Abstract
The opioid crisis represents a major worldwide public health crisis that has accelerated the search for safer and more effective opioids. Over the past few years, the identification of biased opioid ligands capable of eliciting selective functional responses has provided an alternative avenue to develop novel therapeutics without the side effects of current opioid medications. However, whether biased agonism or other pharmacological properties, such as partial agonism (or low efficacy), account for the therapeutic benefits remains questionable. Here, we provide a summary of the current status of biased opioid ligands that target the μ- and ĸ-opioid receptors and highlight advances in preclinical and clinical trials of some of these ligands. We also discuss an example of structure based biased ligand discovery at the μ-opioid receptor, an approach that could revolutionize drug discovery at opioid and other receptors. Last, we briefly discuss caveats and future directions for this important area of research.
Introduction
Opioids, such as morphine, have traditionally been and continue to be among the most potent painkillers in clinical settings (1, 2). However, the notorious worldwide “opioid epidemic” (3) has ignited the search for safer opioids, bringing this area to the forefront of novel drug discovery. A major problem with current opioids is that, at high doses, they suppress respiration and, with overdose, can be lethal. In the past few decades, the use of prescribed opioids (morphine, oxycodone, and hydrocodone) or nonpharmaceutical drugs (heroin and carfentanil) has risen at an alarming rate (2) for pain relief and various recreational uses. Common outcomes due to repeated or long-term licit and illicit use of these opioids are tolerance, dependence, and death. A potential framework to overcome opioid side effects has emerged in the last decade based on the design and discovery of functionally selective ligands at opioid receptors. In this review, we discuss the current status, major challenges, and future perspectives of functionally selective or biased opioid ligands as potential therapeutics to overcome the problems associated with conventional opioid-based drugs.
Opioid Receptors: Signaling And Regulation
Although opioid receptors were postulated to mediate the actions of drugs such as morphine and nalorphine more than 60 years ago (4), it was not until the 1970s that scientists were able to biochemically demonstrate the existence of opioid receptors (5). Several groups used radioligand-binding techniques to devise simple biochemical assays to distinguish agonists from antagonists based on their differential sensitivity to physiological amounts of sodium (6). Subsequently, multiple opioid receptors, which were postulated earlier based on elegant in vivo studies (7), were directly validated by radioligand-binding studies (8–10) and other approaches (11). Eventually, opioid receptors were isolated through molecular cloning (12–15), resulting in the identification of a small subfamily of receptors that are widely expressed on the surface of central and peripheral neuronal cells.
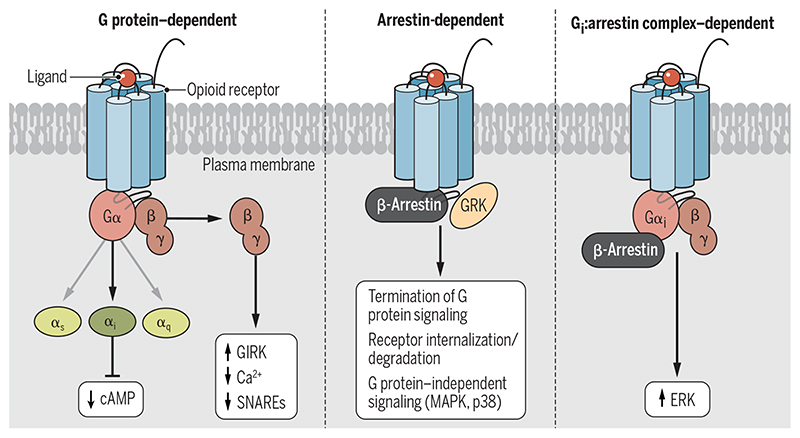
Opioid receptors belong to the class A subgroup of seven-transmembrane domain, G protein–coupled receptors (GPCRs) (16). They include three main subtypes referred to as the μ-, δ-, and κ-opioid receptors (μ-OR, δ-OR, and ĸ-OR, respectively), together with the nonclassical nociceptin opioid receptor (NOP). Once the opioid receptor is activated by opioids, conformational changes from the extracellular ligand-binding site to the intracellular end of the receptor occur (17–19) and rapidly lead to the coupling and activation of intracellular heterotrimeric Gi/o family proteins (Fig. 1). The general paradigm of signaling downstream of opioid receptors mediated by heterotrimeric G proteins includes the inhibition of cyclic adenosine monophosphate (cAMP) production, which attenuates the activity of effector protein kinases, for example, cAMP-dependent protein kinase, and decreases neuronal firing (20). In addition, the Gβγ subunits modulate calcium channels to suppress Ca2+ influx and therefore attenuate the excitability of neurons and inhibit the release of pronociceptive neuropeptides (21–23). Moreover, Gβγ subunits can also activate G protein–coupled inwardly rectifying potassium channels (GIRKs), leading to the hyperpolarization of the cell membranes and, thereby, repression of neuronal excitation (24). Furthermore, Gβγ subunits directly interact with the soluble N-ethylmaleimide–sensitive factor-attachment protein receptor (SNARE) complexes, inhibiting the presynaptic release of neurotransmitters for several Gį/o-coupled receptors, such as the α2-adrenergic and 5-hydroxytryptamine 1B (5-HT1B) serotonin receptors (25–27). However, whether the Gβγ-SNARE interaction plays a similar role downstream of opioid receptor activation requires further study.
Fig. 1. Signaling and regulatory paradigms of opioid receptors.
(Left) Agonist stimulation leads to the coupling of opioid receptors to heterotrimeric G proteins, resulting in a reduction in cAMP abundance, a decreased Ca2+ response, and the activation of GIRK channels. (Middle) Subsequently, the receptor is phosphorylated by GRKs, which results in β-arrestin recruitment, receptor desensitization, and internalization. β-Arrestins also mediate the activation of various signaling pathways, including those of the MAPKs, ERK1/2, and p38. (Right) Gαi protein and β-arrestins can also interact with each other and form a complex to mediate downstream signaling, such as ERK activation.
Similar to most studied GPCRs, activated opioid receptors are phosphorylated on their cytoplasmic loops and C terminus by GPCR kinases (GRKs) (28–32), which is followed by the recruitment of multifunctional proteins called β-arrestins. Initially, β-arrestins were considered as “negative” regulators that attenuate G protein signaling through steric inhibition of G protein binding (desensitization) and promotion of receptor endocytosis through a clathrin-coated mechanism (internalization and degradation) (33). Since 1999, however, it has been evident that β-arrestins can also serve as scaffolding proteins to mediate downstream signaling independently of G protein signaling (34), and studies have provided detailed insights into the structural and molecular details responsible for the activation of β-arrestins and subsequent signaling (35–39). Briefly, phosphorylation of the cytoplasmic tail of an activated GPCR can first confer high-affinity binding to β-arrestins through engagement of both the cytoplasmic tail and intracellular core region (36). In addition, GPCRs can form low-affinity complexes with β-arrestins through the receptor core alone (35). Both interaction modes could trigger arrestin-mediated signaling, including perhaps activation of extracellular signal–regulated kinases 1 and 2 (ERK1/2), members of the family of mitogen-activated protein kinases (MAPKs). Note that although ERK activation has been identified as a frequent consequence of GPCR activation, the role of G proteins and arrestins in ERK activation remains controversial (40–43). Smith et al. (44) found that ERK activation downstream of Gi-coupled receptors requires the engagement of both G protein and β-arrestin. Their data suggest that the Gi:β-arrestin complex directly interacts with and activates ERKs because disruption of the Gi:β-arrestin interaction impaired ERK activation. This study thus introduced an additional noncanonical signaling mechanism mediated by the Gi:β-arrestin complex, which is distinct from the classical pathways of G protein– and arrestin-mediated signaling. Although opioid receptors are also Gi-coupled receptors, whether the Gi:β-arrestin complex plays a similar role in the presence of opioids remains to be studied.
Whereas the role of G proteins in opioid-elicited analgesia has been well characterized, how arrestins are involved in both therapeutic effects and side effects remains controversial. One of the main challenges is that, so far, no unambiguous readout of signaling specific for arrestins is available. β-Arrestins nucleate many downstream signaling mediators, including MAPKs, Akt, the transcriptional regulator nuclear factor κB, and phosphoinositide 3-kinases, and thereby could regulate a diverse array of signaling pathways (Fig. 1) (45). Many of these pathways have been implicated in mediating some forms of signal transduction distinct from G protein signaling. For example, β-arrestin recruitment mediates activation of the kinase Src and subsequently regulates the activity of ERK1/2 (34). This signaling pathway is potentially important for many consequences in vivo because it is involved in regulating dopaminergic neurotransmission and behaviors (46). The arrestin-dependent Akt pathway could, on the other hand, stimulate mechanistic target of rapamycin (mTOR) signaling and subsequent protein translation (47). In this regard, the mTOR pathway was implicated through phosphoproteomic studies of ĸ-OR agonist–mediated aversion (48). Remarkably, mTOR inhibitors were subsequently demonstrated to repress ĸ-OR agonist–induced aversion (48). Again, it is important to recognize that most of these signaling pathways can also be activated by GPCR-independent receptors, such as growth factors and integrins. Ultimately, then, the dependence of a particular opioid-dependent signaling function on arrestins remains unclear.
Biased Agonism At Opioid Receptors
Some ligands that can equally activate G protein and β-arrestin pathways are referred to as “balanced” agonists. These balanced agonists include endogenous peptides (for example, endomorphins, enkephalins, and dynorphins) and small-molecule ligands (for example, fentanyl and salvinorin A). Correspondingly, ligands that can preferentially activate G protein or β-arrestin pathways at a single receptor are called “functionally selective” or “biased” agonists. Several opioid ligands, such as TRV130 (oliceridine), PZM21, RB-64, and triazole 1.1, display G protein–biased activity in vitro. This type of functional selectivity has been proposed to be due to the distinct receptor conformations that can be stabilized by the biased agonists, thereby promoting differential coupling to signaling effectors (49). A key to understanding the molecular mechanisms for GPCR functional selectivity will be to obtain structures of receptors bound to biased agonists in multiple transition states. There are no structures of opioid receptors bound to G protein or arrestin-biased ligands currently available. With the stabilization of a llama-derived nanobody, we identified an inactive-state ĸ-OR (50) that displays distinct conformational features from the previously solved agonist- or antagonist-bound ĸ-OR structures (18, 51), which supports the existence of conformational dynamics in opioid receptors. Structural and computational studies of angiotensin II type 1, D2-dopamine, and 5-HT2A and 5-HT2B serotonin receptors suggest that arrestin-biased ligands could induce or stabilize specific conformational states that favor the coupling of arrestin but not G protein (52–56).
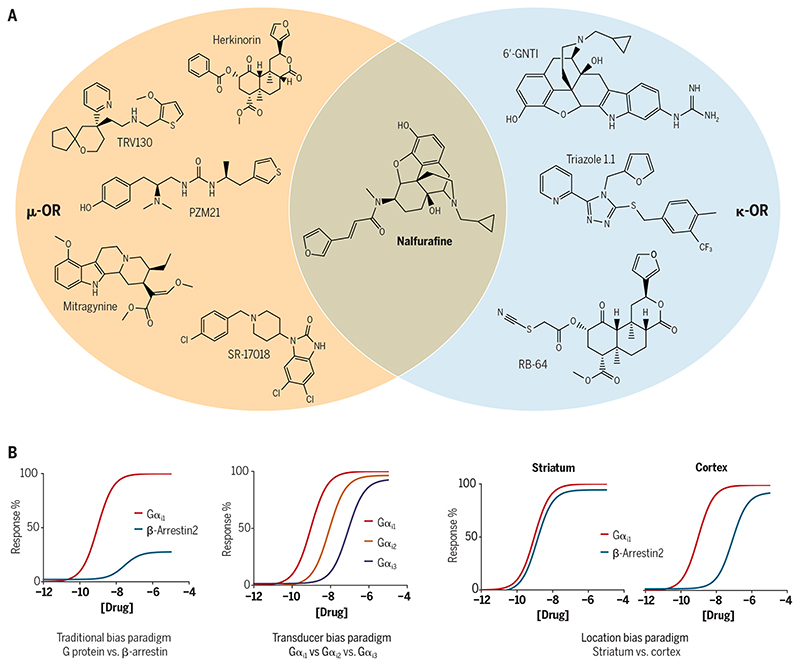
Although biased agonists have demonstrated therapeutic potential (for example, G protein–biased μ-OR agonists show analgesia with reduced side effects), how this may translate into different physiological responses in vivo in humans is still not well understood. In particular, traditional bias paradigms focusing on G proteins and β-arrestins are based on the assumption that a GPCR predominantly couples to one G protein and one β-arrestin protein (Fig. 2). However, mammalian cells encode 16 different G protein subtypes and 4 major β-arrestins. Opioid receptors, such as μ-OR, have long been known to couple to multiple G proteins (Gi1, Gi2, Gi3, GoA, GoB, Gz, and Gustducin) and arrestins (β-arrestin1 and β-arrestin2) with varying efficacies and kinetics (57, 58). Therefore, it is likely that GPCRs may produce unexpected effects by coupling to other G proteins over the main subtypes through a process known as transducer bias (Fig. 2). For example, Gz is predominantly expressed in neuronal cells and couples to ĸ-OR and μ-OR (57). Whether Gz is involved in any opioid-mediated effects remains undefined, although reductions in the actions of morphine were observed in Gz knockout mice (59). Interestingly, Gz could selectively compete with the binding of Gαi/o to Gi-coupled GPCRs (for example, D2-dopamine or opioid family), antagonizing the function of the other Gi proteins (60). Consistent with this observation, a transducerome analysis of ĸ-OR agonists using cell-based assays showed that Gz is more preferred than other G protein subtypes in terms of potency and efficacy (58). On the other hand, β-arrestin1 and β-arrestin2 also mediate separate cellular responses, which suggests that their roles may be nonredundant (61). Thus, measurements of ligand bias should clarify which G protein or β-arrestin subtype is being evaluated. In vivo, another manifestation of biased signaling is secondary or so-called “system bias,” which is due to variations in the relative abundances of receptor, G protein, and arrestin subtypes between different cellular or tissue environments, for example, plasma membrane versus nucleus membrane and striatum versus cortex (Fig. 2). System bias imposes unique bias paradigms because the stoichiometry between receptors and G proteins or β-arrestins can determine distinct signaling profiles (62, 63).
Fig. 2. Chemical space shared by the currently reported G protein–biased ligands at μ-OR and ĸ-OR.
(A) Chemical structures of currently described G protein–biased ligands at the μ-OR and ĸ-OR. Of these, nalfurafine shows moderate selectivity at ĸ-OR compared with μ-OR and δ-OR, whereas the others exhibit substantial selectivity for the different receptors. (B) The different types of ligand bias that can be potentially manifested by opioid receptors.
Another well-studied example of system bias relates to GRKs, which display cell type– and tissue type–specific expression patterns. It is Ligands may elicit differential coupling of heterotrimeric G proteins versus β-arrestins, as well as differential coupling of G protein subtypes and β-arrestin isoforms. In addition, some ligands may also elicit context-specific bias, for example, in different tissues expressing opioid receptors.not known at present which GRKs (for example, GRK2, GRK3, and GRK5) are coexpressed with the localized receptors at specific neurons. Given that GRK-mediated phosphorylation is subtype specific (64), differential GRK accessibility could, conceivably, directly affect the degree of arrestin coupling and therefore shift signaling in either a balanced or biased direction (65). Accordingly, the consequence of GPCRs coupling to one GRK or arrestin subtype can be distinct, similar to the aforementioned consequences of distinct G protein subtype engagement. Future evaluation of biased agonism needs to take into consideration the potential complexities of the signal transduction pathway.
Therapeutic Potential And Challenges Of Biased Agonism In Opioid Receptors
Studies focused on the traditional bias (G proteins versus β-arrestins) have suggested distinct functional outcomes of each pathway when activated by opioid receptors. In particular, opioid-related analgesia is proposed to be mediated by the Gi-dependent pathway, whereas several adverse effects, such as tolerance, addiction, and respiratory depression, are mediated by the β-arrestin pathway at μ-OR. For example, Bohn and colleagues reported that β-arrestin2 knockout mice display enhanced morphine-mediated analgesia but have reduced side effects (66, 67). Similarly, Bruchas et al. and Ehrich et al. (68, 69) reported that the analgesic actions of ĸ-OR agonists require different signaling pathways from those responsible for their aversive effects. Additional studies showed that β-arrestin2 knockout mice display differential responses to ĸ-OR agonists when compared with their wild-type littermates (70).
Inspired by these studies, the search for safer and more efficacious opioids has focused on G protein–biased agonists at opioid receptors. Several such ligands, including natural products (her-kinorin and mitragynine), synthetic small molecules (TRV130, SR-17018, and PZM21), and peptides (cyclopeptide) have been shown in vitro by some groups to display preference toward G protein signaling at the μ-OR (Fig. 2). A few of these compounds have advanced to animal or clinical studies and they have maintained their therapeutic potential in vivo (Table 1). In particular, TRV130 (oliceridine), as a G protein–biased agonist at μ-OR, was approved by the Food and Drug Administration (FDA) in 2020 for the treatment of moderate to acute pain. Similarly, a number of studies have identified, characterized, and optimized G protein–biased ĸ-OR ligands over the past several years, such as 6-GNTI, RB-64, triazole 1.1, and nalfurafine (Fig. 2 and Table 2). ĸ-OR agonists produce analgesia without the side effects associated with μ-OR agonists, such as euphoria or respiratory depression. However, ĸ-OR activation in vivo is frequently associated with other side effects, such as dysphoria and psychotomimesis (71). Preclinical studies of G protein–biased ĸ-OR agonists suggest that they could avoid such side effects, which supports their therapeutic potential as nondysphoric (for example, triazole 1.1) or nonhallucinogenic (for example, nalfurafine) analgesics (72, 73). Furthermore, coadministration of nalfurafine and morphine could substantially enhance the analgesic activity but inhibit the addictive potential of morphine (74), which provides an additional option in the applications of G protein–biased agonists.
Table 1. G protein-biased ligands at the μ-OR.
ND, no data available.
| G protein activation | β-Arrestin recruitment | Administration and dose | Outcome | ||
|---|---|---|---|---|---|
| Oliceridine (TRV130) | EC50 = 7.94 nM Efficacy = 84% | EC50 = 5.01 nM Efficacy = 15% | ND | (81) | |
| EC50 = 8 nM Efficacy = 71% | Efficacy = 14% | C57BL/6J mice | •Peak analgesia in 5 min | (82) | |
| Subcutaneous, 1 mg/kg | •Reduced central nervous system depression and gastrointestinal dysfunction | ||||
| ND | ND | Phase I trial | •Well tolerated | (102) | |
| 18 healthy volunteers | •Nausea and vomiting at 7 mg limited further dose escalation | ||||
| Intravenous, dose range 0.15 to 7 mg | |||||
| ND | ND | Phase II trial | •2 and 3 mg mitigated severe acute pain over 48 hours | (103) | |
| Pilot phase: 144 patients | |||||
| After pilot phase: 195 patients | |||||
| Intravenous, 0.5, 1, 2, or 3 mg every 3 hours | |||||
| ND | ND | Phase III trial | •Superior analgesia | (104) | |
| 375 patients | •Reduced respiratory side effects and increased gastrointestinal tolerability | ||||
| Intravenous: 1.5 mg loading dose followed by 0.1-mg, 0.35-mg, or 0.5-mg doses | |||||
| Morphine (4-mg loading dose; 1-mg demand dose) | |||||
| Mitragynine pseudoindoxyl | EC50 = 1.7 nM Efficacy = 84% | No recruitment at 10 μM | CD-1 mice | •Analgesia | (105) |
| Subcutaneous, 0.76 mg/kg | •Limited respiratory depression and constipation | ||||
| SHR9352 | EC50 = 0.77 nM Efficacy = 96% | EC50 = 2.5 nM Efficacy = 18% | C57BL/6J mice and Wistar rats | •Analgesia | (106) |
| Subcutaneous, 0.1 mg or | •No constipation | ||||
| Intravenous, 0.3 mg | |||||
| SR-17018 | EC50 = 97 nM Efficacy = 75% | No recruitment at 10 μM | C57BL/6J mice | •Analgesia | (107) |
| Intraperitoneal, 6 mg/kg | •No respiratory suppression | ||||
| Herkinorin | EC50 = 0.5 μM | No recruitment at 10 μM | No blood-brain barrier penetration | (108) | |
| PZM21 | EC50 = 4.6 nM Efficacy = 76% | No recruitment at 10 μM | C57BL/6J mice | •Dose dependent response | (97) |
| Subcutaneous, 40, 20, and 10 mg/kg | •Long-lasting analgesia | ||||
| •Decreased respiratory depression and constipation | |||||
| Cyclopeptide | EC50 = 5.2 nM Efficacy = 80% | No recruitment at 10 μM | ND | (109) |
Table 2. G protein–biased ligands at the ĸ-OR.
| G protein activation | β-Arrestin recruitment | Administration and dose | Outcome | ||
|---|---|---|---|---|---|
| 6’-GNTI | EC50 = 1.6 nM Efficacy = 64% | No recruitment activity | C57BL/6J mice | •Analgesia | (110) |
| Spinal cord injection, 10 to 30 nmol | •No aversion | ||||
| •Tolerance | |||||
| RB-64 (22-thiocyanatosalvinorin A) | EC50 = 5.22 nM Efficacy = 99% | EC50 = 1130 nM Efficacy = 126% | C57BL/6J mice | •Long lasting analgesic | (70) |
| Subcutaneous, 3 mg/kg | •No sedative effect | ||||
| •Aversive | |||||
| Triazole 1.1 | EC50 = 77 nM Efficacy = 101% | EC50 = 4955 nM Efficacy = 98% | C57BL/6J mice | •Analgesia | (72) |
| Subcutaneous dose | •Antipruritic | ||||
| Analgesia: 5, 15, and 30 mg/kg | •No sedation or dysphoria observed | ||||
| Antipruritic: 1 and 3 mg/kg | |||||
| HS666 | EC50 = 35.7 nM Efficacy = 50% | EC50 = 449 nM Efficacy = 24% | CD-1 mice | •Time and dose dependent | (111, 112) |
| Intracerebroventricular, 6.02 nmol | •Antinociceptive response | ||||
| •Respiratory suppression | |||||
| Nalfurafine | EC50 = 1.4 nM (pERK1/2) | EC50 = 110 nM (p38) | Rats and primates | •Analgesic | (113) |
| Subcutaneous, 1 mg/kg | •Antipruritic | ||||
| •No dysphoria or aversion | |||||
| EC50 = 0.11 nM Efficacy = 111% | EC50 = 1.4 nM Efficacy = 129% | CD-1 mice | •Analgesic | (73) | |
| Subcutaneous, 10 μg/kg | •Antipruritic | ||||
| •No aversion |
Several independent studies focused on μ-OR agonists have questioned the role of arrestin signaling in mediating μ-OR–related side effects. Because the recruitment of β-arrestin to μ-OR is dependent on receptor phosphorylation, Kliewer et al. (75) generated phosphorylation-deficient μ-OR mutants that fail to recruit β-arrestin and subsequently generated knock-in mouse lines expressing these mutant receptors. Compared to wild-type mice, these phosphorylation-deficient knock-in mice exhibit a substantially greater analgesic response to fentanyl and morphine. This enhanced response presumably arises from reduced receptor desensitization because of the lack of β-arrestin recruitment. Surprisingly, however, both fentanyl and morphine induce profound respiratory depression, constipation, and hyperlocomotion in these knock-in mice, which were the opposite responses to what one would predict based on the biased signaling paradigm discussed earlier. Interestingly, fentanyl- and morphine-induced tolerance was markedly blunted in these knock-in models, albeit at different levels. In another study, Kliewer et al. (76), together with two other groups, in experiments with β-arrestin2 knockout mice found that morphine- or fentanyl-induced respiratory depression is maintained. Regarding G protein–biased μ-OR agonists, Gillis et al. (77) showed that TRV130, PZM21, and SR-17018 had low intrinsic efficacies compared with drugs such as fentanyl and that their reduced side effect profile was correlated with partial agonism rather than biased signaling per se. Accordingly, efforts aimed at only reducing β-arrestin recruitment to μ-OR might not improve the safety profile of opioids (78). In considering these studies, however, one must acknowledge that many of the aforementioned experiments relied on mice in which these genetic manipulations were maintained over the entire life span of the animal and that subsequent compensatory changes could complicate the interpretation of the results.
Whereas the studies described earlier originate from a number of independent laboratories and use rigorous experimental frameworks to establish the therapeutic potential of biased agonism, additional studies will be required to better understand and corroborate the intriguing findings described here. In particular, we advocate for an even more careful and extensive analysis of biased signaling going forward (Fig. 2) because opioid receptors can interact with multiple G proteins and arrestins (58). The consequences of signaling by individual G proteins or arrestins remain understudied. In addition, the available G protein–biased agonists are not particularly biased with respect to G protein signaling, and extremely biased tool compounds will be needed to test the hypothesis definitively. Note also that almost all of the currently marketed opioid drugs exert their analgesic effects through the μ-OR (79). Thus, it is likely that ligands targeting other pain-related opioid receptors (for example, ĸ-OR, δ-OR, and NOP) together with nonopioid receptors (for example, cannabinoid receptors) could be potential alternatives to current opioids. Although not within the scope of this review, G protein–biased δ-OR agonists also demonstrate therapeutic potential without proconvulsive activity or analgesic tolerance as typical δ-OR agonists do (80).
Approaches To Identify New Scaffolds For Biased Ligands At Opioid Receptors
There have been two major approaches for discovering biased ligands at opioid receptors and other pain-related therapeutic targets. The first involves physically screening available libraries of compounds and ultimately testing derivatives on cells and animals to identify those with the desired characteristics (81–84). Although this approach is time-consuming, almost all of the clinically approved drugs were found in this way, including most of the biased opioid ligands described earlier. The advantage of this approach is that the tested ligands can be directly evaluated in assays to examine pathway selectivity. Several assays are now available to investigate the signaling pathways of interest, such as G protein activation, for example, with cAMP inhibition or bioluminescence resonance energy transfer (BRET) assays, and β-arrestin recruitment, for example with parallel receptorome expression and screening via transcriptional output, with transcriptional activation following arrestin translocation (PRESTO-Tango) or BRET assays (85–87). The advantages and disadvantages of these assays have been previously discussed (88). Again, these assays can robustly monitor G protein association or arrestin recruitment. When using these assays, similar levels of amplification should be used to test the same ligand, and a known unbiased ligand should be included in parallel as a reference (for example, DADLE for δ-OR, U50,488 for ĸ-OR, and DAMGO for μ-OR) to minimize systematic bias introduced by the different assays. The potencies and efficacies of the tested ligands in each signaling pathway are then normalized to the reference ligand to minimize the influence of system bias. This normalized curve (G protein or arrestin pathway) is then integrated into pharmacological models to quantify bias propensity toward one signaling pathway over the other (also referred to as a biased factor; for example, the biased factor of a reference ligand is 1) (89–91). Thus, this bias factor is a parameter that enables one not only to evaluate preference for a pathway (G protein or β-arrestin) but also to compare the bias potential between multiple ligands at a single receptor.
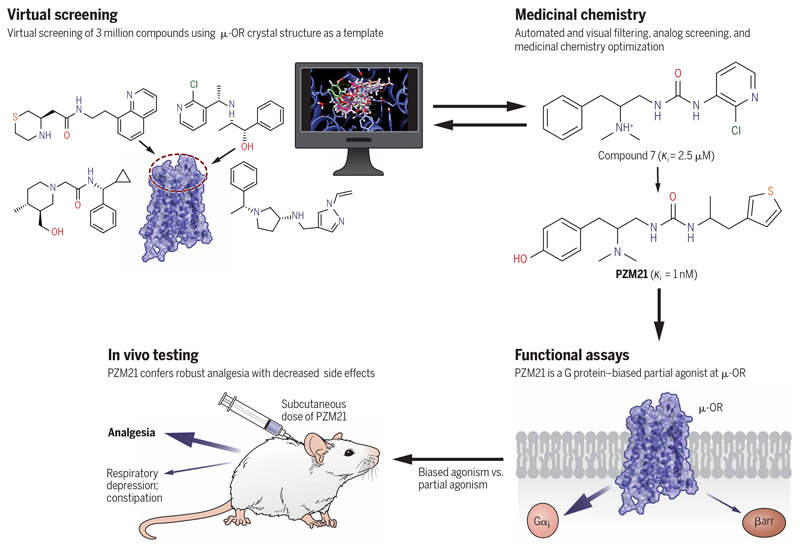
The second approach for the rapid discovery of biased ligands involves structure-guided ligand screening and optimization (Fig. 3). In this approach, hundreds of thousands (92) to more than 100 million compounds (93) can be docked into the binding pocket of a GPCR structure (92, 94) or a homology model (95, 96), and the top-ranking compounds can be physically tested in vitro (96) and in vivo (95). Because this is a computation-based approach, some potential ligands might be missed during the triaging of potentially active compounds. Nonetheless, the explosion of high-resolution GPCR structures provides the potential that this method represents a fruitful approach to discover and optimize novel ligands using a structure-guided approach. Thus, from such a large-scale docking campaign followed by rational design, Manglik et al. (97) identified a new chemotype, PZM21 (Fig. 2), which is a G protein–biased agonist at μ-OR that is very similar to TRV130. Briefly, PZM21 reduces affective pain responses in mice and is devoid of both respiratory depression and constipation. It also does not produce conditioned place preference or stimulate locomotion activity (97). With partial efficacy in the hot plate assay, however, PZM21 does not show effectiveness in the tail-flick test or spinal reflexive response. Together, these findings suggest that the actions of PZM21 are complicated, which may involve other pathways or targets. For example, PZM21 also displays antagonist activity at the ĸ-OR. Another study (98) reported that PZM21 acts as a balanced agonist at μ-OR and causes respiratory depression similarly to morphine. Thus, the in vivo actions of PZM21 and its pharmacodynamics require further study (99). Furthermore, Ehrlich et al. (100) reported that PZM21 retains G protein–biased activity in native neurons. Note that both PZM21 and TRV130 have modest degrees of assay-dependent G protein bias (97) and that compounds with more extreme bias would be better compounds to ultimately test the hypothesis.
Fig. 3. Structure-based discovery and optimization of a G protein–biased μ-OR ligand, PZM21.
Schematic representation of the discovery and optimization pipeline using structure-guided virtual screening. Using the crystal structure of the μ-OR, a large set of chemical compounds was virtually screened, which was followed by the identification of a handful of lead compounds for further testing. Subsequent optimization and structure-function relationship studies yielded PZM21, which is a G protein–biased μ-OR partial agonist, and produced desirable analgesic activity in vivo without the typical side effects observed with other μ-OR agonists, such as morphine.
Another study used structure-based virtual screening approaches with the crystal structure of ĸ-OR and identified 11 previously uncharacterized submicromolar ĸ-OR ligands (101). Of these, the best ligand exhibited a binding affinity of approximately 100 nM for the ĸ-OR, and another ligand identified in this screen, referred to as “compound 81,” was identified as a potent G protein–biased agonist with minimal β-arrestin2 recruitment [median effective concentration (EC50) = 0.53 μM for G protein activation and an EC50 = 8.1 μM for β-arrestin2 recruitment]. Together, these two examples underscore the inherent potential of the structure-based discovery of biased opioid receptor ligands and the likelihood that it will emerge as an even more powerful approach when more structures of opioid receptors in complex with biased ligands become available. Note that both the physical and virtual screening approaches usually do not directly lead to the identification of biased ligands and that the compounds identified typically require further extensive medicinal chemistry optimization.
Concluding Remarks
G protein–biased opioid receptor ligands provide a potentially valuable framework to develop novel therapeutics with minimized side effects aimed at overcoming the increasing burden of the opioid crisis. The approval of TRV130 by the FDA, although with safety concerns, together with other promising preclinical studies at ĸ-OR or δ-OR will continue to make biased agonists as candidates for potential opioid alternatives. Going forward, a more detailed understanding of opioid receptor signaling and regulation, particularly using more profoundly biased ligands for in vivo studies, will be of crucial importance to ensure that the hypothesis is adequately tested in clinical trials. Assays that unambiguously reflect G protein or β-arrestin–dependent signaling are also urgently needed for screening purposes before these probes can be advanced to animal studies or clinical trials. The rapid emergence of structures of GPCRs in complex with ligands of distinct efficacy profiles and specific signal transducers is likely in the next few years. Information from the atomic-level images will improve our current understanding of the structural determinants that drive biased signaling and therefore are likely to be a major guiding platform for the efficient discovery of biased ligands. For example, the identification of specific ligand-receptor interactions that control the equilibrium between receptor functional states can critically facilitate the design of biased ligands. Strong evidence for this approach is already emerging (54, 55). Because GPCRs share many features for activation-related conformational changes, it is likely that the knowledge gained from nonopioid receptor systems will also be applied and translated to opioid receptors for the design of highly biased ligands.
Acknowledgements
We apologize to those authors whose work may have skipped our attention or could not be cited because of space constraints.
Funding
Research in the laboratory of A.K.S. is supported by the Senior Fellowship of the Wellcome Trust/DBT India Alliance (IA/S/20/1/504916) awarded to A.K.S., the Swarnajayanti Fellowship of the Department of Science and Technology (DST/SJF/LSA-03/2017-18), the Department of Biotechnology (DBT BT/PR29041/BRB/10/1697/2018), the Science and Engineering Research Board (EMR/2017/003804), the Young Scientist award from the Lady TATA Memorial Trust, and the Indian Institute of Technology, Kanpur. A.K.S. is EMBO Young Investigator and Joy Gill Chair Professor. This work is also supported by NIH grants RO1MH61887 and U19MH82441, the NIMH Psychoactive Drug Screening Program Contract, and the Michael Hooker Distinguished Chair of Pharmacology, as well as DA035764 (all to B.L.R.). T.C. is supported by NIDA (grant PO1 DA035764).
Footnotes
Competing interests: UNC has licensed a patent with B.L.R. listed as an inventor on biased opioid compounds. The other authors declare that they have no competing interests.
References and Notes
- 1.Brownstein MJ. A brief history of opiates, opioid peptides, and opioid receptors. Proc Natl Acad Sci USA. 1993;90:5391–5393. doi: 10.1073/pnas.90.12.5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volkow ND, Collins FS. The role of science in addressing the opioid crisis. N Engl J Med. 2017;377:391–394. doi: 10.1056/NEJMsr1706626. [DOI] [PubMed] [Google Scholar]
- 3.Skolnick P. The opioid epidemic: Crisis and solutions. Annu Rev Pharmacol Toxicol. 2018;58:143–159. doi: 10.1146/annurev-pharmtox-010617-052534. [DOI] [PubMed] [Google Scholar]
- 4.Woods LA. The pharmacology of nalorphine (N-allylnormorphine) Pharmacol Rev. 1956;8:175–198. [PubMed] [Google Scholar]
- 5.Pert CB, Snyder SH. Opiate receptor: Demonstration in nervous tissue. Science. 1973;179:1011–1014. doi: 10.1126/science.179.4077.1011. [DOI] [PubMed] [Google Scholar]
- 6.Pert CB, Pasternak G, Snyder SH. Opiate agonists and antagonists discriminated by receptor binding in brain. Science. 1973;182:1359–1361. doi: 10.1126/science.182.4119.1359. [DOI] [PubMed] [Google Scholar]
- 7.Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- 8.Lord JA, Waterfield AA, Hughes J, Kosterlitz HW. Endogenous opioid peptides: Multiple agonists and receptors. Nature. 1977;267:495–499. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- 9.Chang KJ, Cuatrecasas P. Multiple opiate receptors. Enkephalins and morphine bind to receptors of different specificity. J Biol Chem. 1979;254:2610–2618. [PubMed] [Google Scholar]
- 10.Robson LE, Kosterlitz HW. Specific protection of the binding sites of D-Ala2-D-Leu5-enkephalin (δ-receptors) and dihydromorphine (μ-receptors) Proc R Soc Lond B Biol Sci. 1979;205:425–432. doi: 10.1098/rspb.1979.0076. [DOI] [PubMed] [Google Scholar]
- 11.Spain JW, Roth BL, Coscia CJ. Differential ontogeny of multiple opioid receptors (mu, delta, and kappa) J Neurosci. 1985;5:584–588. doi: 10.1523/JNEUROSCI.05-03-00584.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans CJ, Keith DE, Jr, Morrison H, Magendzo K, Edwards RH. Cloning of a delta opioid receptor by functional expression. Science. 1992;258:1952–1955. doi: 10.1126/science.1335167. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Mestek A, Liu J, Hurley JA, Yu L. Molecular cloning and functional expression of a mu-opioid receptor from rat brain. Mol Pharmacol. 1993;44:8–12. [PubMed] [Google Scholar]
- 14.Chen Y, Mestek A, Liu J, Yu L. Molecular cloning of a rat kappa opioid receptor reveals sequence similarities to the mu and delta opioid receptors. Biochem J. 1993;295(Pt. 3):625–628. doi: 10.1042/bj2950625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, Caput D, Vassart G, Meunier JC. ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett. 1994;341:33–38. doi: 10.1016/0014-5793(94)80235-1. [DOI] [PubMed] [Google Scholar]
- 16.Wacker D, Stevens RC, Roth BL. How ligands illuminate GPCR molecular pharmacology. Cell. 2017;170:414–427. doi: 10.1016/j.cell.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang W, Manglik A, Venkatakrishnan AJ, Laeremans T, Feinberg EN, Sanborn AL, Kato HE, Livingston KE, Thorsen TS, Kling RC, Granier S, et al. Structural insights into μ-opioid receptor activation. Nature. 2015;524:315–321. doi: 10.1038/nature14886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Che T, Majumdar S, Zaidi SA, Ondachi P, McCorvy JD, Wang S, Mosier PD, Uprety R, Vardy E, Krumm BE, Han GW, et al. Structure of the nanobody-stabilized active state of the kappa opioid receptor. Cell. 2018;172:55–67.:e15. doi: 10.1016/j.cell.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koehl A, Hu H, Maeda S, Zhang Y, Qu Q, Paggi JM, Latorraca NR, Hilger D, Dawson R, Matile H, Schertler GFX, et al. Structure of the μ-opioid receptor-Gi protein complex. Nature. 2018;558:547–552. doi: 10.1038/s41586-018-0219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedarzani P, Storm JF. PKA mediates the effects of monoamine transmitters on the K+ current underlying the slow spike frequency adaptation in hippocampal neurons. Neuron. 1993;11:1023–1035. doi: 10.1016/0896-6273(93)90216-e. [DOI] [PubMed] [Google Scholar]
- 21.Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 22.Wang H-B, Zhao B, Zhong Y-Q, Li K-C, Li Z-Y, Wang Q, Lu Y-J, Zhang Z-N, He S-Q, Zheng H-C, Wu S-X, et al. Coexpression of delta- and mu-opioid receptors in nociceptive sensory neurons. Proc Natl Acad Sci USA. 2010;107:13117–13122. doi: 10.1073/pnas.1008382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein C. Opioid receptors. Annu Rev Med. 2016;67:433–451. doi: 10.1146/annurev-med-062613-093100. [DOI] [PubMed] [Google Scholar]
- 24.Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- 25.Blackmer T, Larsen EC, Bartleson C, Kowalchyk JA, Yoon E-J, Preininger AM, Alford S, Hamm HE, Martin TFJ. G protein βγ directly regulates SNARE protein fusion machinery for secretory granule exocytosis. Nat Neurosci. 2005;8:421–425. doi: 10.1038/nn1423. [DOI] [PubMed] [Google Scholar]
- 26.Gerachshenko T, Blackmer T, Yoon EJ, Bartleson C, Hamm HE, Alford S. Gβγ acts at the C terminus of SNAP-25 to mediate presynaptic inhibition. Nat Neurosci. 2005;8:597–605. doi: 10.1038/nn1439. [DOI] [PubMed] [Google Scholar]
- 27.Zurawski Z, Thompson Gray AD, Brady LJ, Page B, Church E, Harris NA, Dohn MR, Yim YY, Hyde K, Mortlock DP, Jones CK, et al. Disabling the Gβγ-SNARE interaction disrupts GPCR-mediated presynaptic inhibition, leading to physiological and behavioral phenotypes. Sci Signal. 2019;12:eaat8595. doi: 10.1126/scisignal.aat8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pei G, Kieffer BL, Lefkowitz RJ, Freedman NJ. Agonist-dependent phosphorylation of the mouse delta-opioid receptor: Involvement of G protein-coupled receptor kinases but not protein kinase C. Mol Pharmacol. 1995;48:173–177. [PubMed] [Google Scholar]
- 29.McLaughlin JP, Myers LC, Zarek PE, Caron MG, Lefkowitz RJ, Czyzyk TA, Pintar JE, Chavkin C. Prolonged kappa opioid receptor phosphorylation mediated by G-protein receptor kinase underlies sustained analgesic tolerance. J Biol Chem. 2004;279:1810–1818. doi: 10.1074/jbc.M305796200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulz S, Mayer D, Pfeiffer M, Stumm R, Koch T, Hollt V. Morphine induces terminal micro-opioid receptor desensitization by sustained phosphorylation of serine-375. EMBO J. 2004;23:3282–3289. doi: 10.1038/sj.emboj.7600334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miess E, Gondin AB, Yousuf A, Steinborn R, Mosslein N, Yang Y, Goldner M, Ruland JG, Bunemann M, Krasel C, Christie MJ, et al. Multisite phosphorylation is required for sustained interaction with GRKs and arrestins during rapid μ-opioid receptor desensitization. Sci Signal. 2018;11:eaas9609. doi: 10.1126/scisignal.aas9609. [DOI] [PubMed] [Google Scholar]
- 32.Arttamangkul S, Heinz DA, Bunzow JR, Song X, Williams JT. Cellular tolerance at the μ-opioid receptor is phosphorylation dependent. eLife. 2018;7 doi: 10.7554/eLife.34989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shenoy SK, Lefkowitz RJ. β-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci. 2011;32:521–533. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, Caron MG, et al. β-Arrestin-dependent formation of β2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 35.Eichel K, Jullie D, Barsi-Rhyne B, Latorraca NR, Masureel M, Sibarita JB, Dror RO, von Zastrow M. Catalytic activation of β-arrestin by GPCRs. Nature. 2018;557:381–386. doi: 10.1038/s41586-018-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Latorraca NR, Wang JK, Bauer B, Townshend RJL, Hollingsworth SA, Olivieri JE, Xu HE, Sommer ME, Dror RO. Molecular mechanism of GPCR-mediated arrestin activation. Nature. 2018;557:452–456. doi: 10.1038/s41586-018-0077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang Y, Zhou XE, Gao X, He Y, Liu W, Ishchenko A, Barty A, White TA, Yefanov O, Han GW, Xu Q, et al. Crystal structure of rhodopsin bound to arrestin by femtosecond X-ray laser. Nature. 2015;523:561–567. doi: 10.1038/nature14656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Staus DP, Hu H, Robertson MJ, Kleinhenz ALW, Wingler LM, Capel WD, Latorraca NR, Lefkowitz RJ, Skiniotis G. Structure of the M2 muscarinic receptor-β-arrestin complex in a lipid nanodisc. Nature. 2020;579:297–302. doi: 10.1038/s41586-020-1954-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang W, Masureel M, Qu Q, Janetzko J, Inoue A, Kato HE, Robertson MJ, Nguyen KC, Glenn JS, Skiniotis G, Kobilka BK. Structure of the neurotensin receptor 1 in complex with β-arrestin 1. Nature. 2020;579:303–308. doi: 10.1038/s41586-020-1953-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ. β-Arrestin-dependent, G protein-independent ERK1/2 activation by the β2 adrenergic receptor. J Biol Chem. 2006;281:1261–1273. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- 41.Grundmann M, Merten N, Malfacini D, Inoue A, Preis P, Simon K, Ruttiger N, Ziegler N, Benkel T, Schmitt NK, Ishida S, et al. Lack of beta-arrestin signaling in the absence of active G proteins. Nat Commun. 2018;9:341. doi: 10.1038/s41467-017-02661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Hayre M, Eichel K, Avino S, Zhao X, Steffen DJ, Feng X, Kawakami K, Aoki J, Messer K, Sunahara R, Inoue A, et al. Genetic evidence that β-arrestins are dispensable for the initiation of β2-adrenergic receptor signaling to ERK. Sci Signal. 2017;10 doi: 10.1126/scisignal.aal3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luttrell LM, Wang J, Plouffe B, Smith JS, Yamani L, Kaur S, Jean-Charles PY, Gauthier C, Lee MH, Pani B, Kim J, et al. Manifold roles of β-arrestins in GPCR signaling elucidated with siRNA and CRISPR/Cas9. Sci Signal. 2018;11:eaat7650. doi: 10.1126/scisignal.aat7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith JS, Pack TF, Inoue A, Lee C, Zheng K, Choi I, Eiger DS, Warman A, Xiong X, Ma Z, Viswanathan G, et al. Noncanonical scaffolding of Gαi and β-arrestin by G protein-coupled receptors. Science. 2021;371:eaay1833. doi: 10.1126/science.aay1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shukla AK, Xiao K, Lefkowitz RJ. Emerging paradigms of β-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem Sci. 2011;36:457–469. doi: 10.1016/j.tibs.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/β-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 47.Kendall RT, Lee MH, Pleasant DL, Robinson K, Kuppuswamy D, McDermott PJ, Luttrell LM. Arrestin-dependent angiotensin AT1 receptor signaling regulates Akt and mTor-mediated protein synthesis. J Biol Chem. 2014;289:26155–26166. doi: 10.1074/jbc.M114.595728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu JJ, Sharma K, Zangrandi L, Chen C, Humphrey SJ, Chiu YT, Spetea M, Liu-Chen LY, Schwarzer C, Mann M. In vivo brain GPCR signaling elucidated by phosphoproteomics. Science. 2018;360:eaao4927. doi: 10.1126/science.aao4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 50.Che T, English J, Krumm BE, Kim K, Pardon E, Olsen RHJ, Wang S, Zhang S, Diberto JF, Sciaky N, Carroll FI, et al. Nanobody-enabled monitoring of kappa opioid receptor states. Nat Commun. 2020;11:1145. doi: 10.1038/s41467-020-14889-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu H, Wacker D, Mileni M, Katritch V, Han GW, Vardy E, Liu W, Thompson AA, Huang XP, Carroll FI, Mascarella SW, et al. Structure of the human ĸ-opioid receptor in complex with JDTic. Nature. 2012;485:327–332. doi: 10.1038/nature10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wingler LM, Skiba MA, McMahon C, Staus DP, Kleinhenz ALW, Suomivuori CM, Latorraca NR, Dror RO, Lefkowitz RJ, Kruse AC. Angiotensin and biased analogs induce structurally distinct active conformations within a GPCR. Science. 2020;367:888–892. doi: 10.1126/science.aay9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suomivuori CM, Latorraca NR, Wingler LM, Eismann S, King MC, Kleinhenz ALW, Skiba MA, Staus DP, Kruse AC, Lefkowitz RJ, Dror RO. Molecular mechanism of biased signaling in a prototypical G protein-coupled receptor. Science. 2020;367:881–887. doi: 10.1126/science.aaz0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCorvy JD, Wacker D, Wang S, Agegnehu B, Liu J, Lansu K, Tribo AR, Olsen RHJ, Che T, Jin J, Roth BL. Structural determinants of 5-HT2B receptor activation and biased agonism. Nat Struct Mol Biol. 2018;25:787–796. doi: 10.1038/s41594-018-0116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCorvy JD, Butler KV, Kelly B, Rechsteiner K, Karpiak J, Betz RM, Kormos BL, Shoichet BK, Dror RO, Jin J, Roth BL. Structure-inspired design of β-arrestin-biased ligands for aminergic GPCRs. Nat Chem Biol. 2018;14:126–134. doi: 10.1038/nchembio.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim K, Che T, Panova O, Di Berto JF, Lyu J, Krumm BE, Wacker D, Robertson MJ, Seven AB, Nichols DE, Shoichet BK, et al. Structure of a hallucinogen-activated Gq-coupled 5-HT2A serotonin receptor. Cell. 2020;182:1574–1588.:e19. doi: 10.1016/j.cell.2020.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Masuho I, Ostrovskaya O, Kramer GM, Jones CD, Xie K, Martemyanov KA. Distinct profiles of functional discrimination among G proteins determine the actions of G protein-coupled receptors. Sci Signal. 2015;8:ra123. doi: 10.1126/scisignal.aab4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olsen RHJ, Di Berto JF, English JG, Glaudin AM, Krumm BE, Slocum ST, Che T, Gavin AC, McCorvy JD, Roth BL, Strachan RT. TRUPATH, an open-source biosensor platform for interrogating the GPCR transducerome. Nat Chem Biol. 2020;16:841–849. doi: 10.1038/s41589-020-0535-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang J, Wu J, Kowalska MA, Dalvi A, Prevost N, O’Brien PJ, Manning D, Poncz M, Lucki I, Blendy JA, Brass LF. Loss of signaling through the G protein, Gz, results in abnormal platelet activation and altered responses to psychoactive drugs. Proc Natl Acad Sci USA. 2000;97:9984–9989. doi: 10.1073/pnas.180194597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jeong SW, Ikeda SR. G protein alpha subunit G alpha(z) couples neurotransmitter receptors to ion channels in sympathetic neurons. Neuron. 1998;21:1201–1212. doi: 10.1016/s0896-6273(00)80636-4. [DOI] [PubMed] [Google Scholar]
- 61.Gurevich VV, Gurevich EV. Biased GPCR signaling: Possible mechanisms and inherent limitations. Pharmacol Ther. 2020;211:107540. doi: 10.1016/j.pharmthera.2020.107540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Irannejad R, Pessino V, Mika D, Huang B, Wedegaertner PB, Conti M, von Zastrow M. Functional selectivity of GPCR-directed drug action through location bias. Nat Chem Biol. 2017;13:799–806. doi: 10.1038/nchembio.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stoeber M, Jullie D, Lobingier BT, Laeremans T, Steyaert J, Schiller PW, Manglik A, von Zastrow M. A genetically encoded biosensor reveals location bias of opioid drug action. Neuron. 2018;98:963–976.:e5. doi: 10.1016/j.neuron.2018.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zidar DA, Violin JD, Whalen EJ, Lefkowitz RJ. Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. Proc Natl Acad Sci USA. 2009;106:9649–9654. doi: 10.1073/pnas.0904361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Latorraca NR, Masureel M, Hollingsworth SA, Heydenreich FM, Suomivuori CM, Brinton C, Townshend RJL, Bouvier M, Kobilka BK, Dror RO. How GPCR phosphorylation patterns orchestrate arrestin-mediated signaling. Cell. 2020;183:1813–1825.:e18. doi: 10.1016/j.cell.2020.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 67.Raehal KM, Walker JKL, Bohn LM. Morphine side effects in β-Arrestin 2 knockout mice. J Pharmacol Exp Ther. 2005;314:1195–1201. doi: 10.1124/jpet.105.087254. [DOI] [PubMed] [Google Scholar]
- 68.Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, Chavkin C. Stress-induced p38 mitogen-activated protein kinase activation mediates ĸ-opioid-dependent dysphoria. J Neurosci. 2007;27:11614–11623. doi: 10.1523/JNEUROSCI.3769-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ehrich JM, Messinger DI, Knakal CR, Kuhar JR, Schattauer SS, Bruchas MR, Zweifel LS, Kieffer BL, Phillips PE, Chavkin C. Kappa opioid receptor-induced aversion requires p38 MAPK activation in VTA dopamine neurons. J Neurosci. 2015;35:12917–12931. doi: 10.1523/JNEUROSCI.2444-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.White KL, Robinson JE, Zhu H, Di Berto JF, Polepally PR, Zjawiony JK, Nichols DE, Malanga CJ, Roth BL. The G protein-biased ĸ-opioid receptor agonist RB-64 is analgesic with a unique spectrum of activities in vivo. J Pharmacol Exp Ther. 2015;352:98–109. doi: 10.1124/jpet.114.216820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- 72.Brust TF, Morgenweck J, Kim SA, Rose JH, Locke JL, Schmid CL, Zhou L, Stahl EL, Cameron MD, Scarry SM, Aube J, et al. Biased agonists of the kappa opioid receptor suppress pain and itch without causing sedation or dysphoria. Sci Signal. 2016;9:ra117. doi: 10.1126/scisignal.aai8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu JJ, Chiu YT, Di Mattio KM, Chen C, Huang P, Gentile TA, Muschamp JW, Cowan A, Mann M, Liu-Chen LY. Phosphoproteomic approach for agonist-specific signaling in mouse brains: mTOR pathway is involved in ĸ opioid aversion. Neuropsychopharmacology. 2019;44:939–949. doi: 10.1038/s41386-018-0155-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaski SW, White AN, Gross JD, Trexler KR, Wix K, Harland AA, Prisinzano TE, Aube J, Kinsey SG, Kenakin T, Siderovski DP, et al. Preclinical testing of nalfurafine as an opioid-sparing adjuvant that potentiates analgesia by the Mu opioid receptortargeting agonist morphine. J Pharmacol Exp Ther. 2019;371:487–499. doi: 10.1124/jpet.118.255661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kliewer A, Schmiedel F, Sianati S, Bailey A, Bateman JT, Levitt ES, Williams JT, Christie MJ, Schulz S. Phosphorylation-deficient G-protein-biased μ-opioid receptors improve analgesia and diminish tolerance but worsen opioid side effects. Nat Commun. 2019;10:367. doi: 10.1038/s41467-018-08162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kliewer A, Gillis A, Hill R, Schmidel F, Bailey C, Kelly E, Henderson G, Christie MJ, Schulz S. Morphine-induced respiratory depression is independent of β-arrestin2 signalling. BR J Pharmacol. 2020;177:2923–2931. doi: 10.1111/bph.15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gillis A, Gondin AB, Kliewer A, Sanchez J, Lim HD, Alamein C, Manandhar P, Santiago M, Fritzwanker S, Schmiedel F, Katte TA, et al. Low intrinsic efficacy for G protein activation can explain the improved side effect profiles of new opioid agonists. Sci Signal. 2020;13:eaaz3140. doi: 10.1126/scisignal.aaz3140. [DOI] [PubMed] [Google Scholar]
- 78.Gillis A, Kliewer A, Kelly E, Henderson G, Christie MJ, Schulz S, Canals M. Critical assessment of G protein-biased agonism at the μ-opioid receptor. Trends Pharmacol Sci. 2020;41:947–959. doi: 10.1016/j.tips.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 79.Matthes HWD, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the μ-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- 80.Conibear AE, Asghar J, Hill R, Henderson G, Borbely E, Tekus V, Helyes Z, Palandri J, Bailey C, Starke I, von Mentzer B, et al. A novel G protein-biased agonist at the δ opioid receptor with analgesic efficacy in models of chronic pain. J Pharmacol Exp Ther. 2020;372:224–236. doi: 10.1124/jpet.119.258640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen XT, Pitis P, Liu G, Yuan C, Gotchev D, Cowan CL, Rominger DH, Koblish M, Dewire SM, Crombie AL, Violin JD, et al. Structure-activity relationships and discovery of a G protein biased μ opioid receptor ligand, [(3-methoxythiophen-2-yl)methyl]({2-[(9R)-9-(pyridin-2-yl)-6-oxaspiro-[4.5]decan-9-yl]ethyl})amine (TRV130), for the treatment of acute severe pain. J Med Chem. 2013;56:8019–8031. doi: 10.1021/jm4010829. [DOI] [PubMed] [Google Scholar]
- 82.De Wire SM, Yamashita DS, Rominger DH, Liu G, Cowan CL, Graczyk TM, Chen XT, Pitis PM, Gotchev D, Yuan C, Koblish M, et al. A G protein-biased ligand at the μ-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther. 2013;344:708–717. doi: 10.1124/jpet.112.201616. [DOI] [PubMed] [Google Scholar]
- 83.Lovell KM, Frankowski KJ, Stahl EL, Slauson SR, Yoo E, Prisinzano TE, Aube J, Bohn LM. Structure-activity relationship studies of functionally selective kappa opioid receptor agonists that modulate ERK 1/2 phosphorylation while preserving G protein over βArrestin2 signaling bias. ACS Chem Nerosci. 2015;6:1411–1419. doi: 10.1021/acschemneuro.5b00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.White KL, Scopton AP, Rives M-L, Bikbulatov RV, Polepally PR, Brown PJ, Kenakin T, Javitch JA, Zjawiony JK, Roth BL. Identification of novel functionally selective ĸ-opioid receptor scaffolds. Mol Pharmacol. 2014;85:83–90. doi: 10.1124/mol.113.089649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Breton B, Sauvageau E, Zhou J, Bonin H, Le Gouill C, Bouvier M. Multiplexing of multicolor bioluminescence resonance energy transfer. Biophys J. 2010;99:4037–4046. doi: 10.1016/j.bpj.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kroeze WK, Sassano MF, Huang XP, Lansu K, McCorvy JD, Giguere PM, Sciaky N, Roth BL. PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome. Nat Struct Mol Biol. 2015;22:362–369. doi: 10.1038/nsmb.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kenakin T, Christopoulos A. Signalling bias in new drug discovery: Detection, quantification and therapeutic impact. Nat Rev Drug Discov. 2013;12:205–216. doi: 10.1038/nrd3954. [DOI] [PubMed] [Google Scholar]
- 88.Smith JS, Lefkowitz RJ, Rajagopal S. Biased signalling: From simple switches to allosteric microprocessors. Nat Rev Drug Discov. 2018;17:243–260. doi: 10.1038/nrd.2017.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rajagopal S, Ahn S, Rominger DH, Gowen-MacDonald W, Lam CM, Dewire SM, Violin JD, Lefkowitz RJ. Quantifying ligand bias at seven-transmembrane receptors. Mol Pharmacol. 2011;80:367–377. doi: 10.1124/mol.111.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Griffin MT, Figueroa KW, Liller S, Ehlert FJ. Estimation of agonist activity at G protein-coupled receptors: Analysis of M2 muscarinic receptor signaling through Gi/o,Gs, and G15 . J Pharmacol Exp Ther. 2007;321:1193–1207. doi: 10.1124/jpet.107.120857. [DOI] [PubMed] [Google Scholar]
- 91.Kenakin T, Watson C, Muniz-Medina V, Christopoulos A, Novick S. A simple method for quantifying functional selectivity and agonist bias. ACS Chem Nerosci. 2012;3:193–203. doi: 10.1021/cn200111m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carlsson J, Coleman RG, Setola V, Irwin JJ, Fan H, Schlessinger A, Sali A, Roth BL, Shoichet BK. Ligand discovery from a dopamine D3 receptor homology model and crystal structure. Nat Chem Biol. 2011;7:769–778. doi: 10.1038/nchembio.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lyu J, Wang S, Balius TE, Singh I, Levit A, Moroz YS, O’Meara MJ, Che T, Algaa E, Tolmachova K, Tolmachev AA, et al. Ultra-large library docking for discovering new chemotypes. Nature. 2019;566:224–229. doi: 10.1038/s41586-019-0917-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang S, Wacker D, Levit A, Che T, Betz RM, McCorvy JD, Venkatakrishnan AJ, Huang XP, Dror RO, Shoichet BK, Roth BL. D4 dopamine receptor high-resolution structures enable the discovery of selective agonists. Science. 2017;358:381–386. doi: 10.1126/science.aan5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang XP, Karpiak J, Kroeze WK, Zhu H, Chen X, Moy SS, Saddoris KA, Nikolova VD, Farrell MS, Wang S, Mangano TJ, et al. Allosteric ligands for the pharmacologically dark receptors GPR68 and GPR65. Nature. 2015;527:477–483. doi: 10.1038/nature15699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lansu K, Karpiak J, Liu J, Huang XP, McCorvy JD, Kroeze WK, Che T, Nagase H, Carroll FI, Jin J, Shoichet BK, et al. In silico design of novel probes for the atypical opioid receptor MRGPRX2. Nat Chem Biol. 2017;13:529–536. doi: 10.1038/nchembio.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, Levit A, Kling RC, Bernat V, Hübner H, Huang X-P, et al. Structure-based discovery of opioid analgesics with reduced side effects. Nature. 2016;537:185–190. doi: 10.1038/nature19112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hill R, Disney A, Conibear A, Sutcliffe K, Dewey W, Husbands S, Bailey C, Kelly E, Henderson G. The novel μ-opioid receptor agonist PZM21 depresses respiration and induces tolerance to antinociception. BR J Pharmacol. 2018;175:2653–2661. doi: 10.1111/bph.14224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Araldi D, Ferrari LF, Levine JD. Mu-opioid receptor (MOR) biased agonists induce biphasic dose-dependent hyperalgesia and analgesia, and hyperalgesic priming in the rat. Neuroscience. 2018;394:60–71. doi: 10.1016/j.neuroscience.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ehrlich AT, Semache M, Gross F, Da Fonte DF, Runtz L, Colley C, Mezni A, Le Gouill C, Lukasheva V, Hogue M, Darcq E, et al. Biased signaling of the Mu opioid receptor revealed in native neurons. iScience. 2019;14:47–57. doi: 10.1016/j.isci.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zheng Z, Huang XP, Mangano TJ, Zou R, Chen X, Zaidi SA, Roth BL, Stevens RC, Katritch V. Structure-based discovery of new antagonist and biased agonist chemotypes for the kappa opioid receptor. J Med Chem. 2017;60:3070–3081. doi: 10.1021/acs.jmedchem.7b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Soergel DG, Subach RA, Sadler B, Connell J, Marion AS, Cowan CL, Violin JD, Lark MW. First clinical experience with TRV130: Pharmacokinetics and pharmacodynamics in healthy volunteers. J Clin Pharmacol. 2014;54:351–357. doi: 10.1002/jcph.207. [DOI] [PubMed] [Google Scholar]
- 103.Viscusi ER, Webster L, Kuss M, Daniels S, Bolognese JA, Zuckerman S, Soergel DG, Subach RA, Cook E, Skobieranda F. A randomized, phase 2 study investigating TRV130, a biased ligand of the μ-opioid receptor, for the intravenous treatment of acute pain. Pain. 2016;157:264–272. doi: 10.1097/j.pain.0000000000000363. [DOI] [PubMed] [Google Scholar]
- 104.Viscusi ER, Skobieranda F, Soergel DG, Cook E, Burt DA, Singla N. APOLLO-1: A randomized placebo and active-controlled phase III study investigating oliceridine (TRV130), a G protein-biased ligand at the μ-opioid receptor, for management of moderate-to-severe acute pain following bunionectomy. J Pain Res. 2019;12:927–943. doi: 10.2147/JPR.S171013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Varadi A, Marrone GF, Palmer TC, Narayan A, Szabo MR, Le Rouzic V, Grinnell SG, Subrath JJ, Warner E, Kalra S, Hunkele A, et al. Mitragynine/Corynantheidine pseudoindoxyls as opioid analgesics with mu agonism and delta antagonism, which do not recruit β-Arrestin-2. J Med Chem. 2016;59:8381–8397. doi: 10.1021/acs.jmedchem.6b00748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li X, He W, Chen Y, Yang G, Wan H, Zhang L, Hu Q, Feng J, Zhang Z, He F, Bai C, et al. Discovery of SHR9352: A highly potent G protein-biased μ-opioid receptor agonist. ACS Omega. 2017;2:9261–9267. doi: 10.1021/acsomega.7b01452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schmid CL, Kennedy NM, Ross NC, Lovell KM, Yue Z, Morgenweck J, Cameron MD, Bannister TD, Bohn LM. Bias factor and therapeutic window correlate to predict safer opioid analgesics. Cell. 2017;171:1165–1175.:e13. doi: 10.1016/j.cell.2017.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xu H, Partilla JS, Wang X, Rutherford JM, Tidgewell K, Prisinzano TE, Bohn LM, Rothman RB. A comparison of noninternalizing (herkinorin) and internalizing (DAMGO) mu-opioid agonists on cellular markers related to opioid tolerance and dependence. Synapse. 2007;61:166–175. doi: 10.1002/syn.20356. [DOI] [PubMed] [Google Scholar]
- 109.Piekielna-Ciesielska J, Ferrari F, Calo G, Janecka A. Cyclopeptide Dmt-[D-Lys-p-CF3-Phe-Phe-Asp]NH2, a novel G protein-biased agonist of the mu opioid receptor. Peptides. 2018;101:227–233. doi: 10.1016/j.peptides.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 110.Rives ML, Rossillo M, Liu-Chen LY, Javitch JA. 6’-guanidinonaltrindole (6’-GNTI) is a G protein-biased ĸ-opioid receptor agonist that inhibits arrestin recruitment. J Biol Chem. 2012;287:27050–27054. doi: 10.1074/jbc.C112.387332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Spetea M, Berzetei-Gurske IP, Guerrieri E, Schmidhammer H. Discovery and pharmacological evaluation of a diphenethylamine derivative (HS665), a highly potent and selective ĸ opioid receptor agonist. J Med Chem. 2012;55:10302–10306. doi: 10.1021/jm301258w. [DOI] [PubMed] [Google Scholar]
- 112.Spetea M, Eans SO, Ganno ML, Lantero A, Mairegger M, Toll L, Schmidhammer H, McLaughlin JP. Selective ĸ receptor partial agonist HS666 produces potent antinociception without inducing aversion after icv administration in mice. BR J Pharmacol. 2017;174:2444–2456. doi: 10.1111/bph.13854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schattauer SS, Kuhar JR, Song A, Chavkin C. Nalfurafine is a G-protein biased agonist having significantly greater bias at the human than rodent form of the kappa opioid receptor. Cell Signal. 2017;32:59–65. doi: 10.1016/j.cellsig.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]