Abstract
Purpose
Pre-clinical data indicate that DNA methyltransferase inhibition will circumvent cisplatin resistance in various cancers.
Experimental Design
SPIRE comprised a dose escalation phase for incurable metastatic solid cancers, followed by a randomised dose expansion phase for neoadjuvant treatment of T2-4a N0 M0 bladder urothelial carcinoma. The primary objective was a recommended phase II dose (RP2D) for guadecitabine combined with gemcitabine and cisplatin (GC). Treatment comprised 21-day GC cycles (cisplatin 70 mg/m2, IV, day 8; gemcitabine 1000 mg/m2, IV, days 8+15). Guadecitabine was injected, SC, days 1-5, within escalation phase cohorts, and to half of 20 patients in the expansion phase. Registration: ISRCTN 16332228.
Results
Within the escalation phase, dose limiting toxicities related predominantly to myelosuppression requiring G-CSF prophylaxis from cohort 2 (guadecitabine 20 mg/m2, days 1-5). Commonest grade ≥3 adverse events in 17 dose escalation phase patients were neutropenia (76.5%); thrombocytopenia (64.7%) leukopenia (29.4%) and anaemia (29.4%). Addition of guadecitabine to GC in the expansion phase resulted in similar rates of severe haematological adverse events, similar cisplatin dose intensity, but modestly reduced gemcitabine dose intensity. Radical treatment options post-chemotherapy were not compromised. Pharmacodynamic evaluations indicated guadecitabine maximal target effect at the point of cisplatin administration. Pharmacokinetics were consistent with prior data. No treatment related deaths occurred.
Conclusions
The guadecitabine RP2D was 20 mg/m2, days 1-5, in combination with GC and requires GCSF prophylaxis. Gene promotor methylation pharmacodynamics are optimal with this schedule. Addition of guadecitabine to GC was tolerable, despite some additional myelosuppression, and warrants further investigation to assess efficacy.
Introduction
Urothelial carcinoma (UC) accounts for approximately 550,000 new diagnoses and 200,000 deaths annually.(1) Cisplatin based combination chemotherapy is the standard of care therapy for UC, for both radical peri-operative treatment, and as palliative first line treatment for advanced disease.(2–4) Standard regimens for UC combine cisplatin with gemcitabine (GC), or methotrexate, vinblastine and doxorubicin.(5) For metastatic UC, this results in a median survival and time to progressive disease of approximately 14 months and 7 months, respectively.(4) For locally advanced muscle invasive bladder cancer (MIBC), cisplatin based chemotherapy contributes an absolute survival advantage of 5-6% to overall cure rates.(3) Cisplatin resistance remains a critical barrier to therapeutic advance in UC.(6) For example, in a key randomised trial comparing cisplatin based regimens for advanced disease, by 3 years only 13% were alive and progression free and 17% had primary refractory disease.(7) UC progression or relapse is associated with a dismal prognosis. Second line immunotherapy, or chemotherapy, after prior platinum-based treatment, results in median survival outcomes under one year.(5)
Altered cancer gene expression may arise through structural genomic change, or as a result of reversible epigenetic regulation. Epigenetic control includes biochemical modifications, both to histone proteins within chromatin, and also to DNA itself.(8) DNA CpG di-nucleotide methylation is the most widely studied cancer epigenetic change. Dysregulation of CpG methylation in cancer cells, leads to genomic instability, activation of previously silent oncogenes or silencing of tumour suppressor genes (TSG). In solid malignancies, many genes undergo promoter hypermethylation. Hypermethylation reversal, for example through DNA methyltransferase inhibition, allows TSG re-expression with potential for anti-cancer therapy.
Abnormal DNA methylation patterns exist in UC, associated with disease phenotype (stage, grade, histology), and clinical outcomes.(9) Hyper- and hypo-methylation are associated respectively with invasive and non-invasive tumours, potentially through FOXA1 activation, indicating an epigenetic divergence, in addition to a genetic distinction, between lethal and non-lethal UC.(10–12) Various gene targets, microRNAs and mirtrons have been associated with cisplatin resistance and a poor prognosis when hypermethylated in UC.(13–16) An epigenetic field defect characterised by hypermethylation has also been described in normal bladder from UC patients that is hypothesised to predispose to carcinogenesis.(12) DNA methylation patterns are also linked to cisplatin resistance in pre-clinical UC models, and in other cancers, and have been validated in translational studies.(14,17–20) Cisplatin resistance through epigenetic mechanisms may be associated with specific marks, such as HOXA9 promoter methylation, and to cell subset phenotype such as ‘stemness’ of a the UC stem cell population.(18,21) Furthermore, genetic silencing in pre-clinical models, resulting from acquired cisplatin resistance, has been demonstrated to be reversible through DNA methyltransferase inhibition with reinstatement of cisplatin responsiveness.(17,19,21,22) The DNA methyltransferase inhibitors decitabine, azacitidine and zebularine have single agent activity in multiple UC cell line and xenograft models.(22–27) Synergistic inhibition of cell proliferation, and reversal of cisplatin resistance, occurs with co-administration with cisplatin in UC cell line models.(19,21,26) Data also support investigation of a DNA hypomethylating agent with gemcitabine, including in UC.(21, 28–32)
Guadecitabine (SGI-110, Astex Pharmaceuticals) is a DNA methyltransferase inhibitor pro-drug, composed of a decitabine and deoxyguanosine dinucleotide to allow for optimised drug like properties. A maximum tolerated dose of 90 mg/m2 daily, on a 28 day cycle, for patients with myelodysplastic syndrome, was established in a first in human study, but was not reached in patients with acute myeloid leukaemia (AML).(33) DNA demethylation was dose dependent, but plateaued at 60 mg/m2 daily, for 5 days, which was designated as the biologically effective dose recommended for phase II development. Febrile neutropenia, pneumonia, thrombocytopenia, anaemia and sepsis were the most frequent grade ≥3 adverse events. Clinical activity was demonstrated in this setting from monotherapy doses as low as 6 mg/m2 for 5 days, and in a subsequent trial in AML.(33,34)
We hypothesised that cisplatin resistance might be reversed through co-administration with a DNA hypomethylating agent, such as guadecitabine. SPIRE was a phase Ib/IIa trial, in UC and other solid malignancies, to determine a safe dose and schedule of guadecitabine in combination with GC.
Patients And Methods
Study design
SPIRE was an open-label trial comprising a dose escalation phase Ib component for advanced solid cancers, followed by a randomised dose expansion phase IIa component as neoadjuvant treatment for MIBC. Patients eligible for the dose escalation phase had incurable, histologically or cytologically confirmed, locally advanced or metastatic, solid cancer, for which GC was clinically appropriate treatment. Any number of prior systemic chemotherapy lines were permitted.
The dose expansion included patients with T2-4a N0 M0 MIBC, planned for neoadjuvant GC prior to a planned radical cystectomy. Key inclusion criteria for both phases included Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1, 16 years or older, glomerular filtration rate (GFR) estimation ≥60 ml/min, haemoglobin ≥ 90 g/L, neutrophil count ≥1.5 x109/L, platelets ≥100 x109/L, bilirubin ≤ 1.5 x the institutional upper limit of normal (ULN), alanine transaminase and alkaline phosphatase ≤2.5 x ULN (ALP ≤5 x ULN if caused by liver or bone metastases) and life expectancy over 3 months. Key exclusion criteria included unresolved toxicities from prior therapy greater than Common Terminology Criteria for Adverse Events (CTCAE) v4.03 grade 1 (except alopecia), prior radiotherapy to >30% of bone marrow and major surgery, or an investigational medicinal product, within 30 days. Full eligibility criteria are within the protocol (supplementary appendices) and as previously described.(35) The study was undertaken in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines, and approved by North West Haydock Research Ethics Committee (15/NW/0936). Patients provided written informed consent.
Procedures
Baseline assessment included physical examination, full blood count, serum biochemistry (renal, liver and bone profiles) and GFR estimation. Disease evaluation was undertaken in accordance with local routine practice for the relevant cancer type. Treatment cycle assessments were as per the baseline visit, plus assessment of adverse events (CTCAE v4.03) and blood sampling for pharmacodynamics, and in the dose escalation phase, guadecitabine pharmacokinetics, analyses. Dose modifications for predefined adverse event parameters are described in the protocol (supplementary appendices).
In all patients, GC was given as a 21-day cycle (Supplementary Figure 1) with cisplatin 70 mg/m2 on day 8 and gemcitabine 1000 mg/m2 on days 8 and 15 by IV infusions. Supportive medication, including anti-emetics and an intravenous hydration schedule, were administered according to local institutional policy. Guadecitabine was administered by sub-cutaneous injection, preferably within the abdominal area. For the dose escalation phase, up to four dose level patient cohorts of guadecitabine were planned, of 20, 30, 45 and 60 mg/m2, on each of days 1 to 5, for up to 6 treatment cycles. In the randomised dose expansion phase, patients were allocated to GC chemotherapy alone, or in combination with guadecitabine at the recommended phase II dose (RP2D). Planned treatment duration was prospectively defined for either 3 or 4 cycles according to individual institutional practice.
For the dose escalation phase, an evaluable patient was defined as one that, during cycle 1, completed study assessments, received guadecitabine on days 1-5, and cisplatin and gemcitabine on day 8, and where applicable, at least one dose of G-CSF and/or had experienced a dose limiting toxicity (DLT). DLT was defined as any of the following occurring during cycle 1, if deemed definitely or probably related to treatment: >14 day delay in cycle 2 due to treatment induced toxicity; grade 4 neutropenia ≥7 days; grade 3 or 4 neutropenia and temperature ≥38.5°C; grade 3 or 4 neutropenia and bacteriologically proven sepsis; grade 4 thrombocytopenia ≥7 days; grade 3 thrombocytopenia and non-traumatic bleeding; other clinically significant grade ≥3 events except nausea or vomiting.(35) Dose level cohorts enrolled 3-6 evaluable patients with a modified Rolling 6 design.(36) Initial dose level cohorts did not include G-CSF prophylaxis, however, if DLTs occurred, specifically due to neutropenia or its complications, the protocol allowed for repeat of the current dose level cohort with G-CSF at 300μg, SC, daily, on days 15-21 in all future patients. If none of 3, or 1 of 6, patients experienced a DLT then escalation to the next dose cohort was permitted. If ≥2 patients experienced a DLT then this dose was deemed not tolerated. If ≤1 of 6 evaluable patients experienced a DLT and higher doses were not tolerable then this dose level was deemed the Maximum Tolerated Dose (MTD). The RP2D incorporated the MTD with consideration given by a Safety Review Committee (SRC) to the maximally biologically effective dose (MBED) based on circulating cell free DNA (cfDNA) LINE-1 promotor methylation and haemoglobin F (HbF) re-expression. Full criteria for dose escalation decisions, and determination of MTD and RP2D, are within the protocol (supplementary appendices) and are previously described.(35) The RP2D was expanded to include 6 patients with advanced UC. In the dose expansion phase, patients were randomly allocated (1:1) to GC alone, or combined with guadecitabine at the RP2D.
Translational blood samples for pharmacodynamic effect of guadecitabine were taken on days 1, 8 and 15 in the escalation phase and days 1 and 8 in the expansion phase. Promotor methylation status of LINE-1, NBL2, D4Z4, SAT2 and LTR12C was determined by EpigenDx (Hopkinton, MA) through pyrosequencing of bisulphite treated cfDNA and experimental details are provided in the supplementary appendices. HbF level, as a percentage of total haemoglobin, was determined by HPLC using the VARIANT II Hemoglobin Testing System (Bio-Rad, Hertfordshire, UK) according to the manufacturer’s instructions within a United Kingdom Accreditation Service accredited UK National Health Service Department of Haematology & Blood Transfusion.
Objectives and endpoints
The primary objective was to determine a guadecitabine RP2D when combined with GC for future investigation. Primary endpoints were the MTD based on defined criteria for DLT assessed by CTCAE v4.03 and the MBED based on circulating cfDNA LINE-1 methylation and HbF re-expression. Secondary endpoints included the toxicity profile (CTCAE v4.03) of guadecitabine combined with GC, including at the RP2D within a randomised comparison to GC alone, pharmacokinetics of guadecitabine when combined with GC, the pathological complete response (pCR) rate of bladder cancer patients enrolled in the dose expansion phase of the trial (the trial was not formally statistically powered for this) and selected pharmacodynamic endpoints.
Statistical analysis
Statistical analyses were specified a priori in the Statistical Analysis Plan. The dose escalation phase analysis focused on DLT incidence, summarised by dose cohort, within the evaluable patient population. A descriptive summary of the cycle number received, dose intensity and dose modification are presented by treatment allocation with adverse events summarised by CTCAE grade. Pharmacokinetic analysis are presented by dose received for guadecitabine and decitabine, including AUC, Cmax and Tmax. The randomised dose expansion phase was not powered for formal statistical comparisons of efficacy and sample size was set at 20. Analysis was conducted within the intention-to-treat population comprising all randomised patients. pCR was determined by local specialist uro-pathologist assessment summarised by treatment arm. Pharmacodynamic data are presented in graphs as mean change from cycle 1, day 1, by treatment allocation. Analyses were done with SAS (version 9.4) and Stata (version 16.0). Data were analysed by Southampton Clinical Trials Unit statisticians. LD and GS had full access to the raw data. SC and GG had final responsibility for the decision to submit for publication.
Results
Trial Cohorts
40 eligible patients were enrolled between May 2016 and September 2019 (Supplementary Figure 2). Three patients in the dose escalation phase became non-evaluable due to rapid disease progression leading to death (one before treatment allocation, two during treatment cycle 1) and were replaced. The remaining 17 patients represent the evaluable patient population within the dose escalation phase. 20 MIBC patients were randomly assigned within the dose expansion phase.
Baseline characteristics are presented in Table 1. Median age was 59 (range 38 to 76) in the dose escalation phase and 68 (range 34 to 76) in the dose expansion phase. 11 (64.7%) and 19 (95.0%) were male, and 9 (52.9%) and 13 (65.0%) had an ECOG performance status of 0 respectively. Patient characteristics within the dose expansion phase were balanced between treatment arms.
Table 1. Patient characteristics.
| Trial phase | Dose escalation phase | Dose expansion phase | |||||
|---|---|---|---|---|---|---|---|
| Patient cohort | 1 | 2 | 3 | Total | GC + guadecitabine | GC | Total |
| Guadecitabine dose | 20 mg/m2, day 1-5 | 20 mg/m2, day 1-5 + G-CSF | 30 mg/m2, day 1-5 + G-CSF | 20 mg/m2, day 1-5 + G-CSF | |||
| n | 4 | 8 | 5 | 17 | 10 | 10 | 20 |
| Age | |||||||
| Median (IQR) | 63 (57.5 to 70) | 56 (52.5 to 65) | 68 (47 to 70) | 59 (54 to 68) | 68 (58 to 75) | 68 (59 to 71) | 68 (59 to 72) |
| Range | 56 to 73 | 44 to 71 | 38 to 76 | 38 to 76 | 51 to 76 | 34 to 74 | 34 to 76 |
| Gender (%) | |||||||
| Male | 2 (50) | 5 (62.5) | 4 (80) | 11 (64.7) | 9 (90) | 10 (100) | 19 (95%) |
| Female | 2 (50) | 3 (37.5) | 1 (20) | 6 (35.3) | 1 (10) | 0 | 1 (5%) |
| ECOG performance status (%) | |||||||
| 0 | 2 (50) | 5 (62.5) | 2 (40) | 9 (52.9) | 7 (70.0) | 6 (60) | 13 (65) |
| 1 | 2 (50) | 3 (37.5) | 3 (60) | 8 (47.1) | 3 (30.0) | 3 (30) | 6 (30) |
| Missing | 0 | 0 | 0 | 0 | 0 | 1 (10) | 1 (5) |
| Primary tumour site (%) | |||||||
| Urinary tract | 4 (100) | 6 (75) | 1 (20) | 11 (41.2) | 10 (100) | 10 (100) | 20 (100) |
| Pleura | 0 | 0 | 2 (40) | 2 (11.8) | 0 | 0 | 0 |
| Ovary | 0 | 0 | 1 (20) | 1 (5.9) | 0 | 0 | 0 |
| Mediastinum* | 0 | 1 (12.5) | 0 | 1 (5.9) | 0 | 0 | 0 |
| Testis | 0 | 0 | 1 (20) | 1 (5.9) | 0 | 0 | 0 |
| Unknown^ | 0 | 1 (12.5) | 0 | 1 (5.9) | |||
| Histopathology (%) | 0 | 0 | |||||
| Adenocarcinoma | 0 | 0 | 1 (20) | 1 (5.9) | 0 | 0 | 0 |
| Carcinoma* | 0 | 1 (12.5) | 1 (20) | 2 (11.8) | 0 | 0 | 0 |
| Mesothelioma | 0 | 0 | 2 (40) | 2 (11.8) | 0 | 0 | 0 |
| Small cell carcinoma | 0 | 0 | 1 (20) | 1 (5.9) | 0 | 0 | 0 |
| Transitional Cell Carcinoma | 3 (75) | 6 (75) | 0 | 9 (53) | 10 (100) | 10 (100) | 20 (100) |
| Clear cell carcinoma | 1 (25) | 0 | 0 | 1 (5.9) | 0 | 0 | 0 |
| Melanoma | 0 | 1 (12.5) | 0 | 1 (5.9) | 0 | 0 | 0 |
| Tumour stage(%) | |||||||
| T2 | - | - | - | - | 8 (80) | 9 (90) | 17 (85) |
| T3 | - | - | - | - | 2 (20) | 1 (10) | 3 (15) |
| Locally advanced | 0 | 1 (12.5) | 2 (40) | 3 (17.7) | 0 | 0 | 0 |
| Metastatic | 4 (100) | 7 (87.5) | 3 (60) | 14 (82.3) | 0 | 0 | 0 |
| Prior surgery(%) | |||||||
| Yes | 4 (100) | 5 (62.5) | 4 (80) | 13 (76.5) | 0 | 0 | 0 |
| No | 0 | 3 (37.5) | 1 (20) | 4 (23.5) | 10 (100) | 10 (100) | 20 (100) |
| Prior radiotherapy(%) | |||||||
| Yes | 0 | 3 (37.5) | 2 (40) | 5 (29.4) | 0 | 0 | 0 |
| No | 4 (100) | 5 (62.5) | 3 (60) | 12 (70.6) | 10 (100) | 10 (100) | 20 (100) |
| Prior systemic therapy(%) | |||||||
| Yes | 2 (50) | 7 (87.5) | 5 (100) | 14 (82.4) | 0 | 0 | 0 |
| No | 2 (50) | 1 (12.5) | 0 | 3 (17.6) | 10 (100) | 10 (100) | 20 (100) |
| Prior intravesical BCG(%) | |||||||
| Yes | 0 | 0 | 0 | 0 | 0 | 1 (100) | 1 (100) |
| No | 4 (100) | 8 (100) | 5 (100) | 17 (100) | 10 (100) | 9 (90) | 19 (95) |
| Haemoglobin(g/L) | |||||||
| Median (IQR) | 125.0 (105.5 to 140.5) | 133.5 (115.0 to 149.0) | 123.0 (109.0 to 130.0) | 130.0 (110.0 to 141.0) | 139.0 (132.0 to 142.0) | 142.5 (136.0 to 149.0) | 140.0 (134.0 to 145.5) |
| Range | 101.0 to 141.0 | 95.0 to 160.0 | 105.0 to 143.0 | 95.0 to 160.0 | 127.0 to 147.0 | 107.0 to 155.0 | 107.0 to 155.0 |
| Albumin(g/L) | |||||||
| Median (IQR) | 32.0 (28.5 to 37.0) | 41.5 (40.0 to 43.5) | 38.0 (33.0 to 40.0) | 39.0 (33.0 to 41.0) | 41.5 (38.0 to 45.0) | 43.0 (40.0 to 44.0) | 43.0 (39.0 to 44.0) |
| Range | 28.0 to 39.0 | 33.0 to 46.0 | 28.0 to 41.0 | 28.0 to 46.0 | 28.0 to 46.0 | 31.0 to 47.0 | 28.0 to 47.0 |
| GFR(mL/min) | |||||||
| Median (IQR) | 94.7 (87.3 to 122.5) | 89.1 (78.0 to 120.9) | 102.0 (75.0 to 118.0) | 97.2 (79.0 to 120.3) | 78.0 (67.0 to 96.0) | 94.0 (81.0 to 107.0) | 88.5 (69.5 to 97.0) |
| Range | 87.3 to 143.0 | 65.0 to 151.0 | 71.0 to 122.0 | 65.0 to 151.0 | 64.0 to 109.0 | 57.0 to 113.0 | 57.0 to 113.0 |
GC, gemcitabine and cisplatin; G-CSF, granulocyte colony stimulating factor; IQR, inter quartile range; ECOG, Eastern Cooperative Oncology Group; BCG, Bacillus Calmette–Guérin; GFR, glomerular filtration rate; *primary mediastinal germ cell carcinoma; ^biopsy proven melanoma lung metastases with no primary site ever identified
Dose Escalation Phase
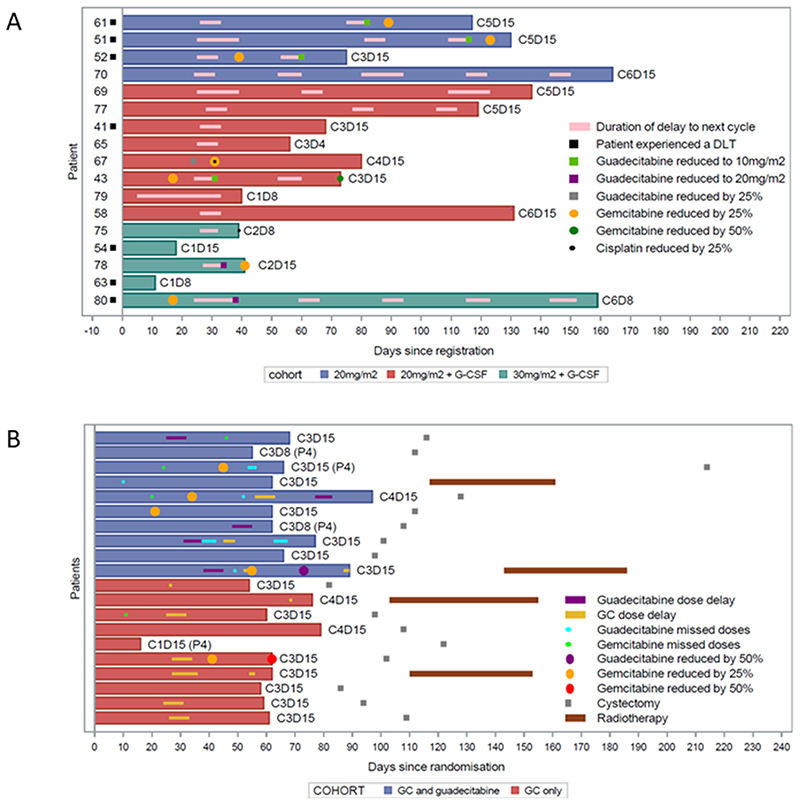
DLTs occurring within the dose escalation phase are summarised in table 2. As three patients within cohort 1 (20 mg/m2 guadecitabine, days 1-5) experienced a DLT, and two of these related to neutropenia, this dose level was repeated (cohort 2) with G-CSF prophylaxis in all subsequent patients (and remaining treatment cycles within cohort 1). Following one DLT in an initial 6 patients recruited to cohort 2, guadecitabine dose was escalated to 30 mg/m2, days 1-5 (cohort 3). Three patients experienced at least one DLT in cohort 3, which was therefore deemed not tolerated. Cohort 2 was designated as the MTD, and after review of pharmacodynamic and pharmacokinetic data (described below), also the MBED. Per protocol, cohort 2 was expanded to 8 patients to include 6 with advanced UC. Adverse events within the dose escalation phase for all treatment cycles are shown in table 3 (grade 3 or higher) and Supplementary Tables 1 to 5. Higher grade (≥ grade 3) toxicities were predominantly haematological and related to neutropenia and thrombocytopenia. Treatment duration and all dose alterations are indicated by treatment cohort in Figure 1A and Supplementary Table 6. Of the 17 dose escalation patients, 7 (41%) discontinued due to treatment related toxicity, 16 (94%) had a delay of at least one treatment dose, and dose reductions were required for guadecitabine in 7 (41%), gemcitabine in 7 (41%), and cisplatin in 2 (12%). Dose delays and alterations were almost entirely related to haematological toxicity through neutropenia and thrombocytopenia. Two patients treated within cohort 2 with refractory germ cell cancer, having received multiple prior lines of chemotherapy (for each including two separate cisplatin containing regimens and carboplatin based high dose chemotherapy with autologous stem cell transplantation) achieved clinical stabilisation of disease and tumour marker responses, as previously reported.(37) Patients with UC in the escalation phase who were cisplatin naïve (n=4) had time to disease progression of between 10 and 46 months, and for those with prior cisplatin exposure (n=7) of between 2 to 10 months (Supplementary Table 7). There were no treatment related deaths.
Table 2. Dose limiting toxicities (DLT) observed within the dose escalation phase by treatment cohort.
| Cohort | Patient | DLT criteria met (and associated details) |
|---|---|---|
| 1 (n=4) | 61 | Other clinically significant grade 3 or above toxicity except nausea or vomiting (grade 3 pulmonary embolism) |
| 51 | Grade 4 neutropenia ≥ 7 days duration | |
| 52 | Grade 3-4 neutropenia associated with a temperature ≥38.5°C | |
| 2 (n=8) | 41 | Grade 3-4 neutropenia associated with a temperature ≥38.5°C |
| 3 (n=5) | 54 | Grade 3-4 neutropenia associated with a temperature ≥38.5°C Grade 4 thrombocytopenia ≥ 7 days duration Greater than 14 days of delay in commencing a second cycle of treatment due to drug toxicity |
| 80 | Grade 4 thrombocytopenia ≥ 7 days duration Grade 4 neutropenia ≥ 7 days duration Other clinically significant grade 3 or above toxicity except nausea or vomiting (grade 3 dental infection) | |
| 63 | Other clinically significant grade 3 or above toxicity except nausea or vomiting (grade 3 diarrhoea and grade 3 hypokalaemia) |
Table 3. Grade 3 and higher adverse events for all cycles in evaluable patients in the dose escalation phase.
| Patient cohort | 1 | 2 | 3 | Total |
|---|---|---|---|---|
| Guadecitabine dose | 20 mg/m2, day 1-5 | 20 mg/m2, day 1-5 + G-CSF | 30 mg/m2, day 1-5 + G-CSF | |
| n | 4 | 8 | 5 | 17 |
| Patients that experienced at least one AE graded 3 or above | 4 (100%) | 8 (100%) | 5 (100%) | 17 (100%) |
| Blood and lymphatic system disorders | ||||
| Anaemia | 1 (25.0%) | 2 (25.0%) | 2 (40.0%) | 5 (29.4%) |
| Febrile neutropenia | 1 (25.0%) | 1 (12.5%) | 0 (0.0%) | 2 (11.8%) |
| Neutropenia | 3 (75.0%) | 5 (62.5%) | 5 (100.0%) | 13 (76.5%) |
| Leucopenia | 1 (25.0%) | 1 (12.5%) | 3 (60.0%) | 5 (29.4%) |
| Pancytopenia | 0 (0.0%) | 1 (12.5%) | 0 (0.0%) | 1 (5.9%) |
| Thrombocytopenia | 4 (100.0%) | 4 (50.0%) | 3 (60.0%) | 11 (64.7%) |
| Ear and labyrinth disorders | ||||
| Hypoacusis | 1 (25.0%) | 0 (0.0%) | 0 (0.0%) | 1 (5.9%) |
| Tinnitus | 0 (0.0%) | 0 (0.0%) | 1 (20.0%) | 1 (5.9%) |
| Gastrointestinal disorders | ||||
| Diarrhoea | 0 (0.0%) | 0 (0.0%) | 1 (20.0%) | 1 (5.9%) |
| Melaena | 0 (0.0%) | 1 (12.5%) | 0 (0.0%) | 1 (5.9%) |
| Nausea | 1 (25.0%) | 1 (12.5%) | 0 (0.0%) | 2 (11.8%) |
| Vomiting | 0 (0.0%) | 1 (12.5%) | 0 (0.0%) | 1 (5.9%) |
| General disorders and administration site conditions | ||||
| Fatigue | 1 (25.0%) | 0 (0.0%) | 0 (0.0%) | 1 (5.9%) |
| Pyrexia | 0 (0.0%) | 0 (0.0%) | 1 (20.0%) | 1 (5.9%) |
| Infections and infestations | ||||
| Corona virus infection | 0 (0.0%) | 1 (12.5%) | 0 (0.0%) | 1 (5.9%) |
| Infection | 0 (0.0%) | 0 (0.0%) | 1 (20.0%) | 1 (5.9%) |
| Pneumonia | 0 (0.0%) | 1 (12.5%) | 0 (0.0%) | 1 (5.9%) |
| Tooth Infection | 0 (0.0%) | 0 (0.0%) | 1 (20.0%) | 1 (5.9%) |
| Urinary Tract Infection | 0 (0.0%) | 1 (12.5%) | 0 (0.0%) | 1 (5.9%) |
| Metabolism and nutrition disorders | ||||
| Dehydration | 0 (0.0%) | 1 (12.5%) | 0 (0.0%) | 1 (5.9%) |
| Hypokalaemia | 0 (0.0%) | 0 (0.0%) | 1 (20.0%) | 1 (5.9%) |
| Hypomagnesaemia | 0 (0.0%) | 0 (0.0%) | 1 (20.0%) | 1 (5.9%) |
| Hyponatraemia | 1 (25.0%) | 0 (0.0%) | 0 (0.0%) | 1 (5.9%) |
| Renal and urinary disorders | ||||
| Ureteric Obstruction | 0 (0.0%) | 1 (12.5%) | 0 (0.0%) | 1 (5.9%) |
| Vascular disorders | ||||
| Embolism | 1 (25.0%) | 1 (12.5%) | 0 (0.0%) | 2 (11.8%) |
| Peripheral Ischaemia | 0 (0.0%) | 0 (0.0%) | 1 (20.0%) | 1 (5.9%) |
Figure 1.
Swimmers plots representing time on treatment and dose alterations within (A) the dose escalation phase and (B) the dose expansion phase. Within the expansion phase, P4 indicates patients were planned for 4 cycles of chemotherapy, with other patients planned for 3 cycles. Note: one patient within the GC only control arm discontinued trial treatment, per protocol, due to emergent renal impairment but completed 3 further cycles of GC chemotherapy prior to cystectomy
Dose Expansion Phase
10 patients per arm were allocated to GC alone, or GC combined with guadecitabine and G-CSF, for the dose expansion phase. Adverse events are presented in table 4 and Supplementary Tables 8 to 12. Again, high grade toxicity was predominantly haematological but balanced between treatment arms, in terms of severity overall, and the nature of the adverse events experienced, and for those events deemed as guadecitabine related. Intended cycle number, dose administration and intensity by treatment arm are shown in Figure 1B and Supplementary Table 13, indicating similar administration of cisplatin but a modest reduction in gemcitabine dose intensity in the guadecitabine arm. The latter was primarily though omission of the day 15 gemcitabine dose, or gemcitabine dose reduction, on the basis of per protocol criteria for neutropenia and thrombocytopenia. All patients, received at least 3 cycles of treatment except one in the GC alone arm (who discontinued trial treatment per protocol after 1 cycle due to a GFR <60 mL/min but continued through 3 further cycles of chemotherapy prior to cystectomy). However, 1 of 4 (25%) in the GC and guadecitabine arm, versus 2 of 3 (67%) in the GC alone arm, had been planned for 4 cycles. Therefore, a greater number of patients discontinued treatment prior to the planned duration of treatment if receiving guadecitabine. Of the 20 randomised expansion patients, 1 (10%) per treatment arm discontinued due to treatment related toxicity and 5 (50%) per arm required delay of at least one treatment dose. Guadecitabine dose reduction was required for 1 (10%) patient. Gemcitabine dose reduction occurred in 1 (10%) patient in the GC alone arm and 4 (40%) in the guadecitabine arm. No cisplatin dose reductions occurred in either arm.
Table 4. Grade 3 and higher adverse events for all cycles in the dose expansion phase.
| Characteristic | GC + guadecitabine | GC | Total |
|---|---|---|---|
| n | 10 | 10 | 20 |
| Patients that experienced at least one AE graded 3 or above | 8 (80.0%) | 7 (70.0%) | 15 (75.0%) |
| Blood and lymphatic system disorders | |||
| Febrile neutropenia | 1 (10.0%) | 0 (0.0%) | 1 (5.0%) |
| Neutropenia | 4 (40.0%) | 5 (50.0%) | 9 (45.0%) |
| Leukopenia | 1 (10.0%) | 0 (0.0%) | 1 (5.0%) |
| Thrombocytopenia | 4 (40.0%) | 3 (30.0%) | 7 (35.0%) |
| General disorders | |||
| Pyrexia | 1 (10.0%) | 1 (10.0%) | 2 (10.0%) |
| Infections and infestations | |||
| Urinary tract infection | 1 (10.0%) | 1 (10.0%) | 2 (10.0%) |
| Respiratory, thoracic and mediastinal disorders | |||
| Pulmonary embolism | 1 (10.0%) | 1 (10.0%) | 2 (10.0%) |
| Skin and subcutaneous tissue disorders | |||
| Rash | 1 (10.0%) | 1 (10.0%) | 2 (10.0%) |
GC, gemcitabine and guadecitabine; AE, adverse event
8 patients in each treatment arm proceeded to radical cystectomy. The remaining 2 patients in each arm opted for radical chemo-radiotherapy. No patients were delayed in proceeding to either cystectomy or radiotherapy through addition of guadecitabine. One patient in each arm underwent cystectomy >90 days from trial treatment for reasons of patient choice (guadecitabine arm) and completion of GC off trial due to a lowered GFR (control arm). 6 of 16 patients had a pCR at cystectomy, 2 in the guadecitabine arm and 4 in the chemotherapy arm. Time from randomisation to completion of radical treatment to the bladder, and post-cystectomy peri-operative morbidity (Clavien-Dindo classification) were similar between treatment arms (Supplementary Table 14). All patients within the dose expansion phase remain alive at a median duration of follow up of 7.6 months (IQR 6.7 to 11.5, GC and guadecitabine) and 8.6 months (IQR 6.8 to 12.4, GC alone) by arm. One patient, in the GC alone arm, has had a UC metastatic relapse diagnosed to date.
Pharmacodynamic and pharmacokinetic endpoints
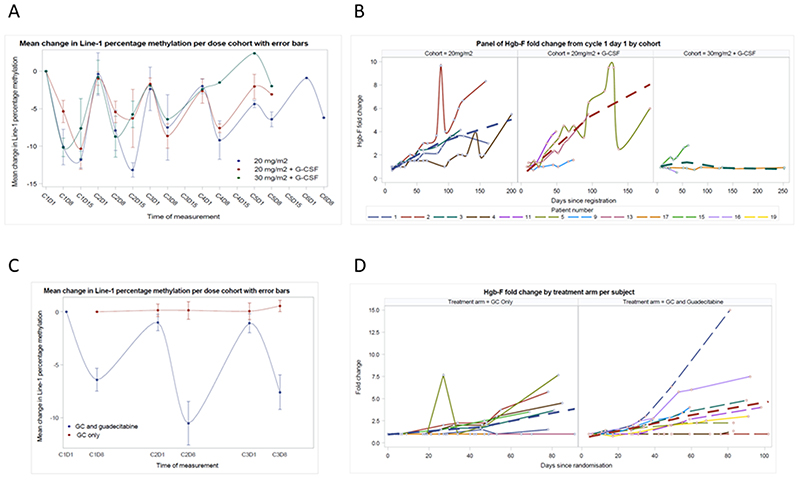
cfDNA LINE-1 promotor methylation and HbF re-expression status are shown for each trial phase in Figure 2. Promotor methylation status for selected other genes is shown in Supplementary Figure 3. Results were consistent with guadecitabine target effect. Pharmacokinetic parameters for guadecitabine are shown in Supplementary Figure 4 and Supplementary Table 15 and were consistent with the single agent experience to date for guadecitabine.(33)
Figure 2.
Pharmacodynamic data for guadecitabine effect with respect to (A, B) dose level cohort within the dose expansion phase and (C, D) randomised treatment allocation within the dose expansion phase for (A, C) mean cfDNA LINE-1 promotor methylation with respect to dose level cohort or treatment arm respectively and (B, D) HbF re-expression status for individual patients and line of best fit (dashed lines, Loess method)
Discussion
We have established a dose and schedule for the DNA methyltransferase inhibitor guadecitabine, for combination with GC chemotherapy at conventional doses for UC. As anticipated, addition of guadecitabine to GC produces some additional treatment related toxicity, manifesting predominantly as neutropenia, thrombocytopenia, and complications of these adverse events. In respect to neutropenia, it is clear that G-CSF prophylaxis is required for all patients to reduce risk of infective complications and impact on dose intensity (which many oncologists would already consider a reasonable addition to GC alone). Beyond this, we detected limited evidence for additional symptomatic adverse events, above that which would be anticipated for GC alone. The randomised dose expansion phase of the trial, for neoadjuvant treatment of MIBC, allowed assessment of relative dose intensity. We found no impact on the cumulative delivery of cisplatin to patients, although gemcitabine dose intensity was reduced modestly. Similar numbers of patient received up to 3 cycles of treatment between arms. However, some patients planned for 4 cycles had the final cycle omitted, and so we cannot exclude a cumulative effect of treatment such that later cycles might be compromised. Future studies should assess carefully the impact on dose delivery over multiple cycles of treatment. Our data were reassuring within the dose expansion phase with respect to timeliness and completion of radical treatment options, with cystectomy and radical radiotherapy delivered to similar time lines despite addition of guadecitabine.
A 28 day treatment cycle has been used in all prior clinical investigation of guadecitabine, either as monotherapy or in therapeutic combinations.(33,38,39) Our schedule utilised day 1 to 5 guadecitabine administration, but for the first time within a 21 day chemotherapy cycle, to accommodate a typical GC dosing schedule for UC, and to maintain cisplatin dose intensity which is considered critically important for this disease.(5) This may have been relevant to the need to incorporate G-CSF, and the impact on gemcitabine delivery on day 15 in some patients. We did not test guadecitabine doses below 20 mg/m2 day 1-5. This was primarily guided by monotherapy pharmacodynamic data that guadecitabine reliably depletes LINE-1 promotor methylation from 18 mg/m2 day 1-5 (28 day cycle) and upwards, and maximally at around day 8 for cisplatin administration in our schedule.(33). We acknowledge that lower, less myelosuppressive, guadecitabine dosing remains a hypothesis to explore for a cisplatin response optimisation strategy, although with a potential sacrifice of guadecitabine monotherapy efficacy seen in pre-clinical UC models.(22–27)
We undertook pharmacodynamic evaluation of guadecitabine effect in cell free DNA. LINE-1 promotor methylation has been used most frequently in this setting, as an on target effect of DNA methyltransferase activity. We found this to decrease in a cyclical fashion, with a nadir at around day 8 to 15 of treatment, and meeting our intention to coincide this with cisplatin administration. One question for future study will be the degree to which magnitude of demethylation correlates to treatment efficacy. Whether this effect requires dose to be escalated to tolerance in chemotherapy combinations remains open to clinical evaluation.(21) Promotor methylation of a panel of other genes demonstrated similar patterns of cyclical demethylation with, subjectively, greatest magnitude between day 8 and 15. We did see a greater variability around the timing of this nadir, and perhaps its duration, than for guadecitabine monotherapy. This is despite pharmacokinetic parameters for guadecitabine that were unaltered compared to prior data as a result of this chemotherapy combination.(33) Arguably there may be benefit in guadecitabine effect covering all three chemotherapy doses in this combination however. Further assessment of this issue would require tumour biopsies, if feasible, in future investigation. We also assessed HbF re-expression as a readily measurable DNA methyltransferase inhibitor effect. Our findings suggest that, although there were 2-6 fold increases seen in HbF percentage in blood, the impact within the randomised expansion was subjectively similar within the control arm. This endpoint would therefore seem to have less utility to monitor treatment induced target effect, at least in a chemotherapy combination.
This trial was not intended to formally assess clinical efficacy and it would be premature to form firm conclusions regarding this surrogate endpoint. With this caveat, we did establish clinical benefit in some patients within the dose escalation phase of the trial. Of note, as previously described, two patients with multiply pre-treated, platinum resistant, germ cell cancers achieved significant clinical benefit which warrants consideration for development in this rare disease.(37) This is consistent with pre-clinical, and limited clinical data, in germ cell cancer that implies supporting this strategy.(40–42) In addition, we saw comparable pCR rates between the two arms of the MIBC dose expansion phase. Elsewhere, clinical data supporting a similar approach has recently been presented for a study of guadecitabine with carboplatin in platinum resistant ovarian cancer. This randomised phase II trial did not meet its progression free survival primary endpoint (16.3 weeks, versus 9.1 weeks, for a chemotherapy of choice control arm, p=0.07). However, the 6-month progression free rate was significantly higher in the guadecitabine with carboplatin group (37% versus 11%; p=0.003). Questions remain for the development of a DNA methyltransferase/platinum combination regarding optimal dose and schedule, optimal platinum agent, optimal degree of demethylation impact and its measurement.
A further practical aspect of this combination regimen is an increase in drug administrations over chemotherapy alone, with multiple subcutaneous administrations of guadecitabine and G-CSF. G-CSF was self-administered at home whereas guadecitabine, required clinic attendance for research nurse administration on days 1-5. We found this to be acceptable to patients and we did not find skin reactions, or multiple subcutaneous administrations, to be problematic. However, patient acceptability and the option of guadecitabine self-administration should be evaluated in future studies.
In conclusion, we have defined a recommended dose and schedule for guadecitabine in combination with GC. This modestly increases the adverse event profile, but appears deliverable over at least 3 to 4 cycles of treatment. Pharmacodynamic parameters are supportive of on-target effect for guadecitabine. Future studies are now warranted to formally test the efficacy of this combination in both platinum refractory, and platinum naive patients.
Supplementary Material
Statement of Translational Relevance.
Treatment options for a cisplatin resistant phenotype remains an important unmet clinical need. Gene promoter methylation patterns are linked to cisplatin resistance and are therapeutically targetable in pre-clinical cancer models. This phase Ib/IIa trial established a recommended dose and schedule for combining the DNA methyltransferase inhibitor guadecitabine with cisplatin and gemcitabine chemotherapy. Translational endpoints confirmed that this schedule delivers optimal reversal of gene promotor methylation at the point of cisplatin administration. The schedule is tolerable over multiple treatment cycles compared to chemotherapy alone and the key adverse events relating to myelosuppression are manageable. The data presented here therefore provide a basis to undertake prospective randomised trials of this therapeutic approach which holds potential relevance for a variety of solid malignancies.
Acknowledgements
We thank the patients and their families for participating in this study, along with all investigators and site personnel. We thank Cancer Research UK and Astex Pharmaceuticals for provision of research funding to undertake this trial. The authors also wish to acknowledge Prof John Staffurth, Dr Anthony Kong and Jacqueline Birks as members of the independent Data Monitoring and Ethics Committee. RAH and BJ acknowledge the support of the Institute of Cancer Research/ Royal Marsden FT NIHR biomedical research centre. This work was supported by Cancer Research UK [grant number C9317/A19903]; and Astex Pharmaceuticals.
Financial Support
This work was supported by Cancer Research UK [grant number C9317/A19903]; and Astex Pharmaceuticals.
Footnotes
Conflicts of Interest:
SJC reports consulting or advisory roles for Roche, Janssen Cilag, MSD, Astellas, Bayer and AstraZeneca, and research funding provision from Astex Pharmaceuticals, AstraZenaca and Clovis Oncology. SD reports consultancy or advisory roles for GSK, Incanthera and Boehringer Ingelheim, and research funding from Amgen. JWFC has received reimbursement for consultancy from Astra Zeneca, Roche and Janssen, speaker fees from BMS, MSD, Nucleix and Roche, and honoraria for membership of an advisory board for Ferring. NS has received speaker fees from Pfizer, EUSA Pharma and BMS. DE has received speaker fees from AstraZeneca, Pfizer, Janssen and MSD. RAH has received honoraria related membership from advisory boards from MSD, Roche, Nektar pharmaceuticals, Janssen, BMS Bayer and Astellas and speakers fees from Roche and Janssen. DC is employed by Astex Pharmaceuticals, Inc. All remaining authors have declared no conflicts of interest.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Birtle A, Johnson M, Chester J, Jones R, Dolling D, Bryan RT, et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): a phase 3, open-label, randomised controlled trial. Lancet. 2020;395(10232):1268–77. doi: 10.1016/S0140-6736(20)30415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International collaboration of trialists on behalf of the Medical Research Council Advanced Bladder Cancer Working Party, EORTC Genito-Urinary Group ABCSG, Group NCIoCCT, Finnbladder NBCSG, group CUEdTOC. Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: a randomised controlled trial. International collaboration of trialists. Lancet. 1999;354(9178):533–40. [PubMed] [Google Scholar]
- 4.von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18(17):3068–77. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 5.Crabb SJ, Douglas J. The latest treatment options for bladder cancer. Br Med Bull. 2018;128(1):85–95. doi: 10.1093/bmb/ldy034. [DOI] [PubMed] [Google Scholar]
- 6.Drayton RM, Catto JW. Molecular mechanisms of cisplatin resistance in bladder cancer. Expert Rev Anticancer Ther. 2012;12(2):271–81. doi: 10.1586/era.11.201. [DOI] [PubMed] [Google Scholar]
- 7.von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23(21):4602–8. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 8.Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358(11):1148–59. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 9.Catto JW, Azzouzi AR, Rehman I, Feeley KM, Cross SS, Amira N, et al. Promoter hypermethylation is associated with tumor location, stage, and subsequent progression in transitional cell carcinoma. J Clin Oncol. 2005;23(13):2903–10. doi: 10.1200/JCO.2005.03.163. [DOI] [PubMed] [Google Scholar]
- 10.Drayton RM, Peter S, Myers K, Miah S, Dudziec E, Bryant HE, et al. MicroRNA-99a and 100 mediated upregulation of FOXA1 in bladder cancer. Oncotarget. 2014;5(15):6375–86. doi: 10.18632/oncotarget.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudziec E, Goepel JR, Catto JW. Global epigenetic profiling in bladder cancer. Epigenomics. 2011;3(1):35–45. doi: 10.2217/epi.10.71. [DOI] [PubMed] [Google Scholar]
- 12.Wolff EM, Chihara Y, Pan F, Weisenberger DJ, Siegmund KD, Sugano K, et al. Unique DNA methylation patterns distinguish noninvasive and invasive urothelial cancers and establish an epigenetic field defect in premalignant tissue. Cancer Res. 2010;70(20):8169–78. doi: 10.1158/0008-5472.CAN-10-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dudziec E, Miah S, Choudhry HM, Owen HC, Blizard S, Glover M, et al. Hypermethylation of CpG islands and shores around specific microRNAs and mirtrons is associated with the phenotype and presence of bladder cancer. Clin Cancer Res. 2011;17(6):1287–96. doi: 10.1158/1078-0432.CCR-10-2017. [DOI] [PubMed] [Google Scholar]
- 14.Drayton RM, Dudziec E, Peter S, Bertz S, Hartmann A, Bryant HE, et al. Reduced expression of miRNA-27a modulates cisplatin resistance in bladder cancer by targeting the cystine/glutamate exchanger SLC7A11. Clin Cancer Res. 2014;20(7):1990–2000. doi: 10.1158/1078-0432.CCR-13-2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Baquero R, Puerta P, Beltran M, Alvarez-Mujica M, Alvarez-Ossorio JL, Sanchez-Carbayo M. Methylation of tumor suppressor genes in a novel panel predicts clinical outcome in paraffin-embedded bladder tumors. Tumor Biol. 2014;35(6):5777–86. doi: 10.1007/s13277-014-1767-6. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Wang J, Xiao W, Xia D, Lang B, Wang T, et al. Epigenetic inactivation of KLF4 is associated with urothelial cancer progression and early recurrence. J Urol. 2014;191(2):493–501. doi: 10.1016/j.juro.2013.08.087. [DOI] [PubMed] [Google Scholar]
- 17.Chang X, Monitto CL, Demokan S, Kim MS, Chang SS, Zhong X, et al. Identification of hypermethylated genes associated with cisplatin resistance in human cancers. Cancer Res. 2010;70(7):2870–9. doi: 10.1158/0008-5472.CAN-09-3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xylinas E, Hassler MR, Zhuang D, Krzywinski M, Erdem Z, Robinson BD, et al. An Epigenomic Approach to Improving Response to Neoadjuvant Cisplatin Chemotherapy in Bladder Cancer. Biomolecules. 2016;6(3) doi: 10.3390/biom6030037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramachandran K, Gordian E, Singal R. 5-azacytidine reverses drug resistance in bladder cancer cells. Anticancer Res. 2011;31(11):3757–66. [PubMed] [Google Scholar]
- 20.Tada Y, Yokomizo A, Shiota M, Tsunoda T, Plass C, Naito S. Aberrant DNA methylation of T-cell leukemia, homeobox 3 modulates cisplatin sensitivity in bladder cancer. Int J Oncol. 2011;39(3):727–33. doi: 10.3892/ijo.2011.1049. [DOI] [PubMed] [Google Scholar]
- 21.Wu M, Sheng L, Cheng M, Zhang H, Jiang Y, Lin S, et al. Low doses of decitabine improve the chemotherapy efficacy against basal-like bladder cancer by targeting cancer stem cells. Oncogene. 2019;38(27):5425–39. doi: 10.1038/s41388-019-0799-1. [DOI] [PubMed] [Google Scholar]
- 22.Khandelwal M, Anand V, Appunni S, Seth A, Singh P, Mathur S, et al. Decitabine augments cytotoxicity of cisplatin and doxorubicin to bladder cancer cells by activating hippo pathway through RASSF1A. Mol Cell Biochem. 2018;446(1-2):105–14. doi: 10.1007/s11010-018-3278-z. [DOI] [PubMed] [Google Scholar]
- 23.Ben-Kasus T, Ben-Zvi Z, Marquez VE, Kelley JA, Agbaria R. Metabolic activation of zebularine, a novel DNA methylation inhibitor, in human bladder carcinoma cells. Biochem Pharmacol. 2005;70(1):121–33. doi: 10.1016/j.bcp.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Cheng JC, Matsen CB, Gonzales FA, Ye W, Greer S, Marquez VE, et al. Inhibition of DNA methylation and reactivation of silenced genes by zebularine. J Natl Cancer Inst. 2003;95(5):399–409. doi: 10.1093/jnci/95.5.399. [DOI] [PubMed] [Google Scholar]
- 25.Christoph F, Kempkensteffen C, Weikert S, Kollermann J, Krause H, Miller K, et al. Methylation of tumour suppressor genes APAF-1 and DAPK-1 and in vitro effects of demethylating agents in bladder and kidney cancer. Br J Cancer. 2006;95(12):1701–7. doi: 10.1038/sj.bjc.6603482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shang D, Liu Y, Matsui Y, Ito N, Nishiyama H, Kamoto T, et al. Demethylating agent 5-aza-2’-deoxycytidine enhances susceptibility of bladder transitional cell carcinoma to Cisplatin. Urology. 2008;71(6):1220–5. doi: 10.1016/j.urology.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Qi F, Cao Y, Zu X, Chen M, Li Z, et al. 5-Aza-2’-Deoxycytidine Enhances Maspin Expression and Inhibits Proliferation, Migration, and Invasion of the Bladder Cancer T24 Cell Line. Cancer Biother Radiopharm. 2013;28(4):343–50. doi: 10.1089/cbr.2012.1303. [DOI] [PubMed] [Google Scholar]
- 28.Clouser CL, Holtz CM, Mullett M, Crankshaw DL, Briggs JE, O’Sullivan MG, et al. Activity of a novel combined antiretroviral therapy of gemcitabine and decitabine in a mouse model for HIV-1. Antimicrob Agents Chemother. 2012;56(4):1942–8. doi: 10.1128/AAC.06161-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuller M, Klein M, Schmidt E, Rohde C, Gollner S, Schulze I, et al. 5-azacytidine enhances efficacy of multiple chemotherapy drugs in AML and lung cancer with modulation of CpG methylation. Int J Oncol. 2015;46(3):1192–204. doi: 10.3892/ijo.2014.2792. [DOI] [PubMed] [Google Scholar]
- 30.Gray SG, Baird AM, O’Kelly F, Nikolaidis G, Almgren M, Meunier A, et al. Gemcitabine reactivates epigenetically silenced genes and functions as a DNA methyltransferase inhibitor. Int J Mol Med. 2012;30(6):1505–11. doi: 10.3892/ijmm.2012.1138. [DOI] [PubMed] [Google Scholar]
- 31.Samulitis BK, Pond KW, Pond E, Cress AE, Patel H, Wisner L, et al. Gemcitabine resistant pancreatic cancer cell lines acquire an invasive phenotype with collateral hypersensitivity to histone deacetylase inhibitors. Cancer Biol Ther. 2015;16(1):43–51. doi: 10.4161/15384047.2014.986967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valdez BC, Nieto Y, Murray D, Li Y, Wang G, Champlin RE, et al. Epigenetic modifiers enhance the synergistic cytotoxicity of combined nucleoside analog-DNA alkylating agents in lymphoma cell lines. Exp Hematol. 2012;40(10):800–10. doi: 10.1016/j.exphem.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Issa JJ, Roboz G, Rizzieri D, Jabbour E, Stock W, O’Connell C, et al. Safety and tolerability of guadecitabine (SGI-110) in patients with myelodysplastic syndrome and acute myeloid leukaemia: a multicentre, randomised, dose-escalation phase 1 study. Lancet Oncol. 2015;16(9):1099–110. doi: 10.1016/S1470-2045(15)00038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kantarjian HM, Roboz GJ, Kropf PL, Yee KWL, O’Connell CL, Tibes R, et al. Guadecitabine (SGI-110) in treatment-naive patients with acute myeloid leukaemia: phase 2 results from a multicentre, randomised, phase 1/2 trial. Lancet Oncol. 2017;18(10):1317–26. doi: 10.1016/S1470-2045(17)30576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crabb S, Danson SJ, Catto JWF, McDowell C, Lowder JN, Caddy J, et al. SPIRE-combining SGI-110 with cisplatin and gemcitabine chemotherapy for solid malignancies including bladder cancer: study protocol for a phase Ib/randomised IIa open label clinical trial. Trials. 2018;19(1):216. doi: 10.1186/s13063-018-2586-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skolnik JM, Barrett JS, Jayaraman B, Patel D, Adamson PC. Shortening the timeline of pediatric phase I trials: the rolling six design. J Clin Oncol. 2008;26(2):190–5. doi: 10.1200/JCO.2007.12.7712. [DOI] [PubMed] [Google Scholar]
- 37.Crabb SJ, Huddart RA, Brown E, Dunkley D, Downs N, McDowell C, et al. Response to guadecitabine (SGI-110) combined with cisplatin and gemcitabine (GCG) in platinum refractory germ cell tumors (GCTs) J Clin Oncol. 2020;38(15_suppl):e17057-e [Google Scholar]
- 38.Lee V, Wang J, Zahurak M, Gootjes E, Verheul HM, Parkinson R, et al. A Phase I Trial of a Guadecitabine (SGI-110) and Irinotecan in Metastatic Colorectal Cancer Patients Previously Exposed to Irinotecan. Clin Cancer Res. 2018;24(24):6160–7. doi: 10.1158/1078-0432.CCR-18-0421. [DOI] [PubMed] [Google Scholar]
- 39.Oza AM, Matulonis UA, Alvarez Secord A, Nemunaitis J, Roman LD, Blagden SP, et al. A Randomized Phase II Trial of Epigenetic Priming with Guadecitabine and Carboplatin in Platinum-resistant, Recurrent Ovarian Cancer. Clin Cancer Res. 2020;26(5):1009–16. doi: 10.1158/1078-0432.CCR-19-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Albany C, Hever-Jardine MP, von Herrmann KM, Yim CY, Tam J, Warzecha JM, et al. Refractory testicular germ cell tumors are highly sensitive to the second generation DNA methylation inhibitor guadecitabine. Oncotarget. 2017;8(2):2949–59. doi: 10.18632/oncotarget.13811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Albany C, Spinella MJ, Adra N, Hanna NH, Einhorn L. A phase I study of guadecitabine (SGI-110) plus cisplatin in patients with platinum refractory germ cell tumors. J Clin Oncol. 2020;38(suppl 6; abstr 408) [Google Scholar]
- 42.Beyrouthy MJ, Garner KM, Hever MP, Freemantle SJ, Eastman A, Dmitrovsky E, et al. High DNA methyltransferase 3B expression mediates 5-aza-deoxycytidine hypersensitivity in testicular germ cell tumors. Cancer Res. 2009;69(24):9360–6. doi: 10.1158/0008-5472.CAN-09-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.