Abstract
The generation of phosphoinositides (PIs) with spatial and temporal control is a key mechanism in cellular organization and signalling. The synthesis of PIs is mediated by phosphoinositide kinases, proteins that are able to phosphorylate unique substrates at specific positions on the inositol headgroup to generate signalling molecules. Phosphatidylinositol 5 phosphate 4 kinase (PIP4K) is one such lipid kinase that is able to specifically phosphorylate phosphatidylinositol 5 phosphate (PI5P), the most recently discovered PI to generate the well-known and abundant PI, phosphatidylinositol 4,5 bisphosphate [PI(4,5)P2]. PIP4K appear to be encoded only in metazoan genomes and several genetic studies indicate important physiological functions for these enzymes in metabolism, immune function and growth control. PIP4K have recently been reported to localize to multiple cellular compartments including the nucleus, plasma membrane, endosomal systems and autophagosome. However, the biochemical activity of these enzymes that is relevant to these physiological functions remains elusive. We review recent developments in this area and highlight emerging roles for these enzymes in cellular organization.
Introduction
Phosphatidylinositol 4,5 bisphosphate [PI(4,5)P2] is the most abundant of the phosphoinositides, the phosphorylated derivatives of phosphatidylinositol (PI) that regulate numerous sub-cellular processes in eukaryotic cells [1]. In addition to its well-known requirement as the substrate for receptor activated phospholipase C activity, it is now well recognised that PI(4,5)P2 binds to and regulates the activity of a large number of proteins in eukaryotic cells; through the activity of these proteins, this lipid is able to regulate numerous sub-cellular events including vesicular transport, cytoskeletal function, ion channel and transporter activity and gene regulation within the nucleus. Each of the seven phosphoinositides shows a characteristic sub-cellular distribution in eukaryotic cells and more recently, it has also been noted that that even within a single organelle membrane, phosphoinositide species may show a domain specific distribution [2]. Although PI(4,5)P2 was originally thought to be restricted to the plasma membrane, it is now recognized that this lipid is present at multiple locations in the cell including the nucleus, Golgi complex, late endosomes and lysosomes. Given this scenario, the localized generation and metabolism of PI(4,5)P2 by relevant enzymes is a key factor in its ability to control sub-cellular processes and cellular functions.
PIP5K enzymes
PI(4,5)P2 can be synthesized in cells by the activity of one of two classes of lipid kinases: phosphatidylinositol 4 phosphate 5-kinase (PIP5K), also previously referred to as Type I PIP kinase and phosphatidylinositol 5 phosphate 4-kinase (PIP4K), previously known as Type II PIP kinase. Biochemical analysis in human cell lines indicate that the PIP5K enzymes, that use phosphatidylinositol 4 phosphate (PI4P) as substrate, are responsible for the major proportion of the PI(4,5)P2 in eukaryotes [4,5]. This enzymatic activity is encoded in all eukaryotic genomes and the enzymes are distributed at multiple cellular membranes including the plasma membrane, Golgi, endosomes and lysosomes ([6,7] and reviewed in [8]) and the activity of this enzyme has been shown to regulate numerous sub-cellular processes [9].
PIP4K enzymes
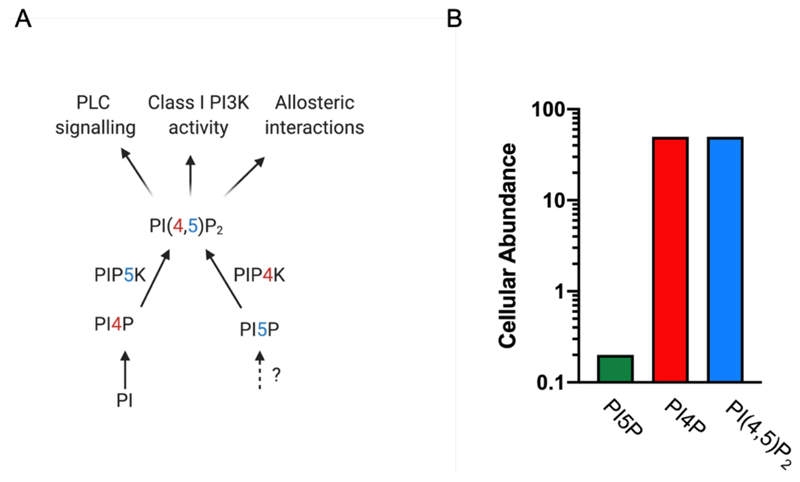
By contrast, the PIP4K enzymes use phosphatidylinositol 5 phosphate (PI5P) as substrate through the addition of a 4 phosphate but are unable to use PI4P as a substrate [10]. Since the total amount of PI5P in cells is only about 1-2 % of PI4P levels [11], PIP4K enzymes likely generate a quantitatively minor pool of PI(4,5)P2. Thus, total PI(4,5)P2 levels, measured biochemically using mass assays remain unaltered in cells depleted of PIP4K activity (reviewed in [12]). However, PI5P levels rise when PIP4K is depleted leading to the idea that the principal function of this enzyme might be to regulate the levels of its substrate PI5P [12] rather than produce a quantitatively small but functionally relevant pool of PI(4,5)P2. The PIP4K enzymes are found only in metazoan genomes being conspicuously absent from the genomes of unicellular eukaryotes (Padinjat lab, unpublished). However genetic studies using model organisms indicate important physiological functions for PIP4K enzymes in growth and development [13], immune function [14], metabolism [15,16] and tumour growth control [17–19]. The manner in which PIP4K regulates these physiological functions remains a matter of active investigation. Polymorphisms in human PIP4K genes have been linked to many disorders such a neuropsychiatric syndromes (reviewed in [20]).
The biochemical activity of PIP4K
The biochemical activity of PIP4K enzymes has been studied using in vitro assays. In all studies to date, a clear preference for PI5P as a substrate has been noted [10,13,21,22]. In addition, some studies have also noted the ability of the enzyme to utilise phosphatidylinositol 3 phosphate (PI3P) as a substrate [23] although with poor efficiency in vitro. An intriguing aspect of PIP4K proteins is the broad range of enzyme activity that is reported for polypeptides encoded by these genes. Typically, in vitro activity is assayed using recombinant protein supplied with pure substrate (PI5P) and ATP and the formation of the product PI(4,5)P2 is quantified. When studied using this approach, each of the three isoforms of PIP4K in mammalian genomes show vastly differing extents of biochemical activity, PIP4K2A is ca. 2000 fold more active than PIP4K2B [24]; PIP4K2C is inactive under equivalent conditions [25]. It has been reported that the three isoforms can form heterodimers and that this may change the activity and localization of a given isoform [26,27]. The intrinsic ATPase activity of all three polypeptides is quite comparable indicating that they are all potentially active kinases [22]; thus the varying PIP4K activity using PI5P as substrate likely reflects the ability of these polypeptides to use it as a substrate in vitro. Remarkably, the only PIP4K in the Drosophila genome shows high activity comparable to that of mammalian PIP4K2A [13,22] whereas the only PIP4K encoded in the C.elegans genome shows minimal activity that is comparable to that of PIP4K2C [22,28]. A recent study explored the relationship between in vitro kinase activity and in vivo function [29]. They studied a null mutant of the only PIP4K in Drosophila that shows a reduction of cell size in the salivary gland. This phenotype can be rescued in a cell-autonomous manner by reconstitution with the Drosophila PIP4K gene; a kinase dead (non-ATP binding) version fails to rescue cell size, reflecting a requirement of kinase activity for this function. Despite their widely varying in vitro activity, each of the three human PIP4K isoforms 2A, 2B and 2C were able to rescue the cell size defect equally well. Interesting a kinase dead version of PIP4K2C was unable to rescue the cell size defect implying that in vivo, there is a requirement for its kinase activity in rescuing cell size [29]. Overall, these findings suggest that there is little correlation between the in vitro PI5P 4-kinase activity of PIP4K and its in vivo activity. Perhaps PIP4K has other substrates in vivo that remain to be identified or there are additional mechanisms that regulate the activity of PIP4K isoforms in vivo. It has also been reported in a study that PIP4K2B binds to GTP better than ATP and preferably hydrolyses GTP than ATP in an in vitro kinase assay [30]. The same study noted that inhibiting cellular GTP utilisation affected the levels of cellular PI5P hinting towards a paradigm where PIP4Ks could prefer GTP over ATP in vivo [30].
Sub-cellular events and physiological processes regulated by PIP4K
The subcellular distribution of PIP4K proteins has been studied by multiple approaches. Each of the mammalian isoforms is distributed across multiple cellular compartments. In mammalian systems each isoform shows a distinctive distribution; while PIP4K2A is found mainly at the plasma membrane, PIP4K2B is found both at the plasma membrane and the nucleus; PIP4K2C appears distributed across a vesicular compartment within cells [24]. In Drosophila, that contains only a single isoform of PIP4K, the endogenous protein co-fractionates with markers of multiple cellular compartments including the plasma membrane, early and late endosomal compartments as well the Golgi complex; it is however excluded from the nucleus [13] and the Drosophila enzyme lacks essential elements of the nuclear localization signal described for PIP4K2B [31]. Interestingly, a biochemical fractionation study of mammalian cells found that although PI5P, the best described substrate of PIP4K, was found primarily at the plasma membrane, it was also distributed across vesicular compartments of the cell [11]. Thus it seems likely that PIP4K performs its function at multiple sub-cellular locations; the manner in which it is targeted to these compartments and its activity regulated at these locations remains an open and very interesting question. PIP4K regulated functions have been described at multiple sub-cellular locations within cells; these are detailed below. Interestingly in some compartments such as the plasma membrane and the nucleus, PIP4K is present along with PIP5K. This represents a situation in which pools of PI(4,5)P2 made by two independent biosynthetic pathways are generated at a single sub-cellular location. If these pools of PI(4,5)P2 have distinctive functions, the mechanism by which they are sensed and segregated at cellular membranes remains to be determined.
Plasma membrane
Several studies have implicated PIP4K function in the regulation of receptor activated Class I PI3K signalling that occurs at the plasma membrane. Early studies had suggested that in mammalian cells PIP4K can regulate Class I PI3K signalling activated by cell surface receptors [15,32]. However the mechanisms through which this regulation occurs were not understood. Recent studies have cast light on this topic. Studies in Drosophila have found that plasma membrane phosphatidylinositol 3,4,5 trisphosphate (PIP3) levels are elevated in cells depleted of PIP4K and that the rise in PIP3 levels following insulin stimulation is greater in cells lacking PIP4K compared to controls [16]; this appears to be a cell-autonomous phenomenon. The enhanced PIP3 production in Drosophila cells lacking PIP4K could be rescued by reconstitution with a PIP4K protein selectively targeted to the plasma membrane [16]. This finding indicates that PIP4K at the plasma membrane in necessary to regulate Class I PI3K activity during receptor activated signalling. Using simultaneous imaging of PI(4,5)P2 and PIP3, this study also found that the enhanced levels of PIP3 during insulin stimulation in PIP4K null cells was also accompanied by a rise in PI(4,5)P2 levels. The mechanisms underlying this remain unknown but a recent analysis in mammalian cells has noted an equivalent result and suggested that a heterodimer of PIP5K and PIP4K leading to enhanced PI(4,5)P2 generation may underlie this phenomenon [33]. Interestingly, previous studies in muscle cells has reported an insulin stimulated rise in PI5P levels that was abolished by overexpression of PIP4K [34]. The raison d’étre for this type of regulation remains to be determined but represents a rare example when plasma membrane PI(4,5)P2 levels rise above normal.
Early endosomal compartment
PIP4K has also been reported to regulate membrane transport. In Drosophila cells depleted of PIP4K, the endocytic trafficking of plasma membrane receptors is multiple cell types was disrupted [35]. This study reported that in fly photoreceptors, rhodopsin, the G-protein coupled receptor for light accumulated in an expanded early endocytic Rab5 positive compartment. Likewise, trafficking was disrupted in cultured Drosophila S2R cells and also in haemocytes depleted of PIP4K. These accumulations of rhodopsin in the early endocytic compartment could be rescued by reconstituting with PIP4K selectively targeted to the early endocytic compartment but not when reconstituted exclusively to the plasma membrane. PIP4K was found to co-localize with Rab5 both in photoreceptors and in Drosophila S2R+ cells [35]. Together these findings imply a function for PIP4K at the early endocytic compartment. Interestingly, a previous study has implicated PI5P, the substrate of PIP4K produced by the activity of S.flexneri IpgD in the regulation of EGF receptor trafficking through the early endosomal compartment [36] and a biochemical analysis has identified the adaptor protein TOM1, as a PI5P binding protein [37].
Autophagosome biogenesis
In addition to a role in the early endocytic compartment, PIP4Ks are reported to localize to the to the autophagy compartment in cultured human cells [38] and depletion of each of the human isoforms increased the number of autophagosome structures. This study also reported that knockdown of PIP4K2C reduced the accumulation of huntingtin aggregates in cultured human cells whereas overexpression enhanced the level of aggregates [38]. A recent study using mouse knockout models of PIP4K2A and PIP4K2B has reported that in the liver, PIP4K2A and 2B are required to regulate autophagy in a p53 depleted background [28]. During starvation, cells with this genetic background (PIP4K2A-/-;PIP4K2B-/-;shTrp53) show an expansion of LC3 positive autophagic and LAMP1 positive lysosomal compartments with evidence of a block in fusion between the autophagosome and lysosomal compartment. Together these findings suggest a function for PIP4K enzymes in the control of autophagosome maturation, likely at the step of fusion of these vesicles with the lysosomal compartment. Interesting in Drosophila, PIP4K mutants which are homozygous viable as adults, have a growth and developmental defect that is associated with reduced TORC1 output [13] and these phenotypes are rescued by the overexpression of Rheb, a key positive regulator of TORC1. TORC1 is a key regulator of autophagy and collectively these findings imply a function of PIP4K in the regulation of autophagy. It is however interesting to reflect on the point that while autophagy as a process is conserved across eukaryotes, PIP4K genes are a feature of metazoan genomes. Thus, the requirement of PIP4K in autophagy must reflect a need for further regulation of this process in the context of metazoan biology. At a physiological level, loss of PIP4K results in altered metabolism in a range of species including C.elegans [28], Drosophila [13,16] and mammals [15,28].
Nucleus
A large body of work implicates PIP4K and its substrate PI5P in the regulation of the DNA damage response and has been summarized in recent reviews [39,40]. Briefly, the isoform PIP4K2B contains a nuclear localization signal that allows it to localize to the nucleus where it regulates PI5P levels in response to signals such UV irradiation and oxidative stress. PI5P then binds to and regulates the activity of several proteins that regulate gene expression such as the PHD domain containing proteins such as ING2, TAF3 and other regulatory molecules such as UHRF1. The functional significance of these molecular observations is underscored by several physiological findings (i) PIP4K2A transcripts are upregulated in leukaemias [41,42][43] (ii) A double knockout of PIP4K2A and PIP4K2B is able to slow the growth of tumours in p53 null mice [17] (iii) overexpression of PIP4K2A can suppress the growth of PTEN induced glioblastoma [19].
Relationship of biological function to PIP4K enzyme activity
An intriguing and related aspect of PIP4K function is question of whether its catalytic activity is required for in vivo function. Typically, this has been addressed in studies where cells lacking PIP4K protein have been reconstituted with a non-ATP binding, kinase dead version of the enzyme and assaying for rescue of function. For example in the Drosophila PIP4K mutant, there appear to be some phenotypes that show dependency on the kinase activity of protein (e.g. elevated PI5P levels [13], cell size defect [29], altered growth & development [16] whereas other appear not to require the kinase activity of the enzyme (e.g. enhanced insulin induced PIP3 production [16] and altered GPCR trafficking in photoreceptors [35]). In mammalian systems, the enhanced sensitivity to insulin stimulation of cells lacking PIP4K can be rescued by a kinase dead version of the enzyme [33]. The reasons for such a variable context dependent requirement for kinase activity in rescuing phenotypes remains unresolved and will be an area for active investigation in the immediate future.
Concluding Summary
PIP4K have emerged as a new class of phosphoinositide kinases with important roles in metazoan physiology and operating at multiple sub-cellular locations in cells. However, important questions remain on substrate specificity of these enzymes and the relationship of in vitro activity to in vivo function. Although phosphoinositide signalling in broadly conserved across eukaryotes, some elements remain a unique feature of metazoans; PIP4K is one such example and it will be interesting to determine the reasons why this emerging class of enzymes is required for normal metazoan physiology.
Figure 1.
(A) Routes of synthesis and metabolism of phosphatidylinositol 4,5 bisphosphate [PI(4,5)P2]. Enzymes and substrates involved in this process and their metabolic relationship are shown. PI-phosphatidylinositol, PI4P-phosphatidylinositol 4 phosphate, PI5P-phosphatidylinositol 5 phosphate, PIP5K-phosphatidylinositol 4 phosphate 5-kinase, PIP4K-phosphatidylinositol 5 phosphate 4-kinase. The functions of PI(4,5)P2 are indicated. PLC-phospholipase C, PI3K-phosphatidylinostiol 3 kinase.? indicates route of synthesis unresolved. (B) Relative abundance of PI4P, PI(4,5)P2 as estimated from mass measurements in eukaryotic cells. Y-axis is in log units. Primary data on abundance from [3]
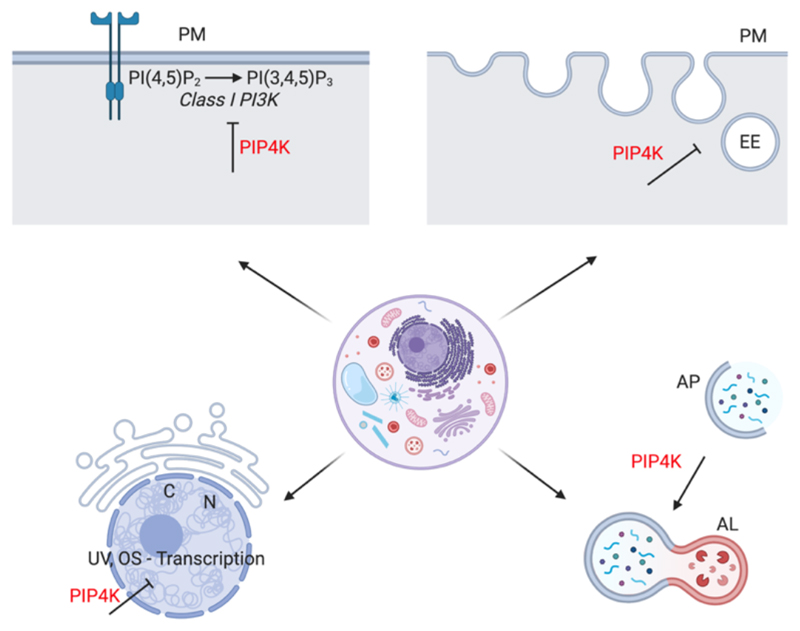
Figure 2. PIP4K regulates function at multiple cellular compartments.
(A) Plasma membrane (B)early endosomal compartment (C) nucleus (D) autophagosome maturation. PM-plasma membrane, PI(4,5)P2-phosphatidylinositol 4,5 bisphosphate, PI(3,4,5)P3 -phosphatidylinositol 3,4,5 trisphosphate, EE-early endosome, AP-autophagosome, AL-autophagolysosome, N-nucleus, C-chromatin, UV, OS-Transcription-ultraviolet radiation and oxidative stress induced transcription. PIP4K-phosphatidylinositol 5 phosphate 4-kinase. Blunt arrowheads imply inhibition.
Acknowledgements
This work was supported by the Department of Atomic Energy, Government of India, under Project Identification No. RTI 4006, a Wellcome-DBT India Alliance Senior Fellowship (IA/S/14/2/501540) to PR. I thank Avishek Ghosh, Harini Krishnan and Aishwarya Venugopal for comments on this manuscript.
References
- 1.Balla T. Phosphoinositides: Tiny Lipids With Giant Impact on Cell Regulation. Physiol Rev. 2013;93:1019–1137. doi: 10.1152/physrev.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallroth A, Haucke V. Phosphoinositide conversion in endocytosis and the endolysosomal system. J Biol Chem. 2018;293:1526–1535. doi: 10.1074/jbc.R117.000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lemmon MA. Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol. 2008;9:99–111. doi: 10.1038/nrm2328. [DOI] [PubMed] [Google Scholar]
- 4.King CE, Hawkins PT, Stephens LR, Michell RH. Determination of the steady-state turnover rates of the metabolically active pools of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in human erythrocytes. Biochem J. 1989;259:893–6. doi: 10.1042/bj2590893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stephens LR, Hughes KT, Irvine RF. Pathway of phosphatidylinositol(3,4,5)-trisphosphate synthesis in activated neutrophils. Nature. 1991;351:33–9. doi: 10.1038/351033a0. [DOI] [PubMed] [Google Scholar]
- 6.Watt SA, Kular G, Fleming IN, Downes CP, Lucocq JM. Subcellular localization of phosphatidylinositol 4,5-bisphosphate using the pleckstrin homology domain of phospholipase C delta1. Biochem J. 2002;363:657–66. doi: 10.1042/0264-6021:3630657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammond GRV, Schiavo G, Irvine RF. Immunocytochemical techniques reveal multiple, distinct cellular pools of PtdIns4P and PtdIns(4,5)P(2) Biochem J. 2009;422:23–35. doi: 10.1042/BJ20090428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun Y, Thapa N, Hedman AC, Anderson RA. Phosphatidylinositol 4,5-bisphosphate: targeted production and signaling. Bioessays. 2013;35:513–22. doi: 10.1002/bies.201200171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van den Bout I, Divecha N. PIP5K-driven PtdIns(4,5)P2 synthesis: regulation and cellular functions. 2009;122:3837–3850. doi: 10.1242/jcs.056127. [DOI] [PubMed] [Google Scholar]
- 10.Rameh LE, Tolias KF, Duckworth BC, Cantley LC. A new pathway for synthesis of phosphatidylinositol-4,5-bisphosphate. Nature. 1997;390:192–196. doi: 10.1038/36621. [DOI] [PubMed] [Google Scholar]
- 11.Sarkes D, Rameh LE. A novel HPLC-based approach makes possible the spatial characterization of cellular PtdIns5P and other phosphoinositides. Biochem J. 2010;428:375–84. doi: 10.1042/BJ20100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolay S, Basu U, Raghu P. Control of diverse subcellular processes by a single multi-functional lipid phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] Biochem J. 2016;473:1681–92. doi: 10.1042/BCJ20160069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta A, Toscano S, Trivedi D, Jones DR, Mathre S, Clarke JH, Divecha N, Raghu P. Phosphatidylinositol 5-phosphate 4-kinase (PIP4K) regulates TOR signaling and cell growth during Drosophila development. Proc Natl Acad Sci U S A. 2013;110:5963–5968. doi: 10.1073/pnas.1219333110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shim H, Wu C, Ramsamooj S, Bosch KN, Chen Z, Emerling BM, Yun J, Liu H, Choo-Wing R, Yang Z, et al. Deletion of the gene Pip4k2c, a novel phosphatidylinositol kinase, results in hyperactivation of the immune system. Proc Natl Acad Sci. 2016;113:7596–7601. doi: 10.1073/pnas.1600934113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamia KA, Peroni OD, Kim YB, Rameh LE, Kahn BB, Cantley LC. Increased insulin sensitivity and reduced adiposity in phosphatidylinositol 5-phosphate 4-kinase beta-/- mice. Mol Cell Biol. 2004;24:5080–5087. doi: 10.1128/MCB.24.11.5080-5087.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma S, Mathre S, Ramya V, Shinde D, Raghu P. Phosphatidylinositol 5 Phosphate 4- Kinase Regulates Plasma-Membrane PIP3 Turnover and Insulin Signaling. Cell Rep. 2019;27:1979–1990.:e7. doi: 10.1016/j.celrep.2019.04.084. [*** This paper demonstrates the requirement for PIP4K in regulating PI(3,4,5)P3 production and PI(4,5)P2 during receptor tyrosine kinase signalling in Drosophila ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emerling BM, Hurov JB, Poulogiannis G, Tsukazawa KS, Choo-Wing R, Wulf GM, Bell EL, Shim HS, Lamia KA, Rameh LE, et al. Depletion of a putatively druggable class of phosphatidylinositol kinases inhibits growth of p53-Null tumors. Cell. 2013;155:844–857. doi: 10.1016/j.cell.2013.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitagawa M, Liao PJ, Lee KH, Wong J, Shang SC, Minami N, Sampetrean O, Saya H, Lingyun D, Prabhu N, et al. Dual blockade of the lipid kinase PIP4Ks and mitotic pathways leads to cancer-selective lethality. Nat Commun. 2017;8 doi: 10.1038/s41467-017-02287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin YJ, Sa JK, Lee Y, Kim D, Chang N, Cho HJ, Son M, Oh MYT, Shin K, Lee JK, et al. PIP4K2A as a negative regulator of PI3K in PTEN-defìcient glioblastoma. J Exp Med. 2019;216:1120–1134. doi: 10.1084/jem.20172170. [** This paper is an unbiased cell-culture based genetic screen that demonstrates the function of PIP4K2A in tumor growth in a glioblastoma model.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raghu P, Joseph A, Krishnan H, Singh P, Saha S. Phosphoinositides: Regulators of Nervous System Function in Health and Disease. Front Mol Neurosci. 2019;12:208. doi: 10.3389/fnmol.2019.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kunz J, Wilson MP, Kisseleva M, Hurley JH, Majerus PW, Anderson RA. The activation loop of phosphatidylinositol phosphate kinases determines signaling specificity. Mol Cell. 2000;5:1–11. doi: 10.1016/s1097-2765(00)80398-6. [DOI] [PubMed] [Google Scholar]
- 22.Clarke JH, Irvine RF. Evolutionarily conserved structural changes in phosphatidylinositol 5-phosphate 4-kinase (PI5P4K) isoforms are responsible for differences in enzyme activity and localization. Biochems J. 2013;454:49–57. doi: 10.1042/BJ20130488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Loijens JC, Boronenkov IV, Parker GJ, Norris FA, Chen J, Thum O, Prestwich GD, Majerus PW, Anderson RA. Phosphatidylinositol-4-phosphate 5-kinase isozymes catalyze the synthesis of 3-phosphate-containing phosphatidylinositol signaling molecules. J Biol Chem. 1997;272:17756–61. doi: 10.1074/jbc.272.28.17756. [DOI] [PubMed] [Google Scholar]
- 24.Wang M, Bond NJ, Letcher AJ, Richardson JP, Lilley KS, Irvine RF, Clarke JH. Genomic tagging reveals a random association of endogenous PtdIns5P 4-kinases IIalpha and IIbeta and a partial nuclear localization of the IIalpha isoform. Biochem J. 2010;430:215–21. doi: 10.1042/BJ20100340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clarke JH, Emson PC, Irvine RF. Localization of phosphatidylinositol phosphate kinase IIgamma in kidney to a membrane trafficking compartment within specialized cells of the nephron. Am J Physiol Renal Physiol. 2008;295:F1422–30. doi: 10.1152/ajprenal.90310.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bultsma Y, Keune W-J, Divecha N. PIP4Kβ interacts with and modulates nuclear localization of the high-activity PtdIns5 P-4-kinase isoform PIP4Kα. Biochem J. 2010;430:223–235. doi: 10.1042/BJ20100341. [DOI] [PubMed] [Google Scholar]
- 27.Wang M, Bond NJ, Letcher AJ, Richardson JP, Lilley KS, Irvine RF, Clarke JH. Genomic tagging reveals a random association of endogenous PtdIns5P 4-kinases IIα and IIβ and a partial nuclear localization of the IIα isoform. Biochem J. 2010;430:215–221. doi: 10.1042/BJ20100340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lundquist MR, Goncalves MD, Loughran RM, Possik E, Vijayaraghavan T, Yang A, Pauli C, Ravi A, Verma A, Yang Z, et al. Phosphatidylinositol-5-Phosphate 4-Kinases Regulate Cellular Lipid Metabolism By Facilitating Autophagy. Mol Cell. 2018;70:531–544.:e9. doi: 10.1016/j.molcel.2018.03.037. [*** This paper is detailed analysis of the function of PIP4K in regulating metabolism and autophagy using genetic models in mice using biochemical and cell-biological approaches.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathre S, Reddy KB, Ramya V, Krishnan H, Ghosh A, Raghu P. Functional analysis of the biochemical activity of mammalian phosphatidylinositol 5 phosphate 4-kinase enzymes. Biosci Rep. 2019;39:BSR20182210. doi: 10.1042/BSR20182210. [*** This paper reports a comparative analysis of the in vitro activity of PIP4K isoforms with their ability to rescue function in vivo.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sumita K, Lo YH, Takeuchi K, Senda M, Kofuji S, Ikeda Y, Terakawa J, Sasaki M, Yoshino H, Majd N, et al. The Lipid Kinase PI5P4Kβ Is an Intracellular GTP Sensor for Metabolism and Tumorigenesis. Mol Cell. 2016;61:187–198. doi: 10.1016/j.molcel.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ciruela A, Hinchliffe KA, Divecha N, Irvine RF. Nuclear targeting of the beta isoform of type II phosphatidylinositol phosphate kinase (phosphatidylinositol 5-phosphate 4-kinase) by its alpha-helix 7. 2000;346(Pt 3):587–91. [PMC free article] [PubMed] [Google Scholar]
- 32.Carricaburu V, Lamia KA, Lo E, Favereaux L, Payrastre B, Cantley LC, Rameh LE. The phosphatidylinositol (PI)-5-phosphate 4-kinase type II enzyme controls insulin signaling by regulating PI-3,4,5-trisphosphate degradation. Proc Natl Acad Sci USA. 2003;100:9867–9872. doi: 10.1073/pnas.1734038100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang DG, Paddock MN, Lundquist MR, Sun JY, Mashadova O, Amadiume S, Bumpus TW, Hodakoski C, Hopkins BD, Fine M, et al. PIP4Ks Suppress Insulin Signaling through a Catalytic-Independent Mechanism. Cell Rep. 2019;27:1991–2001.:e5. doi: 10.1016/j.celrep.2019.04.070. [*** This paper describes a catalytic activity independent mechanism by which PIP4K can regulate Class I PI3K signalling in mammalian cells] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grainger DL, Tavelis C, Ryan AJ, Hinchliffe K. a: Involvement of phosphatidylinositol 5-phosphate in insulin-stimulated glucose uptake in the L6 myotube model of skeletal muscle. Pflugers Arch. 2011;462:723–32. doi: 10.1007/s00424-011-1008-4. [DOI] [PubMed] [Google Scholar]
- 35.Kamalesh K, Trivedi D, Toscano S, Sharma S, Kolay S, Raghu P. Phosphatidylinositol 5- phosphate 4-kinase regulates early endosomal dynamics during clathrin-mediated endocytosis. J Cell Sci. 2017;130:2119–2133. doi: 10.1242/jcs.202259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramel D, Lagarrigue F, Pons V, Mounier J, Dupuis-Coronas S, Chicanne G, Sansonetti PJ, Gaits-Iacovoni F, Tronchere H, Payrastre B. Shigella flexneri Infection Generates the Lipid PI5P to Alter Endocytosis and Prevent Termination of EGFR Signaling. Sci Signal. 2011;4:ra61-ra61. doi: 10.1126/scisignal.2001619. [DOI] [PubMed] [Google Scholar]
- 37.Boal F, Mansour R, Gayral M, Saland E, Chicanne G, Xuereb J-M, Marcellin M, Burlet-Schiltz O, Sansonetti PJ, Payrastre B, et al. TOM1 is a PI5P effector involved in the regulation of endosomal maturation. J Cell Sci. 2015;128:815–27. doi: 10.1242/jcs.166314. [DOI] [PubMed] [Google Scholar]
- 38.Vicinanza M, Korolchuk VI, Ashkenazi A, Puri C, Menzies FM, Clarke JH, Rubinsztein DC. PI(5)P regulates autophagosome biogenesis. Mol Cell. 2015;57:219–234. doi: 10.1016/j.molcel.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fiume R, Faenza I, Sheth B, Poli A, Vidalle MC, Mazzetti C, Abdul SH, Campagnoli F, Fabbrini M, Kimber ST, et al. Nuclear phosphoinositides: Their regulation and roles in nuclear functions. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20122991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poli A, Zaurito AE, Abdul-Hamid S, Fiume R, Faenza I, Divecha N. Phosphatidylinositol 5 phosphate (Pi5p): From behind the scenes to the front (nuclear) stage. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20092080. [** A review of the regulation of gene expression by PIP4K through its function in the nucleus.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsu L-I, Briggs F, Shao X, Metayer C, Wiemels JL, Chokkalingam AP, Barcellos LF. Pathway analysis of genome-wide association study in childhood leukemia among Hispanics. Cancer Epidemiol Biomarkers Prev. 2016 doi: 10.1158/1055-9965.EPI-15-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang S, Li Z, Yan X, Bao L, Deng Y, Zeng F, Wang P, Zhu J, Yin D, Liao F, et al. Regulatory network and prognostic effect investigation of PIP4K2A in leukemia and solid cancers. Front Genet. 2019;10 doi: 10.3389/fgene.2018.00721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jude JG, Spencer GJ, Huang X, Somerville TDD, Jones DR, Divecha N. Somervaille TCP: A targeted knockdown screen of genes coding for phosphoinositide modulators identifies PIP4K2A as required for acute myeloid leukemia cell proliferation and survival. Oncogene. 2015;34:1253–62. doi: 10.1038/onc.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]