Abstract
Triple-negative breast cancers (TNBC) are resistant to standard of care chemotherapy and lack known targetable driver gene alterations. Identification of novel drivers could aid the discovery of new treatment strategies for this hard-to-treat patient population, yet studies using high-throughput and accurate models to define the functions of driver genes in TNBC to date have been limited. Here we employed unbiased functional genomics screening of the 200 most frequently mutated genes in breast cancer, using spheroid cultures to model in vivo like conditions, and identified the histone-acetyltransferase CREBBP as a novel tumor suppressor in TNBC. CREBBP protein expression in patient tumor samples was absent in eight percent of TNBC and at a high frequency in other tumors, including squamous lung cancer where CREBBP inactivating mutations are common. In TNBC, CREBBP alterations were associated with higher genomic heterogeneity and poorer patient survival and resulted in upregulation and dependency on a FOXM1 proliferative program. Targeting FOXM1-driven proliferation indirectly with clinical CDK4/6 inhibitors selectively impaired growth in spheroids, cell line xenografts, and patient-derived models from multiple tumor types with CREBBP mutations or loss of protein expression. In conclusion, we have identified CREBBP as a novel driver in aggressive TNBC and identified an associated genetic vulnerability in tumour cells with alterations in CREBBP and provide a pre-clinical rationale for assessing CREBBP alterations as a biomarker of CDK4/6 inhibitor response in a new patient population.
Keywords: tumour microenvironment, spheroids, triple negative breast cancer, functional genomics screen, CREBBP, FOXM1, CDK4/6 inhibitors
Introduction
The genetic landscape of human cancers has been comprehensively mapped by large sequencing efforts such as The Cancer Genome Atlas (TCGA) (1–3), which have revealed many of the recurrent mutation events that are present in different tumour types. However, most of these mutations have not as yet been established as bona fide ‘drivers’ or exploited therapeutically. It remains a formidable challenge to investigate the ‘long tail’ of driver mutations in relevant cancer models, however the identification of novel cancer genes and resultant cancer-specific vulnerabilities are needed, in particular for aggressive tumour types that are resistant to current treatment options.
It is now appreciated that more complex models of cancer are required to fully appreciate the contributing factors that drive tumorigenesis in vivo and increase the efficacy of novel therapies that make the transition from pre-clinical models to clinical trials. One high-throughput way of achieving this is through the use of 3D spheroid cultures, which more accurately recapitulate the in vivo features of cancer such as hypoxia, altered cell-cell contacts and metabolism (4,5), and allows high-throughput assessment of novel genetic dependencies involved in cancer progression.
Current therapeutic strategies for the treatment of human cancers using precision medicine approaches have achieved clinical success through either direct targeting of oncogenic dependencies (e.g. HER2 targeted therapy in HER2 amplified breast cancer), or through synthetic-lethal approaches (e.g. the use of PARP inhibitors in BRCA1/2-mutant ovarian and breast cancers) (6,7). Synthetic lethality is an attractive strategy for many cancer-associated genomic alterations however, it is reliant on distinct genetic alterations in cancer cells that can act as predictive biomarkers to enable upfront patient stratification. Identification of additional patient populations who would benefit from treatment with clinically approved therapies would fast track these indications through clinical trials. This is a desirable strategy in particular for the most aggressive subtype of breast cancer, triple negative breast cancer (TNBC) that lacks expression of oestrogen receptor (ER), progesterone receptor (PR) or human epidermal growth factor receptor-2 (HER2) receptors. TNBCs are heterogeneous with a significant number of patients having a high risk of early metastatic relapse, which are commonly resistant to standard of care chemotherapy treatments (8,9). TNBCs show a distinct repertoire of copy number alterations, mutations and mRNA expression compared to hormone receptor positive tumours (HR+) and are characterised with higher levels of genomic instability (1,2). TNBCs display a high frequency of mutations in TP53, while they also display a significant burden of mutations in a myriad of other genes albeit at a lower frequency (1). However, despite progress in characterising the genomic landscape of TNBC’s, the majority of these mutations have not been established as ‘drivers’ i.e. have not been functionally tested, meaning targetable biological dependencies remain elusive (10). Together, this highlights the urgent need to identify molecular drivers of TNBC disease progression in order to identify actionable alterations that would increase the therapeutic options for these patients.
In this study we aimed to i) establish the functional impact of recurrently mutated genes in TNBC, ii) identify how to target these through synthetic-lethal approaches and iii) assess whether these findings could be extended to other hard to treat aggressive cancers. Using a functional genomics approach under conditions more similar to those encountered in the unfavourable tumour microenvironment - multicellular spheroid cultures - we silenced the 200 most frequently mutated genes in breast cancer, and identified that inactivation of the histone acetyltransferase CREBBP significantly increased cell growth in cancer cells that experience nutrient stress, such as hypoxia. We report that CREBBP protein expression is reduced in around one fifth of TNBCs, and is associated with a poorer survival. CREBBP protein expression was also reduced in multiple other solid tumours, including bladder, endometrial, and squamous lung cancers, which harbour high frequencies of CREBBP mutations. We identify and validate a mechanism whereby loss of CREBBP activity results in the upregulation of and dependency on a FOXM1-driven transcriptional proliferative program that renders cells selectively sensitive to CDK4/6 inhibition. This is seen in multiple tumour types with CREBBP alterations, which we validate both using in vitro and in vivo models. On the basis of this data, we highlight that CREBBP loss plays an important role in driving the aggressive behaviour of TNBC and other tumour types and propose that CREBBP should be assessed as a biomarker of CDK4/6i sensitivity in clinical trials, particularly in those tumour types where CREBBP genomic alterations are seen at high frequency.
Materials and Methods
Reagents and cell lines
The MCF10a and cell line was purchased from ATCC (USA), (RRID:CVCL_0598). The MCF10DCIS.com cell line was purchased from Asterand, Inc. (Herts, UK), (RRID:CVCL_5552). MCF10NeoT, MCF10AT1, MCF10Ca1a, MCF10Ca1d and MCF10Ca1h RRID:CVCL_5555, RRID:CVCL_6675, RRID:CVCL_6683, RRID:CVCL_5554, RRID:CVCL_6681, RRID:CVCL_6679)were kindly provided by The Barbara Ann Karmanos Cancer Institute (Detroit, MI, USA), (. All progression series cell lines were grown as described previously (11). WT and CREBBP-mutant (HZGHC001109c005) cell lines were purchased from Horizon Discovery, and grown in IMDM media supplemented with 10% fetal bovine serum and penicillin–streptomycin. DV-90, EVSA-T, SU-DHL-6 and NU-DHL-1 cell lines (RRID:CVCL_1184, RRID:CVCL_1207, RRID:CVCL_2206, RRID:CVCL_1876) were purchased from DSMZ (Germany) and grown in RPMI media supplemented with 10% fetal bovine serum and penicillin–streptomycin. AN3CA, NCI-H520, NALM-6, A549, H1299, HCT116, BT20, CAL-51, HCC70, HCC1937, HCC1806, Hs578t and MDA-MB-157 cell lines (RRID:CVCL_0028, RRID:CVCL_1566, RRID:CVCL_0023, RRID:CVCL_0060, RRID:CVCL_0291, RRID:CVCL_0178, RRID:CVCL_1110, RRID:CVCL_1270, RRID:CVCL_0290, RRID:CVCL_1258, RRID:CVCL_0332 RRID:CVCL_0618) were obtained from ATCC and grown in RPMI media supplemented with 10% fetal bovine serum and penicillin–streptomycin. All cell lines were growth in a humidified 37°C incubator with 5% CO2. Cell lines were periodically tested to confirm no mycoplasma infection using MycoalertTM ®Mycoplasma Detection Kit as per manufacturer’s instructions (Lonza, Slough UK). Cells were authenticated by short tandem repeat typing with the Geneprint10 Kit (Promega). Cells were kept for a maximum of 10-15 passages from time of thawing to experimentation.
siRNA spheroid screen
The gene library for the siRNA screen was chosen from a meta-analysis of breast cancer published sequencing studies (1,12–16), choosing the top 200 recurrently mutated genes that encompassed the top 100 genes in ER+, HER2+ and TNBC respectively (Table S1). Reverse transfection siRNA spheroid screens were performed as previously described in triplicate (5,11). MCF10a, MCF10NeoT, MCF10AT1, MCF10DCIS.com, MCF10Ca1a, MCF10Ca1d, MCF10Ca1h cell lines were reverse-transfected with 37.5nM of Dharmacon siGENOME siRNA using Lullaby reagent (Oz biosciences). BT20, CAL-51, HCC70, HCC1937, HCC1806, Hs578t and MDA-MB-157 cell lines were reverse-transfected with 37.5nM of Dharmacon siGENOME siRNA using Viromer (lipocalyx). Cell viability was measured after 5 days by CellTiter-Glo. The progression series screen was analysed using z-score analysis. The TNBC cell line targeted screen was analysed by plate median normalised values. Spheroids were imaged using a Nikon TE 2000 inverted wide field microscope fitted with a motorised stage, filter wheels and a Pro-Scan controller (Prior Scientific), Shutter (Sutter instruments), Orca R2 camera (Hamamatsu), 84000v2 DAPI/FITC/TRITC/Cy5 Quad (Chroma technology). The microscope is operated by HCI imaging software 4.3.1.33. Experiments are carried out at 370C (Solent Scientific) and 10% CO2.
shRNA cell line generation
Generation of doxycycline-inducible TetOnPLKO-shRNA cell lines shRNA sequences targeting CREBBP or non-targeting control (NTC) were cloned into the TetOn-pLKO-puro lentiviral vector as previously described (17). Clone IDs for shRNAs are as follows: shCREBBP #27 (TRCN0000011027) and shCREBBP #81 (TRCN0000356081). Lentiviruses were produced by co-transfecting HEK293T cells with lentiviral and packaging plasmids pCMVΔR8.91 and pMD.G. Supernatants were collected 72 hours after transfection, mixed with polybrene (8μg/mL) and used to infect cells. Cells were selected in medium containing puromycin (2μg/mL).
Tissue microarrays
Tissue microarray (TMA) slides (BR10011a(n=100), BL802a(n=60), EMC1021(n=97), HLug-Squ150CS-01(n=75), OV2084a(n=193)) were purchased from US Biomax, Inc. (Rockville, MD, USA). An additional TNBC series “Belgrade” was assembled from a consecutive series of primary untreated cases (n=175) from The Institute of Oncology and Radiology of Serbia, with three representative cores per tumour. Written informed consent was obtained where appropriate from the patients, and studies were conducted in accordance with recognized ethical guidelines (Declaration of Helsinki, CIOMS) and the studies were approved by an institutional review board. Antibody staining was performed as described above. TMAs were assessed for nuclear CREBBP protein expression in the malignant epithelium only, using a modified Allred score. Only technically sound cores containing >20% invasive tumour cells were included in the analysis. Cores were evaluated by consultant pathologists (HC and IR) for both intensity (0 = no stain; 1 = mild; 2 = moderate; 3 = strong), and percentage of epithelial cells that stained positive (0 = absent; 1 = background; 2 = 1–25%; 3 = 26–50%; 4 = 51-75%; 5 = > 75%), Fig. 1. Only technically sound cores containing >20% invasive tumour cells were included in the analysis. Scores were derived from a sum of the intensity and percentage of immunoreactivity cells; an average score of 0 for each tumour was considered negative/absent, and a score of 7 or 8, high, and a score of 4-6 as intermediate expression and <4 low expression.
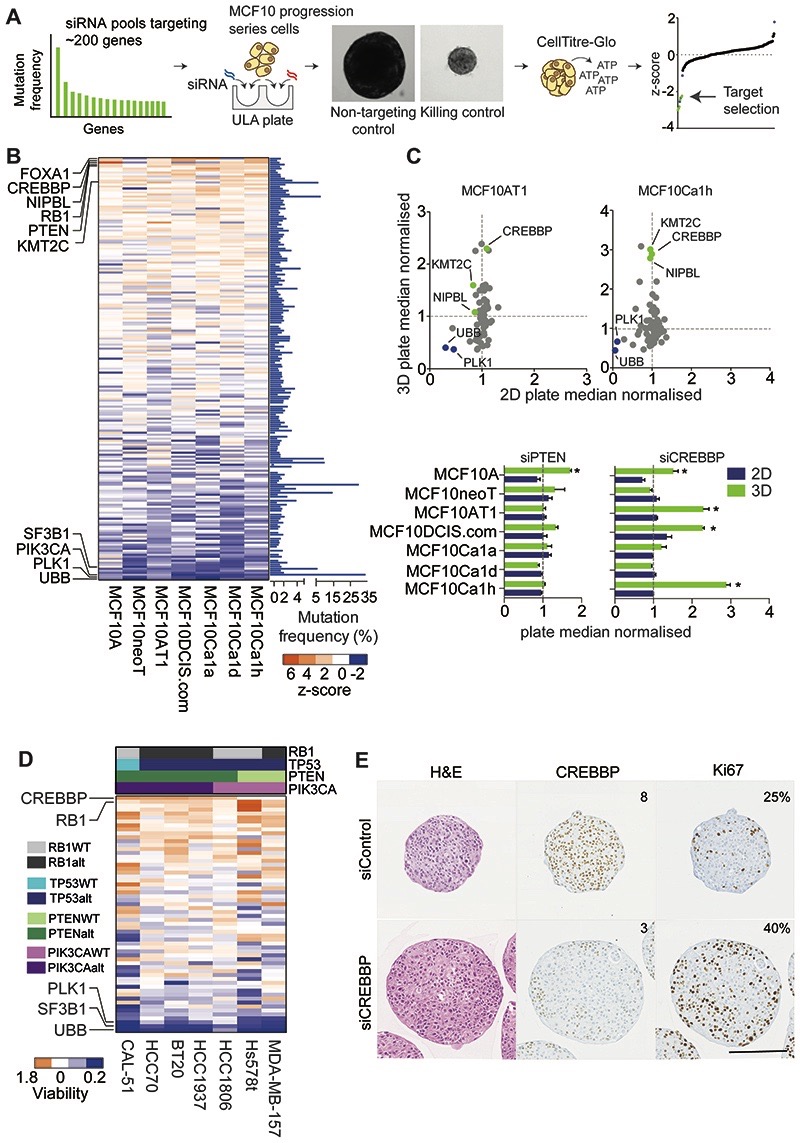
Figure 1. A targeted functional genomics screen in cancer cell line spheroids identifies CREBBP as a tumour suppressor in TNBC.
(A) Schematic of reverse transfection protocol of spheroids ULA (ultra-low attachment plates). (B) Heatmap of the z scores of the MCF10 progression series screen silencing the most frequently mutated genes (n=199) in multicellular spheroids (3D cultures). Known killing controls (essential genes are highlighted (SF3B1, PLK1, UBB). (C) Scatter plot of the spheroid viability of the MCF10 progression series under 2D and 3D conditions with the top fifty siRNAs relative to non-targeting control siRNA (NTC), depicting CREBBP, KMT2C and NIPBL as 3D specific hits. Bar chart of plate median normalised values for depicted genes from are also shown. (D) Heatmap of the plate median normalised values of the validation screen of the spheroid viability of a panel of TNBC spheroids after siRNAs silencing of the top fifty genes identified from (B) Common genomic alterations in the cell lines are depicted. (E) Representative micrographs of H&E and CREBBP and Ki67 protein expression evaluated by immunohistochemistry from 7-day spheroids of MCF10DCIS.com. Text depicts Allred scores for CREBBP IHC and % Ki67 positive cells (Scale bar represents 200μm).
In Vivo Assessment of Palbociclib Efficacy
All animal work was carried out with UK Home Office approval. 1x106 NCI-H520 cells or 2x106 SU-DHL-6 cells were injected subcutaneously with matrigel into right flank of 8 week old female NOD scid gamma (NSG) nude mice. Once tumours showed an increase in tumour size (caliper measurement) animals were randomised into two groups, which were treated orally with 100mg/kg of Palbociclib (Pfizer) or vehicle control (sodium lactate), daily. Operators were blinded to which cohort received Palbociclib and which received vehicle. Tumour burden was calculated using the following equation (v= 0.52 x length x width2, the length should be recorded as the longest diameter). Animals were sacrificed when the humane endpoint was reached (12mm in diameter). Statistical analysis was performed using Prism. In vivo efficacy for CTG-0869 and CTG-2055 were performed by Champions Oncology where cells were implanted into 8 week old female nu/nu nude mice. A surrogate of animal survival was determined when the tumour size reached a predefined volume of 500mm3 (SU-DHL-6 and NCI-H520) or 1000mm3 (CTG-0869). Survival curves were analysed by the method of Kaplan-Meier, with a logrank Mantel-Cox test p-value <0.05 being considered significant. Tumours were formalin-fixed and paraffin-embedded, and slides were stained with H&E, or immunohistochemistry with antibodies against Ki67 as described above.
Results
Identification of CREBBP as a novel driver in TNBC
In order to establish which recurrently mutated genes in TNBC operate as “drivers”, we established a small interfering RNA (siRNA) library that targeted the most frequently mutated genes (n=199) in unselected breast cancers (Fig. 1A and Supplementary Table S1). We performed unbiased functional genomics screens with a two-pronged approach; using the isogenic MCF10 model of TNBC breast cancer progression (MCF10 cell line progression series) and validation screen in a non-isogenic panel of TNBC cell lines, grown as 3D spheroids in order to recapitulate in vivo-like conditions such as hypoxia and nutrient depletion (4,5,11,31,32) (Fig. 1B and Supplementary Table S2). We chose the MCF10 progression series for an initial screen as we have previously shown this is a good model to identify novel genetic dependencies involved in the progression of breast cancer when cells are grown in 3D, and although this model harbours an activating HRAS mutation, these have recently been found to be present in aggressive breast cancers (5,11,33). Analysis of the initial screen identified distinct known oncogenic dependencies including PIK3CA where reduced cell viability was seen only in cells harbouring the H1047R hotspot mutation and a potential novel oncogenic dependency in cells harbouring a SZT2 T211R mutation (Supplementary Fig. S1A). siRNA’s targeting the known tumour suppressor genes RB1 and PTEN caused a significant increase in viability in one or more cell lines, as well as a series of genes not previously implicated in TNBC, including KMT2C, NIPBL and CREBBP (Fig. 1B, Supplementary Fig. S1B), suggesting loss of these genes may affect multiple stages of TNBC progression. Although we observed differences in the effect of gene silencing with some of these, perhaps due to genetic or epigenetic differences, the observed phenotypic effect in multiple cell lines however, suggested these are bona fide driver genes. The majority of these effects were specific to 3D culture conditions (Fig. 1C, Supplementary Table S3), including KMT2C, NIPBL and CREBBP, which promoted growth in multiple cell line spheroids, whereas silencing of PTEN had a smaller relative effect. To ascertain which alterations could be driving growth in diverse genetic backgrounds, we assessed the top 50 target gene siRNAs in a heterogeneous panel of TNBC cell line spheroids. This showed that silencing the lysine acetyltransferase CREBBP and RB1 had the greatest impact on increasing viability across multiple TNBC cell lines (Fig. 1D, Supplementary Fig. S1C). In contrast to our findings in a 3D culture setting, assessment of CREBBP silencing from multiple large scale publicly available 2D CRISPR and shRNA screens, did not show increased cellular viability when CREBBP was silenced (Supplementary Figure S1D-F). Together these results validate the primary screen in the MCF10 cells and show that CREBBP silencing also promotes growth in a 3D-specific manner in cancer cells derived from TNBC patients. Interestingly, those cell lines that showed a significant growth advantage upon CREBBP silencing harboured an enrichment of PIK3CA/PTEN genomic alterations (p=0.0476, Fisher’s exact test).
We next validated this finding using a number of orthogonal approaches. Deconvolution of siRNAs targeting CREBBP showed that all individual sequences resulted in a reduction in CREBBP mRNA and protein levels and an equivalent increase in spheroid size (Supplementary Fig. S2A-D). CREBBP-silenced spheroids displayed a reduction in CREBBP protein expression and concomitant increase in Ki67 staining (Fig. 1E), suggesting that this increase in size was due to an increased proliferative capacity. Silencing of CREBBP with two distinct doxycycline-inducible shRNA hairpins increased MCF10DCIS.com spheroid growth after 14 days (Supplementary Fig. S2E), and was maintained up to 30 days, showing that that continued suppression of CREBBP expression results in a sustained increase in spheroid growth, (p<0.05, t-test), (Supplementary Fig. S2F).
CREBBP loss promotes growth under nutrient stress conditions, leads to global acetylation differences and increased transcriptomic heterogeneity
Our findings demonstrating CREBBP loss specifically promoted growth in 3D conditions, is in agreement with a recent study using whole genome CRISPR screening in lung cancer, who also found CREBBP knockout had a marked 3D specific increase on spheroid size (4). These results hence suggest that environmental factors could impact its capacity as a tumour suppressor gene. Silencing of CREBBP in 2D culture under full serum normoxic (FS/Norm) conditions resulted in a reduction of cell number, whereas cell viability was increased in CREBBP silenced cells grown under low serum conditions (LS/Norm) (Fig. 2A). Growth under both hypoxia and low serum conditions (LS/Hyp) showed a substantial increase in cell number relative to siControl cells (Fig. 2A), suggesting that both nutrient stress and hypoxia are required for CREBBP silenced cells to acquire a growth advantage. The phenotypic effect of CREBBP was further confirmed in a CREBBP mutant (CREBBPmut) and wild type (WT) isogenic cell line model derived from the haploid leukaemia cell line HAP1 (Supplementary Fig. S2G). HAP1 CREBBP mutant cells had a marked four-fold increase in cell viability and significant increase in spheroid volume (p<0.001, t-test), but a significantly lower cell viability in 2D culture in keeping with our observations in the MCF10a cell lines, and thus highlighting the utility of this model (Fig. 2B).
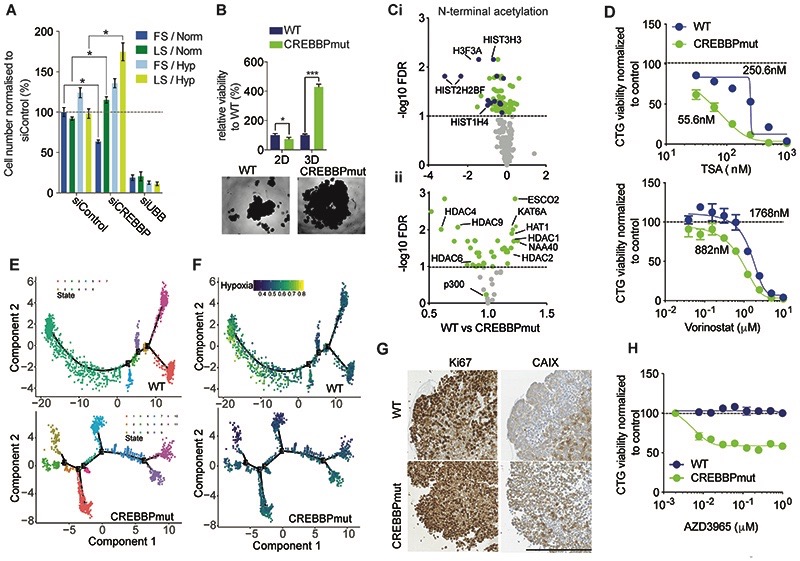
Figure 2. CREBBP loss promotes growth under nutrient stress and hypoxia and confers sensitivity to HDAC and MCT-1 inhibition.
(A) Barchart depicting normalised cell viability assessed with Cell Titre Glo of MCF10AT1 cells that were grown in 2D under differing serum (Full/10% and Low/1% FBS) and oxygen (Normoxia/20% and Hypoxia/1% O2) and in combination for four days. siUBB was used as a positive cell killing control. (B) Bar chart representing isogenic HAP1 cell growth in 2D and 3D for seven days. Representative bright-field images of WT and CREBBPmut spheroids are also shown. (C) i) Volcano plot of the significantly altered N-terminal acetylated peptides between CREBBPmut and WT cells. HAP1 WT and CREBBPmut spheroids were grown for five days. Log2 fold change is plotted against -log10 of FDR corrected p value. Histone proteins are highlighted in blue. ii) Volcano plot of fold change of total protein expression of histone acetylase and deacetylases plotted against FDR corrected p-value. (D) Dose response curves of HAP1 WT and CREBBPmut spheroids were treated with increasing concentrations of HDAC inhibitors, Tricostatin A (TSA) and Vorinostat for five days. (E) Single cell RNAseq analysis after seven days of growth. DDRTree visualization and 2-D embedding showing constructed pseudo-time transcriptional states for WT and CREBBPmut spheroid cells, depicting an increased number of branches in the CREBBPmut cells indicative of differential transcriptional programmes over time. (F) Single cell RNAseq from (E) was also analysed for the presence of a hypoxia gene signature. (G) Representative micrographs of CREBBP, Ki67, carbonic anhydrase CAIX expression in HAP1 WT and CREBBPmut spheroids. Scale bar represents 100μm, indicating increased proliferation in CREBBPmut cells and increased levels of the hypoxia marker CAIX. (H) Dose response curves of HAP1 WT and CREBBPmut spheroids treated with increasing concentrations of the selective Monocoarboxylase transporter 1 inhibitor (MCT-1) AZD3965 for 5 days, showing that CREBBPmut cells are selectively sensitive to MCT-1 inhibition. Spheroid viability was assessed using CellTiter-Glo and normalised to DMSO treated cells.
CREBBP is a histone acetyltransferase (HAT) that regulates the acetylation status of both histone and non-histone proteins (34) and loss of function mutations drive B cell lymphoma (BCL), small cell lung cancer (SCLC) and paediatric Acute Lymphoblastic Leukemias (ALLs) (35–38). We thus sought to characterise acetylation changes at the protein level induced by CREBBP mutations using mass spectrometry in the HAP1 WT and CREBBPmut cell model due to the fact CREBBP loss had the largest phenotypic effect in this model. This analysis identified 52 internal acetylated and 267 N-terminal acetylated peptides mapping to known proteins that were detected in CREBBPmut and WT cells (Supplementary Table S4). Of these 2/52 internal acetylated peptides were differentially expressed between CREBBPmut and WT cells, whereas 92/267 N-terminal acetylated peptides were differentially expressed in CREBBPmut cells compared to WT (FDR corrected p value <0.1, t-test) (Supplementary Table S4). These included dysregulation of internal acetylated peptides of the non-histone proteins including ZNF846 and HSP90AB1. The most significantly down-regulated N-terminal lysine residues mapped to histone H3 (HIST3H3 and H3F3A) and histone H2 and histone H4 in line with CREBBP’s known function (Fig. 2Ci). Furthermore, pathway analysis of these differential N-terminal acetylated proteins highlighted HDAC Class III signalling (involved in the deacetylation of acetyl lysine substrates) to be the most significantly enriched pathway (Supplementary Table S4). In agreement with this, we identified that the majority 38/56 (68%) of all detected HDAC and HAT proteins showed a significant difference in total protein expression in CREBBPmut cells. These included a number of proteins with acetyltransferase and deacetylase activity (e.g. downregulation of HDAC4, 6 and 9, upregulation of HDAC1 and 2 as well as upregulation of a number of proteins with N-terminal de/acetylase activity (Fig. 2Cii, Supplementary Data Table S4), including ESCO2, and the H2A and H4 specific N-terminal transferase NAA40). As a consequence of this HAT/HDAC dysregulation, cells were significantly more sensitive to the broad range histone deacetylase inhibitors Tricostatin A (TSA) and Vorinostat than WT cells (Fig. 2D). Taken together these results indicate that loss of CREBBP results in global dysregulation of N-terminal acetylation of lysine acetylation modulators suggesting an irreversible rewiring of epigenetic patterns that subsequently lead to changes in the total and phospho-proteome.
To further quantitatively assess whether loss of CREBBP led to diverse/global changes at the transcriptomic level, we performed single cell RNA sequencing of CREBBPmut and WT HAP1 spheroids using the 10X Genomics platform. By performing unsupervised pseudo time reconstruction analysis to study the gene expression dynamics in heterogeneous cell populations (22), we found that CREBBPmut cells clustered into eleven distinct transcriptional states compared to seven in WT cells, suggesting loss of CREBBP results in global transcriptomic alterations, again in agreement with its known function (Fig. 2E). By overlaying the published ‘Buffa’ hypoxia signature (39) onto these trajectories, we found that the CREBBPmut cells displayed a divergent response to hypoxia compared to WT cells, with WT cells showing a distinct canonical response to hypoxia as there was a single but large subpopulation that showed an increase in hypoxic gene signature expression. In contrast, cells from CREBBPmut spheroids did not display transcriptional changes in response to hypoxia (Fig. 2F). Indeed, when we analysed the protein expression of the proliferative marker Ki67, CREBBPmut spheroids had sustained expression of Ki67 towards the centre of the sphere while WT spheroids did not, suggesting that CREBBPmut cells were more able to proliferate under an unfavourable environment (Fig. 2G). Moreover, CREBBPmut spheroids displayed ubiquitous staining of the hypoxic marker carbonic anhydrase IX (CAIX), whereas WT spheroids showed only an increase in CAIX towards the core, supporting the hypothesis that CREBBPmut spheroids are distinct in how they respond to low oxygen. As hypoxic cells heavily rely on glycolysis for energy production rather than lactate (as in normoxic cells), inhibition of monocarboxylase transporter 1 (MCT1) that controls bi-directional transport of lactate across the extracellular membrane has been shown to be preferentially kill hypoxic cells through glucose deprivation (40). Indeed, treatment with the MCT1 inhibitor AZD3965 significantly reduced viability in CREBBPmut spheroids, while having no effect on WT spheroids (Fig. 2H). Together, these results suggest that loss of CREBBP induces hypoxia in cells and promotes their survival when they encounter nutrient stress and low oxygen conditions.
CREBBP protein expression is associated with a poor outcome in TNBC and protein loss is common in other tumour types that harbour CREBBP mutations
To investigate the clinical implications of CREBBP alterations in TNBC, we evaluated patient data from The Cancer Genome Atlas (TCGA) (1,2) and Molecular Taxonomy of Breast Cancer International Consortium (METABRIC) cohorts (1,41). The mutational frequency of CREBBP in breast cancer (TCGA) identified an enrichment of mutations in TNBCs, compared to ER-positive disease (Fig. 3A). In addition, heterozygous CREBBP copy number losses were observed in 25-33% of patients from the TCGA and METABRIC and MSKCC cohorts (1,33,41) (Fig. 3B). Stratifying patients as WT or with CREBBP mutations and/or copy number losses (CREBBPaltered) showed that CREBBPaltered tumours had a significant reduction in CREBBP mRNA expression (p<0.05, t-test) (Fig. 3C), indicating a dose-dependent correlation between copy number and gene expression. Assessment of CREBBP protein expression on tissue microarrays (TMAs) from two independent cohorts of TNBCs (n=174), identified a reduction in CREBBP protein levels in 48.3% of TNBC’s, with 20.1% showing either low or no CREBBP protein expression (11.5 low and 8.6% no expression, respectively) (Fig. 3D). We also observed a significant correlation between CREBBP mRNA expression and protein expression as detected by IHC (p=0.004, Mann Whitney U test). (Supplementary Figure S3A). TNBCs that had lower levels of CREBBP mRNA expression had a significantly reduced disease-specific free survival (DSS), compared to high expressing tumours in the METABRIC cohort (p=0.021, Wald test, HR=0.48, 95% CI=0.26-0.90, multivariable analysis) (Fig. 3E). Corroborating these results, a trend towards an association of reduced CREBBP protein levels and poorer distant metastasis-free survival (DMFS) was observed using immunohistochemistry in TNBC patients (p=0.11, Wald test, HR=0.52, 95% CI= 0.23-1.16, multivariable analysis) (Fig. 3F). A significant association with DMFS was also observed at the mRNA level in the Belgrade cohort (p=0.04, Wald test, HR=0.48, 95% CI=0.24-0.97, multivariable analysis) (Fig. 3G).
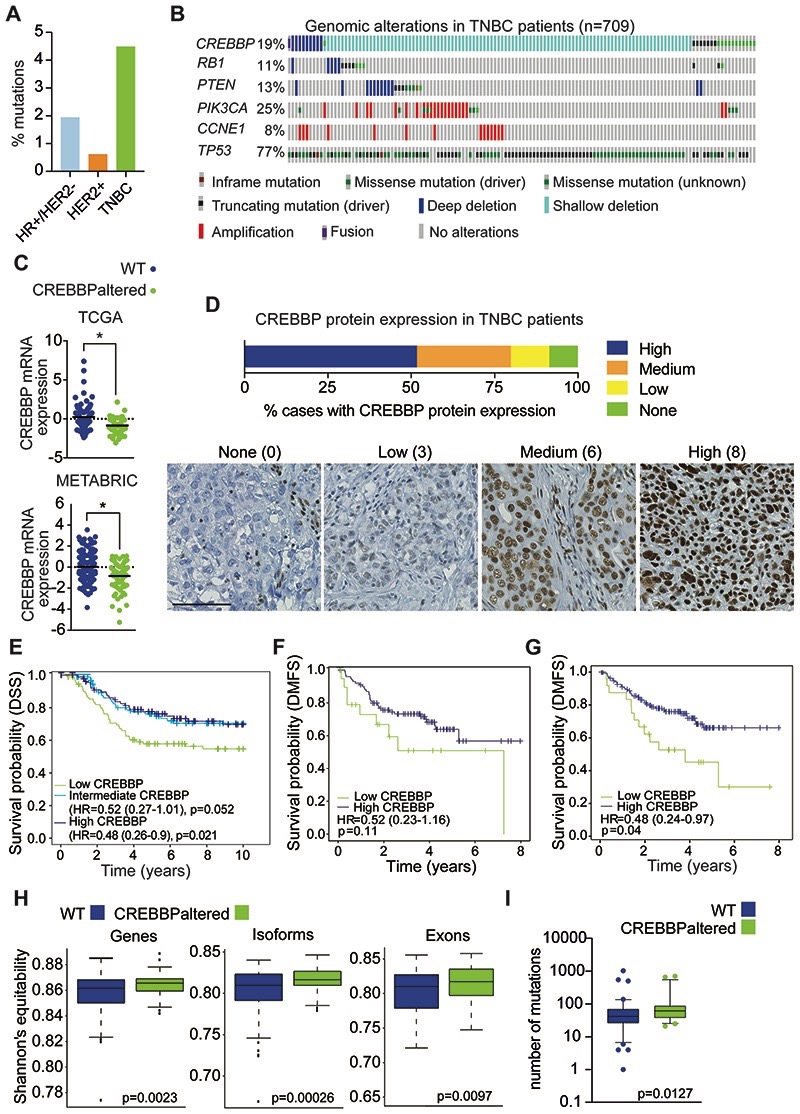
Figure 3. CREBBP protein expression is associated with a poor prognosis in TNBC.
(A) Frequency plot of CREBBP mutations in breast cancer (from TCGA) stratified on subtype. (B) Oncoprint plot of genomic alterations in CREBBP in a combined analysis of TNBC (n=709) from TCGA, METABRIC and MSKCC cohorts. Frequencies of additional genomic alterations in TNBC are also depicted. (C) CREBBP mRNA expression in TNBCs stratified on CREBBP status in TCGA and METABRIC. (D) Bar chart depicting frequency of CREBBP protein expression in TNBCs from tissue microarrays of the Belgrade and BR10011a cohorts combined (n = 174). Representative micrographs of CREBBP protein expression are shown from Belgrade and corresponding Allred scores. Scale bar represents 100μm. (E) Kaplan-Meier plots for disease specific survival (DSS) in triple negative breast cancers from METABRIC (n=276) and that were stratified on their CREBBP mRNA expression (high, intermediate and low). Multivariable survival analysis was performed by taking into account CREBBP mRNA expression status, age, tumour size, node status and grade. (F) Kaplan-Meier plots for distant metastasis-free survival (DMFS) in triple negative breast cancers that were stratified on their CREBBP protein expression (high and low) from the Belgrade cohort that had associated outcome data. Low CREBBP expression was defined as Allred score <4. Multivariable survival analysis was performed by taking into account CREBBP protein expression status, age, TNM stage and grade. (G) Kaplan-Meier plots for distant metastasis-free survival (DMFS) in triple negative breast cancers that were stratified on their CREBBP mRNA expression (high and low) from the Belgrade cohort that had associated outcome data. Multivariable survival analysis was performed by taking into account CREBBP mRNA expression status, age, TNM stage and grade. (H) Box plots of the diversity of gene, isoform and exon expression in CREBBP WT and CREBBPaltered TNBCs. Diversity index was calculated using Shannon’s equitability index. (I) Box plots of the mutational burden in CREBBP WT and CREBBPaltered TNBCs.
Additional analysis of TCGA data identified that a considerable proportion of other solid tumours had a high frequency of alterations in CREBBP including uterine, ovarian, squamous lung and bladder cancers (Supplementary Fig. S3B). Moreover, protein assessment with IHC on TMAs of endometrial, squamous lung, bladder and high grade serous ovarian cancers identified a substantial proportion of these tumour types to have reduced CREBBP protein levels (Allred score <4) (Supplementary Fig. S3C), highlighting that loss of CREBBP protein expression is a recurrent alteration that occurs in multiple tumour types.
Recent data has highlighted the importance of cellular phenotypic heterogeneity in therapeutic resistance in ER+ breast cancer mediated through disrupted expression in the lysine deacetylase protein KDM5 (24). Akin to this, we hypothesised that alterations in CREBBP may also lead to changes in tumour heterogeneity in TNBC. Indeed, patients with CREBBP alterations displayed a higher transcriptional diversity at the gene, isoform and exon level (p<0.01, Welch’s t-test) and higher mutational burden (Fig. 3H-I, Supplementary Fig. S3D). These observations suggest that CREBBP status in patients influences transcriptomic heterogeneity, a known factor, that may determine treatment response (42), in keeping with our observed correlations with a poor patient outcome.
CREBBP alterations lead to increased proliferation via FOXM1 expression and activity and are selectively sensitive to CDK4/6 inhibitors
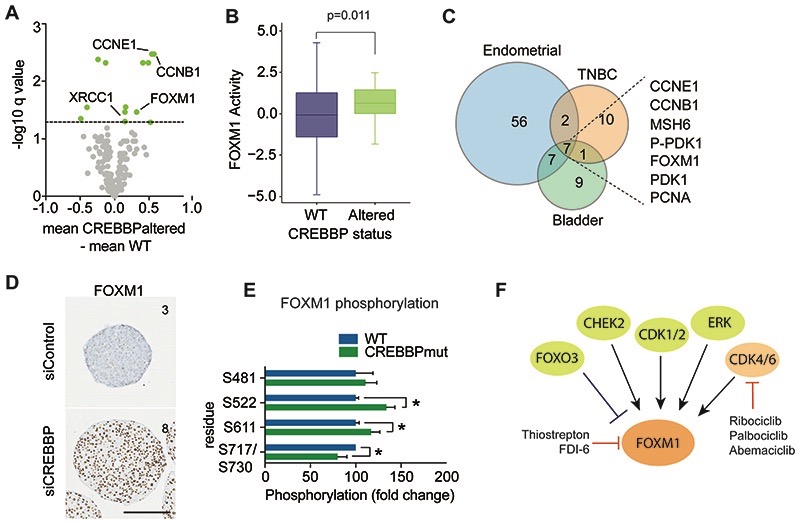
To elucidate altered pathways driving the aggressive nature of CREBBPaltered TNBCs, we compared the transcriptomic profiles and reverse phase protein array (RPPA) proteomic profiles from patients with CREBBPaltered TNBC to TNBCs without CREBBP alterations (WT) from TCGA. In total 1062 transcripts and 21 proteins (total or phosphorylated) were significantly up or down regulated in CREBBPaltered TNBCs, (Fig. 4A, Supplementary Table S5). Common pathways enriched at both the gene expression and protein level in CREBBPaltered TNBC identified the oncogenic FOXM1 transcription factor network to be the most significantly enriched pathway in CREBBPaltered tumours (protein level q value =7.01E-9 and transcript level q value =2.28E-6). This unbiased analysis also identified enrichment of a number of pathways involving G1/S cell cycle control (Supplementary Fig. S4A) (43), highlighting that in TNBC patients CREBBP alterations lead to increased proliferation, in keeping with our observations in vitro. Further analysis of TCGA patient RNA-seq data using unbiased transcription factor activity module analysis (30) also highlighted that CREBBPaltered TNBC’s showed significantly higher FOXM1 transcriptional activity, (p=0.011, t-test), (Fig. 4B). FOXM1, and known downstream targets were also significantly upregulated at the protein level in bladder and endometrial cancers with genomic alterations in CREBBP, demonstrating that increased proliferation is a general feature of CREBBPaltered tumours (q value <0.05), (Fig. 4C, Supplementary Fig. S4B-C). FOXM1 upregulation was further validated in CREBBP-silenced MCF10DCIS.com spheroids (Fig. 4D) as well as HAP1 CREBBPmut cells (Supplementary Fig. S4D) using immunohistochemistry. Assessment of phosphoproteome analysis of WT and CREBBPmut spheroids, highlighted a significant increase in FOXM1 phosphorylation on residues S522 and S611, in CREBBPmut spheroids (Supplementary Table S4), which are two sites that have been shown to be exclusively regulated by CDK4/6 (20) (Fig. 4E). In addition, we identified an enrichment of CDK4 and CDK6 kinase motifs in the CREBBPmut spheroids, suggesting that loss of CREBBP leads to enhanced CDK4 and CDK6 activity (Supplementary Fig. S4E) (20,21).
Figure 4. CREBBPaltered cancers show increased FOXM1 driven proliferation.
(A) Volcano plot of fold change in protein expression (x-axis) from reverse phase protein RPPA data of TNBCs stratified on CREBBP status, plotted against –log10P value (y-axis). Significant alterations in protein expression are highlighted in orange (FDR corrected p values <0.1). (B) Box plot of FOXM1 gene activity in CREBBPaltered TNBC. mRNA expression from TCGA was used to calculate FOXM1 activity utilizing a rank-based enrichment analysis (see Supplementary methods). (C) Venn diagram of significantly altered proteins between CREBBP-altered and WT tumours from RPPA data of TNBCs, endometrial and bladder cancers. (D) Representative micrographs of FOXM1 protein expression from 7-day spheroids of MCF10DCIS.com after siRNA silencing of CREBBP or non-targeting control. Text depicts IHC quantification (Allred scores). (Scale bar represents 200μm). (E) Bar plot of fold change in phosphorylated protein expression (x-axis) from mass spectrometry assessment of day 4 spheroids of HAP1 WT and CREBBPmut spheroids, plotted against –log10P value (y-axis). (F) A diagram of FOXM1 and the proteins that regulate its activity. Direct and indirect inhibitors are highlighted.
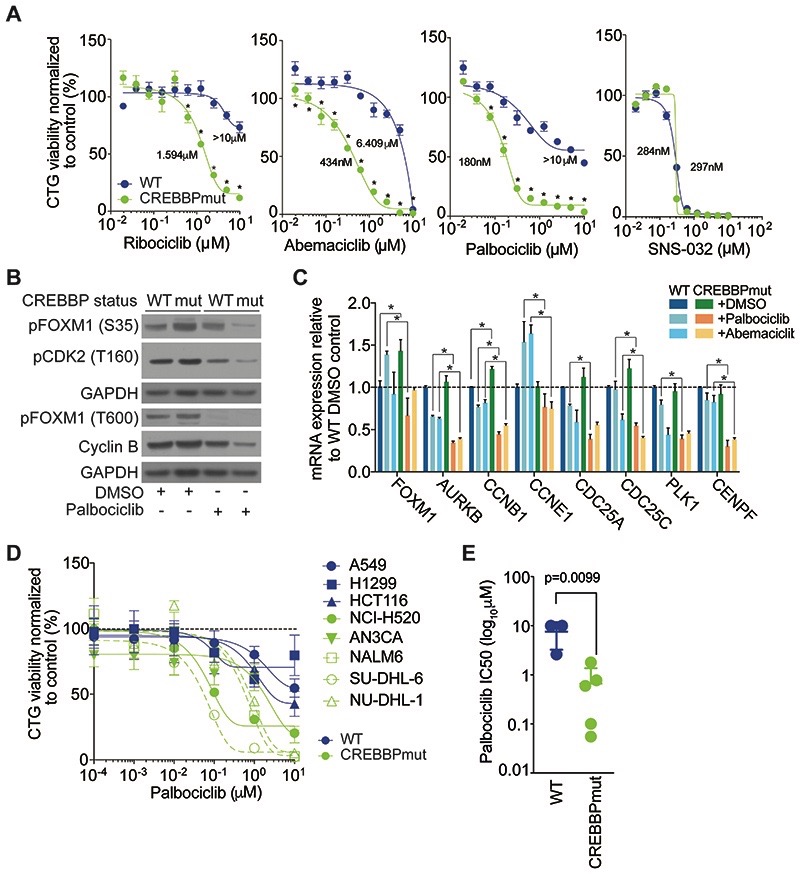
FOXM1 can be indirectly targeted through suppression of CDK4/6 phosphorylation sites using CDK4/6i (20) (Fig 4F). Indeed, treatment of WT and CREBBPmut spheroids with three structurally distinct clinical CDK4/6i’s (Ribociclib, Palbociclib and Abemaciclib) demonstrated a marked increase in sensitivity in CREBBPmut cells compared to WT cells (CREBBPmut SF50 were 180, 434 and 1594nM, WT SF50 were >10, 6.4 and >10μM for Palbociclib, Abemaciclib and Ribociclib, respectively) (Fig. 5A). No difference was observed in sensitivity upon treatment with the CDK2/7/9 inhibitor SNS-032, highlighting the specificity of targeting CDK4 and CDK6 in these cells. Confirming our phosphoproteomic analysis, we observed an increase in FOXM1 phosphorylation (S35) in CREBBPmut spheroids, which was reduced with exposure to the CDK4/6i Palbociclib in CREBBPmut spheroids, while little effect was detected in WT spheroids (Fig. 5B). Treatment of cells with CDK4/6i, also led to a concomitant decrease in Cyclin B protein expression, and reduced mRNA expression of known direct FOXM1 target genes AURKB, CCNB1, CCNE1, CDC25C and CENPF (Fig. 5C), demonstrating that CDK4/6i could selectively target the proliferative transcriptional activity of FOXM1 in CREBBPaltered cells. We further validated the CREBBP/CDK4/6i association in a histologically diverse panel of tumour cell lines. Compared to WT cell lines (lung; A549, H1299 and colon: HCT116) additional CREBBP mutant lung, endometrial, leukaemia and B-cell lymphoma cell lines (NCI-H520, AN3CA, NALM-6, SU-DHL-6 and NU-DHL-1) were significantly more sensitive to Palbociclib, (p=0.0099, t-test), indicating that this effect is likely not restricted to cancer cells from just one tumour type (Fig. 5D-E). In addition, in TNBC cell lines where CREBBP silencing leads to increased growth, it also induces a greater sensitivity to Palbociclib compared to the non-targeting control (Supplementary Fig. S5A). In line with the 3D specific effect of CREBBP silencing on growth, we also find that sensitivity to CDK4/6i is specific to spheroid cultures (Supplementary Fig. S5B-D).
Figure 5. CREBBP loss sensitises cells to CDK4/6i.
(A) Dose response curves of HAP1 WT and CREBBPmut cells treated with increasing concentrations of the clinical CDK inhibitors Ribociclib, Abemaciclib, Palbociclib and SNS-023 at the concentrations indicated. (B) Western blot of protein lysates of HAP1 WT and CREBBPmut cells treated with 1μM of Palbociclib or DMSO for 48hrs showing suppression of specific phospho sites in CREBBPmut cells. (C) Bar chart of relative mRNA expression of FOXM1 target genes in HAP1 WT and CREBBPmut cells +/- Palbociclib (250nM), Abemaciclib (500nM) or DMSO treatment at 24 hours after cell seeding for 72hrs. Gene expression was quantified using RT-PCR. (D) Dose response curves of a panel of non-isogenic cancer cell lines (WT= lung: A549, H1299, colorectal: HCT116, and CREBBPmut lung NCI-H520, endometrial AN3CA, leukaemia NALM-6, and lymphoma SU-DHL-6 and NU-DHL-1) spheroids treated with increasing concentrations of Palbociclib for five days and displayed according to CREBBP status. (E) Bar chart of SF50 values of non-isogenic CREBBPmut and WT cell lines grown as spheroids and treated with Palbociclib for 5 days.
CREBBPaltered cells show selective sensitivity to CDK4/6 inhibitors in vivo
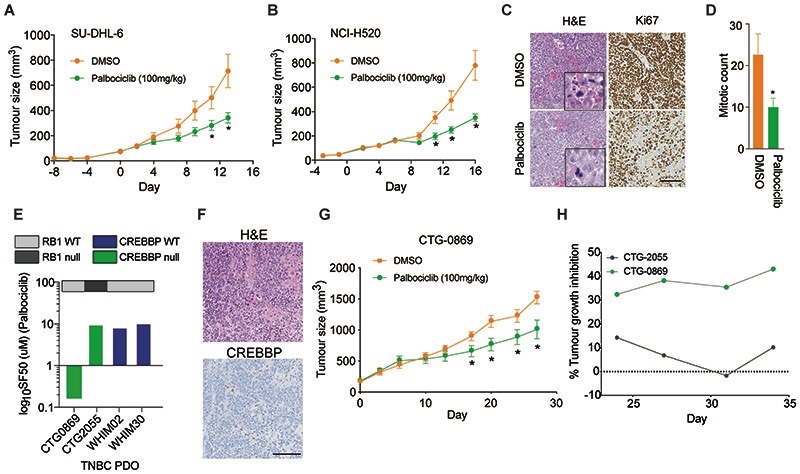
We next assessed whether the clinically approved CDK4/6i, Palbociclib, could inhibit CREBBPaltered tumours in vivo. To do this, we generated cohorts of mice with established xenograft tumours derived from either the high-grade lymphoma cell line SU-DHL-6 that harbours an inactivating frameshift mutation (L470*) in CREBBP (36) or the aggressive lung squamous cell carcinoma cell line NCI-H520, which harbours the recurrent CREBBP hotspot mutation R1446C, resulting in inactivation of HAT domain activity (44). Continuous treatment of tumours with Palbociclib (100mg/kg) daily for 14-16 days had a marked effect on tumour growth and weight, (p<0.05, t-test) (Fig. 6A-B) and significant increase in survival (p=0.0178 SU-DHL-6 and p=0.0002 NCI-H520, Supplementary Fig. S6A-E). Palbociclib-treated tumours displayed a significant reduction in the proliferative fraction of the tumour measured by Ki67 expression (p=0.0089, t-test) and mitotic index (p=0.0160, t-test), (Fig. 6C-D). To model the clinical scenario, a chemotherapy pre-treated TNBC (CTG-0869) patient derived organoid (PDO) negative for CREBBP protein expression that showed no clinical response to Docetaxel or Capecitabine/Bevacizumab, grown ex vivo was sensitive to Palbociclib treatment (SF50 = 160nM) (Fig. 6E), whereas CREBBPnull RB1 deficient and CREBBPWT RB1 proficient TNBC PDO’s were not (Fig. 6E, Supplementary Fig. S7). Treatment of the CREBBPnull RB1 proficient model CTG-0869 grown in vivo also showed a significant reduction in tumour growth and extended survival, an effect which was superior to the chemotherapy Gemcitabine (Fig. 6F-H, Supplementary Fig. S8A-C). Moreover, Palbociclib treatment of an additional CREBBPnull RB1null TNBC PDX CTG-2055 in vivo showed no response (Supplementary Fig. S8D). Together, our results demonstrate that CREBBP is a novel tumour suppressor gene in TNBC and suggest that CDK4/6i could have potential as treatments for aggressive CREBBPaltered cancers, who have failed standard treatment.
Figure 6. CREBBPaltered tumours are sensitive to CDK4/6i in vivo.
(A) Chart depicting tumour volume of the therapeutic response to Palbociclib treatment in immunocompromised mice bearing CREBBP-mutant SU-DHL-6 tumours over time. Tumour volumes after the initiation of treatment are shown. (B) Chart depicting tumour volume of the therapeutic response to Palbociclib treatment in immunocompromised mice bearing CREBBP–mutant NCI-H520 tumours over time. Tumour volumes after the initiation of treatment are shown. (C) Immunohistochemical staining of representative tumours harvested from CREBBPmutant NCI-H520 tumours for Ki67 and H&E (scale bar represents 100μm). (D) Bar chart showing the quantification of mitotic counts in Palbociclib and control treated animals from (C). (E) Barchart depicting SF50 values of Palbociclib in a panel of TNBC PDO’s. CREBBP and RB1 status are shown. (F) Representative micrographs of H&E and CREBBP protein expression of the TNBC PDX CTG-0869 depicting protein loss of CREBBP. Scale bar represents 100μm. (G) Chart depicting CTG-0869 tumour volume of the therapeutic response to Palbociclib treatment in immunocompromised mice. Tumour volumes after the initiation of treatment are shown. (H) Chart depicting tumour growth inhibition (TGI) as percentage of DMSO treated mice bearing CTG-0869 and CTG-2055 TNBC xenografts treated with Palbociclib 100mg/ml after 3 weeks of treatment until experiment endpoint.
Discussion
Our approach using 3D spheroid models to identify novel cancer drivers identified a number of genes not previously implicated in TNBC pathogenesis. Our data deconvoluting the phenotypic effect of CREBBP silencing suggests that this is likely to be regulated via hypoxia and nutrient stress. This is in keeping with recent studies demonstrating that histone demethylases directly sense oxygen to control chromatin and cell fate (45). It is thus likely additional histone acetylases and deacetylases also have similar oxygen sensing roles. Our data is in agreement with a recent pan cancer analysis study that highlighted CREBBP mutations to be one of the most highly correlated mutated genes in tumours with elevated hypoxic signatures (46). Additionally, in support of our overall approach, a recent unbiased 3D CRISPR loss of function screen in lung cancer cell line spheroids identified an enrichment of genes that had 3D specific phenotypic effects that more closely mirrored in vivo phenotypes. Moreover, these were highly enriched for commonly mutated genes in lung cancer including CREBBP (4). This is in keeping with studies in GEMM models which have also highlighted that CREBBP deletions enhance tumorigenesis in B-cell lymphoma and small cell lung cancer models (35,47) and suggests that CREBBP inhibitors currently being tested in patients may not have clinical utility to treat these cancers (48).
Recent studies have demonstrated synthetic-lethal interactions between CREBBP and its orthologue EP300, highlighting the potential utility of EP300 inhibitors for CREBBP deficient tumours (49). In addition, HDAC inhibitors have been shown to be effective in CREBBP mutant tumours, which act via restoration of acetylation and expression of cellular adhesion proteins and increased dependency on HDAC3 (47). Our data also suggest that changes in N-terminal acetylation of proteins involved in HDAC3 activity may lead to increased sensitivity to HDAC inhibition through resultant increase in expression of multiple HDAC proteins as consequence of CREBBP loss. However, in our models, we found that the CREBBP/CDK4/6i selectivity was more profound than these other proposed CREBBP-targeted agents, highlighting that the effect with HDACi may have less efficacy in patients.
In agreement with the loss of function mutation of other epigenetic modulators (24), we identified CREBBP alterations to result in higher transcriptional heterogeneity while maintaining a capacity for cells to survive under nutrient stress conditions i.e. those that you would find in tumours that display hypoxia and have a poor prognosis. This phenotype was validated in TNBC CREBBPaltered patients, where CREBBP mutations or copy number losses resulted in a greater transcriptional diversity. Thus, we propose a model whereby CREBBP loss imparts a more aggressive disease trajectory in TNBC as a result of increased transcriptomic diversity. This is in agreement with the clinical observations that CREBBPaltered tumours are less sensitive to established anticancer therapies and patients are more likely to relapse. Interestingly, CREBBP mutations have been found to be enriched in metastatic ER+ breast cancers, suggesting a role in therapy resistance in agreement with our data in TNBC (33). We identified a substantial proportion of patients with depletion of CREBBP expression through immunohistochemical assessment across multiple tumour cohorts represented on TMAs, including endometrial, bladder, ovarian and squamous lung cancer. For instance, only 8% of squamous lung cancers are known to harbour mutations in CREBBP (3), however, we observed protein depletion in up to 73% of cases suggesting multiple mechanisms exist associated with reduced expression (3). This highlights that protein loss may be a useful for patient stratification in the context of biomarker driven clinical trials, and demonstrates that alterations of CREBBP are common in multiple aggressive cancers.
To date CDK4/6 inhibitors are licensed for use in metastatic ER+ breast cancer and not used in TNBC, but their clinical efficacy is thought to be dependent on a functional RB axis (50) and in the context of TNBC and squamous cell lung cancers RB1 inactivation is relatively common (1,3). Although our in vitro and in vivo data suggest a functional RB1 axis is also required for response in TNBC, the majority of CREBBPaltered TNBC (78%) and squamous lung cancers (79%) harbour a functional RB1 axis. In addition, there is some evidence to suggest that CDK4/6 can drive FOXM1-mediated transcription in the absence of RB1 (20,51,52). For instance, silencing RB1 in FOXM1 amplified osteosarcoma patient derived cells in vivo did not reverse CDK4/6i sensitivity suggesting that in the context of FOXM1 amplification, CDK4/6 inhibition is effective even in the absence of RB1. These data suggest that other mechanisms of FOXM1 upregulation outside the context of CREBBP loss can also sensitise cells to CDK4/6i (52). Of note we observed only 22% of RB1 loss of function alterations or CCNE1 amplifications (known mechanisms of resistance to CDK4/6i) in CREBBPaltered TNBC patients.
In summary, the results from our study demonstrate that CREBBP alterations impart a survival advantage for cancer cells via enhanced proliferation. This is mediated through global changes in acetylation and subsequent transcriptional alterations including upregulation of the FOXM1 proliferative signalling axis. As a consequence, this increased proliferative capacity renders CREBBPaltered cells sensitive to CDK4/6 inhibition, via inhibition of CDK4/6 mediated FOXM1 phosphorylation; a synthetic-lethal association that was demonstrated in multiple tumour types both in vitro and in vivo. The highly recurrent nature of CREBBP alterations in treatment resistant TNBC, lung, bladder and lymphomas and the availability of FDA approved CDK4/6i suggest this clinically translatable approach could benefit a wide range of cancer patients as single agent or in combination with immunotherapeutic or other targeted approaches. Indeed, proof of concept trials such as NCT03130439 which is evaluating the efficacy of single agent Abemaciclib in RB1 proficient TNBC will be crucial to interpreting our results further. Of note although a recent phase 2 study in squamous lung cancers testing single agent Palbociclib in CCND1, CCND2, CCND3 or CDK4 amplified patients failed to demonstrate the prespecified criteria for advancement to phase III, data from TCGA suggests only a small overlap between these alterations and CREBBP mutations; indicating the population may have been missed in the study (53).
Recent data has suggested that the use of CDK4/6i induces an intra-tumoral T cell inflammatory signature that results in an enhanced response to PD-L1 (54,55). Of note we observed a significantly higher tumour mutational burden (a known biomarker of immunotherapy response) in CREBBPaltered TNBC, bladder and squamous lung patients as well as in a pan cancer analysis. The mechanism behind this increased mutational burden is unclear, but may be an indirect consequence of survival in hypoxic environments, which has been linked with downregulation of effective DNA repair mechanisms and accumulation of mutations (56). Although it is tempting to speculate that CREBBPaltered tumours may respond well to combinations of immune checkpoint blockade and CDK4/6i treatments, this needs to be tested in the clinical trial setting. However, our data provide the rationale for a molecularly stratified clinical trial.
Supplementary Material
Significance.
This study demonstrates that CREBBP genomic alterations drive aggressive TNBC, lung cancer, and lymphomas and may be selectively treated with clinical CDK4/6 inhibitors.
Acknowledgements
This work was funded by Programme Grants from Breast Cancer Now as part of Programme Funding to the Breast Cancer Now Toby Robins Research Centre (B Peck, P Bland, G Muirhead, SL Maguire, D Kriplani, A Gibson, R Marlow, R Buus, D Novo, E Knight, N Guppy, P Gazinska, I Roxanis, LA Martin, EM Holgersen, F Noor, F Daley, F Wallberg, S Haider, AN Tutt, R Natrajan) and by funding from Breast Cancer Now’s Catalyst Programme (Grant Ref:2018NovPR100; I Mavrommati, R Natrajan) which is supported by funding from Pfizer; The Institute of Cancer Research (G Falgari, PT Wai, L Yu, J Choudhary, R Natrajan); MRC Confidence in Concept award (R Natrajan); Breast Cancer Now PhD studentship (2013NovPhD185, EM); NC3Rs NC/P001262/1 (AN Tutt); Breast Cancer Now project grant (2014NovPR36, L Krasny, P Huang). PDO generation was funded by Programme Grant funding to ANT from Breast Cancer Now (CTR-Q4) as part of funding to the Breast Cancer Now Toby Robins Research Centre. ICR private donations funds were used to support the PDO work (R Marlow, AN Tutt). In addition, the Breast Cancer Now Research Unit at King’s College London obtained funding from the National Institute for Health Research Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London (AN Tutt). We thank Charlotte Ng for input into generation of the gene list. We thank Charles Mein, Eva Wozniak and Hothri Ananyambica Moka at the Barts sequencing facility for running the single cell RNA-sequencing and to Clare Isacke and Chris Jones for useful discussions. The results shown here are in part based upon data generated by the TCGA Research Network: http://cancergenome.nih.gov/. This study makes use of METABRIC data generated by the Molecular Taxonomy of Breast Cancer International Consortium with funding provided by Cancer Research UK and the British Columbia Cancer Agency Branch. We acknowledge NHS funding to the ICR/Royal Marsden Hospital Biomedical Research Centre.
Footnotes
Author’s Contributions
Conception and design: BP and RN
Development of methodology: BP, PB, FW, PG, FD.
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): BP, PB, IM, HC, PTW, HB, EM, DK, LY, AG, GF, KB, GF, RB, RM, DN, EK, NG, DK, SS, NMM, KN, PG, IR, SP, LAM, FN, SPV, FD, JC, ANT, RN.
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): BP, PB, IM, GM, SLM, MCUC, RM, DN, EK, EMH, GQ, SMcD, SPV, LK, PH, SH and RN
Writing, review, and/or revision of the manuscript: all authors
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): GM, SH
Study supervision: BP and RN.
Disclosure of Potential Conflicts of Interest.
ANT has served on advisory boards for Pfizer. SPV is a Chief investigator on a number of clinical trials involving other compounds with Pfizer. LAM receives academic funding from Pfizer. No potential conflicts of interest were disclosed by the other authors.
Data availability
Raw proteomic data is available in PRIDE (RRID:SCR_012052) accession PXD0120978. Single cell data has been deposited in the European Nucelotide Archive (ENA) with accession number PRJEB32846. Code used in the analysis of this data is provided https://zenodo.org/record/4020438.
References
- 1.Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciriello G, Gatza ML, Beck AH, Wilkerson MD, Rhie SK, Pastore A, et al. Comprehensive Molecular Portraits of Invasive Lobular Breast Cancer. Cell. 2015;163:506–19. doi: 10.1016/j.cell.2015.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research N. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–25. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han K, Pierce SE, Li A, Spees K, Anderson GR, Seoane JA, et al. CRISPR screens in cancer spheroids identify 3D growth-specific vulnerabilities. Nature. 2020;580:136–41. doi: 10.1038/s41586-020-2099-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrison E, Wai P, Leonidou A, Bland P, Khalique S, Farnie G, et al. Utilizing Functional Genomics Screening to Identify Potentially Novel Drug Targets in Cancer Cell Spheroid Cultures. J Vis Exp. 2016 doi: 10.3791/54738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashworth A, Lord CJ. Synthetic lethal therapies for cancer: what’s next after PARP inhibitors? Nat Rev Clin Oncol. 2018;15:564–76. doi: 10.1038/s41571-018-0055-6. [DOI] [PubMed] [Google Scholar]
- 7.Lord CJ, Tutt AN, Ashworth A. Synthetic lethality and cancer therapy: lessons learned from the development of PARP inhibitors. Annu Rev Med. 2015;66:455–70. doi: 10.1146/annurev-med-050913-022545. [DOI] [PubMed] [Google Scholar]
- 8.Masuda H, Baggerly KA, Wang Y, Zhang Y, Gonzalez-Angulo AM, Meric-Bernstam F, et al. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin Cancer Res. 2013;19:5533–40. doi: 10.1158/1078-0432.CCR-13-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Symmans WF, Wei C, Gould R, Yu X, Zhang Y, Liu M, et al. Long-Term Prognostic Risk After Neoadjuvant Chemotherapy Associated With Residual Cancer Burden and Breast Cancer Subtype. J Clin Oncol. 2017;35:1049–60. doi: 10.1200/JCO.2015.63.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–5. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maguire SL, Peck B, Wai PT, Campbell J, Barker H, Gulati A, et al. Three-dimensional modelling identifies novel genetic dependencies associated with breast cancer progression in the isogenic MCF10 model. J Pathol. 2016;240:315–28. doi: 10.1002/path.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banerji S, Cibulskis K, Rangel-Escareno C, Brown KK, Carter SL, Frederick AM, et al. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature. 2012;486:405–9. doi: 10.1038/nature11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding L, Ellis MJ, Li S, Larson DE, Chen K, Wallis JW, et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010;464:999–1005. doi: 10.1038/nature08989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah SP, Morin RD, Khattra J, Prentice L, Pugh T, Burleigh A, et al. Mutational evolution in a lobular breast tumour profiled at single nucleotide resolution. Nature. 2009;461:809–13. doi: 10.1038/nature08489. [DOI] [PubMed] [Google Scholar]
- 15.Shah SP, Roth A, Goya R, Oloumi A, Ha G, Zhao Y, et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486:395–9. doi: 10.1038/nature10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephens PJ, Tarpey PS, Davies H, Van Loo P, Greenman C, Wedge DC, et al. The landscape of cancer genes and mutational processes in breast cancer. Nature. 2012;486:400–4. doi: 10.1038/nature11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiederschain D, Wee S, Chen L, Loo A, Yang G, Huang A, et al. Single-vector inducible lentiviral RNAi system for oncology target validation. Cell Cycle. 2009;8:498–504. doi: 10.4161/cc.8.3.7701. [DOI] [PubMed] [Google Scholar]
- 18.Hornbeck PV, Zhang B, Murray B, Kornhauser JM, Latham V, Skrzypek E. PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res. 2015;43:D512-20. doi: 10.1093/nar/gku1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He Z, Yang C, Guo G, Li N, Yu W. Motif-All: discovering all phosphorylation motifs. BMC Bioinformatics. 2011;12(Suppl 1):S22. doi: 10.1186/1471-2105-12-S1-S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anders L, Ke N, Hydbring P, Choi YJ, Widlund HR, Chick JM, et al. A systematic screen for CDK4/6 substrates links FOXM1 phosphorylation to senescence suppression in cancer cells. Cancer Cell. 2011;20:620–34. doi: 10.1016/j.ccr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitagawa M, Higashi H, Jung HK, Suzuki-Takahashi I, Ikeda M, Tamai K, et al. The consensus motif for phosphorylation by cyclin D1-Cdk4 is different from that for phosphorylation by cyclin A/E-Cdk2. EMBO J. 1996;15:7060–9. [PMC free article] [PubMed] [Google Scholar]
- 22.Qiu X, Mao Q, Tang Y, Wang L, Chawla R, Pliner HA, et al. Reversed graph embedding resolves complex single-cell trajectories. Nat Methods. 2017;14:979–82. doi: 10.1038/nmeth.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hinohara K, Wu HJ, Vigneau S, McDonald TO, Igarashi KJ, Yamamoto KN, et al. KDM5 Histone Demethylase Activity Links Cellular Transcriptomic Heterogeneity to Therapeutic Resistance. Cancer Cell. 2018;34:939–53.:e9. doi: 10.1016/j.ccell.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waggott D, Chu K, Yin S, Wouters BG, Liu FF, Boutros PC. NanoStringNorm: an extensible R package for the pre-processing of NanoString mRNA and miRNA data. Bioinformatics. 2012;28:1546–8. doi: 10.1093/bioinformatics/bts188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li S, Shen D, Shao J, Crowder R, Liu W, Prat A, et al. Endocrine-therapy-resistant ESR1 variants revealed by genomic characterization of breast-cancer-derived xenografts. Cell Rep. 2013;4:1116–30. doi: 10.1016/j.celrep.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–4. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamburov A, Wierling C, Lehrach H, Herwig R. ConsensusPathDB--a database for integrating human functional interaction networks. Nucleic Acids Res. 2009;37:D623-8. doi: 10.1093/nar/gkn698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Alonso L, Iorio F, Matchan A, Fonseca N, Jaaks P, Peat G, et al. Transcription Factor Activities Enhance Markers of Drug Sensitivity in Cancer. Cancer Res. 2018;78:769–80. doi: 10.1158/0008-5472.CAN-17-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schug ZT, Peck B, Jones DT, Zhang Q, Grosskurth S, Alam IS, et al. Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell. 2015;27:57–71. doi: 10.1016/j.ccell.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peck B, Schug ZT, Zhang Q, Dankworth B, Jones DT, Smethurst E, et al. Inhibition of fatty acid desaturation is detrimental to cancer cell survival in metabolically compromised environments. Cancer Metab. 2016;4:6. doi: 10.1186/s40170-016-0146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Razavi P, Chang MT, Xu G, Bandlamudi C, Ross DS, Vasan N, et al. The Genomic Landscape of Endocrine-Resistant Advanced Breast Cancers. Cancer Cell. 2018;34:427–38.:e6. doi: 10.1016/j.ccell.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bannister AJ, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–3. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Vlasevska S, Wells VA, Nataraj S, Holmes AB, Duval R, et al. The CREBBP Acetyltransferase Is a Haploinsufficient Tumor Suppressor in B-cell Lymphoma. Cancer Discov. 2017;7:322–37. doi: 10.1158/2159-8290.CD-16-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pasqualucci L, Dominguez-Sola D, Chiarenza A, Fabbri G, Grunn A, Trifonov V, et al. Inactivating mutations of acetyltransferase genes in B-cell lymphoma. Nature. 2011;471:189–95. doi: 10.1038/nature09730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peifer M, Fernandez-Cuesta L, Sos ML, George J, Seidel D, Kasper LH, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet. 2012;44:1104–10. doi: 10.1038/ng.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mullighan CG, Zhang J, Kasper LH, Lerach S, Payne-Turner D, Phillips LA, et al. CREBBP mutations in relapsed acute lymphoblastic leukaemia. Nature. 2011;471:235–9. doi: 10.1038/nature09727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buffa FM, Harris AL, West CM, Miller CJ. Large meta-analysis of multiple cancers reveals a common, compact and highly prognostic hypoxia metagene. Br J Cancer. 2010;102:428–35. doi: 10.1038/sj.bjc.6605450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sonveaux P, Vegran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930–42. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–52. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGranahan N, Swanton C. Clonal Heterogeneity and Tumor Evolution: Past, Present, and the Future. Cell. 2017;168:613–28. doi: 10.1016/j.cell.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 43.Sanders DA, Ross-Innes CS, Beraldi D, Carroll JS, Balasubramanian S. Genome-wide mapping of FOXM1 binding reveals co-binding with estrogen receptor alpha in breast cancer cells. Genome Biol. 2013;14:R6. doi: 10.1186/gb-2013-14-1-r6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–7. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chakraborty AA, Laukka T, Myllykoski M, Ringel AE, Booker MA, Tolstorukov MY, et al. Histone demethylase KDM6A directly senses oxygen to control chromatin and cell fate. Science. 2019;363:1217–22. doi: 10.1126/science.aaw1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhandari V, Li CH, Bristow RG, Boutros PC, Consortium P. Divergent mutational processes distinguish hypoxic and normoxic tumours. Nat Commun. 2020;11:737. doi: 10.1038/s41467-019-14052-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jia D, Augert A, Kim DW, Eastwood E, Wu N, Ibrahim AH, et al. Crebbp Loss Drives Small Cell Lung Cancer and Increases Sensitivity to HDAC Inhibition. Cancer Discov. 2018;8:1422–37. doi: 10.1158/2159-8290.CD-18-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lasko LM, Jakob CG, Edalji RP, Qiu W, Montgomery D, Digiammarino EL, et al. Discovery of a selective catalytic p300/CBP inhibitor that targets lineage-specific tumours. Nature. 2017;550:128–32. doi: 10.1038/nature24028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogiwara H, Sasaki M, Mitachi T, Oike T, Higuchi S, Tominaga Y, et al. Targeting p300 Addiction in CBP-Deficient Cancers Causes Synthetic Lethality by Apoptotic Cell Death due to Abrogation of MYC Expression. Cancer Discov. 2016;6:430–45. doi: 10.1158/2159-8290.CD-15-0754. [DOI] [PubMed] [Google Scholar]
- 50.Lv C, Zhao G, Sun X, Wang P, Xie N, Luo J, et al. Acetylation of FOXM1 is essential for its transactivation and tumor growth stimulation. Oncotarget. 2016;7:60366–82. doi: 10.18632/oncotarget.11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubio C, Martinez-Fernandez M, Segovia C, Lodewijk I, Suarez-Cabrera C, Segrelles C, et al. CDK4/6 Inhibitor as a Novel Therapeutic Approach for Advanced Bladder Cancer Independently of RB1 Status. Clin Cancer Res. 2019;25:390–402. doi: 10.1158/1078-0432.CCR-18-0685. [DOI] [PubMed] [Google Scholar]
- 52.Sayles LC, Breese MR, Koehne AL, Leung SG, Lee AG, Liu HY, et al. Genome-Informed Targeted Therapy for Osteosarcoma. Cancer Discov. 2019;9:46–63. doi: 10.1158/2159-8290.CD-17-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edelman MJ, Redman MW, Albain KS, McGary EC, Rafique NM, Petro D, et al. SWOG S1400C ( NCT02154490)-A Phase II Study of Palbociclib for Previously Treated Cell Cycle Gene Alteration-Positive Patients with Stage IV Squamous Cell Lung Cancer (Lung-MAP Substudy) J Thorac Oncol. 2019;14:1853–9. doi: 10.1016/j.jtho.2019.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Teo ZL, Versaci S, Dushyanthen S, Caramia F, Savas P, Mintoff CP, et al. Combined CDK4/6 and PI3Kalpha Inhibition Is Synergistic and Immunogenic in Triple-Negative Breast Cancer. Cancer Res. 2017;77:6340–52. doi: 10.1158/0008-5472.CAN-17-2210. [DOI] [PubMed] [Google Scholar]
- 55.Schaer DA, Beckmann RP, Dempsey JA, Huber L, Forest A, Amaladas N, et al. The CDK4/6 Inhibitor Abemaciclib Induces a T Cell Inflamed Tumor Microenvironment and Enhances the Efficacy of PD-L1 Checkpoint Blockade. Cell Rep. 2018;22:2978–94. doi: 10.1016/j.celrep.2018.02.053. [DOI] [PubMed] [Google Scholar]
- 56.Hassan Venkatesh G, Bravo P, Shaaban Moustafa Elsayed W, Amirtharaj F, Wojtas B, Abou Khouzam R, et al. Hypoxia increases mutational load of breast cancer cells through frameshift mutations. Oncoimmunology. 2020;9:1750750. doi: 10.1080/2162402X.2020.1750750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw proteomic data is available in PRIDE (RRID:SCR_012052) accession PXD0120978. Single cell data has been deposited in the European Nucelotide Archive (ENA) with accession number PRJEB32846. Code used in the analysis of this data is provided https://zenodo.org/record/4020438.