Abstract
Proton pump inhibitors (PPIs) are commonly used as a supplement to cancer therapy. Yet, their effect on cancer mortality is largely unknown. Using data from Danish nationwide registries and Cox proportional hazards models, we estimated hazard ratios with 95% confidence intervals (CIs) for cancer-specific and non-cancer death among PPI users (≥2 prescriptions within six months after diagnosis; n=36,066) compared with nonusers (<2 prescriptions, n=311,853) or users of histamine H2-receptor antagonists (H2RA; n=5,152). Multivariable-adjusted HRs for cancer-specific mortality among post-diagnostic PPI users as compared with nonusers or H2RA users were 1.46 (95% CI, 1.43-1.49) and 1.25 (95% CI, 1.20-1.31), respectively. HRs for cancer mortality were highest for ovarian and lowest for esophageal cancer. The associations were largely independent of gender, year of diagnosis and cancer therapy, but stronger with younger age, less comorbidity and advanced cancer stage as well as among new PPI users. To test the effect of PPIs on tumor growth in a model system free for confounding factors, we investigated the effect of pantoprazole on tumor growth in mice. Pantoprazole (5 mg/kg/day) enhanced tumor growth (P = 0.033) and reduced the anti-tumor activity of gemcitabine (P = 0.008) in fibrosarcoma-bearing Balb/c mice, but not in immunodeficient Balb/c nude mice. In breast carcinoma-bearing FVB/N mice, pantoprazole had no effect on tumor growth alone but it reduced the life-prolonging effect of doxorubicin significantly (P = 0.007). Taken together, these data raise concerns about the increasing use of PPIs and calls for further studies addressing their safety among cancer patients.
Keywords: Proton pump inhibitors, pharmacoepidemiology, mortality, tumor immunity, murine cancer model
Introduction
Proton pump inhibitors (PPIs) are the most potent drugs for inhibition of gastric acid production among patients with peptic ulcer and gastroesophageal reflux [1]. Since the approval of omeprazole, in 1989, PPIs have largely replaced histamine H2-receptor antagonists (H2RAs) in the treatment of acid peptic diseases (Supplementary Figure 1A), and are now among the most frequently prescribed drugs worldwide [1, 2]. In spite of the generally excellent safety profile of PPIs, their rapidly increasing use has raised concerns about potential long-term health hazards related to their appropriate and suspected inappropriate use [3–6].
PPIs inactivate proton pumps in the parietal cells of the stomach through covalent binding to cysteine sulfhydryl groups [7, 8]. They can also inhibit proton pumps and other cysteine-containing enzymes in other acidic tissues or in acidic cellular organelles, such as lysosomes [1, 9]. Due to the importance of acidic microenvironment for cancer progression, PPIs have been suggested to possess anticancer activity [10–12]. The drug-mediated neutralization of the normally acidic tumor microenvironment can enhance the activity of tumor-infiltrating lymphocytes upon adoptive immunotherapy [13]. Moreover, high concentrations of PPIs have the ability to kill some types of cancer cells or sensitize them to chemotherapy in vitro as well as in tumor xenografts in immunodeficient mice [14–17]. A recent phase II trial showed increases in objective responses and time to progression among chemotherapy-treated metastatic breast cancer patients upon addition of intermittent high-dose esomeprazole [18]. Besides the suggested anticancer activity, PPI-mediated inhibition of lysosomal enzymes in immune cells compromises antigen presentation and leukocyte transmigration [1, 19], which may explain the reported inhibitory effect of omeprazole on active cancer immunotherapy in immunocompetent mice [9].
Only a few studies have evaluated the association between PPI use and cancer prognosis in humans [12]. In a cohort of 596 patients with previously untreated head and neck squamous cell cancer, use of either PPIs or H2RAs was associated with improved overall survival [20]. The scarcity of epidemiologic results, rapidly increasing use of PPIs (Supplementary Figure 1), and existence of high-quality registry data in Denmark inspired us to conduct a large retrospective cohort study of the association between PPI use and mortality among Danish cancer patients. Our subsequent finding of a strong association between PPI use and increased cancer mortality prompted us to set up experimental mouse models to test the effect of pantoprazole alone and in combination with chemotherapy on tumor growth. In line with the data from the cohort study, pantoprazole significantly promoted tumor growth in immunocompetent mice.
Materials and Methods
Tumor allografts in mice
Murine WEHI-164 fibrosarcoma (ATCC®) and MT2 breast carcinoma [21] cells were maintained as detailed in Supplementary Methods online. FVB/NCtr (FVB/N) mice were purchased from Charles River (www.criver.com) and Balb/c mice from Taconic Biosciences (www.taconic.com). Balb/c nude mice were bread at the host institute. WEHI-164 cells in 100 μl phosphate-buffered saline (PBS) were inoculated subcutaneously into the flank of 6-9-week old female Balb/c (5-10 × 106 cells/mice) or female and male (divided equally into treatment groups) Balb/c nude (1.5 × 106 cells/mice) mice. MT2 cells (1.5 × 106 cells in 100 μl PBS) were inoculated into mammary fat pad (position 3) of 7-week old female FVB/N mice treated with 0.67 mg/ml estrone in drinking water starting a week prior to the inoculation. Pantoprazole (Sigma-Aldrich, Y0001001), gemcitabine (Eli Lilly, Gemzar®) and doxorubicin (Sigma-Aldrich, D1515) were administered in 200 μL PBS i.p. Tumor diameters (d) were measured using a caliper, and volumes were estimated according to the formula: V = 4/3 * π * (d/2)3. The animal studies were approved by the Danish authorities and carried out in accordance with the NIH guidelines.
Epidemiological Study Setting and Data Sources
The study was designed as an inception cohort study using data from nationwide health and demographic registries [22]. Supplementary Methods and Supplementary Table 1 provide a detailed description of the registries and codes used to identify cancer patients, drug use, mortality outcomes and covariates. All data sources were linked by means of the civil registry number assigned to all Danish residents [23].
The study was approved by the Danish Data Protection Agency. Approval from the Danish Scientific Ethical committee was not required, as the study did not involve any patient contact [22].
Study Population and Clinical Parameters
Eligible cohort members were all Danish residents aged 30 years or more at the time of diagnosis with a histo-or cytologically verified first cancer (except non-melanoma skin cancer) diagnosis from 1995 to 2011; ascertained from the Cancer Registry [24], which also provided data on clinical stage at diagnosis. From the National Patient Registry [25], we obtained information on oncologic therapy within six months of the diagnosis.
Assessment of Drug Use
We defined post-diagnostic PPI use as ≥2 prescriptions filled on separate dates within six months following the cancer diagnosis and nonuse as <2 prescriptions. For use in sensitivity analyses, we applied the same exposure definition to a six-month period prior to the diagnosis. Analogously defined H2RA use was included as comparator due to similar indications with PPIs. In Denmark, most (>90%) of the total sales of PPIs are prescriptions, whereas a larger proportion of H2RAs is sold over-the-counter [26].
Follow-up and Mortality Outcomes
The cancer patients were followed from six months after the diagnosis until death, emigration, or end of study (31 December 2013) by linkage to the Civil Registration System [23]. Patients dying during the first six months after the diagnosis were excluded. The outcomes were cancer-specific or non-cancer deaths as recorded in the Registry of Causes of Death [27].
Statistical, Sensitivity and Secondary Analyses
We used Cox proportional hazards regression analysis to estimate multivariable-adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) for total and 1-year cancer-specific or non-cancer death associated with post-diagnostic use of PPIs as primary exposure. Time since baseline (six months after the diagnosis) was used as the underlying time-scale. In separate analyses, we compared users of PPIs with nonusers or users of H2RAs. The multivariable-adjusted models included age, gender, year of cancer diagnosis, clinical stage, Charlson Comorbidity Index score [28], medical history of diabetes, highest achieved education, disposable income, and use (≥2 prescriptions) of aspirin, non-aspirin non-steroidal anti-inflammatory drugs, angiotensin-converting-enzyme inhibitors, angiotensin receptor blockers, glucocorticoids, and statins (Table 1).
Table 1. Descriptive statistics for PPI users and nonusers 0-6 months after cancer diagnosis.
| Variable | Level | Non-user1 N (% of nonusers) | User2 N (% of users) |
|---|---|---|---|
| Patients [Median follow-up, interquartile range] | 311853 (100) [3.49, 1.29-7.42] | 36066 (100) [1.52, 0.50-3.89] | |
| Cancer deaths | 105382 (34) | 16281 (45) | |
| Non-cancer deaths | 33281 (11) | 3981 (11) | |
| Age at diagnosis | <60 | 100143 (32) | 7781 (22) |
| 60-68 | 80176 (26) | 9381 (26) | |
| 69-77 | 75736 (24) | 10056 (28) | |
| >77 | 55798 (18) | 8848 (25) | |
| Year of diagnosis | 1995-2000 | 98751 (32) | 5615 (16) |
| 2001-2005 | 89171 (29) | 10023 (28) | |
| 2006-2011 | 123931 (40) | 20428 (57) | |
| Gender | Male | 147705 (47) | 18088 (50) |
| Female | 164148 (53) | 17978 (50) | |
| Disposable income2 | 1 | 65366 (21) | 9484 (26) |
| 2 | 76030 (24) | 10891 (30) | |
| 3 | 54078 (17) | 6010 (17) | |
| 4 | 50722 (16) | 4680 (13) | |
| 5 | 65657 (21) | 5001 (14) | |
| Education | Higher | 45009 (14) | 4222 (12) |
| Vocational | 119109 (38) | 13738 (38) | |
| Basic | 71332 (23) | 11266 (31) | |
| Unknown | 76403 (24) | 6840 (19) | |
| Clinical stage | Localized | 146339 (47) | 12589 (35) |
| Non-localized | 103462 (33) | 15444 (43) | |
| Unknown | 62052 (20) | 8033 (22) | |
| Aspirin3 | User | 25738 (8) | 5326 (15) |
| Non-aspirin non-steroidal anti-inflammatory drugs3 | User | 25027 (8) | 4982 (14) |
| Angiotensin-converting-enzyme inhibitors or angiotensin receptor blockers3 | User | 36991 (12) | 6418 (18) |
| Statins3 | User | 20905 (7) | 4411 (12) |
| Steroids3 | User | 12286 (4) | 5180 (14) |
| Proton pump inhibitors, pre-diagnostic4 | User | 7790 (2) | 15814 (44) |
| Histamine H2-receptor antagonists3 | User | 4647 (1) | 505 (1) |
| Charlson Comorbidity Index5 | 0 | 225346 (72) | 18525 (51) |
| 1 | 55192 (18) | 9126 (25) | |
| 2+ | 31315 (10) | 8415 (23) | |
| Diabetes diagnosis | Yes | 15463 (5) | 3461 (10) |
| Cancer sites | Lung | 25142 (8) | 5491 (15) |
| Colorectal | 42355 (14) | 4833 (13) | |
| Breast | 61039 (20) | 3876 (11) | |
| Ovary | 6379 (2) | 901 (2) | |
| Stomach | 2376 (1) | 2348 (7) | |
| Prostate | 40187 (13) | 3359(9) | |
| Esophagus | 1726 (1) | 1536 (4) | |
| Pancreas | 3036 (1) | 1213 (3) | |
| Uterus6 | 9457 (3) | 640 (2) | |
| Proton pump inhibitor subtypes3 | Omeprazole | 0 (0) | 8488 (24) |
| Pantoprazole | 0 (0) | 10295 (29) | |
| Lansoprazole | 0 (0) | 8453 (23) | |
| Rabeprazole | 0 (0) | 232 (1) | |
| Esomeprazole | 0 (0) | 8031 (22) | |
| Registered oncological therapy7 | Chemo only | 31478 (16)8 | 5882 (20)9 |
| Radiation only | 22590 (12)8 | 2926 (10)9 | |
| Endocrine only | 8151 (4)8 | 922 (3)9 | |
| Chemo+Radiation | 13166 (7)8 | 2844 (10)9 | |
| None | 113520 (58)8 | 15738 (54)9 |
Less than two prescriptions 0-6 months after diagnosis
Disposable income categorized according to quintiles from lowest (1) to highest (5)
Two prescriptions 0-6 months after diagnosis
Two prescriptions 0-6 months before diagnosis
Modified Charlson Comorbidity Index (excluding cancer)
Cervix and corpus uteri
Data available from 2002 for 195997 nonusers and 28925 users of PPIs. Note that all treatments have not been registered
Percentage of cancer patients (PPI nonusers) diagnosed in 2002-2011
Percentage of cancer patients (PPI users) diagnosed in 2002-2011
In secondary analyses, we stratified the PPI users according to pre-diagnostic use, clinical stage, registered oncologic therapy (data available from 2002), three calendar periods, three categories of comorbidity, four age-groups and five individual types of PPIs (Table 1). Finally, as sensitivity analyses, we repeated the main analyses defining PPI use as ≥1 prescription versus no prescriptions as sensitivity analyses.
In the animal studies, we used t-tests to compare means of tumor volume for various groups separately for each day with measurements. Probabilities of tumor volumes reaching 380 mm3 or reducing >50% were estimated using the Kaplan-Meier estimator. Differences in probability were tested using the Log-rank test.
The proportional hazards assumption was obtained by testing for trends in the scaled Schoenfeld residuals. All analyses were performed using R version 3.0.2.
Results
Cohort characteristics
The study cohort included 347,919 patients with a first cancer diagnosis. Baseline characteristics of the PPI users and nonusers are presented in Table 1. Approximately 10% (N = 36,066) of the patients were post-diagnostic PPI users, while 1.5% (N = 5,152) were post-diagnostic H2RA users. Among the PPI users, 20,252 were new users and 15,814 were continuing users, i.e., also filled ≥ PPI prescriptions within six months prior to the cancer diagnosis. PPI users were slightly younger than nonusers (average ages of 65.0 and 68.7 years, respectively), while the gender distribution was equal among users and nonusers. PPI users had a higher prevalence of diabetes and other comorbidities and were more likely to have used aspirin, non-aspirin non-steroidal anti-inflammatory drugs, angiotensin-converting-enzyme inhibitors or angiotensin receptor blockers, statins and glucocorticoids, whereas no substantial differences were seen for socioeconomic attributes (education and income) or registered oncological therapy. A larger proportion of PPI users than nonusers had non-localized cancer disease (43% versus 33%) at diagnosis, while the proportions of unknown clinical stage were similar (20-22%) between PPI users and nonusers.
PPI use and mortality
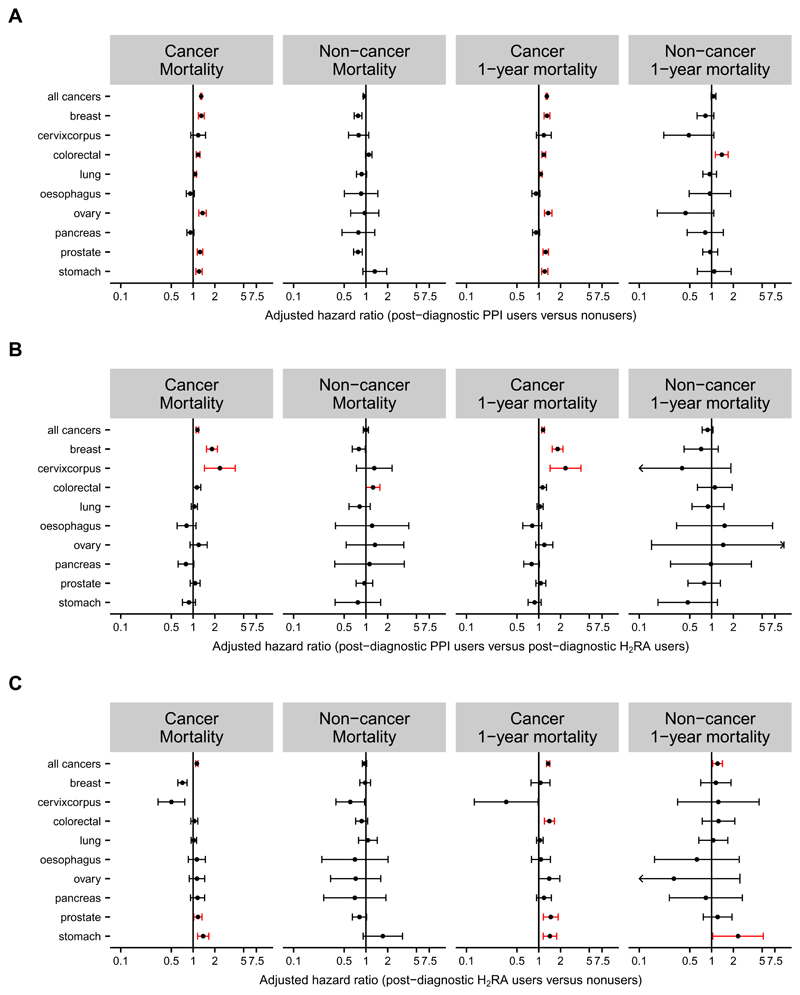
During the 1,623,038 person-years of follow-up, we identified 158,925 deaths, of which 121,663 were cancer-specific (Table 1). The crude rate of cancer-specific death was 2.39 times higher among post-diagnostic PPI users than among nonusers (16.5 vs. 6.9 deaths/100 patient-years), and non-cancer death rates were 1.85 higher among PPI users than among nonusers (4.05 vs. 2.18 deaths/100 patient-years). In adjusted analyses, postdiagnostic PPI use was associated with 46% increased risk of overall (HR, 1.46; 95% CI, 1.43 to 1.49) and 67% increased risk of 1-year (HR, 1.67; 95% CI, 1.62 to 1.72) cancer-specific death compared to nonuse (Figure 1A and Supplementary Table 2). In comparisons with post-diagnostic use of H2RAs, the adjusted HRs for overall (1.25; 95% CI, 1.20 to 1.31) and 1-year (1.27; 95% CI, 1.18 to 1.37) cancer-specific mortality were lower than in comparisons with nonusers, albeit still statistically significantly increased (Figure 1B and Supplementary Table 2). Post-diagnostic H2RA use compared to nonuse was associated with adjusted HRs of 1.17 (95% CI, 1.12 to 1.21) for total and 1.25 (95% CI, 1.18 to 1.32) for 1-year cancer-specific mortality (Figure 1C).
Figure 1. HRs for cancer-specific and non-cancer mortalities among users of PPIs or H2RAs.
Forest plots show adjusted HRs and 95% CIs for cancer-specific and non-cancer mortality among Danish cancer patients estimated using Cox proportional hazards regression models and stratified according to nine major cancer sites.
(A) Values for PPI users with ≥2 PPI prescriptions within 0-6 months after the cancer diagnosis compared to PPI nonusers with <2 PPI prescriptions in the same period.
(B) Values for PPI users with ≥2 PPI prescriptions within 0-6 months after the cancer diagnosis compared to H2RA users with ≥2 H2RA prescriptions in the same period.
(C) Values for H2RA users with ≥2 H2RA prescriptions within 0-6 months after the cancer diagnosis compared to nonusers with <2 H2RA prescriptions in the same period.
The corresponding values for figures A and B are shown in Supplementary Table 2 and unadjusted estimates for cancer mortality for post-diagnostic PPI and H2RA users versus nonusers in Supplementary Figures 2A and B.
Stratification according to nine major cancer sites revealed statistically significantly increased risks for cancer-specific mortality among post-diagnostic PPI users compared to nonusers for all sites except for esophagus (Figure 1A and Supplementary Table 2). Cancers of the ovary (1.59; 95% CI, 1.38 to 1.84), uterus (1.43; 95% CI, 1.08 to 1.88) and breast (1.35; 95% CI, 1.24 to 1.47) exhibited the highest adjusted HRs (Figure 1A). Notably, H2RA use was not associated with increased cancer mortality risk in these three cancer types (Figure 1C).
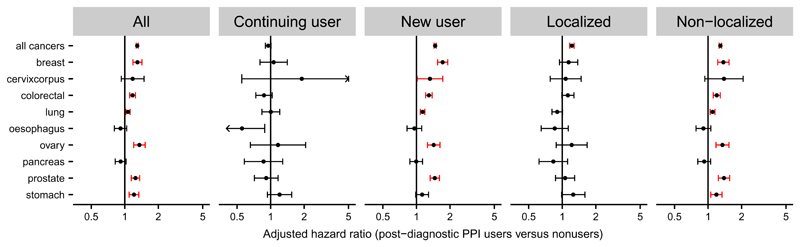
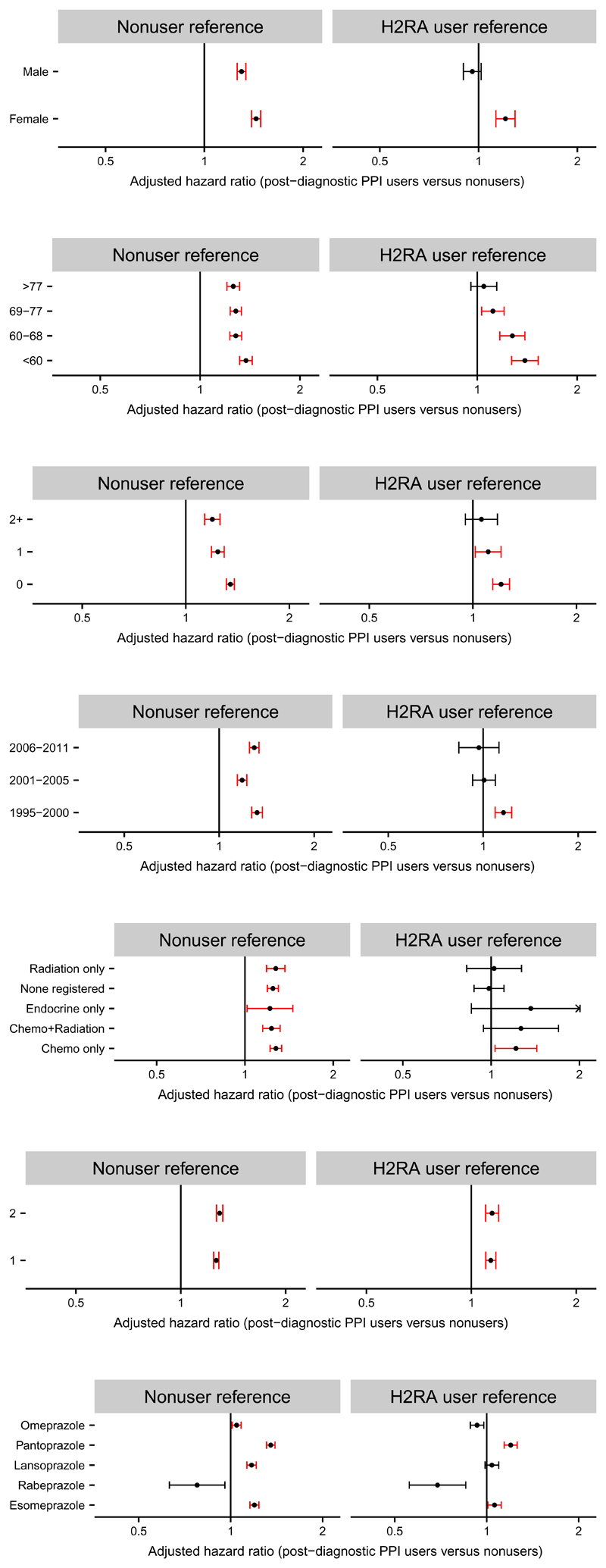
In order to further test the robustness of our results, we performed secondary analyses, as described above. We found that use of PPIs before cancer diagnosis had substantial influence on the association between post-diagnostic use and cancer-specific mortality, with a higher adjusted HR for cancer-specific mortality observed among new post-diagnostic users (1.67; 95% CI, 1.63 to 1.70) than among continuing users (1.20; 95% CI, 1.16 to 1.23) (Figure 2). The adjusted HR for cancer-specific death was also higher among patients with non-localized cancer than among those with localized disease (Figure 2). Moreover, PPI users in the youngest age group (<60 years) or with lowest comorbidity level (score 0) had statistically significantly higher HRs for cancer-specific mortality than elderly subjects (≥69 years) or those with higher comorbidity (scores ≥1), respectively, compared to nonusers (Figure 3). Similar tendencies, albeit not statistically significant, were seen for age and comorbidity in comparisons of post-diagnostic PPI users with postdiagnostic H2RA users (Figure 3). Stratification according to gender, year of diagnosis or type of oncologic therapy revealed only marginal differences in the HRs (Figure 3). Finally, applying one prescription as user definition and no prescriptions as comparison did not substantially influence the associations, although the majority of the risk estimates were slightly lower than those in the main analyses (Figure 3).
Figure 2. HRs for cancer-specific mortality among PPI users stratified on the timing of PPI use and clinical stage.
Forest plots show adjusted HRs and 95% CIs for cancer-specific mortality among Danish cancer patients estimated using Cox proportional hazards regression model and stratified according to nine major cancer sites and PPI use (continuing and new user, middle) or clinical stage (localized and non-localized, right). Values for all cancer patients from figure 1A are shown for comparison (left). Patients with ≥2 prescriptions of PPIs within 0-6 months after the cancer diagnosis were compared to nonusers (<2 prescriptions) of PPIs in the same period (left and right). Continuing (≥2 prescriptions both 0-6 months before and after the cancer diagnosis) and new (<2 prescriptions 0-6 months before and ≥2 prescriptions 0-6 months after the cancer diagnosis) users were compared to nonusers (<2 prescriptions both 0-6 months before and after the cancer diagnosis) (middle).
Figure 3. HRs for cancer-specific mortality among PPI users stratified on patient and therapy.
Forest plots show adjusted HRs and 95% CIs for cancer-specific mortality among Danish cancer patients estimated using Cox proportional hazards regression model and stratified according to gender, age at diagnosis, Charlson Comorbidity Index (CCI) score, calendar year of diagnosis, registered cancer treatment (data available from 2002; note that the group “none registered” also include patients that have received oncological therapy, which has not been registered), number of post-diagnostic PPI prescriptions and type of PPI. Danish cancer patients with ≥2 prescriptions of PPIs 0-6 months after the cancer diagnosis were compared to nonusers (<2 prescriptions) of PPIs or users (≥2 prescriptions) of H2RAs in the same period. Finally, as senitivity analyses, users with one prescription were compared to nonusers with no prescriptions 0-6 months after the cancer diagnosis.
PPI users in the study cohort included users of pantoprazole (29%), omeprazole (24%), lansoprazole (23%), esomeprazole (22%; since 1999) and rabeprazole (<1%; since 1999). Separate analyses of individual drugs exhibited the highest adjusted HRs for cancer-specific mortality among pantoprazole users compared to pantoprazole nonusers (1.58; 95% CI, 1.53-1.63) or to H2RA users (1.35; 95% CI, 1.28-1.42) (Figure 3). These values were statistically significantly higher than corresponding values among omeprazole users (P < 0.001). Use of rabeprazole was not associated with increased HR for cancer-specific mortality, however, the number of users in this group was low.
Pantoprazole enhances tumor growth and inhibits the efficacy of chemotherapy in immunocompetent mice
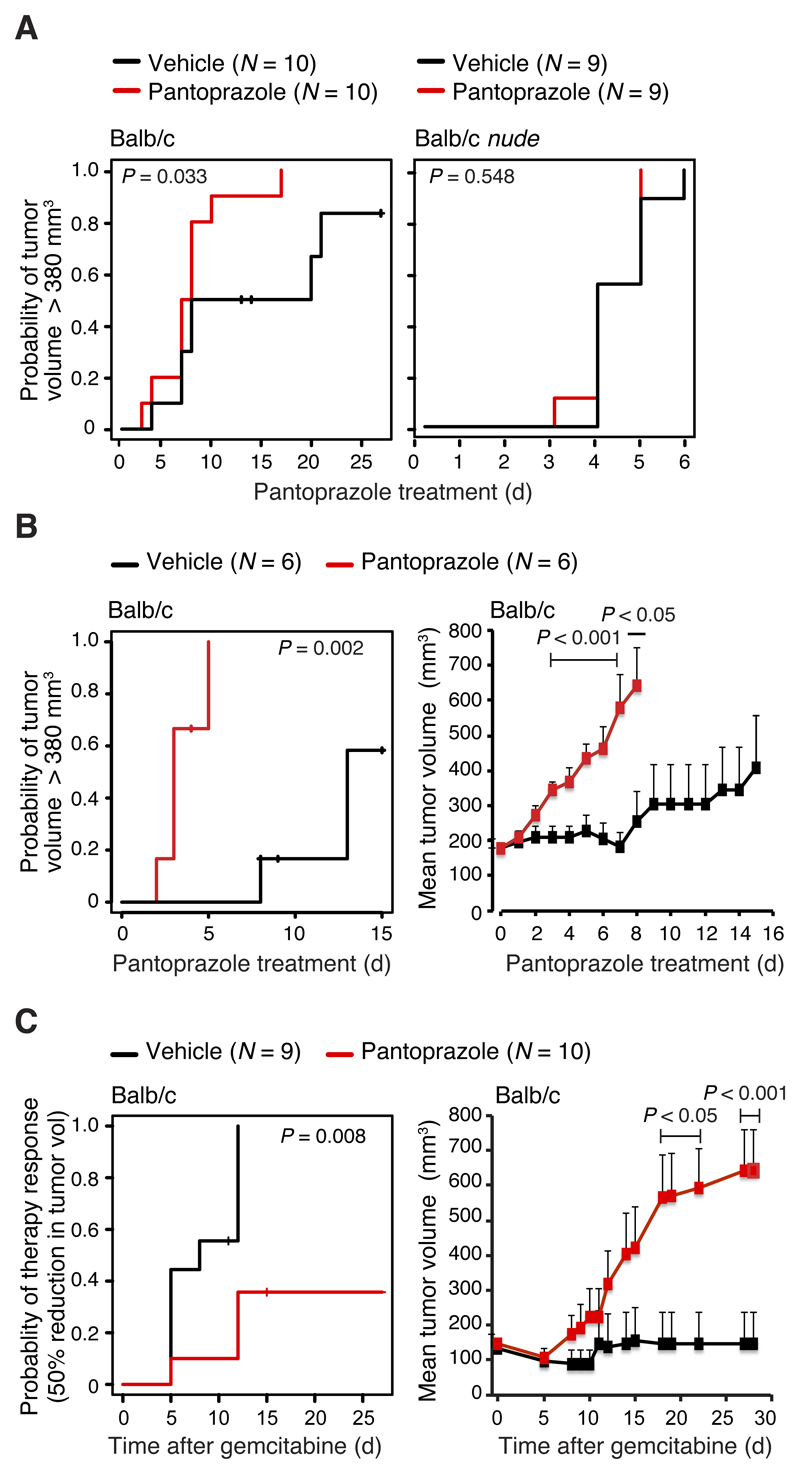
To test the effect of PPIs on tumor growth more directly and without putative confounding factors, we inoculated WEHI-164 fibrosarcoma cells into immunocompetent and immunodeficient (nude) Balb/c mice and treated them daily with pantoprazole (5 mg/kg) or vehicle after the tumors reached a volume of 110 mm3. When corrected for the 7-fold faster metabolic rate of mice than humans, the chosen pantoprazole dose roughly corresponds to the regular daily pantoprazole dose (40 mg) recommended for patients [9]. Pantoprazole treatment significantly enhanced tumor growth in immunocompetent mice in two independent experiments (P values: 0.033 and 0.002) but had no effect on tumor growth in immunodeficient mice (Figures 4A and B). These data suggest that the tumor-promoting effect of pantoprazole may be due to its inhibitory effect on anti-tumor immunity as suggested previously [9]. Thus, we tested the effect of pantoprazole on the anti-tumor activity of gemcitabine, a cytidine analog whose anti-tumor activity in vivo depends on the adaptive immunity of the host [29]. Daily pantoprazole treatment inhibited effectively the anti-tumor activity of a single dose of gemcitabine in WEHI-164 allograft-bearing Balb/c mice (Figure 4C). Tumors in 22% of vehicle- and 80% of pantoprazole-treated mice showed progressive growth after gemcitabine treatment. At the end of the 28-day follow-up, 56% of vehicle- and 20% of pantoprazole-treated mice were alive and tumor-free.
Figure 4. Effect of pantoprazole on WEHI-164 fibrosarcoma allograft growth in immunocompetent and immunodeficient Balb/c mice.
(A) Kaplan Meier plots for the probability of tumors reaching a volume greater than 380 mm3 show the effect of pantoprazole on WEHI-164 tumor allograft growth in immunocompetent (left) and immunodeficient (nude; right) Balb/c mice. Mice were treated intraperitoneally with vehicle or 5 mg/kg/day pantoprazole from the day tumor volume reached 110 mm3 (day 0), and followed until tumor volume reached 380 mm3 or the end of the experiment (day 25). Two mice in the Balb/c vehicle group were sacrificed and censored due to skin wounds on days 12 and 13. P values were calculated using log-rank test.
(B) Kaplan Meier plots for the probability of tumors volumes reaching 380 mm3 (left) and mean tumor volumes + SEM (right) show the effect of pantoprazole on tumor allograft growth in immunocompetent Balb/c mice treated as in (A) from the day tumor volume reached 180 mm3 (day 0), and followed until tumors reached a volume of 905 mm3 or the end of follow-up (day 15). One mouse in the pantoprazole group (day 3) and three in the vehicle group (days 7, 7 and 8) were sacrificed and censored due to skin wounds. P values comparing the two groups were calculated using log-rank test (left) or two-tailed, homoscedastic Student’s t-test (right).
(C) Kaplan-Meier plot representing the probability of a tumor volume reduction by >50% (left) and mean tumor volumes + SEM (right) show the effect of pantoprazole on gemcitabine responsiveness of WEHI-164 tumor allografts in Balb/c mice. Mice were treated with pantoprazole or vehicle as in (A) starting three days after the tumor inoculation. Five days later (day 0), all mice were treated with a single intraperitoneal dose of gemcitabine (100 mg/kg). Mice were followed until tumor volumes reached 700-900 mm3 or the end of the experiment (day 28). One mouse in the vehicle group (day 11) and one mouse in the pantoprazole group (day 15) were sacrificed and censored due to skin wounds. P values were calculated as in (B).
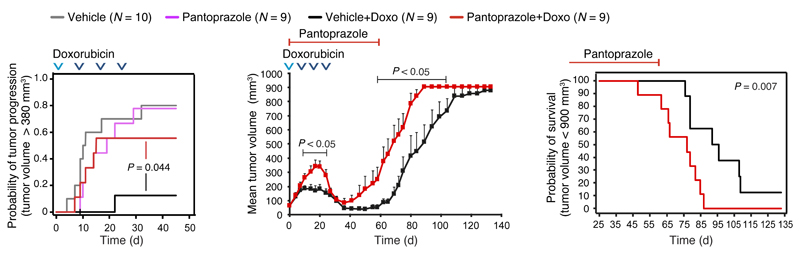
To validate these findings in an independent model system, we compared the effect of corresponding pantoprazole and vehicle treatments on the growth of orthotopic MT2 breast carcinoma allografts in immunocompetent FVB/N mice left otherwise untreated or treated with four cycles of doxorubicin, an anthracycline that induces immunogenic cell death in tumors [30, 31]. Pantoprazole alone had no significant effect on tumor growth, but it effectively reduced the anti-tumor activity of doxorubicin (Figure 5). During the 24-day doxorubicin treatment, tumors in “vehicle + doxorubicin” group either stopped growing or regressed, whereas tumors in “pantoprazole+ doxorubicin” group continued a progressive growth similar to that in mice treated with vehicle or pantoprazole alone (Figure 5). During the two weeks after the last doxorubicin treatment, tumors in both groups regressed, but the pantoprazole-treated ones regained aggressive growth more rapidly than the vehicle-treated ones and had 6.25 times higher HR (95% CI, 1.64-23.81) for mortality (defined as the maximum allowed tumor volume) than vehicle-treated mice (Figure 5). Notably, pantoprazole treatment affected neither growth, survival nor chemotherapy sensitivity of WEHI-164 and MT2 cells in vitro (Supplementary Figure 3).
Figure 5. Effect of pantoprazole on MT2 breast carcinoma allograft growth in FVB/N mice.
Kaplan-Meier plots representing the probability of tumor progression to a volume of 380 mm3 (left) or the survival of the mice (tumor volume > 900 mm3) (middle), and mean tumor volumes + SEM (right) show the effect of pantoprazole on doxorubicin responsiveness of MT2 breast carcinoma allografts in immunocompetent FVB/N mice. Day 0 is the day tumors reached 65 mm3. Mice were treated intraperitoneally with vehicle or 5 mg/kg/day pantoprazole starting on day 0 (“doxorubicin groups”) or on days 1-3 when tumor volume reached 113 mm3 (“no doxorubicin” groups). Intraperitoneal doxorubicin treatment was given on days 0 (2 mg/kg), 8 (3 mg/kg), 16 (3 mg/kg) and 24 (3 mg/kg) as indicated by arrow heads. Mice were followed until tumor volumes reached 900 mm3 or the end of the experiment (day 133). One mouse in the “Vehicle+Doxo” group was sacrificed and censored due to an injection injury on day 9. P values were calculated using log-rank test (Kaplan-Meier plots) or two-tailed, homoscedastic Student’s t-test (mean tumor volumes).
Discussion
The increasing use of PPIs has raised concerns about their potential long-term health hazards, especially among older patients [3–6]. In line with this, our findings suggest that the use of PPIs may worsen the prognosis of cancer patients. This conclusion is based on statistically significant associations between the post-diagnostic use of PPIs and mortality. The primary limitation of our study is the potential for confounding by indication associated with PPI use. Thus, we included several provisions to manage confounding into the study design and analyses. Even though the data were adjusted by clinical stage and oncological therapy, we remained concerned about a conceivable bias induced by differential use of PPIs due to gastric symptoms being associated with more advanced cancer stage or chemotherapy. Our analyses lack information on the specific indications for use of PPIs and drug use during hospitalization. Conceivably, PPIs were used to treat serious complications during hospitalization in some cancer patients. As we were mainly interested in the influence of PPIs on cancer-specific mortality beyond the primary diagnosis and treatment, we defined post-diagnostic use as ≥2 prescriptions filled at pharmacies in the primary health care sector after hospitalization and primary management of the cancer diseases. This definition may have introduced some misclassification of PPI use, however, we believe that patients who filled ≥2 prescriptions were less likely to introduce serious confounding by indication and prescribing bias. Nevertheless, we observed that the post-diagnostic users of PPIs with <2 prescription prior to the cancer diagnosis experienced higher excess HRs for mortality than those who filled minimum two prescriptions both before and after the cancer diagnosis. Thus, we cannot exclude that some of the excess mortality observed among users of PPIs were due to confounding by indication and diseases associated with increased mortality.
To further evaluate the possibility of confounding by indication, we included users of H2RAs, which have similar anti-peptic indications as PPIs, as an additional comparison group in all the main analyses. The statistically significantly increased HRs for mortality among PPI users compared to users of H2RAs support an impact of the PPI effect per se. Moreover, the significantly higher HRs for mortality among pantoprazole users compared to omeprazole users further point to drug-specific effects. Finally, to have a study set-up free for confounding factors, we tested the effect of pantoprazole in two independent cancer models employing inbred Balb/c and FVB/N mice. A drug-specific effect was reinforced by the pantoprazole-induced acceleration of fibrosarcoma growth in otherwise untreated mice and significantly weaker chemotherapy responses in both fibrosarcoma and breast carcinoma-bearing mice.
PPIs have been reported to possess anticancer activity in tumor xenografts in immunodeficient mice [14–17]. We reasoned that our results showing cancer promoting effectsin immunocompetent mice could be attributed to the reported inhibitory effect of PPIs on the host immune system [1, 9, 32, 33]. To test this hypothesis, we took advantage of a WEHI-164 fibrosarcoma allograft model in Balb/c mice. In this model, the primary tumor allograft growth is greatly limited by the host immune system, especially CD8+ and CD4+ T lymphocytes [34]. Thus, the ability of pantoprazole to enhance allograft growth in wild type Balb/c mice but not in Balb/c nude mice lacking mature CD8+ and CD4+ T lymphocytes, supports the idea that pantoprazole enhances tumor growth by interfering with the immune system. This hypothesis is further supported by the pantoprazole-induced potent inhibition of the anti-tumor activities of immunogenic cell death-inducing anti-neoplastic agents, gemcitabine and doxorubicin [29, 31, 35]. Finally, a common target essential for most cancers, such as the immune system, could explain the lack of cancer site specificity in our epidemiological data. It should, however, be noted that further mechanistic studies are needed to test this hypothesis.
Taken together, our finding of an association between post-diagnostic PPI use and increased cancer mortality raises concerns about the growing use of PPIs in general, and their suggested use as a supplement to cancer therapy in particular [12]. Further studies addressing the association of PPI use and cancer mortality in study settings where confounding by indication can be minimized are urgently needed to clarify this issue. Meanwhile, clinicians should carefully consider indication and necessity when prescribing PPIs to cancer patients.
Supplementary Material
Figure 6.
Funding
This work was supported by grants from the European Research Council (ERC AdG 340751), the Danish National Research Foundation (DNRF125), the Danish Cancer Society (R90-A5783) and the Novo Nordisk Foundation (NNF15OC0016914) to M.J. The funding agencies played no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
Novelty and impact:
The observed association between post-diagnostic PPI use and increased cancer mortality among Danish cancer patients together with the PPI-induced acceleration of tumor growth in mouse models raises concerns about the growing use of PPIs. Further studies addressing the safety of PPI use among cancer patients are urgently needed to examine this connection in more detail.
Author contributions:
S.A.T. designed and performed the cell culture and animal experiments, analyzed the data and contributed to the writing of the manuscript. C.D. designed and performed the analysis of all registry-based data. K.Ø. provided important insight into the clinical practice and possible confounding factors. S.F. designed the epidemiological analyses, analyzed the data and contributed to the writing the manuscript. M.J. designed the overall study, acquired funding, analyzed the data and wrote the first draft of the manuscript. All authors contributed to the final text and approved it.
Conflict of interest disclosures:
The authors disclose no potential conflicts of interest.
Additional contributions:
We thank Lene Bregnholt Larsen, Helle Vestergaard Petersen, Lene Jørgensen, Theresa Brøndsted Sten, Lotte Svane Drifte and Christian Bang for technical assistance in the animal facility and Monika Mortensen for invaluable discussions.
References
- 1.Mullin JM, Gabello M, Murray LJ, et al. Proton pump inhibitors: actions and reactions. Drug Discov Today. 2009;14(13–14):647–60. doi: 10.1016/j.drudis.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Mazer-Amirshahi M, Mullins PM, van den Anker J, et al. Rising rates of proton pump inhibitor prescribing in US emergency departments. Am J Emerg Med. 2014;32(6):618–22. doi: 10.1016/j.ajem.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Maggio M, Corsonello A, Ceda GP, et al. Proton pump inhibitors and risk of 1-year mortality and rehospitalization in older patients discharged from acute care hospitals. JAMA Intern Med. 2013;173(7):518–23. doi: 10.1001/jamainternmed.2013.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ. 2008;336(7634):2–3. doi: 10.1136/bmj.39406.449456.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasina L, Nobili A, Tettamanti M, et al. Prevalence and appropriateness of drug prescriptions for peptic ulcer and gastro-esophageal reflux disease in a cohort of hospitalized elderly. Eur J Intern Med. 2011;22(2):205–10. doi: 10.1016/j.ejim.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Gomm W, von Holt K, Thome F, et al. Association of Proton Pump Inhibitors With Risk of Dementia: A Pharmacoepidemiological Claims Data Analysis. JAMA Neurol. 2016;73(4):410–6. doi: 10.1001/jamaneurol.2015.4791. [DOI] [PubMed] [Google Scholar]
- 7.Olbe L, Carlsson E, Lindberg P. A proton-pump inhibitor expedition: the case histories of omeprazole and esomeprazole. Nat Rev Drug Discov. 2003;2(2):132–9. doi: 10.1038/nrd1010. [DOI] [PubMed] [Google Scholar]
- 8.Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10(6):528–34. doi: 10.1007/s11894-008-0098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu W, Baker SS, Trinidad J, et al. Inhibition of lysosomal enzyme activities by proton pump inhibitors. J Gastroenterol. 2013;48(12):1343–52. doi: 10.1007/s00535-013-0774-5. [DOI] [PubMed] [Google Scholar]
- 10.Harguindey S, Arranz JL, Wahl ML, et al. Proton transport inhibitors as potentially selective anticancer drugs. Anticancer Res. 2009;29(6):2127–36. [PubMed] [Google Scholar]
- 11.Fais S, Venturi G, Gatenby B. Microenvironmental acidosis in carcinogenesis and metastases: new strategies in prevention and therapy. Cancer Metastasis Rev. 2014;33(4):1095–108. doi: 10.1007/s10555-014-9531-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fais S. Evidence-based support for the use of proton pump inhibitors in cancer therapy. J Transl Med. 2015;13:368. doi: 10.1186/s12967-015-0735-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calcinotto A, Filipazzi P, Grioni M, et al. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res. 2012;72(11):2746–56. doi: 10.1158/0008-5472.CAN-11-1272. [DOI] [PubMed] [Google Scholar]
- 14.Scaringi L, Cornacchione P, Ayroldi E, et al. Omeprazole induces apoptosis in jurkat cells. Int J Immunopathol Pharmacol. 2004;17(3):331–42. doi: 10.1177/039463200401700313. [DOI] [PubMed] [Google Scholar]
- 15.Luciani F, Spada M, De Milito A, et al. Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs. J Natl Cancer Inst. 2004;96(22):1702–13. doi: 10.1093/jnci/djh305. [DOI] [PubMed] [Google Scholar]
- 16.Yeo M, Kim DK, Kim YB, et al. Selective induction of apoptosis with proton pump inhibitor in gastric cancer cells. Clin Cancer Res. 2004;10(24):8687–96. doi: 10.1158/1078-0432.CCR-04-1065. [DOI] [PubMed] [Google Scholar]
- 17.Azzarito T, Venturi G, Cesolini A, et al. Lansoprazole induces sensitivity to suboptimal doses of paclitaxel in human melanoma. Cancer Lett. 2015;356(2 Pt B):697–703. doi: 10.1016/j.canlet.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Wang BY, Zhang J, Wang JL, et al. Intermittent high dose proton pump inhibitor enhances the antitumor effects of chemotherapy in metastatic breast cancer. J Exp Clin Cancer Res. 2015;34:85. doi: 10.1186/s13046-015-0194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Namazi MR, Jowkar F. A succinct review of the general and immunological pharmacologic effects of proton pump inhibitors. J Clin Pharm Ther. 2008;33(3):215–7. doi: 10.1111/j.1365-2710.2008.00907.x. [DOI] [PubMed] [Google Scholar]
- 20.Papagerakis S, Bellile E, Peterson LA, et al. Proton pump inhibitors and histamine 2 blockers are associated with improved overall survival in patients with head and neck squamous carcinoma. Cancer Prev Res (Phila) 2014;7(12):1258–69. doi: 10.1158/1940-6207.CAPR-14-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan W, Zhang W, Strasner A, et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470(7335):548–53. doi: 10.1038/nature09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thygesen LC, Daasnes C, Thaulow I, et al. Introduction to Danish (nationwide) registers on health and social issues: structure, access, legislation, and archiving. Scand J Public Health. 2011;39(7 Suppl):12–6. doi: 10.1177/1403494811399956. [DOI] [PubMed] [Google Scholar]
- 23.Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(7 Suppl):22–5. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 24.Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39(7 Suppl):42–5. doi: 10.1177/1403494810393562. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt M, Schmidt SA, Sandegaard JL, et al. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–90. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt M, Hallas J, Laursen M, et al. Data Resource Profile: Danish online drug use statistics (MEDSTAT) Int J Epidemiol. 2016;45(5):1401–1402g. doi: 10.1093/ije/dyw116. [DOI] [PubMed] [Google Scholar]
- 27.Helweg-Larsen K. The Danish Register of Causes of Death. Scand J Public Health. 2011;39(7 Suppl):26–9. doi: 10.1177/1403494811399958. [DOI] [PubMed] [Google Scholar]
- 28.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki E, Sun J, Kapoor V, et al. Gemcitabine has significant immunomodulatory activity in murine tumor models independent of its cytotoxic effects. Cancer Biol Ther. 2007;6(6):880–5. doi: 10.4161/cbt.6.6.4090. [DOI] [PubMed] [Google Scholar]
- 30.Casares N, Pequignot MO, Tesniere A, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202(12):1691–701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zitvogel L, Galluzzi L, Smyth MJ, et al. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39(1):74–88. doi: 10.1016/j.immuni.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Hofbauer R, Losert H, Gmeiner B, et al. Inhibitory effect of omeprazole on transmigration of leukocytes through endothelial cell monolayers and leukocyte adhesion. Microvasc Res. 2000;59(1):169–71. doi: 10.1006/mvre.1999.2196. [DOI] [PubMed] [Google Scholar]
- 33.Fisher L, Fisher A. Acid-Suppressive Therapy and Risk of Infections: Pros and Cons. Clin Drug Investig. 2017 doi: 10.1007/s40261-017-0519-y. [DOI] [PubMed] [Google Scholar]
- 34.Jäättelä M. Overexpression of hsp70 confers tumorigenicity to mouse fibrosarcoma cells. Int J Cancer. 1995;60:689–693. doi: 10.1002/ijc.2910600520. [DOI] [PubMed] [Google Scholar]
- 35.Mortara L, Orecchia P, Castellani P, et al. Schedule-dependent therapeutic efficacy of L19mTNF-alpha and melphalan combined with gemcitabine. Cancer Med. 2013;2(4):478–87. doi: 10.1002/cam4.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.