Abstract
Adrenoleukodystrophy is a neurometabolic disorder caused by a defective peroxisomal ABCD1 transporter of very long-chain fatty acids (VLCFAs). Its pathogenesis is incompletely understood. Here we characterize a nematode model of X-ALD with loss of the pmp-4 gene, the worm orthologue of ABCD1. These mutants recapitulate the hallmarks of X-ALD: i) VLCFAs accumulation and impaired mitochondrial redox homeostasis and ii) axonal damage coupled to locomotor dysfunction. Furthermore, we identify a novel role for PMP-4 in modulating lipid droplet dynamics. Importantly, we show that the mitochondria targeted antioxidant MitoQ normalizes lipid droplets size, and prevents axonal degeneration and locomotor disability, highlighting its therapeutic potential. Moreover, peroxisomes appear undetectable in neurons, implying the existence of cell non autonomous mechanisms governing axonal maintenance. Indeed, PMP-4 acting solely in the hypodermis rescues axonal and locomotion abnormalities, suggesting a myelin-like role for the hypodermis in providing essential peroxisomal functions for the nematode nervous system.
Keywords: X-linked adrenoleukodystrophy, axonal degeneration, hypodermis, mitochondria redox imbalance, lipid droplets, peroxisomes
Introduction
Peroxisomes are single membrane-bound ubiquitous organelles that play key roles in redox homeostasis and the metabolism of lipids, in particular fatty acid β-oxidation, ether phospholipids, and bile acids (1). Impairments in any of these essential pathways are associated with major clinical signs and symptoms, usually involving the nervous system (2). X-linked adrenoleukodystrophy (X-ALD), McKusick no. 300100) is the most common peroxisomal disease and leukodystrophy with an incidence of 1:14700 births (3). X-ALD is caused by a loss of function of the ABCD1 gene located on Xq.28, which encodes a peroxisomal transporter that imports very long-chain fatty acids (VLCFAs) into the peroxisome for degradation by β-oxidation (4). As a consequence, VLCFAs, especially hexacosanoic acid or C26:0, accumulate in tissues and plasma and constitute a pathognomonic biomarker for diagnosis. Despite being a single-gene disease, X-ALD is a complex inherited syndrome in which the same mutation in the ABCD1 gene can lead to highly divergent clinical phenotypes, such as childhood cerebral adrenoleukodystrophy (cALD) or chronic progressive adult-onset adrenomyeloneuropathy (AMN) (5–7), accounting for marked variability of phenotypic expression. AMN patients present with spastic paraparesis caused by cortical motor neuron and corticospinal tract involvement often associated with peripheral neuropathy. Approximately 20% of all AMN patients develop cAMN, which is an inflammatory condition similar to cALD that occurs at a later stage (8). Therapeutic options are scarce, and when diagnosed early, the cerebral forms of the disease (cALD and cAMN) are only adequately treatable with an allogeneic bone marrow transplant (9–11) or recently, with haematopoietic stem cell gene therapy for cALD (12, 13). However, no pharmacological treatment has been shown to be beneficial for either form of the disease (14), although several repurposed drugs have been proposed (15–18), and initial encouraging results from a pilot trial with a combination of antioxidants have very recently been reported (19).
The two mouse models of X-ALD (Abcd1- and double mutant Abcd1-/Abcd2-/- mice) develop late-onset axonopathy, with signs and symptoms resembling AMN visible at 20 and 12 months of age, respectively (20, 21). Using these mouse models and patient samples, several studies have indicated that VLCFA-induced oxidative stress is a critical, early pathogenic factor in X-ALD (22–24), although the exact mechanisms by which redox imbalance causes neurodegeneration in X-ALD are incompletely understood.
Here, we established a cost-effective disease model with the aim of identifying critical steps leading to axonal demise and establishing a rapid and amenable platform for high-throughput drug screening in the nematode Caenorhabditis elegans. PMP4 is the worm orthologue of ABCD1, and its function has thus far been unexplored. Despite the fact that the C. elegans nervous system is not myelinated (25), thus precluding the study of the physiopathology of the infantile form of X-ALD (cALD), this work indicates that pmp-4(ok396) worms may constitute a valuable model of the axonopathy occurring in the adult form of the disease, AMN. A study of this model sheds light on the mechanisms leading to mitochondrial and lipid droplet (LD) metabolism impairment and their contributions to axonal degeneration while highlighting the prominent role of the hypodermis in axonal maintenance in the nematode
Results
pmp-4 encodes the peroxisomal ABCD1 orthologue, and pmp-4(ok396) loss of function mutants recapitulate the main hallmarks of X-ALD
Phylogenetic analysis identified pmp-4 as the orthologue and ancestor of mammalian peroxisomal transporters ABCD1 and ABCD2 in C. elegans (26); at the protein level PMP-4 and ABCD1 show 75% similarity (Supplementary Fig. S1A).
To establish a model of X-ALD in the nematode, we used a strain harbouring the pmp-4(ok396) allele, which contains an 867 bp deletion encompassing exons 6 to 10 (www.wormbase.org) (Supplementary Fig. S1B). pmp-4(ok396) worms did not show any obvious defects in growth or maturation.
We generated a polyclonal antibody using the last 21 amino acids of the C-terminal part of PMP-4 (Fig. S1A) and performed western blot (WB) experiments that detected a band above 75 kDa in wild-type (WT, N2 strain) homogenates. This molecular weight is expected for a protein of 734 amino acids, while no protein was observed in pmp-4(ok396) extracts (Fig. 1A). As a positive control, we generated a transgenic strain expressing the PMP-4 protein fused to GFP at the C-terminus under the control of its own promoter in pmp-4(ok396) animals (pmp-4(ok396); Ex042 [Ppmp-4::pmp-4::gfp]) and used the homogenates for the WB (Fig. 1A). PMP-4 was not detected in pmp-4(ok396) animals by immunofluorescence (Fig. 1B–C), demonstrating that pmp-4(ok396) is a null allele. Furthermore, we observed that PMP-4 is well expressed from the first larval stage (L1) to adulthood, with higher expression from L3 onwards, whereas no expression was detected in embryos (Supplementary Fig. S1C).
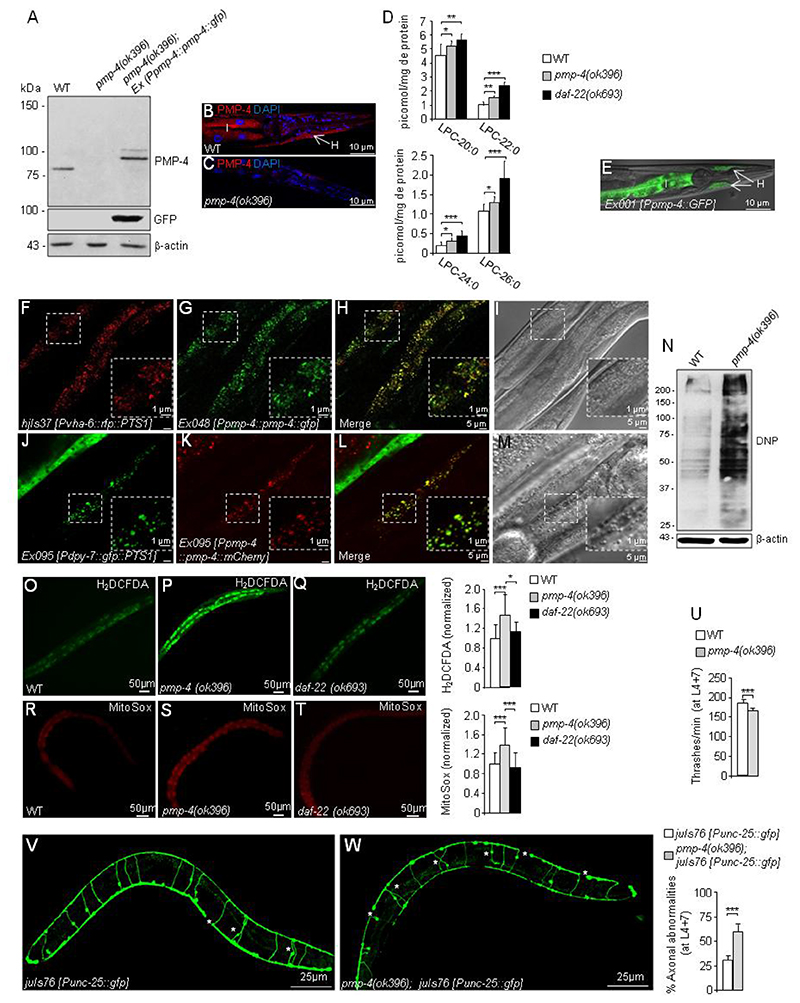
Figure 1. C. elegans pmp-4(ok396) mutants recapitulate the main hallmarks observed in X-ALD.
(A) PMP-4 and PMP-4::GFP protein levels in wild-type (WT), pmp-4(ok396) and pmp-4(ok396) animals expressing PMP-4::GFP under the control of the pmp-4 promoter (pmp-4(ok396); Ex042 [Ppmp-4::pmp-4::gfp]) at the L4 larval stage. β-actin was used as a loading control (bottom panel) (n=4 pools of worms by condition). (B-C) Immunofluorescence staining of formaldehyde-fixed (B) WT and (C) pmp-4(ok396) worms incubated with polyclonal anti-PMP-4 (red) and counterstained with DAPI (blue) at the L4 larval stage. The posterior part of the worm is depicted. I=intestine and H=hypodermis. Scale = 10μm. (D) Lysophosphatidylcholine fatty acid levels (LPC-20:0, LPC-22:0, LPC-24:0 and LPC-26:0) of L4 worm lysates of WT (n=12), pmp-4(ok396)(n=10) and daf-22(ok693) animals (n=6). The daf-22(ok693) mutant was used as a positive control. (E) Tissular expression of GFP under the control of the pmp-4 promoter (Ex001 [Ppmp-4::gfp]) in the intestine=I and hypodermis=H at L4. The worm is oriented head to the left, dorsal face-up. The same results were obtained by three independent transgenic lines. Scale = 10μm. (F-I) The construct containing PMP-4::GFP under the control of the pmp-4 promoter was injected into the strain labelling peroxisomes with RFP in the intestine (hjIs37 [Pvha-6p::rfp::PTS1]; Ex048 [Ppmp-4::pmp-4::gfp]). Images of (F) RFP::PTS1 and (G) PMP-4::GFP staining. (H) Merge of images F and G. In yellow, colocalization of RFP::PTS-1 with PMP-4::GFP. (I) DIC-Nomarsky pictures corresponding to the fluorescence images (n=30). (J-M) The constructs containing GFP::PTS1 under the control of a specific hypodermal promoter (Pdpy-7) and PMP-4::mCherry under the control of the pmp-4 promoter were coinjected in the N2 strain (Ex095 [Pdpy-7::gfp::PTS1 + Ppmp-4::pmp-4::mCherry]). Here, the peroxisomes are labelled with GFP in the hypodermis. Images of (J) GFP::PTS1 and (K) PMP-4::mCherry staining. (L) Merge of images J and K. In yellow, colocalization of GFP::PTS-1 with PMP-4::mCherry. (M) DIC-Nomarsky images corresponding to the fluorescence images (n=30). Scale = 5 μm in each figure except for the expanded pictures in the squares, in which scale = 1 μm. The worms in the figures are oriented head to the left, dorsal face-up and only a portion of the central part of the body is depicted. (N) Dinitrophenol (DNP) protein levels in WT and pmp-4(ok396) animals at the L4 larval stage. The quantification of these blots by densitometry was performed and normalized to β-actin (n=4 pools of worms by condition). Relative total ROS levels (H2DCFDA) were measured by quantifying the fluorescence emission of the H2DCFDA probes in living animals in (O) WT (n=51), (P) pmp-4(ok396)(n=66) and (Q) daf-22(ok693) (n=40) L4 nematodes maintained in a liquid medium. Values are normalized to WT. Relative mitochondrial ROS levels in living animals in (R) WT (n=66), (S) pmp-4(ok396) (n=88) and (T) daf-22(ok693) (n=66) L4 nematodes quantified with MitoSox probes. Values are normalized to WT worms. Scale = 50 μm. (U) The thrashing behaviour of WT and pmp-4(ok396) nematodes was analysed in liquid medium at L4+7 days (n=20 animals/condition). Representative fluorescence images of GFP-labelled GABAergic neurons showing axonal abnormalities and quantitative analysis in living worms in (V) juIs76 [Punc-25::gfp] (n=20) and (W) pmp-4(ok396); juIs76 [Punc-25::gfp] (n=20) at L4+7 days. Scale = 25 μm. Data represent the mean ± standard deviation (SD). Statistical analysis was carried out with one-way ANOVA, followed by Tukey’s post hoc test (*P<0.05; **P<0.01; ***P<0.001) for D, O-S. Statistical analysis was carried out with Student’s t-test (*P<0.05; **P<0.01; ***P<0.001) for U-W.
The main biochemical hallmark of X-ALD is the accumulation of VLCFAs in complex lipids, especially in lysophosphatidylcholine (LPC) (3, 7, 27, 28), which is used as a robust diagnostic marker of X-ALD and, recently, in newborn screening (3, 27, 28). We performed a tandem mass spectrometry analysis of LPC-VLCFAs from WT, pmp-4(ok396) and daf-22(ok693) worms, the latter was used as a positive control for VLCFA accumulation (Fig. 1D). daf-22(ok693) mutants lack the last enzyme of the peroxisomal β-oxidation pathway called SCPx/thiolase and therefore accumulate VLCFAs (29). Here, we observed that LPC-C20:0, LPC-C22:0, LPC-C24:0 and LPC-C26:0 were increased in both pmp-4(ok396) and daf-22(ok693) worms in approximately the same range (Fig. 1D), indicating a role for PMP-4 in importing VLCFAs into peroxisomes for degradation, similar to its mammalian orthologue.
To identify the tissues in which pmp-4 is expressed, we used N2 worms harbouring the Ex001 [Ppmp-4::gfp] transcriptional reporter that expresses GFP under the control of the pmp-4 promoter and found that PMP-4 is mainly expressed in both the intestines and hypodermis (Fig. 1E), which are the main tissues for fat storage in C. elegans (30). Using an in silico mining tool for peroxisomal targeting sequences available at the peroxisome database (www.peroxisomedb.org) (31), we predicted the presence of the PEX19 binding site, a PTS signal for membrane proteins (32), between residues 140 to 151 of the PMP-4 coding sequence (Supplementary Fig. S1A), suggesting a peroxisomal localization for this transporter in the nematode. To validate the in silico prediction of the peroxisomal localization of PMP-4, we took advantage of another peroxisomal targeting signal, PTS type-1 (PTS1), which has been shown to target matrix proteins to peroxisomes in C. elegans (33). Thus, using the strain hjIs37 [Pvha-6::rfp::PTS1], in which the peroxisomes are labelled with RFP in the intestine, we expressed PMP-4::GFP under the control of its own promoter and found the colocalization (yellow labelling) of both reporter proteins in the gut (Fig. 1F–I). Similarly, using the strain Ex095 [Pdpy-7::gfp::PTS1] that labels peroxisomes with GFP in the hypodermis, we expressed PMP-4::mCherry under the control of its own promoter and found colocalization in the hypodermis (yellow labelling)(Fig. 1J–M). These results confirm the presence of PMP-4 in peroxisomes in the two main peroxisomal-containing tissues in C. elegans, the gut and the hypodermis (34).
We next investigated whether impaired redox homeostasis is a phenotypic trait of pmp-4(ok396) nematodes. Redox immunoblotting with an antibody that recognizes dinitrophenol (DNP) modified protein carbonyles (24) showed that pmp-4(ok396) worms had significantly more oxidatively damaged proteins than did WT nematodes (Fig. 1N). This finding is correlated with higher levels of total reactive oxygen species (ROS) measured with H2DCFDA probes (35) (Fig. 1O–P), in agreement with evidence obtained in cellular, in vivo and ex vivo models of X-ALD (36–38). Of note, the daf-22(ok693) strain did not display increased ROS production (Fig. 1O and 1Q). To investigate whether the origin of ROS in the absence of PMP-4 was mitochondrial, in a similar manner as in the fibroblasts of X-ALD patients (39), we incubated the pmp-4(ok396) worms with the MitoSOX probe, which specifically reacts with mitochondrial ROS (35, 40). The results indicated increased mitochondrial ROS in the mutant strain (Fig. 1R–S). In contrast, daf-22(ok693) did not produce higher mitochondrial ROS than WT (Fig. 1R and 1T), consistent with the data obtained when using a probe labelling total ROS (Fig. 1Q).
X-ALD is characterized by the axonal degeneration of corticospinal tracts leading to spastic paraparesis in patients and locomotor disability in mouse models, as evidenced by rotarod, treadmill and bar-cross tests (20, 21, 41). Here, we evaluated the motility of pmp-4(ok396) and WT strains by a trashing assay, in which nematodes were placed in liquid, and the frequency of lateral swimming or thrashing movements was estimated (42). Thrashing assays were performed blindly at L4 (Supplementary Fig. S2A) and L4+7 days (Fig. 1U, and Supplementary movies 1 and 2), well after the worms reached adulthood. The pmp-4(ok396) mutants consistently thrashed at a significantly reduced rate when compared to the behaviour of WT at both ages tested in a comparable manner, thus indicating a defect in motility (Fig. 1U, Supplementary Fig. S2A, and Supplementary movies 1 and 2). Finally, we examined axonal abnormalities by analysing the structural integrity of GABAergic D-type motor neurons and their processes in pmp-4(ok396) animals to evaluate whether locomotor phenotypes were associated with axonal degeneration, as noted in X-ALD mouse models and patients (6, 20, 21). To address this question, we used the juIs76 [Punc-25::gfp] strain with GFP-labelled GABAergic axons and cell bodies of the ventral cord (43). We found significant axonal damage in pmp-4(ok396); juIs76 [Punc-25::gfp] worms compared to juIs76 [Punc-25::gfp] control animals, both before and after reaching adulthood, at L4 (Supplementary Fig. S2B–D) and L4+7 days, respectively (Fig. 1 V–W).
The loss of pmp-4 induces lipid droplet accumulation in the nematode
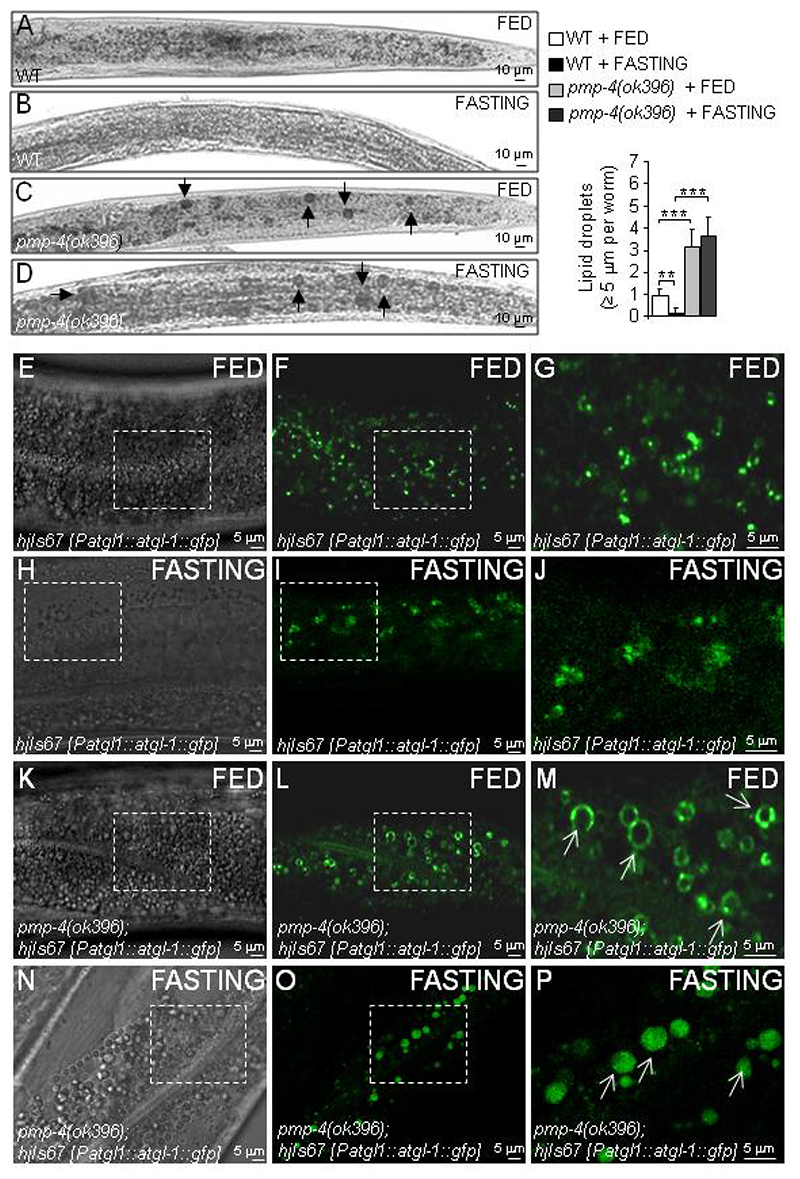
Lipid droplets (LDs) are dynamic organelles, such as peroxisomes, that emerge from the endoplasmic reticulum membrane and serve as a site for the storage of neutral lipids, such as triglycerides and cholesterol (44–46). Cells can form LDs in response to stress conditions, such as inflammation or nutrient deprivation (47–49). In C. elegans, the majority of lipids are stored in LDs and are mainly located in gut and hypodermal cells (50). In other peroxisomal mutants, such as the dhs-28(hj8) and daf-22(ok693) worms, which lack peroxisomal dehydrogenase and SCPx/thiolase, the third and fourth enzymes of the peroxisomal β-oxidation pathway, respectively, an increase in LDs is observed in the gut (33, 51). Thus, we hypothesized that LD formation could also be altered in pmp-4(ok396) mutants. We studied the presence of LDs using Sudan Black, a neutral lipid dye that stains LDs (52, 53) and found that the number of lipid granules with a diameter >5 μm was significantly increased in pmp-4(ok396) worms compared to WT animals (Fig. 2A and 2C). We next applied a 24 h fasting period to the worms. Fasting is a physiological stress condition that leads to the lipolysis of LDs and the intracellular release of free fatty acids, which are subsequently degraded as an energy source. This metabolic route was blunted in pmp-4(ok396) worms, which could not use their lipid reservoirs when most needed (Fig. 2A–D). To complement these results with a specific marker of LDs, we chose adipose triglyceride lipase 1 (ATGL-1), which is a coating component of phospholipid monolayer delimiting LDs (54). ATGL-1 fused to GFP protein localizes at the surface of large LDs in dhs-28(hj8) peroxisomal dehydrogenase mutant worms (33). For this study, we used the strain hjIs67 [Patgl-1::atgl-1::gfp] expressing ATGL-1::GFP (33), which was crossed with the pmp-4(ok396) mutation. in the results showed the improved visualization of the enlarged LDs when PMP-4 was absent (Fig. 2E–P, white arrows in the expanded picture), similar to dhs-28(hj8) peroxisomal mutants (33), corroborating the inability of the pmp-4(ok396) worms to degrade LDs upon fasting (Fig. 2E–P). In conclusion, pmp-4(ok396) mutants recapitulate the increased size of LDs observed in peroxisomal β-oxidation mutants, which is also refractory to fasting-induced lipolysis.
Figure 2. pmp-4(ok396) worms display lipid droplet accumulation.
(A-P) At the L4 larval stage, WT and pmp-4(ok396) nematodes were fed or fasted for 24 h. (A-D) Bright field images of the posterior part of the worm stained with Sudan Black in (A) WT + FED (n=46), (B) WT + FASTING (n=60), (C) pmp-4(ok396) + FED (n=60), and (D) pmp-4(ok396) + FASTING (n=66). Lipids are evident as black droplets, labelled by a black arrow. Scale = 10 μm. Semi-quantitative analysis of the lipid droplets with a diameter >= 5 μm under the indicated conditions. (E-P) Localization of ATGL-1::GFP protein, driven by the atgl-1 promoter at L4, in (E-G) hjIs67 [Patgl-1::atgl-1::gfp] + FED, (H-J) hjIs67 [Patgl-1::atgl-1::gfp] + FASTING, (K-M) pmp-4(ok396); hjIs67 [Patgl-1::atgl-1::gfp] + FED and (N-P) pmp-4(ok396); hjIs67 [Patgl-1::atgl-1::gfp] + FASTING animals. (E) DIC-Nomarsky image and (F-G) GFP staining in WT. G is an enlarged image of F. (H) DIC-Nomarsky image and (I-J) GFP staining in WT after 24 h of fasting. J is an enlarged image of I. (K) DIC-Nomarsky image and (L-M) GFP staining in pmp-4(ok396). M is an enlarged image of L. (N) DIC-Nomarsky image and (O-P) GFP staining in pmp-4(ok396); hjIs67 [Patgl-1::atgl-1::gfp] after 24 h of fasting. P is an enlarged image of O. ATGL-1::GFP protein localized to the surface of large lipid droplets are indicated with white arrows (n=30 by conditions). The worms in the figures are oriented head to the left, dorsal face-up and only a portion of the central part of the body is depicted. Scale = 5 μM. Data represent the mean ± SD. Statistical analysis was carried out with two-way ANOVA, followed by Tukey’s post hoc test (*P<0.05; **P<0.01; ***P<0.001).
Loss of PMP-4 increases vulnerability to mitochondrial ROS
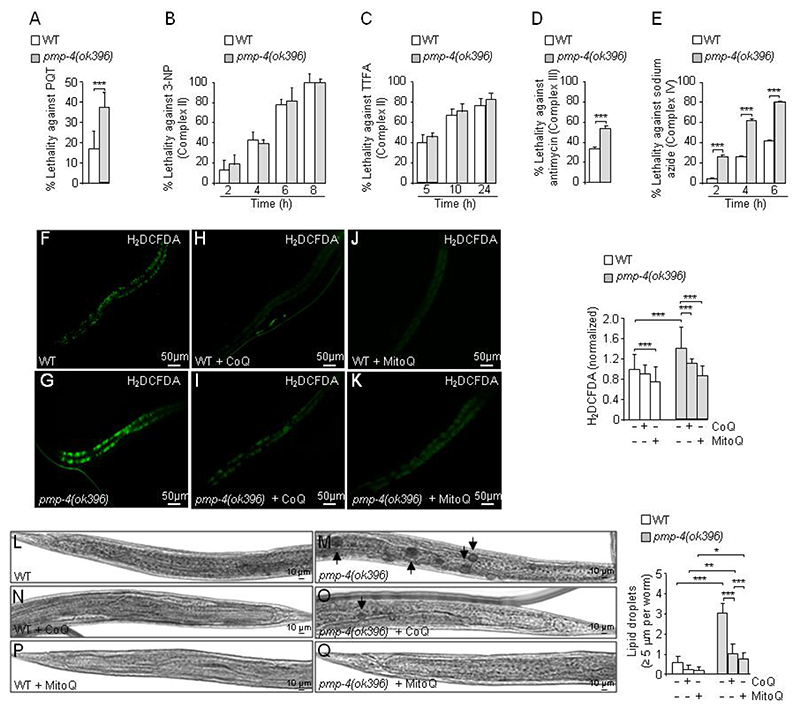
To directly test the sensitivity of pmp-4(ok396) worms to mitochondrial ROS, we incubated the animals with the pro-oxidant paraquat (PQT, 1, 1’-dimethyl-4,4’-bipyridinium dichloride), a redox cycler that stimulates superoxide production in mitochondria at complex I (55). Compared to WT animals, the pmp-4(ok396) worms showed increased lethality under these conditions (Fig. 3A). Furthermore, we tested the impact of the inhibition of complexes II, III, and IV (56) on viability, revealing that pmp-4(ok396) mutants were more sensitive to inhibitors of complexes III and IV but not to inhibitors of complex II (Fig. 3B–E). We interpret these results as pmp-4(ok396) animals showing impaired defences against specific mitochondrial stressors.
Figure 3. Mitochondrial dysfunction in pmp-4(ok396) animals.
WT and pmp-4(ok396) worms were incubated at the L4 larval stage with (A) the mitochondrial complex I inhibitor Paraquat (PQT) (n=51 to 54 animals by condition), the mitochondrial complex II inhibitors (B) 3-nitropropionic acid (3-NP) (n=15 to 20 animals by condition), (C) thenoyltrifluoroacetone (TTFA) (n=18 to 20 animals by condition), (D) the complex III inhibitor (antimycin A) (n=20 animals by condition) and (E) the complex IV inhibitor (sodium azide)(n=20 animals by condition). The lethality of the worms after the treatment was evaluated as described in the methodology. (F-Q). At L4, WT and pmp-4(ok396) nematodes were treated with CoQ (1 mg/ml) or MitoQ (5 μg/ml) for 7 days. Total ROS levels were measured by quantifying the fluorescence emission of the H2DCFDA probes in living animals in (F) WT (n=42), (G) pmp-4(ok396)(n=35), (H) WT + CoQ (n=32), (I) pmp-4(ok396) + CoQ (n=30), (J) WT + MitoQ (n=47), and (K) pmp-4(ok396) + MitoQ (n=32) animals at L4+7 days. Values are normalized to the untreated WT nematodes. Scale = 50 μm. Bright field images of the posterior part of the worm stained with Sudan Black in (L) WT (n=46), (M) pmp-4(ok396)(n=61), (N) WT + CoQ (n=60), (O) pmp-4(ok396) + CoQ (n=60), (P) WT + MitoQ (n=30), and (Q) pmp-4(ok396) + MitoQ (n=27) worms at L4+7 days. Lipids are evident as black droplets, labelled by a black arrow. Scale = 10 μm. Semi-quantitative analysis of the lipid droplets with a diameter >= 5 μm under the indicated conditions. Data represent the mean ± SD. Statistical analysis was carried out with Student’s t-test (*P<0.05; **P<0.01; ***P<0.001) for A-E. Statistical analysis was carried out with two-way ANOVA, followed by Tukey’s post hoc test (*P<0.05; **P<0.01; ***P<0.001) for F-Q.
The accumulation of lipid droplets in pmp-4(ok396) animals is reversed by mitochondrial antioxidants
To determine which of the phenotypes due to loss of PMP-4 were dependent on mitochondrial redox imbalance, we used two antioxidants targeting mitochondria, Coenzyme Q10 (CoQ, ubiquinone) and MitoQ. MitoQ is a modified form of ubiquinol attached to a lipophilic cation that enables it to cross cell membranes and accumulate preferentially on the matrix facing the surface of the mitochondrial inner membrane, where it is optimally positioned to decrease most types of mitochondrial ROS (57, 58). We showed that ROS levels were restored by treatment with both compounds, although much lower doses of MitoQ were necessary (200-fold less compared to CoQ) (Fig. 3F–K), underscoring the mitochondrial origin of the redox imbalance in the absence of PMP-4. Next, we incubated pmp-4(ok396) with CoQ and MitoQ to investigate whether the observed increase in LDs involved a mitochondrial redox-dependent mechanism. Remarkably, the enlargement of LDs could be normalized upon treatment with these two mitochondrial antioxidants (Fig. 3 L–Q).
Axonal degeneration in pmp-4(ok396) mutants is caused by mitochondrial ROS
Next, we examined the effect of CoQ and MitoQ on axonal defects and locomotion disabilities in the pmp-4(ok396) worms. Thrashing abnormalities were restored by CoQ, but more efficiently by the mitochondrial-targeted MitoQ, at the two developmental stages tested L4 (Supplementary Fig. S2E) and L4+7 days (Fig. 4A). This effect was correlated with an improvement of axonal damage with CoQ and MitoQ at L4+7 days (Fig. 4B–G).
Figure 4. Mitochondrial-targeted antioxidant rescue axonal abnormalities and locomotor dysfunction at old stages of development.
(A-G) At the L4 larval stages, WT and pmp-4(ok396) nematodes were treated with CoQ (1 mg/ml) or MitoQ (5 μg/ml) for 7 days. (A) The thrashing behaviour was carried out in WT and pmp-4(ok396) nematodes upon CoQ or MitoQ at L4+7 days (n=15 to 27 animals by condition). Representative fluorescence images showing axonal abnormalities and quantitative analysis at the indicated genotypes upon CoQ or MitoQ at L4+7 days in (B) juIs76 [Punc-25::gfp] (n=20), (C) pmp-4(ok396); juIs76 [Punc-25::gfp] (n=20), (D) juIs76 [Punc-25::gfp] + CoQ (n=18), (E)pmp-4(ok396); juIs76 [Punc-25::gfp] + CoQ (n=15), (F) juIs76 [Punc-25::gfp] + MitoQ (n=26) and (G) pmp-4(ok396; juIs76 [Punc-25::gfp]) + MitoQ (n=27). White asterisks indicate axonal abnormalities. All worms are oriented with the anterior end left and ventral side down. Scale = 25 μm. (H-N) At the L4 larval stage, juIs76 [Punc-25::gfp]and pmp-4(ok396); juIs76 [Punc-25::gfp] nematodes were treated with paraquat (PQT) (0.2 mM) and/or MitoQ (5 μg/ml) for 7 days. (H) The thrashing behaviour was analysed at stage L4+7 days in WT and pmp-4(ok396) nematodes upon PQT and/or MitoQ (n=20 to 31 animals by condition). Representative fluorescence images showing axonal abnormalities and quantitative analysis at stage L4+7 days in (I) juIs76 [Punc-25::gfp]) (n=29), (J) pmp-4(ok396); juIs76 [Punc-25::gfp] (n=31), (K) juIs76 [Punc-25::gfp] + PQT(n=25), (L) pmp-4(ok396); juIs76 [Punc-25::gfp] + PQT (n=20), (M) juIs76 [Punc-25::gfp] + PQT + MitoQ (n=21), (N) pmp-4(ok396; juIs76 [Punc-25::gfp] + PQT + MitoQ (n=28). Scale = 25μm. All worms are oriented with the anterior end left and ventral side down. Data represent the mean ± standard deviation (SD). Statistical analysis was carried out with two-way ANOVA, followed by Tukey’s post hoc test (*P<0.05; **P<0.01; ***P<0.001) for A-N.
We further investigated the role of mitochondrial ROS production in the maintenance of locomotion and axonal health. To address a cause-effect relationship, we evaluated the thrashing behaviour and axonal morphology in N2 and pmp-4(ok396) worms exposed to PQT at a 200 μM sublethal dose at L4+7 days (Fig. 4H–N). PQT treatment reduced the locomotion fitness and increased axonal damage in both N2 and pmp-4(ok396) worms, with an exacerbated effect in pmp-4(ok396) worms (Fig. 4H–L). Importantly, MitoQ normalized these abnormalities (Fig. 4H–N).
To provide orthogonal evidence for the axonal defects caused by damage to the mitochondrial oxidative phosphorylation (OXPHOS) system, we performed RNAi knockdown of the mitochondrial gene nuo-1, encoding the NDUFB4/B15 subunit of complex I required for oxidative phosphorylation, in WT worms (59). We found that nuo-1 RNAi worms recapitulated the axonal defects present in the GABAergic neurons of pmp-4(ok396) and PQT-treated worms at L4+7 days (Supplementary Fig. S2F–G). Altogether, these results indicated that mitochondrial ROS generated by the loss of PMP-4 are detrimental for axonal integrity, causing defects in locomotion.
PMP-4 is not expressed in C. elegans neurons or glial-like cells
Given the axonal pathology characterizing X-ALD (6, 22, 60) and the ubiquitous distribution of ABCD1 in different tissues and organs in mammalian organisms (61), we next investigated the distribution of PMP-4 more specifically in the nematode nervous system, as our first analysis showed no obvious staining. We performed immunostaining for PMP-4 in a reporter strain harbouring GFP-labelled mitochondria in all neurons, (Ex129 [Prgef-1::mito::gfp]). We found that endogenous PMP-4 was localized in the hypodermis (Fig. 5A, red dots) surrounding the neuronal cells (Fig. 5A, green dots) but sparing the neurons.
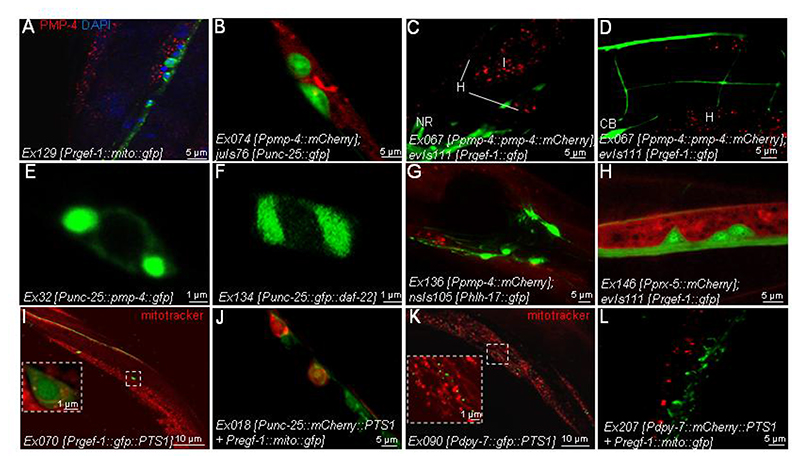
Figure 5. Subcellular localization and tissue distribution of PMP-4.
(A) mito::GFP was expressed under the control of the pan-neuronal promoter rgef-1 (Prgef-1) in WT animals (Ex129 [Prgef-1::mito::gfp]). Here, mitochondria located in all neurons are labelled with GFP. Next, worms were fixed and double immunostained against PMP-4 (red). Nuclei were counterstained with DAPI (blue) (n=30). Scale = 5 μm. (B) mCherry driven by the pmp-4 promoter was expressed in the GABAergic strain juIs76 [Punc-25::gfp], where GABAergic neurons are stained with GFP (juIs76 [Punc-25::gfp]; Ex074 [Ppmp-4::mCherry])(n=30). Scale = 5 μm. (C-D) PMP-4::mCherry driven by the pmp-4 promoter was expressed in a pan-neuronal strain where all neurons are stained with GFP (evIs111 [Prgef-1::gfp]; Ex067 [Ppmp-4::pmp-4::mCherry]) (n=30). Next, PMP-4::mCherry and GFP staining was observed in nematodes. Cell bodies (CB), NR: Nerve ring, H: hypodermis, I: intestine. Scale = 5μm. (E) PMP-4::GFP (Ex132 [Punc-25::pmp-4::gfp]) and (F) GFP::DAF-22 (Ex134 [Punc-25::gfp::daf-22]) under the control of a GABAergic promoter (Punc-25) were expressed in WT worms (n=30 by condition). (E) PMP-4::GFP and (F) GFP::DAF-22 staining was observed in nematodes. Scale = 1 μm. (G) mCherry driven by the pmp-4 promoter was expressed in glial-like cells strain nsIs105 [Phlh-17::gfp], where glial CEPsh cells are labelled with GFP (nsIs105 [Phlh-17::gfp]; Ex 136 [Ppmp-4::mCherry]) (n=30). Next, mCherry and GFP staining was observed in nematodes. Scale = 5 μm. (H) mCherry driven by the peroxisomal prx-5 promoter (Pprx-5) was expressed in the pan-neuronal strain evIs111 [Prgef-1::gfp], where all neurons are labelled with GFP (evIs111 [Prgef-1::gfp]; Ex146 [Pprx-5::mCherry]) (n=30). Next, mCherry and GFP staining were observed in nematodes. Scale = 5μm. We then used a construct containing PTS1 to target the peroxisomes. (I) GFP::PTS1 driven by a pan-neuronal promoter (Prgef-1) was expressed in WT worms (Ex070 [Prgef-1::gfp::PTS1]). Next, mitochondria were stained with Mitotracker probes, and GFP staining was visualized (n=30). Scale = 10 μm. (J) mCherry::PTS1 driven by a GABAergic promoter (Punc-25) and mito::GFP under the pan-neuronal promoter rgef-1 (Prgef-1) were co-expressed in WT animals (Ex218 [Punc-25::mCherry::PTS1 + Prgef-1::mito::gfp]). Next, mCherry and GFP staining were observed in nematodes (n=30). Scale = 5μm. (K) GFP::PTS1 driven by a hypodermal promoter (Pdpy-7) was expressed in WT worms (Ex090 [Pdpy-7::gfp::PTS1]). Next, mitochondria were stained with Mitotracker probes, and GFP staining was visualized (n=30). Scale = 10 μm. (L) mCherry::PTS1 driven by a hypodermal promoter (Pdpy-7) and mito::GFP under the pan-neuronal promoter rgef-1 (Prgef-1) were co-expressed in WT animals (Ex207 [Pdpy-7::mCherry::PTS1 + Prgef-1::mito::gfp]). Next, mCherry and GFP staining was observed in nematodes (n=30). Scale = 5 μm.
Similar results were obtained when expressing mCherry under the control of the pmp-4 promoter in a reporter strain harbouring GFP-labelled GABAergic motor neurons (juIs76 [Punc-25::gfp]) (43). Thus, mCherry labelling was found in the hypodermis surrounding GABAergic neurons but was undetectable within the neurons (Fig. 5B). This result was further confirmed when expressing the fusion protein PMP-4::mCherry under the control of its own promoter in a strain in which all neurons were labelled with GFP (evIs111 [Prgef-1::gfp]). The typical peroxisomal punctate pattern was detected in the tissues surrounding neurons, such as the intestine (I) and hypodermis (H) but was undetectable in neurons (Fig. 5C–D).
To further investigate the presence or absence of PMP-4 in neurons, we expressed PMP-4::GFP under the control of a GABAergic promoter (Punc-25). in the results showed an expression pattern consisting of large fluorescent bodies (Fig. 5E) instead of the typical punctate pattern described above, thus suggesting an aberrant/artefactual subcellular distribution of PMP-4 when ectopically expressed in neurons. We observed similar cytoplasmic accumulation when the peroxisomal β-oxidation protein DAF-22 was expressed in the same subset of neurons (Fig. 5F).
We next explored the possibility that PMP-4 could be expressed in glial cells. In C. elegans, glial-like cells are concentrated in the amphid organ, the principal chemosensory organ located in the head of the nematode (62). We expressed mCherry under the control of the pmp-4 promoter in a reporter strain where the four glial-like cells, called cephalic sheath (CEPsh) cells, of the cephalic sensilla, as well as some motor neurons of the ventral cord, were labelled with GFP (nsIs105 [Phlh-17::gfp]; Ex136 [Ppmp-4::mCherry]) (63). Importantly, no colocalization was detected, indicating that PMP-4 was also not expressed in these glial-like cells (Fig. 5G). These results suggest that neurons or CEPsh cells do not contain peroxisomes.
Peroxisomes are not detected in neurons
Intrigued by the lack of PMP-4 expression in neurons and glial cells, concomitant with an axonal degenerative phenotype, we next conducted an in-depth investigation of the presence of peroxisomes in the nervous system. Indeed, it is possible that specific metabolic routes, such as the beta-oxidation of fatty acids and therefore, PMP-4, would be missing in neural cells but the peroxisomes still would be present in neuronal cells, although with a different set of functions and pathways as compared with peroxisomes in other worm tissues.
Therefore, we examined prx-5, the worm orthologue of mammalian PEX5, a component of the peroxisomal membrane that imports peroxisomal enzymes into the matrix (64). We expressed mCherry under the control of the prx-5 promoter in the evIs111 [Prgef-1::gfp] strain that labels all C. elegans neurons with GFP. Using this strategy, we failed to detect any peroxisome staining in neurons (Fig. 5H).
We next used the peroxisomal targeting signal-1 (PTS1) to generate a strain expressing peroxisomal GFP in all neurons (Ex070 [Prgef-1::gfp::PTS1]) (Fig. 5I), which also failed to show any GFP labelling resembling peroxisomes. Moreover, we used an mCherry::PTS1 fusion expressed in GABAergic neurons, showing a non-overlapping pattern with pan-neuronal GFP-labelled mitochondria (Fig. 5J). Only a diffuse staining pattern well differentiated from the expected punctuate peroxisomal pattern was obtained in both cases. We interpret these results as a mislocalization of the peroxisomal signal in all neurons (Fig. 5I) or in GABAergic cells (Fig. 5J). As a positive control, we used the same PST1 constructs and exchanged the neuronal promoters with a hypodermal promoter, which yielded the classical peroxisomal punctate pattern in the strain Ex090 [Pdpy-7::gfp::PTS1] (Fig 5K) and in the strain Ex207 [Pdpy-7::mCherry::PTS1 + Prgef-1::mito::gfp]) (Fig. 5L). The peroxisome dot-like pattern was clearly separated from the mitochondrial network in these strains (Fig. 5K–L). Taken together, these data strongly suggest the absence of peroxisomes in C. elegans neurons.
PMP-4 expression in the hypodermis rescues locomotor disability and axonal degeneration through cell non-autonomous mechanisms
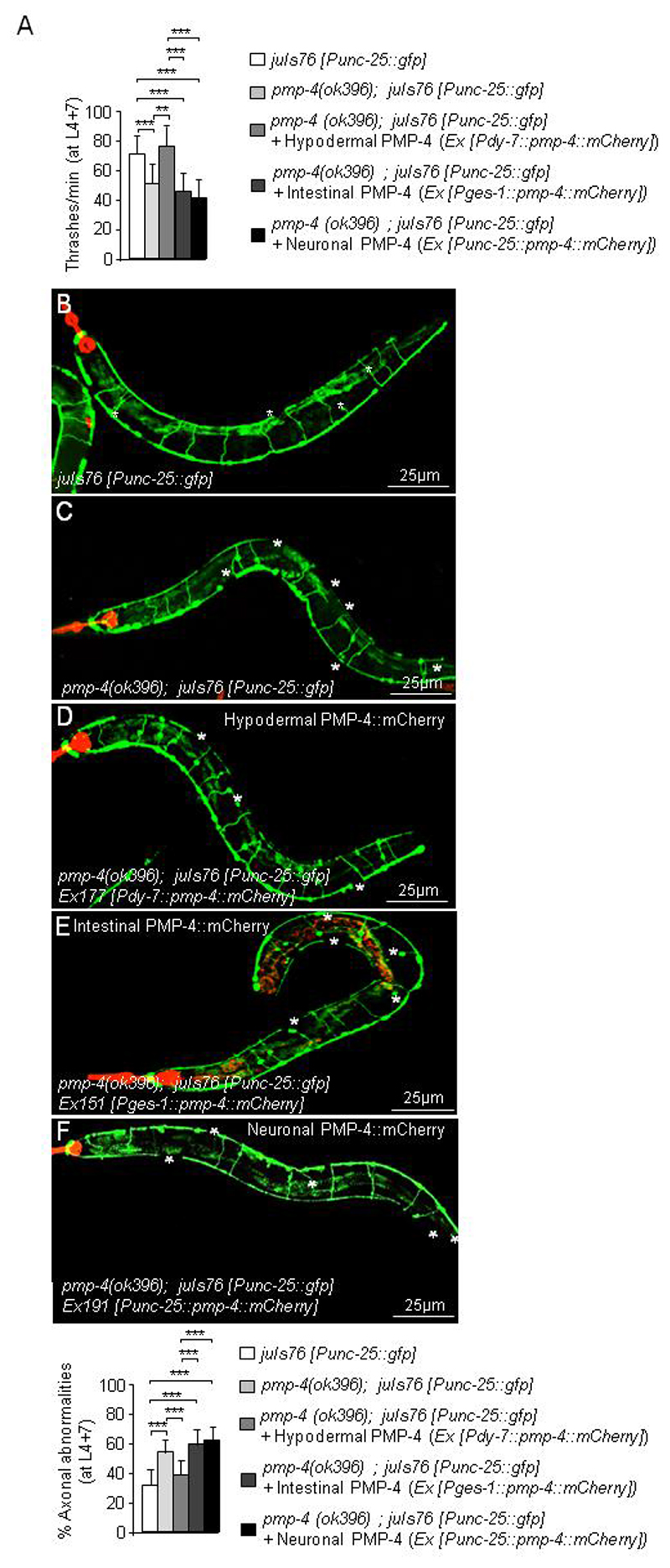
We next aimed to identify the cell non-autonomous mechanisms and cell types underlying axonal degeneration, given that peroxisomes were not present in neurons or glial cells. We then attempted to restore PMP-4 in the tissues where its endogenous expression was higher by expressing PMP-4::mCherry in the hypodermis (Ex177 [Pdpy-7::pmp-4::mCherry]) or in the intestine (Ex151 [Pges-1::pmp-4::mCherry]), using the strain previously used for characterizing the axonal defects, pmp-4(ok396); juIs76 [Punc-25::gfp], as background (Fig. 6A–E). We also expressed PMP-4::mCherry in GFP-labelled GABAergic cells (pmp-4(ok396); juIs76 [Punc-25::gfp]; Ex191 [Punc-25::pmp-4::mCherry]), as additional confirmation (Fig. 6A, 6F). Locomotion defects were only rescued when PMP-4::mCherry was expressed in the hypodermis but not in the intestine or in GABAergic neurons (Fig. 6A, and Supplementary movies S3–S7). Axonal damage was also rescued by restoring the expression of PMP-4::mCherry in hypodermal cells (Fig. 6B–D) but not in intestinal cells (Fig. 6B–C and 6E) or in GABAergic cells (Fig. 6B–C and 6F). Collectively, these results provide evidence that restoring the function of PMP-4 in the hypodermis is necessary and sufficient to maintain axonal integrity and locomotion in a cell non-autonomous manner.
Figure 6. Specific PMP-4 expression in the hypodermis is essential to maintain axonal integrity and locomotion.
(A) Locomotion behaviour expressed as thrashes per minute at L4+7 days in juIs76 [Punc-25::gfp], pmp-4(ok396); juIs76 [Punc-25::gfp] and pmp-4(ok396; juIs76 [Punc-25::gfp]) animals where PMP-4::mCherry is expressed in hypodermis (pmp-4(ok396); juIs76 [Punc-25::gfp]; Ex177 [Pdpy-7::pmp-4::mCherry]), in intestine (pmp-4(ok396); juIs76 [Punc-25::gfp]; Ex151 [Pges-1::pmp-4::mCherry]) or in neurons (pmp-4(ok396); juIs76 [Punc-25::gfp]; Ex191 [Punc-25::pmp-4::mCherry]) (n= 19 to 23 animals by condition). Representative confocal pictures showing axonal damage and quantitative analysis in (B) juIs76 [Punc-25::gfp] (n=22), (C) pmp-4(ok396); juIs76 [Punc-25::gfp] animals (n=19), pmp-4(ok396) animals where PMP-4::mCherry is expressed in (D) hypodermis (pmp-4(ok396); juIs76 [Punc-25::gfp]; Ex177 [Pdpy-7::pmp-4::mCherry]) (n=23), (E) intestine (pmp-4(ok396); juIs76 [Punc-25::gfp]; Ex151 [Pges-l::pmp-4::mCherry]) (n=22) or (F) neurons (pmp-4(ok396); juIs76 [Punc-25::gfp]; Ex191 [Punc-25::pmp-4::mCherry]) (n=19). The red colour in all pictures corresponds to the strong expression in the pharynx of the co-injection marker Pmyo-2::mCherry. White asterisks label the axonal abnormalities. Worms are oriented head to the left and dorsal face-up. Scale = 25 μm. Data represent the mean ± standard deviation (SD). Statistical analysis was carried out with two-way ANOVA, followed by Tukey’s post hoc test (*P<0.05; **P<0.01; ***P<0.001).
Rescue of key phenotypes
To unequivocally demonstrate whether the loss of PMP-4 was directly responsible for the phenotypes shown in this study, we attempted rescue experiments using different approaches. First, we rescued the increased ROS production by using a construct containing PMP-4::mCherry driven by the pmp-4 promoter and injected into pmp-4(ok396) worms (pmp-4(ok396); Ex050 [Ppmp-4::pmp-4::mCherry]) (Supplementary Fig. S3A), in which H2DCFDA staining was normalized. Next, the increase in LDs visualized by Sudan black was no longer detected in this same strain (Supplementary Fig. S3B). Finally, we recovered axonal defects by injecting PMP-4 fused to mCherry into pmp-4(ok396) worms with GFP-labelled GABAergic cells (pmp-4(ok396); juIs76 [Punc-25::gfp]; Ex065 [Ppmp-4::pmp-4::mCherry]) (Supplementary Fig. S3C).
Discussion
This work provides a novel animal model for a dreadful neurometabolic disorder, which, beyond the practicalities of cost-beneficial in vivo drug screening, delivers fundamental insights for an improved understanding of its molecular pathogenesis. pmp-4 deficient worms recapitulate the main hallmarks observed in human AMN patients and Abcd1- mice, i.e., the accumulation of VLCFAs, the redox imbalance of mitochondrial origin, and most importantly, the axonal degeneration and associated locomotor dysfunction (6, 22, 60), indicating that the mechanisms leading to disease upon PMP-4 dysfunction are evolutionarily conserved. We have previously shown that oxidative damage is a direct consequence of fatty acid excess and appears well in advance of the onset of symptoms (36, 39) and that the combination of antioxidants N-acetylcysteine, lipoic acid and vitamin E could halt axonal degeneration in the mouse model (38). Our working hypothesis is based on the interference of intracellular excess VLCFAs or membrane lipidic components containing lateral chains of VLCFAs with OXPHOS assembly or interaction, which leads to the generation of excess ROS and diminish ATP at this site (65, 66). The study of PMP-4 function in C. elegans expands these findings and provides precise evidence of enhanced mitochondrial vulnerability, since inhibitors of complexes I, III and IV dramatically increase pmp-4(ok396) lethality. We also show a direct causative role for mitochondrial redox dysfunction in axonal demise, since the selective mitochondrial antioxidant mitoQ prevented axonal degeneration. Altogether these results strengthen the rationale for using antioxidants targeting mitochondria to modify the progression of this peroxisomal disease. As MitoQ has been used safely in phase II trials for Parkinson’s (67) and has shown clinical efficacy in vascular function in healthy elderly individuals (68), an actual therapeutic possibility for X-ALD derives from this work.
It is worth noting that we did not observe redox imbalance in the peroxisomal thiolase, beta-oxidation defective daf-22(ok693) mutant. A plausible explanation may be that, in this mutant, VLCFA enter peroxisomes and presumably remain inside the organelles, thus preserving mitochondria membranes.
An emerging role for LDs in the pathogenesis of neurodegenerative disease is evidenced by the identification of several key regulators as causative genes of corticospinal axonal demise, such as Spastin, Reep1, or Atlastin-1 (69, 70). Reports on the increase or enlargement of LDs in peroxisomal disorders are scarce but include a mouse mutant of the peroxisomal biogenesis factor PEX5, causing Zellweger syndrome (71). However, a direct connection between peroxisomal malfunction and LD accumulation has remained elusive until very recently. Elegant work in HeLa cells showed that ABCD1 directly interacts with the Spastin 1 protein complex and is responsible for the formation of contact sites between the LD and peroxisomes, for the docking of LD and channelling of fatty acids for degradation via β-oxidation (72). This finding suggests that, in the absence of PMP-4, the transfer of some fatty acids from LDs to peroxisomes may be hampered, as these fatty acids may be retained in LDs. Indeed, VLCFAs are selectively degraded in peroxisomes (2). During fasting, when the main fuel arrives from fatty acid stores, the loss of PMP-4 may impede their degradation in peroxisomes. C. elegans responds to starvation by activating a number of genes involved in lipid metabolism, including the mitochondrial and peroxisomal β-oxidation genes, as these organelles cooperate in LD degradation (26, 73, 74). We propose that mitochondria may compensate for the loss of peroxisomal function in regard to LD catabolism when homeostasis is preserved. This compensation may explain why treatment with antioxidants, which may exert protective effects on mitochondrial function and boost β-oxidation (75), normalizes the expanded LDs, bypassing the loss of LD-peroxisome contact sites. This relationship appears reciprocal, as the production of mitochondrial ROS in neurons activates pathways inducing LD formation in glial cells, in a cell non-autonomous manner, in Drosophila and mouse models of OXPHOS impairment (76). Moreover, increased LDs have been shown to be detrimental under conditions of marked mitochondrial oxidative stress, causing the enhancement of lipid peroxidation and neurodegeneration in Drosophila and mouse models overexpressing the fatty acid transporter FATP (77). Based on the strong body of evidence implicating mitochondrial dysfunction and particularly the OXPHOS system in the pathogenetic cascade in X-ALD (17, 37, 39, 41), we posit that the negative impacts of mitochondrial redox imbalance may be exacerbated by LD accumulation, leading to axonal degeneration. Elucidation of the precise mechanism orchestrating the regulation of LD formation by redox signalling may bear therapeutic potential and impact for neurodegenerative conditions in which mitochondria and LD dyshomeostasis converge.
Another intriguing observation of this work is that peroxisomes appear to be absent from neurons and glial-like CEPsh cells. Indeed, the PTS1 peroxisomal marker does not recognize the organelle in neurons or CEPsh cells, and neither PMP-4 nor the peroxisomal proteins PRX-5 and DAF-22 appear to be expressed in these cells in nematodes. This finding is surprising since peroxisomes are considered ubiquitous organelles of eukaryotic cells (78) and, therefore, indispensable for life in a way that biogenesis defects or malfunctions of peroxisomes lead to severe neurodevelopmental or neurodegenerative syndromes in humans (79). Moreover, the human and mouse orthologues of PMP-4, the ABCD1 and ABCD2 transporters, are ubiquitously expressed in neural cells or show high expression in neurons for the latter in mammals (80). While this paper was in preparation, Park and Paik reported that the DAF-22/thiolase is expressed and located in only one specific neuron called ASK during development, from L1 to L4 (81). In our study, we used two promoters to detect peroxisomes in neurons, one targeting GABAergic cells (Punc-25) and another targeting the total population of neurons (Prgef-1), with negative results. Instead, we observed that GFP::DAF-22 and PMP-4::GFP form large cytoplasmic granules in the cytoplasm of GABAergic cells, not resembling the dotted pattern characteristic of peroxisomes. This shape is also observed in recycling endosomes labelled with GFP::RAB-11 in touch receptor neurons (82). Therefore, we hypothesized that both PMP-4::GFP and GFP::DAF-22 reporter proteins may be degraded via the endocytic pathway when peroxisome proteins are expressed in neural cells. On the other hand, we cannot formally exclude the presence of these organelles in a specialized subtype of neurons at stages earlier than L4, which may have been missed by labelling with pan-neuronal, GABAergic or glial promoters. These intriguing results raised questions about the mechanisms and the specific cell types driving axonal damage in pmp-4(ok396) worms. The ectopic expression of PMP-4::mCherry in the hypodermis of pmp-4(ok396) nematodes was sufficient to halt axonal degeneration, while no effect was observed when PMP-4::mCherry was expressed in the intestine or in neurons. This evidence indicates a fundamental metabolic role for the peroxisome compartment in the hypodermis that is necessary to maintain axonal integrity via a cell non-autonomous mechanism.
Indeed, C. elegans hypodermal cells participate in guiding neuronal migration and share characteristics with vertebrate glial cells (62). Hence, worm neuronal cell bodies and their processes are completely enveloped by hypodermal tissue, which in turn supports neuronal function and architecture (83). During embryogenesis, the hypodermis is implicated in neuronal migration (84) and later in development, axons grow completely embedded into the hypodermis (83, 84). Some evidence of a cell non-autonomous mechanism exists, implicating the hypodermis in the maintenance of axonal integrity, in a model of laser-induced axotomy (85). Thus, it is likely that the strict physical contact between the hypodermis and neurons may facilitate the passage of signalling or nurturing metabolites, such as essential lipids, from peroxisomes in the hypodermis towards axons for axonal maintenance. For instance, DHA (C22:6ω3) is synthetized in peroxisomes by the β-oxidation of C24:6ω3, which is imported into peroxisomes by ABCD1 and ABCD2 transporters, the mouse orthologues of PMP-4 (26). Thus, we posit that hypodermis may play a key supporting role in the protection and maintenance of neurons and axons in nematodes, similar to myelin in mammals, underscoring the importance of this tissue in models of axonal or neuronal degeneration in C. elegans.
Material and Methods
A more detailed explanation of the methodology, strains, and plasmid information is provided in the Supplementary Methodology section.
Products
MitoQ (mitoquinone mesylate:10-(4,5-dimethoxy-2-methyl-3,6-dioxo-1,4 cyclohexadienyl) decyltriphenyl- phosphonium methanesulfonate) was provided by. MP Murphy. CoQ10, paraquat (PQT), 2,4-Dinitrophenylhydrazine, thenoyltrifluoroacetone (TTFA), antimycin, sodium azide and dimethylsulfoxide (DMSO) were purchased from Sigma-Aldrich. H2DCFDA and MitoSOX Red were obtained from Life Technologies.
Strains
Worm strains were maintained under standard conditions on Escherichia coli OP50 at 20° C. All the strains used were provided by the Caenorhabditis Genetic Center (CGC) unless otherwise indicated. N2 (Bristol) was used as the WT strain (N2). The partial deletion strain RB675, pmp-4(ok396), was backcrossed 10 times into the N2 strain prior to experimental work.
All the strains mentioned in the manuscript are detailed in Supplementary Table S1. The strain nsls105 [Phlh-17::gfp] I was kindly provided by Dr. Shai Shaham (The Rockefeller University, New York, NY, USA). Plasmids HYM772 (for targeting peroxisomes in hypodermis and neuron) and HYM433 (to express daf-22 within neurons) were kindly provided by Dr. Ho Yi Mak (Stowers Institute for Medical Research, Kansas City, MO, USA).
In silico sequence analysis
The alignment of protein sequences between the indicated species was performed by ClustalW software, version 2 (86). The Pex19 binding site was predicted by using the PTS predictor tool in the peroxisome database: www.peroxisomedb.org
Plasmids and nematode transgenesis
The vectors pPD95.79, pPD95.77 and pPD96.32 (Addgene, Teddington, UK) were used as backbones for all the plasmids generated in this study. The constructs are listed in Supplementary Table S2, and detailed cloning information will be provided upon request. Transgenic strains were generated using standard microinjection techniques (87) and are listed in Table S1. Plasmids were injected into worm adult gonads alone or together with the plasmid pRF4 (100 ng/μl), which encodes a mutant collagen [rol-6(su1006)] that induces a dominant “roller” phenotype or the plasmid pCFJ90 (2.5 ng/μl), which expresses mCherry under the control of the myo-2 promoter [Pmyo-2::mCherry::unc-54utr], as co-injection markers depending on the experiments. In all cases, at least 3 independent transgenic lines were generated and analysed, all showing similar results.
Generation of the PMP-4 antibody
A PMP-4 antibody was obtained following the standard procedures at the IGBMC core facilities, Strasbourg, France. The PMP-4 peptide CQLLGGNEDHLNMTIDTDDSE (see Supplementary Fig. S1A) was coupled with maleimide-activated ovalbumin (Thermo Scientific, Madrid, Spain) and injected into rabbits. The polyclonal anti-PMP-4 antibody was purified from rabbit serum through a gel matrix (Sulfolink * coupling gel). Fractions of 1 ml were eluted from the column, tested for activity by immunoblotting, aliquoted and stored at -20°C until use.
Analysis of PMP-4 expression by western blotting
PMP-4 expression was performed in synchronized populations of worms obtained by bleaching. To collect L1 worms, eggs were allowed to hatch on Nematode growth medium (NGM)-seeded plates and collected after 4-6 h of feeding at 20°C. To isolate the other larval stages, the eggs were allowed to hatch on M9 O/N at 20°C, and the resulting synchronized L1 worms were added to NGM-seeded plates; the animals were harvested at the appropriate developmental stage after 10 h (for L2 animals), 24 h (for L3 animals), 48 h (for L4 animals), and 7 days (for post-reproductive adult animals). The worms were washed extensively with M9 to remove bacteria, and the worm pellets were frozen at -80°C.
The western blot analysis was performed as described (18). Eight micrograms of protein was separated by 8% SDS–polyacrylamide gel electrophoresis (SDS–PAGE) and blotted onto nitrocellulose membranes (Bio-Rad, Barcelona, Spain). The membranes were then incubated with anti-PMP-4 (1:500 rabbit polyclonal) and anti-rabbit secondary antibodies (1:5000. DAKO, Barcelona, Spain), and the proteins were visualized with an ECL plus detection reagent (GE Healthcare, Barcelona, Spain). The same process was performed for the detection of DNP-derived oxidative damage with slight modifications specific for the detection of oxidized proteins and detailed below in the oxidative stress section. Densitometric analysis was performed using ImageJ software.
Immunocytochemistry
Whole-mount immunofluorescent staining for PMP-4 and/or GFP was performed as described (88). The samples were probed with primary antibodies against PMP-4 described in the previous section at a 1:100 dilution and/or anti-GFP (Abcam, Cambridge, UK) at a 1:200 dilution. Secondary antibodies conjugated to Alexa Fluor 555 (PMP-4) dye and/or Alexa Fluor 555 (GFP) (Molecular Probes, Barcelona, Spain) were used at a 1:1000 dilution. Images were acquired on a Leica Confocal SP5 microscope. Nuclei were detected by DAPI counterstaining.
Imaging
Protein blot images were acquired using the Fujifilm Image Reader (NIH, ver. 1.43). The quantification of protein levels was performed using Multigage software v3.0 (Fujifilm).
Fluorescent microscopy was performed using a Nikon Eclipse E800 epifluorescence microscope equipped with an Endow GFP HYQ filter cube (Chroma Technology). Confocal microscopy was performed using a Leica TCS-SP5 confocal spectral microscope (Barcelona, Spain) and analysed by ImageJ software (NIH, ver. 1.43). Video recordings of the thrashing behaviour were performed on a stereomicroscope (SteREO Lumar. V12, Carl Zeiss, Madrid, Spain) coupled to a digital camera (Jenoptik, model ProgRes CF scan).
Lipid extraction and fatty acid quantification
Newly hatched L1 larvae were grown on regular NGM plus OP50 for 48 h at 20°C. L4 staged animals were washed and collected from 10 plates of 10 cm in diameter and cleaned from bacteria with M9 buffer. Subsequently, the worms were washed twice with S Basal buffer and finally with Optimal LC/MS H2O. The worms were settled by gravity on ice, and the water was carefully removed. The worm pellets were quickly frozen in liquid nitrogen and stored frozen at -80°C until analysis.
For homogenization, the frozen cell pellets were briefly thawed, and 0.5 ml MilliQ water was added, mixed by vortexing and transferred to a “ball sonicator tube”. The original tube was rinsed with 0.5 ml MilliQ water, and the rinse was also transferred to the “ball sonicator tube”. A small steel ball was added, and the tube was capped. The tubes were mixed in the cold for 2-3 minutes 25 times per 1 s in the Qiagen TissueLyser II. The samples appeared to be well mixed and broken into fine particles. Five-microliter aliquots were removed for total protein, 10-μl aliquots were removed for LC/MS/MS, and 0.5 ml x 2 aliquots were removed for total lipid extract by the Folch method (89). The Lyso-PC FIA-MS/MS analysis was performed as previously shown (28) (WT, n=12 and pmp-4(ok396), n=10). The daf-22(ok693) strain accumulates VLCFA (29) and was therefore used as a positive control (n= 6). The results are expressed as pmol/mg protein.
Thrashing assay
Thrashing is a rhythmic pattern of activity where the worm oscillates side-to-side around its midpoint. A single thrash was defined by a movement through the midpoint and back. The thrashing assay was performed blindly and in liquid medium as described previously (42). A drop of 2% agarose was poured over the glass slide and allowed to dry. After adding 20 μl of M9, L4 or L4+7 synchronized animals, the worms were placed on the drop and left for 2 minutes. Thrashes were counted for 30 s, and every animal was counted three consecutive times to obtain an average. This value was multiplied by two to obtain an estimate per minute. A single thrash was defined as a complete change in the direction of the body down the midline. Animals who were motionless for 10 s were discarded from the analysis.
Quantification of GABAergic-associated abnormalities using the juIs76 [Punc-25::gfp] reporter strain
Worms were grown at 20°C for at least two generations before the experiments. Animal age refers to the adult age measured in days, calculated by adding one day consecutively from the given animal went through L4 (90). The animals were transferred daily to avoid mixing populations, and the animals were considered dead when they stopped pharyngeal pumping and failed to respond to mild touch by a platinum wire picker. After synchronization, F1 hermaphrodite animals were maintained at 20°C until the late L4 larval stage and then transferred to the final assay plates (50 worms/plate) supplemented or not with the indicated concentration of drugs (PQT 0.2 mM, CoQ 1 mg/ml and MitoQ, 5 μg/ml) when required.
On the actual day of the experiment, worms of the appropriate age and genotype, with or without drugs, were washed, anaesthetized in 10 mM sodium azide on 6% agar pads and subsequently mounted for image analysis. The worms were scored for the number of GABAergic neurons, gaps in the ventral cord, gaps in the dorsal cord and defects in axonal morphology (91).
The applied formula used to quantify axonal damage is: % of axonal damage = [((number of commissural abnormalities + number of gaps/animal))/number of axons detected) x100].
The experiment was performed blindly and by different investigators to guarantee the reliability of the obtained measurements.
Fasting experiments
L4 animals were starved for 24 h under standard conditions at 20° C.
Analysis of lipid droplets
Fat accumulation was analysed by fixative and non-fixative live methodology. Sudan Black fixative staining was performed as described (92) with slight modifications. The animals were washed with M9 buffer and fixed with 2% paraformaldehyde. The worms were then subjected to three freeze-thaw cycles to disrupt the cuticles and incubated for 5 minutes and 10 minutes on ice. After 3 washes with cold M9, worms were dehydrated through an ethanol series (25%, 50%, 70%) and 2–5 volumes of 3% Sudan Black B solution (Sigma-Aldrich, Madrid, Spain) were added to the worms, which were then incubated overnight. The worms were rehydrated through an ethanol series (70%, 50%, 25%) and finally with M9. Lipid droplets were detectable as large black granules under a bright-field microscope.
The non-fixative live methodology was performed by using the strain VS20 (hjIs67 [Patgl-1::atgl-1::gfp]), in which ATGL-1 localizes to the surface of the lipid droplets (33). This strain was crossed with pmp-4(ok396) animals (pmp-4(ok396); hjIs67 [Patgl-1::atgl-1::gfp]), and the resulting progeny were analysed by confocal microscopy in parallel with the corresponding age-matched controls.
Oxidative stress assays
The sensitivity to oxidative stress was analysed systematically by using different approaches.
Protein carbonylation: Synchronized L4 animals grown at 20°C were used. Fifteen micrograms of worm proteins were run on an 8% SDS gel, transferred to nitrocellulose and incubated with a solution of 0.2% 2,4-Dinitrophenylhydrazine (DNPH, Sigma-Aldrich, Madrid, Spain). This method is based on the formation of a hydrazone (DNP) resulting from the reaction of protein-bound carbonyl and 2,4-DNPH (24). An antibody against DNP was used to detect carbonylated protein. Carbonylated proteins were finally detected with ECL western blotting analysis systems described previously for PMP-4 western blot detection.
Measurement of intracellular ROS was performed as previously described (35, 93). The amount of ROS was detected with the membrane-permeable non-fluorescent dye 2,7-dichlorodihydrofluorescein-diacetate (H2DCFDA), which can enter the cell where it is converted to a polar non-fluorescent derivative (dichlorofluorescein diacetate, H2DCFDA). Then, H2DCFDA is rapidly oxidized to the highly fluorescent 2’,7’-dichlorofluorescein in the presence of intracellular ROS. To detect changes in the levels of ROS, a stock solution of 10 mM carboxy-H2DCFDA (Molecular Probes, Barcelona, Spain) in DMSO was diluted to 10 μM in M9 buffer. Young adult worms were transferred into the staining solution and stained for 30 minutes at 20°C. The worms were mounted on a thick layer of half-dried agar pad on microscopic glass slides and then subjected to fluorescence microscopy (see above).
Measurement of mitochondrial ROS with MitoSOx probes was performed as previously described in (94). MitoSOX Red (Life Technologies, Madrid, Spain) was diluted in DMSO at 10 mM and frozen at -20°C as stock. Before staining, the stocks were diluted in M9 buffer at a 1:1000 dilution. L4-staged worms were transferred into the staining solution and stained overnight at 20°C in a mixer. After incubation, the worms were washed twice with M9 buffer and mounted on a 6% layer of half-dried agar pad on microscopic glass slides and then subjected to fluorescence microscopy. Pictures were taken by NIS software, and the fluorescence was analysed by ImageJ software.
Sensitivity to mitochondrial inhibitors: L4 synchronized animals were incubated separately at the indicated concentrations of PQT (95), thenoyltrifluoroacetone (TTFA), antimycin or sodium azide (96). The worms were considered dead when they failed to move, either spontaneously or in response to touch.
Antioxidant treatments
CoQ assay
CoQ nematode treatment was performed as previously described (91). CoQ10 (100 mg) was dissolved in 10 ml of distilled water containing 0.06% Tween-80. Ten millilitres of this stock was added to NG agar medium (total 100 ml) after autoclaving and cooling to 60°C. For PQT and CoQ10 combined plates, paraquat was supplemented at a final concentration of 2 mM in NGM agar containing 1 mg/ml CoQ10 (1.158 mM). The lethality test was performed as described above.
MitoQ assay
MitoQ nematode treatment was performed as previously described (58, 97). One milligram of MitoQ was dissolved in 1 ml of distilled water, and 250 μl of this stock was added to 60°C NG agar medium (total 50 ml) autoclaving and cooling to 60°C. We confirmed that C. elegans survival is not affected by TPP+ and MitoQ at this concentration. For PQT and MitoQ10 combined plates, PQT was supplemented at a final concentration of 4 mM in NGM agar containing 5 μg/ml MitoQ (7.3 μM). The lethality test was performed as described previously.
Statistics
All data are presented as the mean ± SD. Group means were compared with either Student’s t-test, one-way or two-way ANOVA, followed by Tukey’s post hoc test. All P values were two-tailed, and a P value of less than 0.05 was considered statistically significant. All statistical analyses were analysed using SPSS software.
Supplementary Material
Acknowledgements
We thank CERCA Program / Generalitat de Catalunya for institutional support. Most of the strains were supplied by the Caenorhabditis Genetic Center (CGC). The strain nsls105 [Phlh-17::gfp] I was kindly provided by Dr. Shai Shaham (The Rockefeller University, NY, USA). Plasmids HYM772 and HYM433 were kindly provided by Dr. Ho Yi Mak (Stowers Institute for Medical Research, Kansas City, MO, USA). We especially thank Dr. Marta Artal, Centro Andaluz de Biología del Desarrollo, Sevilla, Spain, for critical reading the manuscript. This work was supported by grants from the Autonomous Government of Catalonia [2017SGR1206] to A.P., The Spanish Ministry of Science and Competitivity grants (PC0009/003 and PI1100968 to E.D). This study has been funded by Instituto de Salud Carlos III through the grants [Miguel Servet program CPII16/00016] to S.F. (Co-funded by European Social Fund. ESF investing in your future), and the Center for Biomedical Research on Rare Diseases (CIBERER) to M.R. S.G. is a fellow of the Autonomous Government of Catalonia [2014FI_B2 00028]. A.C. and J.P. were fellows of IDIBELL.
Footnotes
Declaration of Interest
The authors declare no conflict of interest.
References
- 1.Van Veldhoven PP, Baes M. Peroxisome deficient invertebrate and vertebrate animal models. Front Physiol. 2013;4:335. doi: 10.3389/fphys.2013.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waterham HR, Ferdinandusse S, Wanders RJ. Human disorders of peroxisome metabolism and biogenesis. Biochim Biophys Acta. 2016;1863(5):922–33. doi: 10.1016/j.bbamcr.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Moser AB, Fatemi A. Newborn Screening and Emerging Therapies for X-Linked Adrenoleukodystrophy. JAMA Neurol. 2018:e. doi: 10.1001/jamaneurol.2018.1585. [DOI] [PubMed] [Google Scholar]
- 4.van Roermund CW, Visser WF, Ijlst L, van Cruchten A, Boek M, Kulik W, Waterham HR, Wanders RJ. The human peroxisomal ABC half transporter ALDP functions as a homodimer and accepts acyl-CoA esters. Faseb J. 2008;22(12):4201–8. doi: 10.1096/fj.08-110866. [DOI] [PubMed] [Google Scholar]
- 5.Engelen M, Kemp S, Poll-The BT. X-linked adrenoleukodystrophy: pathogenesis and treatment. Curr Neurol Neurosci Rep. 2014;14(10):486. doi: 10.1007/s11910-014-0486-0. [DOI] [PubMed] [Google Scholar]
- 6.Ferrer I, Aubourg P, Pujol A. General aspects and neuropathology of X-linked adrenoleukodystrophy. Brain Pathol. 2010;20(4):817–30. doi: 10.1111/j.1750-3639.2010.00390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moser H, Smith KD, Watkins PA, Powers J, Moser AB. X-linked adrenoleukodystrophy. In: Scriver C, editor. The Metabolic and Molecular Bases of Inherited disease. 8th. New-York: McGraw-Hill; 2001. pp. 3257–3301. [Google Scholar]
- 8.Berger J, Forss-Petter S, Eichler FS. Pathophysiology of X-linked adrenoleukodystrophy. Biochimie. 2014;98:135–42. doi: 10.1016/j.biochi.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aubourg P, Blanche S, Jambaque I, Rocchiccioli F, Kalifa G, Naud-Saudreau C, Rolland MO, Debre M, Chaussain JL, Griscelli C, et al. Reversal of early neurologic and neuroradiologic manifestations of X-linked adrenoleukodystrophy by bone marrow transplantation. N Engl J Med. 1990;322(26):1860–6. doi: 10.1056/NEJM199006283222607. [DOI] [PubMed] [Google Scholar]
- 10.Kuhl JS, Suarez F, Gillett GT, Hemmati PG, Snowden JA, Stadler M, Vuong GL, Aubourg P, Kohler W, Arnold R. Long-term outcomes of allogeneic haematopoietic stem cell transplantation for adult cerebral X-linked adrenoleukodystrophy. Brain. 2017;140(4):953–966. doi: 10.1093/brain/awx016. [DOI] [PubMed] [Google Scholar]
- 11.van Geel BM, Poll-The BT, Verrips A, Boelens JJ, Kemp S, Engelen M. Hematopoietic cell transplantation does not prevent myelopathy in X-linked adrenoleukodystrophy: a retrospective study. J Inherit Metab Dis. 2014 doi: 10.1007/s10545-014-9797-1. [DOI] [PubMed] [Google Scholar]
- 12.Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, Vidaud M, Abel U, Dal-Cortivo L, Caccavelli L, Mahlaoui N, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326(5954):818–23. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- 13.Eichler F, Duncan C, Musolino PL, Orchard PJ, De Oliveira S, Thrasher AJ, Armant M, Dansereau C, Lund TC, Miller WP, Raymond GV, et al. Hematopoietic Stem-Cell Gene Therapy for Cerebral Adrenoleukodystrophy. N Engl J Med. 2017;377(17):1630–1638. doi: 10.1056/NEJMoa1700554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berger J, Pujol A, Aubourg P, Forss-Petter S. Current and future pharmacological treatment strategies in X-linked adrenoleukodystrophy. Brain Pathol. 2010;20(4):845–56. doi: 10.1111/j.1750-3639.2010.00393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Launay N, Aguado C, Fourcade S, Ruiz M, Grau L, Riera J, Guilera C, Giros M, Ferrer I, Knecht E, Pujol A. Autophagy induction halts axonal degeneration in a mouse model of X-adrenoleukodystrophy. Acta Neuropathol. 2015;129(3):399–415. doi: 10.1007/s00401-014-1378-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Launay N, Ruiz M, Grau L, Ortega FJ, Ilieva EV, Martinez JJ, Galea E, Ferrer I, Knecht E, Pujol A, Fourcade S. Tauroursodeoxycholic bile acid arrests axonal degeneration by inhibiting the unfolded protein response in X-linked adrenoleukodystrophy. Acta Neuropathol. 2017;133(2):283–301. doi: 10.1007/s00401-016-1655-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morato L, Galino J, Ruiz M, Calingasan NY, Starkov AA, Dumont M, Naudi A, Martinez JJ, Aubourg P, Portero-Otin M, Pamplona R, et al. Pioglitazone halts axonal degeneration in a mouse model of X-linked adrenoleukodystrophy. Brain. 2013;136(Pt 8):2432–43. doi: 10.1093/brain/awt143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ranea-Robles P, Launay N, Ruiz M, Calingasan NY, Dumont M, Naudi A, Portero-Otin M, Pamplona R, Ferrer I, Beal MF, Fourcade S, et al. Aberrant regulation of the GSK-3beta/NRF2 axis unveils a novel therapy for adrenoleukodystrophy. EMBO Mol Med. 2018;10(8) doi: 10.15252/emmm.201708604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casasnovas C, Ruiz M, Schluter A, Naudi A, Fourcade S, Veciana M, Castaner S, Alberti A, Bargallo N, Johnson M, Raymond GV, et al. Biomarker Identification, Safety, and Efficacy of High-Dose Antioxidants for Adrenomyeloneuropathy: a Phase II Pilot Study. Neurotherapeutics. 2019 doi: 10.1007/s13311-019-00735-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pujol A, Ferrer I, Camps C, Metzger E, Hindelang C, Callizot N, Ruiz M, Pampols T, Giros M, Mandel JL. Functional overlap between ABCD1 (ALD) and ABCD2 (ALDR) transporters: a therapeutic target for X-adrenoleukodystrophy. Hum Mol Genet. 2004;13(23):2997–3006. doi: 10.1093/hmg/ddh323. [DOI] [PubMed] [Google Scholar]
- 21.Pujol A, Hindelang C, Callizot N, Bartsch U, Schachner M, Mandel JL. Late onset neurological phenotype of the X-ALD gene inactivation in mice: a mouse model for adrenomyeloneuropathy. Hum Mol Genet. 2002;11(5):499–505. doi: 10.1093/hmg/11.5.499. [DOI] [PubMed] [Google Scholar]
- 22.Fourcade S, Ferrer I, Pujol A. Oxidative stress, mitochondrial and proteostasis malfunction in adrenoleukodystrophy: A paradigm for axonal degeneration. Free Radic Biol Med. 2015;88(Pt A):18–29. doi: 10.1016/j.freeradbiomed.2015.05.041. [DOI] [PubMed] [Google Scholar]
- 23.Fourcade S, Lopez-Erauskin J, Ruiz M, Ferrer I, Pujol A. Mitochondrial dysfunction and oxidative damage cooperatively fuel axonal degeneration in X-linked adrenoleukodystrophy. Biochimie. 2014;98:143–9. doi: 10.1016/j.biochi.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Galino J, Ruiz M, Fourcade S, Schluter A, Lopez-Erauskin J, Guilera C, Jove M, Naudi A, Garcia-Arumi E, Andreu AL, Starkov AA, et al. Oxidative damage compromises energy metabolism in the axonal degeneration mouse model of x-adrenoleukodystrophy. Antioxid Redox Signal. 2011;15(8):2095–107. doi: 10.1089/ars.2010.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Bejjani R, Hammarlund M. Neural regeneration in Caenorhabditis elegans. Annu Rev Genet. 2012;46:499–513. doi: 10.1146/annurev-genet-110711-155550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fourcade S, Ruiz M, Camps C, Schluter A, Houten SM, Mooyer PA, Pampols T, Dacremont G, Wanders RJ, Giros M, Pujol A. A key role for the peroxisomal ABCD2 transporter in fatty acid homeostasis. Am J Physiol Endocrinol Metab. 2009;296(1):E211–21. doi: 10.1152/ajpendo.90736.2008. [DOI] [PubMed] [Google Scholar]
- 27.Theda C, Gibbons K, Defor TE, Donohue PK, Golden WC, Kline AD, Gulamali-Majid F, Panny SR, Hubbard WC, Jones RO, Liu AK, et al. Newborn screening for X-linked adrenoleukodystrophy: further evidence high throughput screening is feasible. Mol Genet Metab. 2014;111(1):55–7. doi: 10.1016/j.ymgme.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turgeon CT, Moser AB, Morkrid L, Magera MJ, Gavrilov DK, Oglesbee D, Raymond K, Rinaldo P, Matern D, Tortorelli S. Streamlined determination of lysophosphatidylcholines in dried blood spots for newborn screening of X-linked adrenoleukodystrophy. Mol Genet Metab. 2015;114(1):46–50. doi: 10.1016/j.ymgme.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Joo HJ, Yim YH, Jeong PY, Jin YX, Lee JE, Kim H, Jeong SK, Chitwood DJ, Paik YK. Caenorhabditis elegans utilizes dauer pheromone biosynthesis to dispose of toxic peroxisomal fatty acids for cellular homoeostasis. Biochem J. 2009;422(1):61–71. doi: 10.1042/BJ20090513. [DOI] [PubMed] [Google Scholar]
- 30.Cunningham KA, Ashrafi K. Fat rationing in dauer times. Cell Metab. 2009;9(2):113–4. doi: 10.1016/j.cmet.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 31.Schluter A, Real-Chicharro A, Gabaldon T, Sanchez-Jimenez F, Pujol A. PeroxisomeDB 2.0: an integrative view of the global peroxisomal metabolome. Nucleic Acids Res. 2010;38((Database issue)):D800–5. doi: 10.1093/nar/gkp935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujiki Y, Matsuzono Y, Matsuzaki T, Fransen M. Import of peroxisomal membrane proteins: the interplay of Pex3p-and Pex19p-mediated interactions. Biochim Biophys Acta. 2006;1763(12):1639–46. doi: 10.1016/j.bbamcr.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 33.Zhang SO, Box AC, Xu N, Le Men J, Yu J, Guo F, Trimble R, Mak HY. Genetic and dietary regulation of lipid droplet expansion in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2010;107(10):4640–5. doi: 10.1073/pnas.0912308107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yokota S, Togo SH, Maebuchi M, Bun-Ya M, Haraguchi CM, Kamiryo T. Peroxisomes of the nematode Caenorhabditis elegans: distribution and morphological characteristics. Histochem Cell Biol. 2002;118(4):329–36. doi: 10.1007/s00418-002-0450-y. [DOI] [PubMed] [Google Scholar]
- 35.Yang W, Hekimi S. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol. 2010;8(12):e1000556. doi: 10.1371/journal.pbio.1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fourcade S, Lopez-Erauskin J, Galino J, Duval C, Naudi A, Jove M, Kemp S, Villarroya F, Ferrer I, Pamplona R, Portero-Otin M, et al. Early oxidative damage underlying neurodegeneration in X-adrenoleukodystrophy. Hum Mol Genet. 2008;17(12):1762–73. doi: 10.1093/hmg/ddn085. [DOI] [PubMed] [Google Scholar]
- 37.Kruska N, Schonfeld P, Pujol A, Reiser G. Astrocytes and mitochondria from adrenoleukodystrophy protein (ABCD1)-deficient mice reveal that the adrenoleukodystrophy-associated very long-chain fatty acids target several cellular energy-dependent functions. Biochim Biophys Acta. 2015;1852(5):925–936. doi: 10.1016/j.bbadis.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Lopez-Erauskin J, Fourcade S, Galino J, Ruiz M, Schluter A, Naudi A, Jove M, Portero-Otin M, Pamplona R, Ferrer I, Pujol A. Antioxidants halt axonal degeneration in a mouse model of X-adrenoleukodystrophy. Ann Neurol. 2011;70(1):84–92. doi: 10.1002/ana.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez-Erauskin J, Galino J, Ruiz M, Cuezva JM, Fabregat I, Cacabelos D, Boada J, Martinez J, Ferrer I, Pamplona R, Villarroya F, et al. Impaired mitochondrial oxidative phosphorylation in the peroxisomal disease X-linked adrenoleukodystrophy. Hum Mol Genet. 2013;22(16):3296–305. doi: 10.1093/hmg/ddt186. [DOI] [PubMed] [Google Scholar]
- 40.Qabazard B, Li L, Gruber J, Peh MT, Ng LF, Kumar SD, Rose P, Tan CH, Dymock BW, Wei F, Swain SC, et al. Hydrogen sulfide is an endogenous regulator of aging in Caenorhabditis elegans. Antioxid Redox Signal. 2013;20(16):2621–30. doi: 10.1089/ars.2013.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morato L, Ruiz M, Boada J, Calingasan NY, Galino J, Guilera C, Jove M, Naudi A, Ferrer I, Pamplona R, Serrano M, et al. Activation of sirtuin 1 as therapy for the peroxisomal disease adrenoleukodystrophy. Cell Death Differ. 2015;22(11):1742–53. doi: 10.1038/cdd.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sleigh JN, Buckingham SD, Esmaeili B, Viswanathan M, Cuppen E, Westlund BM, Sattelle DB. A novel Caenorhabditis elegans allele, smn-1(cb131), mimicking a mild form of spinal muscular atrophy, provides a convenient drug screening platform highlighting new and pre-approved compounds. Hum Mol Genet. 2010;20(2):245–60. doi: 10.1093/hmg/ddq459. [DOI] [PubMed] [Google Scholar]
- 43.Cinar H, Keles S, Jin Y. Expression profiling of GABAergic motor neurons in Caenorhabditis elegans. Curr Biol. 2005;15(4):340–6. doi: 10.1016/j.cub.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 44.Hoepfner D, Schildknegt D, Braakman I, Philippsen P, Tabak HF. Contribution of the endoplasmic reticulum to peroxisome formation. Cell. 2005;122(1):85–95. doi: 10.1016/j.cell.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 45.Schluter A, Fourcade S, Ripp R, Mandel JL, Poch O, Pujol A. The Evolutionary Origin of Peroxisomes: An ER-Peroxisome Connection. Mol Biol Evol. 2006;23(4):838–845. doi: 10.1093/molbev/msj103. [DOI] [PubMed] [Google Scholar]
- 46.Wong LH, Gatta AT, Levine TP. Lipid transfer proteins: the lipid commute via shuttles, bridges and tubes. Nat Rev Mol Cell Biol. 2019;20(2):85–101. doi: 10.1038/s41580-018-0071-5. [DOI] [PubMed] [Google Scholar]
- 47.Bozza PT, Viola JP. Lipid droplets in inflammation and cancer. Prostaglandins Leukot Essent Fatty Acids. 2010;82(4-6):243–50. doi: 10.1016/j.plefa.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 48.Cabodevilla AG, Sanchez-Caballero L, Nintou E, Boiadjieva VG, Picatoste F, Gubern A, Claro E. Cell survival during complete nutrient deprivation depends on lipid droplet-fueled beta-oxidation of fatty acids. J Biol Chem. 2013;288(39):27777–88. doi: 10.1074/jbc.M113.466656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santos CR, Schulze A. Lipid metabolism in cancer. Febs J. 2012;279(15):2610–23. doi: 10.1111/j.1742-4658.2012.08644.x. [DOI] [PubMed] [Google Scholar]
- 50.Mak HY. Lipid droplets as fat storage organelles in Caenorhabditis elegans: Thematic Review Series: Lipid Droplet Synthesis and Metabolism: from Yeast to Man. J Lipid Res. 2012;53(1):28–33. doi: 10.1194/jlr.R021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li S, Xu S, Ma Y, Wu S, Feng Y, Cui Q, Chen L, Zhou S, Kong Y, Zhang X, Yu J, et al. A Genetic Screen for Mutants with Supersized Lipid Droplets in Caenorhabditis elegans. G3 (Bethesda) 2016;6(8):2407–19. doi: 10.1534/g3.116.030866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Artal-Sanz M, Tavernarakis N. Prohibitin couples diapause signalling to mitochondrial metabolism during ageing in C. elegans. Nature. 2009;461(7265):793–7. doi: 10.1038/nature08466. [DOI] [PubMed] [Google Scholar]
- 53.Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 2003;421(6920):268–72. doi: 10.1038/nature01279. [DOI] [PubMed] [Google Scholar]
- 54.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306(5700):1383–6. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 55.Cocheme HM, Murphy MP. Complex I is the major site of mitochondrial superoxide production by paraquat. J Biol Chem. 2008;283(4):1786–98. doi: 10.1074/jbc.M708597200. [DOI] [PubMed] [Google Scholar]
- 56.Heddi A, Stepien G, Benke PJ, Wallace DC. Coordinate induction of energy gene expression in tissues of mitochondrial disease patients. J Biol Chem. 1999;274(33):22968–76. doi: 10.1074/jbc.274.33.22968. [DOI] [PubMed] [Google Scholar]
- 57.Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, Smith RA, Murphy MP. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem. 2001;276(7):4588–96. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- 58.Murphy MP, Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol. 2007;47:629–56. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- 59.Tsang WY, Sayles LC, Grad LI, Pilgrim DB, Lemire BD. Mitochondrial respiratory chain deficiency in Caenorhabditis elegans results in developmental arrest and increased life span. J Biol Chem. 2001;276(34):32240–6. doi: 10.1074/jbc.M103999200. [DOI] [PubMed] [Google Scholar]
- 60.Galea E, Launay N, Portero-Otin M, Ruiz M, Pamplona R, Aubourg P, Ferrer I, Pujol A. Oxidative stress underlying axonal degeneration in adrenoleukodystrophy: a paradigm for multifactorial neurodegenerative diseases? Biochim Biophys Acta. 2012;1822(9):1475–88. doi: 10.1016/j.bbadis.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 61.Hoftberger R, Kunze M, Weinhofer I, Aboul-Enein F, Voigtlander T, Oezen I, Amann G, Bernheimer H, Budka H, Berger J. Distribution and cellular localization of adrenoleukodystrophy protein in human tissues: implications for X-linked adrenoleukodystrophy. Neurobiol Dis. 2007;28(2):165–74. doi: 10.1016/j.nbd.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 62.Oikonomou G, Shaham S. The glia of Caenorhabditis elegans. Glia. 2011;59(9):1253–63. doi: 10.1002/glia.21084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoshimura S, Murray JI, Lu Y, Waterston RH, Shaham S. mls-2 and vab-3 Control glia development, hlh-17/Olig expression and glia-dependent neurite extension in C. elegans. Development. 2008;135(13):2263–75. doi: 10.1242/dev.019547. [DOI] [PubMed] [Google Scholar]
- 64.Thieringer H, Moellers B, Dodt G, Kunau WH, Driscoll M. Modeling human peroxisome biogenesis disorders in the nematode Caenorhabditis elegans. J Cell Sci. 2003;116(Pt 9):1797–804. doi: 10.1242/jcs.00380. [DOI] [PubMed] [Google Scholar]
- 65.Ho JK, Moser H, Kishimoto Y, Hamilton JA. Interactions of a very long chain fatty acid with model membranes and serum albumin. Implications for the pathogenesis of adrenoleukodystrophy. J Clin Invest. 1995;96(3):1455–63. doi: 10.1172/JCI118182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whitcomb RW, Linehan WM, Knazek RA. Effects of long-chain, saturated fatty acids on membrane microviscosity and adrenocorticotropin responsiveness of human adrenocortical cells in vitro. J Clin Invest. 1988;81(1):185–8. doi: 10.1172/JCI113292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Snow BJ, Rolfe FL, Lockhart MM, Frampton CM, O’Sullivan JD, Fung V, Smith RA, Murphy MP, Taylor KM. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson’s disease. Mov Disord. 2010;25(11):1670–4. doi: 10.1002/mds.23148. [DOI] [PubMed] [Google Scholar]
- 68.Rossman MJ, Santos-Parker JR, Steward CAC, Bispham NZ, Cuevas LM, Rosenberg HL, Woodward KA, Chonchol M, Gioscia-Ryan RA, Murphy MP, Seals DR. Chronic Supplementation With a Mitochondrial Antioxidant (MitoQ) Improves Vascular Function in Healthy Older Adults. Hypertension. 2018;71(6):1056–1063. doi: 10.1161/HYPERTENSIONAHA.117.10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klemm RW, Norton JP, Cole RA, Li CS, Park SH, Crane MM, Li L, Jin D, Boye-Doe A, Liu TY, Shibata Y, et al. A conserved role for atlastin GTPases in regulating lipid droplet size. Cell Rep. 2013;3(5):1465–75. doi: 10.1016/j.celrep.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Renvoise B, Malone B, Falgairolle M, Munasinghe J, Stadler J, Sibilla C, Park SH, Blackstone C. Reep1 null mice reveal a converging role for hereditary spastic paraplegia proteins in lipid droplet regulation. Hum Mol Genet. 2016;25(23):5111–5125. doi: 10.1093/hmg/ddw315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hulshagen L, Krysko O, Bottelbergs A, Huyghe S, Klein R, Van Veldhoven PP, De Deyn PP, D’Hooge R, Hartmann D, Baes M. Absence of functional peroxisomes from mouse CNS causes dysmyelination and axon degeneration. J Neurosci. 2008;28(15):4015–27. doi: 10.1523/JNEUROSCI.4968-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chang CL, Weigel AV, Ioannou MS, Pasolli HA, Xu CS, Peale DR, Shtengel G, Freeman M, Hess HF, Blackstone C, Lippincott-Schwartz J. Spastin tethers lipid droplets to peroxisomes and directs fatty acid trafficking through ESCRT-III. J Cell Biol. 2019 doi: 10.1083/jcb.201902061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gemmink A, Daemen S, Kuijpers HJH, Schaart G, Duimel H, Lopez-Iglesias C, van Zandvoort M, Knoops K, Hesselink MKC. Super-resolution microscopy localizes perilipin 5 at lipid droplet-mitochondria interaction sites and at lipid droplets juxtaposing to perilipin 2. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863(11):1423–1432. doi: 10.1016/j.bbalip.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 74.Van Gilst MR, Hadjivassiliou H, Yamamoto KR. A Caenorhabditis elegans nutrient response system partially dependent on nuclear receptor NHR-49. Proc Natl Acad Sci U S A. 2005;102(38):13496–501. doi: 10.1073/pnas.0506234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ilkun O, Wilde N, Tuinei J, Pires KM, Zhu Y, Bugger H, Soto J, Wayment B, Olsen C, Litwin SE, Abel ED. Antioxidant treatment normalizes mitochondrial energetics and myocardial insulin sensitivity independently of changes in systemic metabolic homeostasis in a mouse model of the metabolic syndrome. J Mol Cell Cardiol. 2015;85:104–16. doi: 10.1016/j.yjmcc.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu L, Zhang K, Sandoval H, Yamamoto S, Jaiswal M, Sanz E, Li Z, Hui J, Graham BH, Quintana A, Bellen HJ. Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell. 2015;160(1–2):177–90. doi: 10.1016/j.cell.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Van Den Brink DM, Cubizolle A, Chatelain G, Davoust N, Girard V, Johansen S, Napoletano F, Dourlen P, Guillou L, Angebault-Prouteau C, Bernoud-Hubac N, et al. Physiological and pathological roles of FATP-mediated lipid droplets in Drosophila and mice retina. PLoS Genet. 2018;14(9):e1007627. doi: 10.1371/journal.pgen.1007627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Erdmann R. Assembly, maintenance and dynamics of peroxisomes. Biochim Biophys Acta. 2016;1863(5):787–9. doi: 10.1016/j.bbamcr.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 79.Berger J, Dorninger F, Forss-Petter S, Kunze M. Peroxisomes in brain development and function. Biochim Biophys Acta. 2016;1863(5):934–55. doi: 10.1016/j.bbamcr.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Troffer-Charlier N, Doerflinger N, Metzger E, Fouquet F, Mandel JL, Aubourg P. Mirror expression of adrenoleukodystrophy and adrenoleukodystrophy related genes in mouse tissues and human cell lines. Eur J Cell Biol. 1998;75(3):254–64. doi: 10.1016/S0171-9335(98)80121-0. [DOI] [PubMed] [Google Scholar]
- 81.Park S, Paik YK. Genetic deficiency in neuronal peroxisomal fatty acid beta-oxidation causes the interruption of dauer development in Caenorhabditis elegans. Sci Rep. 2017;7(1):9358. doi: 10.1038/s41598-017-10020-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Troulinaki K, Tavernarakis N. Endocytosis and intracellular trafficking contribute to necrotic neurodegeneration in C. elegans. Embo J. 2012;31(3):654–66. doi: 10.1038/emboj.2011.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shao Z, Watanabe S, Christensen R, Jorgensen EM, Colon-Ramos DA. Synapse location during growth depends on glia location. Cell. 2013;154(2):337–50. doi: 10.1016/j.cell.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kennedy LM, Pham SC, Grishok A. Nonautonomous regulation of neuronal migration by insulin signaling, DAF-16/FOXO, and PAK-1. Cell Rep. 2013;4(5):996–1009. doi: 10.1016/j.celrep.2013.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nichols ALA, Meelkop E, Linton C, Giordano-Santini R, Sullivan RK, Donato A, Nolan C, Hall DH, Xue D, Neumann B, Hilliard MA. The Apoptotic Engulfment Machinery Regulates Axonal Degeneration in C. elegans Neurons. Cell Rep. 2016;14(7):1673–1683. doi: 10.1016/j.celrep.2016.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947–8. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 87.Mello C, Fire A. DNA transformation. Methods Cell Biol. 1995;48:451–82. [PubMed] [Google Scholar]
- 88.Finney M, Ruvkun G. The unc-86 gene product couples cell lineage and cell identity in C. elegans. Cell. 1990;63(5):895–905. doi: 10.1016/0092-8674(90)90493-x. [DOI] [PubMed] [Google Scholar]
- 89.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 90.Toth ML, Melentijevic I, Shah L, Bhatia A, Lu K, Talwar A, Naji H, Ibanez-Ventoso C, Ghose P, Jevince A, Xue J, et al. Neurite sprouting and synapse deterioration in the aging Caenorhabditis elegans nervous system. J Neurosci. 2012;32(26):8778–90. doi: 10.1523/JNEUROSCI.1494-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Earls LR, Hacker ML, Watson JD, Miller DM., 3rd Coenzyme Q protects Caenorhabditis elegans GABA neurons from calcium-dependent degeneration. Proc Natl Acad Sci U S A. 2010;107(32):14460–5. doi: 10.1073/pnas.0910630107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277(5328):942–6. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- 93.Ewald CY, Hourihan JM, Bland MS, Obieglo C, Katic I, Moronetti Mazzeo LE, Alcedo J, Blackwell TK, Hynes NE. NADPH oxidase-mediated redox signaling promotes oxidative stress resistance and longevity through memo-1 in C. elegans. Elife. 2017:6. doi: 10.7554/eLife.19493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Smith SW, Latta LCt, Denver DR, Estes S. Endogenous ROS levels in C. elegans under exogenous stress support revision of oxidative stress theory of life-history tradeoffs. BMC Evol Biol. 2014;14:161. doi: 10.1186/s12862-014-0161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gonzalez-Cabo P, Bolinches-Amoros A, Cabello J, Ros S, Moreno S, Baylis HA, Palau F, Vazquez-Manrique RP. Disruption of the ATP-binding cassette B7 (ABTM-1/ABCB7) induces oxidative stress and premature cell death in Caenorhabditis elegans. J Biol Chem. 2011;286(24):21304–14. doi: 10.1074/jbc.M110.211201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ishiguro H, Yasuda K, Ishii N, Ihara K, Ohkubo T, Hiyoshi M, Ono K, Senoo-Matsuda N, Shinohara O, Yosshii F, Murakami M, et al. Enhancement of oxidative damage to cultured cells and Caenorhabditis elegans by mitochondrial electron transport inhibitors. IUBMB Life. 2001;51(4):263–8. doi: 10.1080/152165401753311816. [DOI] [PubMed] [Google Scholar]
- 97.Ng LF, Gruber J, Cheah IK, Goo CK, Cheong WF, Shui G, Sit KP, Wenk MR, Halliwell B. The mitochondria-targeted antioxidant MitoQ extends lifespan and improves healthspan of a transgenic Caenorhabditis elegans model of Alzheimer disease. Free Radic Biol Med. 2014;71:390–401. doi: 10.1016/j.freeradbiomed.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 98.Kennedy SR, Loeb LA, Herr AJ. Somatic mutations in aging, cancer and neurodegeneration. Mech Ageing Dev. 2012;133(4):118–26. doi: 10.1016/j.mad.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Edgar LG, McGhee JD. Embryonic expression of a gut-specific esterase in Caenorhabditis elegans. Dev Biol. 1986;114(1):109–18. doi: 10.1016/0012-1606(86)90387-8. [DOI] [PubMed] [Google Scholar]
- 100.Ved R, Saha S, Westlund B, Perier C, Burnam L, Sluder A, Hoener M, Rodrigues CM, Alfonso A, Steer C, Liu L, et al. Similar patterns of mitochondrial vulnerability and rescue induced by genetic modification of alpha-synuclein, parkin, and DJ-1 in Caenorhabditis elegans. J Biol Chem. 2005;280(52):42655–42668. doi: 10.1074/jbc.M505910200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, Welchman DP, et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421(6920):231–7. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- 102.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Butcher RA, Ragains JR, Li W, Ruvkun G, Clardy J, Mak HY. Biosynthesis of the Caenorhabditis elegans dauer pheromone. Proc Natl Acad Sci U S A. 2009;106(6):1875–9. doi: 10.1073/pnas.0810338106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lucanic M, Kiley M, Ashcroft N, L’Etoile N, Cheng HJ. The Caenorhabditis elegans P21-activated kinases are differentially required for UNC-6/netrin-mediated commissural motor axon guidance. Development. 2006;133(22):4549–59. doi: 10.1242/dev.02648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.