Abstract
Assessment of measurable residual disease (MRD) has emerged as a powerful prognostic tool in pediatric and adult acute lymphoblastic leukemia (ALL). In this single-centre retrospective study, we evaluated the prognostic relevance of MRD based on BCR-ABL1 copy numbers in Ph + ALL patients between 2006 and 2018. Molecular responses were evaluated at 3, 6, 9 and 12 months after the initiation of treatment. Patients who had their MRD assessed at three or more time points were categorized into MRD good risk or poor risk based on BCR-ABL1/ABL1 copy number ratio. MRD positive patients consistently showed a trend toward poor survival and on multivariate analysis, MRD poor risk patients had adverse outcomes when compared to MRD good risk patients in terms of overall (OS; p =.031) and event-free (EFS; p <.001) survival. In conclusion, molecular MRD based on BCR-ABL1 copy number ratio is an ideal prognostic indicator in Ph + ALL patients undergoing treatment.
Keywords: Ph + ALL, BCR-ABL1, minimal residual disease, RQ-PCR
Introduction
The Philadelphia (Ph) chromosome resulting from the balanced chromosomal translocation t(9;22)(q34;q11.2), is the most frequent recurrent cytogenetic abnormality observed in adult acute lymphoblastic leukemia (ALL) patients [1]. The resulting BCR-ABL1 fusion gene encodes an oncoprotein with constitutive tyrosine kinase activity [2]. Unlike in the pediatric ALL, where it is rare, nearly one-third of adult ALL patients have the Ph + chromosome [3,4]. Prior to the incorporation of tyrosine kinase inhibitors (TKI) into the treatment regimen, the outcome of patients with Ph-positive ALL (Ph + ALL) was very poor with long-term survival rates of 20% in most studies [5–7]. The addition of TKIs has substantially improved outcomes in both pediatric and adult Ph + ALL patients and also increased the percentage of patients who are eligible for allogeneic stem cell transplantation (alloHSCT) in first complete remission [5,8–11].
Various studies have shown that the presence of measurable residual disease (MRD) is a strong and independent prognostic factor in ALL, including Ph + ALL treated with conventional chemotherapy or alloHSCT [12–14]. While molecular responses in ALL are commonly measured with real-time quantitative Polymerase Chain Reaction (RQ-PCR) targeting the immunoglobulin/T cell receptor (Ig/TCR) rearrangements, estimating the BCR-ABL1 copy numbers is an equally sensitive method to quantify the residual disease burden in Ph + ALL patients [15,16]. While the importance of MRD in identifying patients who are likely to benefit from alloHSCT in Ph- ALL is well established, the therapeutic and prognostic utility of MRD in Ph + ALL is not as well defined [5].
Herein, we report the prognostic value of molecular MRD monitoring based on BCR/ABL1 fusion transcripts in the adult, adolescent and young adult (AYA) Ph + ALL patients.
Patients and methods
Patients
Adolescent and young adult (AYA) and adult patients who were diagnosed with Ph + ALL from 2006 to 2018 and had BCR-ABL1 copy numbers evaluated by RQ-PCR at least once before the maintenance phase (n = 94) were included in the study. The BCR-ABL1 translocation was detected by standard karyotype and/or fluorescence in situ hybridization analysis and the fusion transcript type [e1a2 or e13a2 (b2a2) or e14a2 (b3a2)] was characterized by nested Reverse Transcriptase PCR (RT-PCR) [17]. Patients with karyotype abnormalities other than t(9, 22) were further stratified into cytogenetic good risk, poor risk or others as defined earlier [18]. The clinical details including age, WBC count, bone marrow (BM) blast percentage, transcript type, treatment and transplant status, CNS involvement and staging, steroid response, baseline karyotype and immunophenotype were collected from the patients’ records. Molecular responses were evaluated at 3 monthly intervals at 3 (TP1), 6 (TP2), 9 (TP3) and 12 (TP4) months after the initiation of treatment irrespective of the transplant status.
MRD assessment by RQ-PCR
Total cellular RNA was extracted from the leucocytes using the QIAamp RNA blood mini kit (Qiagen GmbH, Hilden, Germany) using the manufacturer’s instructions. The concentration (ng/μL) and purity of RNA were measured by spectrophotometric determination of the A260/A280 ratio. Complementary DNA (cDNA) was synthesized using random hexamers (Invitrogen, Carlsbad, CA, USA) and Superscript II reverse transcriptase (Superscript II first strand cDNA synthesis system, Invitrogen, CA, USA). BCR-ABL1 fusion transcript characterization was done by nested RT-PCR to identify the major (e13a2 or e14a2), minor (e1a2) and micro (e19a2) types of BCR-ABL1 fusion transcripts as reported previously [17]. BCR-ABL1 transcript copy numbers were assessed by quantitative Real-Time PCR using Applied Biosystems® 7500 Real-Time PCR Systems as per the established protocol using both commercial (for e13a2 and e14a2 fusion transcripts) and in-house plasmid (for e1a2 fusion transcripts) standards along with TaqMan probes and PCR master mix (Applied Biosystems, Foster City, CA, USA) [19]. Copy numbers of ABL1 and BCR-ABL1 fusion transcripts were derived by extrapolating the data from the standard curve from which the normalized BCR-ABL1/ABL1 copy number (NCN) ratio was calculated [20].
Treatment strategies
Majority of the patients received the hyper CVAD regimen or BFM based regimen as the frontline chemotherapy along with a TKI [16,21]. BFM based regimen was largely used in adolescents and young adults as reported in our earlier studies [22]. Studies have shown comparable results with both the treatment regimens with no significant difference in the outcomes [21]. Few patients were managed with the standard risk modified GMALL regimen as described earlier by us, owing to their financial constraints [22,23]. The TKI was administered during the entire maintenance phase and continued indefinitely after that in these patients. A myeloablative alloHSCT was offered to all the patients at CR1 taking into consideration the patient’s financial resources and the availability of a suitable donor.
Response and outcome definitions
Complete response/remission (CR) was defined as the presence of less than 5% blasts in the BM with more than 1 x 109/L neutrophils and 100 x 109/L platelets in the peripheral blood (PB) without any extramedullary disease [24]. Relapse was defined by the reappearance of more than 5% blasts in a BM aspirate irrespective of the neutrophil and platelet counts or by the presence of extramedullary disease after achieving CR [24]. Overall survival (OS) was calculated from the time of initiation of treatment until death or the last follow up [25]. Relapse-free survival (RFS) was calculated from the time of achieving a response until relapse as defined above [25]. Event-free survival (EFS) was calculated from the beginning of treatment until an event which includes relapse, death in CR, pre and post-transplant [25]. Major molecular response (MMR) was defined as a BCR-ABL1/ABL transcript copy number ratio of <0.1% in the BM, and molecular CR/Complete molecular response (CMR) or negative MRD was defined by the absence of fusion transcripts with a sensitivity of at least 0.01% (ABL1 copy numbers ≥104) or BCR-ABL1/ABL1 copy number ratio <0.01% [26]. Patients who had their MRD assessed at three different time points were categorized into MRD good risk and poor risk groups based on the BCR-ABL1 copy number ratio [MRD good risk – persistent MRD negativity or decreasing BCR-ABL1/ABL1 copy number ratio and attaining MRD negativity by the third measured time point or earlier. MRD poor risk – persistent MRD positivity at all the three measured time points or increasing BCR-ABL1/ABL1 copy number ratio becoming MRD positive by the third measured time point or earlier].
Statistical analysis
Patient characteristics and laboratory indices were expressed as median with range for numerical variables (age, WBC count, blast percentage) and frequencies (percentages) for categorical variables (transcript type, immunophenotype antigen expression, secondary karyotype abnormalities). Statistical significance for the above was derived from the Mann—Whitney U test and Fischer’s exact test respectively. OS, EFS and RFS were calculated using Kaplan—Meier analysis and survival estimates were compared by using the log-rank test [27]. Univariate Cox proportional hazards regression models were used to assess the association between patient characteristics and survival [28]. Clinically relevant variables including patient characteristics with p < .10 in the univariate analysis were included in the multivariate analysis. For all the tests, a 2-sided p value ≤ .05 was considered to be statistically significant. The analysis was done using IBM SPSS statistics version 24.0 software (SPSS, Chicago, IL).
Results
Patient characteristics
The baseline characteristics of the study cohort are summarized in Table 1. The study cohort included 94 patients with a median age of 33 years [range: 14—70years]. The median WBC count, platelet count and BM blast percentage are shown in Table 1. Pre-transfusion hemoglobin levels (g/dL) were not available for most of the patients and hence hemoglobin levels were not included in the analysis. The BCR-ABL1 fusion transcript type e1a2 was seen in 70% of patients while e13a2 or e14a2 transcripts, which corresponds to p210 fusion protein was seen in 23.5% of patients. The remaining patients had e1a2 fusion transcript co-existing with either e13a2 or e14a2 fusion transcripts. CD10 (96%), CD34 (95%), and HLA-DR (99%) expression were seen in most of the patients while CD20, CD13 and CD33 expression were seen in 42%, 48% and 44% of patients respectively. Conventional karyotyping failed to identify the translocation in 7 patients and the diagnosis was based on RT-PCR or FISH which showed the fusion signals in varying percentages of cells ranging from 27% to 95%. Karyotype results were available for 75 patients and apart from t(9, 22), secondary karyotype abnormalities were seen in 2 or more metaphases in 47 patients. This includes 9 patients with high hyperdiploidy (good risk) and 20 patients with complex karyotype or other established poor risk karyotypes. Among the 94 patients, end induction bone marrow morphology results were available for 88 patients of whom 75 (85.2%) had achieved CR. MRD results by RQ-PCR were available for 94, 70, 37 and 31 patients at TP1, TP2, TP3 and TP4 respectively. Among the 94 and 70 patients who had MRD assessed at TP1 and TP2 respectively, 61 (64.9%) and 39 (55.71%) had a copy number ratio greater than 0.01%. Similarly, among the 37 and 31 patients who had their MRD assessed at TP3 and TP4, 21 (56.75%) and 11 (35.5%) had a copy number ratio greater than 0.01% respectively. Eleven (18.03%) out of the 61 patients who were MRD positive at TP1 became MRD negative at TP2. A total of 62 patients had copy number ratio assessed at three or more time points and were categorized as MRD good risk (n = 31) and MRD poor risk (n = 31) as defined earlier.
Table 1. Baseline demographic characteristics, treatment protocols used and response to therapy (n = 94).
| Parameters | Statistics |
|---|---|
| Age (years) | Median: 33 Range: 14-70 |
| Male/Female | 48/46 |
| BCR-ABL Fusion transcript type | – |
| e1a2 | 66 (70%) |
| others | 28 (30%) |
| WBC count (per μL) | Median: 34,200 Range: 1400–477,000 |
| Blast percentage (%) | Median: 90 Range: 28–100 |
| Platelet count (per μL) | Median: 39,000 Range: 4000–413,000 |
| CD20 expression | – |
| Positive | 28 |
| Negative | 29 |
| Not available | 37 |
| Additional chromosomal abnormalities | 47 (50%) |
| Good risk | 9 (9.5%) |
| Poor risk | 20 (21.3%) |
| Others | 18 (19.1%) |
| CNS Staging [I/II/III/NA] | 71/3/4/16 |
| Treatment regimens | – |
| Hyper CVAD | 55 (58%) |
| Modified GMALL | 16 (17%) |
| BFM based protocol | 23 (25%) |
| End Induction marrow | – |
| Complete remission (CR) | 75 (80%) |
| No CR | 13 (14%) |
| Not available | 6 (6%) |
| Time from diagnosis to transplant (months) | Median: 6 Range: 3–14 months |
WBC: white blood cells; CD: cluster of differentiation; CNS: central nervous system; CVAD: cyclophosphamide, vincristine, doxorubicin (adriamycin), and dexamethasone; GMALL: German multicentre acute lymphoblastic leukemia; BFM: Berlin Frankfurt Munich.
MRD and transplant outcomes
A total of 33 patients underwent transplantation in CR1. The timing of alloHSCT after achieving CR1 varied depending on the donor availability and patient-related factors with a median of 6 months from the time of diagnosis [range: 3–14 months]. Sixteen patients underwent alloHSCT after MRD assessment at TP1 including 11 patients who were MRD positive. Among the 11 pre-transplant MRD positive patients, 7 became MRD negative post-transplant (TP2), while all the 5 patients who were MRD negative prior to the transplant, continued to remain MRD negative at TP2. Four patients from the MRD positive group relapsed with a median time of 11.5 months from the transplant to relapse (range: 4-22 months) while among the 5 patients who were MRD negative, one patient relapsed 16 months after the transplant. There were 5 deaths in the MRD positive group including 2 non-relapse mortality (NRM) and 2 deaths in the MRD negative group (1 NRM). Likewise, among the 15 patients who underwent alloHSCT after MRD assessment at TP2, 12 were MRD positive pre-transplant. There were two relapses and 7 deaths (6 NRM) in this group while among the 3 patients who were MRD negative pre-transplant, no relapse was reported and there were 2 deaths. The remaining 2 patients were transplanted 12 and 14 months respectively from the date of diagnosis.
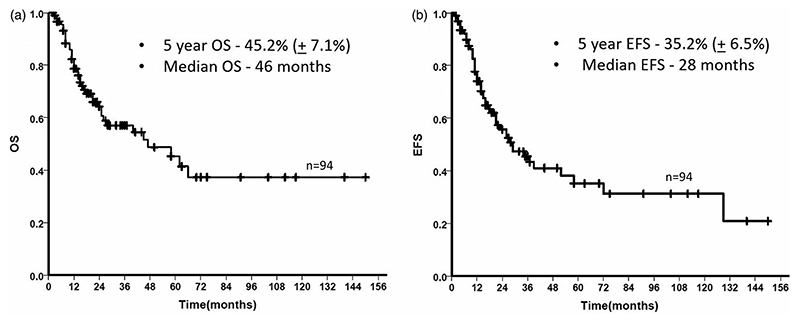
Kaplan Meier survival statistics
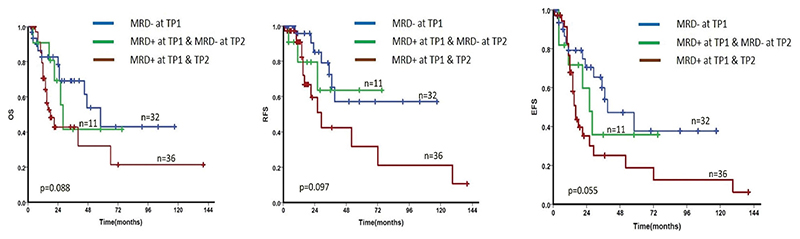
Hyper CVAD regimen was used in 55 patients while 23 AYA patients received the BFM based regimen (Table 1). A total of 27 patients relapsed, including 7 patients who relapsed after alloHSCT and the median time to relapse from the date of diagnosis was 18 months (range: 2-127 months). There was a total of 38 deaths in the entire cohort. The 5-year OS and EFS of the cohort was 45.2% (±7.1%) and 35.2% (±6.5%) respectively and the median OS and median EFS were 46 months and 28 months respectively (Figure 1). The difference in survival at different levels of copy number ratio at TP1 and TP2 are shown in Supplementary Figure 1. The 5-year OS and EFS in patients with high MRD levels (BCR-ABL1/ABL1 copy number ratio >1%) at TP1 were only 25.7% (±11.8%) and 25.3% (±10.7%) respectively whereas at TP2 the 5-year OS and EFS were 16.7% (±14.2%) and 11.4% (±10.7%) respectively when the copy number ratio was >1%. When the patients were segregated into two groups – MRD positive and MRD negative with 0.01% as the cutoff as described earlier, MRD negative patients consistently showed a near significant trend toward improved survival (OS and EFS) when compared to MRD positive patients at TP1 (Table 2). Among the 61 patients who were MRD positive at TP1, 47 had the MRD assessed at TP2. When we compared the kinetics of MRD at successive time points (TP1 and TP2) by including 32 patients who were MRD negative at TP1 (one patient who was MRD negative at TP1 had no follow up after 4 months and was excluded) and the 47 patients who were MRD positive at TP1 and had their MRD assessed at TP2, patients who achieved early MRD negativity (TP1) had the best prognosis while patients who were persistently MRD positive at both the time points had the worst survival (Figure 2 and Table 3).
Figure 1.
Kaplan Meier estimate of the overall survival (OS) and event-free survival (EFS) of the entire cohort. The median OS and EFS were 46 months and 28 months respectively.
Table 2. Study variables with a significant/near significant association with poor overall survival (OS) and event free survival (EFS) on univariate analysis.
| Survival | Characteristics | p-value | Hazards ratio | 95% Confidence interval |
|---|---|---|---|---|
| OS | Additional poor risk karyotype | .009 | 2.5 | 1.25–4.99 |
| MRD at TP1 (n = 94) | .089 | 2.2 | 0.83–3.46 | |
| MRD at TP3 (n = 37) | .026 | 4.9 | 1.08–10.48 | |
| High risk MRD | <.0001 | 4.2 | 1.94–9.33 | |
| EFS | End induction (marrow): No CR | .054 | 2.1 | 0.99–4.69 |
| MRD at TP1 (n = 94) | .069 | 2.9 | 0.91–3.32 | |
| MRD at TP3 (n = 37) | .010 | 6.6 | 1.26–11.66 | |
| High risk MRD | <.0001 | 5.1 | 2.39–10.91 |
MRD: measurable residual disease; TP1: time point 1; TP3: time point 3; CR: complete remission
Figure 2.
Kaplan Meier survival estimate comparing the kinetics of MRD across TP1 and TP2. (Cutoff for MRD positivity is ≥ 0.01%). Patients who were persistently MRD positive at TP1 and TP2 had a poor OS, RFS and EFS when compared to the patients who achieved MRD negativity either at TP1 or at TP2. (p value for the entire cohort is shown here while p value between the individual subgroups in this comparison is shown in Table 3).
Table 3. Survival outcomes based on the kinetics of MRD at TP1 and TP2.
| Survival | MRD | kinetics | p-value | Hazards Ratio | 95% Confidence Interval |
|---|---|---|---|---|---|
| OS | MRD -ve at TP1 vs | MRD -ve at TP2 | .615 | 1.3 | 0.45-3.79 |
| MRD -ve at TP1 vs | MRD + ve at TP1 & TP2 | .037 | 2.2 | 1.05-4.83 | |
| EFS | MRD -ve at TP1 vs | MRD -ve at TP2 | .470 | 1.4 | 0.54-3.77 |
| MRD -ve at TP1 vs | MRD + ve at TP1 & TP2 | .021 | 2.2 | 1.13-4.46 | |
| RFS | MRD -ve at TP1 vs | MRD -ve at TP2 | .626 | 1.4 | 0.36-5.43 |
| MRD -ve at TP1 vs | MRD + ve at TP1 & TP2 | .042 | 2.6 | 1.03-6.71 |
OS: overall survival; EFS: event free survival; RFS: relapse free survival; MRD: measurable residual disease; TP1: time point 1; TP2: time point 2.
Univariate and multivariate analysis of OS and EFS using cox models
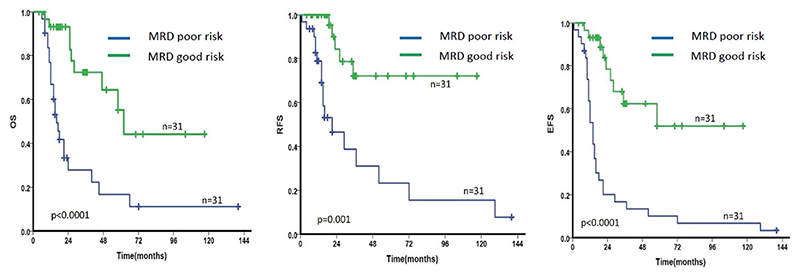
The study variables with a significant (p < .05) or near significant (p < .1) association with OS and EFS were selected for multivariate analysis (Table 2). Although parameters like older age at diagnosis (>40 years), high WBC count (>30,000/μL), high blast percentage (>90%) and low platelet count (<1,00,000/μL) showed a trend toward poor OS and/or EFS, no statistically significant correlation could be achieved. Presence of additional poor risk karyotypes was associated with shorter OS [Hazards Ratio (HR) = 2.5; 95% Confidence Interval (CI) = 1.25-4.99; p = .009] while failure to achieve CR at the end of induction had a near significant association with EFS [HR = 2.1; 95% CI = 0.99-4.69; p = .054]. Persistent/positive MRD (copy number ratio >0.01%) had a significant/near significant association with OS and EFS at TP1 (3 months) and TP3 (9 months)] as shown in Table 2. No definitive conclusion could be drawn from the MRD data at time points TP3 and TP4 due to the small number of patients. Patients categorized as MRD poor risk had a significantly shorter OS, RFS and EFS compared to the MRD good risk patients [OS: (HR = 4.2; CI = 1.94-9.33; p < .0001), RFS: (HR = 5.5; CI = 2.00-15.21; p < .001) and EFS: (HR = 5.1; CI – 2.39-10.91; p < .0001)] (Figure 3, Table 2). Upon multivariate analysis, only the MRD risk stratification was statistically significant for both OS (HR = 2.9; CI = 1.10-7.84; p = .031) and EFS (HR = 5.4; CI = 2.23-13.23; p < .001) while presence of additional poor risk karyotype abnormalities showed a trend toward poor OS (HR = 2.2; CI = 0.89-5.45; p = .086).
Figure 3.
Kaplan Meier survival estimates showing the difference in OS (a), RFS (b) and EFS (c) between MRD good risk and MRD poor risk patients.
Discussion
The potential role of MRD in predicting the treatment outcomes is well established in both pediatric and adult ALL patients [13,29]. Various studies have shown that MRD measured by flow cytometry or PCR based methods has a significant role in predicting the risk of relapse and hence can be used for the refinement of treatment stratification [30,31].
The 5-year OS (45%) and EFS (35%) of our cohort are comparable to various published studies on adult Ph + ALL in the post TKI era [32]. The treatment studies have shown comparable results with both hyper CVAD regimen and the BFM based regimen with no significant difference in the outcomes between the two regimens [33,34]. The molecular responses and the survival rates appear to improve with each successive generation of TKIs [1,29,35,36]. Unlike in CML where the transcript type has a strong impact on the survival outcomes, we did not find any difference in OS or EFS between e1a2 and other fusion transcripts [37]. A subset of Ph + ALL patients (6% in our cohort) may have more than one fusion transcript identified at diagnosis (usually e1a2 in concurrence with either e13a2 or e14a2). The second transcript is usually a result of alternative splicing and in such patients, it is important to identify the dominant transcript for follow up. When the t(9, 22) is not detected by karyotype and/or FISH, it is important to determine the BCR-ABL1/ABL1 copy number ratio by RQ-PCR at diagnosis. In this study, there were 2 patients with normal karyotype who had fusion signals in less than 50% of the cells when analyzed by FISH. Although rare, the possibility of additional clonal events contributing to the leukemogenesis cannot be ruled out in such patients. Flow cytometry could be a better option for MRD detection when compared to stand-alone molecular MRD targeting the fusion transcripts in cases where there is evidence of bi-clonal leukemia.
Although study parameters like older age at diagnosis, high WBC counts, high BM blast percentage and low platelet counts showed a trend toward poor survival (OS and/or EFS), there was no statistical significance for the same unlike our earlier studies in adult ALL patients [23]. This could be because of the sample size limitation and the fact that the current study includes only a poor cytogenetic risk ALL subgroup, defined by BCR-ABL1 translocation, which probably attenuates the impact of the above-mentioned parameters on survival. Interestingly, the presence of additional poor risk karyotypes like near haploidy or complex karyotypes further worsened the overall survival in these patients.
Studies have shown conflicting results regarding the association between rapid molecular clearance (End induction BCR-ABL1 copy numbers <0.01%) OS or EFS in Ph + ALL patients treated with imatinib + chemotherapy or alloHSCT [16,26,38]. The discrepancy could be attributed to the fact that longer time is required to reach a transcript/RNA based BCR-ABL1 response as compared to the DNA-based Ig/TCR response [32]. We did not have the end induction copy number levels for many of our patients and hence it was not included in the analysis. Earlier studies have shown that molecular responses at 3 months had the maximum impact on survival and long term outcomes in Ph + ALL patients [26]. In our study, MRD positivity at TP1 (3 months) showed a trend toward poor outcomes in terms of both OS and EFS (Table 2). When we looked at the MRD kinetics, patients who persistently had BCR-ABL1 copy number ratio >0.01% at both TP1 and TP2 had very poor outcomes when compared to patients who achieved MRD negative status at TP1 or at TP2 (Figure 2 and Table 3). This clearly shows that even in a subgroup of poor risk ALL patients, MRD is an ideal tool to identify patients who will have a better survival outcome with the same line of management.
Conclusion
Quantifying MRD by BCR-ABL1 copy numbers is an ideal tool to monitor treatment responses in Ph + ALL patients and persistent MRD positivity is associated with inferior survival in patients treated with chemotherapy + TKI and/or alloHSCT. Large, prospective trials using MRD-based risk stratification is required to elucidate whether MRD can be used as a tool to decide the ideal management for an individual patient and the need for post-remission alloHSCT in Ph + ALL patients.
Supplementary Material
Supplemental data for this article can be accessed here.
Acknowledgements
The authors would like to thank Ms Senthamizhselvi Anandan and other staffs of leukemia, flow cytometry and cytogenetics labs. The study was presented as an abstract in the 59th Annual Conference of Indian Society of Hematology & Blood Transfusion.
Funding
This study is supported in part by a DBT-COE grant (BT/COE/ 34/SP13432/2015), New Delhi, India and Indian Council of Medical Research Centre for Advanced Research grant 70/ 14/14-CAR to PB. VM is supported by senior fellowship program of Wellcome DBT India Alliance (IA/CPHS/18/1/503930), New Delhi, India. PB is supported by a senior fellowship program of Wellcome DBT India Alliance (IA/S/15/1/501842) New Delhi, India. UPK is supported by an early career fellowship program of Wellcome DBT India Alliance (IA/CPHE/17/1/ 503351), New Delhi, India.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
AKA: Conceptual development of the study, performed research, collected and analyzed the data, wrote the paper. NBJ: performed research, provided cytogenetic inputs. KML: performed research, biostatistics for the study. AK: performed research, clinical data analysis. FNA: performed research, clinical data accrual and analysis. UPK: performed research, clinical data accrual and analysis. AA: performed research, clinical data accrual and analysis. BG: performed research, clinical data accrual and analysis. PB: Conceptual development of the study, performed research, analyzed the data, reviewed the paper. VM: Conceptual development of the study, provided the clinical inputs, analyzed the data, wrote and reviewed the paper.
References
- [1].Ravandi F, O’Brien SM, Cortes JE, et al. Long-Term Follow-up of a phase 2 study of chemotherapy plus dasatinib for the initial treatment of patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer. 2015;121(23):4158–4164. doi: 10.1002/cncr.29646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lugo TG, Pendergast AM, Muller AJ, et al. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science. 1990;247(4946):1079–1082. doi: 10.1126/science.2408149. [DOI] [PubMed] [Google Scholar]
- [3].Schrappe M, Camitta B, Pui CH, et al. Long-term results of large prospective trials in childhood acute lymphoblastic leukemia. Leukemia. 2000;14(12):2193–2194. doi: 10.1038/sj.leu.2401977. [DOI] [PubMed] [Google Scholar]
- [4].Hoelzer D. Advances in the management of Ph-positive ALL. Clin Adv Hematol Oncol. 2006;4(11):804–805. [PubMed] [Google Scholar]
- [5].Fielding AK, Rowe JM, Richards SM, et al. Prospective outcome data on 267 unselected adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia confirms superiority of allogeneic transplantation over chemotherapy in the pre-imatinib era: results from the International ALL Trial MRC UKALLXII/ECOG2993. Blood. 2009;113(19):4489–4496. doi: 10.1182/blood-2009-01-199380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dombret H, Gabert J, Boiron J-M, et al. Outcome of treatment in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia-results of the prospective multicenter LALA-94 trial. Blood. 2002;100(7):2357–2366. doi: 10.1182/blood-2002-03-0704. [DOI] [PubMed] [Google Scholar]
- [7].Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol. 2011;29(5):532–543. doi: 10.1200/JCO.2010.30.1382. [DOI] [PubMed] [Google Scholar]
- [8].Schultz KR, Bowman WP, Aledo A, et al. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a children’s oncology group study. J Clin Oncol. 2009;27(31):5175–5181. doi: 10.1200/JCO.2008.21.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Biondi A, Schrappe M, De Lorenzo P, et al. Imatinib after induction for treatment of children and adolescents with Philadelphia-chromosome-positive acute lymphoblastic leukaemia (EsPhALL): a randomised, open-label, intergroup study. Lancet Oncol. 2012;13(9):936–945. doi: 10.1016/S1470-2045(12)70377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Brissot E, Labopin M, Beckers MM, et al. Tyrosine kinase inhibitors improve long-term outcome of allogeneic hematopoietic stem cell transplantation for adult patients with Philadelphia chromosome positive acute lymphoblastic leukemia. Haematologica. 2015;100(3):392–399. doi: 10.3324/haematol.2014.116954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bassan R, Rossi G, Pogliani EM, et al. Chemotherapy-phased imatinib pulses improve long-term outcome of adult patients with philadelphia chromosome-positive acute lymphoblastic leukemia: Northern Italy Leukemia Group Protocol 09/00. J Clin Oncol. 2010;28(22):3644–3652. doi: 10.1200/JCO.2010.28.1287. [DOI] [PubMed] [Google Scholar]
- [12].Conter V, Bartram CR, Valsecchi MG, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115(16):3206–3214. doi: 10.1182/blood-2009-10-248146. [DOI] [PubMed] [Google Scholar]
- [13].Spinelli O, Peruta B, Tosi M, et al. Clearance of minimal residual disease after allogeneic stem cell transplantation and the prediction of the clinical outcome of adult patients with high-risk acute lymphoblastic leukemia. Haematologica. 2007;92(5):612–618. doi: 10.3324/haematol.10965. [DOI] [PubMed] [Google Scholar]
- [14].Bar M, Wood BL, Radich JP, et al. Impact of minimal residual disease, detected by flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute lymphoblastic leukemia. Leuk Res Treat. 2014;2014:1–9. doi: 10.1155/2014/421723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ravandi F, Jorgensen JL, Thomas DA, et al. Detection of MRD may predict the outcome of patients with Philadelphia chromosome-positive ALL treated with tyrosine kinase inhibitors plus chemotherapy. Blood. 2013;122(7):1214–1221. doi: 10.1182/blood-2012-11-466482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cazzaniga G, De Lorenzo P, Alten J, et al. Predictive value of minimal residual disease in Philadelphia-chromosome-positive acute lymphoblastic leukemia treated with imatinib in the European intergroup study of post-induction treatment of Philadelphia-chromosome-positive acute lymphoblastic leukemia, based on immunoglobulin/T-cell receptor and BCR/ ABL1 methodologies. Haematologica. 2018;103(1):107–115. doi: 10.3324/haematol.2017.176917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].van Dongen J, Macintyre EA, Gabert JA, et al. Standardized RT-PCR analysis of fusion gene transcripts from chromosome aberrations in acute leukemia for detection of minimal residual disease. Leukemia. 1999;13(12):1901–1928. doi: 10.1038/sj.leu.2401592. [DOI] [PubMed] [Google Scholar]
- [18].Moorman AV, Enshaei A, Schwab C, et al. A novel integrated cytogenetic and genomic classification refines risk stratification in pediatric acute lymphoblastic leukemia. Blood. 2014;124(9):1434–1444. doi: 10.1182/blood-2014-03-562918. [DOI] [PubMed] [Google Scholar]
- [19].Gabert J, Beillard E, van der Velden VHJ, et al. Standardization and quality control studies of “‘realtime’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia-a Europe Against Cancer program”. Leukemia. 2003;17(12):2318–2357. doi: 10.1038/sj.leu.2403135. [DOI] [PubMed] [Google Scholar]
- [20].Balasubramanian P, Chendamarai E, Markose P, et al. International reporting scale of BCR-ABL1 fusion transcript in chronic myeloid leukemia: first report from India. Acta Haematol. 2012;127(3):135–142. doi: 10.1159/000334716. [DOI] [PubMed] [Google Scholar]
- [21].Kantarjian HM, O’Brien S, Smith TL, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;18(3):547–561. doi: 10.1200/JCO.2000.18.3.547. [DOI] [PubMed] [Google Scholar]
- [22].Bajel A, George B, Mathews V, et al. Adult ALL: treatment outcome and prognostic factors in an Indian population using a modified German ALL (GMALL) protocol. Leukemia. 2007;21(10):2230–2233. doi: 10.1038/sj.leu.2404785. [DOI] [PubMed] [Google Scholar]
- [23].Jain P, Korula A, Deshpande P, et al. Adult acute lymphoblastic leukemia: limitations of intensification of therapy in a developing country. J Glob Oncol. 2018;4:1–12. doi: 10.1200/JGO.17.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ceppi F, Duval M, Leclerc J-M, et al. Improvement of the outcome of relapsed or refractory acute lymphoblastic leukemia in children using a risk-based treatment strategy. Plos One. 2016;11(9):e0160310. doi: 10.1371/journal.pone.0160310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].NCI Dictionary of Cancer Terms. National Cancer Institute; 2011. [cited 2020 Aug 18]. [Internet] Available from: https://www.cancer.gov/publications/diction-aries/cancer-terms. [Google Scholar]
- [26].Short NJ, Jabbour E, Sasaki K, et al. Impact of complete molecular response on survival in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2016;128(4):504–507. doi: 10.1182/blood-2016-03-707562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- [28].Cox DR. Regression models and life-tables. J R Stat Soc Ser B Methodol. 1972;34(2):187–220. [Google Scholar]
- [29].Lee S, Kim D-W, Cho B-S, et al. Impact of minimal residual disease kinetics during imatinib-based treatment on transplantation outcome in Philadelphia chromosome-positive acute lymphoblastic leukemia. Leukemia. 2012;26(11):2367–2374. doi: 10.1038/leu.2012.164. [DOI] [PubMed] [Google Scholar]
- [30].Bruggemann M, Kotrova M. Minimal residual disease in adult ALL: technical aspects and implications for correct clinical interpretation. Hematology Am Soc Hematol Educ Program. 2017;2017(1):13–21. doi: 10.1182/asheducation-2017.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bar M, Wood BL, Radich JP, et al. Impact of minimal residual disease, as detected by multiparametric flow cytometry pre and post transplantation, on outcome of myeloablative hematopoietic cell transplantation for acute lymphoblastic leukemia. Blood. 2012;120(21):592–592. [Google Scholar]
- [32].Chalandon Y, Thomas X, Hayette S, Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL) et al. Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph-positive acute lymphoblastic leukemia. Blood. 2015;125(24):3711–3719. doi: 10.1182/blood-2015-02-627935. [DOI] [PubMed] [Google Scholar]
- [33].Alacacioglu I, Medeni SS, Ozsan GH, et al. Is the BFM regimen feasible for the treatment of adult acute lymphoblastic leukemia? A retrospective analysis of the outcomes of bfm and hyper-CVAD chemotherapy in two centers. Chemotherapy. 2014;60(4):219–223. doi: 10.1159/000375258. [DOI] [PubMed] [Google Scholar]
- [34].El-Cheikh J, Dika IE, Massoud R, et al. Hyper-CVAD compared With BFM-like chemotherapy for the treatment of adult acute lymphoblastic leukemia. A retrospective single-center analysis. Clin Lymphoma Myeloma Leuk. 2017;17(3):179–185. doi: 10.1016/j.clml.2016.11.002. [DOI] [PubMed] [Google Scholar]
- [35].Kim D-Y, Joo Y-D, Lim S-N, et al. Nilotinib combined with multiagent chemotherapy for newly diagnosed Philadelphia-positive acute lymphoblastic leukemia. Blood. 2015;126(6):746–756. doi: 10.1182/blood-2015-03-636548. [DOI] [PubMed] [Google Scholar]
- [36].Jabbour E, Kantarjian H, Ravandi F, et al. Combination of hyper-CVAD with ponatinib as first-line therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia: a single-centre, phase 2 study. Lancet Oncol. 2015;16(15):1547–1555. doi: 10.1016/S1470-2045(15)00207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Arun AK, Senthamizhselvi A, Mani S, et al. Frequency of rare BCR-ABL1 fusion transcripts in chronic myeloid leukemia patients. Int J Lab Hematol. 2017;39(3):235–242. doi: 10.1111/ijlh.12616. [DOI] [PubMed] [Google Scholar]
- [38].Yanada M, Sugiura I, Takeuchi J, Japan Adult Leukemia Study Group et al. Prospective monitoring of BCR-ABL1 transcript levels in patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia undergoing imatinib-combined chemotherapy. Br J Haematol. 2008;143(4):503–510. doi: 10.1111/j.1365-2141.2008.07377.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.