Abstract
Background
Risk factors for prostate cancer are not well understood. Red blood cell, platelet and white blood cell indices may be markers of a range of exposures that might be related to prostate cancer risk. Therefore, we examined the associations of haematological parameters with prostate cancer risk.
Methods
Complete blood count data from 209,686 male UK Biobank participants who were free from cancer at study baseline were analysed. Participants were followed-up via data linkage. After a mean follow-up of 6.8 years, 5,723 men were diagnosed with prostate cancer and 323 men died from prostate cancer. Multivariable-adjusted Cox regression was used to estimate adjusted hazard ratios (HR) and 95% confidence intervals (CI) for prostate cancer incidence and mortality by haematological parameters, and corrected for regression dilution bias.
Results
Higher red blood cell (HR per 1 SD increase=1.09, 95% CI 1.05-1.13) and platelet counts (HR=1.07, 1.04-1.11) were associated with an increased risk of prostate cancer. Higher mean corpuscular volume (HR=0.90, 0.87-0.93), mean corpuscular haemoglobin (HR=0.90, 0.87-0.93), mean corpuscular haemoglobin concentration (HR=0.87, 0.77-0.97) and mean sphered cell volume (HR=0.91, 0.87-0.94) were associated with a lower prostate cancer risk. Higher white blood cell (HR=1.14, 1.05-1.24) and neutrophil count (HR=1.27, 1.09-1.48) were associated with prostate cancer mortality.
Conclusions
These associations of blood indices of prostate cancer risk and mortality may implicate shared common causes, including testosterone, nutrition and inflammation/infection among several others in prostate cancer development and/or progression.
Impact
These associations provide insights into prostate cancer development and progression.
Keywords: Prostate cancer, complete blood count, UK Biobank, prospective analysis, epidemiology
Introduction
Prostate cancer is the second most common cancer in men and the fifth leading cause of cancer mortality worldwide(1). Established risk factors for prostate cancer include age, family history, ethnicity and genetic factors(2). Although there are large differences in the global incidence of prostate cancer, little is known regarding potential modifiable risk factors.
Testosterone, folate, vitamin B12 and iron are fundamental to the generation of red blood cells, and low concentrations of any of these factors can lead to changes in red blood cell counts and morphologies(3–11). Low free testosterone concentrations may reduce prostate cancer risk(12) and there is some evidence that folate, vitamin B12 and iron may be positively associated with prostate cancer risk, though these associations are inconsistent(13, 14).
Inflammation and infection stimulate increases in platelet and white blood cell production (11, 15). Previous studies indicate that chronic inflammation and/or the immune response may be associated with overall cancer incidence(16, 17), but it is unclear whether these factors are associated specifically with prostate cancer risk(18, 19).
Although some prospective studies have previously examined the associations between a limited number of haematological parameters and overall cancer risk(20–24), few have had the power to examine these exposures in relation to prostate cancer diagnosis or mortality(20, 21, 25). Moreover, these studies have relied on a single measure at study baseline. The UK Biobank has measured complete blood count and reticulocyte indices across the entire cohort, as well as repeat measurements in ~9,000 men, and thus provides a unique opportunity to investigate these associations reliably. Therefore, we aimed to assess the association of haematological indices as possible surrogate markers of exposures including testosterone, dietary factors and inflammation/infection, with risk of prostate cancer incidence and mortality.
Materials and Methods
Study design
UK Biobank is a large prospective study designed to be a resource for research into the causes of disease. Further details of the study protocol and data collection are available online (http://www.ukbiobank.ac.uk/wp-content/uploads/2011/11/UK-Biobank-Protocol.pdf) and in the literature(26).
In brief, all participants were registered with the National Health Service (NHS) and lived within 40 km of one of the UK Biobank assessment centres. Approximately 9.2 million people were initially invited to participate. Overall, about 503,000 men and women aged 40-69 years consented to join the cohort and attended one of 22 assessment centres throughout England, Wales and Scotland between 2006 and 2010, resulting in a participation rate of 5.5%(26).
The UK Biobank study was approved by the North West Multi-Centre Research Ethics Committee (reference number 06/MRE08/65), and at recruitment all participants gave written informed consent to participate and be followed-up.
Baseline assessment
At baseline participants provided data on a range of sociodemographic, physical, lifestyle, and health-related factors and summary diet information (including supplement and medication use) via self-completed touch-screen questionnaires and a computer-assisted personal interview(26). Body mass index (BMI) was measured at the assessment centre.
Blood samples were taken from all participants except for a small proportion who declined, were unable to, or where the attempt was abandoned for either technical or health reasons (0.3%)(26).
To prevent the blood from clotting it was collected into ethylenediaminetetraacetic acid (EDTA) vacutainers and shipped to the central processing laboratory in temperature-controlled shipping boxes (at 4°C)(27). On arrival at the central laboratory, the blood contents were measured on a Beckman automated haematology analyser and reticulocyte parameters were measured on a COULTER LH 750 System, typically within 24 hours of blood draw(27). A maximum of 31 haematological parameters were measured by the machine, including a mixture of measured and calculated values(28) (further details are available from https://biobank.ctsu.ox.ac.uk/crystal/crystal/docs/haematology.pdf).
For red blood cell and platelet parameters, the values analysed here are as measured by the machines. For subtypes of white blood cells (neutrophils, eosinophils, basophils, monocytes, lymphocytes), we calculated the counts from the measured proportions of these cells.
Repeat assessment
Repeat assessment data were collected in a subset of participants (~9,000 men) between August 2012 and June 2013 at the UK Biobank Co-ordinating Centre in Stockport. Participants who lived within a 35 km radius were invited to attend, with an overall response rate of 21%(28, 29) for further details see: https://biobank.ctsu.ox.ac.uk/~bbdatan/Repeat_assessment_doc_v1.0.pdf).
Exclusion criteria
There were up to 209,686 men included in this analysis, after excluding 9,871 men with prevalent malignant cancer (except C44: non-melanoma skin cancer), 213 participants who were identified as being genetically female, and 9,364 men who did not have blood data available.
Participant follow-up
Cancer registration data were provided via record linkage to the NHS Central Register, until the censoring date (31st March 2016 in England and Wales and 31st October 2015 in Scotland). Death data for England and Wales were provided by NHS Digital and for Scotland by the Information and Statistics Division (censoring dates 31st January 2018 and 30th November 2016, respectively).
In the analyses of incident prostate cancer, the endpoint was defined as the first diagnosis of prostate cancer, or death from prostate cancer (International Classification of Diseases Tenth revision code [ICD-10] C61)(30), whichever was recorded first. Prostate cancer cases identified via death records were included only if death from prostate cancer preceded the NHS Central Register censoring date. Person-years were calculated from the date of recruitment to the date of the first cancer registration (excluding non-melanoma skin cancer [ICD-10 C44])(30), death, or censoring date, whichever occurred first. In analyses of prostate cancer mortality, the endpoint was defined as being prostate cancer as the primary cause of death.
Statistical analysis
Partial correlations between the blood indices were estimated after adjustment for region (10 UK regions), age at recruitment (<45, 45–49, 50–54, 55–59, 60–64, and ≥65 years) and BMI (<25, ≥25-<30, ≥30-<35, ≥35 kg/m2, unknown (0.4%)).
Hazard ratios (HRs) and 95% confidence intervals (CIs) of prostate cancer diagnosis and mortality were estimated using Cox proportional hazards models, with age as the underlying time variable. Analyses were stratified by geographic region of recruitment (10 UK regions) and age at recruitment (categories as defined above), and adjusted for Townsend deprivation score (fifths, unknown (0.1%)), racial/ethnic group (white, mixed background, Asian, black, other, and unknown (0.6%)), height (<170, ≥170–<175, ≥175–<180, ≥180 cm, and unknown (0.4%)), lives with a wife or partner (no, yes), body mass index (BMI, categories as defined above), cigarette smoking (never, former, current light smoker (1-14 cigarettes per day), current heavy smoker (≥15 cigarettes per day), current (number of cigarettes per day unknown), and smoking status unknown (0.6%)), alcohol consumption (non-drinkers, <1-≤9, ≥10-<20, ≥20 g ethanol/day, unknown (0.5%)), and self-reported diabetes (no, yes, and unknown (0.5%)). Categories of the adjustment covariates were defined a priori based on previous analyses using UK Biobank data(31).
The blood parameters investigated were chosen a priori. The red blood cells parameters were: red blood cell (erythrocyte) count, red blood cell distribution width, haematocrit, haemoglobin concentration, mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), mean sphered cell volume (MSCV), and reticulocyte count. Platelets parameters were: platelet count, platelet distribution width and mean platelet (thrombocyte) volume. White blood cells parameters were: white blood cell (leukocyte) count, neutrophil count, eosinophil count, basophil count, monocyte count and lymphocyte count. Summary descriptions of haematological parameters and measurements are in Table 1.
Table 1. Summary description of haematological parameters and measurement/calculation methods by UK Biobank.
| Haematological parameter | Description | Measurement/calculation |
|---|---|---|
| Red blood cell | ||
| Red blood cell count (1012 cells/L) | Number of red blood cells in the sample | Measured |
| Red blood cell distribution width (%) | Spread of red blood cell population | Deriveda |
| Haematocrit (%) | Volume occupied by red blood cells in the blood | (MCV x RBC)/10 |
| Haemoglobin concentration (g/dL) | Total haemoglobin concentration in sample | Measured |
| Mean corpuscular volume (pg) | Average volume of red blood cells | Deriveda |
| Mean corpuscular haemoglobin (g/dL) | Mass of haemoglobin in the average red blood cell | (HGB / RBC) x 10 |
| Mean corpuscular haemoglobin concentration (g/dL) | Average mass of haemoglobin per the relative volume of red blood cells in the whole blood sample | (HGB / HCT) x 100 |
| Reticulocyte count (1012 cells/L) | Number of reticulocytes in the red blood cell sample | % Reticulocyte x RBC |
| Platelet | ||
| Platelet count (109 cells/L) | Number of platelets in the sample | Measured |
| Platelet distribution width (%) | Variation in platelet volume | Deriveda |
| Mean platelet volume (fL) | Average volume of individual platelets in the sample | Deriveda |
| White blood cell | ||
| White blood cell count (109 cells/L) | Number of white blood cells in the sample | Measured |
| Neutrophil count (109 cells/L) | Number of neutrophils in the white blood cell sample | (% Proportion of neutrophils/100) x WBC |
| Eosinophil count (109 cells/L) | Number of eosinophils in the white blood cell sample | (% Proportion of eosinophils/100) x WBC |
| Basophil count (109 cells/L) | Number of basophils in the white blood cell sample | (% Proportion of basophils/100) x WBC |
| Monocyte count (109 cells/L) | Number of monocytes in the white blood cell sample | (% Proportion of monocytes/100) x WBC |
| Lymphocyte count (109 cells/L) | Number of lymphocytes in the white blood cell sample | (% Proportion of lymphocytes/100) x WBC |
Measurement/calculation methods were obtained from: http://biobank.ctsu.ox.ac.uk/crystal/docs/haematology.pdf.
Derived values were calculated within the instrument using multiple scatterplots and histograms for each sample.
Abbreviations: HCT=haematocrit; HGB=haemoglobin concentration; MCH=mean corpuscular haemoglobin; MCHC=mean corpuscular haemoglobin concentration; MCV=mean corpuscular volume; MPV=mean platelet volume; PDW=platelet distribution width; PLT=platelet count; RBC=red blood cell count; RCDW=red blood cell distribution width; RET=reticulocyte count; WBC=white blood cell count.
Due to measurement error and within-person variability, using single measures at baseline may lead to substantial under-estimation of potential associations with disease risk (i.e. regression dilution bias)(32). UK Biobank repeated the blood measurements during follow-up in a sub-sample of 8,667 men. HRs for trend were estimated per one unit increase in standard deviation (SD) and were corrected for regression dilution bias using the McMahon-Peto method(33). This method divides the subjects into fifths at baseline measurement and calculates the difference between the mean parameter measurement in the top and bottom quintile groups for both the baseline and a second measurement in order to estimate a ratio of ranges(33). In the categorical analysis, haematological data were categorised into fifths of the distribution of the whole cohort at study baseline. HRs were calculated relative to the lowest fifth of each blood parameter. The variance of the log risk in each group was calculated (from the variances and covariances of the log risk) and used to obtain group-specific 95% CIs, which enabled comparisons across different exposure categories(34).
The proportional hazards assumption was tested using Schoenfeld residuals and revealed no evidence of deviation from the assumption.
Stratified analyses
To examine whether associations with prostate cancer risk differed for cancers diagnosed shortly after recruitment or in men who were diagnosed at a younger age, heterogeneity in the associations of haematological indices with incident prostate cancer was tested by time from recruitment to diagnosis and mortality (<4; ≥4 years), and age at diagnosis (<65; ≥65 years). Stratified Cox models were fitted based on competing risks and heterogeneity in the risk coefficients and standard errors in the two subgroups was tested using the χ2 test of heterogeneity(12).
For non-case defined characteristics, heterogeneity in the associations with incident prostate cancer by age at blood collection (<60, ≥60 years), BMI (<30, ≥30 kg/m2), smoking (never, ever smokers) and diabetes status (yes, no) was tested using the χ2 test of interaction between subgroups.
Sensitivity analyses
Family history of prostate cancer was not included as an adjustment factor in the primary analysis due to the limited availability of data (45.1% missing/unknown). Family history of prostate cancer was further adjusted for as a sensitivity analysis (no, yes (brother or father), and unknown). To adjust for the possibility of laboratory drift, analyses were repeated following additional adjustment for year of blood collection (continuous).
The primary analysis was repeated after: i) excluding men who reported that they were regularly taking folic acid, folate or multivitamin supplementation at baseline (n=41,323); ii) excluding men who reported that they were regularly taking prescription hydroxyurea, interferon, heparin, erythropoietin, anaemia treatment, immunosuppressants, antihistamines or anti-inflammatories at baseline (n=64,284); and iii) after removing outliers (outside the range of [lower quartile – 3*interquartile range, upper quartile + 3*interquartile range]), the number of outliers identified ranged from n=195 for mean platelet volume to n=5,199 for basophils.
All tests of significance were two-sided, and P-values <0.01 were considered statistically significant. Analyses were performed using Stata version 14.1 (Stata Corporation, College Station, TX, USA), and figures were created in R version 3.2.3.
Results
209,686 men were included in this analysis, and after a mean follow-up of 6.8 years (SD=1.3 years) 5,723 (2.7%) were diagnosed with prostate cancer and 323 died from the disease (numbers of incident cases and deaths are smaller for some exposure variables). Table 2 summarises the baseline characteristics of study participants. Mean age at recruitment was 56.6 years (SD=8.2), and men had a mean BMI of 27.8 kg/m2. 12% of men were current smokers, 43% reported drinking ≥20 g alcohol per day. 28% reported having a PSA test prior to baseline and 7.5% had a family history of prostate cancer.
Table 2. Baseline characteristics and blood indices of men free from prostate cancer and men who developed prostate cancer.
| All men (n=209,686) | Men who developed prostate cancer (n=5,723) | |
|---|---|---|
| Sociodemographic | ||
| Age at recruitment (years), mean (SD) | 56.6 (8.2) | 62.2 (5.2) |
| Most deprived quintile, % (n) | 19.8 (41,514) | 15.7 (896) |
| Black ethnicity, % (n) | 1.5 (3,083) | 2.0 (117) |
| Not in paid/self-employment, % (n) | 38.7 (81,179) | 57.1 (3,268) |
| Living with partner, % (n) | 76.3 (160,064) | 79.2 (4,533) |
| Anthropometric, mean (SD) | ||
| Height (cm) | 175.6 (6.8) | 175.1 (6.7) |
| BMI (kg/m2) | 27.8 (4.2) | 27.6 (3.8) |
| Lifestyle, % (n) | ||
| Current cigarette smokers | 12.4 (26,061) | 9.2 (524) |
| Drinking alcohol ≥20 g per day | 43.3 (90,834) | 42.5 (2,432) |
| Low physical activity (0-10 METs per week) | 27.4 (57,517) | 26.2 (1,500) |
| Health history, % (n) | ||
| Hypertension | 52.2 (109,380) | 59.0 (3,376) |
| Diabetes | 6.9 (14,434) | 6.0 (342) |
| Prostate specific factors, % (n) | ||
| Ever had a PSA test | 27.8 (58,279) | 45.9 (2,624) |
| Family history of prostate cancer | 7.5 (15,750) | 13.0 (745) |
| Blood measures at initial assessment, median (interquartile range) | ||
| Red blood cell | ||
| Red blood cell count (1012 cells/L) | 4.75 (4.51 - 4.99) | 4.73 (4.49 - 4.98) |
| Red blood cell distribution width (%) | 13.3 (12.9 - 13.8) | 13.4 (13.0 - 13.9) |
| Haematocrit (%) | 43.4 (41.5 - 45.2) | 43.3 (41.4 - 45.3) |
| Haemoglobin concentration (g/dL) | 15.0 (14.4 - 15.7) | 15.0 (14.3 - 15.6) |
| Mean corpuscular volume (pg) | 91.4 (88.8 - 94.1) | 91.5 (88.9 - 94.2) |
| Mean corpuscular haemoglobin (g/dL) | 31.7 (30.7 - 32.7) | 31.7 (30.7 - 32.6) |
| Mean corpuscular haemoglobin concentration (g/dL) | 34.6 (34.0 - 35.2) | 34.5 (33.9 - 35.2) |
| Mean sphered cell volume (fL) | 82.5 (79.2 - 85.9) | 82.6 (79.4 - 86.1) |
| Reticulocyte count (1012 cells/L) | 0.06 (0.05 - 0.08) | 0.06 (0.05 - 0.08) |
| Platelet | ||
| Platelet count (109 cells/L) | 234 (202 - 269) | 233 (200 - 269) |
| Platelet distribution width (%) | 16.5 (16.2 - 16.9) | 16.5 (16.2 - 16.9) |
| Mean platelet volume (fL) | 9.18 (8.54 - 9.90) | 9.17 (8.53 - 9.93) |
| White blood cell | ||
| White blood cell count (109 cells/L) | 6.69 (5.67 - 7.90) | 6.70 (5.70 - 7.89) |
| Neutrophil count (109 cells/L) | 4.06 (3.29 - 5.00) | 4.15 (3.36 - 5.09) |
| Eosinophil count (109 cells/L) | 0.15 (0.10 - 0.24) | 0.15 (0.09 - 0.24) |
| Basophil count (109 cells/L) | 0.029 (0.019 - 0.044) | 0.028 (0.019 - 0.043) |
| Monocyte count (109 cells/L) | 0.49 (0.39 - 0.61) | 0.50 (0.40 - 0.62) |
| Lymphocyte count (109 cells/L) | 1.81 (1.47 - 2.21) | 1.78 (1.45 - 2.18) |
Abbreviations: BMI= body mass index; MET=metabolic equivalent; PSA=prostate specific antigen; SD=standard deviation.
Median and interquartile range values for haematological data are displayed in Table 2. Characteristics of men by red blood cell, platelet, and white blood cell counts by 1st, 3rd and 5th fifths at baseline are displayed in Table 3. Regression dilution ratios are displayed in Supplementary Table 1. Basophil count and MCHC had the lowest regression dilution ratios (0.20 and 0.24, respectively). Regression dilution ratios for the other haematological parameters ranged between 0.53 - 0.83 (Supplementary Table 1).
Table 3. Baseline characteristics of 209,686 men in UK Biobank according to observed red blood cell, platelet and white blood cell at initial assessment.
| Fifths of observed red blood cell count | Fifths of observed platelet count | Fifths of observed white blood cell count | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 1 | 3 | 5 | 1 | 3 | 5 | |
| No. of men | 41,446 | 41,786 | 41,034 | 41,102 | 41,230 | 41,157 | 41,325 | 41,892 | 41,266 |
| Age at recruitmenta, y | 58.5 (7.7) | 56.3 (8.2) | 55.1 (8.4) | 57.9 (8.1) | 56.5 (8.2) | 55.6 (8.1) | 55.2 (8.2) | 56.8 (8.1) | 57.4 (8.1) |
| BMIa, kg/m2 | 27.1 (4.4) | 27.8 (4.1) | 28.6 (4.2) | 28.0 (4.3) | 27.8 (4.2) | 27.8 (4.3) | 26.7 (3.7) | 27.9 (4.0) | 28.8 (4.9) |
| Height1, cm | 175.2 (6.9) | 175.9 (6.8) | 175.5 (6.9) | 176.2 (6.9) | 175.8 (6.8) | 174.7 (6.8) | 176.7 (6.8) | 175.6 (6.8) | 174.5 (6.8) |
| Smoking, n (%) | |||||||||
| Never | 17,891 (42.6) | 21,231 (49.9) | 22,203 (53.8) | 20,969 (50.0) | 20,816 (49.7) | 18,965 (45.6) | 24,491 (58.2) | 21,593 (50.9) | 14,856 (35.6) |
| Former | 17,686 (42.1) | 16,128 (37.9) | 14,012 (33.9) | 16,087 (38.4) | 15,962 (38.1) | 15,834 (38.1) | 15,099 (35.9) | 16,675 (39.3) | 15,175 (36.4) |
| Current | 6,199 (14.7) | 4,981 (11.7) | 4,779 (11.6) | 4,618 (11.0) | 4,874 (11.6) | 6,531 (15.7) | 2,283 (5.4) | 3,909 (9.2) | 11,320 (27.1) |
| Educational level, n (%) | |||||||||
| No degree | 7,466 (17.8) | 8,034 (18.9) | 7,993 (19.4) | 7,545 (18.0) | 7,831 (18.7) | 8,059 (19.4) | 2,219 (17.9) | 7,915 (18.7) | 8,220 (19.7) |
| Degree | 25,817 (61.4) | 29,400 (64.1) | 27,963 (62.6) | 26,592 (63.4) | 26,816 (63.9) | 25,283 (60.3) | 28,971 (68.9) | 27,044 (63.8) | 23,223 (55.7) |
| Physical activity, n (%) | |||||||||
| Low | 10,684 (25.4) | 11,448 (26.9) | 12,680 (30.7) | 11,228 (26.8) | 11,308 (27.0) | 12,124 (29.1) | 9,644 (22.9) | 11,480 (27.1) | 13,588 (32.6) |
| Moderate | 20,036 (47.7) | 20,606 (48.4) | 19,052 (46.1) | 20,485 (48.8) | 20,147 (48.1) | 19,116 (45.9) | 21,429 (50.9) | 20,422 (48.1) | 18,190 (43.6) |
| High | 9,991 (23.8) | 9,247 (21.7) | 7,958 (19.3) | 8,958 (21.4) | 9,186 (21.9) | 8,874 (21.3) | 9,886 (23.5) | 9,215 (21.7) | 8,295 (19.9) |
| Ethnicity, n (%) | |||||||||
| White | 40,320 (95.9) | 40,481 (96.3) | 36,815 (87.6) | 38,949 (92.9) | 39,514 (94.2) | 39,326 (93.8) | 38,872 (92.4) | 40,164 (94.7) | 39,330 (94.3) |
| Not white | 1,485 (3.5) | 1,870 (4.4) | 4,207 (10.2) | 2,714 (6.5) | 2,164 (5.2) | 2,057 (5.0) | 2,964 (7.0) | 2,026 (4.8) | 2,137 (5.1) |
| Diabetes at baseline, n (%) | |||||||||
| No | 37,762 (89.8) | 39,862 (93.6) | 38,456 (93.1) | 38,097 (90.8) | 39,100 (93.2) | 38,559 (91.9) | 40,243 (95.7) | 39,510 (93.1) | 36,720 (88.0) |
| Yes | 4,089 (9.7) | 2,526 (5.9) | 2,544 (6.2) | 3,601 (8.6) | 2,625 (6.3) | 2,812 (6.8) | 1,652 (3.9) | 2,700 (6.4) | 4,727 (11.3) |
| Marital status, n (%) | |||||||||
| Married | 31,181 (73.9) | 32,935 (77.0) | 31,388 (75.5) | 32,116 (76.6) | 32,328 (77.1) | 30,742 (73.3) | 32,689 (77.4) | 32,865 (77.1) | 29,637 (70.5) |
| Not married | 10,853 (25.7) | 9,638 (22.5) | 9,906 (23.8) | 9,822 (23.4) | 9,565 (22.8) | 10,866 (25.9) | 9,370 (22.2) | 9,553 (22.4) | 12,092 (28.8) |
Percentages may not match due to missing data.
Values are means (SD).
Abbreviations: BMI= body mass index; SD= standard deviation
Correlations between blood indices
Red blood cell count was negatively correlated with MCV and MCH (r=-0.50 and -0.54, respectively). Platelet count was negatively correlated with mean platelet volume and platelet distribution width (r=-0.47 and -0.35, respectively). White blood cell counts were at least weakly positively correlated with each other, and with platelet count (r=0.24) (Supplementary Figure 1).
Associations between haematological data and prostate cancer incidence and mortality
Estimates are corrected for regression dilution bias; uncorrected results are displayed in Supplementary Table 2.
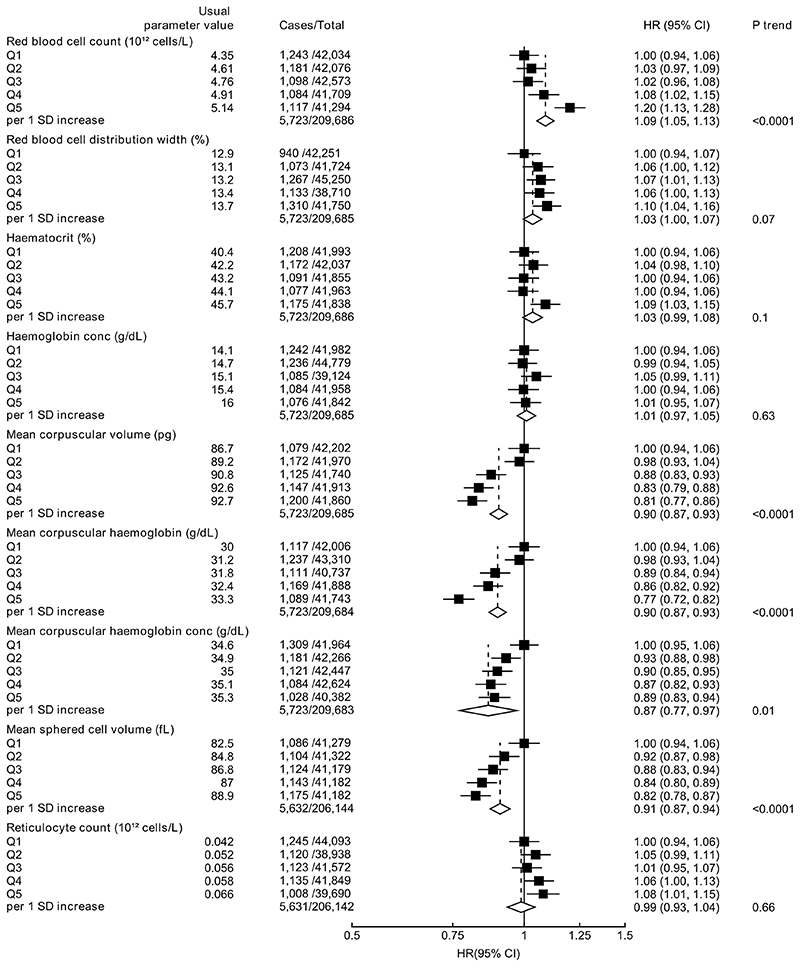
Higher total red blood cell count was associated with an elevated risk of prostate cancer (HR per 1 SD increase=1.09, 95% CI 1.05-1.13; Ptrend<0.001). Higher MCV (HR per 1 SD increase=0.90, 95% CI 0.87-0.93), MCH (0.90, 0.87-0.93), MCHC (0.87, 0.77-0.97) and MSCV (0.91, 0.87-0.94) were associated with a lower prostate cancer risk (Figure 1). Red blood cell indices were not associated with prostate cancer mortality (Supplementary Figure 2).
Figure 1. Hazard ratio (95% CIs) of prostate cancer diagnosis by fifths of red blood cell indices.
HRs are stratified by region and age at recruitment and adjusted for age (underlying time variable), Townsend deprivation score, racial/ethnic group, height, lives with a wife or partner, BMI, cigarette smoking, alcohol consumption and diabetes. HRs per 1 SD increase are adjusted for regression dilution bias. Abbreviations: BMI=body mass index; CI=confidence intervals; HR=hazard ratio; IQR=interquartile range; SD=standard deviation.
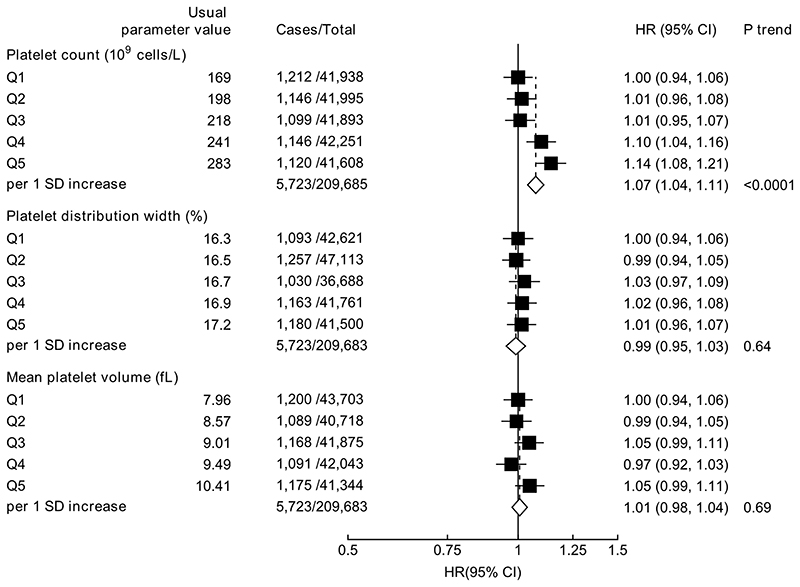
Higher platelet count (HR per 1 SD increase =1.07, 95% CI 1.04-1.11) was associated with increased risk of prostate cancer diagnosis (Figure 2). Platelet indices were not associated with prostate cancer mortality (Supplementary Figure 3).
Figure 2. Hazard ratio (95% CIs) of prostate cancer diagnosis by fifths of platelet indices.
HRs are stratified by region and age at recruitment and adjusted for age (underlying time variable), Townsend deprivation score, racial/ethnic group, height, lives with a wife or partner, BMI, cigarette smoking, alcohol consumption and diabetes.
HRs per 1 SD increase are adjusted for regression dilution bias.
Abbreviations: BMI=body mass index; CI=confidence intervals; HR=hazard ratio; IQR=interquartile range; SD=standard deviation.
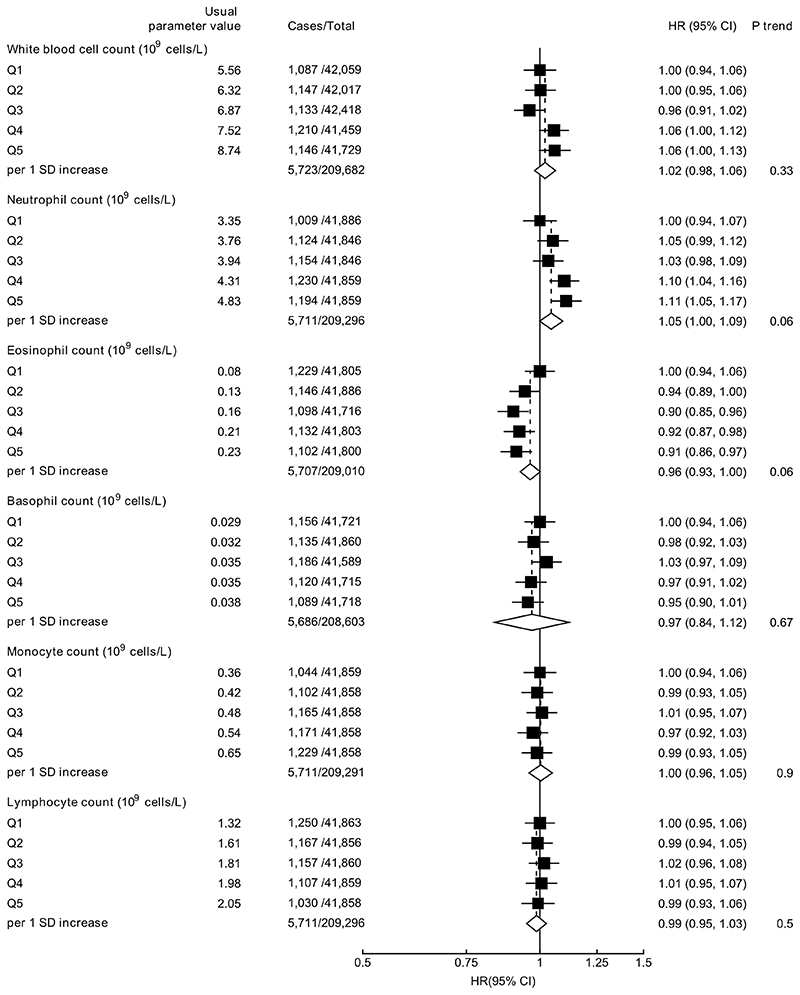
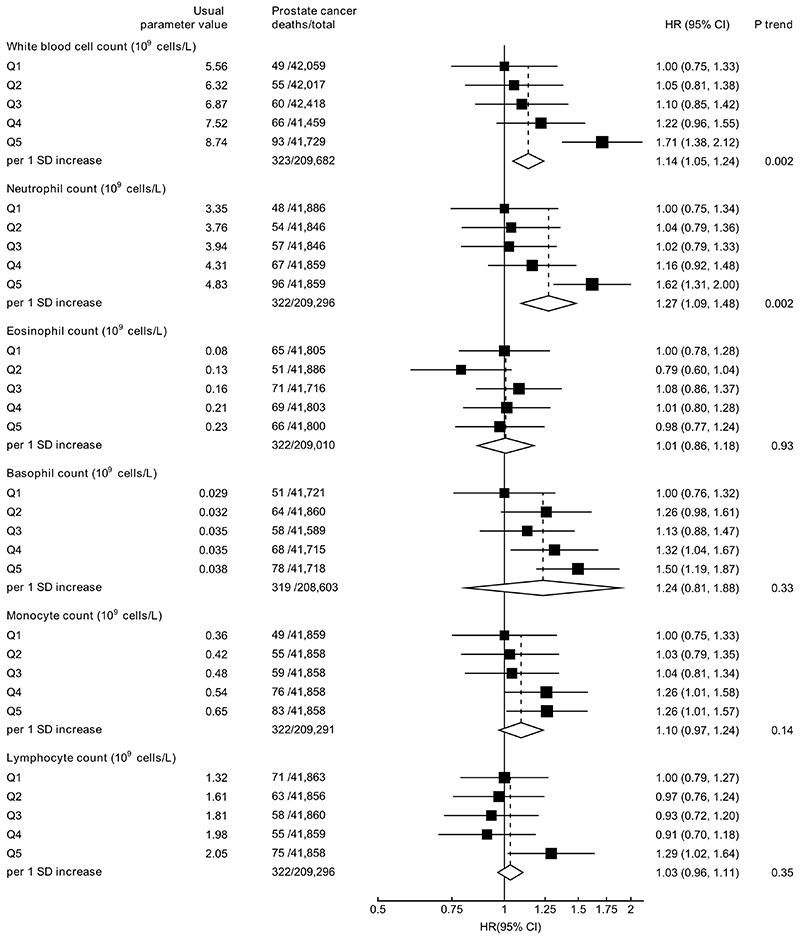
White blood cell counts were not associated with prostate cancer diagnosis (Figure 3), but higher white blood cell (HR per 1 SD increase =1.14, 95% CI 1.05-1.24) and neutrophil counts (1.27, 1.09-1.48) were associated with an increased risk of prostate cancer mortality (Figure 4).
Figure 3. Hazard ratio (95% CIs) of prostate cancer diagnosis by fifths of white blood cell indices.
HRs are stratified by region and age at recruitment and adjusted for age (underlying time variable), Townsend deprivation score, racial/ethnic group, height, lives with a wife or partner, BMI, cigarette smoking, alcohol consumption and diabetes.
HRs per 1 SD increase are adjusted for regression dilution bias.
Abbreviations: BMI=body mass index; CI=confidence intervals; HR=hazard ratio; IQR=interquartile range; SD=standard deviation.
Figure 4. Hazard ratio (95% CIs) of prostate cancer mortality by fifths of white blood cell indices.
HRs are stratified by region and age at recruitment and adjusted for age (underlying time variable), Townsend deprivation score, racial/ethnic group, height, lives with a wife or partner, BMI, cigarette smoking, alcohol consumption and diabetes.
HRs per 1 SD increase are adjusted for regression dilution bias.
Abbreviations: BMI=body mass index; CI=confidence intervals; HR=hazard ratio; IQR=interquartile range; SD=standard deviation.
Stratified analyses
There was some evidence of heterogeneity in the associations of red blood cell and platelet parameters with prostate cancer risk by time to diagnosis, with stronger associations observed in men diagnosed ≥4 years from baseline blood collection for MCH and MSCV parameters (Phet all <0.01 (Table 4). There was also some evidence that associations between white blood cell counts and prostate cancer mortality were stronger with a longer follow-up time, but numbers of prostate cancer deaths were limited (Supplementary Table 3). There was no evidence of heterogeneity in the associations with prostate cancer risk by age at diagnosis (Supplementary Table 4), age at recruitment, smoking, BMI or diabetes status (Phet>0.01 for all.
Table 4. Multivariable-adjusted hazard ratios* (95% CI) for prostate cancer by time to diagnosis in relation to haematological indices.
| Diagnosed < 4 years from baseline | Diagnosed ≥ 4 years from baseline | |||||
|---|---|---|---|---|---|---|
| Cases | HR (95% CI) | Cases | HR (95% CI) | Phet | ||
| Red blood cell | ||||||
| Red blood cell count (1012 cells/L) | 2938 | 1.04(1.01-1.08) | 2785 | 1.10(1.06-1.14) | 0.07 | |
| Red blood cell distribution width (%) | 2938 | 1.02(0.98-1.06) | 2785 | 1.03(0.99-1.07) | 0.77 | |
| Haematocrit (%) | 2938 | 1.01(0.98-1.05) | 2785 | 1.03(0.99-1.07) | 0.52 | |
| Haemoglobin concentration (g/dL) | 2938 | 1.01(0.97-1.04) | 2785 | 1.01(0.97-1.05) | 0.98 | |
| Mean corpuscular volume (pg) | 2938 | 0.95(0.91-0.99) | 2785 | 0.90(0.86-0.93) | 0.04 | |
| Mean corpuscular haemoglobin (g/dL) | 2938 | 0.95(0.91-0.99) | 2785 | 0.88(0.85-0.92) | 0.01 | |
| Mean corpuscular haemoglobin conc (g/dL) | 2938 | 0.99(0.95-1.02) | 2785 | 0.94(0.91-0.98) | 0.13 | |
| Mean sphered cell volume (fL) | 2886 | 0.97(0.93-1.01) | 2746 | 0.90(0.86-0.94) | 0.01 | |
| Reticulocyte count (1012 cells/L) | 2885 | 0.98(0.94-1.03) | 2746 | 1.00(0.96-1.04) | 0.57 | |
| Platelet | ||||||
| Platelet count (109 cells/L) | 2938 | 1.03(0.99-1.06) | 2785 | 1.09(1.05-1.13) | 0.02 | |
| Platelet distribution width (%) | 2938 | 0.99(0.96-1.03) | 2785 | 0.99(0.96-1.03) | 0.96 | |
| Mean platelet volume (fL) | 2885 | 0.99(0.96-1.03) | 2729 | 1.02(0.98-1.05) | 0.42 | |
| White blood cell | ||||||
| White blood cell count (109 cells/L) | 2932 | 0.99(0.95-1.03) | 2778 | 1.04(1.00-1.08) | 0.12 | |
| Neutrophil count (109 cells/L) | 2931 | 1.01(0.97-1.05) | 2780 | 1.05(1.01-1.09) | 0.17 | |
| Eosinophils (109 cells/L) | 2930 | 0.97(0.93-1.01) | 2777 | 0.98(0.94-1.02) | 0.87 | |
| Basophil count (109 cells/L) | 2920 | 1.00(0.96-1.04) | 2766 | 0.98(0.94-1.03) | 0.51 | |
| Monocyte count (109 cells/L) | 2931 | 1.00(0.96-1.04) | 2780 | 1.00(0.96-1.05) | 0.88 | |
| Lymphocyte count (109 cells/L) | 2931 | 0.97(0.93-1.02) | 2780 | 1.00(0.96-1.04) | 0.34 | |
HRs per 1 SD increase are stratified by region and age at recruitment and adjusted for age (underlying time variable), Townsend deprivation score, racial/ethnic group, height, lives with a wife or partner, BMI, cigarette smoking, alcohol consumption and diabetes.
Phet was estimated using stratified Cox models based on competing risks (<4 and ≥4 years from recruitment to diagnosis), and tested using the χ2 test of heterogeneity).
Abbreviations: BMI=body mass index; CI=confidence intervals; HR=hazard ratio; SD=standard deviation.≥
Sensitivity analyses
Further adjustment for family history of prostate cancer and date of blood collection made no appreciable difference to the associations. Associations remained consistent following the exclusion of i) men who reported regularly taking multivitamins/folate supplementation, ii) medications that are known to influence haematological parameters, and iii) following the exclusion of outliers. White blood cell and reticulocyte counts were positively skewed; however log-transformation did not materially alter the results.
Discussion
The findings from this large prospective study in British men provide evidence for the associations of haematological parameters with prostate cancer risk. Higher red blood cell and platelet counts were associated with an elevated risk of prostate cancer diagnosis and higher MCV, MCH, MCHC and MSCV, were associated with a lower risk of prostate cancer diagnosis, but red blood cell and platelet parameters were not associated with prostate cancer mortality. In contrast, white blood cell counts were not associated with risk of prostate cancer diagnosis, but higher white blood cell and neutrophil counts were associated with an increased risk of prostate cancer mortality.
The observed associations of higher red blood cell counts, but lower measures of red blood cell volumes with prostate cancer risk may support the hypothesised roles of testosterone, folate, vitamin B12 and/or iron in prostate cancer development. Testosterone and dietary factors (folate, vitamin B12 and iron) play a role in red blood cell production and deficiencies in any of these factors can cause anaemia(5–11, 35–38). We observed an association between a greater number of red blood cells, but lower red blood cell volumes and an elevated risk of prostate cancer diagnosis. Macrocytic anaemia is a subtype of anaemia which is characterised by larger red cell volumes and is commonly associated with folate and vitamin B12 deficiencies(39), therefore our results are compatible with prior hypotheses that folate and/or B12 may have a positive association with prostate cancer risk, whereas iron deficiencies are more commonly associated with smaller red blood cell volumes i.e. microcytic anaemia(40). Although testosterone is associated with red blood cell production, any possible role in determining red blood cell size is not well described.
Red blood cell production is primarily controlled by the kidneys (via erythropoietin)(3). Previous epidemiological studies have observed a reduced risk of prostate cancer in men with chronic kidney disease(41), therefore, the associations of red blood cell indices with prostate cancer risk may be related to the correlates of kidney function (including testosterone(42), insulin-like growth factor-I(43), iron(44) and metabolic syndrome(45)).
The finding of a positive association of higher platelet count with risk of prostate cancer diagnosis, and higher white blood cell and neutrophil counts with increased risk of prostate cancer mortality may support the hypothesised roles of chronic inflammation and/or infection in prostate cancer development and/or progression(17, 46). Previous studies have reported that inflammatory markers were associated with an increased risk of several cancers(21, 23, 24), but the associations with prostate cancer were inconsistent(21, 24, 47). Furthermore, the associations of intraprostatic inflammation with prostate tumour prognosis remain unclear(48).
It is possible that the associations of blood cells with prostate cancer may indicate causal patterns relating to general cell behaviour(49, 50). Future analyses, such as Mendelian randomization, may be beneficial in identifying the possible causal components that may account for these associations(51).
It is possible that the associations observed in the current study may be at least partially explained by reverse causation. Cancer can cause anaemia by producing cytokines, which lead to iron sequestration(52). Tumours can also increase platelet indices, even during early cancer(53). However, the associations of red blood cell and platelet indices with prostate cancer diagnosis were stronger with a longer follow-up time, which may suggest that they are aetiologically relevant markers. Tumours may also increase the half-life of neutrophils, which can then promote tumour growth and metastasis(54, 55). In this analysis, associations of white blood cell counts with prostate cancer mortality were stronger with longer durations of follow-up. This suggests that these associations with prostate cancer death may not be explained by reverse causation, although there was limited statistical power to assess heterogeneity by time to prostate cancer death.
This analysis has a number of strengths. With data on over 200,000 men, this is the largest prospective study to examine a wide range of haematological measures in relation to prostate cancer specific risk. Blood indices were measured across the entire cohort via standardised methods and participants were well-characterised. The availability of repeat measures in a subsample of men allowed for correction for regression dilution bias, and therefore more accurate estimates of the associations with risk.
A limitation of this analysis was that it was not possible to rule-out detection bias. These blood indices may be associated with health status (for instance, low red blood cell count is associated with hypertension, hypothyroidism and congestive heart failure(56), while high white blood cell count is associated with increased total and cardiovascular mortality(57, 58)). It is also difficult to rule-out the possibility of residual confounding due to other potential lifestyle-related risk factors. Co-morbidities, socioeconomic status and poor health may affect PSA test attendance, but PSA testing attendance after baseline was not known. Prostate tumour stage and grade data are not currently available in UK Biobank. In the absence of data on tumour characteristics, associations with prostate cancer death may be considered more aetiologically relevant than overall diagnosis, although the statistical power to examine associations with death was limited. The associations of white blood cell counts with prostate cancer death may indicate that these indices are associated with more clinically relevant forms of prostate cancer, or may be evidence of their role in tumour progression. UK Biobank participants are healthier and better educated than the sampling population, and have a higher rate of incident prostate cancer(26), which may be related to a greater PSA testing attendance(59). Therefore, risk estimates may not be generalizable to all populations. The UK Biobank prospective cohort study design is underpowered to detect associations with acute short-term infection and inflammation, and associations with inflammation and infection are likely to reflect chronic long-term exposure. However, acute short-term inflammation and infection may decrease regression dilution ratios. Although the blood measurements were generally consistent between baseline measurement and the repeat measurement, MCHC and basophil count had low regression dilution ratios. Lower regression dilution ratios will not affect the p-value, but will increase the uncertainty of the risk estimates. Blood indices were used as surrogate markers of a range of possible risk factors. Circulating folate, vitamin B12, iron and testosterone concentrations or markers of inflammation (such as C-reactive protein) were not available at the time of analysis; therefore, it was not possible to investigate further any potential mechanistic factors which may account for the observed associations.
In conclusion, this analysis of more than 200,000 men observed associations of several haematological parameters and prostate cancer risk. The associations of blood indices with prostate cancer risk and mortality implicate shared common causes with prostate cancer. These relationships are compatible with the hypothesised relationships of testosterone, folate, vitamin B12 and inflammation with prostate cancer development. Future analyses, such as Mendelian randomization may be beneficial in identifying the possible causal components that may account for these associations.
Supplementary Material
Acknowledgements
This research has been conducted using the UK Biobank resource under application number 3282. Data analysis was supported by Cancer Research UK grants C8221/A19170, C8221/A29017 and C8221/A20986. Eleanor Watts was supported by the Nuffield Department of Population Health Early Career Research Fellowship.
Financial support
Data analysis was supported by Cancer Research UK grants C8221/A19170, C8221/A29017 and C8221/A20986. Eleanor Watts was supported by the Nuffield Department of Population Health Early Career Research Fellowship.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- HR
hazard ratios
- ICD-10
International Classification of Diseases Tenth revision
- MCH
mean corpuscular haemoglobin
- MCHC
mean corpuscular haemoglobin concentration
- MCV
mean corpuscular volume
- MSCV
mean sphered cell volume
Footnotes
Conflict of interest: The authors have no conflicts of interest to declare
References
- 1.Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. International Agency for Research on Cancer; Lyon, France: 2018. [Accessed 02/03/2019]. https://gco.iarc.fr/today. [Google Scholar]
- 2.Pernar CH, Ebot EM, Wilson KM, Mucci LA. The epidemiology of prostate cancer. Cold Spring Harbor perspectives in medicine. 2018;8(12) doi: 10.1101/cshperspect.a030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valent P, Büsche G, Theurl I, et al. Normal and pathological erythropoiesis in adults: from gene regulation to targeted treatment concepts. Haematologica. 2018;103(10):1593–603. doi: 10.3324/haematol.2018.192518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coviello AD, Kaplan B, Lakshman KM, Chen T, Singh AB, Bhasin S. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. The Journal of clinical endocrinology and metabolism. 2008;93(3):914–9. doi: 10.1210/jc.2007-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrucci L, Maggio M, Bandinelli S, et al. Low testosterone levels and the risk of anemia in older men and women. Archives of internal medicine. 2006;166(13):1380–8. doi: 10.1001/archinte.166.13.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koury MJ, Ponka P. New insights into erythropoiesis: the roles of folate, vitamin B12, and iron. Annu Rev Nutr. 2004;24(1):105–31. doi: 10.1146/annurev.nutr.24.012003.132306. [DOI] [PubMed] [Google Scholar]
- 7.Ferrucci L, Maggio M, Bandinelli S, et al. Low testosterone levels and the risk of anemia in older men and women. Arch Intern Med. 2006;166(13):1380–8. doi: 10.1001/archinte.166.13.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrero JJ, Barany P, Yilmaz MI, et al. Testosterone deficiency is a cause of anaemia and reduced responsiveness to erythropoiesis-stimulating agents in men with chronic kidney disease. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2012;27(2):709–15. doi: 10.1093/ndt/gfr288. [DOI] [PubMed] [Google Scholar]
- 9.Roy CN, Snyder PJ, Stephens-Shields AJ, et al. Association of testosterone levels with anemia in older men: A controlled clinical trial. JAMA Intern Med. 2017;177(4):480–90. doi: 10.1001/jamainternmed.2016.9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz SO, Flowers VC. Morphologic changes in red blood cell with iron deficiency anemia. JAMA. 1946;130(10):622–4. doi: 10.1001/jama.1946.02870100008002. [DOI] [PubMed] [Google Scholar]
- 11.George-Gay B, Parker K. Understanding the complete blood count with differential. J Perianesth Nurs. 2003;18(2):96–117. doi: 10.1053/jpan.2003.50013. [DOI] [PubMed] [Google Scholar]
- 12.Watts EL, Appleby PN, Perez-Cornago A, et al. Low free testosterone and prostate cancer risk: a collaborative analysis of 20 prospective studies. Eur Urol. 2018;74(5):585–94. doi: 10.1016/j.eururo.2018.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Price AJ, Travis RC, Appleby PN, et al. Circulating folate and vitamin B(12) and risk of prostate cancer: A collaborative analysis of individual participant data from six cohorts including 6875 cases and 8104 controls. Euro Urol. 2016;70(6):941–51. doi: 10.1016/j.eururo.2016.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi JY, Neuhouser ML, Barnett MJ, et al. Iron intake, oxidative stress-related genes (MnSOD and MPO) and prostate cancer risk in CARET cohort. Carcinogenesis. 2008;29(5):964–70. doi: 10.1093/carcin/bgn056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghoshal K, Bhattacharyya M. Overview of platelet physiology: Its hemostatic and nonhemostatic role in disease pathogenesis. Scientific World Journal. 2014;2014:781857. doi: 10.1155/2014/781857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Front Immunol. 2011;2:98. doi: 10.3389/fimmu.2011.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doat S, Marous M, Rebillard X, et al. Prostatitis, other genitourinary infections and prostate cancer risk: Influence of non-steroidal anti-inflammatory drugs? Results from the EPICAP study. International journal of cancer. 2018 doi: 10.1002/ijc.31565. [DOI] [PubMed] [Google Scholar]
- 19.Sfanos KS, Isaacs WB, De Marzo AM. Infections and inflammation in prostate cancer. Am J Clin Exp Urol. 2013;1(1):3–11. [PMC free article] [PubMed] [Google Scholar]
- 20.Adris N, Chua ACG, Knuiman MW, Divitini ML, Trinder D, Olynyk JK. A prospective cohort examination of haematological parameters in relation to cancer death and incidence: the Busselton Health Study. BMC Cancer. 2018;18(1):863. doi: 10.1186/s12885-018-4775-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allin KH, Bojesen SE, Nordestgaard BG. Inflammatory biomarkers and risk of cancer in 84,000 individuals from the general population. International journal of cancer. 2016;139(7):1493–500. doi: 10.1002/ijc.30194. [DOI] [PubMed] [Google Scholar]
- 22.Bailey SER, Ukoumunne OC, Shephard EA, Hamilton W. Clinical relevance of thrombocytosis in primary care: a prospective cohort study of cancer incidence using English electronic medical records and cancer registry data. Br J Gen Pract. 2017;67(659):e405–e13. doi: 10.3399/bjgp17X691109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Margolis KL, Rodabough RJ, Thomson CA, Lopez AM, McTiernan A. Prospective study of leukocyte count as a predictor of incident breast, colorectal, endometrial, and lung cancer and mortality in postmenopausal women. Archives of internal medicine. 2007;167(17):1837–44. doi: 10.1001/archinte.167.17.1837. [DOI] [PubMed] [Google Scholar]
- 24.Van Hemelrijck M, Holmberg L, Garmo H, et al. Association between levels of C-reactive protein and leukocytes and cancer: Three repeated measurements in the Swedish AMORIS study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20(3):428–37. doi: 10.1158/1055-9965.epi-10-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edgren G, Bagnardi V, Bellocco R, et al. Pattern of declining hemoglobin concentration before cancer diagnosis. International journal of cancer. 2010;127(6):1429–36. doi: 10.1002/ijc.25122. [DOI] [PubMed] [Google Scholar]
- 26.Fry A, Littlejohns TJ, Sudlow C, et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. American journal of epidemiology. 2017;186(9):1026–34. doi: 10.1093/aje/kwx246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elliott P, Peakman TC. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. International journal of epidemiology. 2008;37(2):234–44. doi: 10.1093/ije/dym276. [DOI] [PubMed] [Google Scholar]
- 28.Sheard SM, Froggatt J. UK Biobank haematology data companion document. 2017 https://biobank.ctsu.ox.ac.uk/crystal/docs/haematology.pdf.
- 29.UK Biobank. Repeat assessment data. 2013 https://biobank.ctsu.ox.ac.uk/~bbdatan/Repeat_assessment_doc_v1.0.pdf.
- 30.WHO. International statistical classification of diseases and related health problems. 10th revision. 2010 [Google Scholar]
- 31.Perez-Cornago A, Key TJ, Allen NE, et al. Prospective investigation of risk factors for prostate cancer in the UK Biobank cohort study. Br J Cancer. 2017;117(10):1562–71. doi: 10.1038/bjc.2017.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clarke R, Shipley M, Lewington S, et al. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. American journal of epidemiology. 1999;150(4):341–53. doi: 10.1093/oxfordjournals.aje.a010013. [DOI] [PubMed] [Google Scholar]
- 33.MacMahon S, Peto R, Cutler J, et al. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet (London, England) 1990;335(8692):765–74. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- 34.Plummer M. Improved estimates of floating absolute risk. Stat Med. 2004;23(1):93–104. doi: 10.1002/sim.1485. [DOI] [PubMed] [Google Scholar]
- 35.Hunt A, Harrington D, Robinson S. Vitamin B12 deficiency. BMJ. 2014;349 doi: 10.1136/bmj.g5226. [DOI] [PubMed] [Google Scholar]
- 36.Snow CF. Laboratory diagnosis of vitamin b12 and folate deficiency: A guide for the primary care physician. Archives of internal medicine. 1999;159(12):1289–98. doi: 10.1001/archinte.159.12.1289. [DOI] [PubMed] [Google Scholar]
- 37.d’Onofrio G, Chirillo R, Zini G, Caenaro G, Tommasi M, Micciulli G. Simultaneous measurement of reticulocyte and red blood cell indices in healthy subjects and patients with microcytic and macrocytic anemia. Blood. 1995;85(3):818–23. [PubMed] [Google Scholar]
- 38.Barton JC, Bertoli LF, Rothenberg BE. Peripheral blood erythrocyte parameters in hemochromatosis: Evidence for increased erythrocyte hemoglobin content. J Lab Clin Med. 2000;135(1):96–104. doi: 10.1016/S0022-2143(00)70026-6. [DOI] [PubMed] [Google Scholar]
- 39.Aslinia F, Mazza JJ, Yale SH. Megaloblastic anemia and other causes of macrocytosis. Clin Med Res. 2006;4(3):236–41. doi: 10.3121/cmr.4.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uprichard WO, Uprichard J. Investigating microcytic anaemia. Bmj. 2013;346:f3154. doi: 10.1136/bmj.f3154. [DOI] [PubMed] [Google Scholar]
- 41.Wong G, Staplin N, Emberson J, et al. Chronic kidney disease and the risk of cancer: an individual patient data meta-analysis of 32,057 participants from six prospective studies. BMC Cancer. 2016;16:488. doi: 10.1186/s12885-016-2532-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edey MM. Male sexual dysfunction and chronic kidney disease. Front Med (Lausanne) 2017;4:32. doi: 10.3389/fmed.2017.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamenický P, Mazziotti G, Lombès M, Giustina A, Chanson P. Growth hormone, insulin-like growth factor-1, and the kidney: Pathophysiological and clinical implications. Endocrine Reviews. 2014;35(2):234–81. doi: 10.1210/er.2013-1071. [DOI] [PubMed] [Google Scholar]
- 44.Babitt JL, Lin HY. Mechanisms of anemia in CKD. Journal of the American Society of Nephrology : JASN. 2012;23(10):1631–4. doi: 10.1681/ASN.2011111078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pecoits-Filho R, Abensur H, Betônico CCR, et al. Interactions between kidney disease and diabetes: dangerous liaisons. Diabetol Metab Syndr. 2016;8(1):50. doi: 10.1186/s13098-016-0159-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caini S, Gandini S, Dudas M, Bremer V, Severi E, Gherasim A. Sexually transmitted infections and prostate cancer risk: a systematic review and meta-analysis. Cancer epidemiology. 2014;38(4):329–38. doi: 10.1016/j.canep.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 47.Toriola AT, Laukkanen JA, Kurl S, Nyyssonen K, Ronkainen K, Kauhanen J. Prediagnostic circulating markers of inflammation and risk of prostate cancer. International journal of cancer. 2013;133(12):2961–7. doi: 10.1002/ijc.28313. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y, Zhou CK, Rencsok EM, et al. A Prospective Study of Intraprostatic Inflammation, Focal Atrophy, and Progression to Lethal Prostate Cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2019;28(12):2047–54. doi: 10.1158/1055-9965.Epi-19-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gibson CJ, Steensma DP. New Insights from Studies of Clonal Hematopoiesis. Clin Cancer Res. 2018;24(19):4633–42. doi: 10.1158/1078-0432.ccr-17-3044. [DOI] [PubMed] [Google Scholar]
- 50.Wu L, Saxena S, Awaji M, Singh RK. Tumor-Associated Neutrophils in Cancer: Going Pro. Cancers (Basel) 2019;11(4):564. doi: 10.3390/cancers11040564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yarmolinsky J, Wade KH, Richmond RC, et al. Causal inference in cancer epidemiology: what is the role of Mendelian randomization? Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2018;27(9):995–1010. doi: 10.1158/1055-9965.epi-17-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spivak JL. The anaemia of cancer: death by a thousand cuts. Nature reviews Cancer. 2005;5(7):543–55. doi: 10.1038/nrc1648. [DOI] [PubMed] [Google Scholar]
- 53.Menter DG, Tucker SC, Kopetz S, Sood AK, Crissman JD, Honn KV. Platelets and cancer: a casual or causal relationship: revisited. Cancer Metastasis Rev. 2014;33(1):231–69. doi: 10.1007/s10555-014-9498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swierczak A, Mouchemore KA, Hamilton JA, Anderson RL. Neutrophils: important contributors to tumor progression and metastasis. Cancer Metastasis Rev. 2015;34(4):735–51. doi: 10.1007/s10555-015-9594-9. [DOI] [PubMed] [Google Scholar]
- 55.Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nature reviews Cancer. 2016;16:431. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- 56.Gandhi SJ, Hagans I, Nathan K, Hunter K, Roy S. Prevalence, comorbidity and investigation of anemia in the primary care office. J Clin Med Res. 2017;9(12):970–80. doi: 10.14740/jocmr3221w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kabat GC, Kim MY, Manson JE, et al. White blood cell count and total and cause-specific mortality in the Women’s Health Initiative. American journal of epidemiology. 2017;186(1):63–72. doi: 10.1093/aje/kww226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vinholt PJ, Hvas AM, Frederiksen H, Bathum L, Jørgensen MK, Nybo M. Platelet count is associated with cardiovascular disease, cancer and mortality: A population-based cohort study. Thromb Res. 2016;148:136–42. doi: 10.1016/j.thromres.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 59.Littlejohns TJ, Travis RC, Key TJ, Allen NE. Lifestyle factors and prostate-specific antigen (PSA) testing in UK Biobank: Implications for epidemiological research. Cancer epidemiology. 2016;45:40–6. doi: 10.1016/j.canep.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.