Abstract
Inflammation drives colorectal cancer (CRC) development and CRC risk is influenced by dietary factors, including dietary fibre. Hyperactive WNT signalling occurs in CRC and may regulate inflammation. This study investigated i) relationships between the inflammatory potential of diet, assessed using the Energy-adjusted Dietary Inflammatory Index (E-DII™), and markers of WNT signalling, and ii) whether DII status modulated the response to supplementation with two types of dietary fibre.
Seventy-five healthy participants were supplemented with resistant starch (RS) and/or polydextrose (PD) or placebo for 50 days. Rectal biopsies were collected pre and post-intervention and used to assess WNT pathway gene expression and crypt cell proliferation. E-DII scores were calculated from food frequency questionnaire data. High-sensitivity C-reactive protein (hsCRP) and faecal calprotectin concentrations were quantified.
hsCRP concentration was significantly greater in participants with higher E-DII scores (least square means (LSM) 4.7 vs. 2.4mg/L, P=0.03). Baseline E-DII score correlated with FOSL1 (β= 0.503, P=0.003) and WNT11 (β=0.472, P=0.006) expression, after adjusting for age, gender, BMI, endoscopy procedure and smoking status. WNT11 expression was more than two-fold greater in individuals with higher E-DII scores (LSM 0.131 vs. 0.059, P=0.002). Baseline E-DII modulated the effects of PD supplementation on FOSL1 expression (P=0.04).
More pro-inflammatory diets were associated with altered WNT signalling and appeared to modulate the effects of PD supplementation on expression of FOSL1.
This is the first study to investigate relationships between the E-DII and molecular markers of WNT signalling in rectal tissue of healthy individuals.
Keywords: dietary inflammatory index, WNT signalling, dietary fibre, colorectal cancer, inflammation
Introduction
Approximately half of colorectal cancer (CRC) cases are attributable to ‘modifiable’ lifestyle factors e.g. obesity and diet(1, 2). For example, there is “probable” evidence higher consumption of foods containing dietary fibre lowers CRC risk(3). However, because foods and nutrients are not consumed in isolation, it is important to assess diet healthfulness holistically when investigating relationships with disease-related outcomes(4).
Inflammation modulates CRC risk(5–8), and individuals with inflammatory bowel disease (IBD) are at increased risk of CRC(9). The Dietary Inflammatory Index (DII®) quantifies the inflammatory potential of the whole diet(10), and comprises 45 food parameters, including 36 anti-inflammatory components e.g. dietary fibre(10). The DII has been validated in various cohorts and shown to correlate with the expression of inflammatory markers e.g. C-reactive protein (CRP), IL-6 and IL-10(11–15). Furthermore, more pro-inflammatory DII scores are associated with greater risk of all-cause mortality(16) and of cancers(17) including CRC(18). A systematic review and meta-analysis of nine studies revealed that individuals in the highest DII category of exposure had 40% increased risk of CRC compared with those in the lowest category, translating to a 7% increase in CRC risk for each one-point increase in DII score(18). The underlying mechanisms linking DII and CRC risk are not fully understood, but are likely to include effects of the inflammation-related components of the diet on insulin sensitivity, the gut microbiome, local inflammation (which promotes cell proliferation and mutagenesis(19)) and on the production of reactive oxygen species (ROS)(7, 18), as well as modulation of molecular pathways e.g. WNT signalling.
The WNT signalling pathway regulates cellular processes such as proliferation that contribute to the maintenance of homeostasis and tissue self-renewal in the large intestine(20). Aberrant WNT signalling in CRC includes abnormal expression of β-catenin and adenomatous polyposis coli (APC)(21). Furthermore, WNT genes e.g. WNT11 are upregulated in colonic tissue from ulcerative colitis (UC) patients (22). Recent evidence suggests that WNT signalling may influence the inflammatory state via cross-talk with pathways including Nuclear Factor kappa B (NFκB) and Mitogen Activated Protein Kinase (MAPK)(23). WNT signalling may also regulate the activity of inflammatory pathways, e.g. β-catenin inhibits NF-κB signalling(24), and the expression of inflammatory cytokines and chemokines, e.g. WNT5A induces IL-1 and IL-6(25–27). In addition, inflammatory cytokines regulate mucosal WNT signalling via Protein Kinase B (AKT) signalling(28).
The WNT pathway plays an important role in the link between diet, adiposity and physical activity, and gastrointestinal cancers including CRC(29, 30) and several dietary factors modulate WNT pathway activity(31, 32). We have shown that higher adherence to the World Cancer Research Fund (WCRF) Cancer Prevention Recommendations, which includes anti-inflammatory components of the DII such as dietary fibre, was associated with altered expression of WNT pathway components(33). Adherence to the sub-recommendation on dietary fibre intake was associated with significantly lower rectal expression of β-catenin and of WNT11(33). Higher dietary fibre intake protects against CRC(3), and short-chain fatty acids (SCFA) produced by dietary fibre fermentation, primarily butyrate, are chemoprotective and exert anti-inflammatory effects, some of which may be mediated via modulation of WNT signalling(34, 35). In the Dietary Intervention, Stem cells and Colorectal cancer (DISC) Study, we supplemented healthy individuals with two types of dietary fibre, resistant starch (RS) and polydextrose (PD), and observed downregulation of β-catenin, c-MYC, SFRP1 and SFRP2 in the rectal mucosa(36).
Taken together, the evidence suggests that the WNT pathway mediates the effects of diet, including perhaps its inflammatory potential, on CRC risk. Therefore, this study had two aims: i) to test the hypothesis that diet-associated inflammation is related to WNT pathway activity by investigating relationships between DII score and expression of WNT pathway components in the rectal mucosa of healthy individuals; and ii) to investigate whether the inflammatory potential of habitual diet modulated the response to supplementation with RS and/or PD in the DISC Study. We also investigated relationships between DII score and crypt cell proliferative state (CCPS) as a functional outcome of WNT signalling, and biomarker of CRC risk(35, 36).
Materials And Methods
The DISC Study Participants
This study used data and samples from the DISC Study (ClinicalTrials.gov Identifier: NCT01214681), a randomised, placebo-controlled dietary intervention that investigated the effects of two types of dietary fibre (RS and PD) on markers of CRC risk(36, 37). The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Newcastle and North Tyneside Research Ethics Committee (REC No. 09/H0907/77). Healthy participants were recruited from gastroenterology out-patients departments at North Tyneside General Hospital, North Shields, UK and Wansbeck General Hospital, Ashington, UK between May 2010 and July 2011. Written informed consent was obtained from all participants.
Dietary intervention
Participants were supplemented with RS and/or PD or placebo for 50 days in a 2 x 2 factorial design. At least one week after their first endoscopy appointment, participants were randomised to one of four intervention groups: RS (23 g Hi-maize® 260, Ingredion™, Food Innovation), PD (12 g of Litesse® Ultra™ DuPont™ Danisco®), RS and PD or double placebo (12 g of Maltodextrin (RS placebo) and 23 g of Amioca starch (PD placebo)). Randomisation was stratified by endoscopy procedure (flexible sigmoidoscopy or colonoscopy).
Sample collection
Phenotypic data (e.g. height and body weight) and biological samples were collected pre- and post-intervention. Rectal mucosal biopsies were collected at endoscopy (colonoscopy or flexible sigmoidoscopy for baseline samples and rigid sigmoidoscopy for post-intervention samples) using Biobite Biopsy forceps (Medical Innovations) from the mid-rectum (10cm from the ano-rectal verge). For the collection of stool samples, participants were given a sealable bucket pot, a disposable bedpan, two ice packs (to be frozen prior to sample collection) and a cool bag. Participants stored samples in cool bags containing the frozen ice packs. Pre-intervention stool samples were collected at least seven days after the endoscopy appointment and picked up by the research team from the participants’ homes, and post-intervention samples were brought by the participant to the second endoscopy appointment. Samples were divided into aliquots and stored at -80°C until analysis.
Measurement of inflammatory markers
High-sensitivity C-reactive protein (hsCRP) in serum was quantified at Newcastle Laboratories, Freeman Hospital (Newcastle upon Tyne, UK) from blood samples collected in one 5ml BD Vacutainer® SST™ II Advance tube with gold hemogard closure (Becton Dickinson, UK). Faecal calprotectin was quantified in extracts from 100mg of stool using the Faecal Sample Preparation Kit (Calpro AS, Lysaker, Norway). Prior to preparation of faecal extracts, samples were defrosted overnight and mixed using Stomacher®80 Biomaster (Seward Ltd, Worthing, UK). Extracts were diluted 1:20 in sample dilution buffer and used to quantify faecal calprotectin using the Calprolab™ Calprotectin ELISA (ALP) kit (Calpro AS). Optical density was read after 40 minutes incubation with enzyme substrate solution on a FLUOstar® Omega microplate reader (BMG Labtech Ltd, Aylesbury, UK) operated by BMG Omega software version 1.20.
Expression of WNT pathway components
RNA was extracted from rectal mucosal biopsies using the RNeasy Mini Kit (Qiagen) using five 3mm glass beads (VWR) and QiaShredders (Qiagen) for tissue disruption and homogenisation, respectively. cDNA was synthesised from 1μg RNA using the QuantiTect Reverse Transcription Kit (Qiagen). The expression of 12 WNT pathway genes and two reference genes (18S and β2M) was quantified by quantitative PCR (qPCR) using the StepOnePlus™ Real Time PCR system (Applied Biosystems). These target genes were selected by reviewing the literature to identify WNT genes that were a) implicated in colorectal carcinogenesis (selection criterion 1) and b) whose expression is modified by butyrate (a product of dietary fibre fermentation; selection criterion 2) (Supplementary Table 1). In addition, APC was chosen due to its key role in the WNT pathway and in CRC. We have found that the expression 18S and β2M reference genes is stable in rectal mucosal samples(36).
Quantification of CCND1, c-MYC and SFRP1 was performed using primers designed and optimised by Dr. Nigel Belshaw and Dr. Wing Leung (Quadram Institute, Norwich, UK) (Supplementary Table 2). For these three genes, together with two reference genes (18S and β2M), qPCR reactions contained 5μl ImmoMix™ (2x) (Bioline, UK), 0.1 μl MgCl2 (50mM) (Bioline, UK), 1μl BSA (10mg/ml) (Ambion, UK), 0.2μl ROX Reference Dye (50x) (Invitrogen, UK), 0.06μl SYBR Green (100x) (Invitrogen, UK), 0.6μl RNase-free water, 0.02μl each of forward and reverse primers (100μM) and 3μl of cDNA. The programme was run for a 10 minute activation step at 95°C followed by 40 cycles of 30 seconds each, denaturation at 95°C, annealing at 60°C and extension at 72°C. For the remaining nine genes, qPCR was performed using the QuantiTect SYBR Green PCR Kit (Qiagen) and QuantiTect primer assays (Qiagen, Supplementary Table 3), with reactions containing 15μl of master mix and 5μl of the sample cDNA. The programme was run for a 15 minute activation step at 95°C followed by 40 cycles of 15 seconds denaturation at 94°C, 30 seconds annealing at 55°C and 30 seconds extension at 72°C. All samples were run in duplicate. Each plate contained pre- and post-intervention samples for each participant and representatives from each intervention group. Data collection was during the extension stage and melting curve analysis was performed. Gene expression data are expressed as adjusted values (2−ΔCt × 10,000) relative to the geometric mean of 18S and β2M reference genes(38).
Assessment of rectal crypt cell proliferative state (CCPS)
Rectal CCPS was assessed in whole, microdissected, Schiff reagent-stained crypts(37). Briefly, Carnoy’s-fixed rectal mucosal biopsies were hydrated in 50% ethanol, followed by 25% ethanol, for 10 minutes each at room temperature. Biopsies were then hydrolysed in 1M HCl for 10 minutes at 60°C and stained with Schiff reagent (Surgipath™) for one hour at room temperature. The Schiff reagent was replaced with 1ml of 45% acetic acid and whole crypts were microdissected using an Olympus SZ40 dissecting microscope and Leica CLS 150X light source. On a microscope slide with a drop of 45% acetic acid, rows of individual crypts (bases of the crypts facing upwards) were teased apart using fine gauge hypodermic needles (25G × 5/8” Terumo®, Belgium) and covered and sealed with a cover slip (Surgipath®, Leica, UK). Ten intact crypts were selected at random and each divided into ten equal compartments longitudinally, starting from the base of the crypt. The number of mitotic cells in each compartment was counted, and from this the proportion of mitotic cells in the upper half of the crypt was calculated, as well as crypt width and length measurements, from which crypt volumes were calculated.
Quantification of faecal SCFA concentrations
SCFA concentrations were quantified by gas chromatography using pivalic acid as an internal standard as described previously(39). Briefly, 1ml 20mM pivalic acid and 5ml water were added to 1g of faecal sample, mixed thoroughly and centrifuged at 5000xg for 5min. 0.250ml saturated oxalic acid solution was added to 0.5ml of the supernatant and incubated at 48°C for 1 hour. This was centrifuged at 16 000xg for 5min and the supernatant fraction was used for analysis as described previously(40).
Calculation of energy-adjusted DII (E-DII)
Habitual diet was assessed at baseline using a food frequency questionnaire (FFQ) adapted from that used in the EPIC – Norfolk Study (version 6, CAMB/PQ/6/1205)(41), asking participants for their average consumption of foods over the last year. The inflammatory potential of diet was assessed by calculating the DII scores and energy-adjusted DII (E-DII™) scores(10). Dietary intakes of 29 food components (alcohol, beta-carotene, carbohydrates, cholesterol, fibre, total fat, iron, trans fatty acids, folate, energy, magnesium, monosaturated fatty acids, niacin, polyunsaturated fatty acids, protein, retinol, riboflavin, saturated fatty acids, selenium, thiamine, vitamin B6, vitamin B12, vitamin C, vitamin D, vitamin E, zinc, onions, garlic, tea) were included in the calculation(10). Intake from foods only, not supplements, was included in DII calculations. A total E-DII score was calculated by adding the scores for each of the 29 food parameters, and expressed per 1000 kilocalories (4.187 MJ) consumed. Higher E-DII scores indicate more pro-inflammatory diets, whereas lower E-DII scores represent less inflammatory, or more anti-inflammatory, diets.
Statistical analyses
For descriptive statistical analyses, independent sample t-tests and Fisher’s exact tests were used for comparisons between the lower and higher E-DII groups. In cross-sectional analyses, multivariable regression models were used to investigate relationships between E-DII and the measured outcomes, with model 2 adjusting for age, gender, endoscopy procedure, BMI and smoking status as covariates. For categorical analyses, participants were divided into a low E-DII (more anti-inflammatory) and a high E-DII (more pro-inflammatory) group by dichotomising at the median E-DII (0.700). Differences in the measured outcomes between the low and high E-DII groups at baseline were investigated using the ANOVA General Linear Model (GLM) and adjusting for age, gender, endoscopy procedure, BMI and smoking status as covariates. Models were not adjusted for total energy intake because it is one of the components of the DII and is explicitly accounted for in the calculation of E-DII scores.
For the RCT, interactions between E-DII status at baseline and the effects of the dietary intervention (RS and/or PD) on the measured outcomes post-intervention were investigated using the ANOVA GLM, adjusting for pre-intervention measurement, age, gender, endoscopy procedure, BMI and smoking status as covariates. All statistical analyses were performed using IBM® SPSS® Statistics version 25. P<0.05 was considered statistically significant.
Results
Participant demographics
Seventy-five healthy participants were recruited to the DISC Study (Table 1). The mean age of participants was 52 years (range 30-80 years) and 53% were female. Most of the participants (97%) were White. For more details, see Malcomson et al.(36).
Table 1. DISC Study participant characteristics at baseline according to E-DII group.
| Demographics | Lower E-DII group (≤0.700) | Higher E-DII group (>0.700) | P-value |
|---|---|---|---|
| Total n | 37 | 38 | |
| Female n (%) | 24 (65) | 16 (42) | 0.07 |
| Age (years) | 53.0 (1.9) | 51.9 (2.1) | 0.43 |
| Race/Ethnicity | 1.00 | ||
| White n (%) | 36 (97) | 37 (97) | |
| Black n (%) | 1 (3) | 0 (0) | |
| Mixed race n (%) | 0 (0) | 1 (3) | |
| Endoscopy procedure | 0.15 | ||
| Flexible sigmoidoscopy n (%) | 22 | 30 | |
| Colonoscopy n (%) | 15 | 8 | |
| Anthropometrics | |||
| Height (m) | 1.65 (0.01) | 1.68 (0.02) | 0.20 |
| Weight (kg) | 78.9 (2.5) | 87.0 (2.6) | 0.59 |
| BMI (kg/m2) | 28.9 (0.8) | 31.1 (0.9) | 0.33 |
| Waist circumference (cm) | 95.7 (2.0) | 103.3 (2.1) | 0.48 |
| Hip circumference (cm) | 106.3 (2.0) | 107.8 (1.8) | 0.63 |
| Smoking status | 0.03 * | ||
| Never n (%) | 24 (65) | 14 (37) | |
| Former1 n (%) | 9 (24) | 12 (32) | |
| Current n (%) | 4 (11) | 12 (32) | |
| E-DII | -0.999 (0.229) | 2.425 (0.217) | 0.92 |
Data are presented as means with standard error of the mean in parentheses unless otherwise stated. Independent sample t-tests and Fisher’s exact tests were used for comparisons between the lower and higher E-DII groups,
p<0.05.
Former smokers include participants who had stopped smoking prior to the start of the DISC Study.
Inflammatory potential of diets of DISC Study participants
The mean E-DII score was slightly pro-inflammatory (0.736 ± 0.253) and E-DII scores ranged from -4.480 to 5.030. Table 1 shows the participants’ characteristics according to E-DII group. Participants with more pro-inflammatory diets, i.e. those in the higher-E-DII group, were more likely to be former or current smokers (P= 0.03).
Relationships between E-DII and inflammatory markers
hsCRP concentrations in the higher, more pro-inflammatory, E-DII group were approximately two-fold greater compared with the lower E-DII group (P=0.03) (Table 2). Although faecal calprotectin concentrations were, on average, 32% higher in individuals in the higher E-DII group, the considerable inter-individual variation within groups meant that this difference was not statistically significant (P=0.46). There were no significant relationships between E-DII and faecal calprotectin or hsCRP concentrations when investigated using the regression models (Supplementary Table 4).
Table 2. Inflammatory markers at baseline according to E-DII group.
| Inflammatory marker | All participants | Lower E-DII group (≤0.700) | Higher E-DII group (>0.700) | P-value |
|---|---|---|---|---|
| Faecal calprotectin (mg/kg) | 15.5 (54.0) | 13.2 (35.7) | 17.4 (63.9) | 0.46 |
| hsCRP (mg/L) | 3.6 (0.5) | 2.4 (0.4) | 4.7 (0.9) | 0.03 * |
Data for hsCRP are presented as means and standard error of the mean (SEM) in parentheses, independent sample t-test. Data for faecal calprotectin are presented as medians and interquartile ranges in parentheses, Mann-Whitney.
P<0.05.
Relationships between E-DII and WNT pathway markers
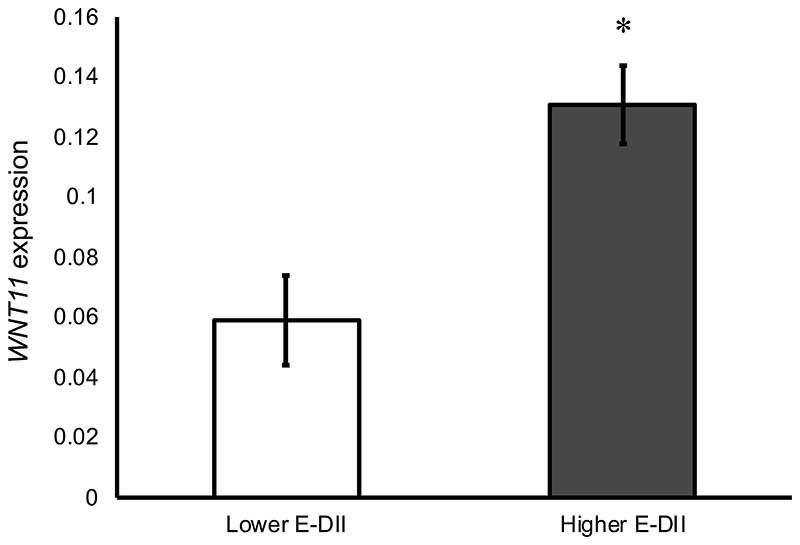
In the unadjusted multilevel linear regression model, E-DII score was significantly associated with baseline (pre-intervention) rectal expression of FOSL1 (β=0.414, P=0.01) and WNT11 (β=0.365, P=0.009) (Table 3). These findings were strengthened in the fully adjusted model (FOSL1 (β=0.503, P=0.003) and WNT11 (β=0.472, P=0.006)). Furthermore, participants in the higher E-DII group had more than two-fold higher expression of WNT11 compared with those in the lower E-DII group (least squares means 0.131 vs. 0.059, P=0.002, Figure 1).
Table 3. Associations between the Dietary Inflammatory Index (E-DII) and expression of WNT pathway genes in the rectal mucosa at baseline.
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| WNT gene | β coefficient | 95% CI | P value | β coefficient | 95% CI | P value |
| APC | 0.147 | -0.066, 0.194 | 0.33 | 0.152 | -0.086, 0.217 | 0.39 |
| AXIN2 | 0.112 | -0.112, 0.295 | 0.37 | 0.016 | -0.207, 0.233 | 0.91 |
| CCND1 | 0.074 | -16.3, 25.8 | 0.65 | -0.034 | -26.6, 22.2 | 0.86 |
| CTNNB1 | 0.090 | -0.638, 1.357 | 0.47 | -0.011 | -1.007, 0.916 | 0.93 |
| FOSL1 | 0.414 | 0.026, 0.186 | 0.01* | 0.503 | 0.046, 0.211 | 0.003* |
| GSK3β | 0.000 | -0.281, 0.280 | 1.00 | -0.105 | -0.407, 0.175 | 0.43 |
| c-JUN | 0.069 | -0.443, 0.775 | 0.59 | 0.035 | -0.548, 0.714 | 0.79 |
| c-MYC | 0.078 | -5.98, 9.69 | 0.63 | 0.013 | -9.04, 9.64 | 0.95 |
| SFRP1 | 0.025 | -4.29, 5.00 | 0.88 | 0.156 | -2.98, 7.34 | 0.90 |
| SFRP2 | 0.088 | -0.003, 0.005 | 0.50 | 0.113 | -0.003, 0.006 | 0.44 |
| WNT5A | 0.111 | -0.015, 0.038 | 0.39 | 0.072 | -0.018, 0.033 | 0.56 |
| WNT11 | 0.365 | 0.003, 0.021 | 0.009 * | 0.472 | 0.005, 0.026 | 0.006 * |
Data are presented as beta (β) coefficients and 95% confidence intervals (CIs). Model 1: unadjusted, Model 2: adjusted for age, gender, BMI, endoscopy procedure and smoking status.
P<0.05 for linear regression model.
Figure 1. Expression of WNT11 in the rectal mucosa of individuals with lower and higher E-DII scores at baseline.
Data are expressed as adjusted copies and presented as least square means following ANOVA GLM adjusted for age, gender, BMI, smoking status and endoscopy procedure. Error bars represent standard error of the mean. *p<0.05
There were no significant associations observed between E-DII and the remaining 10 WNT pathway components (Table 3), nor differences in their expression between the lower and higher E-DII groups (Supplementary Table 5).
Interestingly, there was a weak but significant correlation between rectal mucosal WNT11 expression and faecal calprotectin concentrations (Spearman’s correlation coefficient= 0.362, P=0.01). No such relationship was observed, however, for hsCRP (Spearman’s correlation coefficient= 0.142, P=0.33) and there were no significant correlations between rectal FOSL1 expression and the inflammatory markers measured in this study (hsCRP (Spearman’s correlation coefficient= 0.234, P=0.16) and faecal calprotectin (Spearman’s correlation coefficient= -0.248, P=0.15)).
Relationships between E-DII and rectal crypt cell proliferation state (CCPS) at baseline
There were no significant associations between E-DII score and total mitoses in the rectal epithelium, proportion of mitoses in the top half of the crypts (CCPS outcomes measured in this study) or crypt dimensions (length, width and volume) (Table 4). In addition, crypt dimensions and rectal CCPS outcomes did not differ between participants with lower and higher E-DII scores (Supplementary Table 6). Furthermore, there were no significant correlations between expression of FOSL1 and WNT11 (that were associated with E-DII (Table 3)), and CCPS outcomes or crypt dimensions (Supplementary Table 7).
Table 4. Associations between the Dietary Inflammatory Index (E-DII) and rectal CCPS and crypt dimensions at baseline.
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| Crypt measurement | β coefficient | 95% CI | P value | β coefficient | 95% CI | P value |
| Total mitoses | -0.055 | -0.724, 0.457 | 0.65 | -0.082 | -0.853, 0.450 | 0.52 |
| Proportion of mitotic cells in top half of the crypt | -0.036 | -1.09, 0.802 | 0.77 | -0.029 | -1.17, 0.940 | 0.72 |
| Length | -0.047 | -8.60, 5.82 | 0.70 | -0.084 | -10.5, 5.50 | 0.59 |
| Width | -0.074 | -2.07, 1.10 | 0.54 | -0.119 | -2.55, 0.992 | 0.72 |
| Volume | -0.096 | -3.84 × 105, 2.15 × 105 | 0.57 | -0.136 | -4.37 × 105, 1.99 × 105 | 0.36 |
Data are presented as beta (β) coefficients and 95% confidence intervals (CIs). Model 1: unadjusted, Model 2: adjusted for age, gender, BMI, endoscopy procedure and smoking status. *P<0.05 for linear regression model.
Interaction between baseline E-DII and the effects of supplementation with RS and PD on the measured outcomes
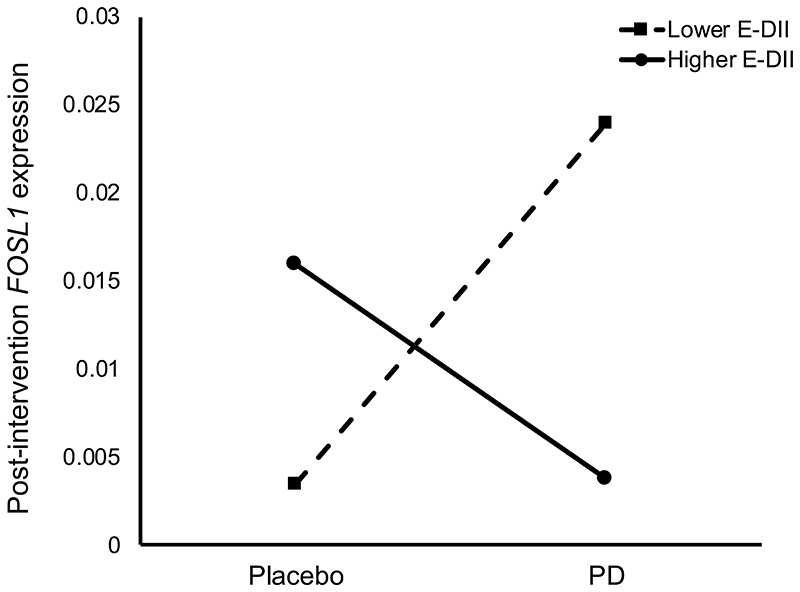
The effects of RS and PD on WNT pathway-related outcomes have been published previously(36, 37). In the present study, we investigated whether E-DII scores, derived from habitual diet data assessed at baseline, modulated the response to RS and PD. There were no significant differences in the inflammatory potential of habitual diet (i.e. E-DII score) according to dietary intervention group at baseline (P=0.64) (Supplementary Table 8). We observed a significant interaction effect of E-DII and PD supplementation on post-intervention rectal FOSL1 expression (P=0.04, Figure 2). Individuals in the higher E-DII group at baseline, with a more pro-inflammatory diet, had a lower post-intervention FOSL1 expression when given PD compared with those with less inflammatory E-DII scores. In individuals given the placebo, individuals with higher E-DII scores had higher post-intervention FOSL1 expression compared with those with less inflammatory diets in the lower E-DII group. There were no interaction effects between EDII and RS and/or PD on the other quantified genes or inflammatory and CCPS markers measured (Supplementary Table 9).
Figure 2. Post-intervention expression of FOSL1 in the rectal mucosa of individuals with lower and higher E-DII scores given PD or placebo.
Data are expressed as adjusted copies and presented as least square means following ANOVA GLM adjusted for age, gender, BMI, smoking status, endoscopy procedure and baseline (pre-intervention) expression.
Discussion
Chronic inflammation is a key risk factor for CRC by causing mutations, chromosomal alterations and aberrant patterns of DNA methylation which lead to oncogene activation, tumour suppressor inactivation, dysregulated DNA repair and chromosomal instability(42). In addition, both inflammatory state and CRC risk are influenced by environmental and lifestyle factors, especially diet(3, 43). Aberrant WNT signalling occurs early in the tumorigenic process(44) and provides both a selective advantage for the initial clonal expansion, and genetic instability for subsequent tumour progression and malignant transformation(45). WNT signalling is modulated by dietary factors including dietary fibre(34) and there may be cross-talk between WNT signalling and inflammatory pathways(24). The inflammatory potential of individual diets can be assessed using the DII(10); higher DII values indicate a more pro-inflammatory diet and have been associated with increased expression of inflammatory markers(11, 12, 14, 15) as well as greater CRC risk (18). However, little is known about the relationships between DII scores and molecular markers of CRC risk. This study is the first to report associations between DII and WNT pathway activity, CCPS and crypt dimensions in the healthy rectal mucosa, and to explore the potential modulation by habitual DII of the response to supplementation with dietary fibre.
E-DII is associated with expression of WNT11 and FOSL1 in the rectal mucosa of healthy adults
We observed significant positive correlations between the E-DII scores and expression of WNT11 and FOSL1 in the rectal mucosa of DISC Study participants. WNT11 is a ligand that regulates the activation of both canonical and non-canonical WNT signalling pathways(46) and its expression is induced by WNT pathway activation and by factors such as TGF-β(47). In the intestinal epithelium, WNT11 regulates cell proliferation, intercellular adhesion and migration and, consequently, is implicated in tumorigenesis(48). WNT11 is upregulated in CRC(49) and is involved in cancer progression(50). Upregulation of WNT11 in colorectal adenocarcinomas may play a role in colorectal tumourigenesis through stimulation of WNT signalling(49) and greater expression of WNT11 has been reported in patients with UC(22). In the present study, a ‘less inflammatory diet’, as assessed by a lower E-DII score, was associated with reduced WNT11 expression. When stratifed by age, the difference between E-DII groups remains statistically significant for the younger (<50 years) group only (p=0.03) (Supplementary Table 10). It is probable that the reduction in group sizes coupled with the greater inter-individual variability in participants aged ≥50 years limited our ability to detect the between E-DII groups among older participants. In addition, rectal WNT11 expression correlated positively with faecal calprotectin, a marker of gastrointestinal inflammation. Interestingly, we have previously reported lower rectal mucosal expression of WNT11 in participants with greater adherence to the WCRF Cancer Prevention Recommendations(33). Furthermore, adherence to the recommendation to consume ≥25g dietary fibre/ day, an anti-inflammatory component of the DII(10), and to the recommendation to be physically active, were associated with lower WNT11 expression(33). These findings suggest that WNT11 may be particularly sensitive to modulation by environmental and lifestyle factors, including diet. Although they will require confirmation in independent studies, our findings suggest that such relationships between lifestyle factors and rectal mucosal markers may be affected by age, which is particularly important as this is the strongest risk factor for CRC. Furthermore, because the molecular characteristics of sporadic CRC cases in early-onset (age <50 years) differ from those developing CRC at an older age(51), and age-dependent effects on other markers of CRC risk have been reported(32, 37, 52), further studies investigating these age-dependent processes are warranted.
Greater expression of FOSL1 (Fos-related antigen 1 (also known as FRA-1)) was also associated with higher E-DII scores, i.e. more inflammatory diets, in the rectal mucosa of healthy individuals. FOSL1 is a member of the FOS oncogene family and a target gene of the WNT pathway. Increased FOSL1 protein and greater β-catenin accumulation occurs in human colorectal adenocarcinomas(53). Interestingly, increased IL-6 secretion as a consequence of activation of signal transducer and activator of transcription 3 (STAT3) signalling promotes FOSL1 deacetylation in CRC cell lines, resulting in increased FOSL1 expression. Furthermore, increased FOSL1 protein was observed in cancer tissue from CRC patients, and this correlated with abundance of the pro-inflammatory cytokine IL-6(54). Aberrant FOSL1 expression has also been reported in patients with mild UC, and expression levels were positively correlated with concentrations of IL-11 in biopsies from UC patients (55). To our knowledge, this is the first study to report relationships between diet quality and FOSL1 expression in the rectal mucosa.
E-DII and expression of other WNT signalling genes in the rectal mucosa
In the present study, we did not detect relationships between E-DII and the expression of the other 10 quantified WNT pathway-related genes. As this is the first study to explore such relationships, we could not be specific about which WNT genes would be modulated by E-DII. Since these target genes were selected because of their potential modulation by dietary fibre(36), it is possible that not all are responsive to differences in the inflammatory potential of diet. However, a previous mouse study suggested that high fat diet-induced inflammation was associated with downregulation of Apc and increased expression of Ctnnb1 and target genes e.g. c-Jun and Ccnd1 in the colon(56). In the present study performed in healthy human adults, we observed no relationships between the inflammatory potential of habitual diet and expression of these four genes in the rectal mucosa.
Dietary fibre supplementation may modulate the relationships between E-DII and FOSL1 in the rectal mucosa
We investigated whether baseline E-DII modulated the effects of supplementing healthy individuals with dietary fibre (provided as RS and/or PD) on WNT pathway-related markers of CRC risk. We observed a significant interaction between E-DII and supplementation with PD on rectal expression of FOSL1, in which those with poorer, more inflammatory diets (i.e. higher E-DII scores) had lower post-intervention FOSL1 expression. Because lower FOSL1 expression may be associated with lower CRC risk, this finding suggests that those with poorer diets may benefit more from supplementation with PD. The opposite was observed for those given placebo; those with higher E-DII scores had higher post-intervention FOSL1 expression. To our knowledge, this is the first study to explore whether baseline E-DII modulates the response to a dietary intervention. However, there is evidence of a poorer response to bariatric surgery (smaller weight and fat mass changes) in individuals with more inflammatory baseline DII scores(57). We explored whether the observed differences in FOSL1 expression in response to PD supplementation between lower and higher E-DII groups could have resulted from differences in faecal SCFA concentrations. We hypothesised that individuals with poorer diets, indicated by higher E-DII scores, may have lower SCFA concentrations at baseline, which may lead to greater relative change in SCFA with PD supplementation, and therefore respond better to the dietary intervention. However, there were no significant differences between individuals with lower and higher E-DII scores in the faecal concentrations or proportions of SCFAs at baseline (Supplementary Table 11) nor in the change in SCFAs post-intervention. The potential mechanisms underpinning the observed effects, and why these were observed for PD supplementation but not for RS, are unclear. Therefore, further research is warranted to substantiate this novel finding.
Greater CCPS and, especially, a higher proportion of mitotic cells in the top half of the crypt, is a biomarker of CRC risk(58, 59). In the present study, for the first time, we investigated relationships between E-DII score and rectal CCPS in healthy participants but we did not observe any significant relationship. Previous studies suggest that dietary components, such as dietary fibre, that modulate inflammation may mediate CRC risk via effects on cell proliferation(37, 52, 60, 61). Butyrate, produced from bacterial fermentation of dietary fibre, activates T-regulatory cells that block pro-inflammatory T-cells, leading to reduced production of cytokines associated with the stimulation of cell proliferation(62). Chronic inflammation is associated with activation of WNT signalling, induced by the STAT3 pathway, which stimulates cell proliferation in the colorectal epithelium(63). In a mouse model of chronic colitis, supplementation with red raspberries, which contain anti-inflammatory compounds, was associated with reduced expression of WNT pathway components that regulate the cell cycle (CCND1, c-MYC) as well as cell proliferation in colonic tissue(64). Furthermore, WNT pathway activity, assessed by the quantification of β-catenin expression, and STAT3 signalling were also reduced by red raspberry supplementation(64).
Strengths and limitations of study
This was a tightly controlled study with careful measurement of exposures, covariates and outcomes. The DISC Study is one of the largest studies assessing a variety of molecular and functional markers of large bowel health and of CRC risk in the macroscopically-normal rectal mucosa, and the largest RCT investigating these effects of dietary fibre in healthy people. All participants were recruited from the same region (two hospitals in the North East of England) using stringent inclusion and exclusion criteria, such as excluding any participants on anti-inflammatory medication, thus minimising the effects of potential confounders. However, this study is limited by its relatively small sample size and lack of ethnic diversity. Whilst the relatively homogenous population within this study reduces the effects of potential confounders, this may limit the generalisability of findings to other populations, with different dietary patterns, socioeconomic status, education, ethnicity and geographical location.
Estimation of habitual dietary intake using self-reported data from FFQs, which are prone to recall bias and misreporting, was used to calculate E-DII scores. At the individual level, BMI has well-recognised limitations as an index of adiposity. Future studies should investigate potential modifying effects of adiposity on E-DII links with CRC risk. Further, baseline biopsies were collected by two different endoscopy procedures, with different bowel preparation requirements. However, for the RCT, randomisation was stratified according to baseline endoscopy procedure, and this was included as a covariate during statistical analyses. In addition, all of the biopsies were collected from the same anatomical site, so reducing potential confounding. Our use of data from a cross-sectional study means that we cannot attribute causality to the observed relationships between E-DII and expression of WNT pathways genes in the rectal mucosa. Such relationships will need to be confirmed in future intervention studies.
Conclusions
Our findings suggest that the WNT signalling pathway may mediate some effects of inflammatory dietary components on markers of large bowel health in the healthy rectal mucosa. More specifically, more pro-inflammatory diets are associated with greater expression of FOSL1 and WNT11, both of which are more highly expressed in CRC tissue and in tissue from IBD patients. Furthermore, individuals with greater E-DII scores had reduced rectal FOSL1 expression after PD supplementation. Expression of both FOSL1 and WNT11 has been associated with levels of inflammatory cytokines such as IL-6(47, 54). Interestingly, we observed a weak but significant correlation between rectal WNT11 expression and the concentration of faecal calprotectin, a local marker of intestinal inflammation. Therefore, FOSL1 and WNT11, putative markers of CRC risk, may be responsive to dietary factors, and may have potential as surrogate endpoints in dietary intervention studies. Since WNT signalling is also modulated by adipose tissue, and obesity-induced inflammation is a risk factor for CRC, further investigations exploring molecular changes in adipose tissue may be of interest(65). Furthermore, investigations into the potential modulation of diet-related inflammation and WNT signalling by obesity and/or body mass change are warranted(29).
To our knowledge, this is the first study to investigate relationships between the inflammatory potential of diet, assessed using the E-DII, and molecular markers in the target tissue (i.e. rectal tissue) of healthy individuals and the first to explore whether E-DII modulates the response to supplementation with dietary fibre. Further investigations, using transcriptome-wide and multi-omic approaches studies, of how the inflammatory potential of habitual diet, assessed using the DII, modulates the response to dietary and other lifestyle interventions are warranted.
Supplementary Material
Acknowledgements
We are very grateful to DISC Study participants without whom this study would have been impossible. We acknowledge the staff at the gastroenterology units at Wansbeck and North Tyneside General Hospitals for their help and support with participant recruitment and study visits. We thank Ingredion™, formerly National Starch, Food Innovation, USA and DuPont™ Danisco®, Finland for supplying the RS and PD, respectively. We are very grateful to Julie Coaker for her very kind assistance with training in the assessment of CCPS.
Financial support
This work was supported by an award from the BBSRC Diet and Health Research Industry Club (DRINC) (grant number BB/H005013/1) to JCM, ITJ, NJB and SK. IM was funded by a fellowship from Northumbria NHS Foundation Trust. The authors acknowledge further support from the Newcastle University Centre for Ageing and Vitality, which is funded by the Medical Research Council and BBSRC as part of the cross-council Lifelong Health and Wellbeing Initiative (grant no. MR/L016354/1), and from the Wellcome Trust. Further funding was awarded by the Wellcome Trust Broadening Our Horizons scheme to support the collaboration between Newcastle University and the University of South Carolina.
Abbreviations
- ANOVA
analysis of variance
- β-catenin
beta-catenin
- β-coefficient
beta coefficient
- BMI
body mass index
- CCPS
crypt cell proliferative state
- CI
confidence intervals
- CRC
colorectal cancer
- CRP
C-reactive protein
- DII
Dietary inflammatory index
- DISC Study
Dietary Intervention, Stem cells and Colorectal Cancer Study
- E-DII
energy-adjusted DII
- GLM
general linear model
- IBD
inflammatory bowel disease
- MAPK
Mitogen Activated Protein Kinase
- NFκB
Nuclear Factor kappa B
- NIH-AARP
National Institutes of Health-American Association of Retired Persons
- PD
polydextrose
- RCT
randomised controlled trial
- RS
resistant starch
- ROS
reactive oxygen species
- SCFA
short-chain fatty acid
- SEM
standard error of the mean
- SFRP
secreted frizzled-related protein
- STAT3
Signal transducer and activator of transcription 3
- UC
ulcerative colitis
Footnotes
Conflict of interest statement: The authors declare no potential conflicts of interest.
Disclosure: Dr. James R. Hébert owns controlling interest in Connecting Health Innovations LLC (CHI), a company that has licensed the right to his invention of the Dietary Inflammatory Index (DII®) from the University of South Carolina in order to develop computer and smart phone applications for patient counselling and dietary intervention in clinical settings. Dr. Nitin Shivappa and Dr. Michael Wirth are employees of CHI. The subject matter of this paper will not have any direct bearing on that work, nor has that activity exerted any influence on this project.
Clinical Trial Registry: The DISC Study is registered with ClinicalTrials.gov (NCT01214681).
References
- 1.Wang X, O’Connell K, Jeon J, Song M, Hunter D, Hoffmeister M, et al. Combined effect of modifiable and non-modifiable risk factors for colorectal cancer risk in a pooled analysis of 11 population-based studies. BMJ Open Gastroenterol. 2019;6(1):e000339. doi: 10.1136/bmjgast-2019-000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown KF, Rumgay H, Dunlop C, Ryan M, Quartly F, Cox A, et al. The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015. Br J Cancer. 2018;118(8):1130–41. doi: 10.1038/s41416-018-0029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WCRF/AICR. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Continuous Update Project Expert Report 2019. 2018 [Google Scholar]
- 4.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 6.Carter AB, Misyak SA, Hontecillas R, Bassaganya-Riera J. Dietary modulation of inflammation-induced colorectal cancer through PPARgamma. PPAR Res. 2009;2009:498352. doi: 10.1155/2009/498352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruce WR, Giacca A, Medline A. Possible mechanisms relating diet and risk of colon cancer. Cancer Epidemiol Biomarkers Prev. 2000;9(12):1271–9. [PubMed] [Google Scholar]
- 8.Malcomson FC. Mechanisms underlying the effects of nutrition, adiposity and physical activity on colorectal cancer risk. Nutrition Bulletin. 2018;43(4):400–15. [Google Scholar]
- 9.Kim ER, Chang DK. Colorectal cancer in inflammatory bowel disease: the risk, pathogenesis, prevention and diagnosis. World J Gastroenterol. 2014;20(29):9872–81. doi: 10.3748/wjg.v20.i29.9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17(8):1689–96. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, et al. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS) Public Health Nutr. 2014;17(8):1825–33. doi: 10.1017/S1368980013002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wirth MD, Burch J, Shivappa N, Violanti JM, Burchfiel CM, Fekedulegn D, et al. Association of a dietary inflammatory index with inflammatory indices and metabolic syndrome among police officers. J Occup Environ Med. 2014;56(9):986–9. doi: 10.1097/JOM.0000000000000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shivappa N, Hebert JR, Rietzschel ER, De Buyzere ML, Langlois M, Debruyne E, et al. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. Br J Nutr. 2015;113(4):665–71. doi: 10.1017/S000711451400395X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corley J, Shivappa N, Hebert JR, Starr JM, Deary IJ. Associations between Dietary Inflammatory Index Scores and Inflammatory Biomarkers among Older Adults in the Lothian Birth Cohort 1936 Study. J Nutr Health Aging. 2019;23(7):628–36. doi: 10.1007/s12603-019-1221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shivappa N, Hebert JR, Marcos A, Diaz LE, Gomez S, Nova E, et al. Association between dietary inflammatory index and inflammatory markers in the HELENA study. Mol Nutr Food Res. 2017;61(6) doi: 10.1002/mnfr.201600707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Arellano A, Martinez-Gonzalez MA, Ramallal R, Salas-Salvado J, Hebert JR, Corella D, et al. Dietary inflammatory index and all-cause mortality in large cohorts: The SUN and PREDIMED studies. Clin Nutr. 2019;38(3):1221–31. doi: 10.1016/j.clnu.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Jayedi A, Emadi A, Shab-Bidar S. Dietary Inflammatory Index and Site-Specific Cancer Risk: A Systematic Review and Dose-Response Meta-Analysis. Adv Nutr. 2018;9(4):388–403. doi: 10.1093/advances/nmy015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shivappa N, Godos J, Hebert JR, Wirth MD, Piuri G, Speciani AF, et al. Dietary Inflammatory Index and Colorectal Cancer Risk-A Meta-Analysis. Nutrients. 2017;9(9) doi: 10.3390/nu9091043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kidane D, Chae WJ, Czochor J, Eckert KA, Glazer PM, Bothwell AL, et al. Interplay between DNA repair and inflammation, and the link to cancer. Crit Rev Biochem Mol Biol. 2014;49(2):116–39. doi: 10.3109/10409238.2013.875514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gregorieff A, Pinto D, Begthel H, Destree O, Kielman M, Clevers H. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129(2):626–38. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Schatoff EM, Leach BI, Dow LE. Wnt Signaling and Colorectal Cancer. Curr Colorectal Cancer Rep. 2017;13(2):101–10. doi: 10.1007/s11888-017-0354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.You J, Nguyen AV, Albers CG, Lin F, Holcombe RF. Wnt pathway-related gene expression in inflammatory bowel disease. Dig Dis Sci. 2008;53(4):1013–9. doi: 10.1007/s10620-007-9973-3. [DOI] [PubMed] [Google Scholar]
- 23.Moparthi L, Koch S. Wnt signaling in intestinal inflammation. Differentiation. 2019;108:24–32. doi: 10.1016/j.diff.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Ma B, Hottiger MO. Crosstalk between Wnt/beta-Catenin and NF-kappaB Signaling Pathway during Inflammation. Front Immunol. 2016;7:378. doi: 10.3389/fimmu.2016.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jang J, Jung Y, Kim Y, Jho EH, Yoon Y. LPS-induced inflammatory response is suppressed by Wnt inhibitors, Dickkopf-1 and LGK974. Sci Rep. 2017;7:41612. doi: 10.1038/srep41612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J, Kim J, Kim DW, Ha Y, Ihm MH, Kim H, et al. Wnt5a induces endothelial inflammation via beta-catenin-independent signaling. J Immunol. 2010;185(2):1274–82. doi: 10.4049/jimmunol.1000181. [DOI] [PubMed] [Google Scholar]
- 27.Pereira C, Schaer DJ, Bachli EB, Kurrer MO, Schoedon G. Wnt5A/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arterioscler Thromb Vasc Biol. 2008;28(3):504–10. doi: 10.1161/ATVBAHA.107.157438. [DOI] [PubMed] [Google Scholar]
- 28.Bradford EM, Ryu SH, Singh AP, Lee G, Goretsky T, Sinh P, et al. Epithelial TNF Receptor Signaling Promotes Mucosal Repair in Inflammatory Bowel Disease. J Immunol. 2017;199(5):1886–97. doi: 10.4049/jimmunol.1601066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ulrich CM, Himbert C, Holowatyj AN, Hursting SD. Energy balance and gastrointestinal cancer: risk, interventions, outcomes and mechanisms. Nat Rev Gastroenterol Hepatol. 2018;15(11):683–98. doi: 10.1038/s41575-018-0053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gunter MJ, Riboli E. Obesity and gastrointestinal cancers-where do we go from here? Nat Rev Gastroenterol Hepatol. 2018;15(11):651–2. doi: 10.1038/s41575-018-0073-y. [DOI] [PubMed] [Google Scholar]
- 31.Tarapore RS, Siddiqui IA, Mukhtar H. Modulation of Wnt/beta-catenin signaling pathway by bioactive food components. Carcinogenesis. 2012;33(3):483–91. doi: 10.1093/carcin/bgr305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holcombe RF, Martinez M, Planutis K, Planutiene M. Effects of a grape-supplemented diet on proliferation and Wnt signaling in the colonic mucosa are greatest for those over age 50 and with high arginine consumption. Nutr J. 2015;14:62. doi: 10.1186/s12937-015-0050-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malcomson FC, Willis ND, McCallum I, Xie L, Kelly S, Bradburn DM, et al. Adherence to the World Cancer Research Fund/American Institute for Cancer Research cancer prevention recommendations and WNT-pathway-related markers of bowel cancer risk. Br J Nutr. 2019;122(5):509–17. doi: 10.1017/S0007114518002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malcomson FC, Willis ND, Mathers JC. Is resistant starch protective against colorectal cancer via modulation of the WNT signalling pathway? Proc Nutr Soc. 2015;74(3):282–91. doi: 10.1017/S002966511500004X. [DOI] [PubMed] [Google Scholar]
- 35.Williams EA, Coxhead JM, Mathers JC. Anti-cancer effects of butyrate: use of micro-array technology to investigate mechanisms. Proc Nutr Soc. 2003;62(1):107–15. doi: 10.1079/PNS2002230. [DOI] [PubMed] [Google Scholar]
- 36.Malcomson FC, Willis ND, McCallum I, Xie L, Ibero-Baraibar I, Leung WC, et al. Effects of supplementation with nondigestible carbohydrates on fecal calprotectin and on epigenetic regulation of SFRP1 expression in the large-bowel mucosa of healthy individuals. Am J Clin Nutr. 2017;105(2):400–10. doi: 10.3945/ajcn.116.135657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malcomson FC, Willis ND, McCallum I, Xie L, Ouwehand AC, Stowell JD, et al. Resistant starch supplementation increases crypt cell proliferative state in the rectal mucosa of older healthy participants. Br J Nutr. 2020:1–12. doi: 10.1017/S0007114520001312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peuranen S, Tiihonen K, Apajalahti J, Kettunen A, Saarinen M, Rautonen N. Combination of polydextrose and lactitol affects microbial ecosystem and immune responses in rat gastrointestinal tract. Br J Nutr. 2004;91(6):905–14. doi: 10.1079/BJN20041114. [DOI] [PubMed] [Google Scholar]
- 40.Holben WE, Williams P, Gilbert M, Saarinen M, Sarkilahti LK, Apajalahti JH. Phylogenetic analysis of intestinal microflora indicates a novel Mycoplasma phylotype in farmed and wild salmon. Microb Ecol. 2002;44(2):175–85. doi: 10.1007/s00248-002-1011-6. [DOI] [PubMed] [Google Scholar]
- 41.Kroke A, Klipstein-Grobusch K, Voss S, Moseneder J, Thielecke F, Noack R, et al. Validation of a self-administered food-frequency questionnaire administered in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: comparison of energy, protein, and macronutrient intakes estimated with the doubly labeled water, urinary nitrogen, and repeated 24-h dietary recall methods. Am J Clin Nutr. 1999;70(4):439–47. doi: 10.1093/ajcn/70.4.439. [DOI] [PubMed] [Google Scholar]
- 42.Chiba T, Marusawa H, Ushijima T. Inflammation-associated cancer development in digestive organs: mechanisms and roles for genetic and epigenetic modulation. Gastroenterology. 2012;143(3):550–63. doi: 10.1053/j.gastro.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 43.Galland L. Diet and inflammation. Nutr Clin Pract. 2010;25(6):634–40. doi: 10.1177/0884533610385703. [DOI] [PubMed] [Google Scholar]
- 44.Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN, et al. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359(6392):235–7. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- 45.Fodde R, Kuipers J, Rosenberg C, Smits R, Kielman M, Gaspar C, et al. Mutations in the APC tumour suppressor gene cause chromosomal instability. Nat Cell Biol. 2001;3(4):433–8. doi: 10.1038/35070129. [DOI] [PubMed] [Google Scholar]
- 46.Mori H, Yao Y, Learman BS, Kurozumi K, Ishida J, Ramakrishnan SK, et al. Induction of WNT11 by hypoxia and hypoxia-inducible factor-1alpha regulates cell proliferation, migration and invasion. Sci Rep. 2016;6:21520. doi: 10.1038/srep21520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uysal-Onganer P, Kypta RM. Wnt11 in 2011-the regulation and function of a non-canonical Wnt. Acta Physiol (Oxf) 2012;204(1):52–64. doi: 10.1111/j.1748-1716.2011.02297.x. [DOI] [PubMed] [Google Scholar]
- 48.Ouko L, Ziegler TR, Gu LH, Eisenberg LM, Yang VW. Wnt11 signaling promotes proliferation, transformation, and migration of IEC6 intestinal epithelial cells. J Biol Chem. 2004;279(25):26707–15. doi: 10.1074/jbc.M402877200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kirikoshi H, Sekihara H, Katoh M. Molecular cloning and characterization of human WNT11. Int J Mol Med. 2001;8(6):651–6. doi: 10.3892/ijmm.8.6.651. [DOI] [PubMed] [Google Scholar]
- 50.Nishioka M, Ueno K, Hazama S, Okada T, Sakai K, Suehiro Y, et al. Possible involvement of Wnt11 in colorectal cancer progression. Mol Carcinog. 2013;52(3):207–17. doi: 10.1002/mc.21845. [DOI] [PubMed] [Google Scholar]
- 51.Holowatyj AN, Gigic B, Herpel E, Scalbert A, Schneider M, Ulrich CM, et al. Distinct Molecular Phenotype of Sporadic Colorectal Cancers Among Young Patients Based on Multiomics Analysis. Gastroenterology. 2020;158(4):1155–8.:e2. doi: 10.1053/j.gastro.2019.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dronamraju SS, Coxhead JM, Kelly SB, Burn J, Mathers JC. Cell kinetics and gene expression changes in colorectal cancer patients given resistant starch: a randomised controlled trial. Gut. 2009;58(3):413–20. doi: 10.1136/gut.2008.162933. [DOI] [PubMed] [Google Scholar]
- 53.Wang HL, Wang J, Xiao SY, Haydon R, Stoiber D, He TC, et al. Elevated protein expression of cyclin D1 and Fra-1 but decreased expression of c-Myc in human colorectal adenocarcinomas overexpressing beta-catenin. Int J Cancer. 2002;101(4):301–10. doi: 10.1002/ijc.10630. [DOI] [PubMed] [Google Scholar]
- 54.Wang T, Song P, Zhong T, Wang X, Xiang X, Liu Q, et al. The inflammatory cytokine IL-6 induces FRA1 deacetylation promoting colorectal cancer stem-like properties. Oncogene. 2019;38(25):4932–47. doi: 10.1038/s41388-019-0763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sabzevary-Ghahfarokhi M, Shohan M, Shirzad H, Rahimian G, Bagheri N, Soltani A, et al. The expression analysis of Fra-1 gene and IL-11 protein in Iranian patients with ulcerative colitis. BMC Immunol. 2018;19(1):17. doi: 10.1186/s12865-018-0257-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Romagnolo DF, Donovan MG, Doetschman TC, Selmin OI. n-6 Linoleic Acid Induces Epigenetics Alterations Associated with Colonic Inflammation and Cancer. Nutrients. 2019;11(1) doi: 10.3390/nu11010171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andrade PA, Hermsdorff HHM, Leite JIA, Shivappa N, Hebert JR, Henriques HKF, et al. Baseline Pro-inflammatory Diet Is Inversely Associated with Change in Weight and Body Fat 6 Months Following-up to Bariatric Surgery. Obes Surg. 2019;29(2):457–63. doi: 10.1007/s11695-018-3530-3. [DOI] [PubMed] [Google Scholar]
- 58.Akedo I, Ishikawa H, Ioka T, Kaji I, Narahara H, Ishiguro S, et al. Evaluation of epithelial cell proliferation rate in normal-appearing colonic mucosa as a high-risk marker for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2001;10(9):925–30. [PubMed] [Google Scholar]
- 59.Lipkin M. Biomarkers of increased susceptibility to gastrointestinal cancer: new application to studies of cancer prevention in human subjects. Cancer Res. 1988;48(2):235–45. [PubMed] [Google Scholar]
- 60.Humphreys KJ, Conlon MA, Young GP, Topping DL, Hu Y, Winter JM, et al. Dietary Manipulation of Oncogenic MicroRNA Expression in Human Rectal Mucosa: A Randomized Trial. Cancer Prev Res (Phila) 2014;7(8):786–95. doi: 10.1158/1940-6207.CAPR-14-0053. [DOI] [PubMed] [Google Scholar]
- 61.van Munster IP, Tangerman A, Nagengast FM. Effect of resistant starch on colonic fermentation, bile acid metabolism, and mucosal proliferation. Digestive diseases and sciences. 1994;39(4):834–42. doi: 10.1007/BF02087431. [DOI] [PubMed] [Google Scholar]
- 62.Chen J, Vitetta L. Inflammation-Modulating Effect of Butyrate in the Prevention of Colon Cancer by Dietary Fiber. Clin Colorectal Cancer. 2018;17(3):e541–e4. doi: 10.1016/j.clcc.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 63.Ibrahem S, Al-Ghamdi S, Baloch K, Muhammad B, Fadhil W, Jackson D, et al. STAT3 paradoxically stimulates beta-catenin expression but inhibits beta-catenin function. Int J Exp Pathol. 2014;95(6):392–400. doi: 10.1111/iep.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bibi S, Kang Y, Du M, Zhu MJ. Dietary red raspberries attenuate dextran sulfate sodium-induced acute colitis. J Nutr Biochem. 2018;51:40–6. doi: 10.1016/j.jnutbio.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 65.Holowatyj AN, Haffa M, Lin T, Scherer D, Gigic B, Ose J, et al. Multi-omics analysis reveals adipose-tumor crosstalk in colorectal cancer patients. Cancer Prev Res (Phila) 2020 doi: 10.1158/1940-6207.CAPR-19-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.