Abstract
SUMMARY
Background:
Infection with Plasmodium falciparum leads to severe malaria and death in approximately 400,000 children each year in sub-Saharan Africa. Blood transfusion may benefit some patients with malaria but could potentially harm others. The aim of this study was to estimate the association between transfusion and death among children admitted to hospital with P. falciparum malaria.
Methods:
We analyzed admissions to six tertiary care hospitals located in Banjul, The Gambia; Blantyre, Malawi; Lambaréné, Gabon; Libreville, Gabon; Kilifi, Kenya; and Kumasi, Ghana that participated in the Severe Malaria in African Children (SMAC) network. Patients were enrolled in the observational study if they were under the age of 15 years and had a blood smear positive for P. falciparum. Blood transfusion was administered at the discretion of the responsible physicians who were aware of local and international transfusion guidelines. Odds of in-hospital death associated with transfusion were estimated using site- and severity-adjusted models. Generalized additive models were used to estimate optimal hemoglobin transfusion thresholds.
Findings:
25,893 patients were admitted to hospital with P. falciparum malaria and enrolled in the study from December 3, 2000 through March 8, 2005. 8,513 (32·8%) of 25,893 received a blood transfusion. Patients were followed until discharge from hospital over a median (IQR) of 2 (1–4) days. Transfusion was associated with decreased odds of death in site-adjusted analysis (OR=0·82 [95%CI 0·71–0·94]) and after adjusting for the increased disease severity of transfused patients (OR=0·50 [95% CI 0·42–0·60]). Among all study participants, transfusion was associated with improved survival when the admission hemoglobin was up to 77 g/L (95% CI: 65–110). Among those with impaired consciousness, transfusion was associated with improved survival at hemoglobin concentrations up to 105 g/L (95% CI 71–115). Among those with hyperlactatemia, the association with improved survival persisted at even greater hemoglobin concentrations (lower bound of 95%CI: 90 g/L).
Interpretation:
Whole blood transfusion was strongly associated with improved survival among children with P. falciparum malaria. Among those with impaired consciousness or hyperlactatemia, transfusion was associated with improved survival at hemoglobin concentrations above the currently recommended transfusion threshold. These findings highlight the need to conduct randomized controlled trials to test higher transfusion thresholds among African children with severe malaria complicated by impaired consciousness or elevated blood lactate.
INTRODUCTION
Despite improvements in access to anti-malarial drugs, treating people with severe malaria remains a global public health challenge. In 2018, there were 228 million cases of malaria worldwide, with many African countries reporting more cases than in 2016.1 The majority of deaths from malaria occurred in sub-Saharan Africa, where 405,000 people died in 2018. Malaria caused by Plasmodium falciparum can progress rapidly and children are often brought to medical attention only after severe complications have developed. Children who present with coma or lactic acidosis have in-hospital mortality rates of 15–21% despite supportive care and treatment with effective parasite-killing drugs.2–5 Most deaths occur within the first twenty-four hours of hospitalization, allowing only a narrow window within which to administer treatments that could reduce mortality.3,5,6
In addition to prompt administration of parasite-killing drugs, supportive care for the child with severe malaria may require correcting hypoglycemia,7,8 managing seizures,9,10 and transfusing blood. In areas where malaria transmission is high, the World Health Organization (WHO) guidelines recommend blood transfusion when the hemoglobin is less than 40 g/L. If anemia is accompanied by acidosis, impaired consciousness, shock or parasitemia greater than 20%, the recommended transfusion threshold is a hemoglobin less than 60 g/L.11,12 These guidelines are based on expert opinion informed by several single-center observational studies,13–15 and two inconclusive randomized-controlled trials.6,16 Despite these recommendations, there remains considerable uncertainty regarding which patients might benefit and which might be harmed from blood transfusion. To address this uncertainty, we examined the relationship between transfusion and death across a range of hemoglobin levels to identify optimal thresholds for transfusing children with severe malaria in sub-Saharan Africa.
METHODS
Study design and participants
This was a multicenter prospective observational study based at six sub-Saharan African hospitals that participated in the Severe Malaria in African Children (SMAC) clinical network in the years 2000–2005: Royal Victoria Hospital (Banjul, The Gambia), Queen Elizabeth Central Hospital (Blantyre, Malawi), Albert Schweitzer Hospital (Lambaréné, Gabon), Université de Médecine et Science de la Santé (Libreville, Gabon), and Kilifi District Hospital (Kilifi, Kenya) (appendix p 15).17–19 Each site was in an area of high malaria transmission.17 The SMAC network prospectively enrolled patients with Plasmodium falciparum malaria using a standardized approach to capture clinical, laboratory, transfusion and outcome data.
The clinical protocol was specifically approved for this study by the scientific steering committees and institutional review boards at each clinical site and country, plus the sponsor (NIAID), and the coordinating center (Michigan State University). We upheld the principles outlined in the Declaration of Helsinki. Each participant’s parent or guardian provided written informed consent.
Any child under the age of 180 months admitted to one of the six study hospitals with a suspicion of malaria was screened with a Giemsa-stained thick blood smear. Those whose blood smears were positive for asexual P. falciparum parasites were enrolled in the study.
Procedures
Patients were treated with intramuscular or intravenous quinine followed by oral sulfadoxine/pyrimethamine. Transfusions were administered at the discretion of the responsible physicians who were aware of local and international guidelines. Whole blood was administered at a target volume of 20 mL per kg of bodyweight. Transfusion was recorded as a binary variable (yes/no); no data were collected on the timing, volume, or duration of the transfusion. No data were recorded on the reasons for giving or withholding transfusions. There were no pre-specified criteria for removing a patient from the study; however, both a clinical monitor and a data safety monitoring board reviewed the study annually.
Blood was obtained via venipuncture at the time of admission. Parasite density was determined from Giemsa-stained thick blood smears. In Blantyre and Kumasi, parasite density was counted per 200 WBCs and an assumption of 8,000 WBC per microliter was applied. At all other sites, parasite density was based on 100 high-powered fields. In Kilifi, the parasite count was calculated by multiplying the parasite count per 100 WBC times the WBC determined by Coulter counter (Coulter MDII). Hemoglobin was determined by Coulter counter (Coulter MDII) in Kilifi, Kumasi, Lambaréné and Libreville. Hematocrit was measured with heparinized capillary tubes using a microcentrifuge in Blantyre and Banjul. Hemoglobin was extrapolated from the hematocrit after determining the relationship between hemoglobin and hematocrit in 17,251 cases in whom both parameters were available (Hb = 0·086 + 0·32 * Hct). Lactate was measured from capillary or venous blood using portable lactate analyzers operated by centrally-trained technicians (Arkay Lactate Pro LT-1710 in Kilifi, Banjul, Blantyre, Lambaréné, and Libreville; 2300 Stat plus analyzer, YSI Corporation, USA in Kumasi).
Outcomes
The primary endpoint was death in hospital. Clinical and laboratory measures were used to define disease severity and were included as covariates in the adjusted analyses. Severe anemia was defined as a hemoglobin concentration less than or equal to 40 g/L. Impaired consciousness was defined as a Blantyre coma score of 4 or less on a scale of 0–5.2 Blood lactate was categorized as normal (less than 3·0 mmol/L), moderately elevated (3·0–4·9 mmol/L) or severely elevated (5·0 mmol/L or greater). Respiratory distress was defined as the presence of deep breathing, irregular breathing, or chest indrawing. Hypoglycemia was defined as a blood glucose of less than 2·2 mmol/L. Nutritional status was assessed by weight-for-age Z-score determined from an appropriate reference population (http://www.who.int/childgrowth/software/en). Hospital outcome was recorded as died, survived or absconded.
The exploratory endpoint was the hemoglobin level at which the association between transfusion and death was neutral, i.e., the odds ratio for death comparing transfused versus not transfused equaled one. This exploratory endpoint was examined in all patients and in subgroups with impaired consciousness or elevated blood lactate.
Statistical analysis
We hypothesized that blood transfusion would be associated with reduced odds of death from malaria. Based on a sample size of 25,893 patients and 689 deaths among the 17,380 patients who were not transfused, we estimated that we would have 80% power to detect an odds ratio of 1.2 or greater at an alpha level of 0.05 (see appendix p 15). All patients enrolled in the study were included in the analysis, except as indicated when data were missing. The baseline characteristics of children across sites were compared through Kruskal-Wallis and Wilcoxon tests when reporting medians and interquartile ranges, and Pearson chi-square or Fisher’s Exact tests when reporting proportions. Logistic regression (LR) was used to estimate the associations between blood transfusion and death and to identify factors that were associated with the clinical decision to transfuse a patient.18–20 LR models were selected using a best subset regression approach. Variables were eliminated if exclusion of the variable: i) did not change the coefficient of blood transfusion when modeling death (p of likelihood ratio test [LRT] < 0·01) and did not change the coefficients of other covariates when modeling blood transfusion; ii) did not change the c-statistic (also known as Harrell’s C)21 by more than 0·01 units; and iii) did not reduce the Akaike and Bayesian Information Criteria (AIC and BIC). The C-statistic corresponds to the area under the receiver operating characteristic curve (AUC-ROC) of the predictions of the model and is a measure of model calibration. Since there was no relevant cut-off point for parasitemia or age, the linearity of the association of each variable with the logit of death was investigated in semiparametric regression using generalized additive models (GAM). Differences across sites in the decision to transfuse blood and in the association between transfusion and death were investigated through interaction terms. Interaction terms were retained in the model if the LRT p-value was less than 0·01 and either the AIC or BIC was reduced.
To investigate optimal transfusion thresholds, we first fitted a logistic GAM with tensor splines to obtain estimates of the effect of transfusion on death (i.e., odds ratio [OR]) over continuous hemoglobin concentration. Using the degrees of freedom estimated by the GAM, a logistic model was fit with a B-spline for the hemoglobin terms (main and interaction) to estimate OR and 95% confidence intervals (CI) of death. At low hemoglobin values, we anticipated the OR of death and transfusion would be less than one, whereas at higher hemoglobin values transfusion may no longer be of benefit, and the OR of death and transfusion would be greater than one. The hemoglobin concentration at which the OR of death and transfusion equals one would be a rational upper limit for the transfusion threshold. The CIs for hemoglobin corresponding to the threshold were estimated through bootstrap. The same GAM and logistic models with B-spline terms were used to examine how individual factors (impaired consciousness, hyperlactatemia, hypoglycemia, respiratory distress, and age) changed the OR of death comparing transfused versus not transfused individuals over a continuous hemoglobin. In all site-specific analyses, confidence intervals across sites within predictors were adjusted for multiple testing using Holms’ approach. Analyses were conducted in the R Computing Environment (mgcv and boot packages, R v3.3)22 and Stata 15 (StataCorp. 2009).
RESULTS
A total of 25,893 patients were admitted to a study hospital with a positive blood smear and enrolled in the study from December 19, 2000 until March 8, 2005 (Figure 1). Patients were followed in-hospital for a median (IQR) of 2 (1–4) days. Clinical features associated with disease severity varied across sites (Table 1 and appendix pp 3–8). Hyperlactatemia was the most common severe feature on admission and was present in 6,456 (26·2%) of 24,635, followed by impaired consciousness in 5,155 (19·7%) of 26,094, respiratory distress in 4,670 (17·9%) of 26,080, severe anemia in 2,803 (10·4%) of 26,022, and hypoglycemia in 1,094 (4·4%) of 24,566.
Figure 1.
Flow diagram of patient enrollment and eligibility for analysis.
Table 1.
Clinical and demographic characteristics of the study population*.
| Banjul | Blantyre | Kilifi | Kumasi | Lambaréné | Libreville | |
|---|---|---|---|---|---|---|
|
| ||||||
| N = 3,318 | N = 5,358 | N = 6,846 | N = 6,925 | N = 1,784 | N = 1,662 | |
| Age, median (Q1, Q3), months | 32 (18, 51) | 26 (14, 46) | 26 (14, 43) | 24 (12, 42) | 28 (16, 56) | 25 (15, 42) |
| Sex, N (%) Female | 1,617 (48) | 2,493 (46) | 3,188 (46) | 3,113 (45) | 841 (47) | 793 (47 |
| Weight, median (Q1, Q3), kg | 11·0 (9·0, 15·0) | 10·2 (8·4, 13·5) | 9·9 (8·0, 12·6) | 10·0 (8·0, 13·5) | 11·0 (9·0, 15·0) | 11·0 (9·0, 14·0) |
| Weight-for-age, median (Q1, Q3), Z-score | −1·36 (−2·30, −0·50) | −1·22 (−2·19, −0·35) | −1·7 (−2·56, −0·86) | −1·15 (−2·04, −0·35) | −1·01 (−1·84, −0·19) | −0·08 (−1·70, 0·05) |
| Temperature, median (Q1, Q3), °C | 37·7 (37·1, 38·6) | 38·5 (37·8, 39·2) | 38·3 (37·3, 39·2) | 37·8 (37·0, 38·6) | 38·5 (37·7, 39·5) | 38·6 (37·9, 39·5) |
| Respirations, median (Q1, Q3), min −1 | 34 (28, 44) | 38 (36, 44) | 36 (30, 46) | 42 (36, 54) | 40 (32, 48) | 42 (36, 52) |
| Respiratory Distress † , N (%) | 692 (20) | 473 (9) | 1,563 (23) | 1,631 (24) | 99 (6) | 212 (12·5) |
| Haemoglobin, median (Q1, Q3), g/L | 63 (45, 86) | 88 (68, 103) | 80 (61, 96) | 62 (47, 85) | 77 (58, 95) | 62 (46, 84) |
| Severe anaemia ‡ , N (%) | 640 (19) | 282 (5) | 489 (7) | 999 (14) | 131 (7) | 262 (15·4) |
| Glucose, median (Q1, Q3), mmol/L | 6·4 (4·8, 8·0) | 5·3 (4·4, 6·2) | 5·2 (4·2, 6·3) | 5·1 (4·2, 6·1) | 4·1 (3·1, 5·1) | 5·3 (4·3, 6·4) |
| Hypoglycemia § , N (%) | 126 (6) | 134 (3) | 322 (5) | 295 (4) | 164 (10) | 53 (3) |
| Parasitaemia, median (Q1, Q3), ul−1 ** | 19,500 (1,500–78,000) | 83,778 (33,333–189,111) | 39,330 (3,140–215,800) | 65,877 (14,281–241,152) | 51,000 (9,000–200,000) | 71,500 (15,500–220,000) |
| Blantyre Coma Score, N (%) | ||||||
| 5 | 2,587 (76) | 4,809 (90) | 5,539 (80) | 5,289(76) | 1,587 (89) | 1,128 (67) |
| 4 | 248 (7) | 113 (2) | 468 (7) | 464 (7) | 55 (3) | 219 (13) |
| 3 | 275 (8) | 147 (3) | 187 (3) | 350 (5) | 56 (3) | 155 (9) |
| 2 | 184 (5) | 128 (2) | 364 (5) | 422 (6) | 64 (4) | 127 (8) |
| 1 | 70 (2) | 85 (2) | 172 (3) | 238 (3) | 27 (2) | 51 (3) |
| 0 | 31 (1) | 81 (2) | 179 (3) | 178 (3) | 1 (0·1) | 16 (1) |
| Impaired consciousness ‖ , N (%) | 808 (24) | 554 (10) | 1,370 (20) | 1,652 (24) | 203 (11) | 568 (33) |
| Lactate, median (Q1, Q3), mmol/L | 3·9 (2·4,7·0) | 3·6 (2·2,6·0) | 2·3 (1·6,3·5) | 3·2 (2·1,5·2) | 3·8 (2·4,5·3) | 3·8 (2·4,5·8) |
| Lactate, N (%) | ||||||
| < 3·0 mmol/L | 990 (35) | 2,108 (40) | 4,517 (67) | 3,104 (45) | 495 (35) | 503 (35) |
| 3·0 – 4·9 mmol/L | 768 (27) | 1,400 (27) | 1,351 (20) | 1,954 (28) | 520 (37) | 469 (33) |
| ≥ 5·0 mmol/L | 1,106 (39) | 1,737 (33) | 866 (13) | 1,886 (27) | 408 (29) | 453 (32) |
| Transfused ¶ , N (%) | 1,526 (46) | 813 (15) | 1,029 (15) | 3,934 (57) | 382 (21) | 829 (50) |
| Outcome | ||||||
| Survived, N | 3,012 | 5,148 | 6,640 | 6,596 | 1,759 | 1,599 |
| Died, N (%) | 316 (7) | 133 (3) | 232 (3) | 313 (5) | 24 (1) | 83 (5) |
| Absconded, N | 24 | 82 | 30 | 35 | 9 | 18 |
Variables were compared across sites using Kruskal-Wallis test for continuous variables, and chi-squared for categorical variables. All variables differed significantly between sites (P< 0·0001), except sex (P = 0·12).
Respiratory distress: presence of deep breathing, irregular breathing, or chest indrawing.
Severe anemia: hemoglobin less than or equal to 40 g/L.
Hypoglycaemia: blood glucose less than 2·2 mmol/L
Impaired consciousness: Blantyre Coma Score less than or equal to 4.
Baseline data separated by transfusion status is available in Table S1.
In Blantyre and Kumasi, parasite density was counted per 200 WBCs and an assumption of 8,000 WBC per microliter was applied. In Kilifi, the parasite count was calculated by multiplying the parasite count per 100 WBC times the WCC determined by Coulter counter (Coulter MDII). At all other sites, parasite density was based on reading 100 high-powered fields. Complete blood counts were not available. This approach may have introduced variability in the estimation of parasite density that may have varied by site.
The percentage of missing values for each variable were as follows: age (0%), sex (0%), weight (0%), temperature (0·2%), respirations (0·4%), respiratory distress (0·1%), haemoglobin (0·3%), severe anaemia (0·3%), glucose (5·9%), hypoglycaemia (5·9%), parasitaemia (0%), Blantyre coma score (0·1%), Impaired consciousness (0·1%), lactate (5·6%), transfused (0·8%), outcome (0·2%).
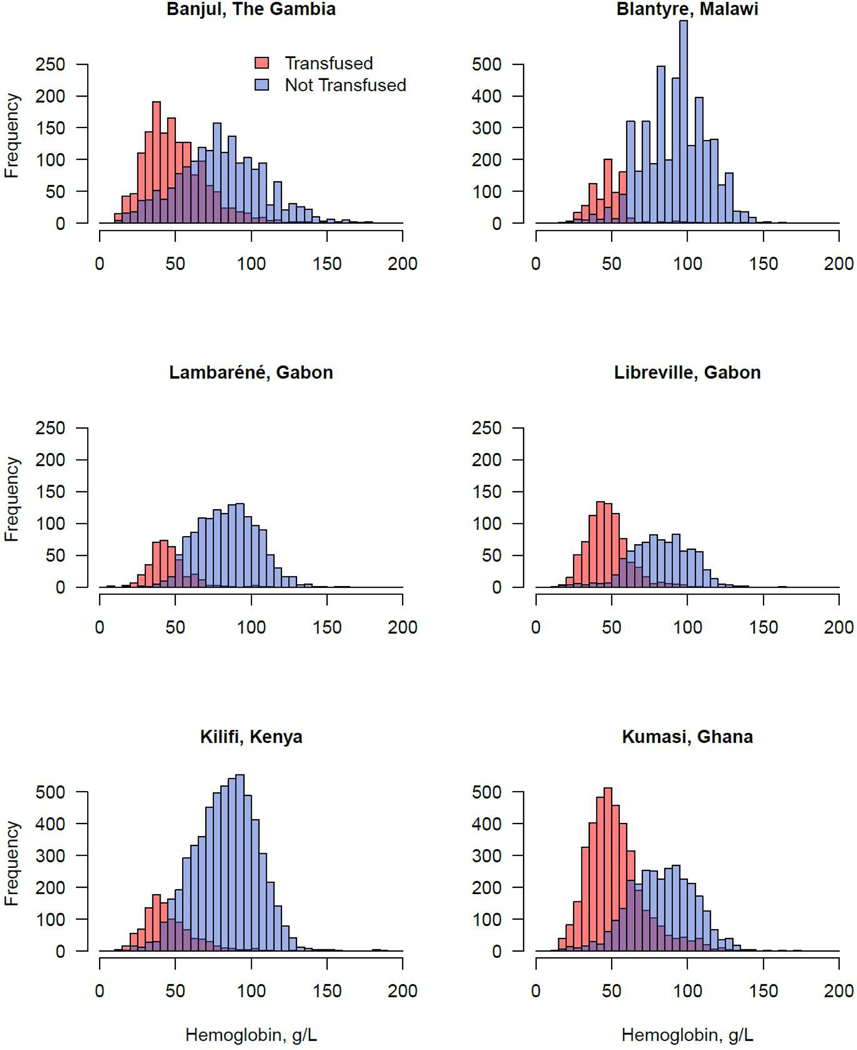
At least one blood transfusion was administered to 8,513 (32·8%) of 25,893 children. This included 4,707 patients for whom transfusion was indicated by the WHO guidelines and 3,806 patients for whom transfusion was administered although not indicated by WHO guidelines. There were 1,078 patients who met criteria for transfusion but were not transfused. The distribution of hemoglobin values among transfused and non-transfused children at each study site are provided in Figure 2. Transfusion frequencies varied across sites (P<0·0001). In Kumasi, 3,934 (57%) of 6,925 were transfused, followed by 829 (50%) of 1,662 in Libreville, 1,526 (46%) of 3,318 in Banjul, 382 (21%) of 1,784 in Lambaréné, 813 (15%) of 5,358 in Blantyre, and 1,029 (15%) of 6,846 in Kilifi.
Figure 2. Distribution of hemoglobin concentrations among transfused and not transfused patients at each hospital.
Red, transfused; Blue, not transfused; Purple indicates superimposition of transfused and not transfused distributions.
The odds of receiving a blood transfusion depended on study site and disease severity (appendix pp 9–13). In site-and severity-adjusted analyses, the following clinical features were associated with greater odds of receiving a blood transfusion: severe anemia, elevated lactate concentration, respiratory distress, and parasite density. Conversely, hypoglycemia, impaired consciousness, elevated temperature, and greater weight-for-age Z-score were associated with lower odds of receiving a transfusion.
To understand the association between transfusion and death, we calculated ORs for death of transfused versus not transfused patients and adjusted for site and disease severity (Table 2 and appendix p 14). In logistic regression analysis adjusted for site only, transfusion was associated with decreased odds of death (OR = 0·82 [95% CI 0·71–0·94], C-statistic = 0·64). After adjustment for site and clinical measures of disease severity (impaired consciousness, lactate, respiratory distress, hypoglycemia, age, severe anemia and parasite density) transfusion was associated with a further reduced odds of death (OR = 0·50 [95% CI 0·42–0·60], C-statistic = 0·85).
Table 2.
Association of blood transfusion with death in site- and severity-adjusted analyses.
| OR | (95% CI) | LRT P* | |
|---|---|---|---|
|
| |||
| Site-Only Adjusted Analysis (N = 25,738) † | |||
| Transfusion | 0·82 | (0·71, 0·94) | p = 0·0030 |
| Study site | < 0·0001 | ||
| c-statistic of the fitted model = 0·64 | |||
|
| |||
| Site and Severity Adjusted Analysis (N = 22,986) ‡ | |||
| Transfusion | 0·50 | (0·42, 0·60) | < 0·0001 |
| Age § | 1·03 | (1·01, 1·04) | < 0·0001 |
| Temperature, °C | 0·83 | (0·78, 0·88) | < 0·0001 |
| Parasite density, ln (parasites/μl of blood) | 0·78 | (0·73, 0·84) | < 0·0001 |
| Lactate, mmol/L | < 0·0001 | ||
| < 3·0 | — | — | |
| 3·0 – 4·9 | 1·43 | (1·14, 1·78) | |
| ≥ 5·0 | 3·22 | (2·64, 3·93) | |
| Severe anaemia | 1·28 | (1·04, 1·57) | 0·028 |
| Impaired consciousness | 4·00 | (3·43, 4·68) | < 0·0001 |
| Respiratory distress | 3·63 | (3·10, 4·27) | < 0·0001 |
| Hypoglycaemia | 3·44 | (2·82, 4·20) | < 0·0001 |
| Study site | < 0·0001 c-statistic of the fitted model = 0·85 | ||
OR, odds ratio; LRT, likelihood ratio test; CI, confidence interval; BCS, Blantyre comma score; c-statistics, area under the receiver operating characteristic curve of the predicted proportions by the model.
P-value of the likelihood ratio test comparing the reduced model (with the corresponding predictor deleted from the model) with the full model.
The crude odds ratio (95% CI) for death and blood transfusion in analysis ignoring study site and including all 25,738 children with information for transfusion and death was 1·09 (0·96, 1·23; P = 0·20). The odds ratio (95% CI) for blood transfusion in analysis adjusting only for study site when analyzing only the 22,986 children with information available for all covariates in the adjusted model was 0·86 (0·75, 1·01; P = 0·07).
Sex was not statistically significant in crude analysis, analysis adjusting by site, analysis including transfusion, and in any of the two selected final models. Exclusion of sex did not affect the OR of transfusion, did not increase the AUC-ROC, and did not decrease the AIC and BIC. In the adjusted model of this table, the OR (95% CI) for male (vs. female) sex and death was 0·96 (0·83, 1·10; P = 0·54). Fully adjusted OR (95% CI) for death by site using Banjul as a reference site: Blantyre, Malawi 0·33 (0·25, 0·43); Lambaréné, Gabon 0·57 (0·41, 0·78); Libreville, Gabon 0·15 (0·08, 0·25); Kilifi, Kenya 0·33 (0·26, 0·42); Kumasi, Ghana 0·43 (0·35, 0·54).
The OR for age is per 6 months change.
To examine the geographic heterogeneity of the association between transfusion and death, we performed an analysis stratified by site and adjusted for disease severity (appendix p 2). After adjustment for disease severity, blood transfusion was associated with decreased odds of death in Banjul, Blantyre, Kumasi, and Libreville, was neutral in Lambaréné and was associated with marginally increased odds of death in Kilifi.
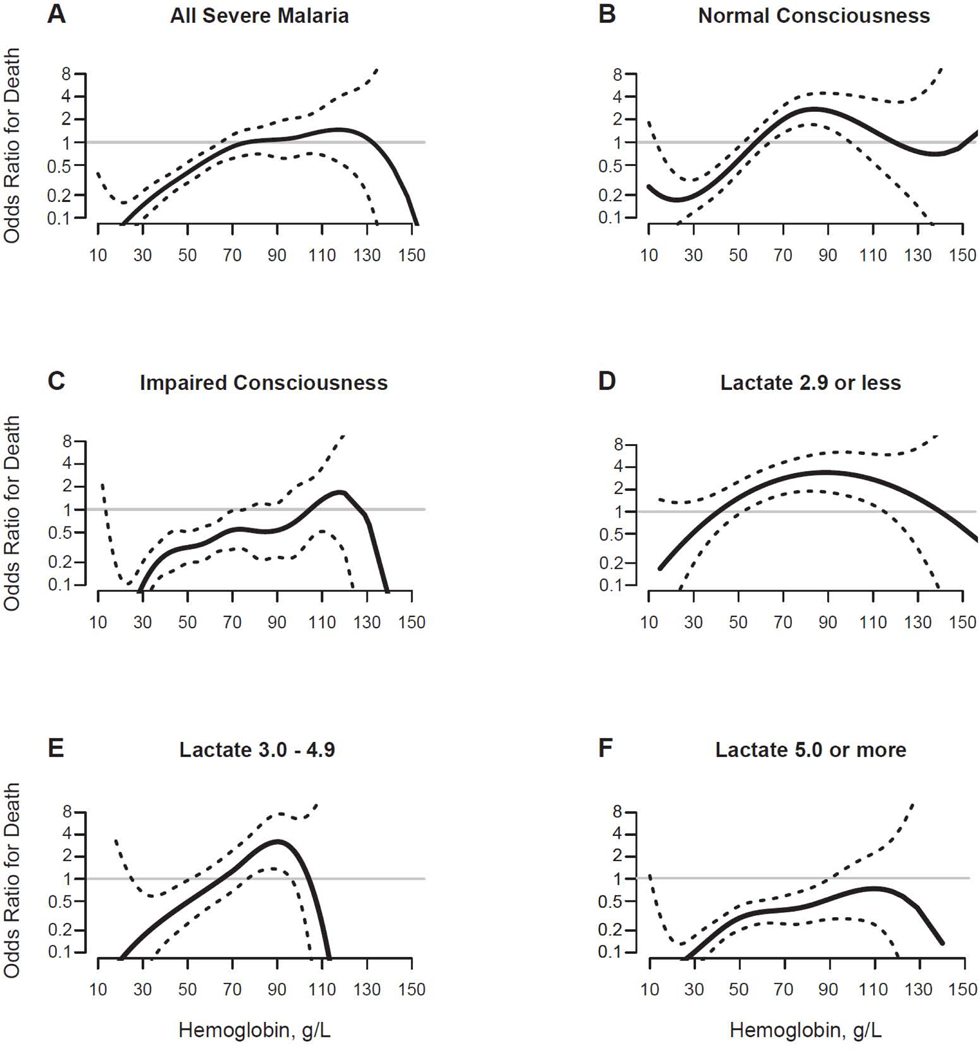
We built generalized additive models to estimate the odds ratio of death and transfusion across a continuous range of hemoglobin values (Figure 2), adjusting for site and malaria severity. The hemoglobin value where transfusion was associated with an OR of death of 1·0 was 77 g/L (95% CI 65–110; Figure 2A). Transfusion was associated with improved outcome when the hemoglobin concentration was less than 77 (95% CI: 65–110) g/L among African children under age 15 years admitted to hospital with P falciparum infection.
Next, we assessed the effect of transfusion on death among children with normal or impaired consciousness, while adjusting for all other covariates (Figures 2B–C). Among those with normal consciousness, transfusion was associated with improved outcome when the hemoglobin was less than 58 (95% CI: 52–66) g/L. Transfusion of children with normal consciousness was associated with worse outcome when the hemoglobin was greater than 58 g/L. In contrast, among children with impaired consciousness, transfusion was associated with improved outcome when the hemoglobin was up to 105 (95% CI: 71–115) g/L. Few transfusions were administered to children whose hemoglobin was greater than 105 g/L, so we were unable to determine whether transfusion at higher hemoglobin levels was associated with adverse outcomes among children with impaired consciousness. Identifying impaired consciousness raised the hemoglobin threshold below which transfusion was associated with improved outcome.
Finally, we assessed the effect of transfusion on death stratified by lactate levels (Figure 2D–F). Among children with normal blood lactate (2·9 mmol/L or less), transfusion was associated with improved outcome when the hemoglobin was less than 42 (95% CI: 28–54) g/L, and associated with worse outcome when the hemoglobin was greater than 42 (95% CI: 28–54) g/L. Among children with moderately elevated blood lactate (3·0 to 4·9 mmol/L), transfusion was associated with improved outcome when the hemoglobin was less than 66 (95% CI: 52–79) g/L, and with worse outcome when the hemoglobin was greater than 66 (95% CI: 52–79) g/L. When the blood lactate was severely elevated (5·0 mmol/L or more), transfusion was associated with improved outcome across a wide range of hemoglobin concentrations. While the OR of death remained less than one, the upper 95% confidence interval of the model crossed one at a hemoglobin of 90 g/L making it hard to exclude a potentially harmful effect of transfusion at higher hemoglobin levels among patients with lactate greater than 5 mmol/L. Overall, an elevated lactate level raised the hemoglobin threshold below which transfusion was associated with improved outcome.
DISCUSSION
We have examined the impact of transfusion on in-hospital mortality among 25,893 children with falciparum malaria who were admitted to hospitals participating in the Severe Malaria in African Children network. Importantly, this study captured transfusion practice for children hospitalized with severe malaria across different countries where adherence to international transfusion guidelines varied, thereby allowing us to compare differences in outcome across a broad range of hemoglobin values. The findings of this study support the current recommendation to administer blood to a child with severe malaria when the hemoglobin is less than 40 g/L while providing compelling new evidence that higher transfusion thresholds may be necessary in a child who has clinical signs or laboratory evidence of impaired perfusion of vital organs.
In light of these results, how should the current recommendations be modified, if at all? These data from a multi-center, prospective observational study suggest that transfusion should be considered for children who meet the WHO definitions of complicated or severe malaria when the hemoglobin level is less than 77 g/L. This transfusion threshold can be further refined based on a child’s level of consciousness. If the child has normal consciousness, transfusion should be considered when the hemoglobin is less than 58 g/L; however, if the child has impaired consciousness, transfusion should be considered when the hemoglobin level is up to 105 g/L. The blood lactate level may be even more helpful in distinguishing patients who could be harmed from those who could be helped by transfusion: if the lactate is normal (less than 3 mmol/L) transfusion should be considered when the hemoglobin is less than 42 g/L; if the lactate is moderately elevated (3–5 mmol/L) transfusion should be considered when the hemoglobin is less than 66 g/L; and if the lactate is severely elevated (greater than 5 mmol/L), transfusion should be considered regardless of the hemoglobin level. Thus, consideration of a child’s blood lactate level along with the hemoglobin level could lead to more efficient use of limited blood resources by prioritizing transfusion for those most likely to benefit and avoiding transfusion in those who could be harmed.
In the future, survival might be improved by better adherence to current guidelines, i.e., by ensuring more children who have hyperlactatemia or impaired consciousness can receive transfusion. However, this prospective observational study highlights the potential benefit of whole blood transfusion when the hemoglobin is higher than the current guideline of 60 g/L -- especially in patients who present with impaired consciousness or hyperlactatemia. These data are of immediate relevance to current transfusion practice in children with severe malaria and may also inform the design of controlled trials of transfusion thresholds for children with severe malaria in sub-Saharan Africa.
This study has limitations which may affect the generalisability of our findings. The study was not designed to evaluate the association between transfusion and mortality and so the time elapsed from admission to transfusion, the precise volume and rate of blood transfusion, and the post-transfusion hemoglobin level were not recorded. These factors could have affected the risks and benefits of transfusion; however, a recent trial of blood transfusion in African children showed that neither immediate transfusion nor increased volume changed the outcome.23,24 Some children may have died before transfusion could be administered, which would make transfusion appear protective. When we excluded participants who died in the first four hours, the protective association of transfusion against death remained significant. In the years since the data were collected, the first-line treatment for severe malaria has changed from quinine to artesunate, and survival has improved.5 We did not have data on bacterial co-infection, which could have modified the response to transfusion. Most study participants were under the age of 60 months, and so the findings might not be applicable to older children or adolescents. The study focused on the endpoint of in-hospital survival, which would capture the immediate life-threatening harms of blood transfusion such as volume overload, transfusion associated acute lung injury, hemolytic transfusion reaction and transmission of bacterial infection. However, the hospital-based study design did not allow us to capture potential longer-term risks of blood transfusion such as viral infection transmission or allo-immunization.25 The level of adherence to WHO transfusion guidelines was approximately 81% in our study, similar to other studies.27 While adherence to WHO transfusion guidelines varied between sites, it also provided the contrasts with which to evaluate the associations of transfusion with outcome in a real-world observational setting.
There are few studies that describe the association of transfusion with outcome in children with malaria and how the outcome might differ across sites in sub-Saharan Africa. A recent analysis concluded that anemia was not associated with increased mortality and that transfusion was not associated with improved survival from severe malaria; however, that study pooled adults and children, and lactate levels were not available on most participants.28 In contrast, the weighted, pooled analysis presented here confirmed a strong association between transfusion and lower mortality across all six sites (OR = 0·50; 95% CI: 0·42–0·60; P < 0·0001). However, our site-specific severity-adjusted analyses revealed that transfusion was neutral in Lambarene, Gabon and associated with higher mortality in Kilifi, Kenya. Our findings in Kilifi are consistent with a separate study in Kilifi over the years 2002–2009 showing no benefit of transfusion in children with malaria25, and a study in Eastern Kenya that estimated transfusion was only beneficial in children with severe malaria and respiratory distress when the hemoglobin was less than 47 g/L.13 In the multi-center study reported here, the clinical observation of respiratory distress was independently associated with death, but it did not effectively identify a subgroup of patients who responded well to transfusion. Therefore, it is unlikely that respiratory distress could be used in place of lactate to identify those who would respond favorably to transfusion. Overall, the heterogeneity of the association between transfusion and death across multiple sub-Saharan African hospitals described here implies that region-specific transfusion guidelines may need to be developed to provide optimal supportive care for children hospitalized with severe malaria.
We must emphasize that the blood product administered in this study was that which was most widely available: whole blood. The apparent benefits of whole blood transfusion might be conferred by mechanisms beyond a simple increase in oxygen carrying capacity provided by red blood cells. Other components of whole blood could potentially affect the course of disease, including protective antibodies29,30, platelets31, and plasma proteins involved in regulating coagulation and scavenging of cell-free hemoglobin.32,33 Future studies should consider not only the availability of whole blood but its biological activities as well.
Ideally, robust evidence for the benefits and harms of higher blood transfusion thresholds in African children with severe malaria would be defined by randomized controlled trials.34,35 For now, the available data from this large, multicenter prospective observational study emphasizes the importance of Blantyre coma score and lactate level in the decision to transfuse. Among pediatric malaria patients with impaired consciousness or hyperlactatemia, a higher transfusion threshold (90 g/L) would need to be compared against the current threshold (60 g/L) in a randomized controlled trial before changes are made to the current guidelines.
Supplementary Material
Figure 3. Odds Ratio for Death Comparing Transfused versus Not Transfused Patients across a Continuous Hemoglobin Concentration with Adjustment for Site and Disease Severity.
Generalized Additive Models of the odds ratio for death were adjusted for study site, hypoglycemia, respiratory distress, impaired consciousness, parasite density, age and temperature. Data are shown for all severe malaria patients (A), and then stratified by normal consciousness (B), impaired consciousness (C), and lactate levels (D-F). In each stratification the stratum variable is not used for adjusting. The upper and lower 95% confidence intervals of the odds ratio for death are indicated by dotted lines. The 95% confidence interval of the hemoglobin concentration at which the odds ratio equals 1 was determined by a bootstrap procedure. All Severe Malaria: 77 (65,110) g/L; Normal Consciousness: 58 (52, 66) g/L; Impaired Consciousness: 105 (71,115) g/L; Lactate 2·9 mmol/L or less: 42 (28, 54) g/L; Lactate 3·0–4·9 mmol/L: 66 (52, 79) g/L; and Lactate 5·0 mmol/L or more: odds ratio model remained less than 1, upper 95% CI crosses 1 at a hemoglobin of 90 g/L.
RESEARCH IN CONTEXT.
Evidence before this study
Blood transfusion is a widely used supportive measure in the treatment of severe malaria; however, it is unclear who might benefit and who might be harmed by blood transfusion. In malaria-endemic areas, the World Health Organization recommends transfusing screened, compatible blood when a child with malaria has a haemoglobin concentration less than 40 g/L or less than 60 g/L when complicated by acidosis, impaired consciousness, shock or hyperparasitaemia. These malaria-specific transfusion thresholds are lower than those for critically ill children in developed countries and yet have never been evaluated in a randomized controlled trial. While observational studies have generally supported a transfusion threshold of 40 g/L for children hospitalized with severe malaria in sub-Saharan Africa, questions remain about what threshold to apply when children present with severe complications such as impaired consciousness or elevated blood lactate.
Added value of this study
These data reveal that among children admitted to hospital for treatment of severe malaria, blood transfusion was associated with improved survival. Among those with impaired consciousness or elevated blood lactate, transfusion was associated with improved survival even at haemoglobin levels well above the currently recommended threshold.
Implications of all the available evidence
This analysis provides evidence to guide transfusion practice for African children with severe malaria and identifies the need to evaluate higher transfusion thresholds for those with impaired consciousness or elevated blood lactate in future randomised trials.
Acknowledgements
The clinical study was supported by a grant from the US National Institutes of Allergy and Infectious Diseases (AI45955). Hans Ackerman was supported by the Intramural Research Programs of the National Institutes of Allergy and Infectious Diseases and the National Heart, Lung, and Blood Institute. David Roberts was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre Programme at Oxford, NIHR Programme Grant NIHR-RP-PG-0310-1004 and research funding from NHS Blood and Transplant (UK). Climent Casals-Pascual was supported by the Medical Research Council, UK (Clinician Scientist Fellowship: G0701885) and the NIHR Oxford Biomedical Research Council (AC14/065).The funding sources did not play a role in the design, conduct, analysis or writing up of this study. The corresponding authors had full access to the data and made the decision to submit for publication.
Footnotes
Data sharing statement
The full data and data dictionary have been deposited here: https://doi.org/10.7910/DVN/0CTWUJ
Access to the data file is restricted as it contains sensitive information on participants. For more detailed information beyond the metadata and study documentation provided, there is a process of managed access requiring submission of a request form for consideration by our Data Governance Committee.
Declaration of interests
The authors each report no potential competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.World malaria report 2019. https://www.who.int/publications-detail/world-malaria-report-2019 (accessed Feb 11, 2020).
- 2.Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q J Med 1989; 71: 441–59. [PubMed] [Google Scholar]
- 3.Marsh K, Forster D, Waruiru C, et al. Indicators of life-threatening malaria in African children. N Engl J Med 1995; 332: 1399–404. [DOI] [PubMed] [Google Scholar]
- 4.Jallow M, Casals-Pascual C, Ackerman H, et al. Clinical features of severe malaria associated with death: a 13-year observational study in the Gambia. PloS One 2012; 7: e45645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dondorp AM, Fanello CI, Hendriksen ICE, et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet Lond Engl 2010; 376: 1647–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bojang KA, Van Hensbroek MB, Palmer A, Banya WA, Jaffar S, Greenwood BM. Predictors of mortality in Gambian children with severe malaria anaemia. Ann Trop Paediatr 1997; 17: 355–9. [DOI] [PubMed] [Google Scholar]
- 7.White NJ, Miller KD, Marsh K, et al. Hypoglycaemia in African children with severe malaria. Lancet Lond Engl 1987; 1: 708–11. [DOI] [PubMed] [Google Scholar]
- 8.Taylor TE, Molyneux ME, Wirima JJ, Fletcher KA, Morris K. Blood glucose levels in Malawian children before and during the administration of intravenous quinine for severe falciparum malaria. N Engl J Med 1988; 319: 1040–7. [DOI] [PubMed] [Google Scholar]
- 9.Gwer SA, Idro RI, Fegan G, et al. Fosphenytoin for seizure prevention in childhood coma in Africa: a randomized clinical trial. J Crit Care 2013; 28: 1086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crawley J, Waruiru C, Mithwani S, et al. Effect of phenobarbital on seizure frequency and mortality in childhood cerebral malaria: a randomised, controlled intervention study. Lancet Lond Engl 2000; 355: 701–6. [DOI] [PubMed] [Google Scholar]
- 11.WHO | Management of severe malaria – A practical handbook. Third edition. WHO. http://www.who.int/malaria/publications/atoz/9789241548526/en/ (accessed Feb 11, 2020). [Google Scholar]
- 12.Pocket Book of Hospital Care for Children: Guidelines for the Management of Common Childhood Illnesses, 2nd edn. Geneva: World Health Organization, 2013. http://www.ncbi.nlm.nih.gov/books/NBK154447/ (accessed Feb 11, 2020). [PubMed] [Google Scholar]
- 13.Lackritz EM, Campbell CC, Ruebush TK, et al. Effect of blood transfusion on survival among children in a Kenyan hospital. Lancet Lond Engl 1992; 340: 524–8. [DOI] [PubMed] [Google Scholar]
- 14.English M, Ahmed M, Ngando C, Berkley J, Ross A. Blood transfusion for severe anaemia in children in a Kenyan hospital. Lancet Lond Engl 2002; 359: 494–5. [DOI] [PubMed] [Google Scholar]
- 15.Brewster DR. Blood transfusions for severe anaemia in African children. Lancet Lond Engl 1992; 340: 917. [PubMed] [Google Scholar]
- 16.Holzer BR, Egger M, Teuscher T, Koch S, Mboya DM, Smith GD. Childhood anemia in Africa: to transfuse or not transfuse? Acta Trop 1993; 55: 47–51. [DOI] [PubMed] [Google Scholar]
- 17.Hay SI, Snow RW. The malaria Atlas Project: developing global maps of malaria risk. PLoS Med 2006; 3: e473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996; 49: 1373–9. [DOI] [PubMed] [Google Scholar]
- 19.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003; 326: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosmer D, Lemeshow S. Assessing the Fit of the Model. In: Applied Logistic Regression. John Wiley & Sons, Ltd, 2005: 143–202. [Google Scholar]
- 21.Harrell F Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis, 2nd edn. Springer International Publishing, 2015. DOI: 10.1007/978-3-319-19425-7. [DOI] [Google Scholar]
- 22.R Development Core Team. a language and environment for statistical computing: reference index. Vienna: R Foundation for Statistical Computing, 2010. http://www.polsci.wvu.edu/duval/PS603/Notes/R/fullrefman.pdf (accessed Feb 11, 2020). [Google Scholar]
- 23.Maitland K, Olupot-Olupot P, Walker AS. Transfusion Timing and Volume in African Children with Severe Anemia. Reply. N Engl J Med 2019; 381: 1687–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maitland K, Kiguli S, Olupot-Olupot P, et al. Immediate Transfusion in African Children with Uncomplicated Severe Anemia. N Engl J Med 2019; 381: 407–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jayaraman S, Chalabi Z, Perel P, Guerriero C, Roberts I. The risk of transfusion-transmitted infections in sub-Saharan Africa. Transfusion (Paris) 2010; 50: 433–42. [DOI] [PubMed] [Google Scholar]
- 26.Maitland K, Olupot-Olupot P, Kiguli S, et al. Transfusion Volume for Children with Severe Anemia in Africa. N Engl J Med 2019; 381: 420–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maitland K, Ohuma EO, Mpoya A, Uyoga S, Hassall O, Williams TN. Informing thresholds for paediatric transfusion in Africa: the need for a trial. Wellcome Open Res 2019; 4: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leopold SJ, Watson JA, Jeeyapant A, et al. Investigating causal pathways in severe falciparum malaria: A pooled retrospective analysis of clinical studies. PLOS Med 2019; 16: e1002858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen S, McGREGOR IA, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature 1961; 192: 733–7. [DOI] [PubMed] [Google Scholar]
- 30.Sabchareon A, Burnouf T, Ouattara D, et al. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am J Trop Med Hyg 1991; 45: 297–308. [DOI] [PubMed] [Google Scholar]
- 31.McMorran BJ, Wieczorski L, Drysdale KE, et al. Platelet factor 4 and Duffy antigen required for platelet killing of Plasmodium falciparum. Science 2012; 338: 1348–51. [DOI] [PubMed] [Google Scholar]
- 32.Elphinstone RE, Riley F, Lin T, et al. Dysregulation of the haem-haemopexin axis is associated with severe malaria in a case-control study of Ugandan children. Malar J 2015; 14: 511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeo TW, Lampah DA, Tjitra E, et al. Relationship of cell-free hemoglobin to impaired endothelial nitric oxide bioavailability and perfusion in severe falciparum malaria. J Infect Dis 2009; 200: 1522–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carson JL, Carless PA, Hebert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev 2012; : CD002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holst LB, Petersen MW, Haase N, Perner A, Wetterslev J. Restrictive versus liberal transfusion strategy for red blood cell transfusion: systematic review of randomised trials with meta-analysis and trial sequential analysis. BMJ 2015; 350: h1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.