Abstract
Copper is crucial for carrying out normal physiological functions in all higher life forms. Copper Transporter 1 (CTR1) is the high-affinity copper importer found in all eukaryotic organisms. The copper transporter family primarily comprises ~ six members (CTR1-6) and the related members share high sequence homology with CTR. However, with the exception of CTR1, not all six CTRs are present in every organism. Despite having a simple trimeric channel structure, CTR1 and other members exhibit some unique regulatory properties. In the present review, we attempt to understand the diversity and similarity of regulation and functioning of the members of this copper transporter family.
Keywords: Copper, Copper Transporter, CTR
Graphic abstract.
Introduction
Copper is essential for growth and development; however, in excess it is harmful. All eukaryotic organisms require copper for various metabolic activities. Copper serves as an essential cofactor for the activity of cytochrome C oxidase in mitochondria, an enzyme essential for respiration, and for Cu, Zn-superoxide dismutase that detoxifies oxygen radicals in the cytosol. In addition, copper is also intrinsically linked to the secretory pathway, where enzymes including dopamine-β-hydroxylase, peptidyl α-monooxygenase, ceruloplasmin, and tyrosinase incorporate copper as a cofactor in their catalytic sites (Lutsenko et al 2007). Key proteins maintaining optimum copper levels in mammalian cells include the homotrimeric plasma membrane copper importer, Copper Transporter 1 (CTR1), and ATP-driven copper export pumps, ATP7 in lower organisms that diverges as ATP7A and ATP7B in chordates (Gupta and Lutsenko 2012). CTR1 is the only high-affinity copper transporter that is primarily localized in the plasma membrane and draws copper from the extracellular space (Clifford et al 2016). CTR1 is a unique and interesting protein, not only because of its biological importance but also in the unconventional fashion that it is regulated. CTR1 belongs to a family of Copper Transporters that has at least six members (CTR1-6) among various organisms. Structural and functional divergence of the CTRs from lower organisms to mammals shed light on how the regulation of this protein family is modulated depending on the requirement and availability of copper. This review article from a phylogenetic viewpoint summarizes and critically analyses the advancements to understand the regulation of CTR1 that has been made over the last two decades.
Transcriptional and Post-Translational Regulation of CTR Family Proteins Exhibit Not only Evolutionary Divergence but also Co-Regulation
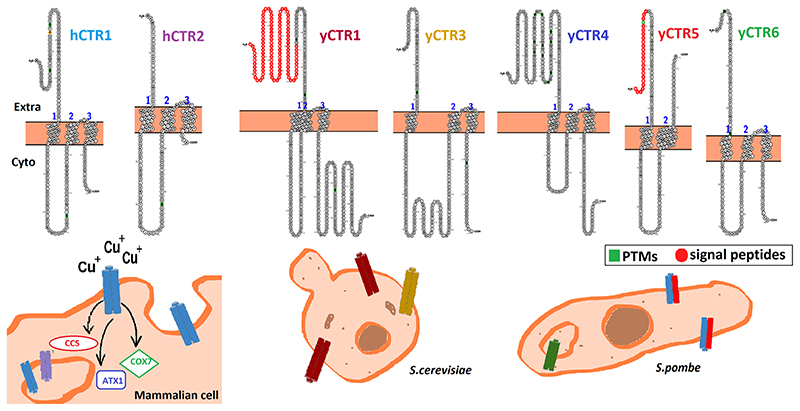
Prokaryotes lack CTR or CTR-like proteins. CTRs were first identified in yeast during genetic screens investigating defects of iron homeostasis (Dancis et al. 1994). Over the next 20 years, the entire family of CTRs was discovered and studied in various organisms. The six known CTR proteins are of variable lengths, from 150 up to 500 residues each with a seemingly characteristic arrangement into 4 major topological domains (1) an extracellular/luminal N-terminal domain, (2) membrane-spanning helices, (3) an intracellular loop between TM1 and TM2, and (4) a cytoplasmic C-ter-minal domain. Possibly meant to impart variation in copper binding and intracellular localization, the extracellular and luminal domains exhibit higher degree of sequence variation among different phyla as compared to the transmembrane domains (Feo et al. 2007).
The baker’s yeast, Saccharomyces cerevisiae, has two high-affinity plasma membrane (PM) residing Cu transporters Ctr1 and Ctr3 (Peña et al. 2000) and the low-affinity vacuolar copper transporter CTR2, that is tightly regulated by the copper-sensing transcription factor Mac1p (Liu et al 2012). Mac1p binding of the copper-responsive elements (CuREs) on the promoters of CTRs is regulated by copper ions. On one hand, the binding of Mac1p to CTR1 promoter is positively regulated by its copper-dependent phosphorylation and on the other hand, exogenous Cu+ disrupts the DNA binding of Mac1p (Heredia et al 2001). Authors hypothesized that Mac1p can sense two different levels of copper ions, physiological and toxic (high copper).
Although Macp1 transcriptionally regulates both proteins in a similar fashion, yet their modes of post-translational regulation differ in response to copper. Under high Cu concentration, Ctr1 undergoes rapid removal from the cell surface while steady-state levels of Ctr3 at the plasma membrane are maintained (Peña et al. 2000; Ooi et al. 1996). In S.cerevisiae Ctr1 is regulated by both endocytosis and proteolytic degradation.
Why does S. cerevisiae require two high-affinity functionally redundant copper transporters CTR1 and CTR3? Interestingly, the post-translational regulation of Ctr3 is distinct from Ctr1. Whereas Ctr1 is degraded rapidly in the presence of physiological levels of copper, the stability and localization of Ctr3 are not affected even by increased non-physiological copper levels. CTR1 is more prone to copper-mediated endocytosis than CTR3. It can be hypothesized that as an adaptive feature, CTR3 possibly evolved in yeast strains growing in severe copper-depleted conditions. In those copper limiting conditions, sustained uptake of copper was necessary that could not have been supported by the rapidly recycling CTR1 but a more stable plasma membrane located CTR3.
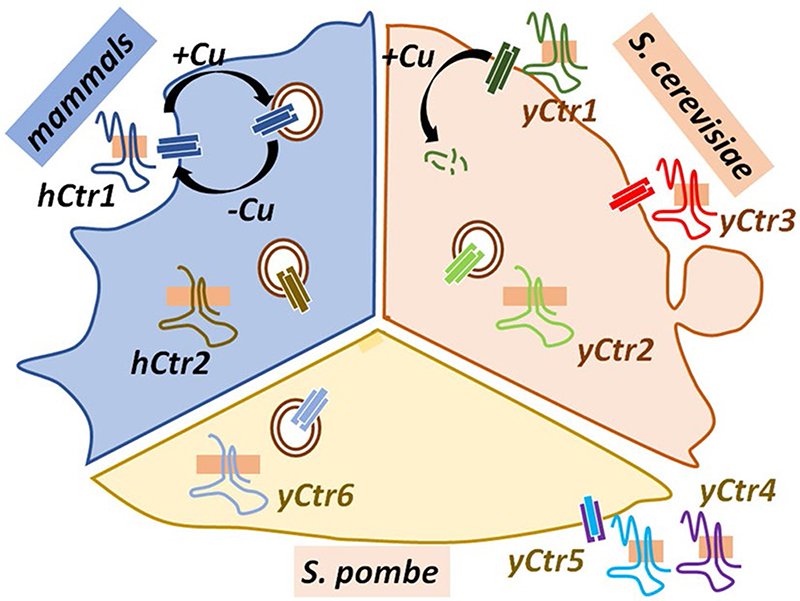
Yeasts exhibit an impressive variation in Copper Transporters in terms of structure and regulation. In Schizosac-charomyces pombe, copper uptake is carried out by the CTR4 and CTR5 proteins in a unique fashion (Ioannoni et al. 2010). In a complementation experiment, Zhou and Thiele showed that S.pombe CTR4 failed to complement CTR1–CTR3 null S.cerevisiae in copper-limiting conditions. However, selection of S. pombe genes, which, when coexpressed with CTR4, restores copper uptake in S. cerevisiae cells resulted in the discovery of CTR5 (Zhou and Thiele 2001). Interestingly, co-expression of CTR5 facilitated the localization of CTR4 to the cell surface that was otherwise mislocalized in an intracellular compartment. The fission yeast, unlike Baker’s yeast, acquires Cu with the help of a CTR heterotrimer consisting of a dimer of CTR4 and a monomer of CTR5. CTR4, a 298 residue long protein harbors five MXXMXM motifs exposed to the extracellular environment in its long N-terminal (Puig et al. 2002; Zhou and Thiele 2001). CTR5 is 173 residues long and structurally very similar to CTR4. Trafficking of the CTR4 and CTR5 to the plasma membrane are interdependent and essential for their functionality (Ioannoni et al. 2010). In contrast to homotrimerizing S. cerevisiae CTR1 and CTR3 and human CTR1, the CTR4 and CTR5 from S. pombe heterotrimerizes. This heterocomplex formation is crucial for their localization at the cell surface and copper import (Ioannoni et al. 2010). Interestingly, similar heteromeric associations between different CTRs have also been observed in higher organisms. Ohrvik and colleagues reported that CTR2 besides being a low-affinity copper transporter also regulates the functioning of Ctr1 to mobilize endosomal copper stores (Ohrvik et al. 2013). This will be elaborated in the later section.
Transcriptional regulation of CTR4 is similar to that of Mac1p-mediated regulation of CTR1–CTR3 in baker’s yeast. Both ctr4 and ctr5 had low basal levels of mRNA expression in rich media, which was further repressed by the addition of copper but increased multifold in copper-chelated conditions. It was further found that the copper-mediated transcription factor Cuf1 regulates the expression of both CTR4 and CTR5 (Zhou and Thiele 2001). Tight regulation of copper uptake at (a) transcriptional and (b) post-translational levels were vividly evident from these studies and signifies the importance of low margin of error in copper handling in early eukaryotes.
Ctr6, another Cu-transport protein found in S.pombe assembles as a trimer and harbors a functionally essential M-X-H-C-X-M motif, very similar to the metal-binding M-X2-M-X-M motif found in other CTRs. This homolog is thought to be involved in mobilizing Cu from vacuole to cytoplasm similar to the function of Ctr2 in S.cerevisiae (Bellemare et al. 2002). Interestingly, transcriptional regulation of CTR4 and CTR6 are linked. The expression of CTR6 is regulated by the promoter element CuSE (copper-signaling element) through its binding to the copper-induced transcription factor Cuf1. Loss of copper-dependent enzymatic activity was observed in CTR6 KO yeasts. Upon CTR6 over-expression, the cells fail to grow in copper excess media. Interestingly, this phenotype is not due to copper toxicity, but due to reduced copper uptake by the other copper transporter CTR4.
The three of these genes encoding ctr4, ctr5, and ctr6 are located under the same promoter CuSE and are transcriptionally regulated by the robust copper-sensing Transcription Factor Cuf1 (Ooi et al. 1996; Bellemare et al. 2002). In S.cerevisiae metalloregulatory transcription factors Ace1 and Mac1 maintain Cu homeostasis in a reciprocal manner. Ace1 gets activated upon sensing high levels of Cu while Mac1 binds Copper Response Elements (CuREs) and upregulates expression of CTR1, CTR3, and Fre1 proteins in Cu-depleted cells (Keller et al. 2005). In flowering plants, e.g., Arabidopsis thaliana, there are six (COPT1-6) known CTR family proteins out of which COPT1 and COPT2 are upregulated in Cu-depleted cells (Puig et al. 2007).
Interesting adaptive features of copper uptake and utilization have been observed in green algae. In low concentration or absence of dissolved oxygen, Cu(II) gets reduced into Cu(I) and metallic Cu and subsequently precipitates (Page et al. 2009). Green algae thriving in swamps show adaptations that enable them to live in hypoxic Cu-depleted environments. The green algae, Chlamydomonas expresses four copper transporters CTR1, CTR2, CTR3, and COPT1 among which only the first three undergo transcriptional upregulation by the Transcription factor CRR1, as a Cu-deficiency response (Page et al. 2009). In addition to the CTR signature motifs, Ctr1 and 2 have remarkably large N-terminal domains (380 and 490 residues long) harboring Cys and Met residues (CxxMxxMxxC-× 5/6-C motif) and fairly large (80 and 190 aa long) C-terminal domains rich in Cys and His residues. C-terminal Cys coordinates with Cu(I) under reducing conditions of the cytoplasm while Met residues, more resistant to oxidation, bind to extracellular Cu. Under hypoxic conditions, the presence of Cys residues increases the affinity of these transporters providing efficient uptake of available Cu. On the other hand, COPT1 has conventional Met–His-rich motif to ensure Cu uptake in a normoxic environment. Ctr3 in Chlamydomonas, a result of partial duplication of Ctr2, does not act as a typical Cu transporter. CTR3 lacks the characteristic transmembrane domains found in the transporters, suggesting that it may be a soluble protein; however, it requires further experimental evidence. In order to survive in Cu-depleted environment, the organism shows interesting adaptive features, i.e., (1) replacement of the major Cu-utilizing protein in Chla-mydomonas, plastocyanin by cytochrome c6; (2) usage of FeSOD and MnSODs instead of Cu/ZnSODs; (3) presence of Cys-rich high-affinity Cu transporters CTR1 and CTR2 to facilitate copper uptake. CTR-type transporters rich in Cys–Met motifs are also found in other green algae such as Volvox carteri and Chlorella, in social amoeba Dictyoste-lium discoideum (P80 protein) as well as pathogenic amoeba Acanthamoeba castellanii (Page et al. 2009).
In mammals, Ohrvik et al. demonstrated a unique mode of regulation of CTR1 by its homolog CTR2. Mammals regulate copper uptake and intracellular mobilization via cleavage of the copper-binding extracellular N-terminal domain of CTR1. Although full-length CTR1 is essential to import copper across the plasma membrane, cleavage of the N-terminal ectodomain is required for CTR1 to mobilize and utilize endosomal copper stores. The biogenesis of this truncated form of CTR1 requires the copper transporter 2 (Ctr2). The ctr2 knockout mice are defective in the production of truncated CTR1 and they exhibit increased tissue copper levels that primarily accumulate at intracellular foci, possibly as non-bioavailable deposits (Ohrvik et al., 2013). Table 1 summarizes the function and regulation of the members of the CTR family that has been characterized in various organisms.
Table 1. The members of the CTR family.
| CTR | Cellular localization | Function (low/high-affin-ity cu transport) | Organism(s) | Regulation (if known) | References |
|---|---|---|---|---|---|
| 1 | Cell membranenucleus(yeast) | High affinity | S.cerevisiae H.sapiens Sus scrofa A.thaliana Chlamydomonas reinhardtii | Endocytosis, proteolytic cleavage | Yeast: (Dancis et al. 1994) Yeast & human: (Puig et al. 2002) Human:(Zhou and Gitschier 1997) Mouse: (Lee et al. 2000) Arabidopsis: (Kampfenkel et al. 1995) Chlamydomonas: (Page et al. 2009) |
| 2 | Vacuolar membrane late endosome recycling endosome | Low affinity (high affinity in A.thaliana) | S.cerevisiae H.sapiens A.thaliana Chlamydomonas reinhardtii | - | Yeast & Arabidopsis: (Kampfenkel et al. 1995) Yeast: (Bellemare et al. 2002) Human: (Zhou and Gitschier 1997) Arabidopsis: (Puig et al. 2007) Chlamydomonas: (Page et al. 2009) |
| 3 | ER, cell membrane | High affinity | S.cerevisiae Chlamydomonas reinhardtii A. thaliana | - | Yeast: (Peña et al. 2000), (Puig et al. 2002) Arabidopsis: (Puig et al. 2007) Chlamydomonas: (Page et al. 2009) |
| 4 | Cell membrane | High affinity | S.pombe Cryptococcus neoformans | Transcriptional, Cuf1 TF, CuRE promoter | Yeast: (Zhou and Thiele 2001) Cryptococcus: (Waterman et al 2007) |
| 5 | Cell membrane ER, fungal-type vacuole | High affinity | S.pombe A.thaliana | Transcriptional, Cuf1 TF, CuRE promoter | Yeast: (Zhou and Thiele 2001) Arabidopsis: (Puig et al. 2007) |
| 6 | Vacuolar membrane, plasma membrane | Mobilizes (low affinity) (high affinity in A.thaliana) | S.pombe, A.thaliana | Transcriptional | Yeast: (Bellemare et al. 2002) Arabidopsis: (Puig et al. 2007) |
Minor Differences in Domain Organization Among CTR Members Allows their Functional Flexibility
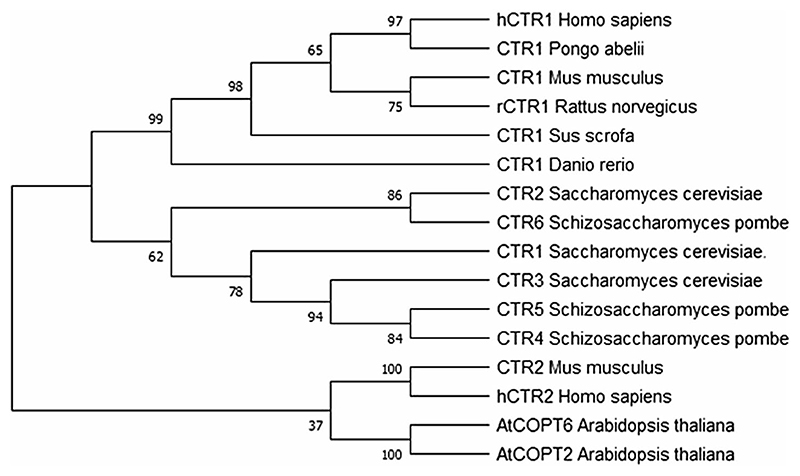
Phylogenetic analysis sheds light on the evolution and divergence of the CTR family members (Fig. 1). The prokaryotes, by virtue of their size and simplicity, do not require Cu-specific importers although they have Cu export pumps to prevent intracellular metal toxicity. Cu utilization is a feature of evolved metabolism which arose after the oxygenation of Earth (Ridge et al. 2008; Gupta and Lutsenko 2012). The phylogenetic study was carried out with sequences from different taxonomic units which are marked reviewed by the UniProt database. The final tree was inferred by the Maximum Likelihood method eliminating partitions with less than 50% bootstrap value. The initial tree was constructed by applying the Neighbor-Joining method on the distance matrix, built according to the JTT model for transmembrane proteins (Jones et al. 1992; Whelan and Goldman 2001; Felsenstein 1985). A discrete Gamma distribution was used to model evolutionary rate differences among sites (5 categories (+ G, parameter = 4.1831)). The rate variation model allowed for some sites to be evolutionarily invariable ([+ I], 0.80% sites). Evolutionary analyses were conducted in MEGA X (Kumar et al. 2018). The phylogram analysis suggests that the different homologs branched early in evolution and evolved independent of each other. In case of higher vertebrates, CTR homologs in a particular species share a lower sequence similarity than orthologs in related species, e.g., human CTR1 and CTR2 share a sequence identity and similarity of 33% and 50%, respectively, while hCTR1 shares a 86% identity and 88% similarity with pig(Sus scrofa) CTR1. Metazoan CTR2 is different from yCTR2 in both structure and origin but they execute a similar function of mobilizing Cu from vacuole to the cytoplasm, an example of evolutionary convergence.
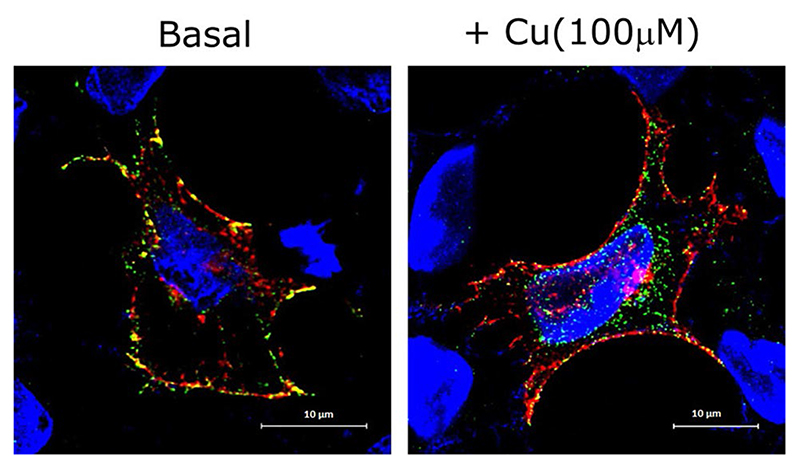
Fig. 2. CTR1 endocytosis in high copper.
Colocalization of wild-type FLAG-CTR1 (Alexa Fluor 488, green) with mCherry-tagged transferrin receptor (red) at the plasma membrane in HEK293T cell line at basal condition (left panel). High copper (100 μM) leads to internalization of FLAG-CTR1 (right panel). DNA was visualized by DAPI (Blue). Bar, 10 μm
A study showed that ctr2 is a result of gene duplication event that occurred during protostome–deuterostome divergence approximately 550 million years ago (Logeman et al. 2017). Logeman and colleagues proposed that ctr2 has undergone more frequent non-synonymous mutations than ctr1 and has lost its Cu-transport property (Logeman et al. 2017). It is known that CTR2 regulates Cu transport by mediating proteolytic cleavage of CTR1 by L-Cathepsin, an endosomal protease, discussed previously. To attain functional flexibility, neofunctionalization of ctr2 during metazoan evolution occurred by ‘gain of function’ mutations. The point mutations, L34F and K47E restored its Cu-transport activity. These two sites are highly conserved in CTR2 suggesting that selection pressure still operates on the current form of CTR2 incapable of Cu transport. Interestingly, Phylum Arthropoda lacks CTR2 altogether (Logeman et al. 2017). As shown in the tree, CTR6 in S.pombe is closely related to CTR2 of S.cerevisiae, 37% residues are identical within the 126 aa overlap between these two proteins (Bellemare et al. 2002). The presence of very low sequence similarity and their varying length point towards functional diversification. There is very little biochemical and structural information available on other CTR family proteins except mammalian CTR1 to further analyze their ancestry.
The amino-terminal sequence is the most variable among the CTRs of different species (Puig et al. 2002). Extensive studies have been done in the field of yeast Cu transporters, their functions, and sequence variability. Studies reflect that in most of the CTRs, the amino-terminal is rich in motifs containing 3–5 methionine residues arranged as MXXMXM, which coordinates the metal atoms, possibly for efficient uptake (Puig et al. 2002; Wu et al. 2009). However, it is not very clear whether the copper ions bound to the N-terminus are ultimately taken up by the transporter or copper binding to this domain only exposes the transmembrane pore enabling copper import. In other words, whether it physically brings the Cu ions close to the pore or just senses Cu availability or does both, is unclear.
Proteins having N-terminal Xxx-His (XH) and Xxx-Zzz-His (XZH, Z can be any amino acid except proline) motifs are known to bind Cu with high affinity, either in a tridentate fashion (3 N coordination), with three nitrogens from the N-terminal amino group (NH2), the first amide (N-) and the imidazole nitrogen at the delta ring position (Nδ), or a 4 N coordination in case of XZH, where Cu(II) can bind to four nitrogens: the N-terminal amine, the first two amides, and the Nδ imidazole nitrogen (Gonzalez 2018). For CTR1 and its other related family members, the abundance of His residues in the N-terminal can strongly implicate the coordination of Cu(II) in either the 3 N or 4 N coordination, based on the position of His and its neighboring residues that are somewhat variable across different metazoans (4 N complexes being more stable than 3 N complexes at physiological pH). The metal-binding affinities of both types of complexes are pH-dependent as it involves deprotonation of amide N. Comparison of Cu-binding affinities for various XZH complexes (X = Z = Asn, Phe, Arg, Lys, Val, Asp, Glu, Tyr, Thr) indicated side chains like Lys and Arg at positions 1 and 2 should increase the affinity of Cu(II), whereas negatively charged residues and H-bond acceptors (like Asp and Glu) would interact (through H-bonding) with the H-N of the amide bond, thereby preventing deprotonation of the amide N–H hence decreasing the affinity for Cu(II). This might shine some light on proposing and testing different copper-coordinated complexes in the N-terminal of several Copper-transporting proteins. The pathway leading to the formation of the thermodynamically stable 4 N complexes via a 2 N intermediate state has recently been reported in another study (Kotuniak et al. 2020). These pieces of information obtained might pave a way to the studies of kinetic and redox properties of ATCUN/NTS(N-terminal site) motifs in hCTR1 and its evolutionarily related counterparts, leading to a better explanation of the mechanism of copper delivery to cells (Kotuniak et al. 2020). Another thing to note is that Cu(I) cannot bind in the same coordination as Cu(II) in XZH, and Cu(I) binds only weakly to XZH. Thus it is very likely that once Cu(II) is reduced to Cu(I), it is going to be rapidly bound by other amino acids within protein (nearby bis-his motif present in hCTR1 and other metazoan species or methionine clusters conserved across all copper transporters) and the Cu-XZH coordination will be lost (Santoro 2017).
In higher eukaryotes, e.g., mammals, structural complexity compensated for the diminishing variety within CTR members of this Copper Transporter family. The presence of CTR3-6 has not been reported in mammals. It is evident that divergence in CTR structure and function is primarily driven by the variations of its copper-binding N-terminus.
Mouse CTR1 contains two Met clusters in the amino-terminal similar to the Human Ctr1, while Arabidopsis thaliana Ctr1 has only one such Met cluster (Kampfenkel et al. 1995; Lee et al. 2000; Zhou and Gitschier, 1997). The last Met residue of the amino-terminal, around 20 amino acids upstream of the first putative TMD, is considered as an important residue for Cu import (Puig et al. 2002). In yCTR1, substitution of this Met127 to Serine or Leucine or Alanine completely abolishes the Cu-import property of the protein. However, any substitution to Cysteine or Histidine restores or partially rescues the function, respectively (Puig et al. 2002). This implies that Cu ion coordination by Met127 residue is crucial for ion transport. Deletion of the amino-terminal Met residues in yeast does not affect the cell surface localization of the protein but decreases the Cu-import property (Puig et al. 2002; Wu et al. 2009). In vitro studies on model peptides have shown that in hCTR1 Cu(II) binds the Amino-Terminal Cu(II)- and Ni(II)-binding site (ATCUN), characterized by a free NH2 and a His residue at the 3rd position. Another spectroscopy-based (EPR, NMR, XAS) study suggests that Cu(II) directly binds bis-His, whereas Met7, 9, and 12 coordinates with Cu(I) (Pushie et al. 2015; Shenberger et al. 2018). Both the studies agree that the reduction of Cu(II) assisted by the microenvironment takes place somewhere near the N-term but the exact residue(s) involved in the reduction in vivo, has not been confirmed yet. Ascorbate-mediated reduction might be a possible hypothesis suggested by Schwab and group (Schwab et al. 2016). In Ctr1 large deletions in N-terminal and C-terminal do not abolish Cu uptake completely, although the former significantly reduces the rate of transport while the later leads to hypersensitivity (Puig et al. 2002; Wu et al. 2009).
The transmembrane (TM) helices form the pore of the CTR proteins, which are essential for their channel-like function. Most of the structural similarity among the CTR family proteins lies in TM2 (with 20–40% identity) and TM3 helices. The highly conserved (> 90%) signature motif of CTR family proteins, MxxM-× 12-GxxxG stretches from the extracellular part of TM2 to mid-TM3 helix in hCTR1. In yCTR1, the MXXXM motif is also located in this region at the interface of the monomers (Puig et al. 2002). This MXXXM motif is conserved throughout different taxonomic units (yeast, Arabidopsis, Drosophila, mouse, rat, human) and performs a crucial role in copper coordination in the TM region (Dancis et al. 1994; Kampfenkel et al. 1995; Labbé et al. 1999; Zhou and Gitschier 1997; Zhou and Thiele 2001). In yeast Ctr1, there are two such motifs in the TMD2, M250XXXM254 and M256XXXM260and between them the later one is critical and located closer to the extracellular milieu. Yeast complementation study of the combination mutations and their effect on growth and import property suggests that M260 is essential for the function of yCtr1. Yeast Ctr3 also contains this crucial motif in the TMD2 as M185XXXM189. Mutagenesis and expression studies of the mutants in different conditions indicate the important role of the Methionine residues in Ctr3 function, similar to hCtr1 M150XXXM154 (Puig et al. 2002; Feo et al. 2009; Rosen-zweig 2000). This particular motif resides closer to the luminal surface and probably helps in the primary acquisition of Cu(I) in the pore. The GxxxG motif appears to be a residual of the Glycine Zipper motif (GxxxGxxxG) found in many transmembrane proteins. This motif is responsible for close packing of neighboring helices in a right-handed direction and mutation in this highly conserved sequence often leads to disruption of the pore structure (Kim et al. 2005). C-α trace model of CTR1 transmembrane helices suggests that this motif indeed helps in close packing. Moreover, computational studies predicted that this can serve as a hinge region conferring conformational flexibility (Schushan 2010). Although this motif is vital for proper folding as well as Cu import, replacement of Glycine by other smaller side-chain amino acids like Ala or Ser does not impair the function of this transporter, whereas a Cys residue at this position is intolerable (Feo et al. 2007). The last portion of TM3 of hCTR1 towards the cytoplasmic domain has a few aromatic residues, they stabilize Cu(I) by electrostatic interaction and can give rise to bends in other helices (Feo et al. 2007; Guo et al. 2004).
In yCTR1, the C-terminal cytosolic tail blocks excess Cu import with the help of several Cys residues and a dileucine motif. C-terminal tails of CTR4 and CTR5 are much shorter than that of yCTR1, CTR4 lacks Cys residues while CTR5 has a Cys–Cys motif. CTR4–CTR5 complex undergoes internalization so the C-terminal might not play a role in gating (Waterman et al 2007). The intracellular 15 amino acid C-terminal tail of hCTR1 harbors the terminal His–Cys–His motif, relaying copper to cytosolic copper chaperons (Feo et al. 2009). Although conserved in CTR1 this motif is absent from other CTR family proteins which indicate the functional diversification as well as different modes of copper delivery to the metalloproteins. The uniqueness of copper uptake mechanism by different CTRs owing to their structural and trafficking differences is still open for research.
Copper Import and Recycling of CTR1 are Intrinsically Linked
Trafficking and copper-mediated regulation of CTR1 have not been fully understood. Under normal physiological copper conditions, in an unpolarized mammalian cell, CTR1 is primarily localized on the plasma membrane (Fig. 2). However, in the case of polarized epithelia with distinct apical–basolateral domains contradicting results have been reported.
Fig. 3. CTR family in Mammals, Saccharomyces cerevisiae and Schizosaccharomyces pombe.
Mammalian CTR1 resides on the plasma membrane in basal condition and transports copper to the copper chaperons, ATOX1, CCS, and COX1. hCTR2 localizes on endosomal membranes. In S. cerevisiae, both yCTR1 and yCTR3 serve as plasma membrane copper importers. In S. pombe, yCTR4 and yCTR5 heterotrimerizes to form a functional channel and localize at the plasma membrane. yCtr6 localizes on endosomal membrane in all copper conditions. The N-terminal regions of the CTRs of different species impart function variability (variable regions of this domain are shown in different colors)
Using immunofluorescence and surface biotinylation in the mouse intestine, Nose et al (2010) have shown that CTR1 localizes on the apical membrane on the intestinal lumen and directly uptakes copper from the diet (Nose et al. 2010). However, previously Zimnicka et al. have reported that CTR1 localizes on the basolateral membrane of polarized MDCK and OK cells (Zimnicka et al. 2007). They further propose a model of intestinal copper uptake in which basolateral CTR1 plays a key role in the physiologically important delivery of copper from blood to intracellular proteins, whereas copper uptake from the diet is possibly indirect or through a different uncharacterized transporter.
Besides plasma membrane, a small fraction of CTR1 is also localized on the vesicular membrane. Barring a few, studies on endogenous protein have been limited by the lack of antibodies against CTR1. Most of the studies have been carried out in epitope-tagged CTR1, overexpressed in mammalian cells. It is likely that localization of the protein in the host cell lines has been partly influenced by tagged epitopes. In studies from our laboratory, we found that C-terminal linked GFP tagged CTR1 fails to fold properly and localizes at the Endoplasmic Reticulum membranes in HEK293T cells (unpublished). However, the same construct was functional in COS-7 cells (Bertinato et al. 2010).
Despite prevailing contradictions on localization of CTR1 in apical vs basolateral membrane under basal or copper-limiting conditions, a consensus has been reached on copper-induced recycling of the protein. Upon copper treatment, CTR1 endocytoses to Early Endosomal Antigen (EEA1) and Rab5 marked compartments. However, it is difficult to differentiate between anterograde and retrograde vesicles. Upon extracellular removal of copper, the protein recycles back to the plasma membrane via the slower recycling endosomes marked by Rab11. No noticeable pool of CTR1 gets accumulated in the late endosomal or lysosomal compartments.
There has been no clear consensus as to whether localization of CTR1 is dictated by extracellular or intracellular copper concentration. The main reason for such a contention is the metal-binding domains of the protein. The extracellular domain, i.e., 67-amino acid long N-terminus of CTR1 contains Histidine and Methionine-rich motifs, similar to other ion transport proteins. Glycosylation signatures are found at Asp15 (N-linked glycosylation) and Thr27 (O-linked glycosylation). Though glycosylation is not directly linked to Cu uptake by CTR1 (Maryon et al. 2007; Tsigelny et al. 2012), it might have a regulatory role in CTR1 trafficking. Human CTR1 amino terminus contains a total of 10 Methionine and 8 Histidine residues forming 4 important clusters which are plausible Cu-interacting sites. There are two Histidine clusters—H1 (contains H3–H6), H2 (contains H22–H24) and two Methionine clusters—M1 (contains M7–M12) and M2 (contains M40–M45). The amino-terminal clustered motifs may have a role in interacting with the Cu ions (Eisses and Kaplan 2002; Larson et al. 2010; Puig et al. 2002) and the N-terminal being dynamic in nature, may fold or unfold in order to bring the ion close to the transmembrane pore but possibly do not play a direct role in the trafficking to PM or copper-induced endocytosis (Guo et al. 2004). Deletion of H1, M1, and H2 did not seem to have any appreciable effect on Cu-induced trafficking in comparison to WT. Under low copper conditions, M2-del mutant shows decreased endo-cytosis but under high Cu conditions, it necessarily behaves similarly to the WT (Eisses and Kaplan 2002; Guo et al. 2004; Puig et al. 2002). The small cytosolic tail of CTR1 is crucial for restricted Cu ion entry into the cell and most of the mutations in this tail increase the Cu uptake rate. But these mutants are unable to perform Cu-induced endocytosis which clearly implies the regulatory role of C-termini in the endocytosis of the protein (Maryon et al. 2013; Tsigelny et al. 2012).
Besides the N and C-termini, the 2nd transmembrane helix harbors the Met150 and Met154 couple that has been shown to be critical for CTR1 endocytosis. In the functional trimeric structure of the protein, within the TM domain, the methionine triad constructs the selectivity filter for the passage of the Cu ion through the pore, into the cell. Interaction of Cu ion with these Met residues is of great importance in the import of the ion as well as endocytosis of the protein (Guo et al. 2004; Klomp et al. 2002). However, mutating any of the Met residues to Cys or His can maintain the ion interaction and allow robust Cu uptake but substitution by Ala or Leu completely abolishes its function. Loss of either one/two methionines of the Met triad completely arrests copper-induced endocytosis (Guo et al. 2004; Liang et al. 2009; Maryon et al. 2013).
H139 on TM2 is of high significance as it participates in the formation of the functional trimeric structure of the protein. H139R mutation alters the pore structure, prevents Cu import as well as prevents endocytosis (Klomp et al. 2002; Maryon et al. 2013; Tsigelny et al. 2012). From the repertoire of data available on mutations, it can be easily implied that any Cu-import property regulates endocytosis and recycling of the protein. However, a point to be noted is that the majority of the mutant phenotypes were studied in the unpolarized cells. Studies in polarized cells will provide much more intricate information on copper-mediated localization of the protein within the cell.
Figure 3 summarizes the localization of the members copper transporter family in mammals and the two most-studied yeast species.
In humans, to have a better insight of the copper physiology and its associated diseases, it is crucial to gather a vivid understanding of how the ion is up-taken in the cells. The present review aims to provide a clearer perspective of its function and regulation in not only an isolated phyla but also how its functional convergence and structural divergence have shaped the intricate and efficient handling of copper along the course of evolution.
Fig. 1. Phylogenetic analysis of CTR proteins.
The evolutionary history was inferred by using the Maximum Likelihood method and the JTT matrix-based model (Jones et al. 1992; Whelan and Goldman 2001). The bootstrap consensus tree inferred from 500 replicates is taken to represent the evolutionary history of the taxa analyzed. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) are shown next to the branches (Felsenstein 1985)
Acknowledgements
This work was supported by Wellcome Trust India Alliance Fellowship (IA/I/16/1/502369) and Early Career Research Award (ECR/2015/000220) from SERB, Department of Science and Technology (DST), Government of India and IISER K intramural funding to AG. SM and SK were supported by Predoctoral fellowships from Council of Scientific and Industrial Research, India. TM and SS were supported by KVPY fellowship from the Govt. of India.
References
- Bellemare DR, Shaner L, Morano KA, Beaudoin J, Langlois R, Labbe S. Ctr6, a vacuolar membrane copper transporter in Schizosaccharomyces pombe. J Biolo Chem. 2002;277(48):46676–46686. doi: 10.1074/jbcM206444200. [DOI] [PubMed] [Google Scholar]
- Bertinato J, Cheung L, Hoque R, Plouffe LJ. Ctr1 transports silver into mammalian cells. J Trace Elements in Medicine and Biology : Organ of the Society for Minerals and Trace Elements (GMS) 2010;24(3):178–184. doi: 10.1016/Jjtemb.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Clifford RJ, Maryon EB, Kaplan JH. Dynamic internalization and recycling of a metal ion transporter: Cu homeostasis and CTR1, the human Cu+uptake system. J Cell Sci. 2016;129(8):1711–1721. doi: 10.1242/jcs173351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancis A, Yuan DS, Haile D, Askwith C, Eide D, Moehle C, Kaplan J, Klausner RD. Molecular characterization of a copper transport protein in S cerevisiae: an unexpected role for copper in iron transport. Cell. 1994;76(2):393–402. doi: 10.1016/0092-8674(94)90345-x. [DOI] [PubMed] [Google Scholar]
- Eisses JF, Kaplan JH. Molecular characterization of hCTR1, the human copper uptake protein. The J Biol Chem. 2002;277(32):29162–29171. doi: 10.1074/jbcM203652200. [DOI] [PubMed] [Google Scholar]
- De Feo CJ, Aller SG, Unger VM. A structural perspective on copper uptake in eukaryotes. Biometals. 2007;20(3-4):705–716. doi: 10.1007/s10534-006-9054-7. [DOI] [PubMed] [Google Scholar]
- De Feo CJ, Aller SG, Siluvai GS, Blackburn NJ, Unger VM. Three-dimensional structure of the human copper transporter hCTR1. Proc Natl Acad Sci USA. 2009;106(11):4237–4242. doi: 10.1073/pnas0810286106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. CONFIDENCE LIMITS ON PHYLOGENIES: AN APPROACH USING THE BOOTSTRAP. Evolution. 1985;39(4):783–791. doi: 10.1111/J1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez P, Bossak K, Stefaniak E, Hureau C, Raibaut L, Bal W, Faller P. N-Terminal Cu-Binding Motifs (Xxx-Zzz-His, Xxx-His) and Their Derivatives: Chemistry, Biology and Medicinal Applications. Chemistry. 2018;24(32):8029–8041. doi: 10.1002/chem201705398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Smith K, Lee J, Thiele DJ, Petris MJ. Identification of methionine-rich clusters that regulate copper-stimulated endocy-tosis of the human Ctr1 copper transporter. The J Biolo Chem. 2004;279(17):17428–17433. doi: 10.1074/jbcM401493200. [DOI] [PubMed] [Google Scholar]
- Gupta A, Lutsenko S. Evolution of copper transporting ATPases in eukaryotic organisms. Curr Genomics. 2012;13(2):124–133. doi: 10.2174/138920212799860661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia J, Crooks M, Zhu Z. Phosphorylation and Cu+ coordination-dependent DNA binding of the transcription factor Mac1p in the regulation of copper transport. The J Biolo Chem. 2001;276(12):8793–8797. doi: 10.1074/jbcM008179200. [DOI] [PubMed] [Google Scholar]
- Ioannoni R, Beaudoin J, Mercier A, Labbé S. Copper-dependent trafficking of the Ctr4-Ctr5 copper transporting complex. PLoS ONE. 2010;5(8):e11964. doi: 10.1371/journalpone.0011964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Computer Applications in the Biosciences: CABIOS. 1992;8(3):275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- Kampfenkel K, Kushnir S, Babiychuk E, Inzé D, VanMontagu M. Molecular characterization of a putative Arabidopsis thaliana copper transporter and its yeast homolog. The J Biolo Chem. 1995;270(47):28479–28486. doi: 10.1074/jbc270.47.28479. [DOI] [PubMed] [Google Scholar]
- Keller G, Bird A, Winge DR. Independent metalloregulation of Ace1 and Mac1 in Saccharomyces cerevisiae. Eukaryot Cell. 2005;4(11):1863–1871. doi: 10.1128/Ec4.11.1863-1871.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Jeon TJ, Oberai A, Yang D, Schmidt JJ, Bowie JU. Transmembrane glycine zippers: physiological and pathological roles in membrane proteins. Proc Natl Acad Sci USA. 2005;102(40):14278–14283. doi: 10.1073/pnas0501234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klomp AE, Tops BB, Van Denberg IE, Berger R, Klomp LW. Biochemical characterization and subcellular localization of human copper transporter 1 (hCTR1) Biochem J. 2002;364(Pt 2):497–505. doi: 10.1042/BJ20011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotuniak R, Strampraad M, Bossak-Ahmad K, Wawrzyniak UE, Ufnal-ska I, Hagedoorn PL, Bal W. Key Intermediate Species Reveal the Copper(II)-Exchange Pathway in Biorelevant ATCUN/ NTS Complexes. Angew Chem. 2020;59(28):11234–11239. doi: 10.1002/anie.202004264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol Biol Evol. 2018;35(6):1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé S, Peña MM, Fernandes AR, Thiele DJ. A copper-sensing transcription factor regulates iron uptake genes in Schizosaccharomyces pombe. The J Biol Chem. 1999;274(51):36252–36260. doi: 10.1074/jbc274.51.36252. [DOI] [PubMed] [Google Scholar]
- Larson CA, Adams PL, Jandial DD, Blair BG, Safaei R, Howell SB. The role of the N-terminus of mammalian copper transporter 1 in the cellular accumulation of cisplatin. Biochem Pharmacol. 2010;80(4):448–454. doi: 10.1016/Jbcp.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Prohaska JR, Dagenais SL, Glover TW, Thiele DJ. Isolation of a murine copper transporter gene, tissue specific expression and functional complementation of a yeast copper transport mutant. Gene. 2000;254(1-2):87–96. doi: 10.1016/s0378-1119(00)00287-0. [DOI] [PubMed] [Google Scholar]
- Liang ZD, Stockton D, Savaraj N, TienKuo M. Mechanistic comparison of human high-affinity copper transporter 1-mediated transport between copper ion and cisplatin. Mol Pharmacol. 2009;76(4):843–853. doi: 10.1124/mol109.056416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Qi J, Yang Z, Peng L, Li C. Low-affinity copper transporter CTR2 is regulated by copper-sensing transcription factor Mac1p in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 2012;420(3):600–604. doi: 10.1016/Jbbrc2012.03.040. [DOI] [PubMed] [Google Scholar]
- Logeman BL, Wood LK, Lee J, Thiele DJ. Gene duplication and neofunctionalization in the evolutionary and functional divergence of the metazoan copper transporters Ctr1 and Ctr2. The J Biol Chem. 2017;292(27):11531–11546. doi: 10.1074/jbcM117.793356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY. Function and regulation of human copper-transporting ATPases. Physiol Rev. 2007;87(3):1011–1046. doi: 10.1152/physrev00004.2006. [DOI] [PubMed] [Google Scholar]
- Maryon EB, Molloy SA, Ivy K, Yu H, Kaplan JH. Rate and regulation of copper transport by human copper transporter 1 (hCTR1) The J Biolo Chem. 2013;288(25):18035–18046. doi: 10.1074/jbcM112.442426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose Y, Wood LK, Kim BE, Prohaska JR, Fry RS, Spears JW, Thiele DJ. Ctr1 is an apical copper transporter in mammalian intestinal epithelial cells in vivo that is controlled at the level of protein stability. J Biol Chem. 2010;285(42):32385–32392. doi: 10.1074/jbcM110.143826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhrvik H, Nose Y, Wood LK, Kim BE, Gleber SC, Ralle M, Thiele DJ. Ctr2 regulates biogenesis of a cleaved form of mammalian Ctr1 metal transporter lacking the copper- and cisplatin-binding ecto-domain. Proc Natl Acad Sci USA. 2013;110(46):E4279–E4288. doi: 10.1073/pnas1311749110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi CE, Rabinovich E, Dancis A, Bonifacino JS, Klausner RD. Copper-dependent degradation of the Saccharomyces cerevisiae plasma membrane copper transporter Ctr1p in the apparent absence of endocytosis. EMBO J. 1996;15(14):3515–3523. [PMC free article] [PubMed] [Google Scholar]
- Page MD, Kropat J, Hamel PP, Merchant SS. Two Chlamydomonas CTR copper transporters with a novel cys-met motif are localized to the plasma membrane and function in copper assimilation. Plant Cell. 2009;21(3):928–943. doi: 10.1105/tpc108.064907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena MM, Puig S, Thiele DJ. Characterization of the Saccharomyces cerevisiae high affinity copper transporter Ctr3. J Biol Chem. 2000;275(43):33244–33251. doi: 10.1074/jbcM005392200. [DOI] [PubMed] [Google Scholar]
- Puig S, Lee J, Lau M, Thiele DJ. Biochemical and genetic analyses of yeast and human high affinity copper transporters suggest a conserved mechanism for copper uptake. J Biol Chem. 2002;277(29):26021–26030. doi: 10.1074/jbcM202547200. [DOI] [PubMed] [Google Scholar]
- Puig S, Andrés-Colás N, García-Molina A, Peñarrubia L. Copper and iron homeostasis in Arabidopsis: responses to metal deficiencies, interactions and biotechnological applications. Plant, Cell Environ. 2007;30(3):271–290. doi: 10.1111/J1365-3040.2Fph007.01642.x. [DOI] [PubMed] [Google Scholar]
- Pushie MJ, Shaw K, Franz KJ, Shearer J, Haas KL. Model Peptide Studies Reveal a Mixed Histidine-Methionine Cu(I) Binding Site at the N-Terminus of Human Copper Transporter 1. Inorg Chem. 2015;54(17):8544–8551. doi: 10.1021/acsinorgchem5b01162. [DOI] [PubMed] [Google Scholar]
- Ridge PG, Zhang Y, Gladyshev VN. Comparative genomic analyses of copper transporters and cuproproteomes reveal evolutionary dynamics of copper utilization and its link to oxygen. PLoS ONE. 2008;3(1):e1378. doi: 10.1371/journalpone.0001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig AC, O’Halloran TV. Structure and chemistry of the copper chaperone proteins. Curr Opin Chem Biol. 2000;4(2):140–147. doi: 10.1016/s1367-5931(99)00066-6. [DOI] [PubMed] [Google Scholar]
- Santoro A, Ewa Wezynfeld N, Vašák M, Bal W, Faller P. Cysteine and glutathione trigger the Cu-Zn swap between Cu(ii)-amyloid-β4-16 peptide and Zn7-metallothionein-3. Chem Com-mun (Camb) 2017;53(85):11634–11637. doi: 10.1039/c7cc06802f. [DOI] [PubMed] [Google Scholar]
- Schushan M, Barkan Y, Haliloglu T, Ben-Tal N. C(alpha)-trace model of the transmembrane domain of human copper transporter 1, motion and functional implications. Proc Natl Acad Sci USA. 2010;107(24):10908–10913. doi: 10.1073/pnas0914717107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab S, Shearer J, Conklin SE, Alies B, Haas KL. Sequence proximity between Cu(II) and Cu(I) binding sites of human copper transporter 1 model peptides defines reactivity with ascorbate and O2. J Inorg Biochem. 2016;158:70–76. doi: 10.1016/Jjinorgbio.2015.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenberger Y, Marciano O, Gottlieb HE, Ruthstein S. Insights into the N-terminal Cu(II) and Cu(I) binding sites of the human copper transporter CTR1. J Coord Chem. 2018;71(11-13):1985–2002. doi: 10.1080/00958972.2018.1492717. [DOI] [Google Scholar]
- Tsigelny IF, Sharikov Y, Greenberg JP, Miller MA, Kouznetsova VL, Larson CA, Howell SB. An all-atom model of the structure of human copper transporter 1. Cell Biochem Biophys. 2012;63(3):223–234. doi: 10.1007/s12013-012-9358-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman SR, Hacham M, Hu G, Zhu X, Park YD, Shin S, Panepinto J, Valyi-Nagy T, Beam C, Husain S, Singh N, et al. Role of a CUF1/CTR4 copper regulatory axis in the virulence of Cryptococcus neoformans. J Clin Investig. 2007;117(3):794–802. doi: 10.1172/JCI30006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan S, Goldman N. A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol Biol Evol. 2001;18(5):691–699. doi: 10.1093/oxfordjournalsmolbeva003851. [DOI] [PubMed] [Google Scholar]
- Wu X, Sinani D, Kim H, Lee J. Copper transport activity of yeast Ctr1 is down-regulated via its C terminus in response to excess copper. J Biol Chem. 2009;284(7):4112–4122. doi: 10.1074/jbcM807909200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Gitschier J. hCTR1: a human gene for copper uptake identified by complementation in yeast. Proc Natl Acad Sci USA. 1997;94(14):7481–7486. doi: 10.1073/pnas94.14.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Thiele DJ. Identification of a novel high affinity copper transport complex in the fission yeast Schizosaccharomy-ces pombe. J Biol Chem. 2001;276(23):20529–20535. doi: 10.1074/jbcM102004200. [DOI] [PubMed] [Google Scholar]
- Zimnicka AM, Maryon EB, Kaplan JH. Human copper transporter hCTR1 mediates basolateral uptake of copper into enterocytes: implications for copper homeostasis. J Biol Chem. 2007;282(36):26471–26480. doi: 10.1074/jbcM702653200. [DOI] [PubMed] [Google Scholar]