Reson Med. 2011;66:1391–9. doi: 10.1002/mrm.22936. [DOI] [PubMed] [Google Scholar]
- 28.Patel AR, Antkowiak PF, Nandalur KR, West AM, Salerno M, Arora V, et al. Assessment of advanced coronary artery disease: Advantages of quantitative cardiac magnetic resonance perfusion analysis. J Am Coll Cardiol. 2010;56:561–9. doi: 10.1016/j.jacc.2010.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jerosch-Herold M, Wilke N, Stillman AE. Magnetic resonance quantification of the myocardial perfusion reserve with a Fermi function model for constrained deconvolution. Med Phys. 1998;25:73–84. doi: 10.1118/1.598163. [DOI] [PubMed] [Google Scholar]
- 30.Zun Z, Wong EC, Nayak KS. Assessment of myocardial blood flow (MBF) in humans using arterial spin labeling (ASL): Feasibility and noise analysis. Magn Reson Med. 2009;62:975–83. doi: 10.1002/mrm.22088. [DOI] [PubMed] [Google Scholar]
- 31.Arnold JR, Karamitsos TD, Pegg TJ, Francis JM, Olszewski R, Searle N, et al. Adenosine stress myocardial contrast echocardiography for the detection of coronary artery disease: A comparison with coronary angiography and cardiac magnetic resonance. JACC Cardiovasc Imaging. 2010;3:934–43. doi: 10.1016/j.jcmg.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Schwitter J, Wacker CM, van Rossum AC, Lombardi M, Al-Saadi N, Ahlstrom H, et al. MR-IMPACT: Comparison of perfusioncardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J. 2008;29:480–9. doi: 10.1093/eurheartj/ehm617. [DOI] [PubMed] [Google Scholar]
- 33.Greenwood JP, Maredia N, Younger JF, Brown JM, Nixon J, Everett CC, et al. Cardiovascular magnetic resonance and singlephoton emission computed tomography for diagnosis of coronary heart disease (CE-MARC): A prospective trial. Lancet. 2012;379:453–60. doi: 10.1016/S0140-6736(11)61335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwitter J, Nanz D, Kneifel S, Bertschinger K, Buchi M, Knusel PR, et al. Assessment of myocardial perfusion in coronary artery disease by magnetic resonance: A comparison with positron emission tomography and coronary angiography. Circulation. 2001;103:2230–5. doi: 10.1161/01.cir.103.18.2230. [DOI] [PubMed] [Google Scholar]
- 35.Ibrahim T, Nekolla SG, Schreiber K, Odaka K, Volz S, Mehilli J, et al. Assessment of coronary flow reserve: Comparison between contrast-enhanced magnetic resonance imaging and positron emission tomography. J Am Coll Cardiol. 2002;39:864–70. doi: 10.1016/s0735-1097(01)01829-0. [DOI] [PubMed] [Google Scholar]
- 36.Parkka JP, Niemi P, Saraste A, Koskenvuo JW, Komu M, Oikonen V, et al. Comparison of MRI and positron emission tomography for measuring myocardial perfusion reserve in healthy humans. Magn Reson Med. 2006;55:772–9. doi: 10.1002/mrm.20833. [DOI] [PubMed] [Google Scholar]
- 37.Morton G, Chiribiri A, Ishida M, Hussain ST, Schuster A, Indermuehle A, et al. Quantification of absolute myocardial perfusion in patients with coronary artery disease: Comparison between cardiovascular magnetic resonance and positron emission tomography. J Am Coll Cardiol. 2012;60:1546–55. doi: 10.1016/j.jacc.2012.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fritz-Hansen T, Hove JD, Kofoed KF, Kelbaek H, Larsson HB. Quantification of MRI measured myocardial perfusion reserve in healthy humans: A comparison with positron emission tomography. J Magn Reson Imaging. 2008;27:818–24. doi: 10.1002/jmri.21306. [DOI] [PubMed] [Google Scholar]
- 39.Costa MA, Shoemaker S, Futamatsu H, Klassen C, Angiolillo DJ, Nguyen M, et al. Quantitative magnetic resonance perfusion imaging detects anatomic and physiologic coronary artery disease as measured by coronary angiography and fractional flow reserve. J Am Coll Cardiol. 2007;50:514–22. doi: 10.1016/j.jacc.2007.04.053. [DOI] [PubMed] [Google Scholar]
- 40.Lockie T, Ishida M, Perera D, Chiribiri A, De Silva K, Kozerke S, et al. High-resolution magnetic resonance myocardial perfusion imaging at 3.0-Tesla to detect hemodynamically significant coronary stenoses as determined by fractional flow reserve. J Am Coll Cardiol. 2011;57:70–5. doi: 10.1016/j.jacc.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 41.Jahnke C, Nagel E, Gebker R, Kokocinski T, Kelle S, Manka R, et al. Prognostic value of cardiac magnetic resonance stress tests: Adenosine stress perfusion and dobutamine stress wall motion imaging. Circulation. 2007;115:1769–76. doi: 10.1161/CIRCULATIONAHA.106.652016. [DOI] [PubMed] [Google Scholar]
- 42.Ingkanisorn WP, Kwong RY, Bohme NS, Geller NL, Rhoads KL, Dyke CK, et al. Prognosis of negative adenosine stress magnetic resonance in patients presenting to an emergency department with chest pain. J Am Coll Cardiol. 2006;47:1427–32. doi: 10.1016/j.jacc.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 43.Klem I, Heitner JF, Shah DJ, Sketch MH, Jr, Behar V, Weinsaft J, et al. Improved detection of coronary artery disease by stress perfusion cardiovascular magnetic resonance with the use of delayed enhancement infarction imaging. J Am Coll Cardiol. 2006;47:1630–8. doi: 10.1016/j.jacc.2005.10.074. [DOI] [PubMed] [Google Scholar]
- 44.Bodi V, Sanchis J, Lopez-Lereu MP, Nunez J, Mainar L, Monmeneu JV, et al. Prognostic value of dipyridamole stress cardiovascular magnetic resonance imaging in patients with known or suspected coronary artery disease. J Am Coll Cardiol. 2007;50:1174–9. doi: 10.1016/j.jacc.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 45.Saraste A, Kajander S, Han C, Nesterov SV, Knuuti J. PET: Is myocardial flow quantification a clinical reality? J Nucl Cardiol. 2012;19:1044–59. doi: 10.1007/s12350-012-9588-8. [DOI] [PubMed] [Google Scholar]
- 46.Schwaiger M, Hutchins G. Quantification of regional myocardial perfusion by PET: Rationale and first clinical results. Eur Heart J. 1995;16:84–91. doi: 10.1093/eurheartj/16.suppl_j.84. [DOI] [PubMed] [Google Scholar]
- 47.Beanlands RS, Muzik O, Melon P, Sutor R, Sawada S, Muller D, et al. Noninvasive quantification of regional myocardial flow reserve in patients with coronary atherosclerosis using nitrogen-13 ammonia positron emission tomography. Determination of extent of altered vascular reactivity. J Am Coll Cardiol. 1995;26:1465–75. doi: 10.1016/0735-1097(95)00359-2. [DOI] [PubMed] [Google Scholar]
- 48.Dayanikli F, Grambow D, Muzik O, Mosca L, Rubenfire M, Schwaiger M. Early detection of abnormal coronary flow reserve in asymptomatic men at high risk for coronary artery disease using positron emission tomography. Circulation. 1994;90:808–17. doi: 10.1161/01.cir.90.2.808. [DOI] [PubMed] [Google Scholar]
- 49.Keereman V, Holen RV, Mollet P, Vandenberghe S. The effect of errors in segmented attenuation maps on PET quantification. Med Phys. 2011;38:6010–9. doi: 10.1118/1.3651640. [DOI] [PubMed] [Google Scholar]
- 50.Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM, et al. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol. 2009;54:150–6. doi: 10.1016/j.jacc.2009.02.069. [DOI] [PubMed] [Google Scholar]
- 51.Tio RA, Dabeshlim A, Siebelink HM, de Sutter J, Hillege HL, Zeebregts CJ, et al. Comparison between the prognostic value of left ventricular function and myocardial perfusion reserve in patients with ischemic heart disease. J Nucl Med. 2009;50:214–9. doi: 10.2967/jnumed.108.054395. [DOI] [PubMed] [Google Scholar]
- 52.Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, et al. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126:1858–68. doi: 10.1161/CIRCULATIONAHA.112.120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Czernin J, Muller P, Chan S, Brunken RC, Porenta G, Krivokapich J, et al. Influence of age and hemodynamics on myocardial blood flow and flow reserve. Circulation. 1993;88:62–9. doi: 10.1161/01.cir.88.1.62. [DOI] [PubMed] [Google Scholar]
- 54.Uren NG, Camici PG, Melin JA, Bol A, de Bruyne B, Radvan J, et al. Effect of aging on myocardial perfusion reserve. J Nucl Med. 1995;36:2032–6. [PubMed] [Google Scholar]
- 55.Kaufmann PA, Gnecchi-Ruscone T, di Terlizzi M, Schafers KP, Luscher TF, Camici PG. Coronary heart disease in smokers: Vitamin C restores coronary microcirculatory function. Circulation. 2000;102:1233–8. doi: 10.1161/01.cir.102.11.1233. [DOI] [PubMed] [Google Scholar]
- 56.Kaufmann PA, Gnecchi-Ruscone T, Schafers KP, Luscher TF, Camici PG. Low density lipoprotein cholesterol and coronary microvascular dysfunction in hypercholesterolemia. J Am Coll Cardiol. 2000;36:103–9. doi: 10.1016/s0735-1097(00)00697-5. [DOI] [PubMed] [Google Scholar]
- 57.Sambuceti G, Parodi O, Marzullo P, Giorgetti A, Fusani L, Puccini G, et al. Regional myocardial blood flow in stable angina pectoris associated with isolated significant narrowing of either the left anterior descending or left circumflex coronary artery. Am J Cardiol. 1993;72:990–4. doi: 10.1016/0002-9149(93)90850-c. [DOI] [PubMed] [Google Scholar]
- 58.Dorbala S, Di Carli MF, Beanlands RS, Merhige ME, Williams BA, Veledar E, et al. Prognostic value of stress myocardial perfusion positron emission tomography: Results from a multicenter observational registry. J Am Coll Cardiol. 2013;61:176–84. doi: 10.1016/j.jacc.2012.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uren NG, Crake T, Lefroy DC, de Silva R, Davies GJ, Maseri A. Delayed recovery of coronary resistive vessel function after coronary angioplasty. J Am Coll Cardiol. 1993;21:612–21. doi: 10.1016/0735-1097(93)90092-f. [DOI] [PubMed] [Google Scholar]
- 60.Schwarz ER, Speakman MT, vom Dahl J, Kloner RA. Hibernating myocardium: Is there evidence for chronic flow reduction? Heart Dis. 1999;1:155–62. [PubMed] [Google Scholar]
- 61.Camici P, Chiriatti G, Lorenzoni R, Bellina RC, Gistri R, Italiani G, et al. Coronary vasodilation is impaired in both hypertrophied and nonhypertrophied myocardium of patients with hypertrophic cardiomyopathy: A study with nitrogen-13 ammonia and positron emission tomography. J Am Coll Cardiol. 1991;17:879–86. doi: 10.1016/0735-1097(91)90869-b. [DOI] [PubMed] [Google Scholar]
- 62.Choudhury L, Elliott P, Rimoldi O, Ryan M, Lammertsma AA, Boyd H, et al. Transmural myocardial blood flow distribution in hypertrophic cardiomyopathy and effect of treatment. Basic Res Cardiol. 1999;94:49–59. doi: 10.1007/s003950050126. [DOI] [PubMed] [Google Scholar]
- 63.Gistri R, Cecchi F, Choudhury L, Montereggi A, Sorace O, Salvadori PA, et al. Effect of verapamil on absolute myocardial blood flow in hypertrophic cardiomyopathy. Am J Cardiol. 1994;74:363–8. doi: 10.1016/0002-9149(94)90404-9. [DOI] [PubMed] [Google Scholar]
- 64.Jorg-Ciopor M, Namdar M, Turina J, Jenni R, Schwitter J, Turina M, et al. Regional myocardial ischemia in hypertrophic cardiomyopathy: Impact of myectomy. J Thorac Cardiovasc Surg. 2004;128:163–9. doi: 10.1016/j.jtcvs.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 65.Cecchi F, Olivotto I, Gistri R, Lorenzoni R, Chiriatti G, Camici PG. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N Engl J Med. 2003;349:1027–35. doi: 10.1056/NEJMoa025050. [DOI] [PubMed] [Google Scholar]
- 66.Opherk D, Schwarz F, Mall G, Manthey J, Baller D, Kubler W. Coronary dilatory capacity in idiopathic dilated cardiomyopathy: Analysis of 16 patients. Am J Cardiol. 1983;51:1657–62. doi: 10.1016/0002-9149(83)90205-9. [DOI] [PubMed] [Google Scholar]
- 67.Stolen KQ, Kemppainen J, Kalliokoski KK, Karanko H, Toikka J, Janatuinen T, et al. Myocardial perfusion reserve and peripheral endothelial function in patients with idiopathic dilated cardiomyopathy. Am J Cardiol. 2004;93:64–8. doi: 10.1016/j.amjcard.2003.08.074. [DOI] [PubMed] [Google Scholar]
- 68.Canetti M, Akhter MW, Lerman A, Karaalp IS, Zell JA, Singh H, et al. Evaluation of myocardial blood flow reserve in patients with chronic congestive heart failure due to idiopathic dilated cardiomyopathy. Am J Cardiol. 2003;92:1246–9. doi: 10.1016/j.amjcard.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 69.Neglia D, Michelassi C, Trivieri MG, Sambuceti G, Giorgetti A, Pratali L, et al. Prognostic role of myocardial blood flow impairment in idiopathic left ventricular dysfunction. Circulation. 2002;105:186–93. doi: 10.1161/hc0202.102119. [DOI] [PubMed] [Google Scholar]
- 70.Jaarsma C, Leiner T, Bekkers SC, Crijns HJ, Wildberger JE, Nagel E, et al. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstructive coronary artery disease: A meta-analysis. J Am Coll Cardiol. 2012;59:1719–28. doi: 10.1016/j.jacc.2011.12.040. [DOI] [PubMed] [Google Scholar]
- 71.Nielsen G, Fritz-Hansen T, Dirks CG, Jensen GB, Larsson HB. Evaluation of heart perfusion in patients with acute myocardial infarction using dynamic contrast-enhanced magnetic resonance imaging. J Magn Reson Imaging. 2004;20:403–10. doi: 10.1002/jmri.20142. [DOI] [PubMed] [Google Scholar]
- 72.Pack NA, DiBella EV, Rust TC, Kadrmas DJ, McGann CJ, Butterfield R, et al. Estimating myocardial perfusion from dynamic contrast-enhanced CMR with a model-independent deconvolution method. J Cardiovasc Magn Reson. 2008;10:52. doi: 10.1186/1532-429X-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Plein S, Kozerke S, Suerder D, Luescher TF, Greenwood JP, Boesiger P, et al. High spatial resolution myocardial perfusion cardiac magnetic resonance for the detection of coronary artery disease. Eur Heart J. 2008;29:2148–55. doi: 10.1093/eurheartj/ehn297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Araoz PA, Glockner JF, McGee KP, Potter DD, Jr, Valeti VU, Stanley DW, et al. 3 Tesla MR imaging provides improved contrast in first-pass myocardial perfusion imaging over a range of gadolinium doses. J Cardiovasc Magn Reson. 2005;7:559–64. doi: 10.1081/jcmr-200060622. [DOI] [PubMed] [Google Scholar]
- 75.Shin T, Hu HH, Pohost GM, Nayak KS. Three dimensional first-pass myocardial perfusion imaging at 3 T: Feasibility study. J Cardiovasc Magn Reson. 2008;10:57. doi: 10.1186/1532-429X-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Machac J. Cardiac positron emission tomography imaging. Semin Nucl Med. 2005;35:17–36. doi: 10.1053/j.semnuclmed.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 77.Salerno M, Beller GA. Noninvasive assessment of myocardial perfusion. Circ Cardiovasc Imaging. 2009;2:412–24. doi: 10.1161/CIRCIMAGING.109.854893. [DOI] [PubMed] [Google Scholar]
- 78.Garcia EV. Physical attributes, limitations, and future potential for PET and SPECT. J Nucl Cardiol. 2012;19:S19–29. doi: 10.1007/s12350-011-9488-3. [DOI] [PubMed] [Google Scholar]
- 79.Algranati D, Kassab GS, Lanir Y. Why is the subendocardium more vulnerable to ischemia? A new paradigm. Am J Physiol Heart Circ Physiol. 2011;300:H1090–100. doi: 10.1152/ajpheart.00473.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Panting JR, Gatehouse PD, Yang GZ, Grothues F, Firmin DN, Collins P, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002;346:1948–53. doi: 10.1056/NEJMoa012369. [DOI] [PubMed] [Google Scholar]
- 81.Muehling OM, Jerosch-Herold M, Panse P, Zenovich A, Wilson BV, Wilson RF, et al. Regional heterogeneity of myocardial perfusion in healthy human myocardium: Assessment with magnetic resonance perfusion imaging. J Cardiovasc Magn Reson. 2004;6:499–507. doi: 10.1081/jcmr-120030570. [DOI] [PubMed] [Google Scholar]
- 82.Muehling OM, Wilke NM, Panse P, Jerosch-Herold M, Wilson BV, Wilson RF, et al. Reduced myocardial perfusion reserve and transmural perfusion gradient in heart transplant arteriopathy assessed by magnetic resonance imaging. J Am Coll Cardiol. 2003;42:1054–60. doi: 10.1016/s0735-1097(03)00924-0. [DOI] [PubMed] [Google Scholar]
- 83.Vermeltfoort IAC, Raijmakers PGHM, et al. Is subendocardial ischaemia present in patients with chest pain and normal coronary angiograms? A cardiovascular MR study. Eur Heart J. 2007;28:1554–8. doi: 10.1093/eurheartj/ehm088. [DOI] [PubMed] [Google Scholar]
- 84.Miyai N, Kawasaki T, Taniguchi T, Kamitani T, Kawasaki S, Sugihara H. Exercise-induced ST-segment depression and myocardial ischemia in patients with hypertrophic cardiomyopathy: myocardial scintigraphic study. J Cardiol. 2005;46:141–7. [PubMed] [Google Scholar]
- 85.Kawasaki T, Akakabe Y, Yamano M, Miki S, Kamitani T, Kuribayashi T, et al. Gated single-photon emission computed tomography detects subendocardial ischemia in hypertrophic cardiomyopathy. Circ J. 2007;71:256–60. doi: 10.1253/circj.71.256. [DOI] [PubMed] [Google Scholar]
- 86.Rajappan K, Rimoldi OE, Dutka DP, Ariff B, Pennell DJ, Sheridan DJ, et al. Mechanisms of coronary microcirculatory dysfunction in patients with aortic stenosis and angiographically normal coronary arteries. Circulation. 2002;105:470–6. doi: 10.1161/hc0402.102931. [DOI] [PubMed] [Google Scholar]
- 87.Vermeltfoort IA, Raijmakers PG, Lubberink M, Germans T, van Rossum AC, Lammertsma AA, et al. Feasibility of subendocardial and subepicardial myocardial perfusion measurements in healthy normals with (15)O-labeled water and positron emission tomography. J Nucl Cardiol. 2011;18:650–6. doi: 10.1007/s12350-011-9375-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaufmann PA, Gnecchi-Ruscone T, Yap JT, Rimoldi O, Camici PG. Assessment of the reproducibility of baseline and hyperemic myocardial blood flow measurements with 15O-labeled water and PET. J Nucl Med. 1999;40:1848–56. [PubMed] [Google Scholar]
- 89.Nagamachi S, Czernin J, Kim AS, Sun KT, Bottcher M, Phelps ME, et al. Reproducibility of measurements of regional resting and hyperemic myocardial blood flow assessed with PET. J Nucl Med. 1996;37:1626–31. [PubMed] [Google Scholar]
- 90.Jerosch-Herold M, Vazquez G, Wang L, Jacobs DR, Jr, Folsom AR. Variability of myocardial blood flow measurements by magnetic resonance imaging in the multi-ethnic study of atherosclerosis. Invest Radiol. 2008;43:155–61. doi: 10.1097/RLI.0b013e31815abebd. [DOI] [PubMed] [Google Scholar]
- 91.Ziadi MC, Dekemp RA, Williams K, Guo A, Renaud JM, Chow BJ, et al. Does quantification of myocardial flow reserve using rubidium-82 positron emission tomography facilitate detection of multivessel coronary artery disease? J Nucl Cardiol. 2012;19:670–80. doi: 10.1007/s12350-011-9506-5. [DOI] [PubMed] [Google Scholar]
- 92.Jogiya R, Kozerke S, Morton G, De Silva K, Redwood S, Perera D, et al. Validation of dynamic 3-dimensional whole heart magnetic resonance myocardial perfusion imaging against fractional flow reserve for the detection of significant coronary artery disease. J Am Coll Cardiol. 2012;60:756–65. doi: 10.1016/j.jacc.2012.02.075. [DOI] [PubMed] [Google Scholar]
- 93.Morton G, Ishida M, Schuster A, Hussain S, Schaeffter T, Chiribiri A, et al. Perfusion cardiovascular magnetic resonance: Comparison of an advanced, high-resolution and a standard sequence. J Cardiovasc Magn Reson. 2012;14:34. doi: 10.1186/1532-429X-14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schroeder MA, Clarke K, Neubauer S, Tyler DJ. Hyperpolarized magnetic resonance: A novel technique for the in vivo assessment of cardiovascular disease. Circulation. 2011;124:1580–94. doi: 10.1161/CIRCULATIONAHA.111.024919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Small GR, Wells RG, Schindler T, Chow BJ, Ruddy TD. Advances in cardiac SPECT and PET imaging: Overcoming the challenges to reduce radiation exposure and improve accuracy. Can J Cardiol. 2013;29:275–84. doi: 10.1016/j.cjca.2012.10.003. [DOI] [PubMed] [Google Scholar]