Abstract
Purpose
At diagnosis, colorectal cancer presents with synchronous peritoneal metastasis in up to 10% of patients. The peritoneum is poorly characterized with respect to its super-specialised microenvironment. Our aim was to describe the differences between peritoneal metastases and their matched primary tumours excised simultaneously at the time of surgery. Also, we tested the hypothesis of these differences being present in primary colorectal tumours and having prognostic capacity.
Experimental design
We report a comprehensive analysis of thirty samples from peritoneal metastasis with their matched colorectal cancer primaries obtained during cytoreductive surgery. We tested and validated the prognostic value of our findings in a pooled series of 660 colorectal cancer primary samples with overall survival (OS) information and 743 samples with disease free survival (DFS) information from publicly available databases.
Results
We identified 20 genes dysregulated in peritoneal metastasis that promote an early increasing role of ‘stemness’ in conjunction with tumour favourable inflammatory changes. When adjusted for age, gender and stage, the 20-gene peritoneal signature proved to have prognostic value for both OS (adjusted-hazard ratio (HR) for the high-risk group (vs low-risk) 2.32 (95% confidence interval (CI) 1.69-3.19; p-value < 0.0001)) and for DFS (adjusted-HR 2.08 (95%CI 1.50-2.91; p-value < 0.0001)).
Conclusions
Our findings indicated that the activation of “stemness” pathways and adaptation to the peritoneal specific environment are key to early stages of peritoneal carcinomatosis. The in-silico analysis suggested that this 20-gene peritoneal signature may hold prognostic information with potential for development of new precision medicine strategies in this setting.
Keywords: colorectal cancer, early peritoneal metastases, stemness, digital spatial profiling, inflammation
Introduction
In 2018, colorectal cancer (CRC) was the third leading cause of cancer mortality according to the World Health Organization GLOBOCAN database. Peritoneal metastases are found in 5 to 10% of colorectal cancer patients at primary surgery, in 4-19% of patients during follow-up after curative surgery, and in 40-80% of patients who die of colorectal cancer (1, 2). Patients with colorectal cancer peritoneal metastases (CRPMs) have significantly shorter median overall survival (OS) (16.3 months) compared with those with liver (19.1 months) or lung (24.6 months) metastases (3).
Recently communicated data from the PRODIGE-7 study comparing Cytoreductive Surgery (CRS) plus Hyperthermic Intraoperative Peritoneal Chemotherapy (HIPEC) or CRS alone in addition to systemic chemotherapy, showed a median OS of 41.2 months (95% CI 35.1-49.7) in the CRS (non-HIPEC) arm and 41.7 months (95% CI: 36.2-52.8) in the CRS+HIPEC arm (4). These findings underscored the fact that CRPMs (M1c CRC in the 8th edition of the American Joint Committee on Cancer TNM classification (5)) in the absence of organ-based metastases could describe a different patient population with a different molecular biology of the disease.
A stepwise model for the development of CRPMs has been proposed (6). Firstly, tumour cells detach from the primary tumour and gain access to the peritoneal cavity. Secondly, these free tumour cells need to acquire the capacity to adhere to the peritoneal surface. Thirdly, they need to penetrate the mesothelial monolayer using the underlying connective tissue as a scaffold for tumour proliferation (6).
The genomic characterisation of CRPMs in a study of 2563 mCRC patients with known BRAF mutation status indicated an association between BRAF status and CRPMs: the primary tumour BRAF mutation rate was 18% in patients with CRPM only and 12% in multi-organ including CRPMs (3). In a more recent report, no significant differences were found for KRAS, BRAF or microsatellite instability when a non-matched pool of primaries (n=617) were compared to CRPMs (n=348) with a similar rate of BRAF mutant in CRPM of about 12% (7).
To date, published data regarding the concordance of genomic alterations between CRPM and their matched primaries is scarce. In a meta-analysis to explore the concordance between primary CRCs and their metastases regarding the main genes used in clinical decision making (e.g. KRAS, NRAS and BRAF), we have demonstrated an overall discordance of 8% (95% CI: 5-10%) (8). However, data on synchronous peritoneal metastases are lacking.
This study presents a comprehensive analysis of thirty matched primary and peritoneal samples from colorectal cancer patients who presented with synchronous CRPMs and underwent CRS and HIPEC. The initial hypothesis was that there would be no significant differences regarding the genomic alterations used to guide clinical management. However, a more profound analyses of gene expression was undertaken to identify potential mechanisms of synchronous CRPM development. Furthermore, we test the possibility of deriving a signature from these differences with prognostic capacity.
Methods
Archival tissue samples
Paired samples (n=30) from 15 consecutive patients with peritoneal only disease that underwent CRS/HIPEC for CRPM (2002-2017) with the primary in situ were identified at a UK national peritoneal tumour centre. The study was approved by the Manchester Cancer Research Centre (MCRC) BioBank institutional review board and ran under ethics approval of the South Manchester Research Ethics Committee (Reference: 18/NW/0092). Written consent was obtained for all patients that were alive at the time of the study and a specific approval from the Health Research Authority (HRA) and Health and Care Research Wales (HCRW) Research Ethics committee was also obtained for the use of samples from deceased patients that were not able to provide written consent before the study initiation (Reference: 19/LO/1339). All clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki and good clinical practice guidelines.
Publicly available data used for validation of findings
Data from 660 colorectal cancer primary samples with overall survival (OS) information and 743 samples with disease free survival (DFS) information from publicly available series (9–12) with expression data for colorectal cancer were selected.
DNA sequencing
The genomic DNA was extracted from 4 FFPE 5μm unstained sections mounted on slides using the Roche cobas® DNA Sample Preparation Kit. Polymerase chain reaction (PCR) based target enrichment was performed. Following this, purification of PCR was done using AMPure XP beads from Agencourt and library preparation followed the custom protocol using Illumina TruSeq PCR Free indexes and reagents for indexing. Purified libraries were sequenced using Illumina sequencing by synthesis chemistry on a MiSeq using 2 x 150bp v2 sequencing chemistry. Following demultiplexing, primer trimming and alignment to the human reference genome variants were called using a clinically validated custom algorithm. All samples have a coverage of >350x. List of genes sequenced in supplementary table S1.
RNA assay
Four 5μm FFPE sections mounted on unstained slides were marked up by a pathologist prior to macro-dissection and then deparaffinised using Qiagen deparaffination solution. Following RNA extraction using the RNeasy FFPE Kit from Qiagen RNA abundance was measured using a Qubit fluorometer. The analysis was done using the NanoString nCounter® mRNA Gene Expression system.
The nCounter® PanCancer IO 360™ Panel includes 750 genes covering key pathways at the interface of the tumour, tumour microenvironment, and immune response; in addition, there are internal reference genes for data normalization and 30 customed probes were added to the nCounter™ CodeSet, all of them shown in supplementary table S2.
Digital spatial profiling (DSP)
A tissue microarray (TMA) containing 0.6 mm cores from primaries and peritoneal metastases was designed. Each sample was represented by 3 cores, active margin (AT), tumour core (TC) and ‘normal’ (surrounding tissue) core. For the purpose of this report, only AT and TC cores were analysed with the GeoMx® DSP system.
The Human Immune Cell Profiling Core Nanostring’s DSP technology combines standard immunofluorescence techniques with digital optical barcoding technology to perform highly multiplexed, spatially resolved profiling experiments.
An automated setup capable of imaging and sample collection was developed by modifying a standard microscope. For protein detection, a multiplexed cocktail of 56 primary antibodies, each with a unique, UV photocleavable indexing oligo, and 3 fluorescent markers (pan-cytokeratin antibody, CD45 antibody, CD68 antibody and nucleic acid staining with Syto 13) was applied to a slide-mounted FFPE tissue section of the TMA.
The regions of interest (ROIs) were then selected based on the fluorescence information and sequentially processed by the microscope automation. Once all ROIs were processed, indexing oligos were hybridized to NanoString optical barcodes for ex situ digital counting and subsequently analysed with an nCounter® Analysis System.
Data analyses and statistics
The plots related to the genomic data were performed using R with GenVisR (13). The expression heatmap and volcanos plots were drawn using the nCounter® Advanced Analysis Software 2.0. Volcano plots display each gene’s -log10(p-value) and log2 fold change with the selected covariate. Horizontal lines indicate various Benjamini-Yekutieli False Discovery Rate (FDR) adjusted p-value thresholds. Highly statistically significant genes fall at the top of the plot above the horizontal lines, and highly differentially expressed genes fall to either side. Genes are coloured if the resulting p-value is below the given FDR. The 40 most statistically significant genes are labelled in each the plot. The loglinear model is applied to perform a multivariable analysis to adjust the gene expression of the hits found when comparing CRPMs versus their matched primaries to relevant clinical variables. The heatmap for the 20 probes with the lowest adjusted p-value was drawn by using the normalised data of gene expression from the previous analysis and then use the Morpheus software (https://software.broadinstitute.org/morpheus). The CMS for each sample was assigned by using the CMSclassifier R package (14). The canonical pathway, the network and functional analyses were developed with the Qiagen Ingenuity Pathway Analysis (IPA). The non-parametric Mann-Whitney U test was used for the DSP expression analysis with IBM SPSS Statistics for Windows, version 19 (IBM Corp., Armonk, N.Y., USA). All the violin plots are developed with R package ggplot2. Benjamini-Hochberg FDR was used to correct for multiple comparisons. Kaplan Meier curves from The Cancer Genome Atlas (TCGA) data are drawn using StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP.
Transcripts in supplementary tables S3 and S4 present the most statistically significantly differentially expressed genes with the selected covariates. ‘Estimated log fold-change’ estimates a gene’s differential expression. For categorical covariates, a gene is estimated to have 2^(log fold change) times its expression in baseline samples, holding all other variables in the analysis constant. If the covariate is continuous, for each unit increase in the selected covariate, a gene’s expression is estimated to increase by 2^(log fold change)-fold, holding all other variables in the analysis constant. The 95% confidence interval for the log fold change is also presented, along with a p-value and FDR. Method column indicates the model used to estimate differential expression. Mixed negative binomial model uses the mle function to run the Wald test. The simplified negative binomial model uses the glm.nb function. The loglinear model uses the lm function. The loglinear model is applied to perform a multivariable analysis to adjust the gene expression of the hits found when comparing Peritoneal metastases versus their matched primaries. Plots for differential expression of each of the 20 lower adjusted p-value probes were performed using GraphPad Prism version 8 for Windows, GraphPad Software, La Jolla California USA.
Prognostic analysis of the 20-gene signature
Data related to all components of the 20-gene signature expression and survival data was downloaded from TCGA (9) and GEO databases GSE17536 (10), GSE17537 (11) and GSE14333 (12). A cox-regression model including all the 20 genes was built and applied to evaluate OS and DFS. The relative hazard ratio for each case was calculated and cases were classified within each database as high or low risk according to their relative risk being above or below the median relative risk. Then, all the cases with information for OS and DFS were pooled together and the Kaplan-Meier analysis and log-rank test was performed using the survminer R package. A multivariable Cox regression analysis was performed adjusting for age, gender and AJCC stage. Cox proportional hazards assumptions of proportionality were checked and verified using the Schoenfeld residual test in each database and in the overall pooled data, using StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP.
For a complete description of methodology, please, see the online supplementary methods section.
Results
Discovery phase
Initial comparison of primary samples and matched CRPMs
Baseline characteristics are summarised in Table S5. Median follow up was 25.3 months (IQR:23.3-37.7) with an estimated median OS of 32 months (95% CI:20.6 - 43.7). Two patients (13.3%) were mismatched-repair deficient (MMRd), one case (6.6%) was BRAF mutated and the mucinous phenotype was reported for 7 patients (46.7%).
We observed 100% concordance between primary and peritoneal tumours for gene mutations in KRAS, NRAS, BRAF, MSH2 and MSH6 (Figure S1).
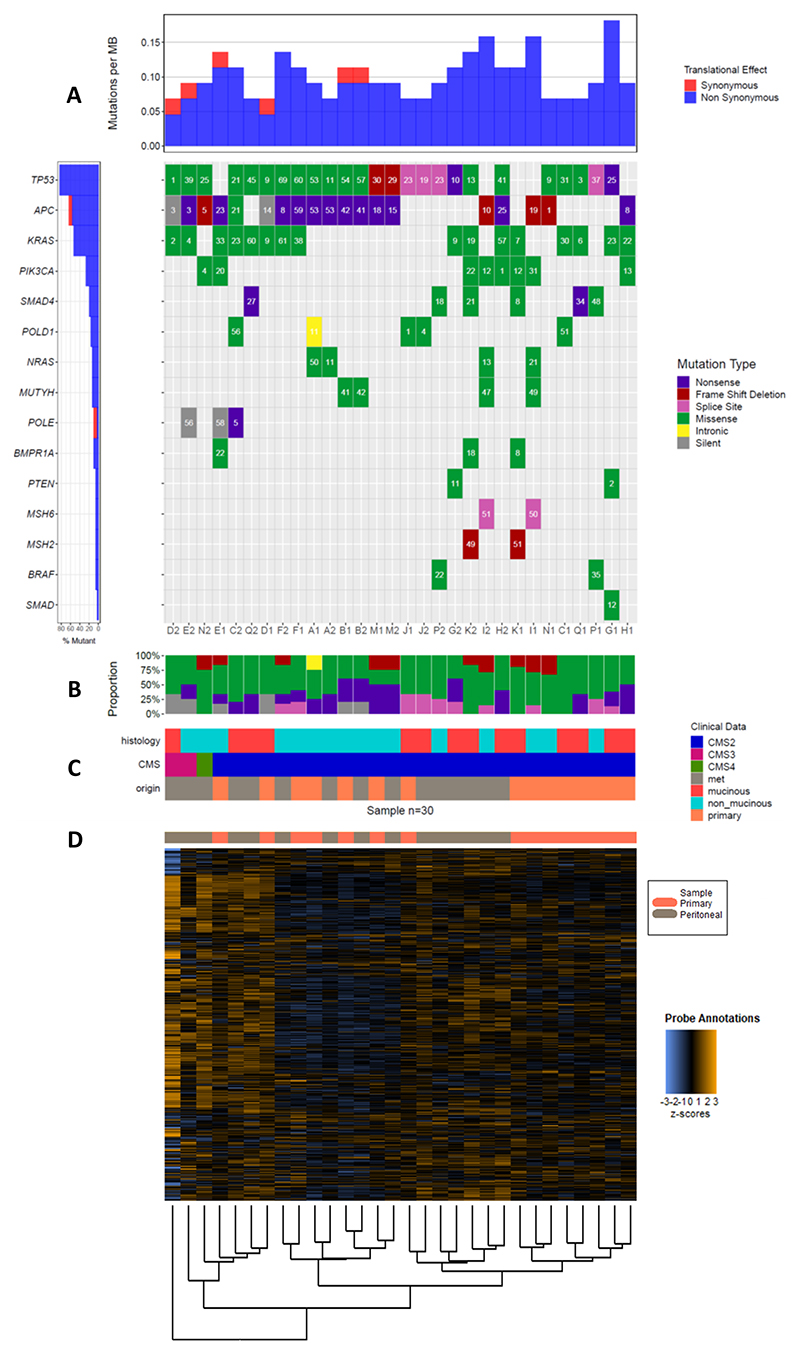
A total of 134 gene variants were identified with a distribution across common drivers of colorectal cancer showing, mutated TP53 in 27.6%, APC in 26.1% and KRAS in 11.9% of the total of variants detected. Median overall VAF was 22%, ranging from 1 to 75% (Figure 1A). The percentages plot in Figure 1A account for gene mutations divided by the total of samples (n=30).
Figure 1. Oncoplot for gene mutations and heatmap with unbiassed hierarchical clustering of the mRNA probes.
1A: The gene mutation analyses displayed as a waterfall plot (commonly called an oncoplot when applied to cancer samples) was done with GenVisR (13). It displays the mutation occurrence and type in the main panel while showing the mutation burden and the percentage of samples with a mutation in the top and side sub-plots. The mutation burden on top is calculated directly from the input via the formula: mutations in sample/coverage space⋆1000000. The coverage space defaults to the size in base pairs of the “SeqCap EZ Human Exome Library v2.0”. This calculation is only meant to be a rough estimate as actual coverage space can vary from sample to sample. 1B: shows the proportion of mutations observed in the cohort. 1C contains the histology mucinous vs non-mucinous, consensus molecular subtypes (CMS) and origin of each sample primary tumour or peritoneal metastases. CMS are derived from mRNA expression to group samples in to 4 possible different categories namely, CMS1 (immune), CMS2 (canonical), CMS3 (metabolic), and CMS4 (mesenchymal). 1D, the samples are organised and aligned to the mRNA heatmap for all the probes used in the Nanostring nCounter® assay listed in the supplementary material with the unbiassed hierarchical clustering at the bottom of the figure.
Initially, discordance was defined as the presence of a given mutation in just one of the paired samples. When all the genes in our NGS panel were considered, a discordance of 53.3% was identified (8 of 15 cases). Expanding our discordance definition to classify any VAF difference > 30% for a given mutation as discordant resulted in all but 2 cases being discordant (B and M) (86.7%) (Figure 1A & B). These data are consistent with the evolutionary landscape of colorectal cancer (15).
To explore for any global discordance between primary and CRPMs, using transcriptomic data, we determined the consensus molecular subtypes (CMS) for each sample (14). All the primary and 12 peritoneal samples were classified as CMS2 (canonical), two of the peritoneal samples were classified as CMS3 (metabolic) and one as CMS4 (mesenchymal) giving an overall concordance of 80% for CMS subtypes as shown in Figure 1C.
The analysis using our 800-probe customised PanCancerIO360° Nanostring panel revealed more profound differences at the mRNA level between primary and matched CRPM samples. In Figure 1D samples are displayed after unsupervised hierarchical clustering with the ‘oncoplot’ of mutational analyses shown above in 1A. Only 4 pairs clustered together, A, B, M and J. Eight primaries (53.3%) clustered together but separately from their corresponding peritoneal samples.
Differential gene expression (DGE) between paired samples
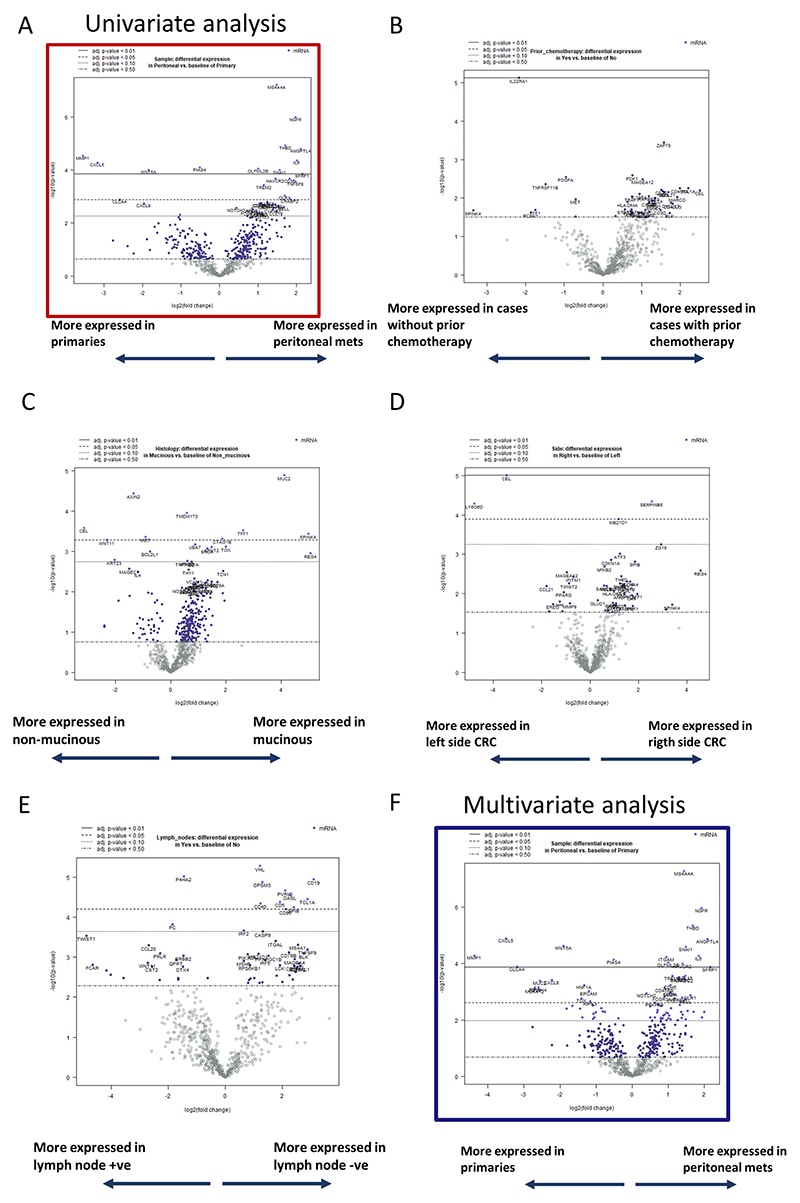
To better understand the differences between primaries and metastases, we used volcano plots representing adjusted p-values of the DGE analyses (Figure 2 & Figure S2). After FDR correction, we focused our downstream analyses on 20 genes with an mRNA differential expression significant at an adjusted p-value of 0.05.
Figure 2. Volcano plots for the differential gene expression (DGE) analysis.
All Figures display different lines with different FDR adjusted p-values as per their legend on top of each volcano plot. Continuous line represents the threshold for an adjusted (adj.) p-value of < 0.01; dashed line for and adj. p-value of < 0.05; dotted line for an adj. p-value of < 0.1; mix dashed and dotted line for an adj. p-value of < 0.5. Blue dots identified probes with an adjusted p-value below 0.50. 2A shows unadjusted DGE based on origin (peritoneal versus primary). 2B represents DGE based on receiving prior chemotherapy. 2C shows DGE based on mucinous histology versus non-mucinous. 2D same analyses for sidedness. 2E similar for lymph node presence. 2F is a similar analysis of 2A but corrected by all the previous studied characteristics in 2B to 2E.
Figure 2A, represents the volcano plot of the 800 probes DGE by their origin (peritoneal metastases vs primary tumour). Figures 2B-E show the DGE by baseline characteristics of interest due to their potential impact on skewing the primary analysis (peritoneal metastases vs primary tumour). including administration of prior chemotherapy (Figure 2B), mucinous histology (Figure 2C), side of primary tumour (Figure 2D) and lymph node spread (Figure 2E). In Figure S2, we analysed the transcriptomic data based on common genomic alterations. Samples were divided by mutant TP53, APC and KRAS alone or a combination including at least one of them. No targets at FDR p-value < 0.05 were found. Thus, we tested for the impact of the clinical parameters on our analyses by performing a multivariable analysis adjusted to the above-mentioned clinical factors (Figure 2F).
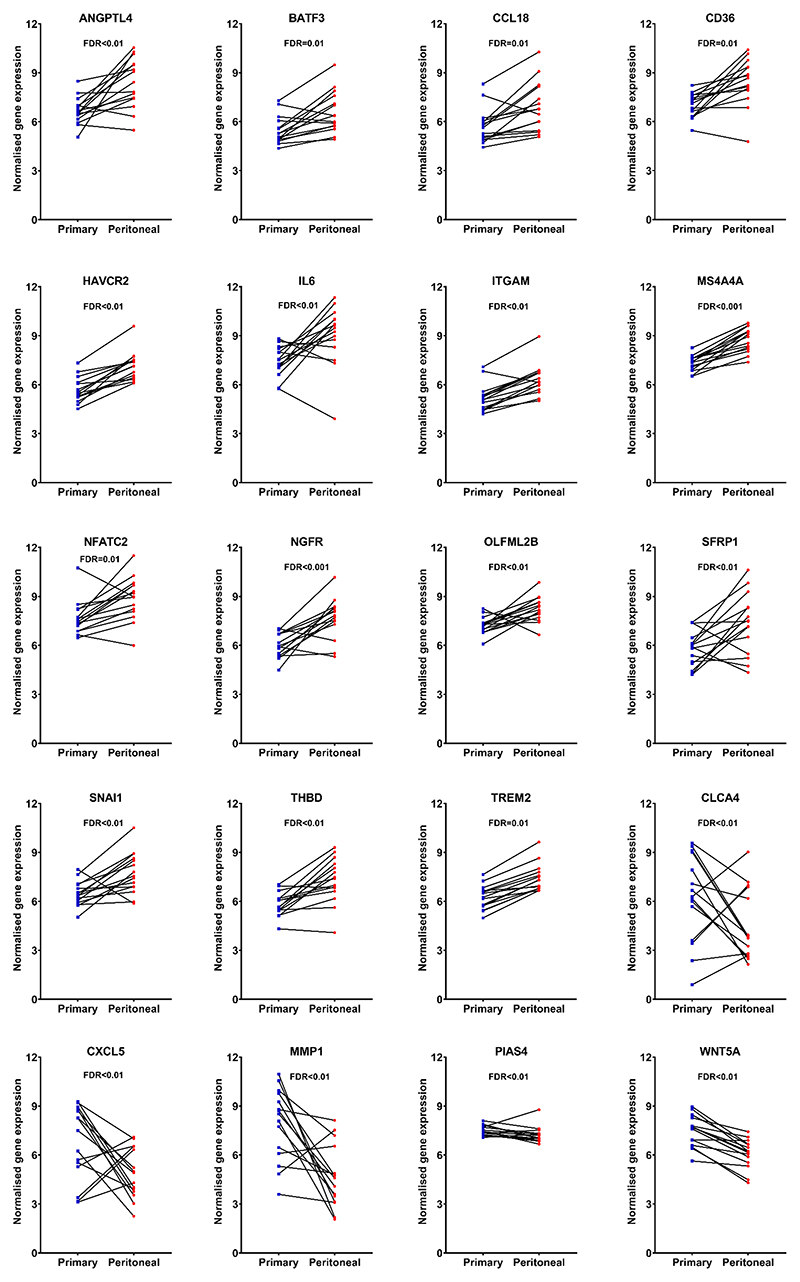
Figure 3 shows the plots of paired analysis of these 20 genes to show the trend of the differential expression for each one of them using Student’s t-test.
Figure 3. Comparison of gene expression in paired primary and peritoneal samples for the 20 transcripts with highest adjusted p-value using Student’s t-test.
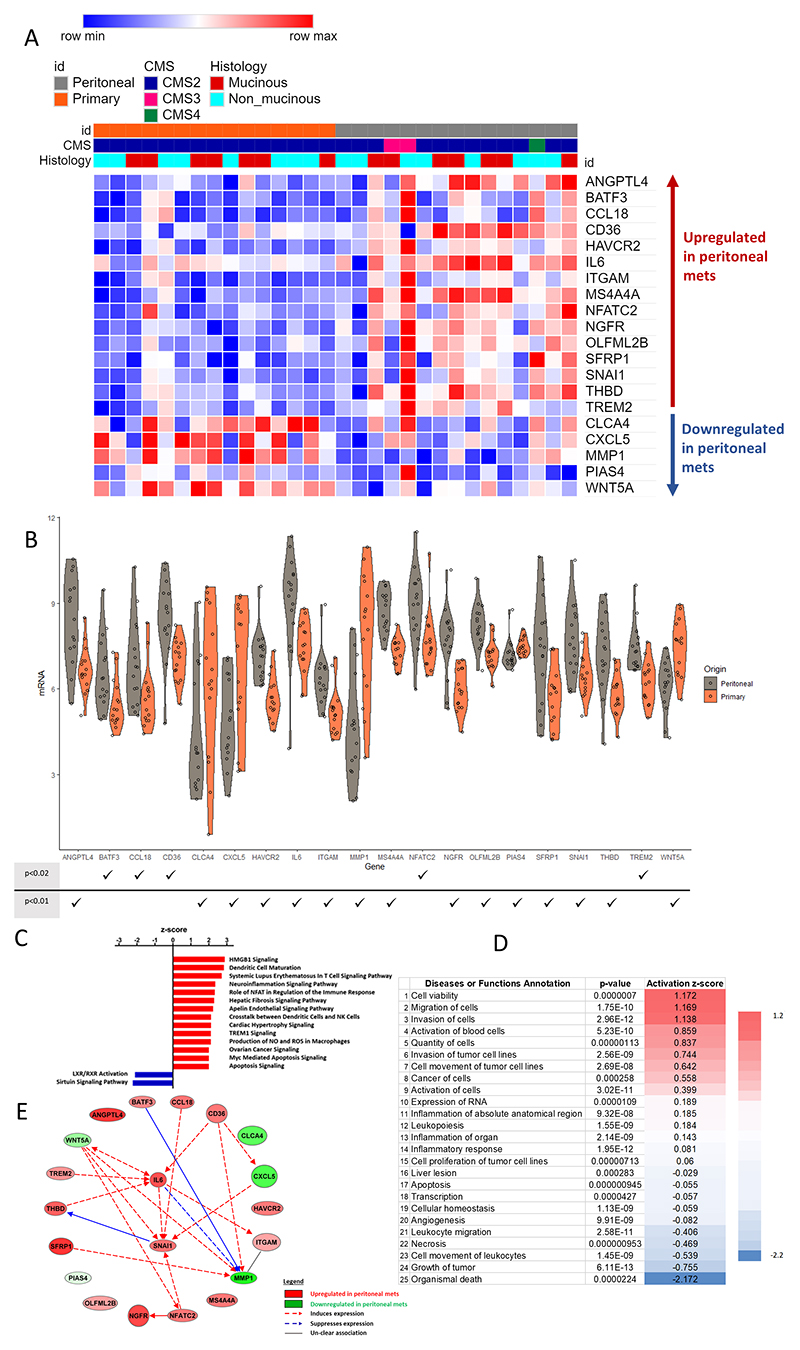
Figure 4A shows the 20-gene signature derived from our DGE analysis between CRPMs and paired primary tumours. We found 15 mRNA probes overexpressed in CRPMs and 5 mRNA probes overexpressed in primary tumours. Twenty genes with the lower FDR adjusted p-value regarding differential expression between peritoneal metastases versus primaries are shown in Figure 4B.
Figure 4. Twenty gene peritoneal signature.
In A, a heatmap for the 20 genes derived from Figure 2F analyses is displayed clustered by origin primary (orange) and peritoneal metastases (grey). In B, the violin plots show the differential expression of each gene between peritoneal (grey) and primary (orange) samples. Below there is a table with p-values for these differences ranked as ≤ 0.02 or ≤ 0.01 (a full list of FDR adjusted p values is provided in table S2). In C, the canonical pathway analysis is displayed. We use Ingenuity Pathway Analysis (IPA) that attributes an activation z-score that assesses the randomness of directionality within a gene set to infer the activation state of a pathway as either activated or inhibited. A negative z-score indicates inhibition and a positive z-score indicates activation, with scores equal to or greater than 2 or equal to or less than −2 being statistically significant (P<0.05), all the about pathways are significant. D shows the functional analyses for the 20 genes using IPA, displaying the top-25 pathways. E is the network map generated using the IPA software for the 20 genes. ANGPTL4, Angiopoietin Like 4; BAFT3, Basic Leucine Zipper ATF-Like Transcription Factor 3; CCL18, C-C Motif Chemokine Ligand 18; CD36, cluster of differentiation 36; HAVCR2, Hepatitis A Virus Cellular Receptor 2; IL6, interleukin 6; ITGAM, Integrin Subunit Alpha M; MS4A4A, Membrane Spanning 4-Domains A4A; NFATC2, Nuclear Factor Of Activated T Cells 2; NGFR, Nerve Growth Factor Receptor; OLFML2B, Olfactomedin Like 2B; SFRP1, Secreted Frizzled Related Protein 1; SNAI1, Snail Family Transcriptional Repressor 1;THBD, thombospondin; TREM2, Triggering Receptor Expressed On Myeloid Cells 2; CLCA4, Chloride Channel Accessory 4; CXCL5, C-X-C Motif Chemokine Ligand 5; MMP1, Matrix Metallopeptidase 1; PIAS4, Protein Inhibitor Of Activated STAT 4; WNT5A, Wnt Family Member 5A.
Understanding differential pathway activation between primaries and CRPMs
A canonical pathway analysis using all the genes identified with a raw p-value ≤ 0.05 (Figure 4C) showed activation of dendritic cell maturation and neuro-inflammation signalling pathways. Functional analysis of the 20 hits described in our CRPM signature is shown in Figure 4D. This demonstrates that compared to paired primary tumours, CRPMs have higher metastatic and invasive potential (rows 2, 3, 6 and 7, Figure 4D), reduced cell death (rows 1 and 22, Figure 4D) and reduced leukocyte and myeloid migration (rows 21 and 23, Figure 4D). Furthermore, network analysis of these 20 genes (Figure 4E) reveals not only the central role of IL-6 in CRPMs but also a relevant axis linked to neuro-inflammation including NGFR, NFATC2 and NCAM.
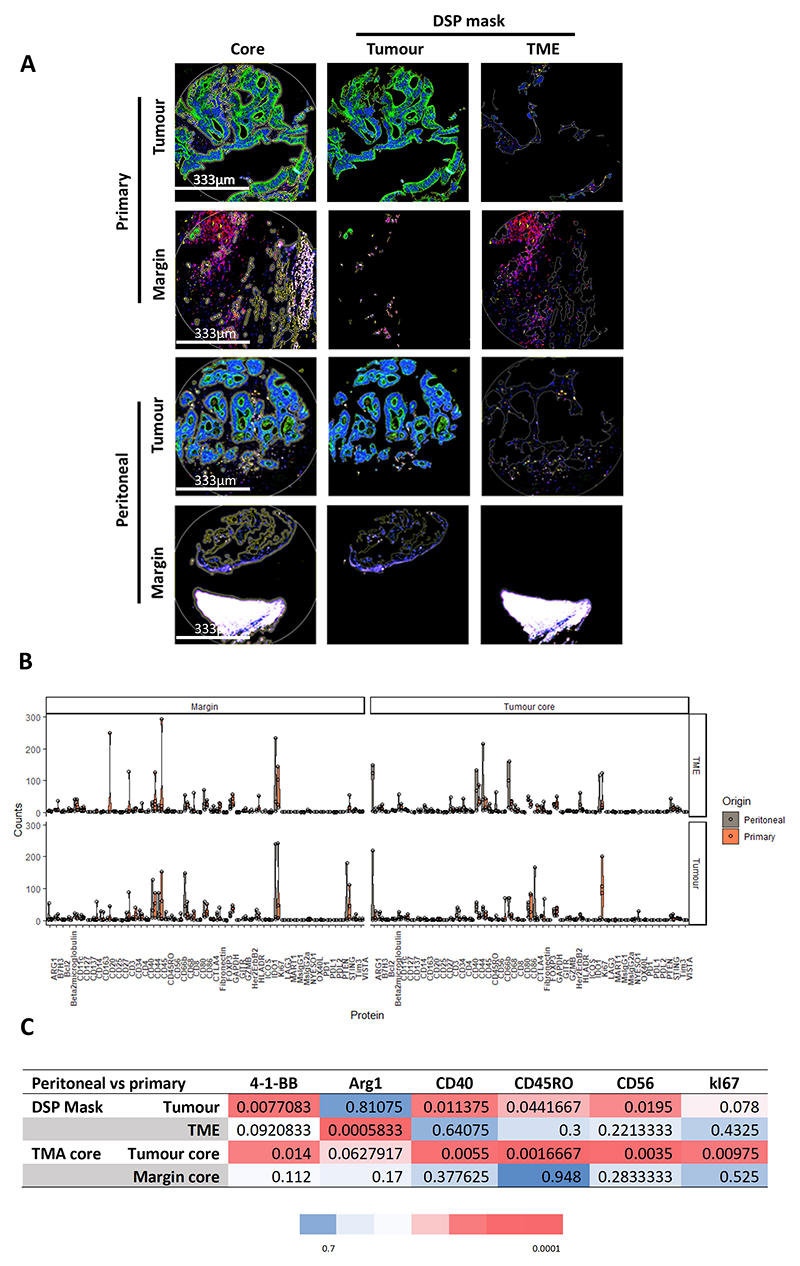
To assess the distribution of different immune related proteins and use a different technology, we used a novel digital spatial profiling (DSP) technology applied to the TMA with 4 paired cases. Each case was represented by 4 cores (CRPM vs primary; CT vs AM). The immunofluorescence images in Figure 5A show an example of all the cores for a single case. We were able to analyse all the cores separately based on their pan-cytokeratin antibody staining (in green), i.e. that for each core we have expression of the different antibodies for tumour enriched areas (or masks) and tumour-microenvironment (TME) enriched areas (full list of antibodies in supplementary table S6). The plot of Figure 5B shows multiple violin plots of the different protein expression profiles in the different areas (CT vs AM cores and TME vs tumour enriched areas). DSP normalised data are shown in supplementary table S7. In Figure 5C, the p-values of the significant differentially expressed proteins are displayed after FDR set at 0.15 (raw and corrected p-values are displayed in supplementary table S8). The DSP findings show a differential expression of Arginase 1 (Arg1) in the TME mask favouring over-expression in CRPMs. This finding agrees with IL-6 being the most relevant pathway in CRPMs linking to an increase in type-2 tumour associated macrophages (TAM2), as Arg1 is a marker of this particular cell type.
Figure 5. Digital spatial profiling.
In A, first there are 12 immunofluorescences images, the first column shows the immunofluorescence staining for the cores without separating tumour microenvironment (TME) from tumour using a pan-cytokeratin antibody (green), CD45 antibody (red), CD68 antibody (yellow) and nucleic acid staining with Syto 13 (blue). The second column shows the TMA cores enriched for tumour cells and the third one the TMA cores enriched for TME components. In the last row, the white area is an artefact of the TMA. The pictures are taken with a complementary metal oxide semiconductor (CMOS) camera at 20x. Below in B, multiple violin plots are displayed for the most relevant protein targets. In C, a table with all the FDR adjusted p-values for the significant targets are displayed for comparisons between CRPMs and primaries the red colour code is correlated with a small corrected p-value and in blue the highest values (n=8). The non-parametric test Mann-Whitney U was used for the DSP expression analysis. Benjamini-Hochberg FDR was used to correct for multiple comparisons. Violin plots are drawn with R package ggplot2. 41BB/CD137, T-Cell Antigen 4-1BB Homolog; ARG1, arginase 1; B7H3, B7 Homolog 3; BCL2, B-Cell CLL/Lymphoma 2; CD, cluster designation; CTLA4, Cytotoxic T-Lymphocyte Associated Protein 4; FOXP3, Forkhead Box P3; GAPDH, Glyceraldehyde-3-Phosphate Dehydrogenase; GITR, Glucocorticoid-Induced TNFR-Related Protein; GZMB, Granzyme B; HER2/ERBB2, Erb-B2 Receptor Tyrosine Kinase 2; HLADR, Major Histocompatibility Complex, Class II, DR; ICOS, Inducible T Cell Costimulator; IDO1, Indoleamine 2,3-Dioxygenase 1; NYESO, Cancer/Testis Antigen 1B; OX40L, Tumor Necrosis Factor Ligand Superfamily Member 4; PD1, Programmed Cell Death 1; PDL2, Programmed Cell Death 1 Ligand 2; PTEN, Phosphatase And Tensin Homolog; STING, Stimulator Of Interferon Response CGAMP Interactor 1; TIM3, T Cell Immunoglobulin Mucin 3; VISTA, V-Set Immunoregulatory Receptor.
External retrospective validation. Prognostic value of the peritoneal signature
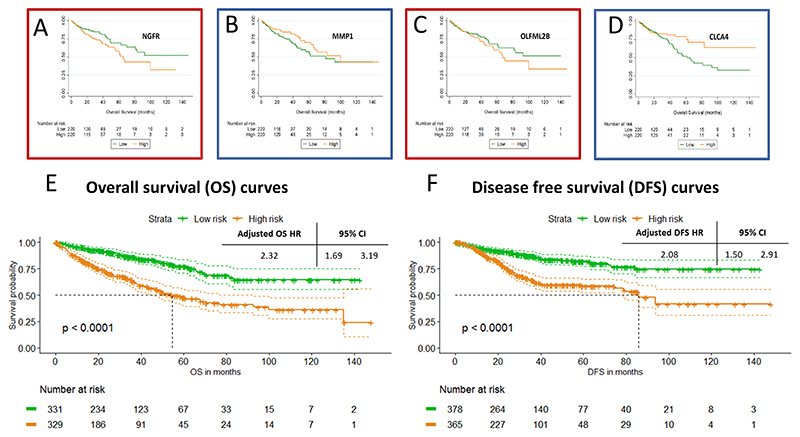
Due to the lack of large series with well characterised transcriptomic data of CRPMs, to better understand the possible clinical utility of our gene signature, we analysed the prognostic capacity of each gene using TCGA database in Figures 6 and S3. In Figure 6A-D, we can see Kaplan-Meier curves for OS dividing the TCGA cohort (n=440) by the median expression of 4 representative genes from the panel including Nerve Growth Factor Receptor (NGFR) (p=0.021, figure 6A).
Figure 6. Survival curves for single genes and 20-gene signature.
Panels A, B, C and D show Kaplan Meier curves for OS for the genes NGFR (p=0.021), MMP1 (p=0.06), OLFML2B (p=0.281) and CLCA4 (p=0.043) respectively. P-values are obtained by log-rank test. E and F show Kaplan Meier curves for the overall 20-gene signature for pooled overall survival (n=660) and disease-free survival (n=743), respectively. Hazard ratios displayed are related to the multivariate analysis of the pool analysis adjusted by age, gender and stage.
To further explore the clinical implication of our results, we hypothesised that our gene-signature, associated with synchronous CRPMs, could be an early event present in primary lesions with detrimental prognosis impact. Initially, we tested the 20-gene model on the TCGA data (9) for OS showing a non-adjusted HR: 4.44 (95%CI 2.70-7.30, p-value <0.0001). We used the online tool PROGgeneV2 (16) with the aim of finding publicly accessible databases with survival and gene expression data for the 20-gene signature. Thus, we selected the colon adenocarcinoma data from GSE17536 (10), GSE17537 (11) and GSE14333 (12). We pooled together the data from all databases for OS (n=660) and for DFS (n=743) analysis (the distribution of cases and events are displayed in supplementary figure S4). Figures 6E-F show the Kaplan-Meier curves for the high-risk and low-risk groups related to OS and DFS. Both median OS and DFS were shorter in patients with high-risk (54.6 months (95%CI 44.3-76.6) and 85.8 months (95%CI 74.6-not calculated), respectively) compared to low-risk group, not reached in both settings. The HR adjusted for age, stage and gender of the high versus low-risk groups was 2.32 (95%CI 1.69-3.19; p-value <0.0001) for OS and 2.08 (95%CI 1.50-2.91; p-value <0.0001) for DFS.
Discussion
The molecular complexity and the difficulty in accessing peritoneal samples has made research in this area challenging. In this communication, we report an in-depth characterisation of synchronous CRPMs when compared with their matched primary colorectal tumours.
Stein et al recently reported, in an unmatched series comparing primaries versus CRPMs, the genes involved in clinical decisions (RAS family and BRAF) showed similar mutational rates in primaries and CRPMs (7). Similarly, our data supports the prediction that for validated and robust biomarkers used in the clinical setting for colon cancer, analysis of either the primary or peritoneal samples will suffice. In agreement with our findings (BRAF: 6.6%), Stein et al also detected a lower than expected BRAF mutation rate of approximately 10%. However, we were able to show a larger number of genomic discrepancies between primary and CRPMs using an expanded NGS panel. Counter-intuitively, the pattern of discrepancies was not related to the mucinous subtype or the CMS categories. Our samples were predominantly classified as CMS2 which was a relatively unexpected finding in view of a recent Dutch series which reported CMS4 in 60% of the primaries and 75% of the CRPMs (17). Nevertheless, APC mutations are known to drive CMS2 and CMS3. The discrepancy with the Dutch study could be partially explained because the authors looked for CMS4 with a specific assay and selecting tumour samples with a minimal threshold of 10% of tumour content. However, our study used macro-dissection to enrich the tumour content. Heterogeneity could, therefore, account for the difference between the two studies. Using a limited panel to define CMS categories could have affected the accuracy of the CMSclassifier. However, other groups have proven that this can be done with even smaller gene panels (18, 19).
The more diverse transcriptomic landscape (Figure 1D) was translated into a 20-gene peritoneal signature. Our peritoneal metastasis signature revealed expected suspects as drivers of metastases in cancer, such us, CD36 (20) and IL-6 (21). Inflammation, metabolic, migration and invasion pathways represent the pathways related to our 20-gene profile. Even though, IL-6 sat at the centre of the network analyses, one novel finding relates to neuro-inflammation and neuronal stemness pathways being highly expressed in CRPMs. Nevertheless, primary tumour gene expression is represented by genes involved in extracellular matrix remodelling, e.g. MMP1 and CLCA4. Although the pathways and genes represented in the signature are well known, this is to our knowledge the first time that they have been presented together as a signature.
The strong IL-6 signal in our data is associated with an increase of TAM2 markers that was observed in the DSP experiment. Mainly, a significant overexpression of Arg1 in the TME of the CRPMs when compared to their matched primaries. In Figure S5, cell subtyping analysis of the transcriptomic data also showed a clear enrichment in all microenvironment cells in CRPM, including macrophages. This clear dominance of macrophage inflammation signals has also been shown experimentally in models of ovarian cancer peritoneal metastasis, in which, ablation of macrophages impacted tumour growth (22). Interestingly, our canonical pathway analyses showed High Mobility Group Box 1 gene (HMGB1) pathway as being the one with highest Z-score when comparing the metastases with primaries. HMGB1 is a prototypic damage-associated molecular pattern (DAMP) /alarmin known to be released by colon cancer cells. This pathway is involved in macrophage infiltration, tumour growth and vascularization (23). Our findings support the hypothesis that involvement of these pathways could be common to other malignancies with a tendency to colonise the peritoneum.
We also found a significant over-expression of CD56 (NCAM) as marker of Natural Killer (NK) cells that was enriched in the tumour core areas of CRPMs (p=0.028). However, the experimental ablation of NK cells in Robinson-Smith’s model seemed to lack a similar degree of tumour growth control when compared to macrophage ablation (22), potentially, ascribing a minor role to NKs compared to macrophages.
CD36, also known as Collagen Type I Receptor or Thrombospondin Receptor, links lipid metabolism with invasiveness and metastatic potential (20). Therefore, it was not unexpected that thrombospondin (THB) was also upregulated in CRPMs. Furthermore, this axis has been reported to confer a survival disadvantage in colon cancer (24).
Nerve growth factor receptor (NGFR, also known as CD271, TNFSFR16 or P75NTR) is a 75 kD single-transmembrane protein without kinase activity and widely expressed in the central and peripheral nervous system (25). Overexpression of NGFR observed in many metastatic cancers promotes tumour migration and invasion in relation to cancer stem cells (26). In addition, it is linked to the IL-6 inflammatory pathway through the upregulation of neurotrophins (27). However, there is conflicting data regarding colorectal cancer, NGFR was initially considered a possible tumour suppressor but recent data showed that NGFR could act as an oncogenic inhibitor of p53 (28). Our NGFR results were validated in the TCGA database (Figure 6) showing that a high expression of NGFR was linked to shorter survival, supporting the hypothesis of this pathway being linked to survival advantage of peritoneal metastatic cells. NGFR findings are a likely link between inflammation and ‘stemness’ due to its relevant role in both pathways (29).
Our findings have implications for the management of CRPMs in relation to the use of immune modulatory agents suggesting a potential role for drugs targeting the IL6 axis, well described ‘stemness’ pathways (e.g. CD36) and less studied ones (e.g. NGFR). Tocilizumab is a recombinant humanized anti-interleukin-6 receptor (IL-6R) monoclonal antibody (30) that could have a role in the management of CRPMs. The attempt to develop anti-CD36 antibodies for metastatic disease has not been successful yet but the axis CD36/THB remains a good target to treat metastasis (31).
Our study suggests a new model for CRPM development (Figure S6). We propose that in early stages of CRPMs, a predominant pattern of chronic inflammation represented by a tumour microenvironment rich in TAM2 with upregulation of IL-6 and rewiring of signal pathways linked to plasticity, ‘stemness’ and metabolism, such as, NGFR and CD36, is observed. In particular, our results showed a shift in expression from components of the Wnt canonical pathway (such as, Wnt5a) (32) and genes related to SUMOylation (33) (process critical for cancer stem cells), such as PIAS4, that are enriched in the primaries, to CD36/THB axis and genes involved in tumour related mesenchymal stem cells, such as, OLFML2B (34) and SNAI1 (35), master regulator of epithelial/mesenchymal transition in CRPMs.
The study has several limitations. First is its small sample size in relation to the paired samples that constituted the discovery set of the study. Despite this, our study represents the largest sample size of its kind analysed to this extent in the literature to date. There was only one rectal case, therefore, no specific remarks could be derived. Second is that our study was not powered to assess phylogenesis and clonal evolution. Our reported VAFs are not bioinformatically corrected for cellularity due to the use of a rather small NGS targeted panel. Tumour mutational burden (TMB) was not calculated due to the use of a small targeted approach. However, an approximation to TMB is shown in Figure 1A. Third is a panel of probes with a specific focus on cancer-related immune and inflammation pathways that could bias the results towards this particular angle. Fourth is the use of FFPE tissue with well-known difficulties to obtain good quality RNA. To overcome this, we selected a panel of technologies with proved robustness in this setting. Nevertheless, our study design could not asses if the inflammatory changes were present in the distant normal tissue. To reduce the impact of a small sample size of our study, we performed an extensive in-silico study of our 20-gene signature in different databases to build a validation set including colorectal cancer transcriptomic data from databases that included assays for all the components of the signature. We also performed a multivariate analysis, although, limited to age, stage and grade because these were the only features common to all 743 cases analysed. This approach indicated to us that the activation of pathways included in the signature might have occurred at a relatively early stage of tumorigenesis. It also showed that our peritoneal-derived gene signature can identify subgroups of patients with worse prognosis and at higher risk of relapsing from samples taken at the initial diagnosis. Setting aside the prognostic use of our peritoneal signature, our results identified NGFR as a new emerging target for CRPM management.
In summary, we present data relating to synchronous CRPM showing early chronic inflammation events in conjunction with rewiring of ‘stemness’ pathways with potential for the development of new treatment strategies. We also derived a peritoneal 20-gene signature with prognostic capacity in non-metastatic colorectal cancer from the study of synchronous peritoneal metastases that could be a good candidate for prospective validation.
Supplementary Material
Statement of translational relevance.
At diagnosis, colorectal cancer presents with synchronous peritoneal metastasis in up to 10% of patients. Despite being a common site of metastasis, peritoneal carcinomatosis is an area in need for novel research. Our study demonstrates that peritoneal metastases acquire a complex and early adaptation, thereby gaining the ability to seed and survive in the super-specialised peritoneal environment by turning it into a favourable inflammatory ‘soil’ for colon cancer cells to grow.
A peritoneal 20-gene signature was derived from the transcriptomic differences between synchronous peritoneal metastases and their matched primaries comprising representatives from inflammation and cancer stem cell pathway. The study reports that this peritoneal signature has prognostic value for patients with colorectal cancer when applied to risk analysis of primary tumours.
The peritoneal signature could be used for a precision medicine approach in patients with synchronous peritoneal metastases. Furthermore, some of the genes included in the signature could be considered drug targets and have available drugs for them.
Acknowledgements & grant support
The results published here are in part based upon data generated by the TCGA Research Network: https://www.cancer.gov/tcga. DGE is supported by the National Institute for Health Research (NIHR) BRC Manchester (Grant Reference Number 1215-200074). RTN is supported by Cancer Research UK Accelarator Award (C64263 / A29365). JB, SO an OA receive funding from the Cancer Research UK Accelarator Award (C64263 / A29365). JB is partially funded by The Christie Charity.
Authors would like to thank the Manchester Cancer Research Centre (MCRC) BioBank, Histology of the CRUK Manchester Institute and NanoString for their support.
Footnotes
Conflicts of interest disclosure statement:
JB reports grants from Cancer Research UK and grants from The Christie Charity during the conduct of the study; grants, personal fees, and non-financial support from Ipsen, personal fees and non-financial support from Pfizer, non-financial support from AAA, personal fees and non-financial support from Novartis, non-financial support from Nanostring, non-financial support from Roche, grants and personal fees from Servier, and personal fees from Nutricia outside the submitted work. AJW personal fees from Roche and personal fees from Astra Zeneca outside the submitted work. CD reports grants and personal fees from AstraZeneca, grants from Astex Pharmaceuticals, grants from Bioven, grants from Amgen, grants from Carrick Therapeutics, grants and personal fees from Merck AG, grants from Taiho Oncology, grants from GSK, grants from Bayer, grants from Boehringer Ingelheim, grants from Roche, grants from BMS, grants from Novartis, grants from Celgene, grants from Epigene Therapeutics Inc, grants from Angle PLC, grants from Menarini, other from Clearbridge Biomedics, and other from Thermo Fisher Scientific outside the submitted work. DGE reports personal fees from Astrazeneca and personal fees from Springworks during the conduct of the study. MPS reports reports personal fees from Servier, personal fees from Merck, and personal fees from Amgen outside the submitted work. The remaining authors declare no conflicts of interest.
Author contributions
Design: JB; DGE; MPS; STO; OA. Data-acquisition: JB; RTN; SB; BC; HS; GB; LF; AJW. Analysis/interpretation: JB; RTN; SB; BC; HS; GB; LF; EK; AJW; MB; CD; DGE; RB; MPS; STO; OA. Drafting: JB; RTN; STO; OA. Manuscript-revision: JB; RTN; SB; BC; HS; GB; LF; EK; AJW; MB; CD; DGE; RB; MPS; STO; OA. Statistics: JB; RTN. Obtained funding: JB; MB; RB; MPS; STO; OA. Technical/material support: JB; RTN; BC; HS; GB; LF; EK; AJW. Supervision: JB; MB; CD; DGE; RB; MPS; STO; OA.
Data availability
Sequencing data are available in Sequence Read Archive (SRA) database through the Bioproject accession number: PRJNA675983
Nanostring nCounter® expression data are available in Gene Expression Omnibus (GEO) database with accession number GSE161097
DSP data are available as supplementary table S7.
Bibliography
- 1.Koppe MJ, Boerman OC, Oyen WJ, Bleichrodt RP. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg. 2006;243(2):212–22. doi: 10.1097/01.sla.0000197702.46394.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glehen O, Osinsky D, Beaujard AC, Gilly FN. Natural history of peritoneal carcinomatosis from nongynecologic malignancies. Surgical oncology clinics of North America. 2003;12(3):729–39. doi: 10.1016/s1055-3207(03)00044-9. xiii. [DOI] [PubMed] [Google Scholar]
- 3.Franko J, Shi Q, Meyers JP, Maughan TS, Adams RA, Seymour MT, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016;17(12):1709–19. doi: 10.1016/S1470-2045(16)30500-9. [DOI] [PubMed] [Google Scholar]
- 4.Quenet F, Elias D, Roca L, Goere D, Ghouti L, Pocard M, et al. A UNICANCER phase III trial of hyperthermic intra-peritoneal chemotherapy (HIPEC) for colorectal peritoneal carcinomatosis (PC): PRODIGE 7. Journal of Clinical Oncology. 2018;36(18_suppl):LBA3503-LBA [Google Scholar]
- 5.Amin MB, American Joint Committee on Cancer., American Cancer Society . AJCC cancer staging manual. Eight. xvii. Chicago IL: American Joint Committee on Cancer, Springer; 2017. 1024. Mahul B. Amin, MD, FCAP; editors, Stephen B. Edge, MD, FACS and 16 others; Donna M. Gress, RHIT, CTR - Technical editor; Laura R. Meyer, CAPM - Managing editor. [Google Scholar]
- 6.Jayne D. Molecular biology of peritoneal carcinomatosis. Cancer treatment and research. 2007;134:21–33. doi: 10.1007/978-0-387-48993-3_2. [DOI] [PubMed] [Google Scholar]
- 7.Stein MK, Williard FW, Xiu J, Tsao MW, Martin MG, Deschner BW, et al. Comprehensive tumor profiling reveals unique molecular differences between peritoneal metastases and primary colorectal adenocarcinoma. J Surg Oncol. 2020 doi: 10.1002/jso.25899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhullar DS, Barriuso J, Mullamitha S, Saunders MP, O’Dwyer ST, Aziz O. Biomarker concordance between primary colorectal cancer and its metastases. EBioMedicine. 2019;40:363–74. doi: 10.1016/j.ebiom.2019.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muzny DM, Bainbridge MN, Chang K, Dinh HH, Drummond JA, Fowler G, et al. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–7. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith JJ, Deane NG, Wu F, Merchant NB, Zhang B, Jiang A, et al. Experimentally derived metastasis gene expression profile predicts recurrence and death in patients with colon cancer. Gastroenterology. 2010;138(3):958–68. doi: 10.1053/j.gastro.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freeman TJ, Smith JJ, Chen X, Washington MK, Roland JT, Means AL, et al. Smad4-mediated signaling inhibits intestinal neoplasia by inhibiting expression of β-catenin. Gastroenterology. 2012;142(3):562–71.:e2. doi: 10.1053/j.gastro.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jorissen RN, Gibbs P, Christie M, Prakash S, Lipton L, Desai J, et al. Metastasis-Associated Gene Expression Changes Predict Poor Outcomes in Patients with Dukes Stage B and C Colorectal Cancer. Clin Cancer Res. 2009;15(24):7642–51. doi: 10.1158/1078-0432.CCR-09-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skidmore ZL, Wagner AH, Lesurf R, Campbell KM, Kunisaki J, Griffith OL, et al. GenVisR: Genomic Visualizations in R. Bioinformatics. 2016;32(19):3012–4. doi: 10.1093/bioinformatics/btw325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guinney J, Dienstmann R, Wang X, de Reynies A, Schlicker A, Soneson C, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21(11):1350–6. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cross W, Kovac M, Mustonen V, Temko D, Davis H, Baker AM, et al. The evolutionary landscape of colorectal tumorigenesis. Nature ecology & evolution. 2018;2(10):1661–72. doi: 10.1038/s41559-018-0642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goswami CP, Nakshatri H. PROGgeneV2: enhancements on the existing database. BMC Cancer. 2014;14:970. doi: 10.1186/1471-2407-14-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ubink I, van Eden WJ, Snaebjornsson P, Kok NFM, van Kuik J, van Grevenstein WMU, et al. Histopathological and molecular classification of colorectal cancer and corresponding peritoneal metastases. Br J Surg. 2018;105(2):e204–e11. doi: 10.1002/bjs.10788. [DOI] [PubMed] [Google Scholar]
- 18.Ragulan C, Eason K, Fontana E, Nyamundanda G, Tarazona N, Patil Y, et al. Analytical Validation of Multiplex Biomarker Assay to Stratify Colorectal Cancer into Molecular Subtypes. Sci Rep. 2019;9(1):7665. doi: 10.1038/s41598-019-43492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piskol R, Huw L, Sergin I, Kljin C, Modrusan Z, Kim D, et al. A Clinically Applicable Gene-Expression Classifier Reveals Intrinsic and Extrinsic Contributions to Consensus Molecular Subtypes in Primary and Metastatic Colon Cancer. Clin Cancer Res. 2019;25(14):4431–42. doi: 10.1158/1078-0432.CCR-18-3032. [DOI] [PubMed] [Google Scholar]
- 20.Pascual G, Avgustinova A, Mejetta S, Martin M, Castellanos A, Attolini CS, et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017;541(7635):41–5. doi: 10.1038/nature20791. [DOI] [PubMed] [Google Scholar]
- 21.Toyoshima Y, Kitamura H, Xiang H, Ohno Y, Homma S, Kawamura H, et al. IL6 Modulates the Immune Status of the Tumor Microenvironment to Facilitate Metastatic Colonization of Colorectal Cancer Cells. Cancer Immunol Res. 2019;7(12):1944–57. doi: 10.1158/2326-6066.CIR-18-0766. [DOI] [PubMed] [Google Scholar]
- 22.Robinson-Smith TM, Isaacsohn I, Mercer CA, Zhou M, Van Rooijen N, Husseinzadeh N, et al. Macrophages Mediate Inflammation-Enhanced Metastasis of Ovarian Tumors in Mice. Cancer Research. 2007;67(12):5708–16. doi: 10.1158/0008-5472.CAN-06-4375. [DOI] [PubMed] [Google Scholar]
- 23.Cottone L, Capobianco A, Gualteroni C, Monno A, Raccagni I, Valtorta S, et al. Leukocytes recruited by tumor-derived HMGB1 sustain peritoneal carcinomatosis. Oncoimmunology. 2016;5(5):e1122860. doi: 10.1080/2162402X.2015.1122860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutton CD, O’Byrne K, Goddard JC, Marshall L-J, Jones L, Garcea G, et al. Expression of Thrombospondin-1 in Resected Colorectal Liver Metastases Predicts Poor Prognosis. Clinical Cancer Research. 2005;11(18):6567–73. doi: 10.1158/1078-0432.CCR-05-0439. [DOI] [PubMed] [Google Scholar]
- 25.Barker PA. p75NTR is positively promiscuous: novel partners and new insights. Neuron. 2004;42(4):529–33. doi: 10.1016/j.neuron.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Civenni G, Walter A, Kobert N, Mihic-Probst D, Zipser M, Belloni B, et al. Human CD271-positive melanoma stem cells associated with metastasis establish tumor heterogeneity and long-term growth. Cancer Res. 2011;71(8):3098–109. doi: 10.1158/0008-5472.CAN-10-3997. [DOI] [PubMed] [Google Scholar]
- 27.Marz P, Heese K, Dimitriades-Schmutz B, Rose-John S, Otten U. Role of interleukin-6 and soluble IL-6 receptor in region-specific induction of astrocytic differentiation and neurotrophin expression. Glia. 1999;26(3):191–200. doi: 10.1002/(sici)1098-1136(199905)26:3<191::aid-glia1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 28.Zhou X, Hao Q, Liao P, Luo S, Zhang M, Hu G, et al. Nerve growth factor receptor negates the tumor suppressor p53 as a feedback regulator. eLife. 2016;5 doi: 10.7554/eLife.15099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.morano n, Garret S, Almo S. Structural and Functional Investigations Into B7-1:NGFR. The Journal of Immunology. 2019;202(1 Supplement):229.7–7. [Google Scholar]
- 30.Sheppard M, Laskou F, Stapleton PP, Hadavi S, Dasgupta B. Tocilizumab (Actemra) Human vaccines & immunotherapeutics. 2017;13(9):1972–88. doi: 10.1080/21645515.2017.1316909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Enciu AM, Radu E, Popescu ID, Hinescu ME, Ceafalan LC. Targeting CD36 as Biomarker for Metastasis Prognostic: How Far from Translation into Clinical Practice? BioMed research international. 2018;2018:7801202. doi: 10.1155/2018/7801202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basu S, Haase G, Ben-Ze’ev A. Wnt signaling in cancer stem cells and colon cancer metastasis. F1000Research. 2016;5 doi: 10.12688/f1000research.7579.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du L, Li YJ, Fakih M, Wiatrek RL, Duldulao M, Chen Z, et al. Role of SUMO activating enzyme in cancer stem cell maintenance and self-renewal. Nat Commun. 2016;7:12326. doi: 10.1038/ncomms12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spitzer TLB, Rojas A, Zelenko Z, Aghajanova L, Erikson DW, Barragan F, et al. Perivascular Human Endometrial Mesenchymal Stem Cells Express Pathways Relevant to Self-Renewal, Lineage Specification, and Functional Phenotype1. Biology of Reproduction. 2012;86(2) doi: 10.1095/biolreprod.111.095885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hojo N, Huisken AL, Wang H, Chirshev E, Kim NS, Nguyen SM, et al. Snail knockdown reverses stemness and inhibits tumour growth in ovarian cancer. Scientific Reports. 2018;8(1):8704. doi: 10.1038/s41598-018-27021-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequencing data are available in Sequence Read Archive (SRA) database through the Bioproject accession number: PRJNA675983
Nanostring nCounter® expression data are available in Gene Expression Omnibus (GEO) database with accession number GSE161097
DSP data are available as supplementary table S7.