Abstract
Macroautophagy (autophagy) is a dynamic intracellular degradation pathway. Monitoring the flux through the autophagy pathway is experimentally challenging but obviously a prerequisite for the proper investigation of the process. Here, we present an indirect autophagy flux assay based on monitoring the degradation of an autophagosome-associated fusion protein Rluc-LC3 by luminescence detection. The method is suitable for screening purposes with a high number of parallel samples and can be used for measurements in cell lysates as well as in living cells. The Rluc-LC3 assay has proven useful for the identification of genes, miRNAs and small molecules that regulate of autophagy flux in mammalian cells.
Keywords: Autophagy, High throughput screening assay, LC3, Luciferace, Real time, Signaling
1. Introduction
1.1. The challenge of measuring autophagic flux
An ideal assay for autophagic flux would quantitatively and continuously track intracellular cargo from its sequestration into autophagosomes to its breakdown in autophagolysomes and release of corresponding breakdown products back to the cytosol. The perhaps closest candidate to this ideal is the classical long-lived protein degradation assay (Bauvy, Meijer, & Codogno, 2009). The assay indeed measures breakdown products; this is achieved by monitoring the release of radioactive isotopes to the media after labeling of long-lived proteins. The definitive assessment of the contribution of autophagy to the readout however relies on the usage of genetic or chemical inhibition of the pathway. Such usage of inhibitors is problematic because of specificity issues and because of potential crosstalk between autophagy and other degradation systems like the proteasomal system (Korolchuk, Menzies, & Rubinsztein, 2010; Mizushima, Yoshimori, & Levine, 2010). Another more practical limitation to the long-lived protein degradation assay is that it is relatively laborious and not well suited to capture the dynamics of autophagy and from a practical point of view, not easily applicable for high throughput analysis. The current experimental limitations in directly measuring bulk autophagic breakdown, combined with an increased understanding of autophagy, on the molecular level has prompted the development of simpler, more indirect, assays based on molecular markers. Among those, assays employing LC3 are the most widely used.
1.2. LC3 as a marker of autophagy
LC3 is a subfamily in the Atg8 family of ubiquitin-like proteins and is the only protein marker described to reliably bind the autophagosomal membrane and the phagophore (Klionsky et al., 2016). LC3 is synthesized as a pro-protein, which is rapidly cleaved leaving a C-terminal glycine. The LC3-I molecule thus formed is diffusely located in the cytoplasm but upon autophagy induction, LC3-I is converted to LC3-II by conjugation of phosphatidylethanolamine to the C-terminus. This lipid serves as an anchor that mediates tight association of LC3-II to the phagophore as well as to the inner and outer autophagosomal membranes. After fusion with lysosomes, LC3-II on the inner membrane is degraded whereas LC3-II on the outer membrane is delipidated and recycled (Tanida, Minematsu-Ikeguchi, Ueno, & Kominami, 2005).
Essentially all the described modifications of LC3 have been exploited in various assays to analyze autophagy (Klionsky et al., 2016). The translocation of LC3 from diffuse into puncta has thus become a standard indicator of autophagosome formation and can be visualized either by antibody staining or by fluorescent microscopy of fusion proteins like enhanced green fluorescent protein (EGFP)–LC3. Due to the dynamics of autophagy, increased puncta formation can however either be caused by increased autophagosome formation or by inhibition of downstream processing, a situation in which autophagy is actually inhibited. A more direct and often used indicator of flux is the degradation of LC3-II associated with the inner autophagosomal membrane; a phenomenon typically investigated by immunoblotting of the distinct LC3-II band. This procedure is however not as straight forward as it may sound. In addition to the usual challenges of normalizing signal to house keeping proteins and the limited dynamic range of LC3-immunoblots, LC3-II is subjected to similar dynamics as the autophagic puncta. To appreciate LC3-II degradation it is therefore often essential to block the turnover by including lysomal inhibitors in the assay. (Klionsky et al., 2016; Mizushima & Yoshimori, 2007; Tanida et al., 2005).
1.3. Cautions in using LC3-II turnover as a marker of autophagic flux
The most basic concern in using LC3-II degradation as a marker of flux is that LC3-II is not a cargo but instead a marker of the autophagic membrane. The exact role of LC3-II on the membrane is furthermore unclear. The correlation between LC3 turnover and flux was addressed in a recent paper; it was shown that it is possible to force an uncoupling of LC3-flux from flux of the cargo marker lactate dehydrogenase by the use of cycloheximide or LC3 siRNA (Szalai et al., 2015).
The quantitative relevance of such uncoupling, when LC3 levels are not artificially lowered, is unclear. But the observations emphasises the importance of corroborating LC3-II turnover data by independent means.
2. The Rluc-LC3 assay
2.1. Description of the assay
The Rluc-LC3 assay is centered on the Rluc-LC3 fusion protein; this protein combines the high dynamic range and sensitivity of the Renilla luciferase with the characteristic ability of LC3 to enter the lysosome in an autophagy dependent manner. Basically, the Rluc-LC3 assay requires two cell populations, one expressing Rluc-LC3wt and another expressing the mutant, Rluc-LC3G120A that cannot undergo lipidation and thus not bind the autophagosomal membranes. For a detailed validation of the Rluc-LC3 assay see (Farkas, Høyer-Hansen, & Jäättelä, 2009).
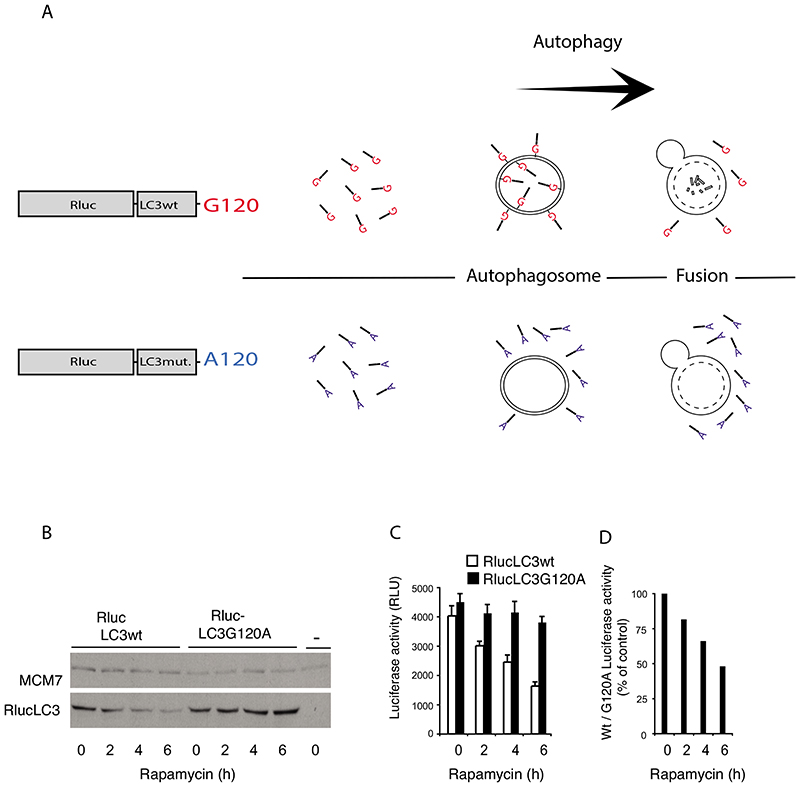
As illustrated in the diagram in fig. 1A and exemplified in the western blot shown fig. 1B, Rluc-LC3wt is degraded when autophagy is induced whereas the reference protein, Rluc-LC3G120A, is stable.
Figure 1. Rapamycin induces autophagy dependent decay of RlucLC3.
A. Illustration of the principle behind the RlucLC3 assay. In the control situation both RlucLC3wt (glycine in position 120 of LC3) and RlucLC3G120A are mainly present soluble in the cytoplasm. During autophagy RlucLC3wt binds both the inner and the outer membrane of the autophagosome whereas RlucLC3G120A stays soluble. After fusion with lysosomes, RlucLC3wt on the inner membrane is degraded.
B. Immunoblotting with antibodies against Renilla luciferase (Chemicon, Mab 4410) and MCM7 (internal control) in total extracts from MCF7 cells stably expressing Rluc-LC3wt or Rluc-LC3G120A and treated with 250 nM rapamycin for 0, 2, 4 or 6 h.
C. Rluc activity in extracts of MCF7 cells stably expressing Rluc-LC3wt or RlucLC3G120A and treated with 250 nM rapamycin for 0, 2, 4 or 6 h. The values represent mean +/- standard deviation of a single experiment performed in sextuplicate.
D. The ratios of the luciferase measurements in (C) were calculated at each time point and expressed as percentages of the ratio obtained from untreated cells. Figure 1 B, C and D are reproduced from Farkas et al 2009 with permission of the publisher.
This figure partially a reproduction of Figure 1 in our previous publication (Farkas et al., 2009) with a permission from the publisher.
The changes in Rluc-LC3wt/G120A levels are mirrored by corresponding changes in luminescence fig. 1C. The increased autophagic flux induced by rapamycin, in the example, is thus displayed as a decrease in the ratio of luciferase activities in extracts from the two cell populations comparing treated to untreated conditions. See figure 1D.
In the example the increased Rluc-LC3 turnover is directly visible as a drop in the Rluc-LC3wt levels. But such simplicity cannot always be assumed. Some treatments might induce changes unrelated to autophagic degradation in for example the rate of synthesis or in lipidation independent stability that affect the level of Rluc-LC3wt. Therefore, to considerably reduce such effects on the readout, it is essential to include the Rluc-LC3G120A data in the assay.
2.2. Applications of the Rluc-LC3 assay
Essentially the Rluc-LC3 assay functions as a sensitive, internally controlled alternative to the classical procedure of estimating changes in endogenous LC3 by western blotting. Notably, the assay, in addition, is readily applied to living cells, as well as extracts, in a microwell format. The Rluc-LC3 assay complements assays based on fluorescent-tagged LC3 such as EGFP-LC3. Such probes are optimized for microscopy analysis and are used to estimate the number of autophagosomes. They provide little information about the rate of autophagic processes. In support of a screen of siRNAs that induce EGFP-LC3 translocation the Rluc-LC3 assay was thus used to distinguish those hits that induced increased flux from those in which the increased translocation more likely was caused by other reasons such as for example reduced autophagosome turnover (Szyniarowski et al., 2011).
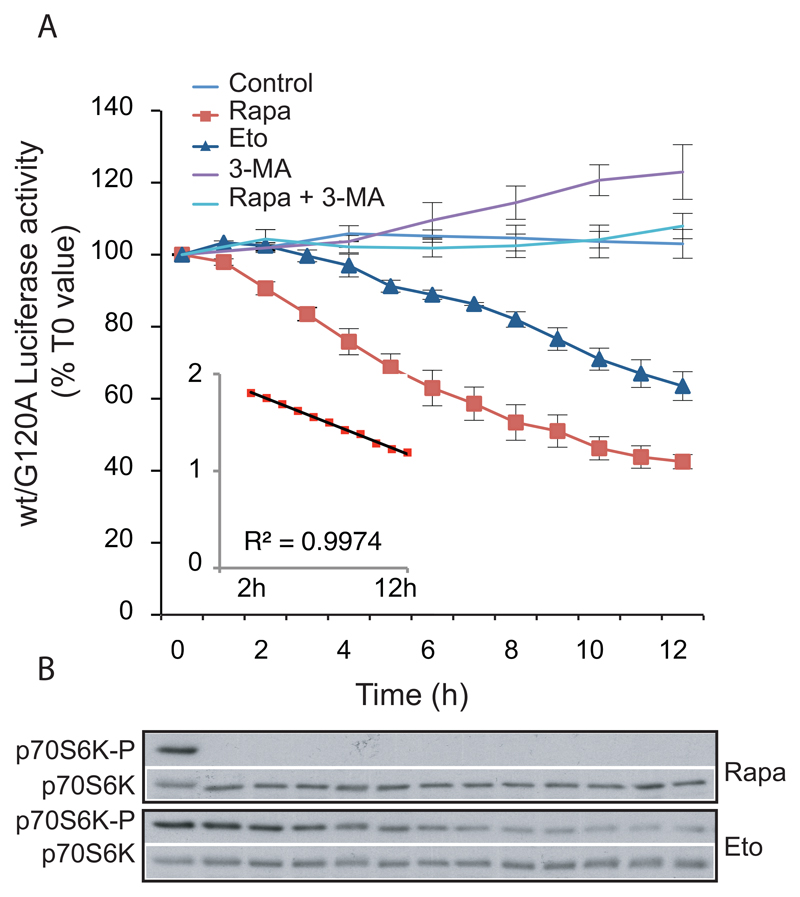
By adding luciferase substrate directly to the growth medium, the Rluc-LC3 assay offers the possibility of making multiple measurements on the same cells thus detecting the propagation of autophagy in real time. This type of analysis can provide valuable information about the molecular events and putative causalities underlying autophagy. An example of a real-time analysis is shown in fig. 2A. By comparing their time courses, the distinct effects of rapamycin and etoposide on Rluc-LC3 turnover are readily revealed. Rapamycin thus induces a fast response that persists with a nearly constant rate from 2h to 12h. Etoposide on the other hand induces a slower response that reaches a maximum rate of Rluc-LC3 turnover after approximately 8h. The distinct pattern of autophagy induction is paralleled by a distinct effect on phosphorylation of the mTorc1 substrate p7OS6K. The Rluc-LC3 assay is suitable for multiwell plate analysis and has been used to identify novel autophagy regulators in small-molecule and microRNA libraries (Farkas, Daugaard, & Jäättelä, 2011; Farkas et al., 2009; Frankel et al., 2014; Frankel et al., 2011).
Figure 2. Analysis of the kinetics of autophagic flux in living cells.
A. MCF-7 cells stably expressing RLuc-LC3wt or Rluc-LC3G120A were plated as described in 6.2 step 1 and incubated with 50 nM of the live cell luciferase substrate EnduRen™ for 2 h prior to addition of either control medium (n = 3) or drug treatments: 250 nM Rapamycin (Rapa; n = 3), 50 μM Etoposide (Eto; n = 3), 10 mM 3-MA (n = 3) or 250 nM Rapamycin + 10 mM 3-MA (Rapa + 3-MA; n = 2). Luciferase activity was measured with 1–2 h intervals as indicated. The values represent the mean ratio ± SEM of luciferase activities from the two cell lines expressed as percentages of the corresponding ratio in untreated cells at T0. The insert is a representation of the logarithm (base 10) of the rapamycin values after subtraction of 27%, as a function of time (from 2 to 12 hours). The trendline and the corresponding R2 were calculated with excel software. The value “27%” represents a hypothetical steady state of wt/G120A luciferase activity in the presence of rapamycin (established as the value giving a trendline with an R2 closest to 1).
B. Immunoblotting with antibodies against p70S6K-P (Cell Signaling, 9206) and p70S6K (Cell Signaling, 9202) of proteins from MCF-7 cells treated with rapamycin (250 nM) or etoposide (50 μM) as above.
The figure is a partial reproduction of Figure 4 in our previous publication (Farkas et al., 2009) with a permission from the publisher.
3. Designing the Rluc-LC3wt and Rluc-LC3G120A fusion proteins
The rat LC3B isoform with 22 amino acids downstream of Glycine 120 was used in Rluc-LC3. This isoform is also widely used as fusion partner in EGFP-LC3 to label autophagosomes (Kabeya et al., 2000). Among the several luciferases described in the scientific literature and in company catalogs, Rluc fulfills two criteria for being a suitable N-terminal fusion partner of LC3. First, protocols exist for assaying Rluc both in live-cells and in extracts. Second, the protein has a long half half-life; to elaborate on this, we use a variant of Rluc in Rluc-LC3 with a substitution (C124A) that increases the half-life of intracellular luciferase-activity from 14h to 52h (Loening, Fenn, Wu, & Gambhir, 2006). A high inherent resistance to inactivation of the luciferase moiety is preferable to support the notion that the instability observed of Rluc-LC3 is mainly dictated by LC3, and thus dependent of autophagy. When creating the lipidation free reference protein, Rluc-LC3G120A, it is important to keep in mind that an alanine substitution in codon 120 not only prevents lipidation but also proper cleavage of pro-LC3 ((Kabeya et al., 2000). Therefore, a stop codon next to codon 120 is also introduced in the expression construct.
4. Establishing Rluc-LC3wt and Rluc-LC3G120A expressing cells
4.1. We have mainly worked with MCF7-cells that express Rluc-LC3wt or Rluc-LC3G120A from stably transfected plasmids
The two expression plasmids pRluc-LC3wt and pRluc-LC3G120A are based on the CMV-promoter controlled pEGFP-Cl (Clontech) but with Rluc-LC3wt/G120A-open reading frames (ORFs) inserted instead of the EGFP ORF. We have also successfully used retrovirally transduced Hela-, U2OS- and MCF7-cells in which the Long Terminal Repeats of pBabehygro controls the expression (Farkas et al., 2011).
4.1.1. Stable transfection of MCF7 cells (Transfections are performed mainly according to the Fugene HD protocol (Promega)
Distribute cells into three 10 cm plates with 106 cells in each. Two plates are needed for transfection of either pRluc-LC3wt or pRluc-LC3G120A. One plate serves as control for G418 sensitivity. Grow cells in RPMI 1640+ GlutaMAX™ supplemented with 6% Fetal Calf Serum (FCS), penicillin 100U/ml and streptomycin 100 μg/ml.
After 40 h. Mix 5 μg pRluc-LC3wt or pRluc-LC3G120A with 250 μl DMEM (31966-021) vortex. Add 15 μl Fugene HD, vortex again. Incubate for 15 min at room temperature.
Add 250 μl of Fugene HD/DNA mixture to cells. Apply below surface of 10 ml freshly shifted growth medium. Shake the plate gently. To reduce toxicity of the transfection mix, the growth medium is shifted again after 12h.
Start antibiotic selection two days after transfection. Use 400 μg/ml G418.
Follow cell death microscopically. Change G418-medium at least every third day. Usually, after two to three weeks of selection cells are ready to be trypsinized, expanded in flasks and frozen according to standard procedures. We use freezing medium containing 10% dimethylsulfoxide (DMSO) +90% FCS.
The cells keep a stable expression of the fusion proteins for more than a month in culture and also keep responding to e.g. rapamycin. But it is advisable to take up fresh cells often to minimize drifting.
5. The Rluc-LC3 assay performed on cell lysates
5.1. Buffers and solutions
Basic buffer: 100 mM Tris-HCL pH 7.4, 300mM Na-Ascorbate. We prepare the basic reaction buffer by mixing 200 ml pH-adjusted Tris-HCL with 11.9 g of Na-Ascorbate.
Coelenterazine stock solution: Dissolve 1mg Coelenterazine (e.g. Synchem s053) in 950 μl acidified methanol (acidified methanol: 49 ml Methanol + 1 ml HCl 1M) to obtain a 2.5 mM stock solution. Keep at −20C.
Reaction buffer: 10 ml Basic buffer + 10 μl coelenterazine stock solution. If several plates are analyzed sequentially prepare fresh reaction buffer immediately before application to each plate.
Luciferase lysis buffer: Dilute 5X Passive lysis buffer (Promega E1941) in water to obtain a 1X solution.
5.2. Protocol
The protocol shown here is adjusted for MCF7-cells in a 96 wells plate format.
Plate Rluc-LC3wt- and Rluc-LC3G120A-cells in the uneven and even numbered wells respectively. Plating this way forms 48 pairs of neighboring wells, each pair establishing a unit inside which cells are treated identically and from which the Rluc-LC3wt/G120A-activity-ratio eventually is calculated. This plating strategy is intended to minimize putative location effects on the readout. We typically plate 8000 cells per well. But due to the high sensitivity of the luciferase assay it is feasible to plate less if needed.
Start the treatments the day after plating. Select a number of Rluc-LC3wt/G120A unit pairs to be used as untreated controls. Remaining pairs can be used for various treatments for example drug testing or starvation. If starvation conditions are tested, it is important to rinse wells twice in starvation medium before final application of starvation medium.
At the end of treatment, remove medium from the wells. Add 40 μl of Luciferase lysis buffer to each well. Freeze plate at −80.
Thaw plate on ice. Mix by vortexing, 2500 rounds per minute for 20 s.
Transfer 6 μl of extract to corresponding wells of a white half volume 96 wells dish.
Add 80 μl of freshly prepared reaction buffer.
Immediately after addition of reaction buffer measure luciferase activity in a plate reader set at room temperature.
6. The Rluc-LC3 assay performed on live cells
6.1. Buffers and solutions
EnduRen™ stock solution: Dissolve 0.34 mg EnduRen™ Live Cell Substrate (Promega E6481) in 6 ml DMSO to obtain a 100 μM stock solution. Heat to 37°C for 10 min and vortex to ensure complete resuspension. We make aliquotes of 100 μl each and store them at −80°C.
Cell growth medium: RPMI-1640 without phenol red (Cat. 11835, Gibco) supplemented with Glutamax™, 6 % FCS, penicillin 100 U/ml and streptomycin 100 μg/ml.
6.2. Protocol
Like above, the protocol is for MCF7-cells in a 96 wells plate format. Here it is described how to apply the method for drug analysis.
Plate cells 10000 cells in 120 μl cell growth medium per well in white cell culture plates (Nunc 136101). Using the same plating strategy as described in step 1 in 5.2. Continue to step 2 the next day.
Take out an aliquot of EnduRen™ stock solution from the −80 freezer. Incubate at 37°C for 10 minutes, vortex briefly. Make a 100 nM EnduRen™ solution in cell growth medium equilibrated to 37°C considering 10 ml per plate. Continue immediately to step 3.
Remove 60 μl of growth medium from each well of the cell culture plate. Add 60 μl of the 100 nM EnduRen™ solution made in step 2. The final concentration of EnduRen™ on cells is consequently 50 nM. Return plate to the cell incubator for a 2h equilibration of the live cell substrate.
Read luminescence in a plate reader (we use a Varioskan Flash) preset at 37°C. Use this initial measurement to calculate the T0 values of the Rluc-LC3wt/G120A activity ratio for each pair of wells. These values can for simplicity be defined to 100%.
After measuring the T0 values, drugs are applied. We dilute drugs to a 5X working concentration in a parallel 96 wells plate, using cell growth medium with 50 nM EnduRen™ as diluent. 30 μl of 5X drug solution is then transferred to the corresponding wells of the cell culture plate.
The luminescence is measured at appropriate intervals, for example once per hour. For each measurement, the Rluc-LC3wt/G120A activity ratio of each pair of wells is calculated and related to the corresponding T0 values. The activity can be measured for at least 12 h.
6.3. Notes
In the initial testing of the live cell substrate EnduRen™ we used 60 μM as suggested by the manufacturer. However, as described in (Farkas et al., 2009), this concentration inhibits the turnover of Rluc-LC3 according to the assay. To eliminate this undesirable effect, we instead use 50 nM final concentration of the live cell substrate. This lower concentration of substrate, in turn, reduces the signal. In some cases, therefore, a cell-line specific optimization of expression vector might be beneficial for consistent detection in the live-cell setting.
Usually the Rluc-LC3 assay shows good correspondence between data obtained in live cells and in lysates. We did however observe a discrepancy using the drug Triciribine (Farkas et al., 2011). This Protein kinase B/Akt inhibitor is known to induce autophagy (Evangelisti et al., 2011). And it does induce flux according to the Rluc-LC3 assay performed in lysates, but not in the live-cell setting. We do not know the reason for this difference, but we notice that the live-cell assay is more complex and possibly influenced by for example the uptake/conversion of EnduRen™ and the intracellular localization of the luciferase and therefore, likely, more prone to generating artifacts. It suggests that it is advisable to make an initial comparison of new drugs in both settings, to avoid this putative artifact of the live-cell assay.
Acknowledgements
This work was supported by a Center of Excellence grant from the Danish National Research Foundation and an Advanced Grant from the European Research Council (LYSOSOME; 340751).
References
- Bauvy C, Meijer AJ, Codogno P. Assaying of autophagic protein degradation. Methods Enzymol. 2009;452:47–61. doi: 10.1016/S0076-6879(08)03604-5. [DOI] [PubMed] [Google Scholar]
- Evangelisti C, Ricci F, Tazzari P, Chiarini F, Battistelli M, Falcieri E, et al. Martelli AM. Preclinical testing of the Akt inhibitor triciribine in T-cell acute lymphoblastic leukemia. J Cell Physiol. 2011;226(3):822–831. doi: 10.1002/jcp.22407. [DOI] [PubMed] [Google Scholar]
- Farkas T, Daugaard M, Jäättelä M. Identification of small molecule inhibitors of phosphatidylinositol 3-kinase and autophagy. J Biol Chem. 2011;286(45):38904–38912. doi: 10.1074/jbc.M111.269134. doi:M111.269134 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas T, Høyer-Hansen M, Jäättelä M. Identification of novel autophagy regulators by a luciferase-based assay for the kinetics of autophagic flux. Autophagy. 2009;5(7):1018–1025. doi: 10.4161/auto.5.7.9443. doi:9443 [pii] [DOI] [PubMed] [Google Scholar]
- Frankel LB, Di Malta C, Wen J, Eskelinen EL, Ballabio A, Lund AH. A non-conserved miRNA regulates lysosomal function and impacts on a human lysosomal storage disorder. Nat Commun. 2014;5:5840. doi: 10.1038/ncomms6840. [DOI] [PubMed] [Google Scholar]
- Frankel LB, Wen J, Lees M, Høyer-Hansen M, Farkas T, Krogh A, et al. Lund AH. microRNA-101 is a potent inhibitor of autophagy. EMBO J. 2011;30(22):4628–4641. doi: 10.1038/emboj.2011.331. doi:emboj2011331 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19(21):5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, et al. Zughaier SM. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12(1):1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korolchuk VI, Menzies FM, Rubinsztein DC. Mechanisms of cross-talk between the ubiquitin-proteasome and autophagy-lysosome systems. FEBS Lett. 2010;584(7):1393–1398. doi: 10.1016/j.febslet.2009.12.047. [DOI] [PubMed] [Google Scholar]
- Loening AM, Fenn TD, Wu AM, Gambhir SS. Consensus guided mutagenesis of Renilla luciferase yields enhanced stability and light output. Protein Eng Des Sel. 2006;19(9):391–400. doi: 10.1093/protein/gzl023. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3(6):542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140(3):313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalai P, Hagen LK, Saetre F, Luhr M, Sponheim M, Overbye A, et al. Engedal N. Autophagic bulk sequestration of cytosolic cargo is independent of LC3, but requires GABARAPs. Exp Cell Res. 2015;333(1):21–38. doi: 10.1016/j.yexcr.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Szyniarowski P, Corcelle-Termeau E, Farkas T, Høyer-Hansen M, Nylandsted J, Kallunki T, Jäättelä M. A comprehensive siRNA screen for kinases that suppress macroautophagy in optimal growth conditions. Autophagy. 2011;7(8):892–903. doi: 10.4161/auto.7.8.15770. doi:15770 [pii] [DOI] [PubMed] [Google Scholar]
- Tanida I, Minematsu-Ikeguchi N, Ueno T, Kominami E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy. 2005;1(2):84–91. doi: 10.4161/auto.1.2.1697. [DOI] [PubMed] [Google Scholar]