Abstract
DNA topoisomerase II (TOP2) poisons induce protein-DNA crosslinks termed TOP2-DNA covalent complexes, in which TOP2 remains covalently bound to each end of an enzyme-induced double strand DNA break (DSB) via a 5’-phosphotyrosyl bond. Repair of the enzyme-induced DSB first requires the removal of the TOP2 protein adduct which, among other mechanisms, can be accomplished through the proteasomal degradation of TOP2. VCP/p97 is a AAA ATPase which utilises energy from ATP hydrolysis to unfold protein substrates, which can facilitate proteasomal degradation by extracting target proteins from certain cellular structures (such as chromatin) and/or by aiding their translocation into the proteolytic core of the proteasome. In this study, we show that inhibition of VCP/p97 leads to the prolonged accumulation of etoposide-induced TOP2A- and TOP2B- DNA complexes in a manner that is epistatic with the proteasomal pathway. VCP/p97 inhibition also reduces the etoposide-induced phosphorylation of histone H2AX, indicative of fewer DSBs. This suggests that VCP/p97 is required for the proteasomal degradation of TOP2-DNA covalent complexes, and is thus likely to be an important mediator of DSB repair after treatment with a TOP2 poison.
Keywords: DNA topoisomerase II, etoposide, proteasome, ubiquitin, VCP
Introduction
DNA topoisomerase II (TOP2) is an important enzyme which mediates topological changes in DNA, including the unwinding of supercoils and the decatenation of sister chromatids. This is achieved by passing an intact double helix of DNA through a double strand DNA break (DSB) introduced by the enzyme. TOP2 forms a covalent 5’-phosphotyrosyl linkage with each end of the DNA break, resulting in a transient intermediate of the TOP2 reaction cycle termed the TOP2-DNA covalent complex. TOP2-DNA covalent complexes are rapidly reversed following strand passage by religation of the DSB. However, TOP2 poisons such as etoposide and mitoxantrone inhibit religation of the DSB and stabilise TOP2-DNA covalent complexes which accumulate on DNA. TOP2-DNA complexes obstruct elongating DNA and RNA polymerases leading to the arrest of replication and transcription, and further processing of trapped TOP2-DNA complexes also leads to the liberation of protein-free DSBs. Therefore, timely repair of TOP2-DNA complexes is crucial for the maintenance of genome stability.
TOP2 is a large protein, and it is well understood that in the presence of TOP2 poisons TOP2-mediated DSB remains concealed within the TOP2-DNA complex until processing of the TOP2 adduct occurs (Martensson et al., 2003). While a number of processing pathways have been described, one major mechanism involves the proteolysis of TOP2. This results in a protein-free DSB linked to short phosphotyrosyl peptides which can then be repaired by the 5’-phosphodiesterase TDP2 prior to DSB repair (Cortes Ledesma et al., 2009; Gao et al., 2014). TOP2-DNA complexes can be degraded by the proteasome in a ubiquitin-dependent manner (Alchanati et al., 2009; Mao et al., 2001; Swan et al., 2020). Indeed, inhibition of the proteasome (or ubiquitination) increases levels of etoposide-stabilised TOP2-DNA complexes (Alchanati et al., 2009; Fan et al., 2008; Lee et al., 2016; Mao et al., 2001; Swan et al., 2020; Tammaro et al., 2013; Zhang et al., 2006) and reduces the activation of etoposide-induced DNA damage response proteins including histone H2AX, RPA and p53 (Swan et al., 2020; Zhang et al., 2006). It has been proposed that TOP2-DNA complexes can be repaired directly by TDP2 through the remodelling of TOP2, which is mediated by the ZATT SUMO ligase when TOP2 is SUMOylated and the proteasome is inhibited (Schellenberg et al., 2017). This pathway has been observed only in the absence of a functional proteasome, further exemplifying the importance of proteolysis in the removal and repair of TOP2-DNA complexes (Lee et al., 2018). In addition upstream SUMOylation may also be required for efficient ubiquitination and targeting of stalled TOP complexes to the proteasome (Sun et al., 2020) Aside from TOP2 proteolysis, TOP2 may be removed by cleavage of the DNA containing the TOP2 adduct, involving the nuclease activity of Mre11, which is stimulated by CtIP (Hartsuiker et al., 2009; Lee et al., 2012; Neale et al., 2005).
In addition to the proteasome, another protease has recently been shown to aid in the proteolysis of TOP1- and TOP2- DNA complexes (Balakirev et al., 2015; Lopez-Mosqueda et al., 2016; Maskey et al., 2017; Stingele et al., 2016; Vaz et al., 2016). SPRTN (Wss1 in yeast) is a replication-associated metalloprotease implicated in the degradation of a number of protein-DNA adducts (Duxin et al., 2014; Stingele et al., 2014). SPRTN forms a complex with TOP1 and TOP2 in vivo (Vaz et al., 2016), and depletion of SPRTN increases the sensitivity to etoposide and prevents the degradation of TOP2A-DNA complexes (Lopez-Mosqueda et al., 2016). Interestingly, Fielden et al show that the degradation of TOP1-DNA complexes by SPRTN requires the ATPase activity of VCP/p97 (Fielden et al., 2020). ATP binding to VCP/p97 induces a major conformational change and subsequent unfolding of the target protein, which then may or may not be degraded. Indeed, VCP/p97 is known to be a key mediator of the ubiquitin-proteasome system where protein unfolding facilitates the translocation of target proteins into the narrow proteolytic core of the 26S proteasome (Beskow et al., 2009; van den Boom and Meyer, 2018; van den Boom et al., 2016). Similarly, it is suggested that the ATPase activity of VCP/p97 enables the remodelling of TOP1, which facilitates the proteolytic cleavage of TOP1-DNA complexes by SPRTN (Fielden et al., 2020). Furthermore, Wei et al reported an increase in levels of ubiquitinated TOP2 in yeast following inactivation of temperature sensitive Cdc48 (the yeast homolog of VCP/p97).
VCP/p97 is implicated in the extraction of protein complexes from various cellular structures. While perhaps best known for its role in endoplasmic reticulum-associated degradation (ERAD), VCP/p97 also enables the removal of protein complexes from chromatin (chromatin-associated degradation, CAD). This includes protein complexes that are otherwise tightly bound to DNA, such as stalled RNA polymerase II (He et al., 2017; Lafon et al., 2015; Verma et al., 2011) and the sterically trapped Ku70/80 complex (van den Boom et al., 2016). In the current study, we investigate the potential role of VCP/p97 in the removal of drug-stabilised TOP2-DNA covalent complexes, and their subsequent processing to protein-free DSBs. We show that both the degradation of TOP2-DNA complexes and the appearance of etoposide-induced DSBs is reduced upon chemical inhibition or siRNA knockdown of VCP/p97.
Materials and methods
Cell culture and reagents
K562 cells were maintained at 37°C, 5% CO2 in RPMI medium containing 10% foetal bovine serum (FBS) and 5% (v/v) penicillin-streptomycin. Cells are regularly checked for mycoplasma. Etoposide and BenzylN-[(2S)-4-methyl-1-[[(2S)-4-methyl-1-[[(2S)-4-methyl-1-oxopentan-2-yl]amino]-1-oxopentan-2-yl]amino]-1-oxopentan-2-yl]carbamate (MG132) were purchased from Sigma-Aldrich (Dorset, UK). 3-(3-(cyclopentylthio)-5-(((2-methyl-4’-(methylsulfonyl)-[1,1’-biphenyl]-4-yl)oxy)methyl)-4H-1,2,4-triazol-4-yl)pyridine (NMS-873) was obtained from Sigma-Aldrich (Missouri, USA), VCP/p97 siRNA was purchased from Qiagen (Maryland, USA, siRNA ID GS7415, containing four siRNAs: SI03019681, SI03019730, S103149657 and S104350444).
Trapped in Agarose DNA Immunostaining (TARDIS) assay
TARDIS assays were performed essentially as described previously (Cowell and Austin, 2018; Swan et al., 2020; Willmore et al., 1998).K562 cells were plated at 2 x 105 cells/mL and incubated overnight before drug treatment. TOP2 antibodies used were 4566-TOP2A and 4555-TOP2B (Atwal et al., 2019) or ubiquitin (FK2, Merck Millipore). Nuclear ghosts were counterstained with Hoechst 33258. Hoechst and immunofluorescent images were captured using an Olympus IX-81 epifluorescence microscope (10X objective) fitted with an Orca-AG camera (Hamamatsu) and suitable narrow band filter. Slides were scored automatically as described previously (Atwal et al., 2019) using Volocity 6.3 software (PerkinElmer Inc.). Statistical analysis was performed using GraphPad Prism 8.2 (Perkin Elmer, San Diego, CA). Each TARDIS experiment contained an additional 100μM etoposide treatment that was used for normalisation. Median integrated fluorescence values per nucleus were calculated and these were converted to a percentage of the median obtained for 100μM etoposide and the mean of the medians ± SD from replica experiments were calculated. Bar charts represent the mean values, and individual median values are plotted for each replicate as blue-lined white circles.
yH2AX immunofluorescent assays
Cell seeding and immunofluorescence was carried out as described previously (Swan et al., 2020). Quantitative immunofluorescence analysis was performed as for the TARDIS assay.
Western blotting
Western blotting was carried out as described previously (Swan et al., 2020) using mouse monoclonal anti VCP/p97 antibody (ab11433, Abcam). Blots were developed on film or using a LI-COR C-DiGit Chemiluminescence Western Blot Scanner.
Data analysis
This is an exploratory study. Statistical analysis was performed using Graph Pad Prism 8.2. The details of tests performed are given in figure legends. For signifying P values, * refers to P < 0.05, ** refers to P < 0.01, *** refers to P< 0.001 and **** refers to P<0.0001. Error bars in bar charts represent SD values. Sample sizes (numbers of replicate experiments) were specified in advance of data acquisition based on prior knowledge of the characteristics of the assays involved and anticipating occasional lost or failed samples.
Results
Chemical inhibition of VCP/p97 slows removal of etoposide induced TOP2A and TOP2B covalent complexes
A number of ubiquitinated proteins are now known to be removed from DNA by VCP/p97, including stalled RNA polymerase (Verma et al., 2011), Ku70/80 (van den Boom et al., 2016), and the polycomb protein L3MBTL1 (Acs et al., 2011). The potential role of VCP/p97 in the processing of etoposide-induced TOP2-DNA covalent complexes was first investigated using the TARDIS assay. TARDIS is an immunofluorescence-based technique which allows the quantification of TOP2 covalently bound to DNA (TOP2-DNA covalent complexes) on a single cell basis (Cowell and Austin, 2018; Willmore et al., 1998). The TARDIS assay was performed in the presence or absence of NMS-873, a small molecule inhibitor of VCP/p97 (Magnaghi et al., 2013), or the proteasome inhibitor MG132 for comparison.
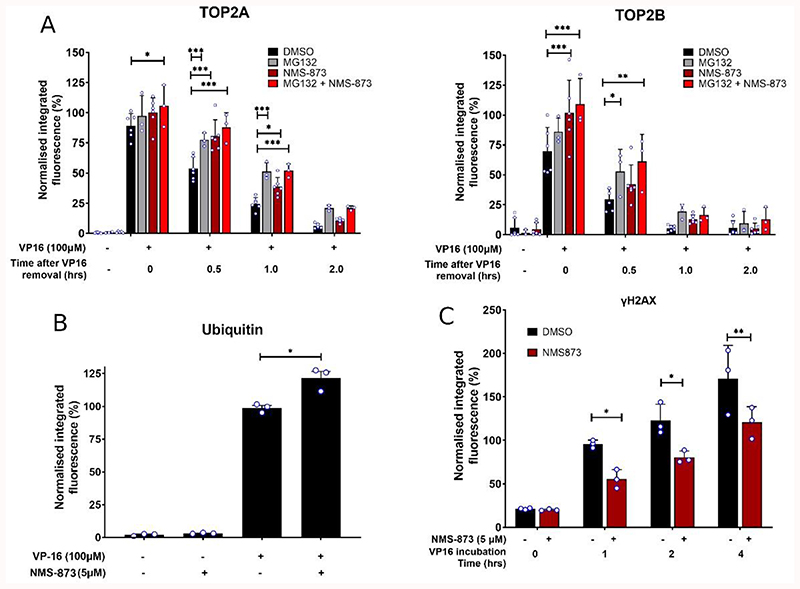
Cells were treated for 2 hours with etoposide alone or etoposide in combination with NMS-873. Following 2 hours of continuous drug exposure, etoposide was removed from the culture medium and cells were incubated for a further 2 hours in the presence of 5 μM NMS-873 or DMSO. Cells were collected at 0, 0.5, 1 and 2 hours after etoposide removal, and levels of TOP2A- and TOP2B- DNA complexes were measured using the TARDIS assay. Where cells were incubated with the proteasome inhibitor MG132, levels of TOP2A-DNA complexes were significantly increased at 0.5, 1 and 2 hours after etoposide removal compared to DMSO-treated cells, and levels of TOP2B-DNA complexes were significantly increased at 0.5 hours as previously shown (Lee et al., 2016; Swan et al., 2020). Levels of TOP2A- and TOP2B- DNA complexes were also significantly increased 0 hours after etoposide removal when cells were maintained in media containing 5 μM NMS-873 (Figure 1A) compared to DMSO-treated cells. Levels of TOP2A-DNA complexes remained significantly increased at 0.5 and 1 hours after etoposide removal in the presence of NMS-873, while TOP2B-DNA complexes returned to normal levels by 0.5 hours after etoposide removal. This suggests that inhibition of VCP/p97 reduces the removal of drug-stabilised TOP2A- and TOP2B- DNA complexes from chromatin, similarly to proteasome inhibition.
Figure 1. Effect of the VCP/p97 inhibitor NMS-873 on the processing of etoposide-induced TOP2-DNA complexes to DSBs.
(A) The TARDIS reversal assay was used to measure levels of TOP2-DNA complexes in K562 cells treated with 100μM etoposide (VP-16) alone or in combination with 5μM NMS-873 (or MG132 for comparison). After 2 hours, etoposide was removed from the culture medium and cells were incubated for up to 2 hours in etoposide-free medium containing DMSO, MG132 or NMS-873. Cells were collected at 0, 0.5, 1 and 2 hours after etoposide removal. Averages are normalised to a 2-hour 100μM etoposide control, and statistical analysis performed by two-way ANOVA. and Bonferroni post hoc test (B) K562 cells were treated with 100μM etoposide (VP-16) alone or in combination with 5μM NMS-873 for 2 hours. Cells were processed as per the TARDIS assay and probed with anti-ubiquitin antibody (clone FK2). All values were normalised to a 100μM VP-16 control, and statistical comparisons made by unpaired t-test. (C) K562 cells were treated with 10μM etoposide (VP-16) alone or in combination with 5μM NMS-873 for up to 4 hours, and protein-free DSBs were measured by yH2AX assay. Statistical significance was determined by two-way ANOVA and Bonferroni post hoc test. Averages were normalised to a 1-hour 10μM etoposide positive control. For each graph bars represent mean values +/- SD, individual values (medians of fluorescence value per cell from individual replicates) are shown as blue-lined circles.
When etoposide-treated cells were co-treated with both NMS-873 and MG132, there was no additional increase in levels of TOP2A- or TOP2B- DNA complexes at any time point tested, suggesting that VCP/p97 and the proteasome operate via the same pathway. This correlated with a significant increase in the levels of ubiquitinated TOP2-DNA complexes as measured by ubiquitin TARDIS (Figure 1B), which may be due to reduced removal of ubiquitinated TOP2 from DNA.
VCP/p97 is involved in the processing of TOP2-DNA complexes to protein-free DSBs
Removal of the TOP2 protein adduct is required for liberation of the TOP2-induced DSB and the subsequent recognition of the break by DNA damage response machinery. In response to a DNA double strand break, the histone variant H2AX becomes phosphorylated, and this leads to the recruitment of repair factors such as BRCA1 and 53BP1. Inhibition of the proteasome reduces the etoposide-induced phosphorylation of histone H2AX (Lee et al., 2016; Swan et al., 2020), consistent with the requirement for the proteasome in the processing of TOP2-DNA complexes to protein-free DSBs that invoke the DNA damage response.
To determine whether VCP/p97 is involved in the processing of etoposide-induced TOP2-DNA complexes to protein-free DSBs, the γH2AX assay was performed in the presence or absence of NMS-873. Levels of etoposide-induced Ser-139-phospho histone H2AX (γH2AX) were significantly reduced with NMS-873 treatment after 1 and 2 hours of continuous drug incubation but returned to normal levels at 4 hours (Figure 1C). This suggests that the appearance of etoposide-induced DSBs is partly VCP/p97-dependent.
Effect of VCP/p97 siRNA on the processing of TOP2-DNA covalent complexes
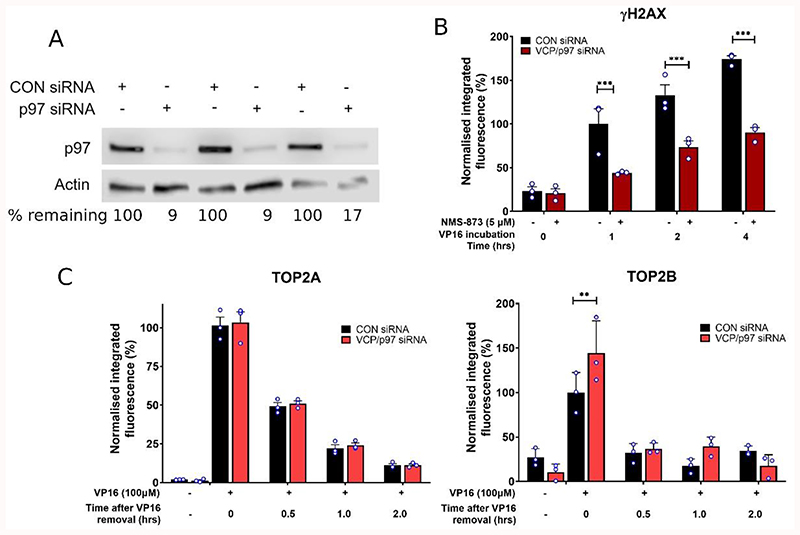
The role of VCP/p97 was further investigated by siRNA knockdown of VCP/p97. This led to a reduction in the level of p97 protein in knockdown cells to ~17% of that in control cells (Figure 2A). VCP/p97 knockdown cells were then treated with etoposide for up to 4 hours, and levels of γH2AX were measured after 0, 1, 2 and 4 hours of continuous etoposide exposure. Consistently with the effect of NSM-873, siRNA knockdown of VCP/p97 reduced the appearance of etoposide-induced DSBs following 1-, 2- and 4-hours etoposide treatment (Figure 2B) supporting the notion that the removal of etoposide-induced TOP2-DNA complexes, and the subsequent appearance of protein-free DSBs, is partly VCP/p97-dependent.
Figure 2. siRNA knockdown of VCP/p97 and the effect on TOP2-DNA complex processing to DSBs.
(A) VCP/p97 siRNA knockdown from triplicate experiments was tested by western blot. Each replicate represents the cells used in yH2AX and TARDIS experiments shown in Figures B and C. (B) Cells treated with VCP/p97 siRNA or control siRNA (CON) were exposed continuously to 10μM etoposide for up to 4 hours, and levels of protein-free DSBs were measured using the yH2AX assay. (C) VCP/p97 siRNA or control (CON) siRNA cells were treated with 100μM etoposide for 2 hours followed by 2 hours incubation in etoposide-free media. The TARDIS assay was used to measure levels of TOP2A- and TOP2B- DNA complexes at 0, 0.5, 1 and 2 hours after etoposide removal. Statistical significance was determined by two-way ANOVA and Bonferroni post hoc test.
In addition, the TARDIS assay was used to investigate the effect of VCP/p97 knockdown on levels of TOP2-DNA complexes. VCP/p97 knockdown cells and control cells were treated with etoposide for 2 hours, followed by incubation in etoposide-free medium for a further 2 hours. Levels of TOP2-DNA complexes after etoposide washout were then measured at 0, 0.5, 1 and 2 hours using the TARDIS assay. As previously shown following chemical inhibition of VCP/p97, levels of TOP2B-DNA complexes were significantly increased in VCP/p97 knockdown cells after 2 hours exposure to etoposide (0 hours after etoposide removal, Figure 2C) but reduced to control levels following etoposide removal. In contrast, the levels of TOP2A-DNA complexes were not significantly affected by VCP/p97 siRNA knockdown either following 2 hours of continuous etoposide exposure or after etoposide removal. It is unclear why VCP/p97 knockdown affected levels of TOP2B but not TOP2A in the TARDIS assay. Notably, the knockdown of VCP/p97 was incomplete in these cells (Figure 2A), which may account for the normal processing of TOP2A-DNA complexes.
Discussion
VCP/p97 is a AAA ATPase implicated in the SPRTN-dependent degradation of TOP1-DNA complexes in human and yeast cells. VCP/p97 is known to facilitate the unfolding of target proteins which may lead to their proteolytic degradation. VCP/p97 promotes the proteasomal degradation of many protein complexes on DNA, including RNAPII and Ku70/80 (van den Boom et al., 2016; Verma et al., 2011). Here we show that inhibition of VCP/p97 slows the processing of etoposide-induced TOP2-DNA complexes to protein-free DSBs.
We show that inhibition or siRNA knockdown of VCP/p97 reduces etoposide-induced histone H2AX phosphorylation. As VCP/p97 is not required for the appearance of irradiation-induced γH2AX foci (Meerang et al., 2011), this implies a specific role for VCP/p97 in the induction of etoposide-induced DSBs. We propose that VCP/p97 is required for the removal of TOP2 adducts from DNA. For example, VCP/p97 may facilitate the extraction of TOP2, thereby improving the efficiency of TOP2 degradation by the proteasome or SPRTN. In the current study, inhibition of VCP/p97 increased levels of TOP2A- and TOP2B- DNA complexes on chromatin in a manner that was epistatic to the proteasomal pathway. While it is unclear how VCP/p97 facilitates the removal of covalently bound TOP2 from DNA, it is plausible that unfolding of the covalently attached TOP2 protein may facilitate the translocation of TOP2 into the catalytic core of proteasomes present on chromatin (Verma et al., 2011). Alternatively, VCP/p97 could be involved in the disassembly of TOP2 dimers where only one TOP2 protomer is covalently attached to the DNA (i.e. etoposide-induced SSBs). It was estimated that only 2-3% of etoposide-induced breaks are DSBs, with the remainder consisting of single stranded DNA breaks (SSBs) (Bromberg et al., 2003; Muslimovic et al., 2009; Yu et al., 2017).
It is important to note that, like proteasome or ubiquitin activating enzyme inhibition, inhibition of VCP/p97 is reported to deplete levels of nuclear ubiquitin (Dantuma et al., 2006; Heidelberger et al., 2018; Xu et al., 2004). Therefore, while the VCP/p97-dependent processing of drug-stabilised TOP2-DNA complexes was epistatic with the proteasomal pathway, we cannot exclude the possibility that the observed proteasome- and VCP/p97- dependent processing of TOP2-DNA complexes is due to inhibition of another ubiquitin-dependent (but proteasome-independent) pathway. For example, ubiquitination regulates the binding of SPRTN to DNA (Stingele et al., 2016), and the proteolysis of TOP2 by SPRTN is increased by the addition of free ubiquitin (but not SUMO-2) in vitro (Lopez-Mosqueda et al., 2016). Therefore, it is plausible that the VCP/p97-dependent pathway described here could be required for the SPRTN-dependent processing of TOP2-DNA complexes. Like SPRTN and the proteasome, VCP/p97 itself can interact directly with ubiquitinated proteins via ubiquitin interacting motifs and ubiquitin adaptor proteins like Ufd1 and Npl4 (Meyer et al., 2000). For instance, VCP/p97 is recruited to stalled replication forks via monoubiquitinated PCNA, where it facilitates translesion synthesis through the extraction and subsequent SPRTN-dependent degradation of translesion polymerase Pol eta (Mosbech et al., 2012). It has also been suggested that SPRTN could serve as a second proteolysis step to further reduce the size of large peptides resulting from the proteasomal degradation of large protein-DNA adducts (Larsen et al., 2019). Further studies are required to investigate the potential involvement of VCP/p97 in the SPRTN-dependent proteolysis of TOP2-DNA complexes.
Extraction of chromatin-associated proteins by VCP/p97 can also lead to protein inactivation without subsequent degradation. For example, the VCP/p97-dependent extraction of ubiquitinated Aurora B from chromatin leads to the inactivation of Aurora B upon exit from mitosis, allowing the decondensation of chromatin and nuclear envelope formation (Ramadan et al., 2007). It is therefore plausible that VCP/p97 could mediate the extraction of TOP2 from chromatin in order to regulate TOP2-specific functions. VCP/p97 is required for the activation of other factors which may indirectly affect TOP2 function, such as NRF1. NRF1 activates transcription via a direct interaction with PARP1, which potentially leads to the recruitment of the PARP1/DNA-PK/Ku80/Ku70/TOP2B-containing protein complex to promoters. NRF1 copurifies with the PARP1/DNA-PK/Ku80/Ku70/TOP2B-containing protein complex (Hossain et al., 2009) The data presented herein suggest that TOP2-DNA complexes can be removed by a VCP/p97-dependent mechanism which may lead to the proteolysis of TOP2 adducts by the proteasome. This is one of many reported pathways which have been shown to facilitate the removal of trapped TOP2-DNA complexes from DNA and subsequent DSB repair. Redundancy between repair pathways increases the timeliness of DNA repair, where the ability to respond rapidly to DNA damage is crucial for genome stability. Choice of processing pathway may depend on various factors such as the availability or proximity of specific repair proteins. For example, the proteasomal pathway may occur where proteasomes are already present on nearby chromatin, such as those involved in the repair of stalled RNAPII after collision with protein adducts, including TOP2-DNA complexes. Alternatively, pathway choice may be determined by the availability of modifying proteins such as ubiquitinating enzymes. For example, Hu et al show that the deubiquitination of VCP/p97 substrates is required for their translocation through the VCP/p97 central pore, and re-ubiquitination may then be required to redirect those proteins to the proteasome for degradation (Hu et al., 2020). Pathway choice may also depend on the context or structure of the DNA adduct, as suggested for SPRTN (Li et al., 2019; Reinking et al., 2020).
VCP/p97 has been implicated in the processing of various protein adducts on DNA, including those sterically trapped or tightly associated with chromatin. With increasing understanding of the diverse functions of VCP/p97 in the cell and dysfunctions in many diseases, there are now a number of VCP/p97 inhibitors in development, including CB 5083 which is currently in phase I clinical trials (Rycenga et al., 2019; Zhou et al., 2015). We show that inhibition of VCP/p97 increases levels of etoposide-induced TOP2-DNA complexes, and reduces levels of potentially leukaemogenic protein-free DSBs. This is new and important observation, that suggests that co-treatment with a VCP/p97 inhibitor may increase the cytotoxicity of TOP2 poisons, while also reducing TOP2 poison genotoxicity. Future investigations could determine whether combinations of VCP/p97 inhibitor and etoposide increase cytotoxicity whilst reducing translocations.
Significance statement.
TOP2 poisons are chemotherapeutic agents used in the treatment of a range of cancers. Better understanding how TOP2 poison-induced DNA damage is repaired could improve therapy with TOP2 poisons by increasing TOP2 poison cytotoxicity and reducing genotoxicity. The results presented herein suggest that repair of TOP2-DNA covalent complexes involves the protein segregase VCP/p97
Acknowledgements
Funding
This study was supported by Bloodwise Research Specialist Program Grant [12031] and by a Bloodwise Gordon Piller Studentship, [13063].
Non-standard abbreviations
- DMSO
Dimethyl sulfoxide
- DSB
DNA double-strand break
- H2AX
histone H2A.X
- yH2AX
S-139 phospho-histone H2A.X
- TOP2A
DNA Topoisomerase IIα
- TOP2B
DNA topoisomerase IIβ
- TOP2
DNA topoisomerase 2
- VCP
valosin-containing protein
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Author contributions
RLS, IGC and CAA participated in research design, RLS conducted experiments. RLS, IGC and CAA wrote the manuscript.
References
- Acs K, Luijsterburg MS, Ackermann L, Salomons FA, Hoppe T, Dantuma NP. The AAA-ATPase VCP/p97 promotes 53BP1 recruitment by removing L3MBTL1 from DNA double-strand breaks. Nat Struct Mol Biol. 2011;18(12):1345–1350. doi: 10.1038/nsmb.2188. [DOI] [PubMed] [Google Scholar]
- Alchanati I, Teicher C, Cohen G, Shemesh V, Barr HM, Nakache P, Ben-Avraham D, Idelevich A, Angel I, Livnah N, Tuvia S, et al. The E3 ubiquitin-ligase Bmi1/Ring1A controls the proteasomal degradation of Top2alpha cleavage complex-a potentially new drug target. PLoS One. 2009;4(12):e8104. doi: 10.1371/journal.pone.0008104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakirev MY, Mullally JE, Favier A, Assard N, Sulpice E, Lindsey DF, Rulina AV, Gidrol X, Wilkinson KD. Wss1 metalloprotease partners with Cdc48/Doa1 in processing genotoxic SUMO conjugates. Elife. 2015;4 doi: 10.7554/eLife.06763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beskow A, Grimberg KB, Bott LC, Salomons FA, Dantuma NP, Young P. A Conserved Unfoldase Activity for the p97 AAA-ATPase in Proteasomal Degradation. Journal of Molecular Biology. 2009;394(4):732–746. doi: 10.1016/j.jmb.2009.09.050. [DOI] [PubMed] [Google Scholar]
- Bromberg KD, Burgin AB, Osheroff N. A two-drug model for etoposide action against human topoisomerase IIalpha. J Biol Chem. 2003;278(9):7406–7412. doi: 10.1074/jbc.M212056200. [DOI] [PubMed] [Google Scholar]
- Cortes Ledesma F, El Khamisy SF, Zuma MC, Osborn K, Caldecott KW. A human 5’-tyrosyl DNA phosphodiesterase that repairs topoisomerase-mediated DNA damage. Nature. 2009;461(7264):674–678. doi: 10.1038/nature08444. [DOI] [PubMed] [Google Scholar]
- Cowell IG, Austin CA. Visualization and Quantification of Topoisomerase-DNA Covalent Complexes Using the Trapped in Agarose Immunostaining (TARDIS) Assay. Methods Mol Biol. 2018;1703:301–316. doi: 10.1007/978-1-4939-7459-7_21. [DOI] [PubMed] [Google Scholar]
- Dantuma NP, Groothuis TAM, Salomons FA, Neefjes J. A dynamic ubiquitin equilibrium couples proteasomal activity to chromatin remodeling. The Journal of Cell Biology. 2006;173(1):19–26. doi: 10.1083/jcb.200510071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duxin JP, Dewar JM, Yardimci H, Walter JC. Repair of a DNA-protein crosslink by replication-coupled proteolysis. Cell. 2014;159(2):346–357. doi: 10.1016/j.cell.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan JR, Peng AL, Chen HC, Lo SC, Huang TH, Li TK. Cellular processing pathways contribute to the activation of etoposide-induced DNA damage responses. DNA Repair (Amst) 2008;7(3):452–463. doi: 10.1016/j.dnarep.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Fielden J, Wiseman K, Torrecilla I, Li S, Hume S, Chiang SC, Ruggiano A, Narayan Singh A, Freire R, Hassanieh S, Domingo E, et al. TEX264 coordinates p97- and SPRTN-mediated resolution of topoisomerase 1-DNA adducts. Nat Commun. 2020;11(1):1274. doi: 10.1038/s41467-020-15000-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Schellenberg MJ, Huang SY, Abdelmalak M, Marchand C, Nitiss KC, Nitiss JL, Williams RS, Pommier Y. Proteolytic degradation of topoisomerase II (Top2) enables the processing of Top2.DNA and Top2.RNA covalent complexes by tyrosyl-DNA-phosphodiesterase 2 (TDP2) J Biol Chem. 2014;289(26):17960–17969. doi: 10.1074/jbc.M114.565374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartsuiker E, Neale MJ, Carr AM. Distinct requirements for the Rad32(Mre11) nuclease and Ctp1(CtIP) in the removal of covalently bound topoisomerase I and II from DNA. Mol Cell. 2009;33(1):117–123. doi: 10.1016/j.molcel.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Zhu Q, Wani G, Wani AA. UV-induced proteolysis of RNA polymerase II is mediated by VCP/p97 segregase and timely orchestration by Cockayne syndrome B protein. Oncotarget. 2017;8(7):11004–11019. doi: 10.18632/oncotarget.14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger JB, Voigt A, Borisova ME, Petrosino G, Ruf S, Wagner SA, Beli P. Proteomic profiling of VCP substrates links VCP to K6-linked ubiquitylation and c-Myc function. EMBO Rep. 2018;19(4) doi: 10.15252/embr.201744754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MB, Ji P, Anish R, Jacobson RH, Takada S. Poly(ADP-ribose) Polymerase 1 Interacts with Nuclear Respiratory Factor 1 (NRF-1) and Plays a Role in NRF-1 Transcriptional Regulation. J Biol Chem. 2009;284(13):8621–8632. doi: 10.1074/jbc.M807198200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Wang L, Wang Y, Ji J, Li J, Wang Z, Li C, Zhang Y, Zhang ZR. RNF126-Mediated Reubiquitination Is Required for Proteasomal Degradation of p97-Extracted Membrane Proteins. Mol Cell. 2020;79(2):320–331.:e329. doi: 10.1016/j.molcel.2020.06.023. [DOI] [PubMed] [Google Scholar]
- Lafon A, Taranum S, Pietrocola F, Dingli F, Loew D, Brahma S, Bartholomew B, Papamichos-Chronakis M. INO80 Chromatin Remodeler Facilitates Release of RNA Polymerase II from Chromatin for Ubiquitin-Mediated Proteasomal Degradation. Molecular Cell. 2015;60(5):784–796. doi: 10.1016/j.molcel.2015.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen NB, Gao AO, Sparks JL, Gallina I, Wu RA, Mann M, Raschle M, Walter JC, Duxin JP. Replication-Coupled DNA-Protein Crosslink Repair by SPRTN and the Proteasome in Xenopus Egg Extracts. Mol Cell. 2019;73(3):574–588.:e577. doi: 10.1016/j.molcel.2018.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KC, Bramley RL, Cowell IG, Jackson GH, Austin CA. Proteasomal inhibition potentiates drugs targeting DNA topoisomerase II. Biochem Pharmacol. 2016;103:29–39. doi: 10.1016/j.bcp.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KC, Padget K, Curtis H, Cowell IG, Moiani D, Sondka Z, Morris NJ, Jackson GH, Cockell SJ, Tainer JA, Austin CA. MRE11 facilitates the removal of human topoisomerase II complexes from genomic DNA. Biol Open. 2012;1(9):863–873. doi: 10.1242/bio.20121834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KC, Swan RL, Sondka Z, Padget K, Cowell IG, Austin CA. Effect of TDP2 on the Level of TOP2-DNA Complexes and SUMOylated TOP2-DNA Complexes. Int J Mol Sci. 2018;19(7) doi: 10.3390/ijms19072056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Raczynska JE, Chen Z, Yu H. Structural Insight into DNA-Dependent Activation of Human Metalloprotease Spartan. Cell Rep. 2019;26(12):3336–3346.:e3334. doi: 10.1016/j.celrep.2019.02.082. [DOI] [PubMed] [Google Scholar]
- Lopez-Mosqueda J, Maddi K, Prgomet S, Kalayil S, Marinovic-Terzic I, Terzic J, Dikic I. SPRTN is a mammalian DNA-binding metalloprotease that resolves DNA-protein crosslinks. 2016;5 doi: 10.7554/eLife.21491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnaghi P, D’Alessio R, Valsasina B, Avanzi N, Rizzi S, Asa D, Gasparri F, Cozzi L, Cucchi U, Orrenius C, Polucci P, et al. Covalent and allosteric inhibitors of the ATPase VCP/p97 induce cancer cell death. Nat Chem Biol. 2013;9(9):548–556. doi: 10.1038/nchembio.1313. [DOI] [PubMed] [Google Scholar]
- Mao Y, Desai SD, Ting CY, Hwang J, Liu LF. 26 S proteasome-mediated degradation of topoisomerase II cleavable complexes. J Biol Chem. 2001;276(44):40652–40658. doi: 10.1074/jbc.M104009200. [DOI] [PubMed] [Google Scholar]
- Martensson S, Nygren J, Osheroff N, Hammarsten O. Activation of the DNA-dependent protein kinase by drug-induced and radiation-induced DNA strand breaks. Radiat Res. 2003;160(3):291–301. doi: 10.1667/0033-7587(2003)160[0291:aotdpk]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Maskey RS, Flatten KS, Sieben CJ, Peterson KL, Baker DJ, Nam HJ, Kim MS, Smyrk TC, Kojima Y, Machida Y, Santiago A, et al. Spartan deficiency causes accumulation of Topoisomerase 1 cleavage complexes and tumorigenesis. Nucleic Acids Res. 2017;45(8):4564–4576. doi: 10.1093/nar/gkx107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerang M, Ritz D, Paliwal S, Garajova Z, Bosshard M, Mailand N, Janscak P, Hubscher U, Meyer H, Ramadan K. The ubiquitin-selective segregase VCP/p97 orchestrates the response to DNA double-strand breaks. Nat Cell Biol. 2011;13(11):1376–1382. doi: 10.1038/ncb2367. [DOI] [PubMed] [Google Scholar]
- Meyer HH, Shorter JG, Seemann J, Pappin D, Warren G. A complex of mammalian Ufd1 and Npl4 links the AAA-ATPase, p97, to ubiquitin and nuclear transport pathways. The EMBO Journal. 2000;19(10):2181–2192. doi: 10.1093/emboj/19.10.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosbech A, Gibbs-Seymour I, Kagias K, Thorslund T, Beli P, Povlsen L, Nielsen SV, Smedegaard S, Sedgwick G, Lukas C, Hartmann-Petersen R, et al. DVC1 (C1orf124) is a DNA damage-targeting p97 adaptor that promotes ubiquitin-dependent responses to replication blocks. Nat Struct Mol Biol. 2012;19(11):1084–1092. doi: 10.1038/nsmb.2395. [DOI] [PubMed] [Google Scholar]
- Muslimovic A, Nystrom S, Gao Y, Hammarsten O. Numerical analysis of etoposide induced DNA breaks. PLoS One. 2009;4(6):e5859. doi: 10.1371/journal.pone.0005859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MJ, Pan J, Keeney S. Endonucleolytic processing of covalent protein-linked DNA double-strand breaks. Nature. 2005;436(7053):1053–1057. doi: 10.1038/nature03872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan K, Bruderer R, Spiga FM, Popp O, Baur T, Gotta M, Meyer HH. Cdc48/p97 promotes reformation of the nucleus by extracting the kinase Aurora B from chromatin. Nature. 2007;450(7173):1258–1262. doi: 10.1038/nature06388. [DOI] [PubMed] [Google Scholar]
- Reinking HK, Kang HS, Gotz MJ, Li HY, Kieser A, Zhao S, Acampora AC, Weickert P, Fessler E, Jae LT, Sattler M, et al. DNA Structure-Specific Cleavage of DNA-Protein Crosslinks by the SPRTN Protease. Mol Cell. 2020;80(1):102–113.:e106. doi: 10.1016/j.molcel.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rycenga HB, Wolfe KB, Yeh ES, Long DT. Uncoupling of p97 ATPase activity has a dominant negative effect on protein extraction. Sci Rep. 2019;9(1):10329. doi: 10.1038/s41598-019-46949-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenberg MJ, Lieberman JA, Herrero-Ruiz A, Butler LR, Williams JG, Munoz-Cabello AM, Mueller GA, London RE, Cortes-Ledesma F, Williams RS. ZATT (ZNF451)-mediated resolution of topoisomerase 2 DNA-protein cross-links. 2017;357(6358):1412–1416. doi: 10.1126/science.aam6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingele J, Bellelli R, Alte F, Hewitt G, Sarek G, Maslen SL, Tsutakawa SE, Borg A, Kjaer S, Tainer JA, Skehel JM, et al. Mechanism and Regulation of DNA-Protein Crosslink Repair by the DNA-Dependent Metalloprotease SPRTN. Mol Cell. 2016;64(4):688–703. doi: 10.1016/j.molcel.2016.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingele J, Schwarz MS, Bloemeke N, Wolf PG, Jentsch S. A DNA-dependent protease involved in DNA-protein crosslink repair. Cell. 2014;158(2):327–338. doi: 10.1016/j.cell.2014.04.053. [DOI] [PubMed] [Google Scholar]
- Sun Y, Miller Jenkins LM, Su YP, Nitiss KC, Nitiss JL, Pommier Y. A conserved SUMO pathway repairs topoisomerase DNA-protein cross-links by engaging ubiquitin-mediated proteasomal degradation. Sci Adv. 2020;6(46) doi: 10.1126/sciadv.aba6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan RL, Poh LLK, Cowell IG, Austin CA. Small Molecule Inhibitors Confirm Ubiquitin-Dependent Removal of TOP2-DNA Covalent Complexes. Mol Pharmacol. 2020;98(3):222–233. doi: 10.1124/mol.119.118893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammaro M, Barr P, Ricci B, Yan H. Replication-dependent and transcription-dependent mechanisms of DNA double-strand break induction by the topoisomerase 2-targeting drug etoposide. PLoS One. 2013;8(11):e79202. doi: 10.1371/journal.pone.0079202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Boom J, Meyer H. VCP/p97-Mediated Unfolding as a Principle in Protein Homeostasis and Signaling. Mol Cell. 2018;69(2):182–194. doi: 10.1016/j.molcel.2017.10.028. [DOI] [PubMed] [Google Scholar]
- van den Boom J, Wolf M, Weimann L, Schulze N, Li F, Kaschani F, Riemer A, Zierhut C, Kaiser M, Iliakis G, Funabiki H, et al. VCP/p97 Extracts Sterically Trapped Ku70/80 Rings from DNA in Double-Strand Break Repair. Mol Cell. 2016;64(1):189–198. doi: 10.1016/j.molcel.2016.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz B, Popovic M, Newman JA, Fielden J, Aitkenhead H, Halder S, Singh AN, Vendrell I, Fischer R, Torrecilla I, Drobnitzky N, et al. Metalloprotease SPRTN/DVC1 Orchestrates Replication-Coupled DNA-Protein Crosslink Repair. Mol Cell. 2016;64(4):704–719. doi: 10.1016/j.molcel.2016.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R, Oania R, Fang R, Smith GT, Deshaies RJ. Cdc48/p97 mediates UV-dependent turnover of RNA Pol II. Mol Cell. 2011;41(1):82–92. doi: 10.1016/j.molcel.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmore E, Frank AJ, Padget K, Tilby MJ, Austin CA. Etoposide targets topoisomerase IIalpha and IIbeta in leukemic cells: isoform-specific cleavable complexes visualized and quantified in situ by a novel immunofluorescence technique. Molecular pharmacology. 1998;54(1):78–85. doi: 10.1124/mol.54.1.78. [DOI] [PubMed] [Google Scholar]
- Xu Q, Farah M, Webster JM, Wojcikiewicz RJ. Bortezomib rapidly suppresses ubiquitin thiolesterification to ubiquitin-conjugating enzymes and inhibits ubiquitination of histones and type I inositol 1,4,5-trisphosphate receptor. Mol Cancer Ther. 2004;3(10):1263–1269. [PubMed] [Google Scholar]
- Yu X, Davenport JW, Urtishak KA, Carillo ML, Gosai SJ, Kolaris CP, Byl JAW, Rappaport EF, Osheroff N, Gregory BD, Felix CA. Genome-wide TOP2A DNA cleavage is biased toward translocated and highly transcribed loci. Genome Res. 2017;27(7):1238–1249. doi: 10.1101/gr.211615.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Lyu YL, Lin CP, Zhou N, Azarova AM, Wood LM, Liu LF. A protease pathway for the repair of topoisomerase II-DNA covalent complexes. J Biol Chem. 2006;281(47):35997–36003. doi: 10.1074/jbc.M604149200. [DOI] [PubMed] [Google Scholar]
- Zhou HJ, Wang J, Yao B, Wong S, Djakovic S, Kumar B, Rice J, Valle E, Soriano F, Menon MK, Madriaga A, et al. Discovery of a First-in-Class, Potent, Selective, and Orally Bioavailable Inhibitor of the p97 AAA ATPase (CB-5083) J Med Chem. 2015;58(24):9480–9497. doi: 10.1021/acs.jmedchem.5b01346. [DOI] [PubMed] [Google Scholar]